Abstract
Background
Despite a higher burden of standard atrial fibrillation (AF) risk factors, African Americans have a lower risk of AF than whites. It is unknown if the higher riskis due to genetic or environmental factors. As African Americans have varying degrees of European ancestry, we sought to test the hypothesis that European ancestry is an independent risk factor for AF.
Methods and Results
We studied whites (n=4,543) and African Americans (n=822) in the Cardiovascular Health Study (CHS) and whites (n=10,902) and Africa Americans (n=3,517) in the Atherosclerosis Risk in Communities (ARIC) Study (n=3,517). Percent European ancestry in African Americans was estimated using 1,747 ancestry informative markers (AIMs) from the Illumina custom ITMAT-Broad-CARe (IBC) array. Among African Americans without baseline AF, 120 of 804 CHS participants and 181 of 3,517 ARIC participants developed incident AF. A meta-analysis from the two studies revealed that every 10% increase in European ancestry increased the risk of AF by 13% (HR 1.13, 95% CI 1.03–1.23, p=0.007). After adjusting for potential confounders, European ancestry remained a predictor of incident AF in each cohort alone, with a combined estimated hazard ratio for each 10% increase in European ancestry of 1.17 (95% CI 1.07–1.29, p=0.001). A second analysis using 3,192 AIMs from a genome wide Affymetrix 6.0 array in ARIC African Americans yielded similar results.
Conclusion
European ancestry predicted risk of incident AF. Our study suggests that investigating genetic variants contributing to differential AF risk in individuals of African versus European ancestry will be informative.
Keywords: Atrial Fibrillation Genetics, Ancestry, African Americans
Although atrial fibrillation (AF) is the most common sustained arrhythmia, the etiology remains incompletely understood.1 African Americans appear to be at a particularly low risk of AF,2, 3 a finding that is paradoxical considering that African Americans have a higher prevalence of many of the risk factors known to increase the likelihood of AF. Compared to whites, African Americans are more frequently hypertensive4 and more commonly exhibit heart failure,5 diabetes,6 and a larger body mass index (BMI).7, 8 Whether environmental or genetic, it would therefore appear that there are novel factors either protecting African Americans from AF or making whites especially prone to AF that remain undetermined. In order to determine if genetic differences are responsible, it would be useful to know if European ancestry is an independent risk factor for AF.
African Americans are a genetically heterogeneous group comprised of both African and European ancestral genomes. Admixture analysis uses ancestry informative markers(AIMs), or genetic markers known to have large allele frequency differences between ancestral populations, to determine the percent European or African ancestry in a given individual.9 Genetic admixture analysis provides a method to investigate whether complex phenotypes may be associated with genetic ancestral background in admixed populations. Thus, genetic heterogeneity of African Americans provides a unique opportunity to determine if ancestry is an important factor in predisposing individuals to AF.10
Because AF is more common in whites, we hypothesized that increasing European ancestry would be associated with elevated risk of AF in African Americans. Therefore, we sought to understand whether genetic ancestry is associated with incident AF in African American participants enrolled in two large cohorts: the Cardiovascular Health Study (CHS) and the Atherosclerosis Risk in Communities (ARIC) study.
Methods
The Cardiovascular Health Study
The design of the Cardiovascular Health Study (CHS) has been previously described.11 Briefly, between 1989 and 1990, 5201 men and women aged 65 years or older were enrolled using random samples from Medicare eligibility lists from 4 communities: Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA. A second cohort of 687 African-American participants was enrolled between 1992 and 1993. All participants underwent a comprehensive examination at baseline, which included a thorough medical history, measurement of height and weight, laboratory testing, a 12-lead ECG, current medications, and assessment of cardiovascular disease status. Alcohol intake defined as number of alcoholic drinks per weeks was determined. Details of the laboratory methods and quality-control procedures and have been reported elsewhere.11–13 Hypertension at baseline was defined as a blood pressure of ≥ 140/90 mm Hgor a physician diagnosis of hypertension. Diabetes was defined as known diabetes requiring insulin or hypoglycemic medication or a fasting blood sugar ≥ 126 mg/dL. ECG diagnosis of left ventricular hypertrophy was determined by the Minnesota Code. A history of MI at baseline was determined by self-report and confirmed by components of the baseline examination or, if necessary, by a validation protocol that included either the review of medical records or surveys of treating physicians.14 Cases of AF were identified by ECG obtained at annual study visits through 1999 and hospital discharge diagnosis during a median of 10 years of follow-up.
Atherosclerosis Risk in Communities Study
The Atherosclerosis Risk in Communities (ARIC) Study has been previously described.15 Briefly, this is a population-based prospective study of cardiovascular disease in a cohort of 15,792 participants, 45 to 64 years of age at enrollment sampled from 4 US communities: suburbs of Minneapolis, MN; Washington County, MD; Jackson, MS; and Forsyth County, NC from 1987 to 1989. After an initial assessment, study participants were examined 3 additional times at intervals of roughly 3 years. In addition, participants were contacted yearly by phone to obtain information about hospital admissions and to ascertain vital status. At baseline, cigarette smoking, blood pressure, antihypertensive medication use, statin use, diabetes, weight, and height were measured using standardized methods.15 Baseline alcohol intake was determined and quantified into grams per week. Hypertension was defined as a blood pressure of ≥140/90 mm Hg or current use of antihypertensive medication. ECG-diagnosed left ventricular hypertrophy was considered present based on Cornell voltage criteria.16 History of myocardial infarction at baseline was defined as a self-reported history of a physician-diagnosed myocardial infarction or evidence of previous myocardial infarction in the baseline ECG. The methods utilized for AF ascertainment have previously been described in detail.17 Briefly, AF diagnoses were obtained from ECGs at baseline and the 3 follow-up visits, from ICD-9 codes from hospital discharge records, and ICD-9 and ICD-10 codes from death certificates. Two previous analyses within this population validated the diagnosis of incident AF based on hospital discharges.17
Those with AF on the baseline ECG were excluded from analysis of incident AF. The study was approved by institutional review boards at each participating center. Written informed consent was obtained from all participants.
Ancestry Informative Marker Selection
Ancestry informative markers (AIMS) were assessed using the Illumina custom IBC array (San Diego, CA) in both cohorts. AIMs from the Affymetrix genome wide human SNP array (Santa Clara, CA) were also analyzed in ARIC.
Illumina IBC Array
As part of the CARe study, a cardiovascular gene-centric 50 K SNP array, called the IBC array (for ITMAT-Broad-CARe), previously was developed and has been described in detail.18 AIMs (n=1,747) passing genotype quality-control criteria were used to estimate African versus European global ancestry. These SNPs were based on panels generated previously,19, 20 excluding SNPs failing Hardy-Weinberg equilibrium (P>0.01). The AIMs panels are listed within the IBC resource site: http://bmic.upenn.edu/cvdsnp/updates/ancestry_informative_markers-ibc-v1.xls. Percent global European ancestry was estimated in African Americans from both cohorts from these AIMs using the structure 2.3.1 software under the default parameters.21 Unrelated Northern European (CEU) and Western African (YRI) samples from the Hap Map Project were also genotyped on the IBC array and included in this analysis to pre-define the parental clusters.
Affymetrix 6.0 Array
After removing related pairs and outlier samples determined by quality control procedures using EIGENSOFT,22 756 YRI samples and 1,178 European American samples were used as parental samples to select the AIMs from the Affymetrix 6.0 genome-wide genotyping platform. TheSmartPCA22 software confirmed that the leading eigenvector reflected genetic differences between Europe and West Africa. A greedy algorithm was used to produce a list of SNPs at least 0.5 cM apart from each other, chosen to have large SNP loadings (the loading is a score of how much a SNP contributes to a given eigenvector), producing a list of 4,917 SNPs. Acheck on pairs of markers for detectable linkage disequilibrium (LD) was then performed: a statistic X, with a chi-square distribution with one degree-of-freedom, was computed at random, and any pair exhibiting a genetic distance of d cM or less was declared to be in LD if X >0.02d. For such a pair, the less informative SNP was deleted.23–25 This strategy produced 3,192 unlinked AIMs. Global European ancestry was estimated in African Americans enrolled in ARIC from these AIMs using the ANCESTRYMAP program under the default parameters.23 The CARe Project did not genotype the CHS African American cohort on Affymetrix 6.0.
Statistical Analyses
Normally distributed variables are presented as means ± SD and were compared using t-tests. Continuous variables that were not normally distributed are presented as medians and interquartile ranges (IQR) and was compared using the Wilcoxon Rank Sum test. Categorical variables were compared using the χ2 test. A Cox proportional hazards model was used to assess predictors of incident AF. Potential confounders were added to the multivariable Cox Proportional Hazards model based on previously established covariates known to be associated with African American race and AF. We assessed the log-linearity of the association between percent European ancestry and risk of AF using restricted cubic splines. Specifically, we used a chi-square test for the joint effect of the three non-linear spline components, adjusting for the linear effect. No evidence for non-linear response was found (all p>0.66), and therefore European ancestry was analyzed as a continuous variable. Hazard ratios are expressed as point estimates and 95% confidence intervals (CI). A random-effects meta-analytic method was used to calculate a pooled estimate of the unadjusted and adjusted hazard ratios for percent European ancestry. This method averages the hazard ratios from each study on the log scale, weighted by the inverse of the variance of each estimate, and calculates conservative confidence intervals under the assumption that the true effects in each study arise from a normal distribution. Heterogeneity of the study-specific estimates from ARIC and CHS was assessed using the Q-statistic.26 Stata version 11 (College Station, Texas) was used to perform statistical analyses. A two tailed p value less than 0.05 was considered statistically significant.
Results
Racial Differences and Cardiovascular Risk Factors
In CHS, of the 5,365 participants included in the CARe study, 4,543 were white and 822 were African American. Of the 14,419 ARIC participants included in the CARe study, 10,902 were white and 3,517 were African American. African Americans were younger than whites in both cohorts and more often female (Table 1). In both cohorts, compared to their white counterparts, African American participants had a higher average BMI, and a larger proportion had hypertension, diabetes, heart failure, and electrocardiographic evidence of left ventricular hypertrophy. One hundred and thirty-three (3%) white and 12 (2%) African American CHS participants had AF on their baseline ECG (p=0.018), and 27(0.3%) white and 6 (0.2%) African Americans ARIC CARe participants had AF on their baseline ECG (p=0.41). These participants with prevalent AF were excluded from the incident AF analyses.
Table 1.
Baseline differences between African Americans and whites enrolled in ARIC and CHS.
| Baseline Characteristic | CHS | ARIC | ||||
|---|---|---|---|---|---|---|
| African Americans | Whites | P value | African Americans | Whites | P value | |
| N | 822 | 4543 | 3,517 | 10,902 | ||
| Age (years) | 73 ± 6 | 73 ± 6 | 0.46 | 53 ± 6 | 54 ± 6 | <0.0001 |
| BMI (kg/m2) | 29 ± 6 | 26 ± 5 | <0.0001 | 30 ± 6 | 27 ± 5 | <0.0001 |
| Male | 310 (38%) | 1,987 (44%) | 0.001 | 1,310 (37%) | 5,119 (47%) | <0.001 |
| Hypertension | 604 (74%) | 2,534 (56%) | <0.001 | 1,942 (56%) | 2,912 (27%) | <0.001 |
| Diabetes | 196 (25%) | 664 (15%) | <0.001 | 675 (20%) | 954 (9%) | <0.001 |
| Heart failure | 55 (7%) | 202 (5%) | 0.006 | 231 (7%) | 412 (4%) | <0.001 |
| LVH* | 73 (9%) | 178 (4%) | <0.001 | 180 (5%) | 105 (1%) | <0.001 |
| MI | 67 (8%) | 450 (10%) | 0.12 | 133 (4%) | 460 (4%) | 0.29 |
Values are expressed as mean ± SD or n (%).
Determined by electrocardiogram using the Cornell voltage criteria.
Abbreviations: BMI, body mass index; LVH, left ventricular hypertrophy; MI, myocardial infarction.
In CHS, a total of 1,172 incident cases of AF were identified over a median 10 years of follow-up (IQR 6–13 years). In ARIC, a total of 1,068incident cases of AF were observed over a median 16 years of follow-up (IQR 15–17 years). In both cohorts, those with incident AF were older, more often male and more frequently exhibited hypertension, diabetes, heart failure, left ventricular hypertrophy, and a history of myocardial infarction (Table 2). Increasing BMI was also associated with AF in ARIC. In both cohorts, African Americans had a significantly lower risk of AF (Table 2), a difference that was more pronounced after adjustment for age, sex, BMI, hypertension, diabetes, heart failure, left ventricular hypertrophy, history of myocardial infarction, and study site. In CHS, African Americans had an adjusted 40% lower risk of AF (HR 0.60, 95% CI 0.49–0.74, p<0.001). In ARIC, African Americans had an adjusted 56% lower risk of AF (HR 0.44, 95% CI 0.27–0.72, p=0.001).
Table 2.
Predictors of incident atrial fibrillation in ARIC and CHS.
| CHS 95% | ARIC | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | Confidence Interval | P value | Hazard Ratio | 95% Confidence Interval | P value | |
| Age (per year) | 1.08 | 1.07–1.09 | <0.001 | 1.12 | 1.11–1.13 | <0.001 |
| BMI (per kg/m2) | 1.01 | 1.0–1.02 | 0.21 | 1.04 | 1.03–1.05 | <0.001 |
| Male | 1.59 | 1.42–1.78 | <0.001 | 1.73 | 1.53–1.96 | <0.001 |
| Hypertension | 1.50 | 1.33–1.70 | <0.001 | 2.11 | 1.87–2.38 | <0.001 |
| Diabetes | 1.53 | 1.32–1.78 | <0.001 | 2.09 | 1.792.45 | <0.001 |
| Heart failure | 3.04 | 2.43–3.81 | <0.001 | 2.93 | 2.39–3.59 | <0.001 |
| LVH* | 2.39 | 1.90–3.00 | <0.001 | 2.67 | 1.95–3.63 | <0.001 |
| History of MI | 2.22 | 1.88–2.61 | <0.001 | 3.56 | 2.90–4.36 | <0.001 |
| AA race | 0.75 | 0.62–0.91 | 0.003 | 0.65 | 0.55–0.76 | <0.001 |
Values are expressed as mean ± SD or n (%).
Determined by electrocardiogram using the Cornell voltage criteria
Abbreviations: LVH, left ventricular hypertrophy; MI, myocardial infarction; AA, African American.
European Ancestry and Risk for Atrial Fibrillation
In CHS, 120 cases of incident AF occurred among 804 African Americans without AF at baseline after a median follow-up of 10 years (IQR 5.5–10.5). The African American participants of CHS exhibited an average of 24 ± 15% European ancestry per the IBC array. Every 10% increase in European ancestry was associated with a 13% increase risk in AF (HR 1.13, 95% CI 1.004–1.27, p=0.043). After adjusting for age, sex, BMI, hypertension, diabetes, heart failure, left ventricular hypertrophy, history of myocardial infarction, and study site, every 10% increase in European ancestry was associated with a 16% increased risk of AF (HR 1.16, 95% CI 1.03–1.31, p=0.015). Adding baseline statin use, antihypertensive drug use, and alcohol intake as potential confounders to the multivariate model did not meaningfully change the results
Of 3,481 African Americans without baseline AF in ARIC followed for a median of 16 years (IQR 15–17), 181 developed incident AF. African Americans in ARIC had an average 17 ± 11% European ancestry. In unadjusted analysis, European ancestry was associated with a higher risk of AF, but this did not reach statistical significance: per the IBC array, the hazard ratio for each 10% increase in European ancestry was 1.12 (95% CI 0.99–1.27, p=0.065). However, after adjustment for age, sex, BMI, hypertension, diabetes, heart failure, left ventricular hypertrophy, history of myocardial infarction, and study site, each 10% increase in European ancestry was found to be associated with a statistically significant 18%increased risk of incident AF (Figure 1). Adding baseline statin use, antihypertensive drug use, and alcohol intake as potential confounders to the multivariate model did not meaningfully change the results.
Figure 1.
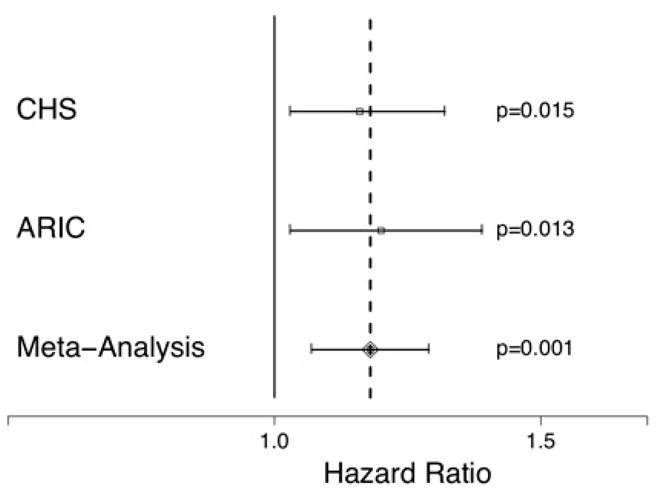
Hazard ratios of AF for each 10% increase in European ancestry as determined by the IBC array after adjusting for age, sex, body mass index, hypertension, diabetes, heart failure, left ventricular hypertrophy, myocardial infarction, and study site in African Americans enrolled in ARIC alone, CHS alone, and in a meta-analysis of the two cohorts. Y error bars denote 95% confidence intervals.
In the ARIC study, we were able to confirm the relationship observed between European ancestry and the risk of AF using a second, higher density genotyping array. Using 3,192 unlinked AIMs from the Affymetrix 6.0 array, we observed similar results to the 1,747 AIMs from the IBC array. With the Affymetrix 6.0 array in ARIC, every 10% increase in European ancestry was associated with a 20% increased risk of AF (95% CI 1.03–1.39, p=0.017) after adjusting for age, sex, BMI, hypertension, diabetes, heart failure, left ventricular hypertrophy, history of myocardial infarction, and study site. Adding baseline statin use, antihypertensive drug use, and alcohol intake as potential confounders to the multivariate model did not meaningfully change the results.
A meta-analysis of the IBC array AIMs in CHS and ARIC revealed statistically significant results for both the unadjusted (HR 1.13, 95% CI 1.03–1.23, p=0.007) and adjusted (Figure 1) associations between European ancestry and incident AF. There was no evidence of heterogeneity in the unadjusted (p=0.92) or adjusted (p=0.73) meta-analysis.
Discussion
In two community based, multi-racial cohorts, African Americans had a lower incidence of AF despite a higher prevalence of many traditional risk factors for AF including hypertension, diabetes, heart failure, and larger BMI. By taking advantage of admixture mapping, we found that an increasing percentage of European ancestry was associated with an increased risk of AF in African Americans. Our results were consistent across two distinct cohorts, genotyping assays, and analytic methods. Thus, racial differences in risk of AF between African Americans and whites appear to be genetically mediated.
Although risk factors for AF are well known, they do not explain all attributable risk for AF.27,28 In fact, it remains a mystery why some individuals with heart failure and large atria never develop AF while others with less cardiovascular disease do. To our knowledge, our study is the first to demonstrate that a quantitative assessment of European ancestry predicts AF onset. The finding persisted after accounting for standard AF risk factors. This suggests that either African ancestry provides some protective effect or that something about European ancestry increases the risk. The fact that African Americans exhibit significantly less AF despite the preponderance of risk factors suggests that other mediators at work are likely quite powerful.
Of particular importance is that African Americans exhibited substantially less AF despite having an average larger BMI and more often having hypertension, diabetes, heart failure, and left ventricular hypertrophy. This apparent paradox between established AF risk factors and AF incidence suggests that the association between European ancestry and AF is due to some as yet unknown factor. However, presumably the consequences of AF, including thromboembolic complications in particular, would follow the same positive correlation with European ancestry. Future research can determine if embolic strokes, myocardial infarctions attributed to thromboemboli, and tachycardia induced cardiomyopathy are more common in white populations and/or those with more European ancestry.
That AF is inherited has been well described in families with rare mutations,29–31 and a family history of AF is known to be a risk factor for the disease, particularly in those with lone AF.32–34 However, the finding that European ancestry itself is an independent risk factor implies that the heritability of AF may be substantially broader in scope than previously recognized.
According to the “out of Africa” hypothesis, there was a single migration of modern Homo sapiens out of Africa, with a subsequent loss of genetic variation as that initial non-African founder population grew and expanded to the North and East.35, 36 Indeed, genetic clusters often correspond closely to collections of geographically and linguistically similar populations.37 Mutations occur at a certain rate, and an uncommon mutation may become a relatively common variant if that mutation occurs in the founder of a group residing in a particular geographic area (such as the European subcontinent). For example, there is now good evidence that the original settlement of the whole region of Australia arose from a single founder group.38 It is therefore possible that a founding population of the European subcontinent brought a genetic variation that increased the propensity to AF. Because AF generally develops at an older age and is not immediately lethal, it is certainly plausible that this genetic propensity could be transmitted to offspring without substantial natural selection against the putative variant(s). However, this genetic difference may in fact have offered some as yet unknown survival advantage in the European environment, with an increased risk of AF in older age representing a consequential byproduct.
Several previous studies have successfully leveraged clinically observed racial disparities in disease prevalence to identify genomicloci in other complex diseases. Admixture mapping studies have revealed loci associated with prostate cancer39 and hypertension40 in African American men. Based on epidemiologic observations that, like AF, multiple sclerosis is more common in those with more European ancestry, a genomic locus for this poorly understood disease was successfully identified.25 A similar approach may be informative in AF.
Our study has several limitations. First, there may be residual confounding. For example, it is possible that more European ancestry resulted in phenotypic differences associated with cultural practices or socio-economic status that may have affected AF risk or detection. One possibility is that those with more European ancestry had lighter skin pigmentation, which itself was associated some behavior or environmental exposure responsible for the increased AF risk. Indeed, as those with more European ancestry by definition had more ancestors from a different continent, there may have been behavioral factors inherent to different social or cultural norms that caused the differential AF risk. Second, our study did not include echocardiographic data. We relied on a clinical history of heart failure and electrocardiographic evidence of left ventricular hypertrophy. However, it is unlikely that data from echocardiograms would have changed our results—in fact, echocardiographic differences might provide information regarding potential mediators of the racial differences. Although a small difference, we previously reported that African Americans have a shorter left atrial diameter than whites, albeit no difference in left atrial volumes.41 While our outcome was restricted to incident AF, prevalent AF for both studies was defined as AF present on the baseline ECG. Therefore, it is possible that some of those deemed to have incident AF in fact had a known previous history of AF. However, as the primary predictor was genetic, it would appear unlikely that the misclassification of some prevalent AF patients as incident AF patients would substantially affect the results. Finally, as AF was ascertained via serial clinic visits and hospitalizations, we can not exclude the possibility that some paroxysmal AF patients or asymptomatic AF patients were misclassified as not having AF. Such misclassification would generally result in a type II error and therefore should not explain our positive findings. However, it is possible that race, or continental ancestry, is associated with different types of AF rather than a simple presence or absence of AF.
Conclusion
We found that European ancestry is a risk factor for incident AF. This suggests that some of the difference in AF risk across races is probably genetic and that unknown factors independent of recognized risk factors likely affect susceptibility to AF. Identification of these factors might reveal new avenues for treating and possibly preventing this important disease.
Clinical Summary.
Although atrial fibrillation (AF) is the most common sustained arrhythmia encountered in clinical practice, the exact etiology of the disease remains poorly understood. Previous data suggest that African Americans have a lower risk of AF despite a higher burden of AF risk factors, and it is not known if this is due to environmental or genetic factors. As African Americans represent a population admixed with African and European ancestry, we sought to test the hypothesis that a greater degree of European ancestry is associated with a greater risk of AF. African Americans in both the Cardiovascular Health Study (CHS) and the Atherosclerosis Risk in Communities (ARIC) study had a significantly lower risk of AF despite more commonly exhibiting AF risk factors. Using ancestral informative markers (AIMs) from the ITMAT-Broad-CARe (IBC) array, we found that increasing European ancestry was independently associated with incident AF in both cohorts. Using AIMs from the Affymetrix 6.0 array in ARIC, we found similar results. This is the first study to demonstrate that European ancestry is an independent risk factor for AF. The paradoxical finding that whites have more AF despite less AF risk factors suggests that something associated with European ancestry, presumably a genetic variant, is a common and powerful component in determining propensity to AF independent of traditional risk factors such as hypertension, diabetes, heart failure, and obesity.
Acknowledgments
The authors wish to acknowledge the support of the National Heart, Lung, and Blood Institute and the contributions of the research institutions, study investigators, field staff and study participants in creating this resource for biomedical research. We would like to thank Drs. Richard S. Cooper and Philip L. De Jager for providing the Nigerian and European American Affymetrix 6.0 genotype datasets, as well as Dr. Nick Patterson and Arti Tandon for curating the list of ancestry informative markers on the Affymetrix 6.0 genotyping platform.
Sources of Funding
This work was made possible by grant number KL2 RR024130 (G.M.M.) from the National Center for Research Resources (NCRR), a component of the NIH. The Candidate-gene Association Resource (CARe) project (N01-HC-65226) involves nine parent studies that contributed parent study data, ancillary study data, and DNA samples through the Massachusetts Institute of Technology -Broad Institute to create this genotype/phenotype data base for wide dissemination to the biomedical research community:
Atherosclerotic Risk in Communities (ARIC): University of North Carolina at Chapel Hill (N01-HC-55015), Baylor Medical College (N01-HC-55016), University of Mississippi Medical Center (N01-HC-55021), University of Minnesota (N01-HC-55019), Johns Hopkins University (N01-HC-55020), University of Texas, Houston (N01-HC-55022), University of North Carolina, Forsyth County (N01-HC-55018); R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. This study was also supported by grant RC1-HL099452 from NIH/NHLBI and grant 09SDG2280087 from the American Heart Association; Cardiovascular Health Study (CHS): University of Washington (N01-HC-85079), Wake Forest University (N01-HC-85080), Johns Hopkins University (N01-HC-85081), University of Pittsburgh (N01-HC-85082), University of California, Davis (N01-HC-85083), University of California, Irvine (N01-HC-85084), New England Medical Center (N01-HC-85085), University of Vermont (N01-HC-85086), Georgetown University (N01-HC-35129), Johns Hopkins University (N01 HC-15103), University of Wisconsin (N01-HC-75150), Geisinger Clinic (N01-HC-45133), University of Washington (N01 HC-55222, U01 HL080295); Cleveland Family Study (CFS): Case Western Reserve University (NIH HL 46380, M01RR00080); Cooperative Study of Sickle Cell Disease (CSSCD): University of Illinois (N01-HB-72982, N01-HB-97062), Howard University (N01-HB-72991, N01-HB-97061), University of Miami (N01-HB-72992, N01-HB-97064), Duke University (N01-HB-72993), George Washington University (N01-HB-72994), University of Tennessee (N01-HB-72995, N01-HB-97070), Yale University (N01-HB-72996, N01-HB-97072), Children’s Hospital-Philadelphia (N01-HB-72997, N01-HB-97056), University of Chicago (N01-HB-72998, N01-HB-97053), Medical College of Georgia (N01-HB-73000, N01-HB-97060), Washington University (N01-HB-73001, N01-HB-97071), Jewish Hospital and Medical Center of Brooklyn (N01-HB-73002), Trustees of Health and Hospitals of the City of Boston, Inc., (N01-HB-73003), Children’s Hospital-Oakland (N01-HB-73004, N01-HB-97054), University of Mississippi (N01-HB-73005), St. Luke’s Hospital-New York (N01-HB-73006), Alta Bates-Herrick Hospital (N01-HB-97051), Columbia University (N01-HB-97058), St. Jude’s Children’s Research Hospital (N01-HB-97066), Research Foundation, State University of New York-Albany (N01-HB-97068, N01-HB-97069), New England Research Institute (N01-HB-97073), Interfaith Medical Center-Brooklyn (N01-HB-97085); Coronary Artery Risk in Young Adults (CARDIA): University of Alabama at Birmingham (N01-HC-48047), University of Minnesota (N01-HC-48048), Northwestern University (N01-HC-48049), Kaiser Foundation Research Institute (N01-HC-48050), University of Alabama at Birmingham (N01-HC-95095), Tufts-New England Medical Center (N01-HC-45204), Wake Forest University (N01-HC-45205), Harbor-UCLA Research and Education Institute (N01-HC-05187), University of California, Irvine (N01-HC-45134, N01-HC-95100); Framingham Heart Study (FHS): Boston University (N01-HC-25195, R01HL092577-01A1, RO1 HL076784, R01 AG028321, 6R01-NS 17950); Jackson Heart Study (JHS): Jackson State University (N01-HC-95170), University of Mississippi (N01-HC-95171), Tougaloo College (N01-HC-95172); Multi-Ethnic Study of Atherosclerosis (MESA): University of Washington (N01-HC-95159), Regents of the University of California (N01-HC-95160), Columbia University (N01-HC-95161), Johns Hopkins University (N01-HC-95162, N01-HC-95168), University of Minnesota (N01-HC-95163), Northwestern University (N01-HC-95164), Wake Forest University (N01-HC-95165), University of Vermont (N01-HC-95166), New England Medical Center (N01-HC-95167), Harbor-UCLA Research and Education Institute (N01-HC-95169), Cedars-Sinai Medical Center (R01-HL-071205), University of Virginia (subcontract to R01-HL-071205); Sleep Heart Health Study (SHHS): Johns Hopkins University (U01 HL064360), Case Western University (U01 HL063463), University of California, Davis (U01 HL053916), University of Arizona (U01 HL053938), University of Minnesota (relocating in 2006 to Univ Arizona) (U01 HL053934), University of Pittsburgh (U01 HL077813), Boston University (U01 HL053941), MedStar Research Institute (U01 HL063429), Johns Hopkins University (U01 HL053937).
This work was also supported by grants from the NIH: RC1-HL01056 to Drs. Benjamin and Alonso; and DA027021 to Dr. Ellinor; R01 HL088456 to Dr. Sotoodehnia. Dr. Lubitz is supported by a training grant in the Epidemiology of Cardiovascular Disease from the NIH (T32HL007575). Dr. Mehra is supported by the following: NHLBI K23 HL079114, American Heart Association National Scientist Development Award 0530188N and Central Society of Clinical Research Career Development Award.
Footnotes
Disclosures: none
References
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the An Ticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Ruo B, Capra AM, Jensvold NG, Go AS. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: the Epidemiology, Practice, Outcomes, and Costs of Heart Failure (EPOCH) study. J Am Coll Cardiol. 2004;43:429–435. doi: 10.1016/j.jacc.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 4.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, Jinagouda S, Shea S. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2004;17:963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter JS, Pugh JA, Monterrosa A. Non-insulin-dependent diabetes mellitus in minorities in the United States. Ann Intern Med. 1996;125:221–232. doi: 10.7326/0003-4819-125-3-199608010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Differences in prevalence of obesity among black, white, and Hispanic adults -United States, 2006–2008. MMWR Morb Mortal Wkly Rep. 2009;58:740–744. [PubMed] [Google Scholar]
- 8.Ford ES, Li C, Zhao G, Pearson WS, Capewell S. Trends in the prevalence of low risk factor burden for cardiovascular disease among United States adults. Circulation. 2009;120:1181–1188. doi: 10.1161/CIRCULATIONAHA.108.835728. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg NA, Li LM, Ward R, Pritchard JK. Informativeness of genetic markers for inference of ancestry. Am J Hum Genet. 2003;73:1402–1422. doi: 10.1086/380416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parra EJ, Marcini A, Akey J, Martinson J, Batzer MA, Cooper R, Forrester T, Allison DB, Deka R, Ferrell RE, Shriver MD. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63:1839–1851. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 12.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 13.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 14.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular diseasein the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 15.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 16.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75:565–572. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 17.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating BJ, Tischfield S, Murray SS, Bhangale T, Price TS, Glessner JT, Galver L, Barrett JC, Grant SF, Farlow DN, Chandrupatla HR, Hansen M, Ajmal S, Papanicolaou GJ, Guo Y, Li M, Derohannessian S, de Bakker PI, Bailey SD, Montpetit A, Edmondson AC, Taylor K, Gai X, Wang SS, Fornage M, Shaikh T, Groop L, Boehnke M, Hall AS, Hattersley AT, Frackelton E, Patterson N, Chiang CW, Kim CE, Fabsitz RR, Ouwehand W, Price AL, Munroe P, Caulfield M, Drake T, Boerwinkle E, Reich D, Whitehead AS, Cappola TP, Samani NJ, Lusis AJ, Schadt E, Wilson JG, Koenig W, McCarthy MI, Kathiresan S, Gabriel SB, Hakonarson H, Anand SS, Reilly M, Engert JC, Nickerson DA, Rader DJ, Hirschhorn JN, Fitzgerald GA. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PloS one. 2008;3:e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haiman CA, Patterson N, Freedman ML, Myers SR, Pike MC, Waliszewska A, Neubauer J, Tandon A, Schirmer C, McDonald GJ, Greenway SC, Stram DO, Le Marchand L, Kolonel LN, Frasco M, Wong D, Pooler LC, Ardlie K, Oakley-Girvan I, Whittemore AS, Cooney KA, John EM, Ingles SA, Altshuler D, Henderson BE, Reich D. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet. 2007;39:638–644. doi: 10.1038/ng2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price AL, Butler J, Patterson N, Capelli C, Pascali VL, Scarnicci F, Ruiz-Linares A, Groop L, Saetta AA, Korkolopoulou P, Seligsohn U, Waliszewska A, Schirmer C, Ardlie K, Ramos A, Nemesh J, Arbeitman L, Goldstein DB, Reich D, Hirschhorn JN. Discerning the ancestry of European Americans in genetic association studies. PLoS Genet. 2008;4:e236. doi: 10.1371/journal.pgen.0030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 23.Patterson N, Hattangadi N, Lane B, Lohmueller KE, Hafler DA, Oksenberg JR, Hauser SL, Smith MW, O’Brien SJ, Altshuler D, Daly MJ, Reich D. Methods for high-density admixture mapping of disease genes. Am J Hum Genet. 2004;74:979–1000. doi: 10.1086/420871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reich D, Patterson N, De Jager PL, McDonald GJ, Waliszewska A, Tandon A, Lincoln RR, DeLoa C, Fruhan SA, Cabre P, Bera O, Semana G, Kelly MA, Francis DA, Ardlie K, Khan O, Cree BA, Hauser SL, Oksenberg JR, Hafler DA. A whole-genome admixture scan finds a candidate locus for multiple sclerosis susceptibility. Nat Genet. 2005;37:1113–1118. doi: 10.1038/ng1646. [DOI] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 28.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–378. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 29.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 30.Brugada R, Tapscott T, Czernuszewicz GZ, Marian AJ, Iglesias A, Mont L, Brugada J, Girona J, Domingo A, Bachinski LL, Roberts R. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905–911. doi: 10.1056/NEJM199703273361302. [DOI] [PubMed] [Google Scholar]
- 31.Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, Ballew JD, de Andrade M, Burnett JC, Jr, Olson TM. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158–165. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox CS, Parise H, D’Agostino RB, Sr, Lloyd-Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA, Benjamin EJ. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 33.Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–184. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 34.Marcus GM, Smith LM, Vittinghoff E, Tseng ZH, Badhwar N, Lee BK, Lee RJ, Scheinman MM, Olgin JE. A first-degree family history in lone atrial fibrillation patients. Heart Rhythm. 2008;5:826–830. doi: 10.1016/j.hrthm.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabatti C, Risch N. Homozygosity and linkage disequilibrium. Genetics. 2002;160:1707–1719. doi: 10.1093/genetics/160.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Risch N, Burchard E, Ziv E, Tang H. Categorization of humans in biomedical research: genes, race and disease. Genome Biol. 2002;3:comment2007.1–2007.12. doi: 10.1186/gb-2002-3-7-comment2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, Feldman MW. Genetic structure of human populations. Science. 2002;298:2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- 38.Hudjashov G, Kivisild T, Underhill PA, Endicott P, Sanchez JJ, Lin AA, Shen P, Oefner P, Renfrew C, Villems R, Forster P. Revealing the prehistoric settlement of Australia by Y chromosome and mtDNA analysis. Proc Natl Acad Sci U S A. 2007;104:8726–8730. doi: 10.1073/pnas.0702928104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freedman ML, Haiman CA, Patterson N, McDonald GJ, Tandon A, Waliszewska A, Penney K, Steen RG, Ardlie K, John EM, Oakley-Girvan I, Whittemore AS, Cooney KA, Ingles SA, Altshuler D, Henderson BE, Reich D. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A. 2006;103:14068–14073. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu X, Luke A, Cooper RS, Quertermous T, Hanis C, Mosley T, Gu CC, Tang H, Rao DC, Risch N, Weder A. Admixture mapping for hypertension loci with genome-scan markers. Nat Genet. 2005;37:177–181. doi: 10.1038/ng1510. [DOI] [PubMed] [Google Scholar]
- 41.Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, Hulley SB, Schiller NB. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375, e1–7. doi: 10.1016/j.amjmed.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


