Abstract
Recent studies have demonstrated that cancer stem cells play an important role in the pathobiology of head and neck squamous cell carcinomas (HNSCC). However, little is known about functional interactions between head and neck cancer stem-like cells (CSC) and surrounding stromal cells. Here, we used Aldehyde Dehydrogenase activity and CD44 expression to sort putative stem cells from primary human HNSCC. Implantation of 1,000 CSC (ALDH+CD44+Lin−) led to tumors in 13 (out of 15) mice, while 10,000 non-cancer stem cells (NCSC; ALDH−CD44−Lin−) resulted in 2 tumors in 15 mice. These data demonstrated that ALDH and CD44 select a sub-population of cells that are highly tumorigenic. The ability to self-renew was confirmed by the observation that ALDH+CD44+Lin− cells sorted from human HNSCC formed more spheroids (orospheres) in 3-D agarose matrices or ultra-low attachment plates than controls and were serially passaged in vivo. We observed that approximately 80% of the CSC were located in close proximity (within 100-µm radius) of blood vessels in human tumors, suggesting the existence of perivascular niches in HNSCC. In vitro studies demonstrated that endothelial cell-secreted factors promoted self-renewal of CSC, as demonstrated by the upregulation of Bmi-1 expression and the increase in the number of orospheres as compared to controls. Notably, selective ablation of tumor-associated endothelial cells stably transduced with a caspase-based artificial death switch (iCaspase-9) caused a marked reduction in the fraction of CSC in xenograft tumors. Collectively, these findings indicate that endothelial cell-initiated signaling can enhance the survival and self-renewal of head and neck cancer stem cells.
Keywords: Tumor microenvironment, perivascular niche, anti-angiogenic therapy, squamous cell carcinoma, stemness
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most prevalent cancer type, accounting for more than 500,000 new cases each year in the world (1). The integration of platinum-based chemotherapy to the curative management of HNSCC resulted in an improvement in the control of local-regional disease and enhanced organ preservation (2). However, as the control of local-regional disease improved, the incidence of distant metastatic disease has risen (3, 4). As a result, the overall survival rate for patients with HNSCC has not improved significantly over the last 30 years and continues to be one of the lowest among the major cancer types. This clinical observation suggests that by creating a non-favorable local environment for head and neck tumor cells with current therapies, these cells acquire a more aggressive phenotype leading to distant metastasis. Better understanding of the pathobiology of HNSCC is urgently needed for the development of more effective therapies.
Cancer stem cells (CSC) constitute a sub-population of cells that are multi-potent, self-renewing, and capable of generating the entire heterogeneous population seen in tumors (5–8). Cancer stem cells are believed to “drive” tumorigenesis of some cancer types, including breast and head and neck tumors (9–11). This implies that the successful growth of a metastasis of tumors that follow the cancer stem cell model requires that at least one cancer stem cell resists to therapy (12). Notably, cancer stem cells are slow-dividing cells that are capable of resisting to current therapies for cancer (13).
Stem cells and cancer stem cells are frequently found in unique microenvironments called the “niche” (14, 15). Cell-to-cell interactions through direct contact or secreted factors support the survival and maintain the stemness of stem cells in cancer and in normal tissues (16). Perivascular niches have been identified in neural stem cells (17–19) and neural tumors (20). However, it is not known if the stem cells of head and neck tumors are localized in close proximity to blood vessels and depend on interactions with the cellular components of vascular niches for their survival and stemness.
Head and neck cancer stem cells were first identified using CD44 (9), a marker of stem cells in epithelial tumors (21, 22). Aldehyde Dehydrogenase (ALDH), an enzyme found to be highly active in stem cells of various origins (23–25), was recently used to identify stem cells in HNSCC (26). Here, we utilized ALDH1 and CD44 to identify a sub-population of cells that exhibit several properties of cancer stem cells, including self-renewal and capacity to regenerate heterogeneous tumors. Analysis of human HNSCC demonstrated that the majority of the cancer stem cells are located in close proximity to blood vessels. Using 3-D models in vitro, we showed that endothelial cell-secreted factors promote proliferation and self-renewal of HNCSC along with increased expression levels of Bmi-1. Notably, selective ablation of tumor-associated endothelial cells with a caspase-based artificial death switch resulted in a significant decrease in the number of cancer stem cells in vivo. Collectively, these data unveil the functional interdependency of cancer stem cells and vascular endothelial cells in head and neck tumors, and show proof-of-principle evidence that therapeutic targeting of tumor blood vessels reduces the number of CSC.
Materials and Methods
Cell Culture
Head and neck squamous cell carcinoma cell lines (UM-SCC-1, UM-SCC-74A, UM-SCC-74B, UM-SCC-17A, UM-SCC-17B, UM-SCC-11B; gift from Dr. Carey, University of Michigan) were cultured in Dulbecco’s Modified Eagle Medium (DMEM), 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin (Invitrogen; Grand Island, NY, USA) and human dermal microvascular endothelial cells (HDMEC; Cambrex, Walkersville, MD) in endothelial cell growth medium-2 (EGM2-MV; Lonza, Walkersville, MD, USA). FACS sorted cells were cultured in low glucose DMEM, 10% fetal bovine serum (Invitrogen) and 100 U/ml Penicillin-streptomycin (Invitrogen) or 100 U/ml Antibiotic Antimycotic Solution (AAA) (Sigma; St. Louis, MO, USA) in ultra-low attachment plates (Corning; New York, NY, USA). Conditioned Medium (CM) from HDMEC was collected in serum free DMEM from 24-hour cultures. HDMEC stably transduced with iCaspase-9 (HDMEC-iCaspase-9) were generated as described (27). The identity of all tumor cell lines was confirmed by genotyping at the University of Michigan DNA sequencing core facility.
Head and neck cancer stem cell sorting
Informed consent was obtained from patients undergoing surgery for removal of HNSCC. Tumor specimens were collected within 30 minutes post-surgery and transported in DMEM Low Glucose, 10%FBS, AAA, at 4°C. Within 12 hours, the tumors were cut into small pieces and minced with a sterile scalpel until they could pass through a 25 ml pipette tip. They were suspended in a 9:1 solution of DMEM-F12 (Hyclone, Waltham, MA) containing collagenase and hyaluronidase (Stem Cell Technologies; Vancouver, BC, Canada). The mixture was incubated at 37°C for one hour and passed through a 10-ml pipette every 15 minutes for mechanical dissociation. Cells were filtered through a 40-µm nylon mesh (BD Falcon; Franklin Lakes, NJ, USA), washed with low glucose DMEM containing 10% FBS, and centrifuged at 800 rpm for 5 minutes. Single cell suspensions obtained from primary specimens (as well as from cell lines or xenografts) were washed, counted, and re-suspended at 1×106cells/ml PBS. The Aldefluor kit (Stem Cell Technologies) was used to identify cells with high ALDH activity. Briefly, cells were suspended with activated Aldefluor substrate (BAA) or the negative control (DEAB, a specific ALDH inhibitor) for 45 minutes at 37°C. Then, cells were exposed to anti-CD44 (clone G44-26BD; BD Pharmingen; Franklin Lakes, NJ, USA) and lineage markers (i.e. anti-CD2, CD3, CD10, CD16, CD18; BD Pharmingen). Mouse cells were identified using anti-H2Kd antibody (BD Biosciences; Franklin Lakes, NJ, USA) and eliminated. Cells were cultured in ultra-low attachment plates overnight to allow for recovery from the flow sorting procedure. Next day, cells were either implanted in mice or plated for in vitro experiments. Anti-CD31 (Biolegend; San Diego, CA, USA), anti-HA (Sigma) and 7-Aminoactinomycin (7-AAD, BD Pharmingen) were used in selected experiments. Propidium iodide staining followed by flow cytometry was used for the identification of apoptotic cells (i.e. sub-G0/G1 cells). Here and throughout this manuscript, studies were done in triplicate specimens per condition and time point, and three experiments were performed to verify reproducibility of the data.
Confocal Microscopy
Frozen sections were treated with peroxidase (Dako, CA) and the antigen retrieval solution (Dako) was used for 45 minutes at 90°C. The anti-ALDH1 (BD Biosciences), CD44 (Abcam, Cambridge, MA, USA), and Factor VIII (Neomarkers; Fremont, CA, USA) were pre-labeled with Alexafluor 488 or 594 using the Zenon labeling kit (Invitrogen) and confocal imaging was performed using Zeiss confocal (Carl Zeiss; Thornwood, NY, USA). Z–stacked images of 8 individual images were generated. The deconvolution was done using Autoquant (Media Cybernetics; Bethesda, MD, USA) and the 3-D reconstruction completed using the Imaris Software (Bitplane AG; Zurich, Switzerland).
Colony formation assay and “orospheres”
Colony formation assays were performed in 3-D suspension cultures, as described (24, 29). Orospheres (i.e. spheroids of HNSCC-derived cells) were generated from 5×103 cells cultured in triplicate in ultra-low attachment plates (Corning). Alternatively, cells were mixed with 0.2% Agarose and layered on plates that were pre-coated with a layer of 0.4% Agarose. Cells were maintained in low glucose DMEM containing or not conditioned medium from HDMEC at a ratio of 3:1. Orospheres generated in ultra-low attachment plates were mechanically dissociated into single cell suspensions and replated to generate secondary and tertiary cultures.
SCID mouse model of human tumor angiogenesis
Xenograft tumors vascularized with functional human microvessels were generated in severe combined immunodeficient (SCID) mice (CB 17 SCID, Taconic; Germantown, NY, USA), as described (30). Briefly, 1,000 CSC (ALDH+CD44+Lin−) or 10,000 NCSC (ALDH−CD44−Lin−) were seeded with HDMEC for a total of 1×106 cells in poly-(L-lactic) acid (PLLA; Medisorb, Nicosia, Cyprus) biodegradable scaffolds. Bilateral scaffolds were implanted subcutaneously in the dorsum of each mouse. Mice were monitored daily for tumor growth for 6 months or until the volume of the tumor reached 0.85 cm3. Alternatively, mice received scaffolds containing 9.99×105 HDMEC-iCaspase-9 and 1×103 CSC, or controls. 24 days after transplantation of the scaffolds, mice received daily intraperitoneal injections of 2 mg/kg AP20187 (ARIAD; Cambridge, MA, USA) for 4 days to activate iCaspase-9 and selectively ablate tumor blood vessels, as described (27).
Statistical Analyses
T-test or one-way ANOVA followed by post-hoc analyses was performed using the SigmaStat 2.0 software (SPSS, Chicago, IL). Statistical significance was determined at P < 0.001 (unless otherwise specified).
Results
ALDH+CD44+Lin− cells retrieved from primary head and neck squamous cell carcinomas are highly tumorigenic
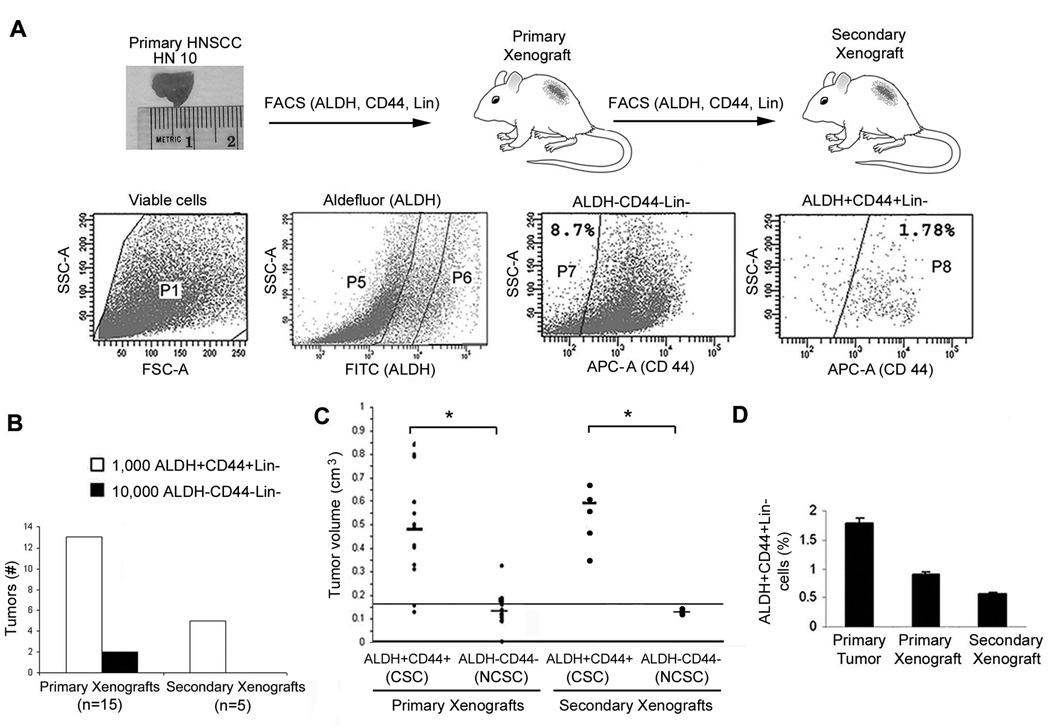
CD44 was used as the marker for stem cells in the study that demonstrated the existence of stem cells in head and neck squamous cell carcinomas (9). However, a relatively large proportion of cells was found to be positive for CD44 in that study. Here, we examined the ability of ALDH activity and CD44 expression to identify cells that have a cancer stem-like phenotype. We observed a “gradient of stemness” ranging from the ALDH+CD44+ cells with the highest number of colonies in soft agar to ALDH−CD44+ and ALDH−CD44− with the least number of colonies (Supplementary Fig. S1). In addition, we observed that ALDH and CD44 co-localize in a sub-population of cells in primary human HNSCC (Supplementary Fig. S2). These data led us to adopt the combined use of ALDH and CD44 for identification of CSC throughout this work. The efficacy of tumor take was compared when putative CSC (ALDH+CD44+Lin−) or non-CSC (ALDH−CD44−Lin−) were implanted in immunodeficient mice (Fig. 1A). Single cell suspensions were prepared from 4 patients with primary human head and neck squamous cell carcinomas immediately after surgical resection (Supplementary Fig. S3). Viable cells were selected with 7-AAD (P1) and then gated in sequence for ALDH activity and CD44 expression, after elimination of lineage (Lin) cells (Fig. 1A). We observed that 1.78% of the cells were ALDH+CD44+Lin− (putative CSC) and 8.7% were ALDH−CD44−Lin− (NCSC) in a representative tumor (HN 10) (Fig. 1A). To evaluate the tumorigenicity of these cells, 1,000 ALDH+CD44+Lin− or 10,000 ALDH−CD44−Lin− (10-fold more cells) were co-implanted with human endothelial cells to generate human xenograft tumors vascularized with human blood vessels in immunodeficient mice, as described (30). 13/15 implants generated tumors (as determined by palpation and confirmed by histological analysis), in the ALDH+CD44+Lin− group as compared to 2/15 of the ALDH−CD44−Lin− group, demonstrating that the combination of ALDH and CD44 allowed for selection of a highly tumorigenic sub-population of cells (Fig. 1B). To evaluate their capacity of self-renewal, viable tumor xenografts were retrieved at six months or when their volume reached 850 mm3, processed into single cell suspensions, and serially transplanted to other mice. All implants containing ALDH+CD44+Lin− cells generated secondary tumors, whereas none of the implants containing ALDH−CD44−Lin− cells generated tumors (Fig. 1B). The volume of the primary and secondary xenograft tumors generated with ALDH+CD44+Lin− cells was higher than with the ALDH−CD44−Lin− cells (P < 0.001) at the end of the experimental period (Fig. 1C). Notably, the fraction of the putative CSC (ALDH+CD44+Lin−) in the primary and secondary xenografts remained low and comparable to the fraction of these cells in the primary human tumors (Fig. 1D).
Figure 1.
Combination of ALDH and CD44 selects highly tumorigenic cells. A, Schematic representation of the approach used for the testing of the tumorigenic potential of cells sorted from primary tumors. ALDH+CD44+Lin− cells were isolated from HNSCC and serially transplanted into immunodefficient mice to generate primary and secondary xenografts. Representative flow cytometry: Viable cells (P1) were isolated from a head and neck squamous cell carcinoma patient (HN 10) using 7AAD. Lineage cells (Lin) were eliminated and remaining cells were gated for positivity to ALDH (P6), using DEAB (ALDH inhibitor) as reference. ALDH− cells are found in P5. ALDH+ and ALDH− cells were gated against CD44 to select ALDH+CD44+Lin− (P8=1.78%) and ALDH−CD44−Lin− (P7=8.7%). Side-scatter area (SSC-A); Forward-scatter area (FSC-A). B, Graph depicting the number of tumors generated by the implantation of 1,000 ALDH+CD44+Lin− or 10,000 ALDH−CD44−Lin− cells. The presence of a tumor was determined clinically by palpation over a period of 6 months (primary xenograft) and additional 6 months (secondary xenograft) and confirmed histologically upon tumor retrieval. C, Graph depicting the volume of xenograft tumors (primary and secondary) obtained by the implantation of 1,000 ALDH+CD44+Lin− or 10,000 ALDH−CD44−Lin− cells sorted from human HNSCC into immunodeficient mice. Asterisk depicts P < 0.001. D, Graph depicting the percentage of putative head and neck cancer stem cells (ALDH+CD44+Lin−) in primary tumors, primary and secondary xenografts.
Xenografts generated with ALDH+CD44+Lin− cells resemble the primary tumors
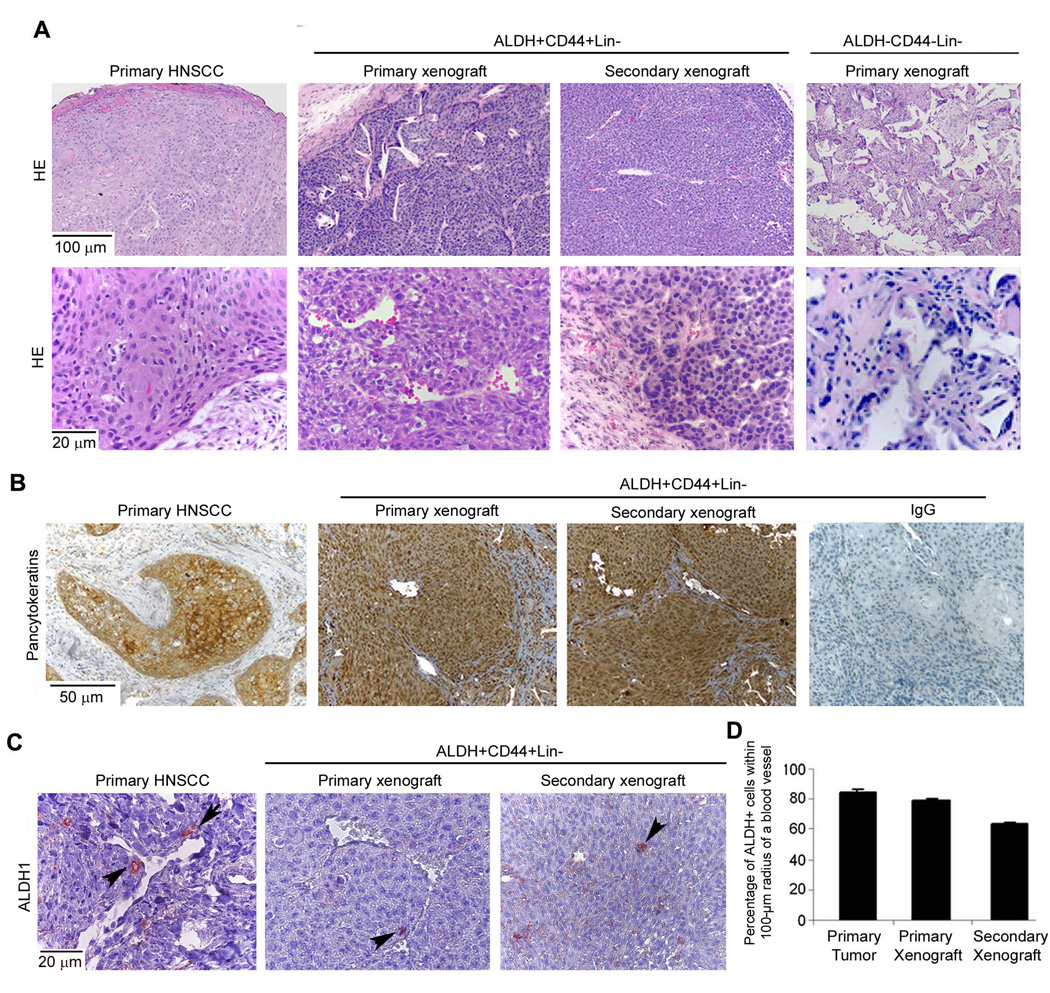
The histological organization of the primary and secondary xenograft tumors generated from the ALDH+CD44+Lin− cells was comparable to the primary tumor from which these cells were retrieved (Fig. 2A). In contrast, most of the implants seeded with ALDH−CD44−Lin− did not generate tumors. And, in the few instances (2 out of 15 implants) that tumors were generated from these cells, they were structurally disorganized and had smaller tumor islands as compared to xenografts generated from ALDH+CD44+Lin− cells (Fig. 2A). These findings, along with the observation that tumor xenografts maintained a complex cellular composition and a proportion of ALDH+CD44+ cells that was comparable with the primary tumor throughout 2 serial passages in vivo, indicated that ALDH+CD44+Lin− cells exhibit features of multipotency. The epithelial origin of the tumor xenografts was confirmed by positive immunostaining for pancytokeratin (Fig. 2B). Analysis of the localization of the stem-like cells (ALDH-positive) within these xenografts revealed that the majority of these cells were found within 100-µm of blood vessels (Fig. 2C,D). This observation led us to a more in depth analysis of the localization of stem-like cells in primary tumors.
Figure 2.
Head and neck cancer stem-like cells generate xenografts with histological features that closely resemble those of the original primary tumors. A, Photomicrographs of a representative primary tumor (HN10), and respective primary and secondary xenograft originated by the transplantation of ALDH+CD44+Lin− cells (HE staining). Control tissues generated by the implantation of ALDH−CD44−Lin− cells. B, Expression of pancytokeratin in the primary tumor, respective primary and secondary xenograft, and IgG control group. C, Representative photomicrographs of ALDH1 immunostaining of primary tumors and respective xenografts. Arrows depict ALDH1 positive cells. D, Graph depicting the percentage of ALDH1 positive cells found within a 100-µm radius of blood vessels in the primary tumors and xenografts generated by the transplantation of ALDH+CD44+Lin− cells.
Head and neck cancer stem-like cells exhibit perivascular localization
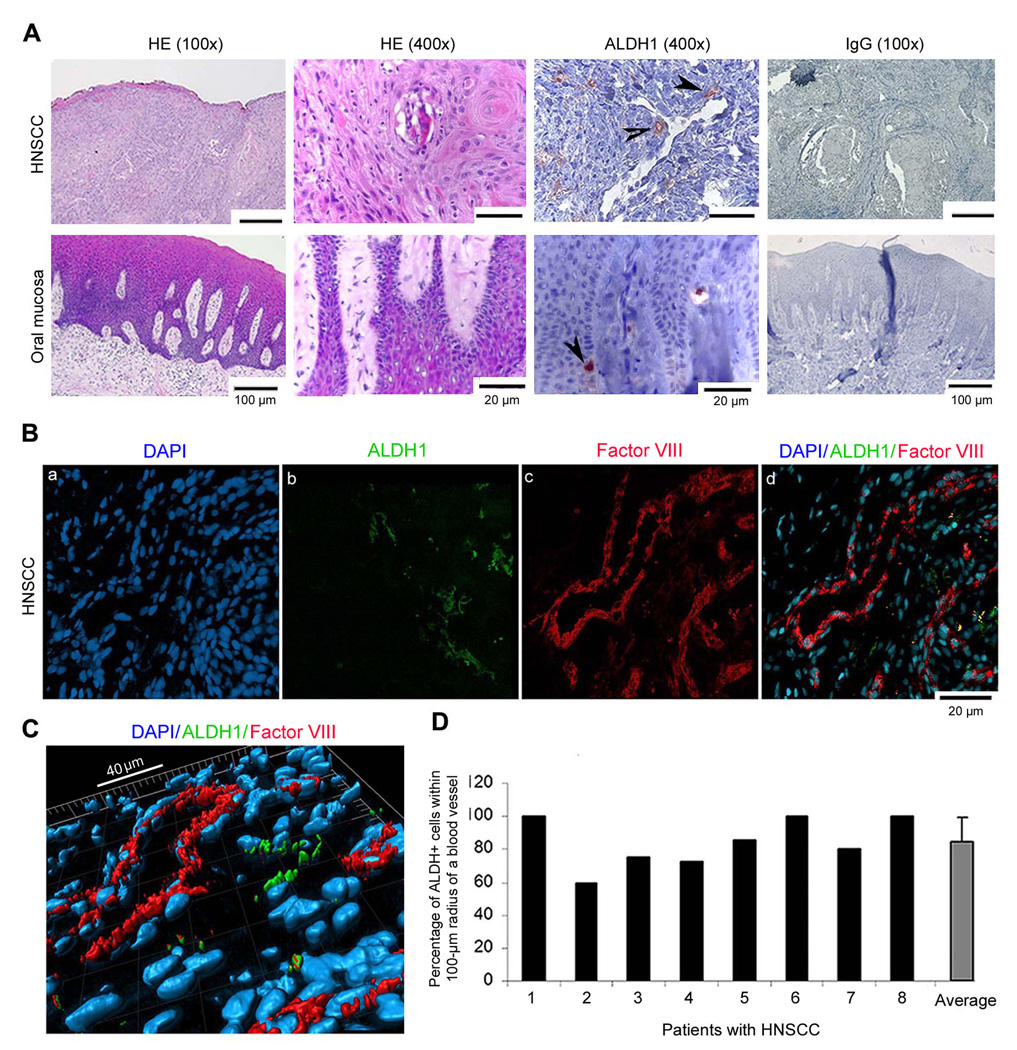
In oral mucosa, the small sub-population of ALDH-positive cells were found primarily in the basal layer of the squamous epithelium (Fig. 3A), the expected localization of stem cells in this tissue. In HNSCC, the ALDH-positive cells were seen in tumor islands, in close proximity to blood vessels (Fig. 3A). To assess the relative percentage of putative stem cells in oral mucosa and HNSCC, we prepared single cell suspensions and sorted them for ALDH and CD44. The proportion of ALDH+CD44+ was lower (P=0.004) in normal human oral mucosa (0.31 ± 0.11%) than in the HNSCC (2.62 ± 0.68%) (N=3). Confocal microscopy and 3-D image reconstruction were used to evaluate the spatial relationship between ALDH-positive cells and blood vessels in 8 patients with HNSCC (Fig. 3B–D). An area with 100-µm radius around each blood vessel was selected as representative of the “perivascular” area, since this is the approximate area of diffusion of oxygen and nutrients around vessels (28). We observed that the majority of the CSC (i.e. approximately 80%) was found in the perivascular area in human HNSCC (Fig. 3D).
Figure 3.
Head and neck cancer stem-like cells are localized in close proximity to blood vessels. A, Representative photomicrographs of tissue sections stained with HE or immunostained for ALDH1 in human HNSCC and control oral mucosa. Arrows depict ALDH1 positive cells. B, Confocal microscopy of human HNSCC immunostained for ALDH1 (green) and Factor VIII (red) for localization of blood vessels. The overlay image shows the perivascular localization of the ALDH-positive cells. C, 3-D reconstruction of the overlay image shown in panel B, depicting the spatial relationship between ALDH positive cells (green) and blood vessels (red). D, Graph depicting the percentage of ALDH+ cells found within a 100-µm radius of a blood vessel. Analysis was performed in 6 random areas of 6 different tissue sections from each individual human HNSCC (N=8).
Endothelial cell-derived growth factor milieu promotes proliferation, survival, and self-renewal of HNCSC
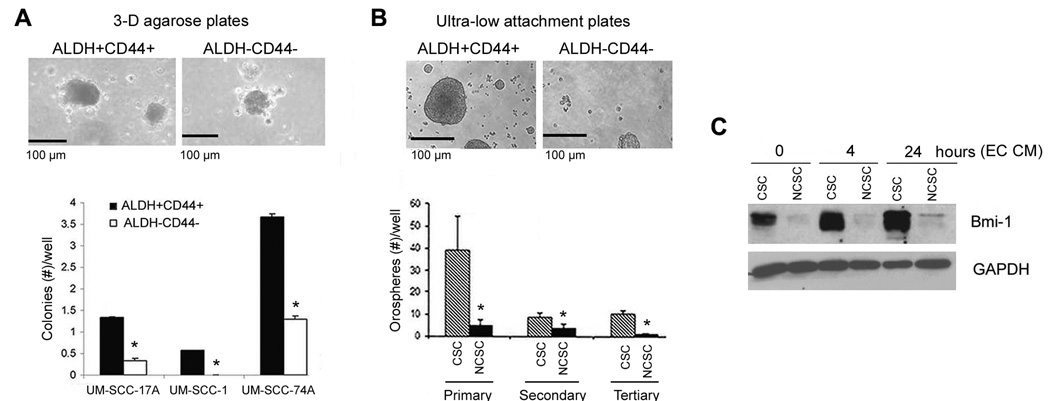
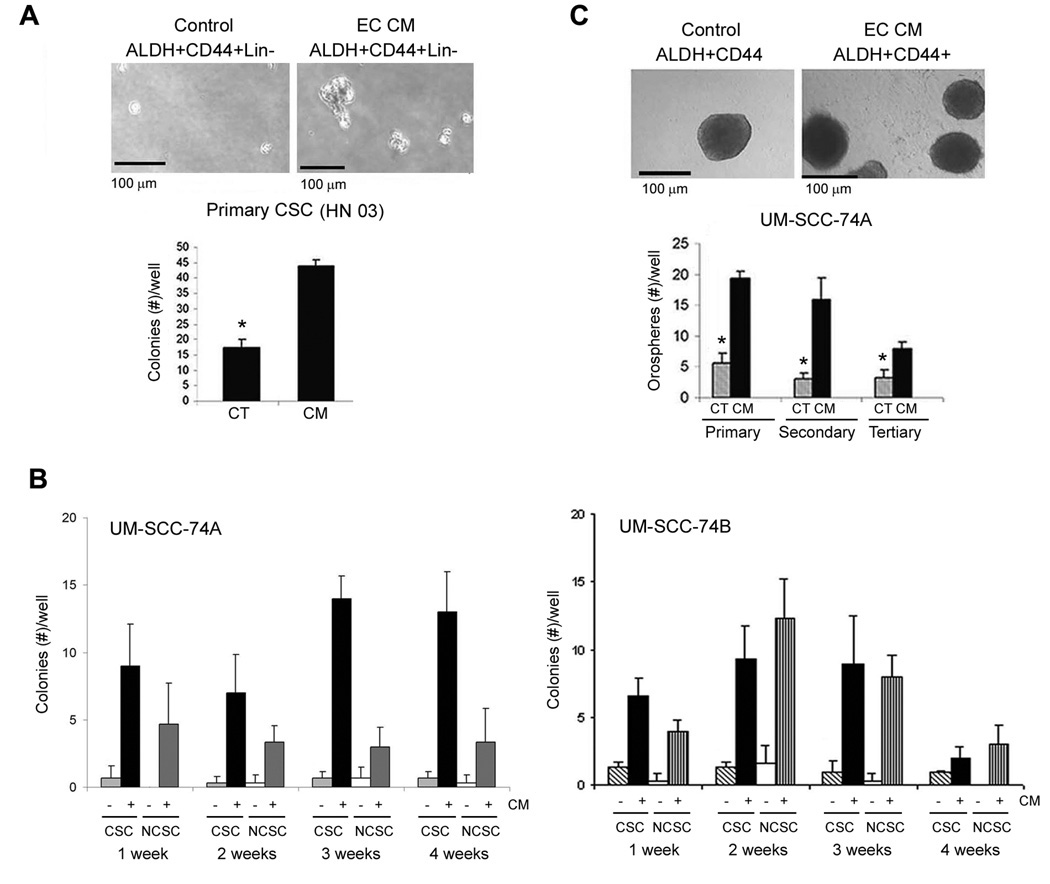
To understand whether endothelial cell-secreted factors have a direct functional effect on HNCSC, we studied the effect of endothelial cell conditioned medium on proliferation, survival and self-renewal of ALDH+CD44+ selected from a panel of established head and neck tumor cell lines in vitro. The proliferation of both, ALDH+CD44+ and ALDH−CD44− cells cultured in low attachment conditions was enhanced by exposure to endothelial cell conditioned medium (Supplementary Fig. S4A). The increase in cell numbers may also be attributed to an enhancement in survival mediated by the endothelial cell-derived factors (Supplementary Fig. S4B). In addition, we performed a series of supporting in vitro experiments using a panel of established HNSCC cell lines. The presence and proportion of putative cancer stem cells is depicted in Supplementary Figure S5. To evaluate the self-renewal potential of CSC, we plated them in agarose and observed the formation of colonies in a three-dimensional culture condition (Supplementary Fig. S6). We observed the formation of sphere-like colonies developed from single cells (Fig. 4A and B), using a method inspired by the work on “mammospheres” (29). These colonies derived from head and neck tumor stem-like cells were named “orospheres”. The number of colonies generated from ALDH+CD44+ cells was greater than ALDH−CD44− cells (P < 0.001), using cells sorted from three established HNSCC cell lines, i.e. UM-SCC-17A, UM-SCC-1 and UM-SCC-74A (Fig. 4A). To evaluate the behavior of these cells over time, the orospheres were dissociated and passed twice. While the overall number of orospheres decreased over time, the ALDH+CD44+ group persistently presented higher number of orospheres than the control group over three serial passages in vitro (Fig. 4B). Notably, ALDH+CD44+ cells strongly express the marker of self-renewal Bmi-1, as compared to control ALDH−CD44− cells (Fig. 4C). In the same experiment, we observed that endothelial-cell secreted factors enhances expression of Bmi-1 in ALDH+CD44+ cells over time, indicating an inductive effect of these factors on the self-renewal properties of the CSC (Fig. 4C). To further understand the effect of endothelial cells on self-renewal and survival of cancer stem cells, we isolated ALDH+CD44+Lin− cells from primary HNSCC and performed the orosphere assay with primary cells. A 3-fold increase in the number of orospheres was observed in the group treated with endothelial cell conditioned medium (P < 0.001), as compared to untreated controls (Fig. 5A). These results were verified in experiments performed with 6 additional head and neck tumor cell lines (Fig. 5B; Supplementary Fig. S6). Notably, the inductive effect of endothelial cell-secreted factors on the number of orospheres generated from ALDH+CD44+ cells was maintained during 3 serial passages in vitro (Fig. 5C).
Figure 4.
ALDH and CD44 select for a sup-population of cells that exhibit self-renewal properties in vitro. A, Representative photomicrographs of colonies (orospheres) arising from ALDH+CD44+ and ALDH−CD44− cells sorted from UM-SCC-74A and grown in 3-D agarose matrices. Graph depicts the quantification of the number of colonies arising from cancer stem-like cells (CSC; ALDH+CD44+) or non-cancer stem cells (NCSC; ALDH−CD44−) sorted from three HNSCC cell lines (UM-SCC-17A, UM-SCC-1, UM-SCC-74A) and cultured in 3-D agarose matrices. B, Representative photomicrographs of orospheres arising from ALDH+CD44+ and ALDH−CD44− cells sorted from UM-SCC-74A and grown in ultra-low attachment plates. Graph depicting the number of “orospheres” from serial passage assays that evaluate self-renewal of CSC (ALDH+CD44+) and NCSC (ALDH−CD44−) cells in vitro. Asterisk depicts P < 0.001. C, Western blot depicting the expression of Bmi-1 in CSC (ALDH+CD44+) and NCSC (ALDH−CD44−) cells treated with endothelial cell conditioned medium (EC CM).
Figure 5.
Endothelial cell-derived factors promote proliferation and self-renewal of head and neck cancer stem-like cells. A, Photomicrographs of representative colonies arising from ALDH+CD44+Lin− cells sorted from a human HNSCC and grown in 3-D Agarose matrices. Cells were treated with endothelial cell conditioned medium (CM) or unconditioned control medium (CT) for one week. Asterisk depicts P < 0.001. B, Time course experiment depicting the number of orospheres arising from CSC (ALDH+CD44+) and NCSC (ALDH−CD44−) cells treated or not with endothelial cell conditioned medium (CM) over a period of 4 weeks. C, Photomicrographs of representative colonies arising from ALDH+CD44+Lin− cells sorted from UM-SCC-74A cells and cultured in ultra-low attachment plates. Cells were treated with endothelial cell conditioned medium (CM) or unconditioned control medium (CT). Graph depicting primary, secondary and tertiary orospheres arising from ALDH+CD44+ treated with endothelial cell conditioned medium (CM) or control medium (CT). Asterisk depicts P < 0.05.
Selective ablation of blood vessels reduces the proportion of cancer stem cells
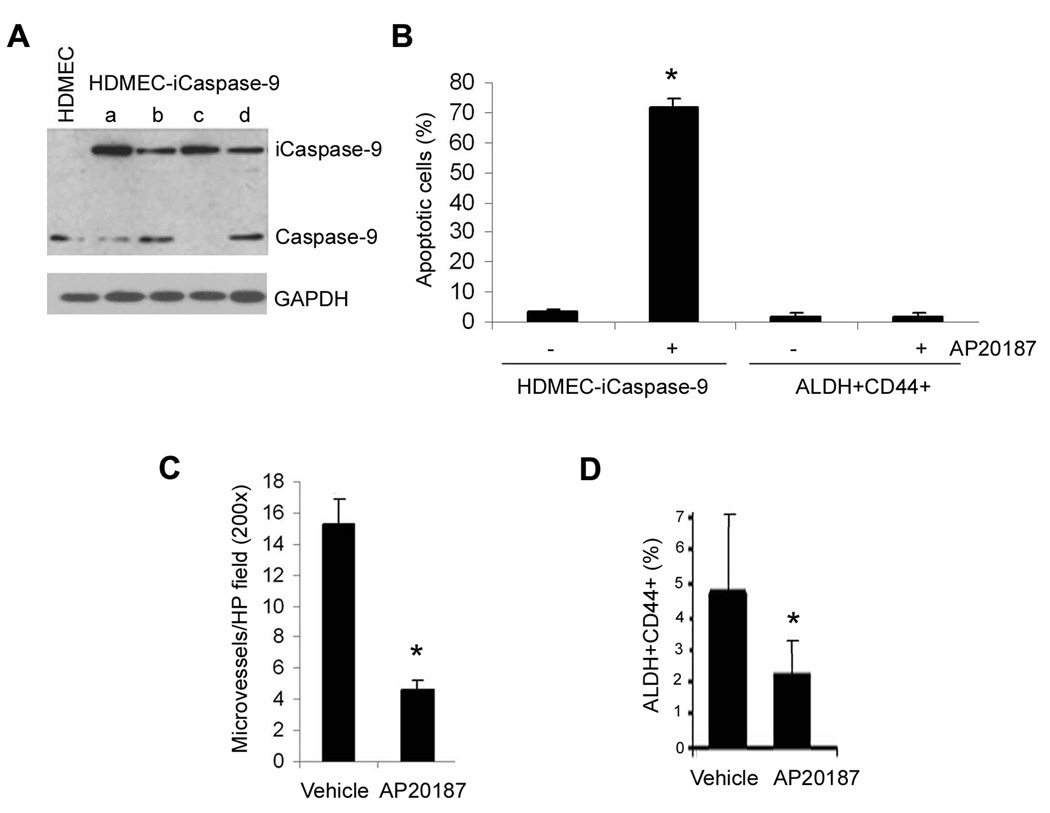
A critical question that remained unanswered was if the effects of endothelial cells on the survival and self-renewal of head and neck tumor stem cells was also observable in vivo. To address this question, we utilized two complementary approaches. Firstly, we performed a serial dilution experiment with 1,000, 100, 10, or 1 ALDH+CD44+Lin− cells from a primary HNSCC seeded in the scaffolds with or without HDMEC. Co-implantation of endothelial cells and CSC resulted in larger tumors than implantation of CSC by themselves (Supplementary Fig. S7). Secondly, endothelial cells were stably transduced with a caspase-based artificial death switch (iCaspase-9) (Fig. 6A). This unique approach allows for selective elimination of endothelial cells transduced with iCaspase-9 upon activation by the dimerizer drug AP20187 and ablation of tumor vasculature in vivo (27, 31). Here, we showed that treatment with AP20187 induces apoptosis of HDMEC-iCaspase-9, but not untransduced cells (i.e. ALDH+CD44+ cells) (Fig. 6B). As expected, AP20187-induced apoptosis of the iCaspase-9-transduced endothelial cells resulted in a significant reduction in the microvessel density of tumors retrieved from mice injected with AP20187, as compared to vehicle-treated controls (Fig. 6C). Due to the short-term nature of the treatment with AP20187 (4 days), we have not observed a significant change in tumor size (data not shown). However, the fraction of ALDH+CD44+Lin− cells within the xenograft tumors was significantly reduced when endothelial cells were selectively ablated by activation of iCaspase-9 as compared to vehicle-treated controls (Fig. 6D).
Figure 6.
Selective ablation of tumor-associated endothelial cells decreases the number of head and neck cancer stem-like cells. A, Western blot showing 4 clones of HDMEC stably transduced with the caspase-based artificial death switch iCaspase9. Clone “d” was selected for remaining experiments depicted in this figure. B, Graph depicting the proportion of apoptotic cells when HDMEC-iCaspase-9 or ALDH+CD44+ cells are exposed to the dimerizer drug AP20187. C, Graph depicting the tumor microvessel density of xenografts generated by the co-transplantation of HDMEC-iCaspase-9 and ALDH+CD44+ cells in immunodeficient mice. Mice were injected with AP20187 to activate Caspase-9 and selectively eliminate the xenograft tumor blood vessels. D, Graph depicting the percentage of ALDH+CD44+ cells in the AP20187-treated tumors compared to vehicle treated controls. Asterisk depicts P < 0.005.
Discussion
The poor survival and high recurrence rates in patients with HNSCC demand a re-assessment of the pathobiology of these cancers. Here, we showed that head and neck cancer stem cells reside in perivascular niches. Notably, we observed that endothelial cell-secreted factors have a major impact on the self-renewal and survival of cancer stem cells. These data suggest that therapeutic targeting of the tumor endothelium may reduce the rate of head and neck tumor recurrence and metastasis by decreasing the proportion of cancer stem cells.
Prince and colleagues (2007) reported that CD44+ cells from primary HNSCC exhibit a cancer stem-cell phenotype, and are capable of initiating tumors at low numbers (9). ALDH1 has been recently described as a putative marker for CSC in head and neck tumors (26). Here, we demonstrate that the combination of ALDH1 and CD44 selects a sub-population of cells with properties of cancer stem cells than if used as single markers. One thousand ALDH+CD44+ cells were capable of initiating tumors much more efficiently than 10,000 ALDH−CD44− cells. ALDH+CD44+ cells could also be transplanted serially and generated secondary xenografts, evidencing the self-renewal nature of these cells. It is noteworthy that although control ALDH−CD44− cells formed a few primary xenografts (2 out of 15), we did not observe any tumor being formed from the serial transplantation experiments with these cells. The two tumors generated from ALDH−CD44− cells could be due to the existence of a few progenitor cells with the capability of tumor initiation or possible inaccuracy of FACS sorting. Moreover, the histology of the xenografts obtained from the ALDH+CD44+ cells resembled that of primary tumors. These findings confirmed that the xenografts were of human epithelial origin and supported the hypothesis that ALDH+CD44+ cells have a behavior that is consistent with the behavior of cancer stem cells.
We observed that ALDH-positive cells are found primarily in the basal layer of the oral epithelium, where stem cells of the skin have been traditionally found (14, 32). In contrast, in the HNSCC the ALDH-positive cells have a more disperse localization within the tumor microenvironment. Of note, the ALDH-positive cells were consistently localized within close proximity of blood vessels. The close association of cancer stem cells and blood vessels has earlier been documented in the nervous system and that these vascular niches helped in the maintenance of stem cells and cancer stem cells (17, 20, 33). However, such association has not been reported yet for head and neck tumors.
The cancer stem cells are believed to escape current therapies like radiation and chemotherapy and possibly lead to recurrences in various cancers. Thereby, identifying and targeting cancer stem cells or their niches might be a novel therapeutic strategy in the clinic (34). However, to be able to target the cancer stem cells or their niches, we have to understand its pathobiology, identify their niches and their effects on the cancer stem cells. Endothelial cells have been implicated in the self-renewal and survival of neural cancer stem cells (20). Studies in hematopoietic stem cells suggest that the vascular niche can promote cell survival signals (18), which could make them resistant to chemotherapies. Other groups have studied the effects of endothelial cell survival and self-renewal on cancer stem cells in neural tumors (20, 35). Anti-angiogenic agents (e.g. bevacizumab) have been shown to mediate a depletion in the cancer stem cells in models of gliomas and medulloblastomas. Here, we used a unique experimental approach to selectively eliminate tumor-associated endothelial cells and evaluate the effect on the stem cell compartment. Unlike previous experimental strategies that were based on anti-angiogenic drugs, the approach used here eliminates the risk of a direct effect of the drug on the viability or stemness of the tumor stem cells. We observed that selective ablation of tumor-associated blood vessels is sufficient to decrease the proportion of head and neck tumor stem cells within 4 days, while no changes were observed in tumor volume in the same time period.
Our work demonstrates that endothelial cells initiate signaling events that enhance the survival and self-renewal of stem cells in head and neck tumors. In addition, the data presented here supports the concept that head and neck cancer indeed follows the cancer stem cell hypothesis, since implantation of few cells consistently gives rise to tumors that can be serially passaged in vivo. Collectively, these data suggest that therapeutic strategies that include anti-angiogenic agents might have the benefit of reducing the proportion of cancer stem cells in head and neck tumors. These results might translate into lower recurrence rates and better survival of head and neck cancer patients.
Supplementary Material
Acknowledgements
We thank the University of Michigan Flow Cytometry Core and the Imaging Core for their assistance. We acknowledge Chris Strayhorn for assistance with the histology, Rodrigo Neiva and Brent Ward for the oral mucosal samples; Fernanda Visioli and Marcia Campos for pathological analysis; Zhaochang Zhang and Kristy Warner for valuable suggestions and for critically reading this manuscript.
Financial support:
Weathermax foundation, University of Michigan Comprehensive Cancer Center; grant P50-CA97248 (University of Michigan Head and Neck SPORE) from the NIH/NCI; grants R01-DE14601, R01-DE15948, R01-DE16586, R21-DE19279 from the NIH/NIDCR.
References
- 1.Chin D, Boyle GM, Porceddu S, et al. Head and neck cancer: past, present and future. Expert Rev Anticancer Ther. 2006;6:1111–1118. doi: 10.1586/14737140.6.7.1111. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA. Chemotherapy in the treatment of locally advanced head and neck cancer. J Surg Oncol. 2008;97:701–707. doi: 10.1002/jso.21012. [DOI] [PubMed] [Google Scholar]
- 3.Genden EM, Ferlito A, Bradley PJ, Rinaldo A, Scully C. Neck disease and distant metastasis. Oral Oncol. 2003;39:207–212. doi: 10.1016/s1368-8375(02)00049-0. [DOI] [PubMed] [Google Scholar]
- 4.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26:645–662. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 6.Tu LC, Foltz G, Lin E, Hood L, Tian Q. Targeting stem cells-clinical implications for cancer therapy. Curr Stem Cell Res Ther. 2009;4:147–153. doi: 10.2174/157488809788167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Setoguchi T, Taga T, Kondo T. Cancer stem cells persist in many cancer cell lines. Cell Cycle. 2004;3:414–415. doi: 10.4161/cc.3.4.799. [DOI] [PubMed] [Google Scholar]
- 8.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 9.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho RW, Clarke MF. Recent advances in cancer stem cells. Curr Opin Genet Dev. 2008;18:48–53. doi: 10.1016/j.gde.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Hill RP, Marie-Egyptienne DT, Hedley DW. Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol. 2009;19:106–111. doi: 10.1016/j.semradonc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Hermann PC, Bhaskar S, Cioffi M, Heeschen C. Cancer stem cells in solid tumors. Semin Cancer Biol. 2010 doi: 10.1016/j.semcancer.2010.03.004. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Fucs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 15.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 16.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 17.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 18.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Veeravagu A, Bababeygy SR, Kalani MY, Hou LC, Tse V. The cancer stem cell-vascular niche complex in brain tumor formation. Stem Cells Dev. 2008;17:859–867. doi: 10.1089/scd.2008.0047. [DOI] [PubMed] [Google Scholar]
- 20.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 23.Corti S, Locatelli F, Papadimitriou D, et al. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24:975–985. doi: 10.1634/stemcells.2005-0217. [DOI] [PubMed] [Google Scholar]
- 24.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YC, Chen YW, Hsu HS, et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385:307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 27.Nör JE, Hu Y, Song W, Spencer DM, Núñez G. Ablation of microvessels in vivo upon dimerization of iCaspase-9. Gene Ther. 2002;9:444–451. doi: 10.1038/sj.gt.3301671. [DOI] [PubMed] [Google Scholar]
- 28.Janssen HL, Haustermans KM, Sprong D, et al. HIF-1A, pimonidazole and iododeoxyuridine to estimate hypoxia and perfusion in human head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2002;54:1537–1549. doi: 10.1016/s0360-3016(02)03935-4. [DOI] [PubMed] [Google Scholar]
- 29.Dontu G, Abdullah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nör JE, Peters MC, Christensen JB, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 31.Dong Z, Zeitlin BD, Song W, et al. Level of endothelial cell apoptosis required for a significant decrease in microvessel density. Exp Cell Res. 2007;313:3645–3657. doi: 10.1016/j.yexcr.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto A, Chikamatsu K, Sakakura K, Hatsushika K, Takahashi G, Masuyama K. Expansion and characterization of cancer stem-like cells in squamous cell carcinoma of the head and neck. Oral Oncol. 2009;45:633–639. doi: 10.1016/j.oraloncology.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.