Abstract
Objectives
The concurrent influence of depressive symptoms, medical conditions, and disabilities in activities of daily living (ADL) upon the rates of decline in older Americans’ cognitive function is unknown.
Design
This study examined a national sample of 6,476 adults born before 1924 to determine differences in cognitive function trajectories by prevalence and incidence of depressive symptoms, chronic diseases, and ADL disabilities. Cognitive performance was tested 5 times between 1993 and 2002 with a multifaceted inventory that we examined as a global measure (range: 0–35, standard deviation (SD) 6.00) and with word recall (range: 0–20, SD 3.84) analyzed separately.
Results
Baseline prevalence of depressive symptoms, stroke, and ADL limitations were each independently and strongly associated with lower baseline cognition scores, but did not predict future cognitive decline. Each incident depressive symptom was independently associated with 0.06 point reduction (95% confidence interval (CI):0.02–0.10) in recall score, incident stroke with 0.59 point reduction in total score (95% CI:0.20–0.98), each new basic ADL limitation with 0.07 point (95% CI:0.01–0.14) reduction in recall score and 0.16 point reduction in total score (95% CI:0.07–0.25), and each incident instrumental ADL limitation with 0.20 point reduction in recall score (95% CI:0.10–0.30) and 0.52 point reduction in total score (95% CI:0.37–0.67).
Conclusion
Prevalent and incident depressive symptoms, stroke, and ADL disabilities contribute independently to reductions in cognitive functioning in older Americans, but do not appear to influence rates of future cognitive decline. Therefore, prevention, early identification, and aggressive treatment of these conditions may ameliorate the burdens of cognitive impairment.
Keywords: depression, cognitive decline, physical health, physical disabilities
INTRODUCTION
Cognitive decline presents an immense burden on older adults, their families, and society. Although mild decrements in cognitive functioning are associated with aging,1–2 the rates of cognitive decline are quite heterogeneous;3,4 thus identification of modifiable factors that hasten decline is a research imperative.
Depression has long been postulated to be one such factor, as cognitive impairment that co-exists with depressive symptoms can improve with symptom resolution.5 However, whether depressive symptoms affect the rate of cognitive decline is unknown; some studies suggest an associated hastening of decline,6–9 while others do not.10–13 Depressive symptoms may hamper cognitive test performance by lowering effort but it remains unclear if rates of cognitive decline are affected.
Chronic diseases and physical limitations may impact rates of cognitive decline in addition to confounding observed associations between depressive symptoms and cognitive functioning. Medical conditions, such as diabetes, stroke, and cardiovascular disease, are causally related to neuronal loss, thus contributing to cognitive decline.14 Yet, in cross-sectional studies, medical conditions account for only a fraction of population variance in cognition scores: diabetes and stroke together explain less than 1% of test score variance15,16 and the four leading medical conditions together explain 1.6% or less.17 Physical disabilities may hasten cognitive decline;18 however, the independent effects of depression, physical disabilities, and chronic diseases upon rates of cognitive decline have not been quantified.
Accordingly, the objective of this study was to determine if, and how much, depression, medical conditions, and physical disabilities contribute to the trajectories of cognitive functioning in older Americans, using a well characterized, nationally representative cohort, serially tested for cognitive performance over nine years.
METHODS
This study used data from The Study of Assets and Health Dynamics Among the Oldest Old (AHEAD), a national United States 1993 survey based on a probability sample of non-institutionalized persons born in 1923 or before.19 Subjects were selected using a multistage area probability design and a dual-frame sample of Medicare recipients. The overall 80 percent response rate yielded a sample of 8,222 individuals from 6,047 households. Sampling weights reflect the probability of selection including the over-sampling of African Americans, Hispanics, and residents of Florida and ensure nationally representative non-institutionalized persons 70 years or older living in the US in 1993. Follow up assessments were made in 1995, 1998, 2000, and 2002.
The Study Sample
The analytic sample consisted of all AHEAD participants who were at least 69 years and 10 months and had non-proxy interviews and valid cognition and depression data at the time of baseline assessment. Of 6,656 age-eligible participants, over 97 percent (6,474) had baseline usable cognition scores (missing no more than one cognition subscale) and depression data. Of those, over 80 percent (5,271) had cognition scores for at least 2 visits and 2,353 participants (36.4%) had cognition scores at all 5 visits. The majority of missing follow-up cognition scores were due to interim deaths (2, 503 deaths over the study period, 557 by first follow up); of those who were alive for testing, 89% had cognition scores for at least 2 visits and 59% had scores for all 5 visits. However, we censored individuals when they performed poorly on cognition testing (i.e., their follow-up data was excluded after the poor performance – see below for cut-off criteria), to avoid confounding by reverse causation (i.e., cognitive impairment leading to depression or physical disability). This left us with 3,874 follow up cognitive measurements in 1995, 2,901 scores in 1998, 2,212 in 2000, and 1,667 in 2002.
Measurements
Demographic and Socioeconomic
Demographic information included gender, age, marital status (married, widowed, separated/divorced, never married), and race/ethnicity (Non-Hispanic White, non-Hispanic Black, Mexican American, non-Mexican Hispanic, and other). Socioeconomic measures were highest year of completed school or college, household wealth (sum of all wealth components minus all debt) and annual household income (e.g., individual and spouse’s earnings, pensions, social security). To allow for non-linear relationships with cognition, we categorized age (70–79, 80–89, ≥90 years), wealth, and annual income (each as ≤ 20th, 21–50 th, 51–80 th, and >80 th percentile). In follow up visits, changes from baseline were recorded for three variables: marital status (dichotomized as lost vs. gained a partner or no change); wealth; and income (each classified 3-ways: a) increase by ≥ 15%, b) decrease by ≥ 15%, and c) change magnitude ≤ 15%).
Depressive Symptoms
Eight self-reported depressive symptom-items, were from the longer Center for Epidemiologic Studies-Depression Scale (CES-D), modified to two responses, yes (1) or no (0) for whether the symptom had been experienced during "much of the time in the past week”19 A summary CES-D score was created as the number (range: 0–8) of symptoms endorsed. The 8-item CES-D’s internal consistency reliability (α=.77) and construct validity have been documented for the AHEAD sample.20 The CES-D score was created for everyone who answered at least 5 of the 8 items, by appropriately scaling the incomplete score. Of 6,474 participants, 76 (1.2%) had baseline CES-D scores imputed.
Sixty-one percent of the study sample endorsed fewer than 2 symptoms. To address this skewed CES-D distribution, we created 3 baseline categories of depressive symptomatology: Low: CES-D<2 (61.1%), Medium: ≥ 2 to < 4 (21.8%), and High: ≥ 4 (17.1%). Change from baseline score (range, −8 to +8) for follow up visits was a continuous measure.
Chronic Diseases
Participants were asked whether a doctor ever told them they had a heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems (combined together as heart disease), a stroke, or diabetes. These medical conditions are most consistently associated with cognitive impairment and dementia, and are thought to contribute to cognitive decline by causing cortical neuronal loss and disruptions in sub-cortical circuitry.21 Interim development of these conditions after baseline visits was recorded at all follow ups.
Physical Disabilities
Participants were asked about difficulties with both basic ADLs (bathing, dressing, eating, getting across a room, getting out of bed, and toileting) and instrumental ADLs (preparing hot meals, grocery shopping, making telephone calls, taking medications, and managing money). Those who received help, had difficulty, or needed special equipment for any difficulty received a score of 1 for that item, otherwise 0. Change in the number of ADL limitations (basic and instrumental, separately) since baseline assessment was recorded at every follow up.
Cognitive Performance
Cognitive performance testing, based on the Telephone Interview for Cognitive Status (a validated assessment tool),22 was administered at all visits. Participants received telephone interviews if 79 years or younger, and in-person interviews if over age 79, but were allowed to switch mode of administration. Testing included immediate and 5-minute delayed recall of ten high-frequency nouns (each scored 0–10); serial 7’s subtractions, as a test of working memory, attention, and calculation (scored 0–5); and other mental status items (scored 0–10) assessing orientation (date, month, year, day of week; 4 points), attention (counting backwards from 20; 2 points), language (object naming; 2 points), and knowledge of current affairs (United States president and vice president; 2 points). The total score (range, 0–35) has been validated,23 has a near-normal distribution, and because of the verbal memory component, is sensitive to early changes, and less susceptible to ceiling effects.24
Total cognition scores for those missing one of four subscales (immediate recall, delayed recall, serial 7’s, and other) were computed as the sum of the three available subscales. This imputation (zero for the missing test) assumes a test was refused for fear of failure,23 and underestimates cognitive ability; therefore an indicator variable for imputed scores was included in all models. The proportion of imputed cognitive scores was 1.8% at baseline (n=119), 6.4% in 1995 (n=321), 5.2% in 1998 (n=215), 5.8% in 2000 (n=191), and 4.6% in 2002 (n=121). To capture early, age-related memory changes, recall scores (range, 0–20) were computed by summing immediate and delayed recall scores
Since cognitive impairment can lead to depression and physical disabilities and is also associated with larger declines in cognitive functioning over time, we censored participants after their first poor cognitive performance (based on a bottom quartile total cognition score in one’s education peer group). We used education-level-specific cut points to identify low performers (total cognition score ≤ 13 if less than high school education, ≤ 18 if only high school education, and ≤ 19 if more than high school education), to account for education’s influence on cognition tests even in the absence of impairment; education-level criteria are recommended for detection of cognitive impairment.25
Statistical Analyses
We fit a 3-parameter growth curve to model cognition score trajectories, with intercept (baseline score), practice effect (step increase after first testing, that reflects learning and increased self-assurance),26,27 and a linear aging-related decline (constant slope, starting at baseline). In the 2,353 participants with scores at all 5 visits, the 3-parameter model fit the mean trajectory well for both total cognition score (R2 =0.89) and recall score (R2 =0.95).
We used linear, mixed effects regression (HLM version 6.01 with full maximum likelihood),28 and allowed trajectory parameters (intercept, practice effect, and slope) to vary by baseline values of predictors and covariates (as fixed effects) and to vary from individual to individual (as random effects). We included education × age interactions because education effects on cognition trajectories appear to vary by age. Additionally, change from baseline in predictors and covariates were modeled as affecting cognition score contemporaneously – also as fixed effects. This allows changes in cognitive performance to be initiated by changes in socioeconomic, marital, health, or functional status, and to persist as long as the change in predictor/covariate status is maintained.
To minimize bias due to differential attrition, we included a binary indicator of continued participation (at least one follow up cognition testing), and the number of cognition assessments (integer, range: 1–5). Both terms were modeled to influence the intercept (baseline score), and the integer term to also influence practice effect and slope. These terms reflect among other factors, proximity to death or serious illness that would prevent continued participation, and have been found by others to be important predictors of cognitive functioning and decline.29
For all analyses, normalized sample weights were used such that findings were representative of the 1993 non-institutionalized older adult population in the US.
RESULTS
Compared to excluded cohort study members, the baseline study sample was younger, more educated, wealthier, and more likely to be female and non-Hispanic White (See Table 1). They also had fewer depressive symptoms and were less likely to have chronic diseases and ADL limitations.
Table I.
Descriptive statistics for study sample and the rest of the AHEAD cohort
| Study Sample* N=6,474 |
Rest of the AHEAD cohort † (N=973) |
p value for test of difference ‡ |
|
|---|---|---|---|
| Baseline (1993) Variables | |||
| Age (years): mean | 77.1 | 80.2 | < .001 |
| Gender | |||
| Male (%) | 38.7 | 49.5 | < .001 |
| Female (%) | 61.3 | 50.5 | |
| Marital status | |||
| Married (%) | 51.8 | 56.4 | < .01 |
| Widow/widower (%) | 39.9 | 38.3 | |
| Separated/divorced (%) | 5.0 | 2.3 | |
| Never married (%) | 3.3 | 3.0 | |
| Ethnicity | |||
| Non-Hispanic White (%) | 88.2 | 80.1 | < .001 |
| Non-Hispanic Black (%) | 7.6 | 10.4 | |
| Mexican American (%) | 1.7 | 4.4 | |
| Other Hispanics (%) | 1.4 | 2.1 | |
| Other (%) | 1.1 | 3.1 | |
| Educational level (years): mean | 11.3 | 9.3 | < .001 |
| Wealth ($): median | 96,000 | 50,200 | < .01 |
| Annual income ($): median | 17,532 | 13,068 | < .01 |
| Depressive Symptoms § | |||
| Low, <2 (%) | 62.3 | 39.6 | < .001 |
| Medium, >=2 but <4 (%) | 21.7 | 30.3 | |
| High, >=4 (%) | 16 | 28.8 | |
| Chronic Diseases | |||
| Heart disease (%) | 29.2 | 34.4 | < .05 |
| Diabetes (%) | 11.8 | 13.7 | |
| Stroke (%) | 6.7 | 18.0 | < .001 |
| Physical Disabilities | |||
| Basic ADL¶ Limitations (%) | 25.7 | 53.7 | < .001 |
| IADL# limitations (%) | 22.1 | 64.9 | < .001 |
Weighted to make AHEAD cohort representative of non-institutionalized persons 70 years and older living in the US in 1993
Remaining age-eligible AHEAD cohort with missing data (791 had proxy interviews, 4 were missing depression data, and 178 were missing 2 or more cognition subscales = 973)
Tests of difference between groups: t-test for age, and education, chi-square for marital status and ethnicity, and rank sum test for wealth and income. All tests were 2-sided
76 (1.2%) had baseline CES-D scores imputed: 67 were missing 1 item, 7 were missing 2 items, and 2 were missing 3 items
Activities of Daily Living
Instrumental Activities of Daily Living
Baseline mean total cognition score (maximum: 35) was 19.84 (95% confidence interval (CI): 19.70, 9.99; standard deviation (SD): 6.04), and mean baseline recall score (maximum: 20) was 7.78 (95% CI: 7.68, 7.88; SD: 4.09). Mean decline with aging was 3.5 points (or 0.68 SD) per decade in total score and 2.1 points (or 0.60 SD) per decade in recall score. Adjusted for demographic and socioeconomic variables, participation length, and imputation of total cognition score, baseline depressive symptoms were strongly associated with baseline total cognition score and recall score, but not practice effect in or the rate of decline (slope) of either score – See Table 2. New depressive symptoms were also associated with lower cognition scores at and after the symptom changes - a step reduction in cognitive performance. Compared to individuals with fewer than 2 depressive symptoms at baseline, those with 4 or more depressive symptoms scored 1.09 lower on total cognition score, 95% confidence interval (CI): 0.74 –1.44, and 0.56 lower on recall score (95% CI: 0.30 –0.81). Cognition scores in individuals with 2 or 3 depressive symptoms at baseline were also significantly lower than in those with fewer than 2 symptoms, but not as low as in individuals with 4 or more symptoms – See Table 2. In addition, each incident depressive symptom was associated with a step reduction of 0.09 in total score (95% CI: 0.04 –0.14) and 0.08 in recall score (95% CI: 0.04 – 0.12).
Table II.
Associations * of depressive symptoms, chronic diseases, and ADL limitations with trajectories of total cognition score and recall score
| Contemporaneous Association with Total Score † |
Contemporaneous Association with Recall Score † |
|
|---|---|---|
| Depressive Symptoms Only Model | ||
| Baseline depressive symptoms ¶ | ||
| Medium (vs. low) | − 0.38 (0.15) # | −0.26 (0.11) # |
| High (vs. low) | − 1.09 (0.18) †† | −0.56 (0.13) †† |
| Incident depressive symptoms | − 0.09 (0.03) ** | −0.08 (0.02) †† |
| Diseases Only Model | ||
| Heart disease at baseline | 0.12 (0.13) | 0.01 (0.10) |
| Diabetes at baseline | −0.04 (0.19) | −0.05 (0.14) |
| Stroke at baseline | −1.23 (0.26) †† | −0.67 (0.17) †† |
| Incident heart disease | −0.29 (0.15) | −0.14 (0.11) |
| Incident diabetes | −0.15 (0.21) | −0.22 (0.17) |
| Incident stroke | −0.88 (0.20) †† | −0.42 (0.15) ** |
| Disabilities Only Model | ||
| ADL limitations at baseline ‡‡ | −0.60 (0.16) †† | −0.32 (0.11) ** |
| IADL limitations at baseline ‡‡ | −1.47 (0.17) †† | −0.68 (0.12) †† |
| Incident ADL limitations §§ | −0.20 (0.05) †† | −0.11 (0.03) ** |
| Incident IADL limitations §§ | −0.54 (0.08) †† | −0.22 (0.05) †† |
| Depressive Symptoms and Diseases Model | ||
| Baseline depressive symptoms ¶ | ||
| Medium (vs. low) | − 0.37 (0.15) # | −0.25 (0.11) # |
| High (vs. low) | − 1.06 (0.18) †† | −0.53 (0.13) †† |
| Heart disease at baseline | 0.19 (0.13) | 0.05 (0.10) |
| Diabetes at baseline | 0.00 (0.19) | −0.03 (0.14) |
| Stroke at baseline | − 1.15 (0.26) †† | −0.63 (0.17) ** |
| Incident depressive symptoms | − 0.08 (0.03) ** | −0.07 (0.02) †† |
| Incident heart disease | −0.26 (0.15) | −0.12 (0.11) |
| Incident diabetes | −0.13 (0.21) | −0.21 (0.17) |
| Incident stroke | − 0.83 (0.20) †† | −0.38 (0.15) # |
| Full Model | ||
| Baseline depressive symptoms ¶ | ||
| Medium (vs. low) | − 0.20 (0.15) | −0.17 (0.11) |
| High (vs. low) | − 0.74 (0.18) †† | −0.38 (0.13) ** |
| Heart disease at baseline | 0.25 (0.13) | 0.08 (0.10) |
| Diabetes at baseline | 0.14 (0.19) | 0.03 (0.14) |
| Stroke at baseline | − 0.80 (0.26) ** | −0.47 (0.18) # |
| ADL limitations at baseline ‡‡ | − 0.45 (0.16) ** | −0.22 (0.12) |
| IADL limitations at baseline ‡‡ | − 1.38 (0.17) †† | −0.62 (0.12) †† |
| Incident depressive symptoms | − 0.04 (0.03) | −0.06 (0.02) ** |
| Incident heart disease | − 0.19 (0.14) | −0.09 (0.11) |
| Incident diabetes | − 0.09 (0.20) | −0.19 (0.17) |
| Incident stroke | − 0.59 (0.20) ** | −0.28 (0.15) |
| Incident ADL limitations §§ | − 0.16 (0.05) ** | −0.07 (0.03) # |
| Incident IADL limitations §§ | − 0.52 (0.07) †† | −0.20 (0.05) †† |
All associations are reported as point estimate (standard error), and are adjusted for age, gender, marital status at baseline and change in marital status, education, age × education interaction, annual household income at baseline and change in annual income, household wealth at baseline and change in wealth, length of participation, and imputation of partially completed cognition tests, as described in the text. Only contemporaneous associations are tabulated. None of the associations with slope and practice effect were statistically significant at the 0.05 level.
For baseline predictors, the association is with baseline cognition score; for change/incident variables, the association is with cognition score at the time the change took place.
For comparison, mean practice effect was +0.87(95% Confidence Interval [CI]: 0.71, 1.04)
For comparison, mean slope was −0.35 per year (95% CI: −0.38, −0.32)
Low: CES-D<2, Medium 2<=CES-D<4, High; CES-D>=4, based on 8-item CES-D scale (range, 0–8).
p < 0.05;
p < 0.01;
p < .001
One or more limitation vs. none (ADL: Activities of Daily Living; IADL: Instrumental Activities of Daily Living)
Per new limitation
Both prevalent and incident strokes were also associated with lower cognition scores, but chronic diseases had no associations with either practice effect or rate of decline. Similarly, basic and instrumental ADL limitations were independently associated with lower baseline total cognition scores, but had no associations with either practice effect or rate of decline. The development of new basic ADL and instrumental ADL (IADL) limitations during the study interval were also associated with lower total cognition scores (Table 2).
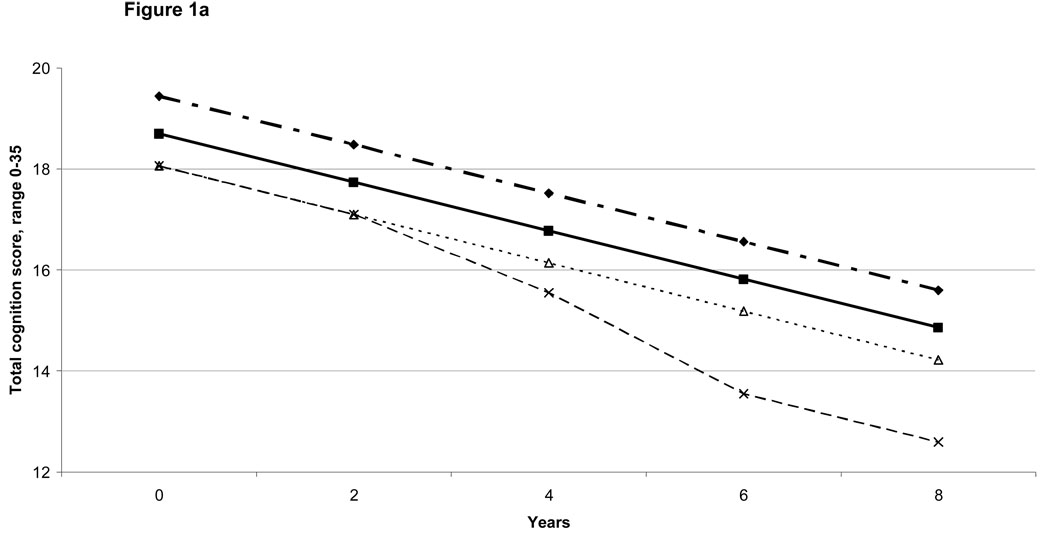
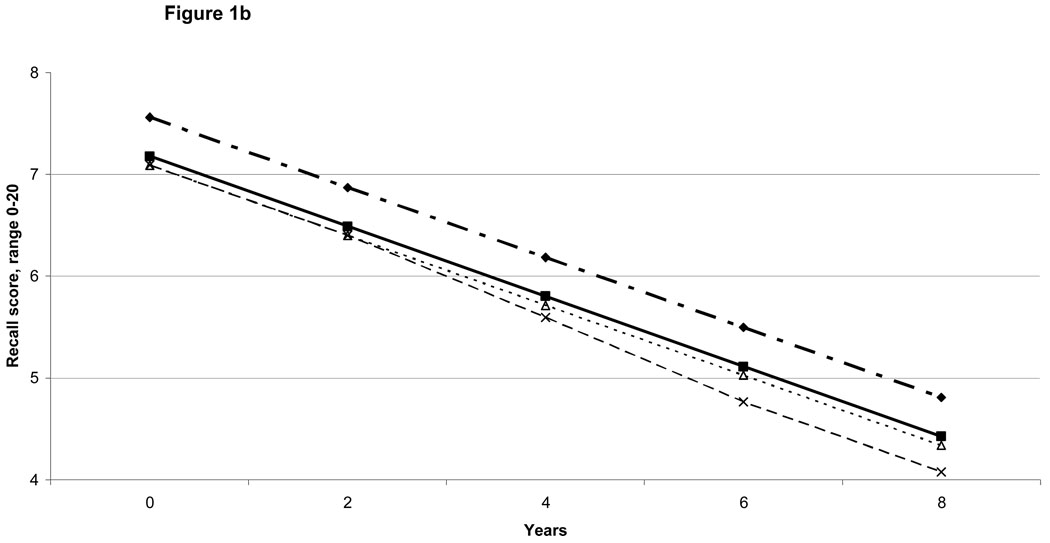
With depressive symptoms and chronic diseases in the same model, depressive symptoms and stroke associations with cognition score trajectories did not substantially weaken. However, with physical disabilities also included (i.e., in the full model), the strength of the depression and stroke associations declined by almost a third, and some became statistically non-significant (Table 2). Even in the full model, incident conditions were independently associated with contemporaneous reductions in cognition scores. Thus, for instance, each incident depressive symptom was independently associated with 0.06 point reduction (95% confidence interval (CI):0.02–0.10) in recall score, incident stroke with 0.59 point reduction in total cognition score (95% CI:0.20–0.98), each new basic ADL limitation with 0.07 point (95% CI:0.01–0.14) reduction in recall score and 0.16 point reduction in total score (95% CI:0.07–0.25), and each incident instrumental ADL limitation with a 0.20 point reduction in recall score (95% CI:0.10–0.30) and 0.52 point reduction in total score (95% CI:0.37–0.67). These associations from the full model are illustrated for specific combinations of conditions in Figures 1a and 1b.
Figure 1.
Figure 1a: Model-predicted Total Cognition Score Trajectories: Illustrative Examples
 Total Score: Reference Group
Total Score: Reference Group
 Total Score if Prevalent High Depressive Symptoms
Total Score if Prevalent High Depressive Symptoms
 Total Score if Prevalent IADL (Instrumental Activities of Daily Living) Limitation
Total Score if Prevalent IADL (Instrumental Activities of Daily Living) Limitation
 Total Score if Prevalent IADL Limitation + Incident Stroke at 4 years + 2 Incident IADL Limitations at 6 years
Total Score if Prevalent IADL Limitation + Incident Stroke at 4 years + 2 Incident IADL Limitations at 6 years
The practice effect is not shown, given that it is mainly an artifact of how cognition was measured. There were no associations between depression, diseases, or disability with the size of the practice effect.
Figure 1b: Model-predicted Recall Score Trajectories: Illustrative Examples
 Recall Score: Reference Group
Recall Score: Reference Group
 Recall Score if Prevalent High Depressive Symptoms
Recall Score if Prevalent High Depressive Symptoms
 Recall Score if Prevalent Stroke
Recall Score if Prevalent Stroke
 Recall Score if Prevalent, Legend for Figure 1b.
Recall Score if Prevalent, Legend for Figure 1b.
Stroke + 2 Incident Depressive Symptoms at 4 years + 2 Incident ADL (Activities of Daily Living) Limitations at 6 years.
The practice effect is not shown, given that it is mainly an artifact of how cognition was measured. There were no associations between depression, diseases, or disability with the size of the practice effect.
The complete model explained 57% of the (null model) variance in intercept, 28% of the practice effect and 36% of the variance in slope. Depressive symptoms, chronic diseases, and physical disabilities together explained 5% of the intercept variance not explained by demographic and socioeconomic variables. The full model for recall score trajectories explained 44% of the variance in intercept, 32% of the variance in practice effect, and 32% of the variance in slope. Depressive symptoms, chronic diseases, and physical disabilities together explained 2.4% of the intercept variance not explained by demographic and socioeconomic variables.
DISCUSSION
In this large, nationally representative cohort of older adults, cognitive performance gradually declined with aging. Prevalent and incident depressive symptoms, stroke, and limitations in basic and instrumental ADLs were each independently associated with additional reductions in cognitive performance whereas heart disease and diabetes were not. However, the rates of longitudinal cognitive decline were not associated with any of these conditions. This suggests that depression, stroke, and physical disabilities negatively affect cognitive functioning at the time of their onset (i.e., a step reduction in cognitive functioning), but probably do not lead to continued deterioration over time.
The causal mechanisms by which strokes and atherosclerotic vascular disease, in general, lead to reduced cognitive functioning have been well studied.30 These same conditions can also contribute to depression and physical disabilities;31 thus, the independent effects of depression, vascular disease, and physical disabilities on cognitive functioning are difficult to tease apart.
Unlike previous studies in which health conditions do not explain much of the variance in cognition scores,15–17 in this study, the full set of predictors explained 44–57% of between-person variance in cognition scores. Inclusion of incident conditions (new development of depressive symptoms, stroke, and physical disabilities) in our models may have improved the prediction ability beyond that seen in previous studies. Inclusion of incident conditions may also partly explain why prevalent conditions did not predict worsening of age-related cognitive decline, unlike previous studies that have found accelerated cognitive declines associated with vascular disease and diabetes.32–34 If diabetes and vascular disease affect cognitive change through ischemic events, incident (new) conditions such as strokes, depressive symptoms, and limitations in ADLs, may capture these events and explain much, if not all, of the effects of prevalent disease on longitudinal change in cognitive functioning. Some previous studies have also failed to uncover associations between either hypertension or diabetes and longitudinal cognitive decline.35,36
Our findings are also consistent with several previous studies that have found that depressive symptoms do not predict future cognitive decline.10–13 Studies that did demonstrate this relationship either examined change in cognition scores over 2 waves (wherein associations may be confounded by practice effects 27 and adjustment for baseline score can induce spurious associations with change),37 or not examined the role of incident depressive symptoms.6–9 This study however, did demonstrate that incident depressive symptoms are associated with step-wise reductions in cognitive performance. Sub-cortical pathology, relative glucocorticoid dysregulation, and chronic inflammation, have all been postulated as mechanistic links between depression and cognitive impairment. Depression may also lower cognitive test effort, explaining the contemporaneous associations seen here.
Some limitations should be noted. Although we found no predictors of the rate of decline for total cognition or recall score, such associations might exist with untested cognitive domains. Secondly, although the AHEAD cognitive tests avoid ceiling effects and detect early change, they may be susceptible to floor effects. However, we censored individuals once they performed poorly, to minimize this effect. Thirdly, depressive symptoms, chronic diseases, physical disabilities and cognitive functioning are likely to have multi-directional relationships, with cognitive impairment contributing to depressive symptoms and worse physical functioning.18, 38–40 We tried to minimize confounding through such reverse pathways, by excluding the low baseline cognitive performers and censoring individuals with low performance at follow up. Our findings thus apply only to the prediction of cognition trajectories in cognitively intact individuals. Nonetheless, while we have demonstrated associations between incident depressive symptoms and step reductions in cognition score, for example, we are unable to address issues of causality (or directionality). We cannot be certain whether incident depression preceded, was followed by, or co-occurred with the deterioration in cognitive function in the 2 years between consecutive study visits. Finally, though over 80% of the original AHEAD sample (89% of those alive) had data for at least 2 visits, only 36% of the original sample (59% of those alive) had cognition data for all 5 visits. Lack of data from those alive at subsequent waves may have been due to cognitive decline or related events. This may limit the generalizability of some of our longitudinal findings. To minimize potential bias from differential attrition, we included controls for attrition in all models.
We submit, however, that these limitations are outweighed by several strengths, including sample size and diversity, the number of follow up assessments, sensitivity of the test measure to early change, modeling of practice effects, controls for differential retention, and assessment of incident conditions’ role in determining cognition trajectories.
In conclusion, health conditions and functional status do not appear to influence rates of declines in cognitive performance in older Americans. However, prevalent as well as incident depressive symptoms, stroke, and basic and instrumental ADL disabilities contribute independently to step reductions in cognitive functioning. Therefore, prevention, early identification, and aggressive treatment of depression, cerebrovascular disease, and physical disabilities in aging adults may ameliorate the burdens of cognitive impairment.
ACKNOWLEDGMENTS
Funding Sources: This research was supported by a grant from the National Institute on Aging (R01 AG022537, Carol S. Aneshensel, Ph.D., Principal Investigator). Dr. Karlamangla was partially supported by the National Institute on Aging’s Older Americans Independence Center at UCLA (5P30 AG028748).
Sponsor’s Role: The grantor played no role in the design, methods, subject recruitment, data collections, analysis or preparation of the paper.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions:
Dr. Chodosh participated in the study concept and design, interpretation of data, and preparation of the manuscript.
Dr. Miller-Martinez participated in the interpretation of data, data analysis, and preparation of the manuscript.
Dr. Aneshensel participated in the study concept and design, acquisition of the data, interpretation of data, data analysis, and preparation of the manuscript.
Dr. Wight participated in the study concept and design, interpretation of data, data analysis, and preparation of the manuscript.
Dr. Karlamangla participated in the study concept and design, interpretation of data, data analysis, and preparation of the manuscript.
REFERENCES
- 1.Kemper S, Greiner LH, Marquis JG, et al. Language decline across the life span: Findings from the Nun Study. Psychol Aging. 2001;16:227–239. [PubMed] [Google Scholar]
- 2.Lamar M, Resnick SM, Zonderman AB. Longitudinal changes in verbal memory in older adults: Distinguishing the effects of age from repeat testing. Neurology. 2003;60:82–86. doi: 10.1212/wnl.60.1.82. [DOI] [PubMed] [Google Scholar]
- 3.Schaie KW. The Seattle Longitudinal Study: A thirty-five-year inquiry of adult intellectual development. Z Gerontol. 1993;26:129–137. [PubMed] [Google Scholar]
- 4.Comijs HC, Dik MG, Deeg DJH, et al. The course of cognitive decline in older persons: Results from the Longitudinal Aging Study Amsterdam. Dement Geriatr Cogn Disord. 2004;17:136–142. doi: 10.1159/000076346. [DOI] [PubMed] [Google Scholar]
- 5.McNeil JK. Neuropsychological characteristics of the dementia syndrome of depression: onset, resolution, and three-year follow-up. Clin Neuropsychol. 1999;13:136–146. doi: 10.1076/clin.13.2.136.1962. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Blackwell T, Gore R, et al. Depressive symptoms and cognitive decline in non-demented elderly women. A prospective study. Arch Gen Psychiatry. 1999;56:425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 7.Comijs HC, Jonker C, Beekman AT, et al. The association between depressive symptoms and cognitive decline in community-dwelling elderly persons. Int J Geriatr Psychiatry. 2001;16:361–367. doi: 10.1002/gps.343. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RF, Mendes de Leon CF, Bennett DA, et al. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]
- 9.Chodosh J, Kado D, Seeman T, et al. Depressive symptoms as a predictor of cognitive decline. MacArthur Studies of Successful Aging. Am J Geriatr Psychiatry. 2007;15:406–415. doi: 10.1097/01.JGP.0b013e31802c0c63. [DOI] [PubMed] [Google Scholar]
- 10.Bassuk SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Arch Gen Psychiatry. 1998;55:1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- 11.Comijs HC, van Tilburg T, Geerlings SW, et al. Do severity and duration of depressive symptoms predict cognitive decline in older persons? Results of the Longitudinal Aging Study Amsterdam. Aging Clin Exp Res. 2004;16:226–232. doi: 10.1007/BF03327388. [DOI] [PubMed] [Google Scholar]
- 12.Vinkers DJ, Gussekloo J, Stek ML, et al. Temporal relation between depression and cognitive impairment in old age: prospective, population based study. BMJ. 2004;329:881. doi: 10.1136/bmj.38216.604664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganguli M, Du Y, Dodge HH, et al. Depressive symptoms and cognitive decline in late life: A prospective epidemiological study. Arch Gen Psychiatry. 2006;63:153–160. doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- 14.Whitmer RA, Sidney S, Selby J, et al. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 15.Zelinksi EM, Gilewski MJ. Effects of demographic and health variables on Rasch scaled cognitive scores. J Aging Health. 2003;15:435–464. doi: 10.1177/0898264303253499. [DOI] [PubMed] [Google Scholar]
- 16.Zelinksi EM, Crimmins A, Reynolds S, et al. Do medical conditions affect cognition in older adults? Health Psychol. 1998;17:504–512. doi: 10.1037//0278-6133.17.6.504. [DOI] [PubMed] [Google Scholar]
- 17.Verhaeghen P, Borchelt M, Smith J. Relation between cardiovascular and metabolic disease and cognition in very old age: Cross-sectional and longitudinal findings from the Berlin Aging Study. Health Psychol. 2003;22:559–569. doi: 10.1037/0278-6133.22.6.559. [DOI] [PubMed] [Google Scholar]
- 18.Black SA, Rush RD. Cognitive and functional decline in adults aged 75 and older. J Am Geriatr Soc. 2002;50:1978–1986. doi: 10.1046/j.1532-5415.2002.50609.x. [DOI] [PubMed] [Google Scholar]
- 19.Soldo BJ, Hurd MD, Rodgers WL, et al. Asset and health dynamics among the oldest old: An overview of the AHEAD study. J Gerontol B Psychol Sci Soc Sci. 1997;52(Special Issue):S1–S20. doi: 10.1093/geronb/52b.special_issue.1. [DOI] [PubMed] [Google Scholar]
- 20.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–148. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 21.Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257:80–87. doi: 10.1016/j.jns.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 22.Ferrucci L, Del Lungo I, Guralnik JM, et al. Is the telephone interview for cognitive status a valid alternative in persons who cannot be evaluated by the Mini Mental State Examination? Aging (Milano) 1998;10:332–338. doi: 10.1007/BF03339796. [DOI] [PubMed] [Google Scholar]
- 23.Herzog AR, Wallace RB. Measures of cognitive functioning in the AHEAD Study. J Gerontol B Psychol Sci Soc Sci. 1997;52B:37–48. doi: 10.1093/geronb/52b.special_issue.37. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Smith GE, Ivnik RJ, et al. Memory function in very early Alzheimer's disease. Neurology. 1994;44:867–872. doi: 10.1212/wnl.44.5.867. [DOI] [PubMed] [Google Scholar]
- 25.Kittner SJ, White LR, Farmer ME, et al. Methodological issues in screening for dementia: The problem of education adjustment. J Chron Dis. 1986;39:163–170. doi: 10.1016/0021-9681(86)90019-6. [DOI] [PubMed] [Google Scholar]
- 26.Morris MC, Evans DA, Hebert LE, et al. Methodological issues in the study of cognitive decline. Am J Epidemiol. 1999;149:789–793. doi: 10.1093/oxfordjournals.aje.a009893. [DOI] [PubMed] [Google Scholar]
- 27.Rabbitt P, Diggle P, Holland F, et al. Practice and drop-out effects during a 17-year longitudinal study of cognitive aging. J Gerontol B Psychol Sci Soc Sci. 2004;59B:84–97. doi: 10.1093/geronb/59.2.p84. [DOI] [PubMed] [Google Scholar]
- 28.Raudenbush SW, Bryk AS, Cheong YF, et al. Hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International; 2000. [Google Scholar]
- 29.Singer T, Verhaeghen P, Ghisletta P, et al. The fate of cognition in very old age: Six-year longitudinal findings in the Berlin Aging Study (BASE) Psychol Aging. 2003;18:318–331. doi: 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- 30.Jellinger KA. The pathology of "vascular dementia": A critical update. J Alzheimers Dis. 2008;14:107–123. doi: 10.3233/jad-2008-14110. [DOI] [PubMed] [Google Scholar]
- 31.Thomas AJ, Kalaria RN, O'Brien JT. Depression and vascular disease: What is the relationship? J Affect Disord. 2004;79:81–95. doi: 10.1016/S0165-0327(02)00349-X. [DOI] [PubMed] [Google Scholar]
- 32.Hassing LB, Grant MD, Hofer SM, et al. Type 2 diabetes mellitus contributes to cognitive decline in old age: A longitudinal population-based study. J Int Neuropsychol Soc. 2004;10:599–607. doi: 10.1017/S1355617704104165. [DOI] [PubMed] [Google Scholar]
- 33.Okereke OI, Kang JH, Cook NR, et al. Type 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. J Am Geriatr Soc. 2008;56:1028–1036. doi: 10.1111/j.1532-5415.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- 34.Maggi S, Limongi F, Noale M, et al. for the ILSA Study Group. Diabetes as a risk factor for cognitive decline in older patients. Dement Geriatr Cogn Discor. 2009;27:24–33. doi: 10.1159/000183842. [DOI] [PubMed] [Google Scholar]
- 35.Stewart R, Prince M, Mann A. Age, vascular risk, and cognitive decline in an older, British, African-Caribbean population. J Am Geriatr Soc. 2003;51:1547–1553. doi: 10.1046/j.1532-5415.2003.51504.x. [DOI] [PubMed] [Google Scholar]
- 36.van den Berg E, de Craen AJ, Biessels GJ, et al. The impact of diabetes mellitus on cognitive decline in the oldest of the old: A prospective population-based study. Diabetologia. 2006;49:2015–2023. doi: 10.1007/s00125-006-0333-1. [DOI] [PubMed] [Google Scholar]
- 37.Glymour MM, Weuve J, Berkman LF, et al. When is baseline adjustment useful in analysis of change? An example with education and cognitive change. Amer J Epidemiol. 2005;162:267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 38.Mehta KM, Yaffe K, Covinsky KE. Cognitive impairment, depressive symptoms, and functional decline in older people. J Am Geriatr Soc. 2002;50:1045–1050. doi: 10.1046/j.1532-5415.2002.50259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fultz NH, Ofstedal MB, Herzog AR, et al. Additive and interactive effects of comorbid physical and mental conditions on functional health. J Aging Health. 2003;15:465–481. doi: 10.1177/0898264303253502. [DOI] [PubMed] [Google Scholar]
- 40.Kelley-Moore JA, Ferraro KF. A 3-D model of health decline: Disease, disability, and depression among black and white older adults. J Health Soc Behav. 2005;46:376–391. doi: 10.1177/002214650504600405. [DOI] [PubMed] [Google Scholar]