Abstract
Exhaled breath condensate (EBC) is a potential rich source for countless biomarkers that can provide valuable information about respiratory as well as systemic diseases. EBC has been studied in a variety of diseases including allergic rhinitis, asthma, chronic obstructive lung disease, cystic fibrosis, lung cancer, and obstructive sleep apnea syndrome. Although numerous biomarkers have been discovered and studied in EBC, the methods of collection and biomarker detection have not been fully standardized. While leaving standardization methods up to individual labs for the present time is optimal for the continued discovery of new biomarkers in EBC, this decreases the reproducibility and generalizability of the findings. In this review we will discuss specific biomarkers studied in specific diseases as well as some of the related technical issues including collection, processing and analysis.
Background
Biomarkers are substances used as indicators of a biologic state—normal or abnormal. In medicine, biomarkers are used to detect disease states. Detection indicates a change in the expression of a biomarker that has been found to correlate with a risk or progression of a disease or with a susceptibility of the disease to a given treatment. In respiratory disease, biomarkers are used to reflect disease processes occurring in the lungs. Biomarkers can be detected in lung tissue, bronchoalveolar lavage, sputum, peripheral blood, urine, exhaled gases and exhaled breath condensate. Physicians use these biomarkers to diagnose and monitor a variety of lung diseases.
Breath analysis, a non-invasive technique, is promising for biomarker detection. Minimally invasive procedures are ones performed with the least amount of damage to surrounding structures. The number of minimally invasive procedures performed has steadily increased in medicine, leading to greater success in the evaluation and treatment of a variety of diseases. Similarly, the field of breath research, a novel non-invasive method of examining the airways, has taken off in the medical community and is being used for diagnosing diseases and monitoring response to treatment.
In the past, invasive tests like lung biopsies were the only way to investigate the lungs and lower airways. Breath monitoring has emerged as a simple way to learn about airways. Nitric oxide (NO), found in exhaled breath, is an established biomarker for lung disease; fractional exhaled NO (FENO) is already being used to make medical decisions regarding the diagnosis and treatment of diseases, particularly asthma [1, 2]. Like spirometry and lung function tests, however, FENO may only tell part of the story of what is going on at the level of the airways. Exhaled breath condensate (EBC), another method of breath monitoring, is a technique that may provide more information about what is happening at the level of the airways.
EBC is more than a biomarker: EBC is a matrix in which countless biomarkers may be identified, similar to those found in blood, urine and the gases found in exhaled breath. EBC is obtained as breath is exhaled from the lungs into a cooled collecting device, thereby condensing the vapor and aerosolized droplets emerging with the breath (figure 1) [3]. All nonvolatile compounds found in EBC originate in the airway lining fluid (ALF) or are reaction products of volatiles that enter EBC from the gas phase. This totally non-invasive procedure has no influence on airway function or inflammation.
Figure 1.
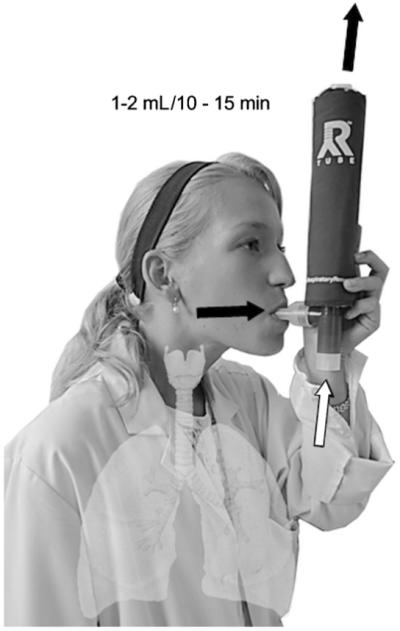
Exhaled breath condensate schematic. As the individual inhales, air flows into the device, bypassing the cooling sleeve, as indicated by the white arrow. During exhalation air moves out through the cooling chamber as indicated by the black arrows.
Guidelines were published by the American Thoracic Society (ATS) for EBC measurement in 2005 [3]. The task force reviewed the most recent studies using EBC in order to establish a consensus of guidelines for standardization of this novel method. Although numerous biomarkers have been discovered in EBC, each group has methods of EBC evaluation optimized for a specific biomarker. The task force concluded with the suggestion that each disease marker studied should be evaluated by the investigators involved. Leaving standardization methods up to individual labs for the present time is optimal for the continued discovery of new biomarkers in EBC but decreases the reproducibility of EBC as a technique.
Factors effecting EBC collection
Many different methods exist for obtaining exhaled breath condensate; these methods are optimized to collect the mediator of interest. Most techniques are a modification of the most common method of EBC collection. Patients are asked to breathe tidally through a mouthpiece; this mouthpiece is connected to a collecting device that is cooled to 0 °C. Patients are usually asked to breath through the device for anywhere from 10 to 30 min to obtain between 1 and 3 ml of condensate. Exhaled breath condensate is usually stored at −70 °C, where it can later be accessed for the detection of specific mediators. Groups that slightly alter the methods may increase the collection time to obtain more condensate or cool the collecting device to lower temperatures in order to preserve markers [4].
EBC has been applied to a variety of diseases including allergic rhinitis, asthma, chronic obstructive lung disease, cystic fibrosis, lung cancer and obstructive sleep apnea syndrome. We will discuss these diseases individually with specific attention to biomarkers that were studied in each particular disease.
EBC in allergic rhinitis, asthma and COPD
Airway inflammation is the most studied process in EBC; inflammation is an underlying process common to allergic rhinitis, asthma and chronic obstructive pulmonary disease. Allergic rhinitis is a clinical syndrome recognized by sneezing, nasal congestion and nasal itching; airway inflammation is involved in the pathogenesis of this condition. Similarly, asthma is a pulmonary disorder characterized by the triad of inflammation, bronchial hyperresponsiveness and reversible airway obstruction. EBC has also been used to learn about the most severe form of airway inflammation: chronic obstructive pulmonary disease (COPD). COPD is characterized by airflow limitation that is not fully reversible; this limitation is usually progressive and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases. The field of non-invasive EBC monitoring has provided insight to the airways of asthmatics (table 1) [5]; EBC has also provided valuable information on the spectrum of allergic rhinitis, asthma and COPD (table 2).
Table 1.
Major findings from studies relating to EBC in asthma. Adapted from Kostikas et al [5]
| pH | NO-related products | Prostanoids | Leukotrienes | 8-Isoprostane | H2O2 | |
|---|---|---|---|---|---|---|
| Stable asthma | ↓ [6, 20] | ↑ NOx [74] | ↔ PGE2 [37-39, 41, 42] | ↑ Cys-LTs [36, 38, 39, 54, 55, 57, 96, 140] | ↑ [6, 42, 67] | ↑ [72-77] |
| ↑ nitrotyrosine [30, 31] | ↑ TXB2 [41] | ↑ LTE4 [38, 41, 42, 55, 60] | ||||
| ↑ S-nitrosothiols [27] | ↑ LTB4 [30, 38, 41, 47, 55, 141] | |||||
| Exacerbation | ↓ [7] | ↑ Cys-LTs [57, 58] | ↑ [6, 67] | ↑ [75] | ||
| Effect of smoking | ↓ [17] | ↓ NOx [25, 75] | ↑ PGE2 [37] | ↑ LTB4 [50] | ↑ [74] | |
| Effect of treatment | ICS ↓ [6,7] | ICS ↓ NOx [25, 74] | ICS ↔ PGE2 [41] | Allergen avoidance ↓ Cys-LTs [33] | ICS ↓ or ↔ [36, 42, 58, 67, 68] | ICS ↓ [74-77] |
| Montelukast ↔ PGE2 [42] | Nasal steroids ↓ Cys-LTs [54] | Proton pump inhibitors ↓ [23] | ||||
| Montelukast ↓ LTE4 [42, 49, 61] | ||||||
| ICS ↓ Cys-LTs [58] | ||||||
| ICS ↓ ↔ LTB4 [41, 47-49] | ||||||
| Atopy | ↓ [16] | ↑ NO2/NO3 [20] | ↑ Cys-LT [54] | ↔ [76] | ||
| ↔ LTB4 [47] |
Abbreviations: nitrite/nitrate (NOx); prostaglandin (PG); thromboxane (TX); cysteinyl leukotrienes (Cys-LTs); leukotrienes (LT); inhaled corticosteroids (ICS).
Table 2.
Major findings from studies relating to EBC in COPD
| pH | NO-related products | Prostanoids | Leukotrienes | 8-Isoprostane | H2O2 | |
|---|---|---|---|---|---|---|
| Stable COPD | ↓ [6] | ↓ NOx [142] | ↑ PGE2, PGF2α [43] | ↑ LTB4 [44, 51] | ↑ [43] | ↑ [80, 81] |
| ↑ nitrite [27] | ||||||
| ↑ S-nitrosothiols [27] | ||||||
| Exacerbation | ↑ LTB4 [51] | ↑ [80, 81] | ||||
| Effect of smoking | ↔ NOx [26] | ↔ [43] | ↔ [81] | |||
| Effect of treatment | ICS ↔ [6] | ICS ↔ NOx [25, 26, 109] | Non-selective COX ↓ PGE2 [45] | Non-selective COX ↑ LTB4 [45] | Antibiotic ↓ [51] | ICS ↔ ↓ [82, 83] |
| ICS ↔ PGE2 [44] | Antibiotic ↓ LTB4 [51] | |||||
| ICS ↔ LTB4 [44] |
Abbreviations: cyclooxygenase inhibition (non-selective COX); nitrite/nitrate (NOx); prostaglandin (PG); thromboxane (TX); cysteinyl leukotrienes (Cys-LTs); leukotrienes (LT); inhaled corticosteroids (ICS).
Several biomarkers have been studied in these diseases including pH, nitric oxide metabolites, eicosanoids, isoprostanes, hydrogen peroxide, proteins and others. We will discuss each biomarker in detail.
pH
Measuring EBC pH after dearation for the removal of CO2 is a validated technique [6, 7] that is a reproducible and relevant marker of disease [8]. A recent study suggests that this method of EBC measurement is the most reproducible method for both healthy and asthmatic subjects [9, 10]. Alteration of airway pH may serve as an innate host defense mechanism but, unregulated, it can lead to the pathological processes underlying inflammatory diseases. Acidification occurs in inflammatory processes throughout the body; it is reasonable to expect the same acidification with other inflammatory diseases like asthma [11, 12]. A decrease in the pH of airways causes bronchoconstriction [11], impairs ciliary motility [13], increases the viscosity of airway mucus [12] and damages airway epithelium [12].
The mean pH of healthy subjects is 7.7, with a normal range of 7.4−8.8 [14]. The pH of EBC collected from children with atopic dermatitis and asthma is lower than that of allergic rhinitis and healthy controls [15]. Patients with stable persistent asthma have lower EBC pH values than healthy controls; patients with severe asthma have more acidic EBC than non-severe asthmatics [6] (table 3). EBC pH values can also reflect acute exacerbations of asthma, which then normalize with anti-inflammatory therapy [7]. Airway acidification has also been found in asthmatic children [166] and children with allergic rhinitis and atopic dermatitis, suggesting that EBC pH may be a useful method for evaluating the progression of atopy to asthma [16].
Table 3.
Comparison of EBC pH in healthy subjects, asthma, allergic rhinitis, atopic dermatitis and COPD. Data are presented as mean ± standard deviation unless otherwise written. Adapted from [6, 16, 17, 23]
| Disease condition | pH |
|---|---|
| Healthy adults [25] | 7.50 ± 0.20 |
| [165] | 7.61 (CI 7.52–7.70) |
| In children [16] | 7.78 (range 7.06–8.10) |
| Asthma exacerbation [25] | 5.23 ± 0.21 |
| Stable asthma [25] | 7.8 ± 0.1 |
| In children [16] | 7.32 (range 6.2–8.02) |
| In children [166] | 6.46 (IQR 5.86–6.72) |
| Mild asthma [25] | 7.6 (CI 7.55–7.65) |
| Moderate asthma without GERD [25] | 7.3 ± 0.60 |
| Moderate asthma with GERD [25] | 7.2 ± 0.1 |
| Smoking asthmatics [17] | 6.3 ± 0.8 |
| Allergic rhinitis in children [16] | 7.48 (range 6.90–7.98) |
| Atopic dermatitis in children [16] | 7.40 (range 7.00–7.97) |
| COPD [165] | 6.97 (CI 6.65–7.29) |
Abbreviations: 95% confidence interval (CI); interquartile range (IQR); gastroesophageal reflux disease (GERD); chronic obstructive pulmonary disease (COPD).
Patients with COPD form more acidic EBC than patients with asthma and healthy controls (table 3) [6]. Unlike the response to treatment seen in asthma, no difference has been observed in EBC pH between patients with COPD who are treated with ICS and those that are steroid-näive [6]. Borrill et al showed that EBC pH changes over time in patients with COPD, a phenomenon not seen in other inflammatory diseases of the lung [165].
Acid stress can also be used to understand the effect of environmental conditions on the airways. EBC pH is decreased in smoking asthma patients compared to non-smoking asthmatics [17]. Smoking asthma patients have features similar to the early stages of COPD [17].
Some studies, however, have found that EBC pH may not correlate with symptoms of inflammation, lung function, airway hyperresponsiveness or airway inflammation expressed by FENO [18-20]. Although EBC pH has good repeatability in long-term assessments, EBC pH in asthmatics fluctuates more than in healthy subjects, leading to pH variability [10]. In addition, preliminary reports suggest that EBC pH may be influenced by environmental temperature and relative humidity [21], suggesting a need for larger investigational studies to standardize techniques. A study of EBC pH in COPD patients found that EBC acidification was affected by the balance of salivary acids and bases, suggesting the impact of another extrapulmonary condition on exhaled breath [22].
EBC has also been used to evaluate the influence of gastroesophageal reflux disease (GERD) on airway acidity in asthma [23]. EBC pH in asthma patients with GERD is lower than those in asthma patients without GERD (table 3) [23].
Nitric oxide (NO) and its metabolites
NO plays an important role in inflammation and in the regulation of smooth muscle tone. NO levels increase in response to pro-inflammatory cytokines and oxidants [24]; this increase is detectable in the breath as fractional exhaled nitric oxide. As a free radical, NO reacts with oxygen to produce nitrogen oxides such as nitrates and nitrites. Total nitrite/nitrate (NOx) levels are elevated in patients with asthma when compared to healthy subjects; atopy is also known to increase nitrite/nitrate levels, but to a smaller degree than asthma [20]. EBC NOx levels decrease after the use of ICS in asthma, but not in COPD [25, 26]. Smoking decreases EBC NOx levels in patients with asthma, but not in COPD [26].
Nitrite levels in EBC are increased in COPD and may reflect the increased nitrative stress in the airways of COPD patients [27]. A strong relationship between EBC nitrite levels and hyperinflation measured by pulmonary function tests exists [28]. This suggests the possibility of using EBC nitrite levels as a biomarker for over-distension in the lungs.
Nitrotyrosine is formed when NO and superoxide anions create peroxynitrite, which can then react with tyrosine residues on proteins [29]. Nitrotyrosine is increased in the EBCs of steroid-naïve adults [30] and children with asthma [31]. Patients with asthma receiving oral steroids, however, have comparatively lower levels of nitrotyrosine in EBC, suggesting that systemic steroids may inhibit the inflammatory response in the airways and lead to a reduction in local oxidative stress [32, 33]. In COPD a significant negative correlation between FEV1 and the amount of nitrotyrosine exists: as lung function declines measured by FEV1, the amount of oxidative stress measured by nitrotyrosine levels in EBC increases [34]. Recent work with more sensitive techniques of detecting nitrotyrosine in EBC, however, found that nitrotyrosine levels are variable and that this biomarker may not be a reproducible and reliable marker for oxidative stress, particularly in children with stable asthma [35].
S-nitrosothiols are produced when peroxynitrate reacts with thiol-containing macromolecules (i.e. cystiene and glutathione). S-nitrosothiols are increased in bronchial asthma, with levels directly correlating with the severity of asthma [27]. Patients with COPD also have higher S-nitrosothiols concentrations in EBC than healthy non-smokers [27].
Eicosanoids (prostaglandins and leukotrienes)
Prostanoids are synthesized by the cyclooxygenase (COX) pathway to produce prostaglandins and thromboxanes. Prostaglandin E2 (PGE2), D2 and F2a, as well as thromboxane B2 (TXB2) have been found in EBC samples [36-40]. Although the role of prostanoids is not fully understood, PGE2 is thought to be protective through its bronchodilative properties. Smoking asthmatics have higher PGE2 levels in EBC than both asthmatic non-smokers and control subjects [37]. No significant difference in PGE2 levels, however, exists between asthmatics and normal controls, despite controlling for steroid use [37]. ICS does not reduce PGE2 in EBCs from asthmatic children [41]; the same is true with leukotriene receptor antagonists (montelukast) [42]. This suggests that PGE2 may be more useful for understanding environmental effects like smoking on airways in asthma, rather than its treatment. Another eicosanoid, TXB2, is also increased in the EBC of asthmatic subjects compared with controls [40], but this finding has not been reproduced in children [41].
PGE2 and PGF2α are also markedly increased in patients with COPD compared with healthy non-smokers [43]. PGE2 may have anti-inflammatory effects in the airways; raised PGE2 levels in the airways of patients with COPD could be a mechanism for counteracting the lung inflammation of this disease. Steroid-naïve and steroid-treated patients with COPD have similar PGE2 concentrations in EBC, again suggesting that PGE2 may not be the best mediator for monitoring treatment with ICS [44]. Studies of the eicosanoid pathway and COX inhibition, however, suggest that this biomarker may be used to understand processes of airway inflammation. Non-selective COX inhibition, therefore, may have important implications for lung inflammation in COPD patients. Oral ibuprofen (non-selective COX inhibition) decreases PGE2 levels in patients with COPD; COX-2 inhibition, however has no effect on PGE2 levels in these patients [45]. This suggests that PGE2 may become useful for investigating airway inflammation and novel treatments for COPD.
Leukotrienes are classified into two classes: LTB4 and the cysteinyl leukotrienes (Cys-LTs: LTC4, LTD4 and LTE4). LTB4 is a chemoattractant for inflammatory cells, including neutrophils and eosinophils [46]; its exact role in allergy and asthma is unknown. LTB4 levels are elevated in the EBC of asthmatic adults [30, 38] but not in atopic children without asthma [47]. The effect of ICS use on LTB4 levels in asthma has varied in the literature [47-49]. Smoking asthmatics have higher levels of LTB4 than smokers without asthma; these values approach those of COPD patients, suggesting a shared characteristic [50].
Although LTB4 is increased in both asthma and COPD, the increase is more pronounced in COPD: LTB4 levels are increased in patients with COPD compared to healthy non-smokers [44]. ICS use has no effect on LTB4 concentrations in EBC collected from patients with COPD [44]. Antibiotic treatment, however, results in a reduction in LTB4 levels after COPD exacerbations [51]. Non-selective COX inhibition increases LTB4 in EBC; selective COX-2 inhibition has no effect on this eicosanoid [45]. The significance of these trends is unknown.
Cys-LTs are generated during the early and late phase allergic reactions, inducing smooth muscle contraction, microvascular leakage and mucous hypersecretion [52]. In fact, Cyst-LTs are 1000-fold more potent than histamine on bronchial smooth muscle tone [53]. Cys-LTs are elevated in patients with allergic rhinitis; intranasal steroid reduce these levels in EBC [54]. Cys-LT levels in EBC are increased in adults [38, 55] and children [55, 56] with asthma and are especially elevated in patients with unstable asthma [57]. These elevated levels in asthmatics approach healthy normal values after treatment with oral corticosteroids [58]. Cys-LTs in EBC have also been used to investigate other types of treatment, including leukotriene receptor antagonists like montelukast. Cys-LTs levels in EBC have not been shown to be related to the severity of exercise-induced bronchospasm or to montelukast response in children with asthma [59].
Asthmatics have elevated levels of LTE4 levels in EBC [38, 41, 42, 55, 60]. Leukotriene receptor antagonists decrease exhaled LTE4 in atopic children with asthma, but not in atopic children without asthma [42]. LTE4 may be useful for distinguishing between allergic rhinitis and asthma. A strong correlation between EBC Cyst-LTs and reticular basement membrane thickness exists, suggesting that cys-LTs may play an important role in airway remodeling [61]. Similarly, increased LTE4 levels in children with mild asthma may be useful as a non-invasive marker for assessing airway inflammation and bronchial hyperresponsiveness in children with asthma [60].
Disorders of the upper respiratory tract, like allergic rhinitis, are commonly associated with bronchial hyperresponsiveness and asthma [62]. Leukotriene concentrations in EBC (LTB4, LTE4) are significantly increased in and after the pollen season in patients with seasonal allergic rhinitis compared to healthy controls [63]. Leukotriene levels collected in EBC decrease after pollen season compared with the seasonal baseline [63]. Patients with the highest in season leukotriene levels also had the highest post-season levels [63]. These findings suggest that leukotrienes may be an early marker of the inflammatory process in the lower airways.
Isoprostanes
Isoprostanes are prostaglandin-like compounds formed by free-radical lipid peroxidation. Many studies have demonstrated that these compounds are accurate markers of oxidative stress in airways [64-66]. 8-Isoprostane is one of the most studied isoprostanes in EBC. Levels of 8-isoprostane are twice as high in mild asthma compared to healthy subjects; this difference is further increased in moderate to severe asthma [42, 67]. Contraindicatory results have been shown for asthma in response to ICS treatment [36, 42, 67, 68]. 8-Isoprostane levels are also elevated in patients with COPD exacerbations, but these levels are decreased after antibiotic treatment [51].
EBC levels of 8-isoprostanes are elevated in cigarette smokers without defined lung disease, but to a much greater extent in patients with COPD [43]. Despite this, 8-isoprostane levels do not differ between current smokers with COPD and ex-smokers with COPD [43]. This suggests that this isoprostane is derived from the oxidative stress specific to the inflammation underlying COPD, rather than from cigarettes alone.
8-Isoprostane levels in EBC are strongly related to small airway function in asthma, suggesting that this biomarker in EBC may be complementary to peak flow measurements currently used to examine airways in asthmatics [69]. 8-Isoprostane levels may also reflect the extension of lung damage in COPD patients [70]. 8-Isoprostane levels are inversely related to lung function in COPD: patients with poorer lung function have higher levels of 8-isoprostane detected in EBC [71].
Hydrogen peroxide (H2O2)
Active inflammatory cells respond to stress with a respiratory burst, resulting in the production of reactive oxygen species such as H2O2. Most studies report elevated levels of H2O2 in steroid-naïve asthmatic subjects, although several studies report levels of H2O2 that are below the limit of detection [72-77]. H2O2 levels are influenced by disease severity [76], smoking habit [74], the presence of symptoms in unstable patients [75], but not atopy [76]. H2O2 levels are thought to be related to bronchial hyperresponsiveness and bronchoconstriction [78]. Evidence that this mediator is related to eosinophilic inflammation [76] and the products of NO metabolism [74] also exists. ICS decrease H2O2 levels: stable asthmatic patients treated with ICS have lower H2O2 levels compared to steroid-naïve subjects and similar to normal subjects [74-76].
Cigarette smoking causes an influx of inflammatory cells into the lower airways; smokers have a five-fold higher level of H2O2 in EBC than non-smokers [79]. Patients with COPD have increased levels of H2O2 compared to normal subjects; these levels are further increased during exacerbations [80, 81]. Despite the known relationship between smoking and the progression to COPD, no significant differences have been found between H2O2 levels in current smokers with COPD and ex-smokers with COPD; similarly, no correlation has been found between H2O2 levels in EBC and daily cigarette consumption [81]. This suggests that oxidative stress is a characteristic feature of COPD that cannot be entirely explained by the oxidants present in tobacco smoke.
H2O2 levels in EBC after the ICS treatment of COPD is controversial [82, 83]. Another treatment, however, has more promising data. Nebulized antioxidant N-acetylcysteine increases exhaled H2O2 levels in the EBC of stable COPD patients [84]. H2O2 levels have also been used as an inflammatory marker to determine the association between tissue inflammation and inflammation-associated priming of neutrophils in the peripheral blood [85]. A strong relationship exists between the levels of H2O2 in EBC and neutrophil priming, suggesting that local inflammation has systemic effects on cells of the innate immune system [85].
Other EBC biomarkers in asthma, allergic rhinitis and COPD
Malondialdehyde is generated by arachidonic and docosa-hexenoic acid; other classes of aldehydes are produced by lipid peroxidation. Malondialdehyde levels in EBC increase during asthma exacerbations but decrease after steroid treatment [86]. COPD have increased levels of malondialdehyde compared with healthy smokers [86]. EBC aldehydes are not strongly related to findings in induced sputum of asthmatics; this suggests that the two techniques should be evaluated independently [87].
Most proteins, including cytokines, are difficult to reliably measure in EBC. Cytokines help mediate inflammatory processes; several cytokines have been detected in EBC [38, 68]. Patients with COPD may have increased concentrations of pro-inflammatory mediators in EBC (IL-1β and IL-12) compared to healthy controls [88]. Patients with acute exacerbations of COPD have increases in IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF-α detected in EBC compared to patients with stable COPD, smokers and healthy volunteers [88]. ICS treatment decreases levels of IL-1β, IL-6, IL-8, IL-10 and IL-12 with dose-dependency for IL-8, IL-1β and IL-12 [88]. TNF-α is significantly higher in the EBC of patients with COPD than non-COPD patients [89]. This group also detected erythropoietin in EBC, although no significant difference for EPO levels or correlation between EPO and TNF-α was found [89]. Despite these promising findings, data reproducibility has been poor for EBC studies in COPD.
Cytokines have also been investigated with asthma. Asthma is associated with eosinophilic airway inflammation and the overproduction of T-helper type 2 (Th2) lymphocyte-related cytokines. An elevated IL-4/IFN-γ ratio is found in the EBC of children with asthma, suggesting a predominance Th2 inflammation in these airways [71]. The following cytokines, chemokines and growth factors are significantly up-regulated in asthmatic airways: IL-4, IL-8, IL-17, TNF-α, RANTES, IFN-γ-inducible protein 10, TGF-β, macrophage-derived chemokine (MDC), eotaxin and macrophage inflammatory proteins 1α and 1β [71, 90, 91]. RANTES levels in EBC strongly correlate with airway caliber, FEV1 and respiratory resistance values in asthmatics [91]. Levels of TNF-α and TGF-β measured in EBC correlate with methacholine threshold and peak expiratory flow variability [90]. EBC eotaxin and macrophage-derived chemokine levels are higher in asthmatics on ICS than the steroid-naïve asthmatics or controls [71].
Atopic dermatitis and allergic rhinitis often coexists with asthma or precede the development of asthma. EBC IL-5 levels are higher in children with atopic dermatitis, allergic rhinitis and asthma than in healthy controls [15]. Another group evaluated the relationship of chemokines (macrophage-derived chemokine, eotaxin) found in EBC with atopy-related indices [92]. They found that atopy-related indices (i.e. IgE levels and eosinophil percentage) should be considered as separate dimensions from airway inflammation in the assessment of airway inflammation. Inflammatory markers in peripheral blood and in EBC were also found to be non-overlapping factors of childhood asthma [92].
Measuring cytokine levels in EBC may be a promising approach to assess the inflammatory status of the airways in order to monitor treatment interventions and to investigate the pathophysiology of asthma in the future. The major limitation of cytokine studies is that these proteins, when actually found in EBC, are very close to the detection limit. Newer more sensitive techniques may be needed in order to make this mediator a reliable and reproducible biomarker in EBC.
Endothelins are pro-inflammatory, bronchoconstrictive and vasoconstrive peptides that are important in airway inflammation and airway remodeling in asthma. Inflammatory cells and cytokines are thought to alter the expression and release of endothelins. Exercise-induced bronchoconstriction leads to an increase in the release of endothelin-I from the bronchial epithelium, which can be detected in EBC; this release may be important for understanding airway inflammation after post-exercise bronchoconstriction in asthmatics [93].
Adenosine, a purine nucleoside expressed in a variety of physiological cells, is present in bronchoalveolar lavage and EBC [94, 95]. Adenosine levels in EBC are significantly increased in asthmatic patients during exercise but not in healthy controls [96]. This exercise-induced change in adenosine concentration strongly correlates with a decline in FEV1. EBC adenosine is also increased in allergic rhinitis, suggesting a subclinical inflammation in lower airways of patients with allergic rhinitis [97].
Long-term exposure to tobacco smoke may increase the uptake of toxic metals in the lungs since these metals are major contaminants in tobacco smoke. Current smokers have higher levels of lead and cadmium in EBC than healthy non-smoking subjects. Smoking COPD patients have higher levels of toxic metals than ex-smokers with COPD; ex-smokers, however, still have higher levels of these toxic metals in COPD than non-smoking controls [98]. Metallic elements in EBC may also be useful in distinguishing asthma from COPD since patients with COPD have elevated levels of toxic metals and transition elements involved in redox systems of oxidative stress [99]. These metals and elements may be useful as biomarkers of exposure and useful in distinguishing similar diseases such as allergic rhinitis, asthma and COPD.
EBC in cystic fibrosis
Cystic fibrosis (CF) is the most common fatal inherited disease in Caucasians. An inherited mutation in a gene encoding a transmembrane protein leads to the clinical manifestations of the disease. This transmembrane protein controls the secretions of the sweat glands and the respiratory, gastrointestinal and reproductive tracts; a non-functional protein leads to altered thick secretions that lead to clinical manifestations of the disease, including chronic lung disease, exocrine pancreatic insufficiency, disease-related diabetes and liver disease. Chronic airway inflammation occurs in CF as a result of repeated bacterial lung infection and an exaggerated host response to this process [100].
Adults [101] and children [102] with cystic fibrosis have acidified airways detectable by EBC pH. This decrease in EBC pH is related to infective exacerbations of CF in children [102]. These patients also have low fractional exhaled nitric oxide (FENO) compared to healthy subjects [103]. Ojoo et al investigated the relationship of airway lining fluid pH and FENO regulation in respiratory disease [104]. This group found that patients with stable CF had lower FENO and EBC pH than healthy controls; NOx levels, however, were significantly higher in CF than that found in healthy controls [104]. CF exacerbations lead to a further decrease in EBC pH but similar FENO and NOx levels in EBC [104]. This group’s findings suggest that EBC pH and FENO could describe different aspects of airway inflammation. Care should be taken to differentiate the difference between what these biomarkers indicate about the pathology behind the inflammatory process.
Patients with CF have elevated levels of nitrite and nitrate in EBC compared to healthy controls [105, 106], during exacerbations and stable disease. The bacterial airway colonization and subsequent chronic airway inflammation may be detected by EBC; early recognition of airway inflammation and airway infection could lead to better clinical outcomes. Horak et al [107] investigated the use of EBC nitrites in monitoring CF lung disease activity in children and found no correlation between EBC nitrite levels and markers of disease severity; in fact, elevated EBC nitrite levels did not predict subsequent pulmonary exacerbations [107]. This suggests that EBC nitrite levels may not be ideal biomarkers for monitoring CF lung disease in children. Patients with CF also have elevated levels of nitrotyrosine in EBC-compared normal subjects [108]. S-nitrosothiols are elevated in EBCs of adults with very severe CF [27].
Chronic pulmonary infections often determine the course and prognosis of CF [109]. Opportunistic bacteria such as Staphylococcus aureus and Psuedomonas aeruginosa are a major cause of the chronic airway neutrophil-dominated inflammation [110]. Neutrophils release many mediators, including eicosanoids, which can be detected in EBC. LTB4 levels in EBC are increased in children with CF compared to healthy controls; this difference is enhanced during a CF exacerbation [102]. LTB4, a biomarker for neutrophilic inflammation, is elevated in the EBC of children with CF with active bacterial airway infections [48]. CF children colonized with P. aeruginosa have higher levels of LTB4 in EBC than those colonized with S. aureus [48].
8-Isoprostane levels are increased three-fold in patients with CF compared to healthy controls [111]. No relationship exists between EBC pH and 8-isoprostane levels in children with CF [102].
H2O2 levels, thought to be indicative of oxidative stress levels, are reduced during the antibiotic treatment of patients with infective exacerbations of CF [112].
IFN-γ levels in EBC are significantly higher in children with CF compared to healthy controls. Robroeks et al used multivariate logistic regression models to evaluate biomarkers in EBC [113]. This group found that the presence of CF is best indicated by 8-isoprostane, nitrite and IFN-γ levels in EBC [113]. An exacerbation of CF, however, is best indicated by 8-isoprostane and nitrite levels. CF severity is best indicated by EBC acidity [113].
IL-10, IL-4, TNF-α and IFN-γ are in the EBCs of CF patients [113]. EBC levels of IL-8 are elevated in CF patients colonized by P. aeruginosa and S. aureus; IL-8 levels are also elevated in CF patients that are not colonized, compared to healthy children [114].
Purines are established biomarkers of airway inflammation in respiratory disease [95]. Purines mediate airway mucus clearance, airway surface liquid volume, ciliary function and mucin secretion [115, 116]. Purines are also involved in inflammatory cell responses: neutrophils release ATP when activated [117, 118]. ATP levels are increased in CF relative to controls; these levels decrease after the treatment of pulmonary exacerbation due to CF [119]. Esther et al developed a sensitive and specific liquid chromatography/tandem mass spectrometry method to detect adenyl purines as biomarkers from EBC; urea was detected as a dilution marker in EBC [120]. The AMP/urea ratio is elevated in EBC collected from patients with CF, compared to that from healthy controls [120]. This group’s findings suggest that EBC can be used to detect a biomarker for airway inflammation and to control for variable dilution [120]. Purines are candidate biomarkers of neutrophilic airway inflammation.
EBC has also been used to evaluate the effectiveness of glucose as a biomarker for airway inflammation in cystic fibrosis [121]. The respiratory tract has low levels of glucose: in animals, glucose concentration in respiratory tract lining fluid is 3–20 times lower than plasma glucose concentration (1, 22). In humans, respiratory tract glucose concentrations are also low, but they are elevated by inflammation and hyperglycemia [121]. Breath glucose from EBC of CF patients is higher than expected for blood glucose in CF patients and in CF-related diabetes [121]. This group’s data suggest that EBC may be a useful estimate of respiratory fluid glucose concentration and that the effects of lung disease and hyperglycemia can be distinguished by this method.
EBC in lung cancers
Lung cancer is the most common cancer in the word. One current limitation in diagnosis is that lung cancer is typically detected through chest x-ray and sputum cytology. As a result, a considerable amount of research has been directed toward the early detection of tumor markers before the cancer becomes clinically observable and unresponsive to treatment. Many different research groups have brought forward potential lung cancer biomarkers; however, none have proven to be useful in a clinical setting for the early diagnosis of lung cancer. Currently, an accurate diagnosis is usually only made when the disease has further progressed and is less responsive or unresponsive to therapeutic intervention. Unfortunately, in lung cancer there are no sensitive and specific biomarkers, such as prostate-specific antigen in prostate cancer. Several biomarkers will probably have to be used together, including P53, ras and the methylation of different genes.
Several articles reported that EBC may present as a simple non-invasive alternative lung cancer biomarkers. Chromate workers with and without lung cancer have higher pulmonary tissue Cr levels than controls [122]. By using electrothermal atomic absorption, Cr can be detected in EBC from patients and controls. Significant increase in the levels of Cr-EBC after surgical intervention in non-small cell lung cancer patients gives new information for sensitivity of Cr-EBC as a biomarker of lung exposure. The problem is that pulmonary Cr levels have never been standardized and different values have been reported in control subjects [123, 124]. Certainly, tissue Cr level is affected by age, gender and region and even by lifestyle therefore before using Cr-EBC as a biomarker for lung cancer, it is necessary to standardize the Cr levels in pulmonary tissue.
The alteration of DNA in EBC have been reported as significantly more frequently than those of blood DNA [125]. This result suggests that the detection of abnormalities in EBC could be a marker and identify patients with high risk of lung cancer. Carpagnano et al showed that whole blood DNA shows significantly lower microsatellite alteration frequency compared with EBC DNA [126]. This alteration in EBC could be a significant marker for tumorgenesis and could become a useful biomarker for early lung cancer diagnosis.
EBC in sleep apnea
Obstructive sleep apnea syndrome is characterized by repetitive episodes of upper airway obstruction during sleep and occur in approximately 9% of men and 4% of women [127]. The inflammation in the airway plays a key role in the pathogenesis of OSAS but still the mechanism is unknown [128]. Recently, researchers are increasingly looking for new markers of airways inflammation although there are very few studies made on EBC in OSAS. Carpagnano et al are the first group that showed the exhaled pH in patients with OSAS [129]. They suggest that obese patients with OSAS and obese patients without OSAS present upper airway inflammation which could be monitored by the exhaled pH in EBC. There are also studies that shows the exhaled breath markers such as 8-isoprostane [130, 131], leukotrienes, nitrates, H2O2 and pH correlates with the severity of OSAS [130]. There are also pro-inflammatory cytokines such as TNF-α and IL-6 which are the strongest predictors of apnea–hypopnea index change. Li et al showed that monitoring IL-6 and TNF-α could be useful for follow-up procedures [131]. Goldbart et al suggest that EBC could be a useful tool as a non-invasive biomarker for upper airway inflammation in children with sleep-disordered breathing. They showed that eicosanoids are measurable in EBC and there is a difference in these inflammatory mediators that emerge among children with and without sleep-disordered breathing which could be used as a non-invasive tool in the clinic.
EBC measurement and analysis techniques
The statement released by the ATS/ERS and several other groups have reviewed the technical aspects of EBC [3, 5, 132]. We focused our review on papers published after ATS/ERS guidelines. Of these 76 recent papers on EBC, 26 papers studied asthma, 15 papers investigated COPD, 7 papers evaluated CF and the remainder evaluated other populations (healthy reference, obstructive sleep apnea, bronchectasis, asbestosis, allergic rhinitis, lung cancer) (table 4). The most popular EBC collection device was the Ecoscreen EBC collecting device (27 studies), followed by the R-tube (12 studies) and glass condensing systems (11 studies) (table 5).
Table 4.
Demographic information of EBC papers reviewed. Papers were grouped by those that studied healthy reference populations (A) asthma, allergic rhinitis, atopic dermatitis and COPD (B); obstructive sleep apnea (C); cystic fibrosis (D); other lung diseases (E)
| First author | Year | Population studied | Sample size |
|---|---|---|---|
| (A) | |||
| Czebe [143] | 2008 | Healthy reference, adult | 12 |
| Do [144] | 2008 | Healthy reference, adult, smokers and non-smokers |
12 |
| Hoffmann [145] | 2008 | Healthy reference, adult | 6 |
| Hu [146] | 2008 | Healthy reference, adults; rheumatoid arthritis |
22 |
| Kullmann [21] | 2008 | Healthy reference, adults | 12 |
| Balbi [147] | 2007 | Healthy reference, adult | Review |
| Bloemen [148] | 2007 | Healthy reference, adult | 21 |
| Conventz [149] | 2007 | Healthy, adults | 27 |
| Gaber [150] | 2006 | Healthy, adults | 34 |
| Paget-Brown [14] | 2006 | Healthy reference, adult and children |
404 |
| Schumann [89] | 2006 | Healthy reference, adult | 22 |
| (B) | |||
| Accordino [10] | 2008 | Asthmatic, adults | 42 |
| Akpinar-Elci [151] | 2008 | Environmental exposure, water damage and asthma |
371 |
| Bayley [152] | 2008 | Bronchiectasis, COPD | 61 |
| Makris [70] | 2008 | COPD, adults | 30 |
| Raissy [59] | 2008 | Asthma, children | 11 |
| Romieu [153] | 2008 | Asthma, pollution exposure, children |
107 |
| Zhao [19] | 2008 | Asthma, adults | 64 |
| Carraro [133] | 2007 | Asthmatic, children | 36 |
| Corhay [134] | 2007 | COPD, adults | 110 |
| Fireman [154] | 2007 | Obstructive lung disease, interstitial lung disease, persistent cough, adults |
75 |
| Gessner [28] | 2007 | COPD, adults | 112 |
| Liu [26] | 2007 | COPD, adults, smoking status |
176 |
| Noble [155] | 2007 | Asthma, adults | 41 |
| Prieto [156] | 2007 | Asthma, allergic rhinitis, healthy adults |
23 |
| Robroeks [157] | 2007 | Asthma, children | 114 |
| Rysz [84] | 2007 | COPD, adults | 19 |
| Shimizu [23] | 2007 | Asthma, GERD adults | Review |
| Vogelberg [158] | 2007 | Bronchitis, children | 94 |
| Zietkowski [93] | 2007 | Asthma, adults | 26 |
| Baraldi [31] | 2006 | Asthma, children | 48 |
| Bodini [33] | 2006 | Asthma, children | 10 |
| Boulet [17] | 2006 | Asthma, smoking status, adults |
49 |
| Brunetti [16] | 2006 | Asthma, allergic rhinitis, atopic dermatitis, children |
186 |
| Effros [22] | 2006 | COPD, adults | 20 |
| Failla [54] | 2006 | Allergic rhinitis, adults | 69 |
| Ko [71] | 2006 | Asthma, adults | 64 |
| Ko [71] | 2006 | COPD, adults | 32 |
| Leung [166] | 2006 | Asthma, children | 58 |
| Lex [61] | 2006 | Asthma, children | 29 |
| Matsunaga [90] | 2006 | Asthma, adults | 36 |
| Montuschi [42] | 2006 | Asthma, children | 33 |
| Mutti [99] | 2006 | COPD and asthma, adults; smokers |
160 |
| Mutti and Corradi [98] | 2006 | COPD, smoking, adults | Review |
| Nicolaou [18] | 2006 | Asthma, children | 630 |
| Oudijk [85] | 2006 | COPD, adults | 10 |
| Profita [15] | 2006 | Asthma, atopic dermatitis, allergic rhinitis, children |
91 |
| Ratnawati [20] | 2006 | Asthma, children | 92 |
| Shibata [60] | 2006 | Asthma, children | 43 |
| Vass [97] | 2006 | Allergic rhinitis, adults | 42 |
| Borrill [165] | 2005 | COPD, adults | 48 |
| Cap [63] | 2005 | Allergic rhinitis, adults | 79 |
| Gessner [88] | 2005 | COPD, smoking, adults | 120 |
| Leung [92] | 2005 | Asthma, children | 92 |
| Montuschi [47] | 2005 | COPD, adults | 30 |
| Ojoo [104] | 2005 | Asthma, CF, adults | 45 |
| (C) | |||
| Carpagnano [129] | 2008 | OSA, obese | 60 |
| Li [159] | 2008 | OSA, smokers | 90 |
| (D) | |||
| Esther [119] | 2008 | CF, children and adults | 58 |
| Esther [120] | 2008 | CF, adults | 14 |
| Robroeks [113] | 2008 | CF, children | 98 |
| Baker [121] | 2007 | CF and DM, adults | 56 |
| Horak [107] | 2007 | Cystic fibrosis, children | 32 |
| Celio [35] | 2006 | Cystic fibrosis, asthma, children |
56 |
| Bodini [114] | 2005 | CF, children | 40 |
| (E) | |||
| Gessner [160] | 2008 | Respiratory failure, ARDS, adults |
30 |
| Gogate [161] | 2008 | Review, children | Review |
| Hunt [132] | 2007 | Review | Review |
| Lehtonen [162] | 2007 | Asbestosis | 30 |
| Hunt [132] | 2007 | Review | Review |
| Kharitonov [139] | 2006 | EBC review | Review |
| ATS [3] | 2005 | Review | Review |
Abbreviations: chronic obstructive pulmonary disease (COPD); obstructive sleep apnea (OSA); cystic fibrosis (CF); acute respiratory distress syndrome (ARDS); diabetes mellitus (DM); gastroesophageal reflux disease (GERD).
Table 5.
EBC techniques of papers reviewed. Papers were grouped by EBC collection device: R-tube (A), Ecoscreen (B) or other (C). Papers that utilized lyophilization are listed in (D)
| First author | Year | EBC collection device | EBC component measured | EBC analysis | Lyophilization |
|---|---|---|---|---|---|
| (A) | |||||
| Bayley [152] | 2008 | R-tube | Leukotriene B4, IL-8, secretory protease inhibitor, α1-antitrypsin, myeloperoxidase |
ELISA; myeloperoxidase by chromogenic substrate assay |
No |
| Czebe [143] | 2008 | Ecoscreen, R-tube, Anacon |
pH, protein, leukotriene | pH; protein via Bradford method; leukotrienes by ELISA |
No |
| Do [144] | 2008 | R-tube | pH, NH4+ | pH meter; HPLC (NH4+) | No |
| Esther [119] | 2008 | R-tube | Purines (ATP, ADP, AMP, adenosine) |
Luciferin–luciferase luminescence assay |
Yes |
| Esther [120] | 2008 | R-tube | Adenyl purines, urea | LC-MS | Yes |
| Kullmann [21] | 2008 | R-tube | pH | pH meter | No |
| Raissy [59] | 2008 | R-tube | Cysteinyl leukotrienes | ELISA | Yes |
| Romieu [153] | 2008 | R-tube | Malondialdehyde | Fluorescence | Unknown |
| Baker [121] | 2007 | R-tube | Glucose | Anion-exchange chromatography with pulsed amperometric detection |
Yes |
| Bloemen [148] | 2007 | R-tube | Variability of pH, volume, protein content |
Protein-fluorescence spectrophotometer; pH, biotrode |
No |
| Prieto [156] | 2007 | R-tube, Ecoscreen | pH | pH meter | No |
| Leung [166] | 2006 | Ecoscreen, R-tube | pH, 8-isoprostane, cysteinyl leukotrienes, leukotriene B4 |
pH meter, ELISA | No |
| Nicolaou [18] | 2006 | R-tube | pH | pH meter | No |
| Paget-Brown [14] | 2006 | R-tube | pH | pH meter | No |
| Leung [92] | 2005 | R-tube | MDC, eotaxin, leukotriene B4 |
ELISA (MDC, eotaxin), LTB4 (acetylcholinesterase competitive enzyme immunoassay) |
No |
| (B) | |||||
| Carpagnano [129] | 2008 | Ecoscreen | pH | pH meter | No |
| Czebe [143] | 2008 | Ecoscreen, R-tube, Anacon |
pH, protein, leukotriene |
pH; protein via Bradford method; ELISA |
No |
| Gessner [28] | 2008 | Ecoscreen | Protein (cytokeratins 2, 9, 10); IL-6, IL-8 |
ELISA (IL-6, IL-8); Western blot (proteins) |
Yes |
| Hoffmann [145] | 2008 | Ecoscreen | Cytokeratins | Mass spectrometry | Yes |
| Li [159] | 2008 | Ecoscreen | IL-6, IL-10, TNF-α, 8-isoprostane |
ELISA | No |
| Zhao [19] | 2008 | Ecoscreen | pH, 8-isoprostane | ELISA (8-isoprostane), pH meter |
No |
| Corhay [134] | 2007 | Ecoscreen | Eosinophil and neutrophil chemotactic activity |
Microchambers and chemotactic index |
No |
| Gessner [28] | 2007 | Ecoscreen | IL-8, IL-1β, IL-6, IL-10, IL-12, TNF-α, nitrite |
GREISS reaction (nitrite), multiplex immunoassay (cytokines) |
No |
| Lehtonen [162] | 2007 | Ecoscreen | Leukotriene B4, 8-isoprostane |
ELISA | No |
| Noble [155] | 2007 | Ecoscreen | pH | pH meter | No |
| Piotrowski [163] | 2007 | Ecoscreen | 8-Isoprostane, cysteinyl leukotrines, luekotriene B4 |
ELISA | Unknown |
| Prieto [156] | 2007 | R-tube, Ecoscreen | pH | pH meter | No |
| Vogelberg [158] | 2007 | Ecoscreen | pH | pH meter | No |
| Zietkowski [93] | 2007 | Ecoscreen | Endothelin-1 | ELISA | No |
| Celio [35] | 2006 | EcoScreen | 3-nitrotyrosine, | GC-NICI-MS; HPLC | Yes |
| Gaber [150] | 2006 | Ecoscreen | Leukotriene B4 | ELISA | No |
| Ko [71] | 2006 | Ecoscreen | Eotaxin, MDC | ELISA | No |
| Ko [164] | 2006 | Ecoscreen | 8-Isoprostane, growth related oncogene-α, monocyte chemoattractant protein-1 |
Sandwich enzyme gimmunoassays |
No |
| Leung [166] | 2006 | Ecoscreen, R-tube | pH, 8-isoprostane, cysteinyl leukotrienes, leukotriene B4 |
pH meter, ELISA | No |
| Lex [61] | 2006 | Ecoscreen | Cysteinyl leukotrienes | ELISA | No |
| Matsunaga [90] | 2006 | Ecoscreen | Cytokines | Protein array | No |
| Montuschi [42] | 2006 | Ecoscreen | Leukotriene E4, 8-isoprostane, prostaglandin E2 |
ELISA (LTE4), HPLC (8-isoprostane, gprostaglandin E2) |
No |
| Profita [15] | 2006 | Ecoscreen | pH, IL-5 | ELISA (IL-5), pH meter | No |
| Schumann [89] | 2006 | Ecoscreen | Erythropoietin, TNF-α | Cytometric bead arrays | No |
| Vass [97] | 2006 | Ecoscreen | Adenosine | HPLC | No |
| Borrill [165] | 2005 | Ecoscreen | pH | pH meter | No |
| Cap [63] | 2005 | Ecoscreen | Leukotrienes B4, C4, D4, E4 | GC-MS | No |
| Gessner [88] | 2005 | Ecoscreen | Cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12, TNF-α) |
Cytometric bead arrays | Yes |
| Montuschi [47] | 2005 | Ecoscreen | PGE2, LTB4 | ELISA (LTB4), Radioimmunoassays (PGE2) |
No |
| (C) | |||||
| Accordino [10] | 2008 | Tygon tube | pH | Dearation, pH meter | No |
| Akpinar-Elci [151] | 2008 | Corr-A-Flex II | IL-8, nitrate | Immunoassay; Chemiluminescence |
No |
| Czebe [143] | 2008 | Ecoscreen, R-tube, Anacon |
pH, protein, leukotriene | pH l; protein via Bradford method; ELISA |
No |
| Hu [146] | 2008 | Unknown | H2O2 | Chemiluminescence | No |
| Makris [70] | 2008 | Glass condensing chamber | 8-Isoprostane | ELISA | No |
| Robroeks [113] | 2008 | Glass condensing chamber | pH, NOx, 8-isoprostane, H2O2, IFN-γ |
pH meter, fluoremetric assay (NOx), ELISA (8-isoprostane), spectrophotometry (H2O2) |
No |
| Carraro [133] | 2007 | Tygon tube | Low molecular weight metabolites |
Metabolomics, NMR spectra |
Yes |
| Fireman [154] | 2007 | Teflon | H2O2 | Colorimetry | No |
| Horak [107] | 2007 | Teflon | Nitrites | Griess assay | No |
| Liu [26] | 2007 | Glass condensing chamber | Nitrite/nitrate | Griess method | No |
| Robroeks [157] | 2007 | Unknown | NOx, H2O2, 8-isoprostane, IFN-γ, TNF-α, IL-2, IL-4, IL-5, IL-10, pH |
Flow cytometry (Cytokines); ELISA (8-isoprostane), fluorometry (NOx) |
No |
| Rysz [84] | 2007 | Glass condensing chamber | H2O2 | Spectrophotometry | No |
| Baraldi [31] | 2006 | Glass condensing chamber | 3-nitrotyrosine | LC-MS | No |
| Bodini [33] | 2006 | Glass condensing chamber | Nitrotyrosine | ELISA | No |
| Boulet [17] | 2006 | Unknown | pH | pH meter | No |
| Brunetti [16] | 2006 | Teflon | pH | pH meter | No |
| Effros [22] | 2006 | Polycarbonate condenser; Corr-a-Flex |
pH, acetate, NH4+ | pH meter | Yes |
| Failla [54] | 2006 | Custom | Cysteinyl leukotrienes | ELISA | No |
| Mutti [99] | 2006 | TURBO DECCS | Metallic elements and serum pneumoproteins |
Inductively coupled plasma-mass spectrometry and electrothermal atomic absorption spectroscopy |
No |
| Oudijk [85] | 2006 | Glass condensing chamber | H2O2 | Spectrophotometry | No |
| Ratnawati [20] | 2006 | Glass condensing chamber | NOx levels, pH | Fluorescence and pH meter | No |
| Shibata [60] | 2006 | Unknown | LTE4 | ELISA | Yes |
| Bodini [114] | 2005 | Glass condensing chamber | pH, LTB4, IL-8 | ELISA (LTB4, IL-8), pH meter |
No |
| Ojoo [104] | 2005 | Glass condensing chamber | pH, NOx | pH meter, colorimetry (NOx) |
No |
| (D) | |||||
| Esther [119] | 2008 | R-tube | Purines (ATP, ADP, AMP, adenosine) |
Luciferin–luciferase luminescence assay |
Yes |
| Esther [120] | 2008 | R-tube | Adenyl purines, urea | LC-MS | Yes |
| Gessner [28] | 2008 | Ecoscreen | Protein (cytokeratins 2, 9, 10); IL-6, IL-8 |
ELISA (IL6, IL8); Western blot (proteins) |
Yes |
| Hoffmann [145] | 2008 | Ecoscreen | Cytokeratins | Mass spectrometry | Yes |
| Raissy [59] | 2008 | R-tube | Cysteinyl leukotrienes | ELISA | Yes |
| Baker [121] | 2007 | R-tube | Glucose | Anion-exchange chromatography with pulsed amperometric detection |
Yes |
| Carraro [133] | 2007 | Tygon tube | Low molecular weight metabolites |
Metabolomics, NMR spectra |
Yes |
| Celio [35] | 2006 | EcoScreen | 3-nitrotyrosine, | GC-NICI-MS; HPLC | Yes |
| Effros [22] | 2006 | Polycarbonate condenser; Corr-a-Flex |
pH, acetate, NH4+ | pH meter | Yes |
| Shibata [60] | 2006 | Unknown | LTE4 | ELISA | Yes |
| Gessner [88] | 2005 | Ecoscreen | Cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12, TNF-α) |
Multiplex chemiluminescent immunoassay system (cytometric bead arrays) |
Yes |
Abbreviations: interleukin (IL); enzyme-linked immunoassay (ELISA); nitrate/nitrite ratio (NOx); not applicable (NA); liquid chromatography-tandem mass spectrometry (LC-MS); gas chromatography/negative ion chemical ionization/mass spectrometry (GC/NICI/MS); high-performance liquid chromatography (HPLC); gas chromatography–mass spectrometry (GC-MS); macrophage-derived chemokine (MDC).
Standard techniques for detecting markers in EBC include enzyme-linked immunoassays, pH measurement and fluorometric assays. We found that several new high-sensitivity techniques are being successfully utilized for biomarker detection in EBC, including liquid chromatography/electrospray ionization tandem mass spectrometry (table 5). These techniques may be more sensitive than previous methods and increase the ability of detecting important markers.
Novel techniques of EBC analysis are promising for learning more about the pathobiology of asthma. A recent group used metabolomics in EBC and discovered novel compounds. This suggests the possibility of creating biochemical fingerprints for each disease in the near future [133]. EBC has also been applied to other types of biomarkers. Corhay et al [134] used EBC to compare the eosinophil and neutrophil chemotactic activity of patients with COPD and healthy subjects. This group found that current smoking favored neutrophil chemotactic activity and that COPD patients had more neutrophil chemotactic activity than eosinophilic activity in EBC, compared to healthy controls.
Lyophilization
Perhaps the most promising methods to increase the utility of EBC is lyophilization. In this method, freeze-drying of EBC is used to concentrate the sample. One of the largest obstacles to the field of EBC research is biomarker detection. Since the majority of the sample consists of condensed water vapor, most biomarkers exist in EBC at very low concentrations. When detected in EBC, these biomarkers are very close to the detection limit for that marker. This proximity decreases the reproducibility and reliability of the use of EBC in biomarkers. The dilution problem has partly been addressed by lyophilization, a freeze-drying process that allows for concentrating a sample. Normally, a solution can be concentrated by adding heat and evaporating free water; this process of heating, however, can significantly damage a sample. Lyophilization is a method of dehydrating a delicate sample that would otherwise be damaged by the drying process. By regulating the temperature and pressure of the sample, freeze-drying brings the sample system around the triple point of a typical phase diagram, thereby avoiding the liquid–gas transition seen in ordinary drying (figure 2). Water is removed from the sample after it is frozen and placed under a vacuum, allowing the ice to change directly from solid to vapor without passing through a liquid phase; this allows for the removal of free water without excessive heating of the product (figure 2).
Figure 2.

Phase diagram illustrating the lyophilization process in detail. Figure adapted from Labconco protocol on freeze-drying [135]. Most samples are frozen below the point at which the entire suspension is completely frozen (eutectic point) (X) and raised to just below the critical temperature (Y). The pressure is lowered (Z) to encourage the free flow of water molecules from the product. The collecting system of the lyophilizer acts as a cold trap with a low temperature (W) to collect moisture, leaving the frozen sample.
The process of lyophilization consists of three steps: pre-freezing, primary drying and secondary drying [135]. Samples are pre-frozen before starting lyophilization at the eutectic temperature, the temperature at which all areas of the concentrated solute are frozen. Eutectics are mixtures of substances that freeze at lower temperatures than water. When an aqueous suspension is cooled, water is separated from the lower freezing solute as it changes to ice. This creates pockets of concentrated solute with a lower freezing temperature than water. The product may appear to be frozen at this point but is not until all of the solute in the suspension is frozen. The eutectic temperature is the temperature at which all of the eutectic mixture is frozen in the suspension. Next, in primary drying free water in the form of ice is removed via sublimation: ice evaporates off as vapor. This is accomplished by decreasing the pressure surrounding the samples and by adding small amounts of energy in the form of heat (figure 2). It is important to note, however, that very little energy is added to prevent the sample from crossing the damaging triple point of the phase diagram; crossing this point would lead to the damage seen in traditional drying. Secondary drying is a final isothermal desorption process that removes residual moisture [135]. After lyophilization is complete, all free water has been removed and solute remains. This solute can then be reconstituted in a solution of choice.
Although lyophilization may be an effective tool for concentrating dilute samples that would be damaged by traditional drying techniques, the process of lyophilization is the most complex, timely and expensive form of drying. Lyophilization has been used by many groups evaluating EBC biomarkers [28, 59]. Despite the growing trend of using this technique to concentrate EBC for biomarker detection, very little is known about the reproducibility and reliability of this method.
Discussion
Exhaled breath condensate is a promising non-invasive technique for learning about the airways. EBC provides a real-time assessment of pulmonary pathobiology. Patients whose condition limits testing ability by other methods (i.e. children) are able to successfully participate in this technique. The simplicity and low cost of the collection device will allow for the use of this technique in large longitudinal studies, such as what the ECLIPSE study did for biomarkers in EBC in evaluating COPD [136].
Airway acidification has also been found in allergic rhinitis and atopic dermatitis, suggesting that EBC pH may be a useful method for evaluating the progression of atopy to asthma [16]. Studies in COPD suggest the need for EBC pH in screening large populations for airway inflammation to better understand how pH changes relate to COPD, particularly with the phenomenon of changing pH with time [165]. The next goal in understanding pH as a biomarker in EBC is to explain the effect of treatment on EBC pH in different diseases. This may help elucidate the differences between inflammatory processes in each disease. Similarly, extrapulmonary conditions may affect the airways and, ultimately, the content of the breath exhaled. These extrapulmonary conditions need to be better understood in order to account for their effect in future studies, thereby eliminating possible confounding effects on measured EBC pH.
Although studies of nitric oxide metabolites have been promising, not enough data exists to understand its relationship with FENO and other parameters of lung function for asthma and COPD. This finding suggests the importance of evaluating oxidative stress in the airways by evaluating several different mediators. Although nitrotyrosine has not proven to be a reliable marker in EBC for asthma, it appears as though the same is not true for COPD. Patients with COPD may produce consistently higher levels of this biomarker in EBC, enough to be useful as a marker for monitoring airway inflammation and its relationship to other markers of lung function. Larger studies should be done to investigate the role of these compounds in oxidative stress and asthma to determine whether these compounds have pathophysiological significance.
LTB4 is promising for investigating the differences in airway inflammation of asthma, atopy and COPD, but more work should be done to evaluate the effect of different treatments for these airway diseases. Cyst-LTs have been well studied in asthma and allergic rhinitis; treatment monitoring with ICS is a promising future for Cyst-LTs in EBC. LTE4 may prove to be especially useful in understanding the pathobiology of inflammation in the airways of patients with asthma. These findings suggest that leukotrienes may be an early marker of the inflammatory process in the lower airways.
8-Isoprostane illustrates an increased oxidative stress in the airways and increased levels have been found in patients with more severe COPD. This biomarker must be assessed to learn about disease progression in COPD patients. Hydrogen peroxide levels in EBC have been used to learn more about the clinical syndrome of asthma and may prove to be an important tool for investigating the pathophysiology of inflammatory lung diseases like asthma. More studies must be done to establish the effects of therapy on H2O2 levels in EBC for patients with COPD.
Further investigations must be done in order to determine a consensus regarding EBC technique in order to reduce the effect of confounders on data interpretation. Guidelines similar to the existing recommendations for FENO measurement [137] and sputum eosinophils collection [138] are needed.
Several things must be improved and many questions must be answered in order to improve the routine use of EBCs. One of the largest problems with EBC is the need for a sensitive and specific assay for detecting biomarkers. Work has been done with novel techniques but high cost and complicated procedures hinder their application. EBC will also need to be standardized as a technique. Since different mediators detected in EBC arrive from different parts of the airway, it is unlikely that one technique will be the best for obtaining all biomarkers. More studies must be done in order to improve the understanding of where these markers originate before techniques can be standardized. Once these issues are resolved, reference values for each biomarker found in EBC should be created in order to use these biomarker levels to aid in diagnosing and treating conditions, similar to what exists for FENO.
Future work for EBC is promising. Many groups [139] have suggested that a combination of biomarkers (FENO, EBC pH, EBC H2O2 levels, etc) would enhance our understanding of the airways; in fact, it is possible that ‘disease breathprints’ can be created for each disease. Although many biomarkers have been detected in EBC, little information exists on how these biomarkers are related to clinical outcomes. The use of EBC in large samples and the future reference values of each biomarker may be used to predict disease progression, disease stability and response to current and novel therapies.
List of abbreviations
- ALF
airway lining fluid
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- ATS
American Thoracic Society
- CF
cystic fibrosis
- COPD
chronic obstructive pulmonary disease
- Cr
chromium
- Cyst-LT
cysteinyl leukotriene
- EBC
exhaled breath condensate
- ECLIPSE
evaluation of COPD longitudinally to identify predictive surrogate end-points
- EPO
erythropoietin
- FENO
fractional exhaled nitric oxide
- FEV1
forced expiratory volume in 1 s
- GERD
gastroesophageal reflux disease
- H2O2
hydrogen peroxide
- ICS
inhaled corticosteroids
- IFN
interferon
- IgE
immunoglobulin E
- IL
interleukin
- IRB
institutional review board
- LT
leukotriene
- MDC
macrophage-derived chemokine
- NO
nitric oxide
- NOx
nitric oxide metabolites
- OSAS
obstructive sleep apnea syndrome
- PG
prostaglandin
- TGF
tumor growth factor
- TNF
tumor necrosis factor
- TX
thromboxane
References
- [1].Grob NM, Dweik RA. Exhaled nitric oxide in asthma: progress since the introduction of standardized methodology. J. Breath Res. 2008;2:037002. doi: 10.1088/1752-7155/2/3/037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Grob NM, Dweik RA. Exhaled nitric oxide in asthma: from diagnosis, to monitoring, to screening: are we there yet? Chest. 2008;133:837–9. doi: 10.1378/chest.07-2743. [DOI] [PubMed] [Google Scholar]
- [3].ATS Workshop Proceedings 2006 Exhaled nitric oxide and nitric oxide oxidative metabolism in exhaled breath condensate: executive summary. Am. J. Respir. Crit. Care Med. 173:811–3. doi: 10.1164/rccm.2601014. [DOI] [PubMed] [Google Scholar]
- [4].Scheideler L, et al. Detection of nonvolatile macromolecules in breath: a possible diagnostic tool? Am. Rev. Respir. Dis. 1993;148:778–84. doi: 10.1164/ajrccm/148.3.778. [DOI] [PubMed] [Google Scholar]
- [5].Kostikas K, et al. Exhaled breath condensate in patients with asthma: implications for application in clinical practice. Clin. Exp. Allergy. 2008;38:557–65. doi: 10.1111/j.1365-2222.2008.02940.x. [DOI] [PubMed] [Google Scholar]
- [6].Kostikas K, et al. pH in expired breath condensate of patients with inflammatory airway diseases. Am. J. Respir. Crit. Care Med. 2002;165:1364–70. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- [7].Hunt JF, et al. Endogenous airway acidification: implications for asthma pathophysiology. Am. J. Respir. Crit. Care Med. 2000;161:694–9. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- [8].Vaughan J, et al. Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. Eur. Respir. J. 2003;22:889–94. doi: 10.1183/09031936.03.00038803. [DOI] [PubMed] [Google Scholar]
- [9].Kullmann T, et al. Exhaled breath condensate pH standardised for CO2 partial pressure. Eur. Respir. J. 2007;29:496–501. doi: 10.1183/09031936.00084006. [DOI] [PubMed] [Google Scholar]
- [10].Accordino R, et al. Long-term repeatability of exhaled breath condensate pH in asthma. Respir. Med. 2008;102:377–81. doi: 10.1016/j.rmed.2007.10.014. [DOI] [PubMed] [Google Scholar]
- [11].Ricciardolo FL, et al. Bronchoconstriction induced by citric acid inhalation in guinea pigs: role of tachykinins, bradykinin, and nitric oxide. Am. J. Respir. Crit. Care Med. 1999;1159:557–62. doi: 10.1164/ajrccm.159.2.9804022. [DOI] [PubMed] [Google Scholar]
- [12].Holma B, Hegg PO. pH- and protein-dependent buffer capacity and viscosity of respiratory mucus: their interrelationships and influence on health. Sci. Total Environ. 1989;84:71–82. doi: 10.1016/0048-9697(89)90371-9. [DOI] [PubMed] [Google Scholar]
- [13].Luk CK, Dulfano MJ. Effect of pH, viscosity and ionic-strength changes on ciliary beating frequency of human bronchial explants. Clin. Sci. (Lond.) 1983;64:449–51. doi: 10.1042/cs0640449. [DOI] [PubMed] [Google Scholar]
- [14].Paget-Brown AO, et al. Normative data for pH of exhaled breath condensate. Chest. 2006;129:426–30. doi: 10.1378/chest.129.2.426. [DOI] [PubMed] [Google Scholar]
- [15].Profita M, et al. Noninvasive methods for the detection of upper and lower airway inflammation in atopic children. J. Allergy Clin. Immunol. 2006;118:1068–74. doi: 10.1016/j.jaci.2006.07.028. [DOI] [PubMed] [Google Scholar]
- [16].Brunetti L, et al. Exhaled breath condensate pH measurement in children with asthma, allergic rhinitis and atopic dermatitis. Pediatr. Allergy Immunol. 2006;17:422–7. doi: 10.1111/j.1399-3038.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- [17].Boulet LP, et al. Smoking and asthma: clinical and radiologic features, lung function, and airway inflammation. Chest. 2006;129:661–8. doi: 10.1378/chest.129.3.661. [DOI] [PubMed] [Google Scholar]
- [18].Nicolaou NC, et al. Exhaled breath condensate pH and childhood asthma: unselected birth cohort study. Am. J. Respir. Crit. Care Med. 2006;174:254–9. doi: 10.1164/rccm.200601-140OC. [DOI] [PubMed] [Google Scholar]
- [19].Zhao JJ, et al. The relationship between oxidative stress and acid stress in adult patients with mild asthma. J. Investig. Allergol. Clin. Immunol. 2008;18:41–5. [PubMed] [Google Scholar]
- [20].Ratnawati, et al. Exhaled breath condensate nitrite/nitrate and pH in relation to pediatric asthma control and exhaled nitric oxide. Pediatr. Pulmonol. 2006;41:929–36. doi: 10.1002/ppul.20469. [DOI] [PubMed] [Google Scholar]
- [21].Kullmann T, et al. Environmental temperature and relative humidity influence exhaled breath condensate pH. Eur. Respir. J. 2008;31:474–5. doi: 10.1183/09031936.00128007. [DOI] [PubMed] [Google Scholar]
- [22].Effros RM. Exhaled breath condensate pH. Am. J. Respir. Crit. Care Med. 2006;173:1047–8. doi: 10.1164/ajrccm.173.9.1047b. [DOI] [PubMed] [Google Scholar]
- [23].Shimizu Y, Dobashi K, Mori M. Exhaled breath marker in asthma patients with gastroesophageal reflux disease. J. Clin. Biochem. Nutr. 2007;41:147–53. doi: 10.3164/jcbn.2007020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eiserich JP, Patel RP, O’Donnell VB. Pathophysiology of nitric oxide and related species: free radical reactions and modification of biomolecules. Mol. Aspects Med. 1998;19:221–357. doi: 10.1016/s0098-2997(99)00002-3. [DOI] [PubMed] [Google Scholar]
- [25].Eu JP, et al. An apoptotic model for nitrosative stress. Biochemistry. 2000;39:1040–7. doi: 10.1021/bi992046e. [DOI] [PubMed] [Google Scholar]
- [26].Liu J, et al. Nitric oxide and exhaled breath nitrite/nitrates in chronic obstructive pulmonary disease patients. Respiration. 2007;74:617–23. doi: 10.1159/000106379. [DOI] [PubMed] [Google Scholar]
- [27].Corradi M, et al. Increased nitrosothiols in exhaled breath condensate in inflammatory airway diseases. Am. J. Respir. Crit. Care Med. 2001;163:854–8. doi: 10.1164/ajrccm.163.4.2001108. [DOI] [PubMed] [Google Scholar]
- [28].Gessner C, et al. Breath condensate nitrite correlates with hyperinflation in chronic obstructive pulmonary disease. Respir. Med. 2007;101:2271–8. doi: 10.1016/j.rmed.2007.06.024. [DOI] [PubMed] [Google Scholar]
- [29].Ricciardolo FL, et al. Reactive nitrogen species in the respiratory tract. Eur. J. Pharmacol. 2006;533:240–52. doi: 10.1016/j.ejphar.2005.12.057. [DOI] [PubMed] [Google Scholar]
- [30].Hanazawa T, Kharitonov SA, Barnes PJ. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. Am. J. Respir. Crit. Care Med. 2000;162:1273–6. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- [31].Baraldi E, et al. 3-Nitrotyrosine, a marker of nitrosative stress, is increased in breath condensate of allergic asthmatic children. Allergy. 2006;61:90–6. doi: 10.1111/j.1398-9995.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- [32].McSharry CP, et al. Short and long-term effects of cigarette smoking independently influence exhaled nitric oxide concentration in asthma. J. Allergy Clin. Immunol. 2005;116:88–93. doi: 10.1016/j.jaci.2005.03.025. [DOI] [PubMed] [Google Scholar]
- [33].Bodini A, et al. Flunisolide decreases exhaled nitric oxide and nitrotyrosine levels in asthmatic children. Mediators Inflamm. 2006;2006:31919. doi: 10.1155/MI/2006/31919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ichinose M, et al. Increase in reactive nitrogen species production in chronic obstructive pulmonary disease airways. Am. J. Respir. Crit. Care Med. 2000;162:701–6. doi: 10.1164/ajrccm.162.2.9908132. [DOI] [PubMed] [Google Scholar]
- [35].Celio S, et al. Free 3-nitrotyrosine in exhaled breath condensates of children fails as a marker for oxidative stress in stable cystic fibrosis and asthma. Nitric Oxide. 2006;15:226–32. doi: 10.1016/j.niox.2006.06.008. [DOI] [PubMed] [Google Scholar]
- [36].Antczak A, et al. Increased exhaled cysteinyl-leukotrienes and 8-isoprostane in aspirin-induced asthma. Am. J. Respir. Crit. Care Med. 2002;166:301–6. doi: 10.1164/rccm.2101021. [DOI] [PubMed] [Google Scholar]
- [37].Kostikas K, et al. Prostaglandin E2 in the expired breath condensate of patients with asthma. Eur. Respir. J. 2003;22:743–7. doi: 10.1183/09031936.03.00000603. [DOI] [PubMed] [Google Scholar]
- [38].Montuschi P, Barnes PJ. Exhaled leukotrienes and prostaglandins in asthma. J. Allergy Clin. Immunol. 2002;109:615–20. doi: 10.1067/mai.2002.122461. [DOI] [PubMed] [Google Scholar]
- [39].Montuschi P, et al. Validation of leukotriene B4 measurements in exhaled breath condensate. Inflamm. Res. 2003;52:69–73. doi: 10.1007/s000110300003. [DOI] [PubMed] [Google Scholar]
- [40].Huszar E, et al. Comparative measurement of thromboxane A2 metabolites in exhaled breath condensate by different immunoassays. Inflamm. Res. 2005;54:350–5. doi: 10.1007/s00011-005-1361-x. [DOI] [PubMed] [Google Scholar]
- [41].Mondino C, et al. Effects of inhaled corticosteroids on exhaled leukotrienes and prostanoids in asthmatic children. J. Allergy Clin. Immunol. 2004;114:761–7. doi: 10.1016/j.jaci.2004.06.054. [DOI] [PubMed] [Google Scholar]
- [42].Montuschi P, et al. Effects of a leukotriene receptor antagonist on exhaled leukotriene E4 and prostanoids in children with asthma. J. Allergy Clin. Immunol. 2006;118:347–53. doi: 10.1016/j.jaci.2006.04.010. [DOI] [PubMed] [Google Scholar]
- [43].Montuschi P, et al. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. Am. J. Respir. Crit. Care Med. 2000;162:1175–7. doi: 10.1164/ajrccm.162.3.2001063. [DOI] [PubMed] [Google Scholar]
- [44].Montuschi P, et al. Exhaled leukotrienes and prostaglandins in COPD. Thorax. 2003;58:585–8. doi: 10.1136/thorax.58.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Montuschi P, et al. Effects of cyclo-oxygenase inhibition on exhaled eicosanoids in patients with COPD. Thorax. 2005;60:827–33. doi: 10.1136/thx.2004.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway: biochemistry and relation to pathobiology in human diseases. N. Engl. J. Med. 1990;323:645–55. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- [47].Montuschi P, et al. Liquid chromatography/mass spectrometry analysis of exhaled leukotriene B4 in asthmatic children. Respir. Res. 2005;6:119. doi: 10.1186/1465-9921-6-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bodini A, et al. Exhaled breath condensate eicosanoids and sputum eosinophils in asthmatic children: a pilot study. Pediatr. Allergy Immunol. 2004;15:26–31. doi: 10.1046/j.0905-6157.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- [49].Biernacki WA, et al. Effect of montelukast on exhaled leukotrienes and quality of life in asthmatic patients. Chest. 2005;128:1958–63. doi: 10.1378/chest.128.4.1958. [DOI] [PubMed] [Google Scholar]
- [50].Kostikas K, et al. Leukotriene B4 in exhaled breath condensate and sputum supernatant in patients with COPD and asthma. Chest. 2005;127:1553–9. doi: 10.1378/chest.127.5.1553. [DOI] [PubMed] [Google Scholar]
- [51].Biernacki WA, Kharitonov SA, Barnes PJ. Increased leukotriene B4 and 8-isoprostane in exhaled breath condensate of patients with exacerbations of COPD. Thorax. 2003;58:294–8. doi: 10.1136/thorax.58.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–75. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- [53].Barnes NC, Piper PJ, Costello JF. Comparative effects of inhaled leukotriene C4, leukotriene D4, and histamine in normal human subjects. Thorax. 1984;39:500–4. doi: 10.1136/thx.39.7.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Failla M, et al. Intranasal steroid reduces exhaled bronchial cysteinyl leukotrienes in allergic patients. Clin. Exp. Allergy. 2006;36:325–30. doi: 10.1111/j.1365-2222.2006.02449.x. [DOI] [PubMed] [Google Scholar]
- [55].Cap P, et al. Gas chromatography/mass spectrometry analysis of exhaled leukotrienes in asthmatic patients. Thorax. 2004;59:465–70. doi: 10.1136/thx.2003.011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Csoma Z, et al. Increased leukotrienes in exhaled breath condensate in childhood asthma. Am. J. Respir. Crit. Care Med. 2002;166:1345–9. doi: 10.1164/rccm.200203-233OC. [DOI] [PubMed] [Google Scholar]
- [57].Zanconato S, et al. Leukotrienes and 8-isoprostane in exhaled breath condensate of children with stable and unstable asthma. J. Allergy Clin. Immunol. 2004;113:257–63. doi: 10.1016/j.jaci.2003.10.046. [DOI] [PubMed] [Google Scholar]
- [58].Baraldi E, et al. Increased exhaled 8-isoprostane in childhood asthma. Chest. 2003;124:25–31. doi: 10.1378/chest.124.1.25. [DOI] [PubMed] [Google Scholar]
- [59].Raissy HH, et al. Pretreatment with albuterol versus montelukast for exercise-induced bronchospasm in children. Pharmacotherapy. 2008;28:287–94. doi: 10.1592/phco.28.3.287. [DOI] [PubMed] [Google Scholar]
- [60].Shibata A, et al. Increased leukotriene E4 in the exhaled breath condensate of children with mild asthma. Chest. 2006;130:1718–22. doi: 10.1378/chest.130.6.1718. [DOI] [PubMed] [Google Scholar]
- [61].Lex C, et al. Exhaled breath condensate cysteinyl leukotrienes and airway remodeling in childhood asthma: a pilot study. Respir. Res. 2006;7:63. doi: 10.1186/1465-9921-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ramsdale EH, et al. Asymptomatic bronchial hyperresponsiveness in rhinitis. J. Allergy Clin. Immunol. 1985;75:573–7. doi: 10.1016/0091-6749(85)90032-6. [DOI] [PubMed] [Google Scholar]
- [63].Cap P, et al. Analysis of exhaled leukotrienes in nonasthmatic adult patients with seasonal allergic rhinitis. Allergy. 2005;60:171–6. doi: 10.1111/j.1398-9995.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- [64].Morrow JD, Roberts LJ., II The isoprostanes: current knowledge and directions for future research. Biochem. Pharmacol. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- [65].Montuschi P, Barnes P, Roberts LJ., II Insights into oxidative stress: the isoprostanes. Curr. Med. Chem. 2007;14:703–17. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- [66].Montuschi P, Barnes PJ, Roberts LJ., II Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- [67].Montuschi P, et al. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am. J. Respir. Crit. Care Med. 1999;160:216–20. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- [68].Shahid SK, et al. Exhaled 8-isoprostane in childhood asthma. Respir. Res. 2005;6:79. doi: 10.1186/1465-9921-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Battaglia S, et al. Small airways function and molecular markers in exhaled air in mild asthma. Thorax. 2005;60:639–44. doi: 10.1136/thx.2004.035279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Makris D, et al. Exhaled breath condensate 8-isoprostane, clinical parameters, radiological indices and airway inflammation in COPD. Respiration. 2008;75:138–44. doi: 10.1159/000106377. [DOI] [PubMed] [Google Scholar]
- [71].Ko FW, et al. Exhaled breath condensate levels of eotaxin and macrophage-derived chemokine in stable adult asthma patients Clin. Exp. Allergy. 2006;36:44–51. doi: 10.1111/j.1365-2222.2006.02398.x. [DOI] [PubMed] [Google Scholar]
- [72].Antczak A, et al. Increased hydrogen peroxide and thiobarbituric acid-reactive products in expired breath condensate of asthmatic patients. Eur. Respir. J. 1997;10:1235–41. doi: 10.1183/09031936.97.10061235. [DOI] [PubMed] [Google Scholar]
- [73].Emelyanov A, et al. Elevated concentrations of exhaled hydrogen peroxide in asthmatic patients. Chest. 2001;120:1136–9. doi: 10.1378/chest.120.4.1136. [DOI] [PubMed] [Google Scholar]
- [74].Ganas K, et al. Total nitrite/nitrate in expired breath condensate of patients with asthma. Respir. Med. 2001;95:649–54. doi: 10.1053/rmed.2001.1117. [DOI] [PubMed] [Google Scholar]
- [75].Horvath I, et al. Combined use of exhaled hydrogen peroxide and nitric oxide in monitoring asthma. Am. J. Respir. Crit. Care Med. 1998;158:1042–6. doi: 10.1164/ajrccm.158.4.9710091. [DOI] [PubMed] [Google Scholar]
- [76].Loukides S, et al. The relationships among hydrogen peroxide in expired breath condensate, airway inflammation, and asthma severity. Chest. 2002;121:338–46. doi: 10.1378/chest.121.2.338. [DOI] [PubMed] [Google Scholar]
- [77].Antczak A, et al. Inhaled glucocorticosteroids decrease hydrogen peroxide level in expired air condensate in asthmatic patients. Respir. Med. 2000;94:416–21. doi: 10.1053/rmed.1999.0801. [DOI] [PubMed] [Google Scholar]
- [78].Hulsmann AR, et al. Oxidative epithelial damage produces hyperresponsiveness of human peripheral airways. Am. J. Respir. Crit. Care Med. 1994;149:519–25. doi: 10.1164/ajrccm.149.2.8306055. [DOI] [PubMed] [Google Scholar]
- [79].Nowak D, et al. Increased content of hydrogen peroxide in the expired breath of cigarette smokers. Eur. Respir. J. 1996;9:652–7. doi: 10.1183/09031936.96.09040652. [DOI] [PubMed] [Google Scholar]
- [80].Dekhuijzen PN, et al. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996;154:813–6. doi: 10.1164/ajrccm.154.3.8810624. [DOI] [PubMed] [Google Scholar]
- [81].Nowak D, et al. Cigarette smoking does not increase hydrogen peroxide levels in expired breath condensate of patients with stable COPD. Monaldi Arch. Chest Dis. 1998;53:268–73. [PubMed] [Google Scholar]
- [82].Ferreira IM, et al. Exhaled nitric oxide and hydrogen peroxide in patients with chronic obstructive pulmonary disease: effects of inhaled beclomethasone. Am. J. Respir. Crit. Care Med. 2001;164:1012–5. doi: 10.1164/ajrccm.164.6.2012139. [DOI] [PubMed] [Google Scholar]
- [83].van Beurden WJ, et al. Effects of inhaled corticosteroids with different lung deposition on exhaled hydrogen peroxide in stable COPD patients. Respiration. 2003;70:242–8. doi: 10.1159/000072004. [DOI] [PubMed] [Google Scholar]
- [84].Rysz J, et al. Increased hydrogen peroxide concentration in the exhaled breath condensate of stable COPD patients after nebulized N-acetylcysteine. Pulm. Pharmacol. Ther. 2007;20:281–9. doi: 10.1016/j.pupt.2006.03.011. [DOI] [PubMed] [Google Scholar]
- [85].Oudijk EJ, et al. Expression of priming-associated cellular markers on neutrophils during an exacerbation of COPD. Respir. Med. 2006;100:1791–9. doi: 10.1016/j.rmed.2006.01.022. [DOI] [PubMed] [Google Scholar]
- [86].Corradi M, et al. Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003;167:1380–6. doi: 10.1164/rccm.200210-1253OC. [DOI] [PubMed] [Google Scholar]
- [87].Corradi M, et al. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. Eur. Respir. J. 2004;24:1011–7. doi: 10.1183/09031936.04.00002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Gessner C, et al. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respir. Med. 2005;99:1229–40. doi: 10.1016/j.rmed.2005.02.041. [DOI] [PubMed] [Google Scholar]
- [89].Schumann C, et al. Detection of erythropoietin in exhaled breath condensate of nonhypoxic subjects using a multiplex bead array. Mediators Inflamm. 2006;2006:18061. doi: 10.1155/MI/2006/18061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Matsunaga K, et al. Airway cytokine expression measured by means of protein array in exhaled breath condensate: correlation with physiologic properties in asthmatic patients. J. Allergy Clin. Immunol. 2006;118:84–90. doi: 10.1016/j.jaci.2006.04.020. [DOI] [PubMed] [Google Scholar]
- [91].Robroeks CM, et al. Cytokines in exhaled breath condensate of children with asthma and cystic fibrosis. Ann. Allergy Asthma Immunol. 2006;96:349–55. doi: 10.1016/S1081-1206(10)61247-1. [DOI] [PubMed] [Google Scholar]
- [92].Leung TF, et al. Clinical and atopic parameters and airway inflammatory markers in childhood asthma: a factor analysis. Thorax. 2005;60:822–6. doi: 10.1136/thx.2004.039321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zietkowski Z, et al. Endothelin-1 in exhaled breath condensate of allergic asthma patients with exercise-induced bronchoconstriction. Respir. Res. 2007;8:76. doi: 10.1186/1465-9921-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Driver AG, et al. Adenosine in bronchoalveolar lavage fluid in asthma. Am. Rev. Respir. Dis. 1993;148:91–7. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- [95].Huszar E, et al. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur. Respir. J. 2002;20:1393–8. doi: 10.1183/09031936.02.00005002. [DOI] [PubMed] [Google Scholar]
- [96].Csoma Z, et al. Adenosine level in exhaled breath increases during exercise-induced bronchoconstriction. Eur. Respir. J. 2005;25:873–8. doi: 10.1183/09031936.05.00110204. [DOI] [PubMed] [Google Scholar]
- [97].Vass G, et al. The effect of allergic rhinitis on adenosine concentration in exhaled breath condensate. Clin. Exp. Allergy. 2006;36:742–7. doi: 10.1111/j.1365-2222.2006.02496.x. [DOI] [PubMed] [Google Scholar]
- [98].Mutti A, Corradi M. Recent developments in human biomonitoring: non-invasive assessment of target tissue dose and effects of pneumotoxic metals. Med. Lav. 2006;97:199–206. [PMC free article] [PubMed] [Google Scholar]
- [99].Mutti A, et al. Exhaled metallic elements and serum pneumoproteins in asymptomatic smokers and patients with COPD or asthma. Chest. 2006;129:1288–97. doi: 10.1378/chest.129.5.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Osika E, et al. Distinct sputum cytokine profiles in cystic fibrosis and other chronic inflammatory airway disease. Eur. Respir. J. 1999;14:339–46. doi: 10.1034/j.1399-3003.1999.14b17.x. [DOI] [PubMed] [Google Scholar]
- [101].Tate S, et al. Airways in cystic fibrosis are acidified: detection by exhaled breath condensate. Thorax. 2002;57:926–9. doi: 10.1136/thorax.57.11.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Carpagnano GE, et al. Breath condensate pH in children with cystic fibrosis and asthma: a new noninvasive marker of airway inflammation? Chest. 2004;125:2005–10. doi: 10.1378/chest.125.6.2005. [DOI] [PubMed] [Google Scholar]
- [103].Lundberg JO, et al. Exhaled nitric oxide in paediatric asthma and cystic fibrosis. Arch. Dis. Child. 1996;75:323–6. doi: 10.1136/adc.75.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ojoo JC, et al. Exhaled breath condensate pH and exhaled nitric oxide in allergic asthma and in cystic fibrosis. Thorax. 2005;60:22–6. doi: 10.1136/thx.2003.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ho LP, Innes JA, Greening AP. Nitrite levels in breath condensate of patients with cystic fibrosis is elevated in contrast to exhaled nitric oxide. Thorax. 1998;53:680–4. doi: 10.1136/thx.53.8.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Linnane SJ, et al. Total sputum nitrate plus nitrite is raised during acute pulmonary infection in cystic fibrosis. Am. J. Respir. Crit. Care Med. 1998;158:207–12. doi: 10.1164/ajrccm.158.1.9707096. [DOI] [PubMed] [Google Scholar]
- [107].Horak F, Jr, et al. Longitudinal monitoring of pediatric cystic fibrosis lung disease using nitrite in exhaled breath condensate. Pediatr. Pulmonol. 2007;42:1198–206. doi: 10.1002/ppul.20719. [DOI] [PubMed] [Google Scholar]
- [108].Balint B, et al. Increased nitrotyrosine in exhaled breath condensate in cystic fibrosis. Eur. Respir. J. 2001;17:1201–7. doi: 10.1183/09031936.01.00072501. [DOI] [PubMed] [Google Scholar]
- [109].Emerson J, et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- [110].Berger M. Inflammation in the lung in cystic fibrosis: a vicious cycle that does more harm than good? Clin. Rev. Allergy. 1991;9:119–42. doi: 10.1007/978-1-4612-0475-6_8. [DOI] [PubMed] [Google Scholar]
- [111].Montuschi P, et al. Exhaled 8-isoprostane as a new non-invasive biomarker of oxidative stress in cystic fibrosis. Thorax. 2000;55:205–9. doi: 10.1136/thorax.55.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Jobsis Q, et al. Hydrogen peroxide and nitric oxide in exhaled air of children with cystic fibrosis during antibiotic treatment. Eur. Respir. J. 2000;16:95–100. doi: 10.1034/j.1399-3003.2000.16a17.x. [DOI] [PubMed] [Google Scholar]
- [113].Robroeks CM, et al. Biomarkers in exhaled breath condensate indicate presence and severity of cystic fibrosis in children. Pediatr. Allergy Immunol. 2008 doi: 10.1111/j.1399-3038.2007.00693.x. online, doi: 10.1111/j.1399-3038.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- [114].Bodini A, et al. Biomarkers of neutrophilic inflammation in exhaled air of cystic fibrosis children with bacterial airway infections. Pediatr. Pulmonol. 2005;40:494–9. doi: 10.1002/ppul.20336. [DOI] [PubMed] [Google Scholar]
- [115].Burnstock G. Purinergic signalling. Br. J. Pharmacol. 2006;147(Suppl. 1):S172–81. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 1993;265:C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- [117].Chen Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–5. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- [118].Lennon PF, et al. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J. Exp. Med. 1998;188:1433–43. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Esther CR, Jr, et al. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur. Respir. J. 2008;31:949–56. doi: 10.1183/09031936.00089807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Esther CR, Jr, et al. A mass spectrometric method to simultaneously measure a biomarker and dilution marker in exhaled breath condensate. Rapid Commun. Mass Spectrom. 2008;22:701–5. doi: 10.1002/rcm.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Baker EH, et al. Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J. Appl. Physiol. 2007;102:1969–75. doi: 10.1152/japplphysiol.01425.2006. [DOI] [PubMed] [Google Scholar]
- [122].Goldoni M, et al. Chromium in exhaled breath condensate and pulmonary tissue of non-small cell lung cancer patients. Int. Arch. Occup. Environ. Health. 2008;81:487–93. doi: 10.1007/s00420-007-0242-8. [DOI] [PubMed] [Google Scholar]
- [123].Kollmeier H, et al. Age, sex, and region adjusted concentrations of chromium and nickel in lung tissue. Br. J. Ind. Med. 1990;47:682–7. doi: 10.1136/oem.47.10.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Takemoto K, et al. Metal concentrations in human lung tissue, with special reference to age, sex, cause of death, emphysema and contamination of lung tissue. Int. Arch. Occup. Environ. Health. 1991;62:579–86. doi: 10.1007/BF00381111. [DOI] [PubMed] [Google Scholar]
- [125].Carpagnano GE, et al. 3p microsatellite alterations in exhaled breath condensate from patients with non-small cell lung cancer. Am. J. Respir. Crit. Care Med. 2005;172:738–44. doi: 10.1164/rccm.200503-439OC. [DOI] [PubMed] [Google Scholar]
- [126].Carpagnano GE, et al. 3p microsatellite signature in exhaled breath condensate and tumor tissue of patients with lung cancer. Am. J. Respir. Crit. Care Med. 2008;177:337–41. doi: 10.1164/rccm.200707-1136OC. [DOI] [PubMed] [Google Scholar]
- [127].Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- [128].Olopade CO, et al. Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest. 1997;111:1500–4. doi: 10.1378/chest.111.6.1500. [DOI] [PubMed] [Google Scholar]
- [129].Carpagnano GE, et al. Exhaled pH, exhaled nitric oxide, and induced sputum cellularity in obese patients with obstructive sleep apnea syndrome. Transl. Res. 2008;151:45–50. doi: 10.1016/j.trsl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [130].Petrosyan M, et al. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath. 2007;12:207–15. doi: 10.1007/s11325-007-0160-8. [DOI] [PubMed] [Google Scholar]
- [131].Li Y, et al. Are biomarker levels a good follow-up tool for evaluating obstructive sleep apnea syndrome treatments? Respiration. 2008;76:317–23. doi: 10.1159/000119542. [DOI] [PubMed] [Google Scholar]
- [132].Hunt J. Exhaled breath condensate: an overview. Immunol. Allergy Clin. North Am. 2007;27:587–96. doi: 10.1016/j.iac.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Carraro S, et al. Metabolomics applied to exhaled breath condensate in childhood asthma. Am. J. Respir. Crit. Care Med. 2007;175:986–90. doi: 10.1164/rccm.200606-769OC. [DOI] [PubMed] [Google Scholar]
- [134].Corhay JL, et al. Granulocyte chemotactic activity in exhaled breath condensate of healthy subjects and patients with COPD. Chest. 2007;131:1672–7. doi: 10.1378/chest.06-2225. [DOI] [PubMed] [Google Scholar]
- [135].Labconco I A Guide to Freeze Drying for the Laboratory. 2008 Available at www.labconco.com.
- [136].Vestbo J, et al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE) Eur. Respir. J. 2008;31:869–73. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- [137].Smith AD, et al. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N. Engl. J. Med. 2005;352:2163–73. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- [138].Green RH, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- [139].Kharitonov SA, Barnes PJ. Exhaled biomarkers. Chest. 2006;130:1541–6. doi: 10.1378/chest.130.5.1541. [DOI] [PubMed] [Google Scholar]
- [140].Carraro S, et al. Exhaled breath condensate cysteinyl leukotrienes are increased in children with exercise-induced bronchoconstriction. J. Allergy Clin. Immunol. 2005;115:764–70. doi: 10.1016/j.jaci.2004.10.043. [DOI] [PubMed] [Google Scholar]
- [141].Montuschi P, et al. Ion trap liquid chromatography/tandem mass spectrometry analysis of leukotriene B4 in exhaled breath condensate. Rapid Commun. Mass Spectrom. 2004;18:2723–9. doi: 10.1002/rcm.1682. [DOI] [PubMed] [Google Scholar]
- [142].Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am. J. Respir. Crit. Care Med. 2001;163:1693–722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- [143].Czebe K, et al. Influence of condensing equipment and temperature on exhaled breath condensate pH, total protein and leukotriene concentrations. Respir. Med. 2008;102:720–5. doi: 10.1016/j.rmed.2007.12.013. [DOI] [PubMed] [Google Scholar]
- [144].Do R, et al. Within- and between-person variability of exhaled breath condensate pH and NH4+ in never and current smokers. Respir. Med. 2008;102:457–63. doi: 10.1016/j.rmed.2007.10.002. [DOI] [PubMed] [Google Scholar]
- [145].Hoffmann HJ, et al. Human skin keratins are the major proteins in exhaled breath condensate. Eur. Respir. J. 2008;31:380–4. doi: 10.1183/09031936.00059707. [DOI] [PubMed] [Google Scholar]
- [146].Hu Y, Zhang Z, Yang C. A sensitive chemiluminescence method for the determination of H2O2 in exhaled breath condensate. Anal. Sci. 2008;24:201–5. doi: 10.2116/analsci.24.201. [DOI] [PubMed] [Google Scholar]
- [147].Balbi B, et al. Bronchoalveolar lavage, sputum and exhaled clinically relevant inflammatory markers: values in healthy adults. Eur. Respir. J. 2007;30:769–81. doi: 10.1183/09031936.00112306. [DOI] [PubMed] [Google Scholar]
- [148].Bloemen K, et al. Determinants of variability of protein content, volume and pH of exhaled breath condensate. Respir. Med. 2007;101:1331–7. doi: 10.1016/j.rmed.2006.10.008. [DOI] [PubMed] [Google Scholar]
- [149].Conventz A, et al. Simultaneous determination of 3-nitrotyrosine, tyrosine, hydroxyproline and proline in exhaled breath condensate by hydrophilic interaction liquid chromatography/electrospray ionization tandem mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2007;860:78–85. doi: 10.1016/j.jchromb.2007.10.031. [DOI] [PubMed] [Google Scholar]
- [150].Gaber F, et al. Saliva is one likely source of leukotriene B4 in exhaled breath condensate. Eur. Respir. J. 2006;28:1229–35. doi: 10.1183/09031936.00151905. [DOI] [PubMed] [Google Scholar]
- [151].Akpinar-Elci M, et al. Respiratory inflammatory responses among occupants of a water-damaged office building. Indoor Air. 2008;18:125–30. doi: 10.1111/j.1600-0668.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- [152].Bayley DL, et al. Validation of assays for inflammatory mediators in exhaled breath condensate. Eur. Respir. J. 2008;31:943–8. doi: 10.1183/09031936.00081707. [DOI] [PubMed] [Google Scholar]
- [153].Romieu I, et al. Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma. J. Allergy Clin. Immunol. 2008;121:903–9. doi: 10.1016/j.jaci.2007.12.004. [DOI] [PubMed] [Google Scholar]
- [154].Fireman E, et al. Hydrogen peroxide in exhaled breath condensate (EBC) vs eosinophil count in induced sputum (IS) in parenchymal vs airways lung diseases. Inflammation. 2007;30:44–51. doi: 10.1007/s10753-007-9020-8. [DOI] [PubMed] [Google Scholar]
- [155].Noble DD, et al. Respiratory heat and moisture loss is associated with eosinophilic inflammation in asthma. Eur. Respir. J. 2007;29:676–81. doi: 10.1183/09031936.00071106. [DOI] [PubMed] [Google Scholar]
- [156].Prieto L, et al. Differences in exhaled breath condensate pH measurements between samples obtained with two commercial devices. Respir. Med. 2007;101:1715–20. doi: 10.1016/j.rmed.2007.02.023. [DOI] [PubMed] [Google Scholar]
- [157].Robroeks CM, et al. Exhaled nitric oxide and biomarkers in exhaled breath condensate indicate the presence, severity and control of childhood asthma. Clin. Exp. Allergy. 2007;37:1303–11. doi: 10.1111/j.1365-2222.2007.02788.x. [DOI] [PubMed] [Google Scholar]
- [158].Vogelberg C, et al. Exhaled breath condensate pH in infants and children with acute and recurrent wheezy bronchitis. Pediatr. Pulmonol. 2007;42:1166–72. doi: 10.1002/ppul.20712. [DOI] [PubMed] [Google Scholar]
- [159].Li Y, et al. Exhaled breath condensate cytokine level as a diagnostic tool for obstructive sleep apnea syndrome. Sleep Med. 2008 doi: 10.1016/j.sleep.2007.11.013. online, doi:10.1016/j.sleep.2007.11.013. [DOI] [PubMed] [Google Scholar]
- [160].Gessner C, et al. Presence of cytokeratins in exhaled breath condensate of mechanical ventilated patients. Respir. Med. 2008;102:299–306. doi: 10.1016/j.rmed.2007.08.012. [DOI] [PubMed] [Google Scholar]
- [161].Gogate S, Katial R. Pediatric biomarkers in asthma: exhaled nitric oxide, sputum eosinophils and leukotriene E4. Curr. Opin. Allergy Clin. Immunol. 2008;8:154–7. doi: 10.1097/ACI.0b013e3282f60f61. [DOI] [PubMed] [Google Scholar]
- [162].Lehtonen H, et al. Increased alveolar nitric oxide concentration and high levels of leukotriene B(4) and 8-isoprostane in exhaled breath condensate in patients with asbestosis. Thorax. 2007;62:602–7. doi: 10.1136/thx.2006.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Piotrowski WJ, et al. Eicosanoids in exhaled breath condensate and BAL fluid of patients with sarcoidosis. Chest. 2007;132:589–96. doi: 10.1378/chest.07-0215. [DOI] [PubMed] [Google Scholar]
- [164].Ko FW, et al. Exhaled breath condensate levels of 8-isoprostane, growth related oncogene alpha and monocyte chemoattractant protein-1 in patients with chronic obstructive pulmonary disease. Respir. Med. 2006;100:630–8. doi: 10.1016/j.rmed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- [165].Borrill Z, et al. Reproducibility of exhaled breath condensate pH in chronic obstructive pulmonary disease. Eur. Respir. J. 2005;25:269–74. doi: 10.1183/09031936.05.00085804. [DOI] [PubMed] [Google Scholar]
- [166].Leung TF, et al. Clinical and technical factors affecting pH and other biomarkers in exhaled breath condensate. Pediatr. Pulmonol. 2006;41:87–94. doi: 10.1002/ppul.20296. [DOI] [PubMed] [Google Scholar]


