Abstract
The prevalence of tobacco use in women has increased over the past century. This has resulted in dramatic increases in smoking-related lung diseases, such as chronic obstructive pulmonary disease (COPD) and lung cancer. There is growing literature suggesting that women may be more susceptible than men to the effects of tobacco and to the development of COPD. Women may also have specific barriers that interfere with smoking cessation. This article addresses possible differences in lung function decline and nicotine metabolism in women compared to men. Differences in COPD between the sexes are discussed. Finally, barriers to smoking cessation in women are presented.
Introduction
Tobacco consumption has contributed considerably to morbidity and mortality. Once thought of as exclusive to men, tobacco use among women has increased dramatically over the last century. The prevalence of tobacco use in women was 6% in 1924, peaked at 33% in 1965,1 and has gradually decreased to about 18% in the United States.2 Worldwide, about 250 million women smoke, and the majority of female smokers are in developed countries.3 This has led to increases in the number of conditions that were once thought of as male-dominated diseases, such as chronic obstructive pulmonary disease (COPD) and lung cancer. In fact, COPD now kills more women than breast cancer,4 and the number of new cases of COPD is increasing three times as fast in women annually as compared to men.5 As late as 1980, the U.S. Surgeon General's Report first highlighted the growing epidemic of tobacco use in women. Smoking has caused an annual average of 178,311 deaths in women in the United States, an increase in women that is in contrast to the decrease seen in men.6
The rise in tobacco use among women has been led by relentless tobacco industry marketing designed to specifically promote smoking in women. In the 1920s, cigarettes became a hallmark of women's liberation, and by the 1930s, cigarettes were considered a symbol of the independent, glamorous American woman.7 Marketing tobacco products toward women has continued and is exemplified by Phillip Morris' advertising of Virginia Slims in the 1960s, the first and most successful brand targeted exclusively to women in the United States.8 The marketing to women of certain cigarettes as light or low tar has sent the false message that these products are safer to smoke.9
Given the epidemic of tobacco use in women, it is important to understand what motivates women to smoke, what effects smoking has on women's health, and what barriers women face when confronting tobacco cessation. A PubMed search using the keywords women, tobacco, lung function, COPD, and smoking cessation was done, and clinical trials, reviews, randomized controlled trials (RCTs), and meta-analyses from 1990 to the present were used to form the basis of this review article. This review not only discusses the role of COPD in women but also focuses on specific issues that are unique to women and tobacco use. The objective of this review is to examine the effects of tobacco on the lung function of women. As nicotine dependence is the underlying mechanism responsible for persistent tobacco use and subsequent smoking-related illness, sex differences in nicotine metabolism are highlighted as well. Because the prevalence of COPD in women is increasing, the unique aspects of this disease in the female population are discussed. Finally, challenges in smoking cessation specific to women are highlighted.
Tobacco and the Effect on Lung Function in Women
The increase in the prevalence of tobacco use in women has led to great interest in sex differences in lung function decline in smokers. Men and women exhibit differences in lung function growth and development very early in life. Throughout the human life span, females tend to have smaller lungs than males and higher forced expiratory flow rates. The concept of dysanaptic growth, which is the disproportionate growth of lung size compared to airway size, may explain this finding, as the larger lungs of males have longer airways and higher airway resistance.10 Also, the growth of lungs in females ceases around the mid-teenage to late teenage years but continues at a slower pace for males until the mid-20s, leading to larger airway diameter compared to females. Finally, women's airways are constantly influenced by variations in hormone levels attributed to menstruation, oral contraceptive (OC) therapy, pregnancy, and menopause.10 These differences in lung growth and development may account for differences in the effects of tobacco use.
The effect of smoking on lung function in adolescent girls and boys aged 10–18 was examined in a prospective cohort study by Gold et al.11 Children who smoked had higher rates of asthma and wheezing compared to never smokers. Wheezing increased according to the amount smoked and was higher in females than males at each level of smoking (p < 0.001). Compared to boys at or beyond their peak height growth, girls who smoked 5 or more cigarettes daily had larger declines in lung function. A cross-sectional study of adolescent girls and boys in Norway showed that girls reported more respiratory symptoms than boys with comparable smoking histories, and a dose-response relation between smoking and reduced lung function was found only in girls.12 Greater decline in pulmonary function in girls has been shown in those exposed to secondhand smoke.13 This difference seems to persist into adulthood, as women have shown a significantly greater decline in forced expiratory volume in 1 second (FEV1) compared to men exposed to passive smoking.14
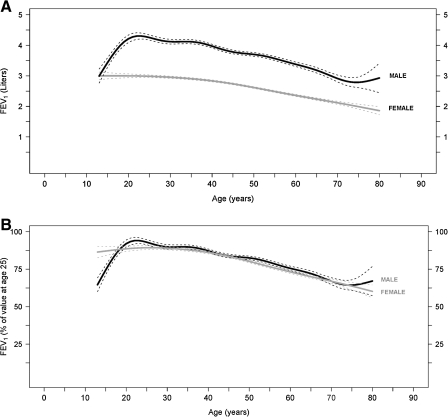
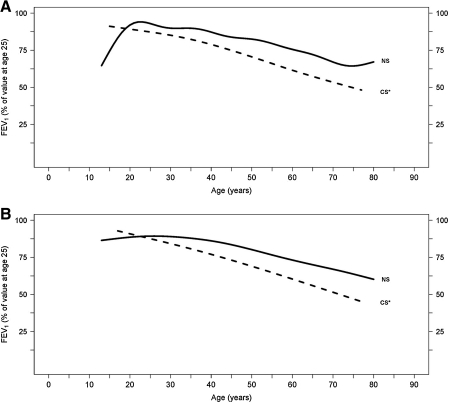
Whereas studies in adolescents show greater effects of cigarette smoke on lung function in girls compared to boys, similar studies in adults have yielded conflicting results. For example, female smokers have an increased prevalence of airway hyperresponsiveness as determined by a higher proportion of positive methacholine challenges. It has been postulated that this difference is a result of smaller female airway caliber compared to men.15 This study is consistent with others demonstrating that female smokers exhibit larger declines in lung function than male smokers16–23; however others have shown opposite results.24–30 For example, a longitudinal study following approximately 4000 males and females demonstrated that males had a greater decline in lung function for a comparable amount of tobacco use.31 Another recent prospective cohort study examined participants of the Framingham offspring cohort who had two or more spirometry measurements. The median follow-up time was 23 years, and male smokers had a mean FEV1 decline of 38.2 mL/year, whereas female smokers had a mean FEV1 decline of 23.9 mL/year (p < 0.001).32 However, this study did not adjust for smoking intensity. Figure 1, from this study, displays FEV1 changes with age in healthy never smoking males and females, and Figure 2 compares FEV1 decline in healthy never smokers and continuous smokers, both male and female.
FIG. 1.
Mean forced expiratory volume in 1 second (FEV1) values by age (and 95% confidence intervals [CI]) in male (black line) and female (gray line) healthy never smokers, expressed (A) in absolute values and (B) as percentage of the FEV1 value at the age of 25. The mean FEV1 decline value (and 95% CI) for males was 19.6 ml (17.1-22.1) and for females was 17.6 ml (13.8-21.4), with a p value of 0.266. (From Kohansal et al.32 Reprinted with permission of the American Thoracic Society.)
FIG. 2.
Mean FEV1 values (expressed as percent of its value at the age of 25) by age, for healthy never smokers (NS) and continuous smokers (CS). Data for (A) males and (B) females. The mean FEV1 decline value (and 95% CIs) for males was 38.2 mL (33.9-42.6) and for females 23.9 mL (20.9-27.0), with a p value <0.001. *p < 0.05 vs. healthy NS. (From Kohansal et al.32 Reprinted with permission of the American Thoracic Society.)
In contrast, participants originally enrolled in the Lung Health Study were reassessed at 11 years, and results showed that for continuing smokers, there was no difference in percent predicted FEV1 decline between the sexes,33 although the percent predicted FEV1 declined less for female sustained quitters than male sustained quitters. A subgroup analysis of the Understanding the Potential Long-term Impact of Tiotropium (UPLIFT) trial34 compared sex differences in the impact of tiotropium on lung function over a 4-year period. Although women in the tiotropium group had a lower incidence of COPD exacerbations compared to women in the control group, both groups had slightly higher exacerbation rates compared to men. According to the authors, smaller airway caliber, lower threshold for women to experience dyspnea, and higher likelihood to seek medical care could explain these differences. There was no difference in lung function decline between men and women in both the placebo and tiotropium groups, although the rates of hospitalization were slightly lower for women in the tiotropium group compared to women in the placebo group but similar for men (p = 0.06).
In summary, there is no clear-cut evidence that women smokers have a greater decline in lung function than men smokers. Underreporting of tobacco use may influence the outcomes of tobacco use and lung function between the sexes as well; however, a meta-analysis of 26 studies found that self-reporting was generally accurate.35 Environmental and occupational exposures may have a greater impact on the respiratory symptoms of women.36 For example, indoor use of solid fuels, such as coal and biomass, is an important cause of COPD in nonsmokers, particularly in women.37 More women than men also report increased respiratory symptoms associated with traffic pollutants.38 Occupations that have high exposures to biological dusts, such as healthcare professions, food and textile workers, artists, and cleaners, appear to have a higher association with COPD in women compared to men.39,40 Puffing behavior may also be different between men and women, as one study revealed that women take smaller puffs of shorter duration but draw more puffs per cigarette.41 This suggests that the increased risk of COPD among women smokers may involve sex interactions with other, nonsmoking environmental factors or genetic factors.
COPD and Women
Given that tobacco use among women has risen over the last several decades, it is not surprising that the prevalence of COPD in women has dramatically increased. COPD is expected to be the fourth most common cause of death by the year 2030.1,4 Between 1980 and 2000, the annual mortality rate for COPD in women increased by 291% compared to 60% in men, and the annual hospitalization rate due to COPD increased by 43% in women compared to 12% in men.5 In 2000 for the first time, the number of women who died from COPD surpassed the number of men.5 Sex differences in COPD are increasingly recognized with regard to susceptibility of disease, disease phenotype, radiographic appearance, and response to pharmacotherapy.
Earlier studies initially suggested that there was equal predisposition to COPD between the sexes. In the British Physicians study, 6,194 women and 34,440 men were followed between 1951 and 1973; male and female smokers showed similar mortality rates from COPD, and the risk of developing COPD was attributed to the amount of tobacco consumed.42,43 More recent data, however, suggest that women who smoke may be more susceptible to the development of COPD. For example, a recent study by Dransfield et al.44 examined patients with COPD who had a >20 pack-year smoking history and showed that men had less loss of lung function per pack-year smoked than women. This study was limited in that it was not population based, thus introducing a possible selection bias. A systematic review and meta-analysis comparing the annual decline of lung function between men and women smokers with COPD followed for at least 3 years found that in current smokers, women had a significantly faster rate of FEV1 decline compared to men.45
Silverman et al.46 investigated sex differences and severe early-onset COPD in 84 early-onset COPD subjects and 348 first-degree relatives. They found a markedly elevated prevalence of females (71.4%) among the group with severe, early-onset COPD. Among first-degree relatives with a current or former smoking history, females had a significantly lower FEV1/forced vital capacity (FVC) ratio and significantly greater bronchodilator response and were more likely to have an FEV1 % predicted <40%. In an observational study that included 10,711 Spanish subjects with stable COPD, of whom 75% were male, women were younger and had less smoking history, more comorbid conditions, lower quality of life scores, and less degree of airflow obstruction than men.47
The phenotype of COPD may differ between males and females. Women appear to have an airway disease-predominant phenotype, whereas men may have an emphysematous phenotype. Based on a retrospective review of 1,438 patients with a diagnosis of COPD, spirometric evidence of airflow obstruction, and computed tomography (CT) scan data, a significantly greater proportion of women had airway disease.48 Women with COPD report greater complaints of dyspnea or breathlessness49–51 as opposed to cough or phlegm production.51,52 de Torres et al.53 examined gender differences in a cohort of stable, mainly stage II and III COPD patients in an outpatient pulmonary clinic that included 53 FEV1-matched men and women. Women were 8 years younger on average, smoked less, and had less comorbidity and more exacerbations than men. Complaint of dyspnea and significantly worse scores for quality of life domains of the St. George Respiratory Questionnaire (SGRQ) were noted for women compared to men.
Martinez et al.54 investigated sex differences in people with severe pulmonary emphysema by studying 1,053 patients, 39% of whom were female, who had been evaluated for lung volume reduction surgery (LVRS) as part of the National Emphysema Treatment Trial (NETT). Women had slightly less airflow obstruction, as measured by FEV1 % predicted, but slightly lower diffusion capacity. Based on high-resolution CT (HRCT) of the chest, women showed a similar proportion of emphysema in the core (more centrally located areas) of the lung but a lower proportion in the peel (peripheral regions) compared to men. Histologically, women also had thicker small airway walls relative to lumen size, supporting the airway-predominant phenotype of COPD in women. Additional data from the National Lung Screening Trial has demonstrated that for a given severity of airflow obstruction, women have less prominent emphysema identified on chest CT. Taken together, these HRCT studies suggest that women have a distinct phenotypic expression of COPD compared to men.
Psychological disorders, such as depression and anxiety, complicate COPD and have been shown to increase rates of exacerbations and hospitalizations.55 These disorders have also been associated with increased mortality in patients with COPD.56,57 This association is crucial in understanding the outcome of COPD in women, as rates of depression and anxiety appear to be increased in this population.49,54,58,59 Laurin et al.60 performed a cross-sectional study evaluating sex differences in the prevalence of mood and anxiety disorders in patients with stable COPD. The prevalence of psychiatric disorders in both men and women with COPD was 49%, which is higher than in the general population, based on published reports.61 Among the COPD patients with a psychiatric comorbidity, women had significantly higher rates of psychiatric disorders compared to men (60% vs. 38%) independent of age, smoking, and COPD duration and severity. Women also exhibited more self-depreciation and anxiety/depression, lower levels of symptom control confidence, and worse health-related quality of life.
Sex differences in COPD also exist with respect to diagnosis, treatment, and response to pulmonary rehabilitation. There appears to be an inherent bias in diagnosing COPD in women. Chapman et al.62 surveyed 192 primary care physicians using a hypothetical case of cough and dyspnea in a smoker to determine if there was a provider bias in the diagnosis of COPD. COPD is more likely to be diagnosed in the hypothetical male patient than the hypothetical female patient (64.6% vs. 49%, p < 0.05); Once spirometry is performed, however, this bias disappears.63 Although spirometry is underused in diagnosing COPD in general,64 this difference is most notable in the female population, as it has been shown that after adjusting for age and pack-year smoking history, women still were less likely than men to have spirometric assessment despite reporting more severe dyspnea.51 There are very few studies examining treatment response between the sexes. One study showed that combination therapy with salmeterol/fluticasone caused improvement in FEV1 similarly in men and women.65 However, women may be less likely to receive prescribed newer dry powder inhalers66 and less likely to use proper technique when using metered-dose inhalers.67
There are mixed data about outcomes for women placed on long-term oxygen therapy (LTOT). A Brazilian prospective cohort study noted significant differences by sex among COPD patients on LTOT for a minimum of 6 months, with women at a significantly higher risk for death than men.68 The difference in survival became apparent approximately 3 years after follow-up. One major limitation of the study was that data were not collected on adherence to oxygen therapy. In contrast, other studies have shown a survival benefit in women on LTOT compared to men.69,70 Women appear to respond to pulmonary rehabilitation differently as well. The literature in this area is scant. One RCT by Foy et al.71 compared sex differences in health-related quality of life between participants exposed to short-term exercise therapy (3 months) or long-term exercise therapy (18 months). The short-term therapy group was instructed to engage in independent, home-based exercise therapy after completion of the 3-month structured program. There was significant improvement in all domains of the Chronic Disease Respiratory Questionnaire in both men and women at 3 months, with women reporting more gains in dyspnea and fatigue. However, men enrolled in the long-term exercise therapy group continued to show improvements at 18 months compared to the short-term exercise group, whereas women did not. These results may be related to the fact that women achieved a much higher benefit at the 3-month follow-up, whereas it took men 18 months to achieve comparable dyspnea scores. Also, there may have been differences between the sexes in adherence to exercise therapy in the short-term group.
Nicotine Metabolism in Women
The possibility that women may be more vulnerable to the effects of tobacco has generated an interest in understanding differences in nicotine metabolism between the sexes. Cigarette smoke contains a variety of chemicals, most notably nicotine, tar, benzo[a]pyrene, benzene, nitrosamines, N-nitroso derivatives, and polycyclic aromatic hydrocarbons (PAH).1 These chemicals are absorbed rapidly and metabolized by oxidation and subsequent conjugation. The first step of the oxidative phase is mediated by cytochrome p450 (CYP),72 which tends to have a greater expression in females.73 CYP2A6, which is the main CYP enzyme responsible for the first step in the oxidative phase of nicotine metabolism, is also expressed more in females.74,75 The expression of CYP1A1, which is involved in the metabolism of PAH, is also upregulated in females.76,77
Higher CYP expression is correlated with faster metabolism of the chemicals contained in cigarette smoke, which allows for metabolic bioactivation, the conversion of relatively benign compounds into toxic chemicals.78 This is true for PAH, benzo[a]pyrene, and naphthalene.78–80 Thus, females have a higher rate of nicotine metabolism, leading to higher levels of these bioactivated compounds. Given that nicotine is the psychoactive component that smokers seek and women metabolize it more rapidly, they must dose themselves accordingly, which puts them at increased risk of exposure to toxins within cigarettes.
Estrogen appears to be involved in nicotine metabolism differences between the sexes. There are two known estrogen receptors in the lungs, estrogen receptor α and estrogen receptor β, with the latter being predominant.78 Estrogen receptor α can upregulate CYP1A1 and CYP1B1 expression, both involved in PAH bioactivation,81 and CYP2A6 has been shown to be directly induced by estradiol via estrogen receptors. When analyzing CYP2A6 protein concentration in human endometrial tissue, the levels were significantly higher in the proliferative phase of the menstrual cycle, which is the estrogen-rich phase of the cycle.82 Thus, upregulation of CYP enzymes appears to be influenced by estrogen concentration.
Clinical studies examining the role of estrogen and nicotine metabolism are not only conflicting but also scarce. One recent study by Benowitz et al.83 observed 270 healthy twins and 16 subjects who were siblings of twins and exposed them to deuterium-labeled nicotine and cotinine. Women had faster plasma clearance of nicotine and cotinine and higher levels of their metabolites compared to men. As the clearance of nicotine to cotinine and generation of cotinine metabolites are thought to be a measure of CYP2A6 activity,84 the authors concluded that women had higher CYP2A6 activity. Interestingly, women on estrogen-only OC pills had a significantly higher metabolite concentration compared to women not on hormonal supplements. The authors concluded that there appears to be a spectrum of nicotine metabolism, with women on estrogen-only or combination pills having the fastest rate of nicotine metabolism, followed by women on progesterone-only pills, then by men and postmenopausal women. Interestingly, pregnant women appear to have the highest rate of nicotine metabolism.85
Smoking Cessation in Women
Women may actually benefit more from smoking cessation than do men, which should make smoking cessation a priority in women's health. As part of the Lung Health Study, a prospective RCT enrolled 3,818 smokers between the ages of 35 and 60 with mild to moderate airflow obstruction into a smoking cessation program vs. usual care.86 In the smoking intervention group, 22% remained abstinent from year 1 to year 5, compared to 5% in the usual care group. In sustained quitters, the rate of decline in FEV1 was 31 ± 48 mL/year, whereas continued smokers had a rate of FEV1 decline of 62 ± 55 mL/year (p < 0.001). Female sustained quitters had an average improvement in FEV1% predicted 2.5 times greater than men. Interestingly, women who continued to smoke had a proportionately greater annual decline in lung function compared to men with a comparable smoking history. Using the 2001 National Health Interview Survey (NHIS), Browning et al.87 showed that smoking cessation assistance by healthcare providers did not differ between men and women. Despite the large beneficial effect of tobacco cessation, numerous studies show that women have more difficulty quitting than men; however, these studies are not population-based studies, increasing the chance of selection bias.88–91
Specific barriers exist for women when trying to abstain from cigarette use. Women appear to be more motivated to smoke when shown images of thin models, and the fear of weight gain is rampant among female smokers trying to quit.89,92 In one study by Perkins et al.,93 women who received cognitive behavioral therapy targeted at reducing weight concerns as part of their smoking cessation treatment were significantly more likely to remain abstinent at 12-month follow-up. Women consistently show less confidence in their ability to quit smoking, have lower levels of quitting motivation, and feel more stress during the cessation period.89,94,95 Women involved in smoking cessation therapy also report more tobacco withdrawal symptoms, such as anxiety, depression, and irritability, compared to men.95
There is some literature highlighting significant differences in success rates of nicotine replacement therapy (NRT) between men and women.96–99 Based on a number of studies, it has been theorized that NRT is not as useful in women, implying that nicotine is not the only reinforcing agent in female smokers. Women seem to be more sensitive to the nonnicotine components of tobacco, such as sight, smell, and the sensation of smoking.100 Men appear to have higher pharmacological dependence on smoking, whereas women have higher behavioral dependence.90 In a study by Perkins et al.,101 30 healthy smokers who consumed at least 10 cigarettes per day with cigarette brands that had a nicotine yield of at least 0.7 mg were studied to determine if the reinforcing effect was dose related. Each participant was given a low-nicotine cigarette brand (0.1 mg nicotine and 1 mg tar) as well as a high-nicotine cigarette brand (0.9 mg nicotine and 12.2 mg tar). Plasma levels of nicotine correlated with the dose of nicotine in each brand and were not different between the sexes. However, women had smaller differences in subjective and reinforcing effects based on dose. Thus, nicotine may not be as strong a component in the addictive nature of tobacco use in women as in men. These findings should be considered when devising smoking cessation plans for women.
An aspect of smoking cessation therapy unique to women is the effect of the menstrual cycle on success rates. Premenstrual symptoms have been associated with higher relapse rates in women attempting smoking cessation.102 In one randomized trial, female smokers were assigned to quit smoking either during the follicular phase (estrogen predominant) or the luteal phase (progesterone predominant) of their menstrual cycle.103 Women who quit during the follicular phase had a significantly higher relapse rate than women quitting during the luteal phase. However, the women who quit during the follicular phase and relapsed did so in 14–20 days, which would have been during the luteal phase, a time of increased premenstrual symptoms. This may have contributed to their desire to start smoking again.
A recent randomized trial by Allen et al.104 studied the withdrawal and premenstrual symptoms in women quitting during the follicular phase or the luteal phase, as well as smoking cessation outcomes; 202 female smokers were randomized to quit during the follicular phase of their menstrual cycle vs. the luteal phase. These women received individualized behavioral counseling and self-help materials as an aid for smoking cessation. More premenstrual symptoms were observed in women quitting during the luteal phase, but this was not significantly associated with smoking cessation outcomes. Craving and anger were the two smoking withdrawal symptoms that were associated with an increased risk of relapse, but only for women who quit during the follicular phase. These studies suggest that the menstrual cycle may have an active role in smoking cessation outcomes and that quitting during the follicular phase may be associated with a higher relapse rate. As previous animal studies have shown, estrogen may be associated with more reinforcing effects of addictive drugs, further complicating the success of smoking cessation in females.105
Conclusions
Tobacco use is responsible for many diseases affecting women today, most notably COPD and lung cancer. With the rise in sex-targeted marketing of tobacco products, there has been an alarming increase in the number of tobacco-attributable diseases in women. The literature addressing sex differences in susceptibility to tobacco and its associated diseases as well as sex differences in smoking cessation is at times contradictory. Although this is likely related to study design and selection bias, there is still intriguing evidence that sex differences may exist. Women may be more susceptible to the effects of tobacco and, thus, more likely to succumb to the ramifications of long-term smoking. More importantly, healthcare practitioners may be unaware of the increased susceptibility of women to smoking-related diseases. Even smoking cessation therapies need to be tailored specifically for women, as the reasons why they smoke and the barriers that interfere with successfully quitting are unique to women. Strategies to help alleviate the epidemic of tobacco use in women include a greater emphasis on patient and physician education, devising smoking cessation programs geared specifically toward women, and increasing sex-specific research in tobacco-related diseases.
Disclosure Statement
S.D.R. has no conflicts of interest to report. P.T.D. has received grants from the National Institutes of Health, MPEX Pharmaceuticals, and Boehringer. M.E.W. has received grants from the National Institutes of Health and the Centers for Disease Control and Prevention.
References
- 1.U.S. Surgeon General. The health consequences of smoking: Chronic obstructive lung disease. Washington, DC: U.S. Government Press Office; 1984. [Google Scholar]
- 2.Cigarette smoking among adults—United States, 2006. MMWR. 2007;56:1157–1161. [PubMed] [Google Scholar]
- 3.Mackay J. Eriksen M. The tobacco atlas. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 4.Mathers CD. Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannino DM. Homa DM. Akinbami LJ. Ford ES. Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- 6.Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States, 1995–1999. MMWR. 2002;51:300–303. [PubMed] [Google Scholar]
- 7.Tanoue LT. Cigarette smoking and women's respiratory health. Clin Chest Med. 2000;21:47–65. doi: 10.1016/s0272-5231(05)70007-1. [DOI] [PubMed] [Google Scholar]
- 8.Toll BA. Ling PM. The Virginia Slims identity crisis: An inside look at tobacco industry marketing to women. Tobacco Control. 2005;14:172–180. doi: 10.1136/tc.2004.008953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services. Monograph 13. Washington, DC: U.S. Department of Health and Human Services, National Cancer Institute; 2001. National Cancer Institute. Risks associated with smoking cigarettes with low tar machine-measured yield of tar and nicotine. [Google Scholar]
- 10.Becklake MR. Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54:1119–1138. doi: 10.1136/thx.54.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold DR. Wang X. Wypij D. Speizer FE. Ware JH. Dockery DW. Effects of cigarette smoking on lung function in adolescent boys and girls. N Engl J Med. 1996;335:931–937. doi: 10.1056/NEJM199609263351304. [DOI] [PubMed] [Google Scholar]
- 12.Holmen TL. Barrett-Connor E. Clausen J. Langhammer A. Holmen J. Bjermer L. Gender differences in the impact of adolescent smoking on lung function and respiratory symptoms. The Nord-Trondelag Health Study, Norway, 1995–1997. Respir Med. 2002;96:796–804. doi: 10.1053/rmed.2002.1350. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y. Rennie DC. Lockinger LA. Dosman JA. Gender, environmental tobacco smoke, and pulmonary function in rural children and adolescents: The Humboldt Study. J Agric Saf Health. 2005;11:167–173. doi: 10.13031/2013.18183. [DOI] [PubMed] [Google Scholar]
- 14.Kauffmann F. Tessier JF. Oriol P. Adult passive smoking in the home environment: A risk factor for chronic airflow limitation. Am J Epidemiol. 1983;117:269–280. doi: 10.1093/oxfordjournals.aje.a113539. [DOI] [PubMed] [Google Scholar]
- 15.Kanner RE. Connett JE. Altose MD, et al. Gender difference in airway hyperresponsiveness in smokers with mild COPD. The Lung Health Study. Am J Respir Crit Care Med. 1994;150:956–961. doi: 10.1164/ajrccm.150.4.7921469. [DOI] [PubMed] [Google Scholar]
- 16.Xu X. Li B. Wang L. Gender difference in smoking effects on adult pulmonary function. Eur Respir J. 1994;7:477–483. doi: 10.1183/09031936.94.07030477. [DOI] [PubMed] [Google Scholar]
- 17.Xu X. Weiss ST. Rijcken B. Schouten JP. Smoking, changes in smoking habits, and rate of decline in FEV1: New insight into gender differences. Eur Respir J. 1994;7:1056–1061. [PubMed] [Google Scholar]
- 18.Chen Y. Horne SL. Dosman JA. Increased susceptibility to lung dysfunction in female smokers. Am Rev Respir Dis. 1991;143:1224–1230. doi: 10.1164/ajrccm/143.6.1224. [DOI] [PubMed] [Google Scholar]
- 19.Prescott E. Bjerg AM. Andersen PK. Lange P. Vestbo J. Gender difference in smoking effects on lung function and risk of hospitalization for COPD: Results from a Danish longitudinal population study. Eur Respir J. 1997;10:822–827. [PubMed] [Google Scholar]
- 20.Buist AS. Ghezzo H. Anthonisen NR, et al. Relationship between the single-breath N test and age, sex, and smoking habit in three North American cities. Am Rev Respir Dis. 1979;120:305–318. doi: 10.1164/arrd.1979.120.2.305. [DOI] [PubMed] [Google Scholar]
- 21.Chinn S. Jarvis D. Melotti R, et al. Smoking cessation, lung function, and weight gain: A follow-up study. Lancet. 2005;365:1629–1635. doi: 10.1016/S0140-6736(05)66511-7. [DOI] [PubMed] [Google Scholar]
- 22.Sherrill DL. Enright P. Cline M. Burrows B. Lebowitz MD. Rates of decline in lung function among subjects who restart cigarette smoking. Chest. 1996;109:1001–1005. doi: 10.1378/chest.109.4.1001. [DOI] [PubMed] [Google Scholar]
- 23.Langhammer A. Johnsen R. Gulsvik A. Holmen TL. Bjermer L. Sex differences in lung vulnerability to tobacco smoking. Eur Respir J. 2003;21:1017–1023. doi: 10.1183/09031936.03.00053202. [DOI] [PubMed] [Google Scholar]
- 24.Rijcken B. Schouten JP. Xu X. Rosner B. Weiss ST. Airway hyperresponsiveness to histamine associated with accelerated decline in FEV1. Am J Respir Crit Care Med. 1995;151:1377–1382. doi: 10.1164/ajrccm.151.5.7735588. [DOI] [PubMed] [Google Scholar]
- 25.Vollmer WM. Enright PL. Pedula KL, et al. Race and gender differences in the effects of smoking on lung function. Chest. 2000;117:764–772. doi: 10.1378/chest.117.3.764. [DOI] [PubMed] [Google Scholar]
- 26.Viegi G. Paoletti P. Prediletto R, et al. Prevalence of respiratory symptoms in an unpolluted area of northern Italy. Eur Respir J. 1988;1:311–318. [PubMed] [Google Scholar]
- 27.Xu X. Dockery DW. Ware JH. Speizer FE. Ferris BG., Jr Effects of cigarette smoking on rate of loss of pulmonary function in adults: A longitudinal assessment. Am Rev Respir Dis. 1992;146:1345–1348. doi: 10.1164/ajrccm/146.5_Pt_1.1345. [DOI] [PubMed] [Google Scholar]
- 28.Camilli AE. Burrows B. Knudson RJ. Lyle SK. Lebowitz MD. Longitudinal changes in forced expiratory volume in one second in adults. Effects of smoking and smoking cessation. Am Rev Respir Dis. 1987;135:794–799. doi: 10.1164/arrd.1987.135.4.794. [DOI] [PubMed] [Google Scholar]
- 29.Tashkin DP. Clark VA. Coulson AH, et al. The UCLA population studies of chronic obstructive respiratory disease. VIII. Effects of smoking cessation on lung function: A prospective study of a free-living population. Am Rev Respir Dis. 1984;130:707–715. doi: 10.1164/arrd.1984.130.5.707. [DOI] [PubMed] [Google Scholar]
- 30.Jedrychowski W. Krzyzanowski M. Wysocki M. Changes in lung function determined longitudinally compared with decline assessed cross-sectionally. The Cracow Study. Eur J Epidemiol. 1986;2:134–138. doi: 10.1007/BF00157025. [DOI] [PubMed] [Google Scholar]
- 31.James AL. Palmer LJ. Kicic E, et al. Decline in lung function in the Busselton Health Study: The effects of asthma and cigarette smoking. Am J Respir Crit Care Med. 2005;171:109–114. doi: 10.1164/rccm.200402-230OC. [DOI] [PubMed] [Google Scholar]
- 32.Kohansal R. Martinez-Camblor P. Agusti A. Buist AS. Mannino DM. Soriano JB. The natural history of chronic airflow obstruction revisited: An analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180:3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 33.Anthonisen NR. Connett JE. Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 34.Tashkin D. Celli B. Kesten S. Lystig T. Decramer M. Effect of tiotropium in men and women with COPD: Results of the 4-year UPLIFT((R)) trial. Respir Med. 2010;104:1495–1504. doi: 10.1016/j.rmed.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 35.Patrick DL. Cheadle A. Thompson DC. Diehr P. Koepsell T. Kinne S. The validity of self-reported smoking: A review and meta-analysis. Am J Public Health. 1994;84:1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy SM. Chambers R. Du W. Dimich-Ward H. Environmental and occupational exposures: Do they affect chronic obstructive pulmonary disease differently in women and men? Proc Am Thorac Soc. 2007;4:692–694. doi: 10.1513/pats.200707-094SD. [DOI] [PubMed] [Google Scholar]
- 37.Torres-Duque C. Maldonado D. Perez-Padilla R. Ezzati M. Viegi G. Biomass fuels and respiratory diseases: A review of the evidence. Proc Am Thorac Soc. 2008;5:577–590. doi: 10.1513/pats.200707-100RP. [DOI] [PubMed] [Google Scholar]
- 38.Sunyer J. Jarvis D. Gotschi T, et al. Chronic bronchitis and urban air pollution in an international study. Occup Environ Med. 2006;63:836–843. doi: 10.1136/oem.2006.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matheson MC. Benke G. Raven J, et al. Biological dust exposure in the workplace is a risk factor for chronic obstructive pulmonary disease. Thorax. 2005;60:645–651. doi: 10.1136/thx.2004.035170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hnizdo E. Sullivan PA. Bang KM. Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the U.S. population: A study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;156:738–746. doi: 10.1093/aje/kwf105. [DOI] [PubMed] [Google Scholar]
- 41.Melikian AA. Djordjevic MV. Hosey J, et al. Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tobacco Res. 2007;9:377–387. doi: 10.1080/14622200701188836. [DOI] [PubMed] [Google Scholar]
- 42.Doll R. Peto R. Mortality in relation to smoking: 20 years' observations on male British doctors. BMJ. 1976;2:1525–1536. doi: 10.1136/bmj.2.6051.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doll R. Gray R. Hafner B. Peto R. Mortality in relation to smoking: 22 years' observations on female British doctors. BMJ. 1980;280:967–971. doi: 10.1136/bmj.280.6219.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dransfield MT. Davis JJ. Gerald LB. Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med. 2006;100:1110–1116. doi: 10.1016/j.rmed.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Gan WQ. Man SF. Postma DS. Camp P. Sin DD. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Respir Res. 2006;7:52. doi: 10.1186/1465-9921-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverman EK. Weiss ST. Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:2152–2158. doi: 10.1164/ajrccm.162.6.2003112. [DOI] [PubMed] [Google Scholar]
- 47.Carrasco Garrido P. de Miguel Diez J. Rejas Gutierrez J, et al. Characteristics of chronic obstructive pulmonary disease in Spain from a gender perspective. BMC Pulm Med. 2009;9:2. doi: 10.1186/1471-2466-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tatsumi K. Kasahara Y. Kurosu K. Tanabe N. Takiguchi Y. Kuriyama T. Clinical phenotypes of COPD: Results of a Japanese epidemiological survey. Respirology. 2004;9:331–336. doi: 10.1111/j.1440-1843.2004.00611.x. [DOI] [PubMed] [Google Scholar]
- 49.Katsura H. Yamada K. Wakabayashi R. Kida K. Gender-associated differences in dyspnoea and health-related quality of life in patients with chronic obstructive pulmonary disease. Respirology. 2007;12:427–432. doi: 10.1111/j.1440-1843.2007.01075.x. [DOI] [PubMed] [Google Scholar]
- 50.Dales RE. Mehdizadeh A. Aaron SD. Vandemheen KL. Clinch J. Sex differences in the clinical presentation and management of airflow obstruction. Eur Respir J. 2006;28:319–322. doi: 10.1183/09031936.06.00138105. [DOI] [PubMed] [Google Scholar]
- 51.Watson L. Vestbo J. Postma DS, et al. Gender differences in the management and experience of chronic obstructive pulmonary disease. Respir Med. 2004;98:1207–1213. doi: 10.1016/j.rmed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Cydulka RK. Rowe BH. Clark S. Emerman CL. Rimm AR. Camargo CA., Jr Gender differences in emergency department patients with chronic obstructive pulmonary disease exacerbation. Acad Emerg Med. 2005;12:1173–1179. doi: 10.1197/j.aem.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 53.de Torres JP. Casanova C. Hernandez C. Abreu J. Aguirre-Jaime A. Celli BR. Gender and COPD in patients attending a pulmonary clinic. Chest. 2005;128:2012–2016. doi: 10.1378/chest.128.4.2012. [DOI] [PubMed] [Google Scholar]
- 54.Martinez FJ. Curtis JL. Sciurba F, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176:243–252. doi: 10.1164/rccm.200606-828OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu W. Collet JP. Shapiro S, et al. Independent effect of depression and anxiety on chronic obstructive pulmonary disease exacerbations and hospitalizations. Am J Respir Crit Care Med. 2008;178:913–920. doi: 10.1164/rccm.200804-619OC. [DOI] [PubMed] [Google Scholar]
- 56.Yohannes AM. Baldwin RC. Connolly MJ. Predictors of 1-year mortality in patients discharged from hospital following acute exacerbation of chronic obstructive pulmonary disease. Age Ageing. 2005;34:491–496. doi: 10.1093/ageing/afi163. [DOI] [PubMed] [Google Scholar]
- 57.de Voogd JN. Wempe JB. Koeter GH, et al. Depressive symptoms as predictors of mortality in patients with COPD. Chest. 2008;135:619–625. doi: 10.1378/chest.08-0078. [DOI] [PubMed] [Google Scholar]
- 58.Gift AG. Shepard CE. Fatigue and other symptoms in patients with chronic obstructive pulmonary disease: Do women and men differ? J Obstet Gynecol Neonatal Nurs. 1999;28:201–208. doi: 10.1111/j.1552-6909.1999.tb01985.x. [DOI] [PubMed] [Google Scholar]
- 59.Di Marco F. Verga M. Reggente M, et al. Anxiety and depression in COPD patients: The roles of gender and disease severity. Respir Med. 2006;100:1767–1774. doi: 10.1016/j.rmed.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 60.Laurin C. Lavoie KL. Bacon SL, et al. Sex differences in the prevalence of psychiatric disorders and psychological distress in patients with COPD. Chest. 2007;132:148–155. doi: 10.1378/chest.07-0134. [DOI] [PubMed] [Google Scholar]
- 61.Kessler RC. Demler O. Frank RG, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapman KR. Tashkin DP. Pye DJ. Gender bias in the diagnosis of COPD. Chest. 2001;119:1691–1695. doi: 10.1378/chest.119.6.1691. [DOI] [PubMed] [Google Scholar]
- 63.Miravitlles M. de la Roza C. Naberan K, et al. [Attitudes toward the diagnosis of chronic obstructive pulmonary disease in primary care.] Arch Bronconeumol. 2006;42:3–8. doi: 10.1016/s1579-2129(06)60106-7. [DOI] [PubMed] [Google Scholar]
- 64.Volkova NB. Kodani A. Hilario D. Munyaradzi SM. Peterson MW. Spirometry utilization after hospitalization for patients with chronic obstructive pulmonary disease exacerbations. Am J Med Qual. 2009;24:61–66. doi: 10.1177/1062860608326417. [DOI] [PubMed] [Google Scholar]
- 65.Vestbo J. Soriano JB. Anderson JA. Calverley P. Pauwels R. Jones P. Gender does not influence the response to the combination of salmeterol and fluticasone propionate in COPD. Respir Med. 2004;98:1045–1050. doi: 10.1016/j.rmed.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Sestini P. Cappiello V. Aliani M, et al. Prescription bias and factors associated with improper use of inhalers. J Aerosol Med. 2006;19:127–136. doi: 10.1089/jam.2006.19.127. [DOI] [PubMed] [Google Scholar]
- 67.Goodman DE. Israel E. Rosenberg M. Johnston R. Weiss ST. Drazen JM. The influence of age, diagnosis, and gender on proper use of metered-dose inhalers. Am J Respir Crit Care Med. 1994;150:1256–1261. doi: 10.1164/ajrccm.150.5.7952549. [DOI] [PubMed] [Google Scholar]
- 68.Machado MC. Krishnan JA. Buist SA, et al. Sex differences in survival of oxygen-dependent patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:524–529. doi: 10.1164/rccm.200507-1057OC. [DOI] [PubMed] [Google Scholar]
- 69.Franklin KA. Gustafson T. Ranstam J. Strom K. Survival and future need of long-term oxygen therapy for chronic obstructive pulmonary disease—Gender differences. Respir Med. 2007;101:1506–1511. doi: 10.1016/j.rmed.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 70.Miyamoto K. Aida A. Nishimura M. Aiba M. Kira S. Kawakami Y. Gender effect on prognosis of patients receiving long-term home oxygen therapy. The Respiratory Failure Research Group in Japan. Am J Respir Crit Care Med. 1995;152:972–976. doi: 10.1164/ajrccm.152.3.7663812. [DOI] [PubMed] [Google Scholar]
- 71.Foy CG. Rejeski WJ. Berry MJ. Zaccaro D. Woodard CM. Gender moderates the effects of exercise therapy on health-related quality of life among COPD patients. Chest. 2001;119:70–76. doi: 10.1378/chest.119.1.70. [DOI] [PubMed] [Google Scholar]
- 72.Cashman JR. Park SB. Yang ZC. Wrighton SA. Jacob P., 3rd Benowitz NL. Metabolism of nicotine by human liver microsomes: Stereoselective formation of transnicotine N′-oxide. Chem Res Toxicol. 1992;5:639–646. doi: 10.1021/tx00029a008. [DOI] [PubMed] [Google Scholar]
- 73.Spivack SD. Hurteau GJ. Fasco MJ. Kaminsky LS. Phase I and II carcinogen metabolism gene expression in human lung tissue and tumors. Clin Cancer Res. 2003;9:6002–6011. [PubMed] [Google Scholar]
- 74.Al Koudsi N. Mwenifumbo JC. Sellers EM. Benowitz NL. Swan GE. Tyndale RF. Characterization of the novel CYP2A6*21 allele using in vivo nicotine kinetics. Eur J Clin Pharmacol. 2006;62:481–484. doi: 10.1007/s00228-006-0113-3. [DOI] [PubMed] [Google Scholar]
- 75.Yamanaka H. Nakajima M. Fukami T, et al. CYP2A6 AND CYP2B6 are involved in nornicotine formation from nicotine in humans: Interindividual differences in these contributions. Drug Metab Dispos. 2005;33:1811–1818. doi: 10.1124/dmd.105.006254. [DOI] [PubMed] [Google Scholar]
- 76.Mollerup S. Ryberg D. Hewer A. Phillips DH. Haugen A. Sex differences in lung CYP1A1 expression and DNA adduct levels among lung cancer patients. Cancer Res. 1999;59:3317–3320. [PubMed] [Google Scholar]
- 77.Mollerup S. Berge G. Baera R, et al. Sex differences in risk of lung cancer: Expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer. 2006;119:741–744. doi: 10.1002/ijc.21891. [DOI] [PubMed] [Google Scholar]
- 78.Ben-Zaken Cohen S. Pare PD. Man SF. Sin DD. The growing burden of chronic obstructive pulmonary disease and lung cancer in women: Examining sex differences in cigarette smoke metabolism. Am J Respir Crit Care Med. 2007;176:113–120. doi: 10.1164/rccm.200611-1655PP. [DOI] [PubMed] [Google Scholar]
- 79.Dix TA. Marnett LJ. Metabolism of polycyclic aromatic hydrocarbon derivatives to ultimate carcinogens during lipid peroxidation. Science. 1983;221:77–79. doi: 10.1126/science.6304879. [DOI] [PubMed] [Google Scholar]
- 80.Wilson AS. Davis CD. Williams DP. Buckpitt AR. Pirmohamed M. Park BK. Characterisation of the toxic metabolite(s) of naphthalene. Toxicology. 1996;114:233–242. doi: 10.1016/s0300-483x(96)03515-9. [DOI] [PubMed] [Google Scholar]
- 81.Han W. Pentecost BT. Pietropaolo RL. Fasco MJ. Spivack SD. Estrogen receptor alpha increases basal and cigarette smoke extract-induced expression of CYP1A1 and CYP1B1, but not GSTP1, in normal human bronchial epithelial cells. Mol Carcinog. 2005;44:202–211. doi: 10.1002/mc.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Higashi E. Fukami T. Itoh M, et al. Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab Dispos. 2007;35:1935–1941. doi: 10.1124/dmd.107.016568. [DOI] [PubMed] [Google Scholar]
- 83.Benowitz NL. Lessov-Schlaggar CN. Swan GE. Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 84.Dempsey D. Tutka P. Jacob P, 3rd, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 85.Dempsey D. Jacob P., 3rd Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301:594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 86.Scanlon PD. Connett JE. Waller LA. Altose MD. Bailey WC. Buist AS. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161:381–390. doi: 10.1164/ajrccm.161.2.9901044. [DOI] [PubMed] [Google Scholar]
- 87.Browning KK. Ferketich AK. Salsberry PJ. Wewers ME. Socioeconomic disparity in provider-delivered assistance to quit smoking. Nicotine Tobacco Res. 2008;10:55–61. doi: 10.1080/14622200701704905. [DOI] [PubMed] [Google Scholar]
- 88.Royce JM. Corbett K. Sorensen G. Ockene J. Gender, social pressure, and smoking cessations: The Community Intervention Trial for Smoking Cessation (COMMIT) at baseline. Soc Sci Med. 1997;44:359–370. doi: 10.1016/s0277-9536(96)00149-9. [DOI] [PubMed] [Google Scholar]
- 89.Ward KD. Klesges RC. Zbikowski SM. Bliss RE. Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addict Behav. 1997;22:521–533. doi: 10.1016/s0306-4603(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 90.Bohadana A. Nilsson F. Rasmussen T. Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: Influence of baseline smoking behavior. Nicotine Tobacco Res. 2003;5:111–116. doi: 10.1080/1462220021000060482. [DOI] [PubMed] [Google Scholar]
- 91.Pauly JR. Gender differences in tobacco smoking dynamics and the neuropharmacological actions of nicotine. Front Biosci. 2008;13:505–516. doi: 10.2741/2696. [DOI] [PubMed] [Google Scholar]
- 92.Lopez EN. Drobes DJ. Thompson JK. Brandon TH. Effects of a body image challenge on smoking motivation among college females. Health Psychol. 2008;27:S243–251. doi: 10.1037/0278-6133.27.3(suppl.).s243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perkins KA. Marcus MD. Levine MD, et al. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J Consult Clin Psychol. 2001;69:604–613. [PubMed] [Google Scholar]
- 94.Etter JF. Prokhorov AV. Perneger TV. Gender differences in the psychological determinants of cigarette smoking. Addiction. 2002;97:733–743. doi: 10.1046/j.1360-0443.2002.00135.x. [DOI] [PubMed] [Google Scholar]
- 95.Wetter DW. Kenford SL. Smith SS. Fiore MC. Jorenby DE. Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- 96.Cepeda-Benito A. Reynoso JT. Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: Differences between men and women. J Consult Clin Psychol. 2004;72:712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- 97.Swan GE. Jack LM. Ward MM. Subgroups of smokers with different success rates after use of transdermal nicotine. Addiction. 1997;92:207–217. [PubMed] [Google Scholar]
- 98.Perkins KA. Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tobacco Res. 2008;10:1245–1250. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- 99.Gourlay SG. Forbes A. Marriner T. Pethica D. McNeil JJ. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. BMJ. 1994;309:842–846. doi: 10.1136/bmj.309.6958.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perkins KA. Donny E. Caggiula AR. Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nicotine Tobacco Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- 101.Perkins KA. Jacobs L. Sanders M. Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl) 2002;163:194–201. doi: 10.1007/s00213-002-1168-1. [DOI] [PubMed] [Google Scholar]
- 102.Allen SS. Allen AM. Pomerleau CS. Influence of phase-related variability in premenstrual symptomatology, mood, smoking withdrawal, and smoking behavior during ad libitum smoking, on smoking cessation outcome. Addict Behav. 2009;34:107–111. doi: 10.1016/j.addbeh.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allen SS. Bade T. Center B. Finstad D. Hatsukami D. Menstrual phase effects on smoking relapse. Addiction. 2008;103:809–821. doi: 10.1111/j.1360-0443.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Allen AM. Allen SS. Lunos S. Pomerleau CS. Severity of withdrawal symptomatology in follicular versus luteal quitters: The combined effects of menstrual phase and withdrawal on smoking cessation outcome. Addict Behav. 2010;35:549–552. doi: 10.1016/j.addbeh.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lynch WJ. Roth ME. Mickelberg JL. Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]