Abstract
It is commonly accepted that the DNA of Drosophila melanogaster does not contain 5-methylcytosine, which is essential in the development of most eukaryotes. We have developed a new, highly specific and sensitive assay to detect the presence of 5-methylcytosine in genomic DNA. The DNA is degraded to nucleosides, 5-methylcytosine purified by HPLC and, for detection by 1D- and 2D-TLC, radiolabeled using deoxynucleoside kinase and [γ-32P]ATP. Using this assay, we show here that 5-methylcytosine occurs in the DNA of D.melanogaster at a level of ∼1 in 1000–2000 cytosine residues in adult flies. DNA methylation is detectable in all stages of D.melanogaster development.
Keywords: DNA methylation/DNA methyltransferase/DNA modification/Drosophila melanogaster/5-methylcytosine
Introduction
Methylation of DNA is a unique way of encoding information because it is heritable but, nevertheless, can be edited. Mammalian DNA contains ∼1–2 mol% of 5-methylcytosine, with ∼2–10% of all cytosine residues being modified (Razin and Riggs, 1980; Ehrlich and Wang, 1981). Similar methylation of the C5 position of cytosine residues at CpG or CNG sites is observed in many species of all phylogenetic kingdoms (Doerfler, 1983; Bestor and Verdine, 1994; Cheng, 1995; Colot and Rossignol, 1999). In mammals, CpG methylation is implicated in the regulation of chromatin structure (Jones and Wolffe, 1999), gene repression (Tate and Bird, 1993; Colot and Rossignol, 1999), parental imprinting (Feil and Khosla, 1999) and X chromosome inactivation in females (Migeon, 1994; Colot and Rossignol, 1999). The importance of DNA methylation is underscored by the finding that mice nullizygous for the DNA methyltransferases Dnmt1 or Dnmt3b are not viable and die during embryogenesis (Li et al., 1992; Okano et al., 1999). Moreover, erroneous DNA methylation is an important factor in human disease because it contributes to tumorigenesis (Jones and Laird, 1999) and ageing (Toyota and Issa, 1999). Mutations in the human Dnmt3b gene cause ICF [immunodeficiency, (para)centromeric instability and facial abnormalities], a hereditary disease (Hansen et al., 1999; Okano et al., 1999; Xu et al., 1999).
The methylation status of the DNA from Drosophila melanogaster has been controversial over decades. Although a small amount of methylcytosine had been detected by methylcytosine-directed antibodies (Achwal et al., 1983, 1984), other studies using restriction site protection, McrBC digestion, nearest neighbor analysis and HPLC did not provide evidence for modified cytosines (Rae and Steele, 1979; Frankel and Smith, 1981; Szyf et al., 1982; Doerfler, 1983; Patel and Gopinathan, 1987; Tweedie et al., 1997, 1999; Lyko et al., 1999). As a consequence of the overwhelming evidence that argues against DNA methylation in D.melanogaster, it has become general opinion that this species does not contain 5-methylcytosine (Alberts et al., 1994; Mathews and van Holde, 1995; Voet and Voet, 1995; Lewin, 1997). Recently, in apparent contrast to this view, a homolog of the Dnmt2 protein has been identified in D.melanogaster (Hung et al., 1999; Tweedie et al., 1999). The Dnmt2 family comprises homologs in Schizosaccharomyces pombe, mouse and human. These proteins contain all amino acid sequence motifs characteristic of cytosine-C5 methyltransferases, but so far catalytic activity could not be detected in vivo or in vitro (Okano et al., 1998; Van den Wyngaert et al., 1998; Yoder and Bestor, 1998). However, it has been shown that a variant of the S.pombe Dnmt2 in which one amino acid residue is deleted is catalytically active (Pinarbasi et al., 1996). Moreover, homologs of proteins known to interact with methylated DNA have been identified in D.melanogaster (Hung et al., 1999; Tweedie et al., 1999). The presence of these proteins in the proteom of D.melanogaster has prompted us to re-investigate the incidence of 5-methylcytosine in the DNA of this species.
Results and discussion
Preparation of DNA and McrBC analysis
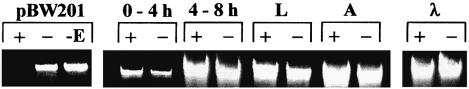
Genomic DNA was isolated from D.melanogaster (oreR) at different stages of development, i.e. from embryos before 4 h of development, between 4 and 8 h of development, from larvae and from adult flies. To detect DNA methylation, McrBC restriction was carried out with each of these samples. The McrBC restriction system, in the presence of GTP, cleaves DNA containing two RmC (R = A or G) sites within a distance of 80–2000 bp (Stewart and Raleigh, 1998). However, no difference was detectable between samples incubated with or without GTP, demonstrating the absence of McrBC restriction (Figure 1). This result is in agreement with similar observations recently made by others (Lyko et al., 1999). It indicates that during all stages of development the level of DNA methylation in D.melanogaster must be lower than in mammals.
Fig. 1. McrBC cleavage of DNA isolated from D.melanogaster (+, with 1 mM GTP; –, in the absence of GTP). As a control, the cleavage of pBW201 plasmid DNA and λ DNA is shown. pBW201 is methylated at CCGG sequences at the first cytosine (+, with 1 mM GTP; –, in the absence of GTP; –E, without McrBC). λ DNA (MBI Fermentas) is not methylated at cytosine residues.
HPLC separation and dNK labeling of nucleosides
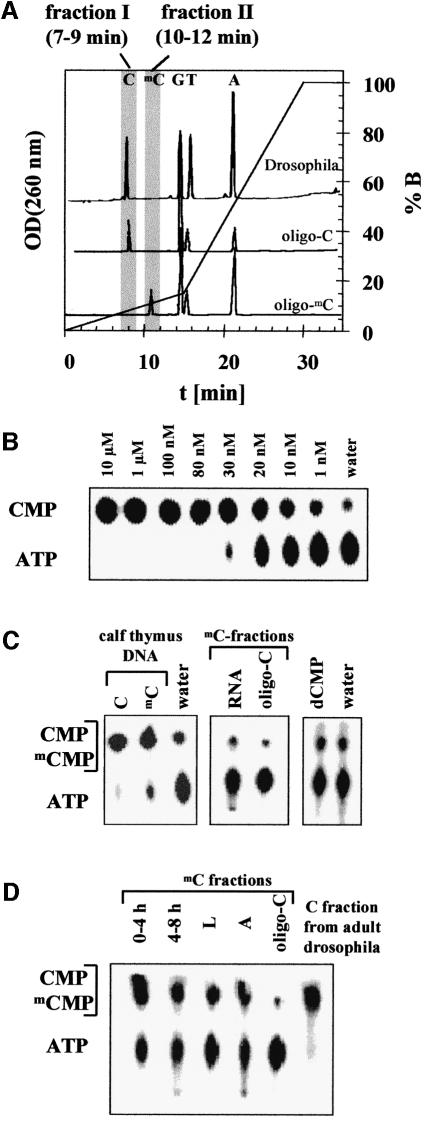
To detect methylcytosine with higher sensitivity, the DNA was enzymatically hydrolyzed to nucleosides. The nucleosides were separated by reversed-phase HPLC and the fractions containing cytidine and methylcytidine were collected (Figure 2A). These samples were incubated with deoxyribonucleoside kinase (dNK) and [γ-32P]ATP to obtain labeled CMP and mCMP. Labeled mononucleotides were separated from the radiolabeled ATP by thin layer chromatography (TLC). To calibrate the HPLC separation, HPLC was carried out with hydrolysates of a PCR product and an oligonucleotide (oligo-C) that do not contain methylcytosine, as well as of calf thymus DNA and a second oligonucleotide (oligo-mC) both containing methylcytosine. As shown in Figure 2A, cytidine elutes after 8 min and methylcytidine elutes from the HPLC column after 11 min. A small methylcytidine peak was also detected after 11 min with calf thymus DNA (data not shown). Therefore, two fractions were collected in preparative HPLC runs: the C-fraction (7–9 min), containing the cytidine, and the mC-fraction (10–12 min), which contains the methylcytidine.
Fig. 2. (A) OD260 nm profiles of the HPLC separation of mononucleosides obtained from an enzymatic hydrolysis of DNA from adult D.melanogaster and two oligonucleotides used as control (oligo-C, which does not contain deoxymethylcytidine, and oligo-mC, which does not contain deoxycytidine). Two fractions were collected to quantify the deoxycytidine and deoxymethylcytidine content of the samples (C-fraction, 7–9 min; mC-fraction, 10–12 min). (B) Sensitivity of the enzymatic detection of deoxynucleosides using dNK. Different concentrations of deoxycytidine (Sigma) were incubated with dNK and analyzed by 1D-TLC as described in Materials and methods. The assay can detect 1 nM deoxycytidine in a 20 µl reaction volume corresponding to 20 fmol. (C) Control reactions of the HPLC/dNK assay. In the left panel, a 1D-TLC analysis of dNK-treated C- and mC-fractions of calf thymus DNA (Sigma) is shown. Methylcytidine is clearly detected in this reaction. In addition, 1D-TLC analyses of a dNK reaction using the mC-fraction of identically treated samples of yeast RNA (Boehringer Mannheim) and of dNK reactions with 200 nM dCMP are displayed, confirming that ribonucleosides and deoxyribonucleotides are not detected in the HPLC/dNK assay. (D) Detection of methylcytosine in DNA isolated from D.melanogaster at different stages of development (after 0–4 and 4–8 h of develop ment; L, from larvae; A, from adult flies). Shown are 1D-TLCs of dNK-treated mC-fractions. As a control, analyses of an identically treated sample of oligo-C that does not contain methylcytosine are shown. For comparison, the result obtained with C-fraction, which contains the cytidine, from adult flies is displayed.
To estimate the sensitivity of the dNK-mediated detection of nucleosides, we carried out phosphorylation of different amounts of cytidine by dNK using [γ-32P]ATP. The reactions were analyzed by 1D-TLC on PEI-cellulose, which easily separates CMP and ATP. As shown in Figure 2B, as little as 20 fmol of deoxycytidine in 20 µl can be detected with this assay (lane labeled ‘1 nM’), demonstrating the high sensitivity of detection that is due to radioactive labeling of the nucleoside.
To check the reliability of the HPLC/dNK assay, several controls were performed. (i) As shown in Figure 2C, methylcytosine is readily detected in the mC-fractions of DNA isolated from calf thymus. Even higher amounts of methylcytosine were detected in the mC-fraction of oligo-mC (data not shown). (ii) In the analyses of the mC-fractions obtained with the PCR product and oligo-C, only background signals were observed (Figure 2C and data not shown). This background signal was also observed in reactions without the addition of substrate (Figure 2B and C, lanes labeled ‘water’). 2D-TLC analyses showed that the background is due to the presence of bound cytidine and thymidine in the enzyme preparation (see below and Figure 3A). (iii) Although no RNA contamination of the DNA samples was detectable on the agarose gels (data not shown), we wanted to exclude the contribution of ribonucleosides to the signals observed with the mC-fraction. Therefore, 20 µg of yeast RNA were digested and subjected to HPLC analysis. Whereas uridine elutes within the C-fraction (7–9 min), none of the major ribonucleosides elutes in the mC-fraction (10–12 min), and in the enzymatic analysis of the mC-fraction from RNA, only a background signal was obtained (Figure 2C). (iv) A similar analysis carried out with dUMP (hydrolysis, HPLC analysis, OD260 nm detection) confirmed that deoxyuridine, which may be present in trace amounts in DNA, elutes after 9 min from the HPLC and does not contribute to the signal observed in the mC-fraction. (v) Finally, we checked whether dNK might be able to catalyze an exchange reaction between nucleotides and ATP. To this end, high concentrations of dCMP and CMP were incubated with dNK, but we did not detect any increase in the background signal (Figure 2C and data not shown). This result confirms that exchange reactions are not detected under our experimental conditions and, hence, trace amounts of nucleotides (either ribo- or deoxy-) in the DNA hydrolysate would not affect the results of the HPLC/dNK analysis.
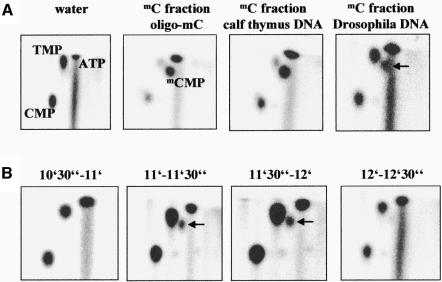
Fig. 3. (A) 2D-TLC analyses of mC-fractions of DNA from calf thymus, D.melanogaster and oligo-mC labeled with dNK. As a control, a 2D-TLC analysis of a reaction in which dNK is incubated with water is displayed. The relative positions of all four major nucleotides as well as of ATP were identified by 2D-TLCs with standard compounds. The mCMP spot in DNA isolated from D.melanogaster is marked with an arrow. (B) 2D-TLC analysis of the elution profile of methylcytidine from the HPLC column. In this experiment, DNA from adult flies was hydrolyzed and subjected to HPLC. Twenty fractions were collected between 5 and 15 min. In this figure, a 2D-TLC analysis of the fractions collected between 10.5–11, 11–11.5, 11.5–12 and 12–12.5 min is displayed, showing that only the fractions collected between 11 and 12 min contain methylcytidine. The mCMP spot in DNA isolated from D.melanogaster is marked with an arrow.
We have employed the HPLC/dNK assay to investigate the presence of methylcytosine in the DNA prepared from D.melanogaster. As shown in Figure 2D, the signal obtained with the mC-fractions of DNA from D.melanogaster was significantly higher than the background. These data suggest that significant amounts of 5-methylcytosine are present in DNA isolated from D.melanogaster in all stages of development, i.e. in early embryos, in late embryos, in the larval stage and in adult flies (Figure 2D). We repeatedly found that DNA from adults contained the lowest amounts of methylcytosine.
Detection of methylcytidine by 2D-TLC
Since the 1D-TLC analysis does not allow the separation of mCMP from CMP, we attempted to visualize directly the presence of 5-methylcytosine in the DNA of D.melanogaster. To this end, DNA from adult flies was hydrolyzed to nucleosides, which were separated by HPLC. C- and mC-fractions were collected and labeled with dNK. To identify the labeled base exactly, we used a 2D-TLC technique, which allows the separation of all natural and modified bases (Kuchino et al., 1987; Oakeley, 1999). In this system, the positions of all natural nucleotides were determined by using dNK-labeled standard nucleosides. mCMP was identified by using mC-fractions of oligo-mC and calf thymus DNA. As shown in Figure 3A, in the 2D-TLC system mCMP is well separated from ATP as well as from CMP and TMP, which were formed by phosphorylation of cytidine and thymidine, which co-purify with the dNK. No mCMP was detected in mC fractions of oligo-C (data not shown). The 2D-TLC analyses clearly demonstrate the presence of mCMP in the dNK-labeled mC-fraction of DNA from adult D.melanogaster (Figure 3A). This result strongly supports the conclusions drawn from the 1D-TLC analyses. Finally, we subjected a new sample of DNA from adult D.melanogaster to HPLC separation, and collected 20 fractions each for 30 s, from 5 to 15 min. The fractions were incubated with dNK and the labeled nucleotides analyzed by 2D-TLC. As shown in Figure 3B, methylcytidine is only present in the two fractions collected between 11 and 12 min, demonstrating that the results of the dNK labeling are in excellent agreement with the elution profile of the bases from the HLPC column as detected by OD260nm (Figure 2A). This result confirms the presence of methylcytosine in the DNA of D.melanogaster.
Estimation of the amount of methylcytosine in the DNA of D.melanogaster
To estimate the degree of DNA methylation in D.melanogaster, we first determined the mC/C ratio of the DNA from calf thymus by comparing the amounts of cytidine and methylcytidine in the corresponding HPLC fractions. To this end, several dilutions of the C- and mC-fractions from each HPLC run were incubated with deoxynucleoside kinase and analyzed by 1D-TLC to find dilutions of each fraction that resulted in the same signal. According to this analysis, ∼1–5% of all cytidines in calf thymus DNA are methylated (data not shown). This amount agrees with the ratio of the areas of the cytidine and methylcytidine peaks in the HPLC analysis (∼2% methylcytidine with respect to cytidine) and to reported amounts of methylcytosine in mammalian DNA (2–5%). To evaluate the amount of methylcytosine in the DNA of D.melanogaster, the relative intensities of the mCMP spots of D.melanogaster DNA and calf thymus DNA on the 2D-TLC plates were compared (Figure 3A). The mCMP spot of D.melanogaster DNA turned out to be 25% of that of calf thymus DNA. Considering the amounts of DNA applied to the HPLC in both cases, a ratio of 1/1300 for methylcytosine/cytosine in D.melanogaster DNA can be estimated—∼50 times lower than in mammals. This low amount is comparable to values determined with an immunological method (1/2500) (Achwal et al., 1984). It explains why the modified base could not be detected in the McrBC cleavage assays, because if only 1 in 1000 cytosines is modified the average distance of modified McrBC recognition sites is 20 000 bp, much too far to induce DNA cleavage by McrBC (Stewart and Raleigh, 1998). Similarly, such low amounts of methylation are not detectable by conventional HPLC or restriction protection analyses.
Conclusions
The results obtained with the D.melanogaster DNA provide strong evidence for the presence of 5-methylcytosine in D.melanogaster. Moreover, the combination of enzymatic hydrolysis, HPLC, dNK labeling and 2D-TLC provides high specificity and sensitivity for the detection of methylcytosine in genomic DNA, which is due to several advantages of this procedure. First, with HPLC, methylcytidine can be quantitatively separated from all other nucleosides, thus allowing the detection of methylcytidine in the virtual absence of other nucleosides. Secondly, the deoxynucleoside kinase allows the radioactive labeling of deoxynucleosides with high specific activity and selectivity. Thirdly, with the 2D-TLC analysis, all labeled bases can be identified unequivocally.
The detection of methylcytosine in the DNA of D.melanogaster is in agreement with several observations: (i) D.melanogaster contains a gene for a putative DNA methyltransferase (Hung et al., 1999; Tweedie et al., 1999); (ii) proteins that resemble methylcytosine binding proteins have been identified in D.melanogaster (Hung et al., 1999; Tweedie et al., 1999); (iii) other insects also contain methylcytosine (Adams et al., 1979; Deobagkar et al., 1982; Patel and Gopinathan, 1987; Field, 1989; Devajyothi and Brahmachari, 1992; Tweedie et al., 1999); (iv) the artificial introduction of high levels of DNA methylation by transgenic expression of the murine Dnmt3a DNA methyltransferase in D.melanogaster leads to lethality during development (Lyko et al., 1999), indicating that methylation causes a biological response; and (v) 5-azacytidine is cytotoxic in D.melanogaster and leads to increased levels of recombination (Katz, 1985; Osgood and Seward, 1989; Pontecorvo et al., 1992). This drug is toxic because it forms covalent complexes between the DNA and DNA methyltransferases (Ferguson et al., 1997; Jackson-Grusby et al., 1997) that require recombination for repair (Barbe et al., 1986; Bhagwat and Roberts, 1987; Lal et al., 1988).
Several roles of cytosine methylation have been suggested in mammals. Whereas some of these functions, like reduction of transcriptional noise (Bird, 1995) or protection from selfish genetic elements (Bestor and Tycko, 1996), require an exhaustive methylation of the genome, others do not. For example, DNA methylation within the promoter regions of a few genes would suffice for an epigenetic control of gene expression during development or to establish a genetic imprint.
Materials and methods
Materials
DNA preparations from 300 adult flies (D.melanogaster oreR) or corresponding amounts of individuals in earlier stages of development underwent seven steps of phenol/chloroform extraction and ethanol precipitation. Oligonucleotides were purchased from Interactiva (Ulm, Germany) in an HPLC-purified form. Calf thymus DNA was from Sigma and yeast total RNA from Boehringer Mannheim. All standard nucleotides and nucleosides were purchased from Sigma.
McrBC digestion
For the McrBC cleavage reactions, 0.5–1 µg of the DNA preparations from D.melanogaster were incubated with 20 U of McrBC (NEB) in an assay buffer provided by the supplier, in the absence or presence of 1 mM GTP for 1 h. Since McrBC is strictly dependent on GTP for DNA cleavage, specific DNA cleavage can only be observed in the presence of GTP (Figure 1). DNA cleavage was analyzed by agarose gel electrophoresis.
HPLC analyses
For the HPLC analyses, samples were treated with RNase A, purified over QiaAmp columns (Qiagen) and precipitated with ethanol. HPLC separation of the nucleosides was carried out as described (Jeltsch et al., 1999). Approximately 5 µg DNA of each preparation were degraded to nucleosides using P1 nuclease, Serratia marcescens nuclease and shrimp alkaline phosphatase (Amersham), and subjected to reversed-phase HPLC. To increase the resolution in the region of the methylcytidine peak, a modified gradient was used: t = 0 min, 100% solvent A (100 mM triethylammonium acetate pH 6.5); linear gradient to t = 15 min, 15% solvent B (100 mM triethylammonium acetate pH 6.5, 30% acetonitrile); linear gradient to t = 30 min, 100% solvent B; t = 35 min, 100% solvent B. As a control, two oligonucleotides [oligo-C: d(AGACGGTGGTCGGGTTCGACG); oligo-mC: d(GAAGmCTGGGAmCTTmCmCGGAGGAGAGTGmCAA)], a PCR product and DNA from calf thymus were hydrolyzed to nucleosides and subjected to HPLC separation. Cytidine elutes after 8 ± 0.5 min and methylcytidine after 11 ± 0.5 min. In the preparative runs, two fractions were collected: a C-fraction (7–9 min), which contains the cytidine, and a mC-fraction (10–12 min), containing the methylcytidine. The fractions were dried in a vacuum, washed twice in water/ethanol (90:10) and finally dissolved in 10 µl of water.
dNK labeling and 1D-TLC analyses
A clone containing the gene for the multisubstrate dNK was obtained form M.Johansson (Stockholm, Sweden) and the enzyme was purified as described (Johansson et al., 1999). To detect deoxycytidine and deoxymethylcytidine in the HPLC fractions, 6 µl of the dissolved samples were incubated with 30 nM dNK in 15 µl of buffer (50 mM Tris–HCl pH 8.5, 5 mM MgCl2) containing 0.25 µl of [γ-32P]ATP (370 MBq/ml; NEN) for 3 h. Reaction mixture (1.5 µl) was spotted onto a TLC plate (Polygram CEL 300 PEI, 5 × 20 cm; Machery-Nagel, Düren, Germany). The TLC was run in 1 M KH2PO4, dried and the radioactivity analyzed using an instant imager (Canberra Packard). Prior to the phosphorylation reaction, the deoxyribonucleotide kinase was incubated with 40 nM unlabeled ATP for 10 min. Comparison of the efficiencies of phosphorylation of the mC-fraction from oligo-mC and the C-fraction from oligo-C confirmed that dNK equally accepts deoxycytidine and deoxymethylcytidine as substrates (data not shown).
2D-TLC analyses
For the 2D-TLC analyses, samples were treated as described, except that only 2 µl of the dissolved HPLC fractions were used for dNK labeling. The 2D-TLCs were carried out on cellulose TLC plates (Cellulose CEL 400-10, 20 × 20 cm; Macherey-Nagel, Düren, Germany). The first dimension was run in isobutyric acid/water/ammonia (66:33:1, v/v/v). The plate was dried in air and developed in the second dimension with isopropanol/concentrated HCl/water (70:15:15, v/v/v). The plate was dried and the radioactivity analyzed using an instant imager (Canberra Packard).
Acknowledgments
Acknowledgements
Expert technical assistance by H.Büngen is gratefully acknowledged. We thank M.Johansson for providing a clone of the multisubstrate dNK from D.melanogaster. Thanks are due to U.Pieper and D.Groll for supplying us with McrBC, M.Henze for help with the handling of D.melanogaster, and A.Pingoud for fruitful discussions and comments. This work was supported by the Deutsche Forschungsgemeinschaft (JE 252/1-1).
References
- Achwal C.W., Iyer,C.A. and Chandra,H.S. (1983) Immunochemical evidence for the presence of 5mC, 6mA and 7mG in human, Drosophila and mealybug DNA. FEBS Lett., 158, 353–358. [DOI] [PubMed] [Google Scholar]
- Achwal C.W., Ganguly,P. and Chandra,H.S. (1984) Estimation of the amount of 5-methylcytosine in Drosophila melanogaster DNA by amplified ELISA and photoacoustic spectroscopy. EMBO J., 3, 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R.L., McKay,E.L., Craig,L.M. and Burdon,R.H. (1979) Methylation of mosquito DNA. Biochim. Biophys. Acta, 563, 72–81. [DOI] [PubMed] [Google Scholar]
- Alberts B., Bray,D., Lewis,J., Raff,M., Roberts,K. and Watson,J.D. (1994) Molecular Biology of the Cell. Garland Publishing, Inc., New York, NY. [Google Scholar]
- Barbe J., Gibert,I. and Guerrero,R. (1986) 5-azacytidine: survival and induction of the SOS response in Escherichia coli K-12. Mutat. Res., 166, 9–16. [DOI] [PubMed] [Google Scholar]
- Bestor T.H. and Tycko,B. (1996) Creation of genomic methylation patterns. Nature Genet., 12, 363–367. [DOI] [PubMed] [Google Scholar]
- Bestor T.H. and Verdine,G.L. (1994) DNA methyltransferases. Curr. Opin. Cell Biol., 6, 380–389. [DOI] [PubMed] [Google Scholar]
- Bhagwat A.S. and Roberts,R.J. (1987) Genetic analysis of the 5-azacytidine sensitivity of Escherichia coli K-12. J. Bacteriol., 169, 1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A.P. (1995) Gene number, noise reduction and biological complexity. Trends Genet., 11, 94–100. [DOI] [PubMed] [Google Scholar]
- Cheng X. (1995) Structure and function of DNA methyltransferases. Annu. Rev. Biophys. Biomol. Struct., 24, 293–318. [DOI] [PubMed] [Google Scholar]
- Colot V. and Rossignol,J.L. (1999) Eukaryotic DNA methylation as an evolutionary device. BioEssays, 21, 402–411. [DOI] [PubMed] [Google Scholar]
- Deobagkar D.N., Muralidharan,K., Devare,S.G., Kalghatgi,K.K. and Chandra,H.S. (1982) J. Biosci., 4, 513–526. [Google Scholar]
- Devajyothi C. and Brahmachari,V. (1992) Detection of a CpA methylase in an insect system: characterization and substrate specificity. Mol. Cell. Biochem., 110, 103–111. [DOI] [PubMed] [Google Scholar]
- Doerfler W. (1983) DNA methylation and gene activity. Annu. Rev. Biochem., 52, 93–124. [DOI] [PubMed] [Google Scholar]
- Ehrlich M. and Wang,R.Y.-H. (1981) 5-methylcytosine in eukaryotic DNA. Science, 212, 1350–1357. [DOI] [PubMed] [Google Scholar]
- Feil R. and Khosla,S. (1999) Genomic imprinting in mammals: an interplay between chromatin and DNA methylation? Trends Genet., 15, 431–435. [DOI] [PubMed] [Google Scholar]
- Ferguson A.T., Vertino,P.M., Spitzner,J.R., Baylin,S.B., Muller,M.T. and Davidson,N.E. (1997) Role of estrogen receptor gene demethylation and DNA methyltransferase. DNA adduct formation in 5-aza-2′-deoxycytidine-induced cytotoxicity in human breast cancer cells. J. Biol. Chem., 272, 32260–32266. [DOI] [PubMed] [Google Scholar]
- Field L.M. (1989) Changes of DNA methylation are associated with loss of insecticide resistance in the peach-potato aphid Myzus persicae. FEBS Lett., 243, 323–327. [Google Scholar]
- Frankel A.D. and Smith,H.O. (1981) Restriction and modification enzymes detect no allosteric changes in DNA with bound lac repressor or RNA polymerase. J. Mol. Biol., 146, 611–619. [DOI] [PubMed] [Google Scholar]
- Hansen R.S., Wijmenga,C., Luo,P., Stanek,A.M., Canfield,T.K., Weemaes,C.M. and Gartler,S.M. (1999) The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc. Natl Acad. Sci. USA, 96, 14412–14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung M.S., Karthikeyan,N., Huang,B., Koo,H.C., Kiger,J. and Shen,C.J. (1999) Drosophila proteins related to vertebrate DNA (5-cytosine) methyltransferases. Proc. Natl Acad. Sci. USA, 96, 11940–11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Grusby L., Laird,P.W., Magge,S.N., Moeller,B.J. and Jaenisch,R. (1997) Mutagenicity of 5-aza-2′-deoxycytidine is mediated by the mammalian DNA methyltransferase. Proc. Natl Acad. Sci. USA, 94, 4681–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch A., Christ,F., Fatemi,M. and Roth,M. (1999) On the substrate specificity of DNA methyltransferases: adenine-N6 DNA methyltransferases also modify cytosine residues at position N4. J. Biol. Chem., 274, 19538–19544. [DOI] [PubMed] [Google Scholar]
- Johansson M., van Rompay,A.R., Degreve,B., Balzarini,J. and Karlsson,A. (1999) Cloning and characterization of the multisubstrate deoxyribonucleoside kinase of Drosophila melanogaster. J. Biol. Chem., 274, 23814–23819. [DOI] [PubMed] [Google Scholar]
- Jones P.A. and Laird,P.W. (1999) Cancer epigenetics comes of age. Nature Genet., 21, 163–167. [DOI] [PubMed] [Google Scholar]
- Jones P.L. and Wolffe,A.P. (1999) Relationships between chromatin organization and DNA methylation in determining gene expression. Semin. Cancer Biol., 9, 339–347. [DOI] [PubMed] [Google Scholar]
- Katz A.J. (1985) Genotoxicity of 5-azacytidine in somatic cells of Drosophila. Mutat. Res., 143, 195–199. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Hanyu,N. and Nishimura,S. (1987) Analysis of modified nucleosides and nucleotide sequence of tRNA. Methods Enzymol., 155, 379–396. [DOI] [PubMed] [Google Scholar]
- Lal D., Som,S. and Friedman,S. (1988) Survival and mutagenic effects of 5-azacytidine in Escherichia coli. Mutat. Res., 193, 229–236. [DOI] [PubMed] [Google Scholar]
- Lewin B. (1997) Genes VI. Oxford University Press, Oxford, UK. [Google Scholar]
- Li E., Bestor,T.H. and Jaenisch,R. (1992) Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell, 69, 915–926. [DOI] [PubMed] [Google Scholar]
- Lyko F., Ramsahoye,B.H., Kashevsky,H., Tudor,M., Mastrangelo,M.A., Orr-Weaver,T.L. and Jaenisch,R. (1999) Mammalian (cytosine-5) methyltransferases cause genomic DNA methylation and lethality in Drosophila. Nature Genet., 23, 363–366. [DOI] [PubMed] [Google Scholar]
- Mathews C.K. and van Holde,K.E. (1995) Biochemistry. The Benjamin/Cummings Publishing Company, Inc., Menlo Park, CA. [Google Scholar]
- Migeon B.R. (1994) X-chromosome inactivation: molecular mechanisms and genetic consequences. Trends Genet., 10, 230–235. [DOI] [PubMed] [Google Scholar]
- Oakeley E.J. (1999) DNA methylation analysis: a review of current methodologies. Pharmacol. Ther., 84, 389–400. [DOI] [PubMed] [Google Scholar]
- Okano M., Xie,S. and Li,E. (1998) Dnmt2 is not required for de novo and maintenance methylation of viral DNA in embryonic stem cells. Nucleic Acids Res., 26, 2536–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M., Bell,D.W., Haber,D.A. and Li,E. (1999) DNA methyl transferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell, 99, 247–257. [DOI] [PubMed] [Google Scholar]
- Osgood C.J. and Seward,S.M. (1989) 5-azacytidine induces sex chromosome loss and interchange in immature germ cells of Drosophila mei-9 males. Environ. Mol. Mutagen., 14, 135–145. [DOI] [PubMed] [Google Scholar]
- Patel C.V. and Gopinathan,K.P. (1987) Determination of trace amounts of 5-methylcytosine in DNA by reverse-phase high-performance liquid chromatography. Anal. Biochem., 164, 164–169. [DOI] [PubMed] [Google Scholar]
- Pinarbasi E., Elliott,J. and Hornby,D.P. (1996) Activation of a yeast pseudo DNA methyltransferase by deletion of a single amino acid. J. Mol. Biol., 257, 804–813. [DOI] [PubMed] [Google Scholar]
- Pontecorvo G., Avitabile,A., Esposito,G., Migliaccio,G. and Carfagna,M. (1992) Induced crossing-over in Drosophila melanogaster germ cells of DNA repair-proficient and repair-deficient (mei-9L1) males following larval feeding with 5-azacytidine and mitomycin C. Mutat. Res., 266, 93–98. [DOI] [PubMed] [Google Scholar]
- Rae P.M. and Steele,R.E. (1979) Absence of cytosine methylation at C-C-G-G and G-C-G-C sites in the rDNA coding regions and intervening sequences of Drosophila and the rDNA of other insects. Nucleic Acids Res., 6, 2987–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A. and Riggs,D. (1980) DNA methylation and gene function. Science, 210, 604–610. [DOI] [PubMed] [Google Scholar]
- Stewart F.J. and Raleigh,E.A. (1998) Dependence of McrBC cleavage on distance between recognition elements. Biol. Chem., 379, 611–616. [PubMed] [Google Scholar]
- Szyf M., Gruenbaum,Y., Urieli-Shoval,S. and Razin,A. (1982) Studies on the biological role of DNA methylation: V. The pattern of E.coli DNA methylation. Nucleic Acids Res., 10, 7247–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate P.H. and Bird,A.P. (1993) Effects of DNA methylation on DNA-binding proteins and gene expression. Curr. Opin. Genet. Dev., 3, 226–231. [DOI] [PubMed] [Google Scholar]
- Toyota M. and Issa,J.P. (1999) CpG island methylator phenotypes in aging and cancer. Semin. Cancer Biol., 9, 349–357. [DOI] [PubMed] [Google Scholar]
- Tweedie S., Charlton,J., Clark,V. and Bird,A. (1997) Methylation of genomes and genes at the invertebrate–vertebrate boundary. Mol. Cell. Biol., 17, 1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie S., Ng,H.-H., Barlow,A.L., Turner,B.M., Hendrich,B. and Bird,A. (1999) Vestiges of a DNA methylation system in Drosophila melanogaster? Nature Genet., 23, 389–390. [DOI] [PubMed] [Google Scholar]
- Van den Wyngaert I., Sprengel,J., Kass,S.U. and Luyten,W.H.M.L. (1998) Cloning and analysis of a novel human putative DNA methyltransferase. FEBS Lett., 426, 283–289. [DOI] [PubMed] [Google Scholar]
- Voet D. and Voet,J.G. (1995) Biochemistry. John Wiley and Sons, Inc., Somerset, NJ. [Google Scholar]
- Xu G.-L. et al. (1999) Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature, 402, 187–191. [DOI] [PubMed] [Google Scholar]
- Yoder J.A. and Bestor,T.H. (1998) A candidate mammalian DNA methyltransferase related to pmt1p of fission yeast. Hum. Mol. Genet., 7, 279–284. [DOI] [PubMed] [Google Scholar]