Abstract
Background
Impairments in component processes of working and episodic memory mark both HIV infection and chronic alcoholism, with compounded deficits often observed in individuals comorbid for these conditions. Remote semantic memory processes, however, have only seldom been studied in these diagnostic groups. Examination of remote semantic memory could provide insight into the underlying processes associated with storage and retrieval of learned information over extended time periods while elucidating spared and impaired cognitive functions in these clinical groups.
Methods
We examined component processes of remote semantic memory in HIV infection and chronic alcoholism in 4 subject groups (HIV, ALC, HIV+ALC, and age matched healthy adults) using a modified version of the Presidents Test. Free recall, recognition, and sequencing of presidential candidates and election dates were assessed. In addition, component processes of working, episodic, and semantic memory were assessed with ancillary cognitive tests.
Results
The comorbid group (HIV+ALC) was significantly impaired on sequencing of remote semantic information compared with age matched healthy adults. Free recall of remote semantic information was also modestly impaired in the HIV+ALC group, but normal performance for recognition of this information was observed. Few differences were observed between the single diagnosis groups (HIV, ALC) and healthy adults, although examination of the component processes underlying remote semantic memory scores elicited differences between the HIV and ALC groups. Selective remote memory processes were related to lifetime alcohol consumption in the ALC group and to viral load and depression level in the HIV group. Hepatitis C diagnosis was associated with lower remote semantic memory scores in all three clinical groups. Education level did not account for group differences reported.
Conclusions
This study provides behavioral support for the existence of adverse effects associated with the cormorbidity of HIV infection and chronic alcoholism on selective component processes of memory function, with untoward effects exacerbated by Hepatitis C infection. The pattern of remote semantic memory function in HIV+ALC is consistent with those observed in neurological conditions primarily affecting frontostriatal pathways and suggests that remote memory dysfunction in HIV+ALC may be a result of impaired retrieval processes rather than loss of remote semantic information per se.
Keywords: Remote memory, semantic knowledge, chronic alcoholism, HIV-infected
Chronic abusive drinking is reported in upwards of 50% of HIV-infected individuals (Conigliaro et al., 2006; Miguez et al., 2003; Samet et al., 2004). Overlapping and dissociable neural systems are affected in HIV infection and chronic alcoholism, indicating the need to examine these conditions separately and together as comorbid diagnoses. It has been consistently reported that HIV infection demonstrates an affinity for basal ganglia structures and associated pathways (e.g., frontostriatal pathways) (Castelo et al., 2007; Kaul et al., 2001; Navia et al., 1986). By contrast, the effect of chronic alcoholism on the brain is generally widespread but with a predilection for prefrontal and cerebellar tissue and structures of Papez circuit (Cardenas et al., 2007; Makris et al., 2008; Pfefferbaum et al., 1997; Pitel et al., 2009; Sullivan and Pfefferbaum, 2009). Processes relying on neural systems implicated in both HIV infection and chronic alcoholism could be at particularly high risk in individuals who are comorbid for both these conditions. The few studies that have investigated the potential additive or synergistic effects of these conditions on cognition report that comorbid individuals often show greater deficits in visuospatial, verbal reasoning, episodic memory, motor, and reaction time processes compared with individuals with HIV infection or alcoholism alone (Fama et al., 2009; Farinpour et al., 2000; Green et al., 2004; Martin et al., 2003; Rothlind et al., 2005; Sassoon et al., 2007).
Specific impairments in component processes of memory are observed in both HIV infection and chronic alcoholism. Working memory and short-term memory impairments have consistently been reported in HIV infection (e.g., Stout et al., 1995; Woods et al. 2009), whereas episodic memory has been consistently shown to be impaired in chronic alcoholism (e.g., Nixon et al., 1987; Oscar-Berman, 1990). Studies investigating processes of visual and verbal working memory (Bartok et al., 1997; Martin et al., 2001) and episodic memory (Fama et al., 2009) in individuals comorbid for HIV infection and substance abuse, including alcoholism, have reported selective and often additive effects of these conditions on memory processes.
Whereas processes of consolidation of new information have been the focus of nearly all mnemonic studies of HIV, chronic alcoholism, or HIV-alcoholism comorbidity, the fate of processes supporting remote memory and related semantic memory processes are relatively unknown in these diseases. Tests of remote semantic memory generally assess recall or recognition of previously learned information, including names of famous people, places, or events. In contrast to episodic memory tasks that assess recently learned information (e.g., word lists or stories presented by examiner at test session), remote memory tasks assess information learned months, years, or even decades ago. Within the domain of remote memory, content (e.g., name of person, place, historical event) and contextual (e.g., the temporal or spatial information associated with person, place, historical event) aspects have been shown to be dissociable (Fama et al., 2000b; Sagar et al., 1988). Further, an array of component processes, both mnemonic (e.g., working and episodic memory processes) and nonmnemonic (e.g., executive functions including ability to search and retrieve information; cf., Kopelman, 1991; Mangels et al., 1996) likely influences these processes. Examining mnemonic and nonmnemonic influences on remote memory processes could provide information about the underlying processes associated with storage and retrieval of remote semantic memories.
Functionally independent brain systems mediate different aspects of memory (Gabrieli, 1998; Squire and Zola-Morgan, 1991). For example, although the hippocampus and related medial temporal structures are principal neural substrates of episodic memory consolidation, these structures do not play a similarly central role in access to remote semantic information (Bright et al., 2006; Fama et al., 2004a; Kopelman, 1993; Squire and Zola, 1997). Rather, remote semantic information is considered to be widely distributed throughout cortical association areas (Bright et al., 2006; Ungerleider, 1995).
Studies of remote semantic memory in alcoholism have typically focused on patients with Korsakoff’s syndrome (KS) because of their notable amnesia (e.g., Albert et al., 1979; Cermak, 1987; Chang et al., 1997; Fama et al., 2004a; Kopelman et al., 2009; Marslen-Wilson and Teuber, 1975; Sanders and Warrington, 1971; Seltzer and Benson, 1974; Verfaellie et al., 1995). These studies have generally reported remote semantic memory impairments with most but not all noting a temporal gradient, a performance pattern characterized by markedly better remote than recent memory.
In HIV-related studies, Sadek and colleagues (Sadek et al., 2004) investigated remote memory processes in HIV infection with concomitant dementia (HIV+dementia), Huntington’s disease (HD), and probable Alzheimer’s disease (AD), using an updated version of the Remote Memory Battery (Albert et al., 1981). They reported that the deficits observed in the HIV+dementia group were similar to those observed in HD and different from the deficit pattern observed in AD. Temporal gradients characteristic of remote memory impairments in AD (cf., Beatty et al., 1988; Fama et al., 2000b; Kopelman, 1989; Sagar et al., 1988; Squire and Cohen, 1984) were not observed in the HIV+dementia group. In addition, recognition of information was relatively spared while free recall was markedly impaired. The authors attributed the remote memory impairments in HIV+dementia patients to dysfunction in subcortical systems. These results are consistent with a number of other studies reporting similarities between the cognitive deficits observed in HIV infection and those observed in conditions primarily affecting subcortical structures and associated pathways (e.g., Heaton et al., 1995; Peavy et al., 1994; Tross et al., 1988).
In this study we used a modification of a test devised by Hamsher and Roberts (Presidents Test; Hamsher and Roberts, 1985) to examine content and contextual information of past public figures and events. This measure has been used successfully to assess remote semantic memory processes in individuals diagnosed with Alzheimer’s disease (Fama et al., 2000a), Parkinson’s disease (Fama et al., 2000b), and Korsakoff’s syndrome (Fama et al., 2004a). Here, we examined remote semantic memory processes and the potential influences of mnemonic and nonmnemonic component processes in individuals with HIV infection, chronic alcoholism, and those with both conditions. We tested the hypotheses that 1) remote semantic memory processes would be impaired in the comorbid group (HIV+ALC) compared with a normal comparison (NC) group of healthy adults; 2) the combined untoward effects of HIV infection and chronic alcoholism would put the comorbid group at liability for performing more poorly than either single diagnosis group (HIV, ALC); 3) recognition scores for remote memory events would be significantly better than free recall scores, particularly in the HIV groups, based on the reported frontostriatal dysfunction associated with HIV infection; 4) HIV and ALC groups would perform below the level of normal comparison group on the remote memory test but at similar levels to each other, and the pattern of component processes underlying these scores would differ between these groups; and 5) greater disease burden measured, for example by total alcohol consumption in the alcoholic and CD4 cell count in the HIV-infected groups would correlate with remote semantic memory scores.
Methods
Study participants included 18 men and 8 women with HIV infection (HIV), 21 men and 7 women with alcoholism (ALC), 23 men and 9 women comorbid for both conditions (HIV+ALC), and 16 healthy adults who made up the normal comparison group (NC; 12 men, 4 women). Clinical participants were recruited from HIV clinics and local substance abuse treatment programs. Subjects for the normal comparison group were recruited from the local community. All participants were screened using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1998) and structured questionnaires on health status. Upon initial assessment subjects were excluded if they had a significant history of psychiatric or neurological disorder not related to their primary diagnosis, a history of past or present alcohol or drug abuse or dependence in the NC group, recent (within the last 3 months) substance dependence other than alcohol in the clinical groups, or a serious medical condition or HIV-related opportunistic infection. The Beck Depression Inventory-II (Beck et al., 1996) assessed severity of depression symptoms in all groups. HIV subjects were also rated on the Karnofsky Scale, which provides a global measure of general abilities in activities of daily living (Karnofsky, 1949). All subjects underwent a semi-structured interview (Skinner, 1982; Skinner and Sheu, 1982) to quantify lifetime alcohol consumption. Blood samples were used to determine HIV status via HIV Ab, plasma viral load and CD4 cell count. At time of recruitment all HIV positive individuals had CD4>100 cells per mm3 and a Karnofsky score >70. Subjects were assigned to one of the following four groups based on clinical assessment: 1) HIV: individuals who were HIV seropositive and had never met criteria for alcohol dependence, 2) ALC: individuals who met lifetime criteria for alcohol dependence within the past 3 years and were seronegative for HIV, 3) HIV+ALC: individuals who were HIV seropositive and also met criteria for alcohol dependence or abuse within the past 3 years at initial visit, and 4) NC: normal comparison individuals who were neither HIV seropositive nor met alcohol dependence or abuse criteria or other Axis-I diagnosis in their lifetime. Neither amount of alcohol drunk (Table 1) nor duration of time since last drink (ALC group 1.2±2.1 years; HIV+ALC group 1.0±2.1 years) was statistically different between the alcohol groups. The HIV-infected groups did not differ on duration of illness or viral load (HIV group: duration 14.9±8.0 years, log viral load 2.2±1.1; HIV+ALC group: duration 15.2±7.7 years; viral load 2.3±1.1).
Table 1.
Demographic characteristics of subjects (mean, standard deviation, range)
| Group | Age (yrs) | Education (yrs) | NART-IQ† | Lifetime Alcohol Consumption (kg) | Beck Depression Inventory-II | CD4 count |
|---|---|---|---|---|---|---|
| Healthy Adults (Normal Comparison) (NC n=16) | 50.1 (5.7) 43 to 62 |
15.9 (1.7) 13 to 18 |
114.1 (9.5) 92 to 126 |
26.7 (36.8) 0 to 148 |
2.3 (2.8) 0 to 11 |
NA |
| HIV (HIV n=26) | 51.7 (6.5) 40 to 64 |
13.8 (2.9) 9 to 19 |
106.2 (9.6) 90 to 123 |
70.3 (63.9) 0 to 280 |
10.8 (9.8) 0 to 31 |
556.0 (259.6) 88 to 1106 |
| Alcoholic (ALC n=28) | 52.1 (7.0) 40 to 66 |
13.9 (2.5) 10 to 21 |
107.7 (9.9) 90 to 124 |
1387.5 (981.3) 244 to 4376 |
8.1 (5.6) 0 to 22 |
NA |
| HIV with Alcoholism (HIV+ALC n=32) | 50.6 (5.9) 41 to 62 |
13.0 (2.0) 9 to 18 |
105.8 (7.7) 92 to 123 |
907.2 (1045.5) 22 to 5937 |
11.5 (8.8) 0 to 36 |
547.6 (340.9) 83 to 1320 |
| Post-hoc t-tests | ns | p=.0014 | p=.024 | p=.0001 | p=.0012 | ns |
| NC>HIV, ALC, HIV+ALCNC>HIV, ALC, HIV+ALCNC, HIV<ALC, HIV+ALCNC<HIV, ALC, HIV+ALC ALC>HIV+ALC | ||||||
NART - National Adult Reading Test
NA - Not applicable
Ethnic Composition of Groups:
HIV: 46.2%-White (Europe, Middle East, North Africa) and 53.8% non-White (11.5%-Hispanic, 42.3%-Black or African American)
ALC: 46.4%-White and 53.6% non-White (10.7%-Hispanic, 3.6%-Native American, 39.3%-Black or African American
HIV+ALC: 15.6%-White and 84.4% non-White (18.8%-Hispanic, 65.6%-Black or African American)
NC: 43.8%-White and 56.2% non-White (25%-Black or African American, 31.25%-Asian)
Five of the HIV and 8 of the HIV+ALC subjects met CDC criteria for AIDS (CD4<200, AIDS defining symptom) sometime during the course of their illness. At the time of testing, 2 of the 26 HIV subjects and 5 of the 32 HIV+ALC subjects had T cell counts below 200, while 13 HIV and 18 HIV+ALC subjects had T cell counts above 500. Of the 26 HIV subjects, 22 individuals were on HAART and 3 others were on other HIV-related medications. Of the 32 HIV+ALC subjects, 27 were on HAART and 4 others were on other HIV-related medications. Eleven of the HIV and 30 of the HIV+ALC subjects met criteria for a non-alcohol substance abuse diagnosis sometime in their lifetime (but not currently). Average number of years since criteria was met for a non-alcohol substance abuse diagnosis was 14.7 years for the HIV group and 7.2 years for the HIV+ALC group. Seven of the HIV, 6 of the ALC, and 12 of the HIV+ALC subjects tested positive for Hepatitis C. All data were obtained in compliance with the regulations of both Stanford University and SRI International through Institutional Review Board review and approval. Written informed consent was obtained from all participants.
Only subjects who were at least 40 years old at the time of testing were included in this study as they would have the advantage of episodic memories associated with elections prior to the most recent time period (cf., Piolino et al., 2007), whereas individuals younger than 40 years old would likely only have episodic memories for presidential elections since 1980, when they were at least 10 years old (cf., Westmacott and Moscovitch, 2003). Demographics for the four subject groups are in Table 1.
Groups did not differ significantly in age (F(3,98)=0.52, p=.67) but did differ in years of formal education (F(3,98)=5.61, p=.0014) and estimated IQ scores based on the NART (National Adult Reading Test; F(3,98)=3.28, p=.024) (Nelson, 1982). Follow-up t-tests indicated that the HIV, ALC, and HIV+ALC groups had significantly fewer years of education and lower estimated IQ scores than the NC group but did not differ significantly from each other.
The Presidents Test
The Presidents Test required subjects to recall, recognize, and sequence the names of elected and defeated presidential candidates and the years of presidential elections. We modified the Presidents Test by Hamsher and Roberts (Hamsher and Roberts, 1985; Roberts et al., 1990) which was designed originally as an episodic memory test. Remote semantic memory processes were assessed here by extending the temporal range of the test to include presidential elections from 1928–2004. The test consisted of three subtests, administered in the following fixed order: free recall, recognition of candidate names and election dates, and sequencing. Subjects were given as much time as needed to complete these subtests.
Items of the Presidents Test are considered to have greater equivalency than those from other remote memory measures (cf., Brandt and Benedict, 1993; Squire and Cohen, 1982; Warrington and Sanders, 1971) because all test items refer to a common category of people and events, presidential candidates and elections, albeit specific presidential candidates or elections may be particularly memorable for specific individuals. Further, compared with one-time episodic events, such as the raising of the flag in Iwo Jima or the September 11, 2001 attacks on the World Trade Center and Pentagon, for which people can often remember where they first learned of these events, presidential elections have generally less autobiographical significance or temporal-spatial context. With loss of temporal-spatial associations with the passing of time, names of presidential candidates likely become more semantic than episodic in nature (cf., Cermak, 1984). Elections were divided into 4 time periods (each time period containing five presidential elections) to differentiate historical knowledge from remote semantic memory processes for this information (Time 1: 1928–1944; Time 2: 1948–1964; Time 3: 1968–1984; Time 4: 1988–2004). Testing took place between 2007 and 2009.
Candidate Recall
Subjects were given a sheet of paper divided into columns and asked to write down as many of the presidential candidates they could recall (Republican and Democratic) who ran in any election between the years 1928 and 2004. Subjects were also asked to write down the year of the election for each candidate recalled. A candidate’s last name was deemed sufficient for credit. Order of names was not important for this subtest. Subjects were given one point for each name correctly recalled; two scores were computed - one denoting the number of correct candidates recalled and one denoting the number of different candidates recalled. Thus, while the maximum number of presidential candidates from the 20 elections was 20 elected and 20 defeated candidates (40 total names) the maximum number of different candidates was 12 elected and 18 defeated candidates (30 total names) because a number of individuals ran for office in more than one election. See Appendix A for a list of presidential candidates and election dates.
Candidate and Election Year Recognition
Each of the 20 items of this subtest consisted of 6 names and 3 dates; subjects were to circle the 2 names that represented presidential candidates who ran against each other in a particular election and the date of the election. The 4 foils for each item were names of high profile individuals who had been politically or socially active during the same time period as the presidential candidates. The 2 election year foils were balanced across items such that one of the incorrect dates was ±4 years and the other incorrect date was ±12 years from the correct election year. For example, one recognition item included the names John Dean, Robert Dole, Nelson Rockefeller, Gerald Ford, Jimmy Carter, and Warren Burger, arranged in a 2 × 3 array, with the dates 1972, 1976, 1964 placed underneath the names. The correct choices for this item are Gerald Ford and Jimmy Carter and the correct date is 1976. Maximum score for this subtest was 40 points for candidate recognition (20 for correct elected candidates, 20 for defeated candidates) and 20 points for date recognition. Only items that were correct for both elected and defeated candidates were eligible to be scored for date recognition.
Candidate Sequencing
Subjects were presented a set of 20 index cards, each card containing two names for a particular election - the elected presidential candidate on top and the defeated candidate below. Subjects were asked to sequence the cards in chronological order. Cards were presented in a fixed random order and a point was given for every card correctly sequenced.
Ancillary Cognitive Measures
Tests assessing component processes of executive functions, working memory, episodic memory, and semantic knowledge were administered to examine the underlying component processes of remote semantic memory for public figures and dates. The Trail Making Test - Part B (Reitan, 1958) assessed component executive functions, namely sequencing and ability to switch set. Subjects connected 25 circles containing numbers and letters in alternate sequence (i.e., 1-A-2-B-3-C). Working memory was assessed with the Letter Number Sequencing subtest of the WAIS-III (Wechsler, 1997). In this test, subjects are presented a sequence of numbers and letters and asked to organize them in ascending order within category, numbers first and then letters. The Logical Memory subtest from the WMS-R (Wechsler, 1987) assessed episodic memory processes. After listening to a short narrative, subjects recalled stories. Semantic fluency (1-minute trials for inanimate objects and animals) assessed semantic knowledge. Subjects produced as many different exemplars as possible for each category (Newcombe, 1969).
Statistical Analyses
Between-group and within-group analyses were conducted. Analysis of variance (ANOVA), analysis of covariance (ANCOVA), and t-tests assessed between group differences. A priori two group analyses were also conducted to investigate hypothesized differences between the comorbid (HIV+ALC) group and the normal comparison (NC) group independent of the four group analyses. It was hypothesized that because of the combined untoward effects of HIV infection and alcoholism the HIV+ALC group would show impairment compared with healthy adults not necessarily detectable in either single diagnosis.
Within-group analyses were conducted with paired t-tests and correlational analyses. Paired t-tests assessed differences between free recall and recognition scores within subject groups. Correlational analyses using Bonferroni adjusted significance levels examined the relationships between President Test scores and other mnemonic and nonmnemonic measures. In addition, relationships between cognitive test scores and demographic variables, including age, years of education, depressive symptoms, and disease-related variables such as lifetime alcohol consumption (ALC and HIV+ALC groups), CD4 cell count, and viral load (HIV and HIV+ALC groups) were assessed. Finally, multiple regression analyses examined selectivity of significant relationships by testing the unique contribution of demographic and cognitive processes to remote semantic memory scores.
Results
Between Group Analyses
Education as a Covariate
Average years of education for the NC group was approximately 2 years more than those of the HIV and ALC groups and almost 3 years more than the HIV+ALC group (Table 1). Education and semantic knowledge have been reported to be positively correlated (Heaton et al., 1986; Lezak et al., 2004) and analyses investigating this association in the present study yielded significant relationships between education level and Presidents Test scores in all subject groups. Years of education was used as a covariate in all between-group analyses that included the normal comparison group. We did not conduct separate ANCOVAs controlling for estimated IQ scores because years of education was highly correlated with estimated IQ scores, which were derived from the NART.
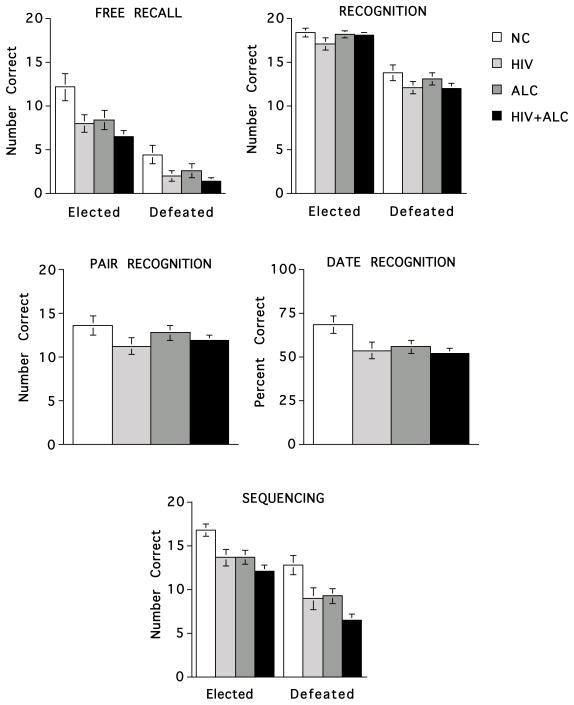
After controlling for education differences, significant group differences were observed, between the HIV+ALC and NC groups on sequencing (F(1,45)=4.63, p=.037) and a statistical trend was observed between these groups on free recall (F(1,45)=3.03, p=.089). Effect sizes (Cohen’s d) were calculated for both raw scores and education-corrected Z scores, which were based on our normal comparison group. The lowest level of education for the normal comparison group was 13 years, whereas the lowest level of education for the clinical groups was 9 years. When standardizing scores on education based on our comparison group we may also be partially controlling for diagnosis; thus, we calculated effect sizes based on the raw scores as well as on the education corrected Z-scores, considering that the true effect size likely falls between these two values. The computed effect size for sequencing ranged from small to moderate (.256 for education-corrected Z scores and .597 for raw scores). A similar pattern for effect size was observed for free recall (.187 for education-corrected Z scores and .481 for raw scores). Group differences were not observed for free recall, recognition, or sequencing in 4-group analyses after controlling for education.
The next set of analyses assessed the potential interaction of HIV infection and chronic alcoholism effects on free recall, recognition, and sequencing of remote semantic information. A 2×2 (ALC − present/absent, HIV present/absent) analysis of variance yielded no significant interactions (Free Recall (F(1.98)=1.12, p=.293, Recognition (F(1,98)=.710. p=.402, Sequencing F(1,97)=.553, p=.459).
Historical Semantic Information vs. Remote Semantic Memory
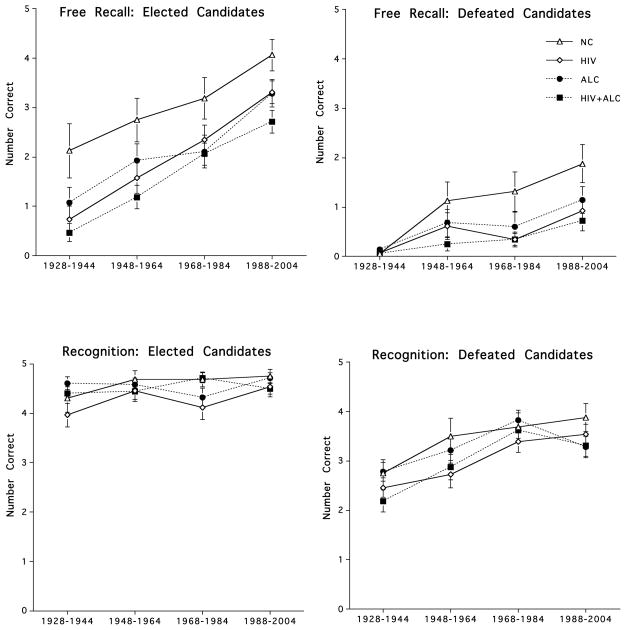
Figure 2 depicts free recall and recognition scores for the Presidents Test over the four designated time periods. Names of presidential candidates and election dates from the most remote time period (1928–1944) is likely a measure of pure semantic knowledge because events took place before the subjects from this study were either born or old enough to incorporate episodic information about these events. By contrast, presidential candidates from the two most recent time periods (1968–2004) likely reflect both semantic knowledge and the influence of episodic based memories as to contextual events surrounding these elections (Westmacott and Moscovitch, 2003).
Figure 2.
Free recall and recognition of elected and defeated presidential candidates over four designated time periods (Time 1: 1928–1944; Time 2: 1948–1964; Time 3 1968–1984; Time 4: 1988–2004) for each subject group.
Repeated measures ANCOVA, controlling for years of education, comparing the most remote time period with the two most recent time periods, failed to identify group × time interactions for free recall or recognition in both the two group (HIV+ALC vs. NC F(1,46)=.380, p=.541; F(1,46)=.482, p=.491) and four group (F(3,98)=.204, p=.893; F(3,98)=1.766, p=.159) analyses, suggesting that all groups showed the same pattern of performance, i.e., free recall and recognition of presidential candidates for the most recent time periods was better than that for the most remote time period.
Correlational analyses, with Bonferroni family-wise correction (p<.016), indicated that age was significantly associated with free recall (r=.682, p=.0036), recognition (r=.633, p=.0085), and sequencing (r=.629, p=.009) scores in the NC group, with older subjects scoring better than younger subjects. These relationships, however, were not consistently observed in the HIV, ALC, or HIV+ALC groups. By contrast to the NC group, age in the clinical groups was not significantly related to free recall, recognition, or sequencing scores.
Free Recall versus Recognition
Repeated measures ANCOVA, controlling for years of education, indicated a significant group (HIV+ALC, NC) × memory score (free recall, recognition) interaction (F(1,56)=8.61, p=.005), demonstrating that although memory scores for both groups were aided by recognition cues, HIV+ALC showed a significantly greater increase on recognition score than NC relative to free recall scores. No group × memory score interaction was forthcoming on the four group analyses (F(3,98)=2.03, p=.115).
Within Group Analyses
Relationships between Presidents Test and Ancillary Cognitive Measures
Correlational analyses were conducted to examine the relationships between scores on the Presidents Test and measures of executive functions, working memory, episodic memory, and semantic fluency. Raw scores for these ancillary cognitive measures are presented in Table 2. Due to the number of comparisons conducted, correlations were deemed significant if p≤0.01. As Table 3 indicates, there were a number of significant relationships between ancillary test scores and scores for the Presidents Test in the HIV and ALC groups. By contrast, the only significant relationships observed in the HIV+ALC group were between Semantic Fluency score and free recall and recognition scores.
Table 2.
Descriptive Statistics for Ancillary Test Measures (mean and se)
| Trail Making - B (number of seconds) | Letter-Number Sequencing (max=21) | Logical Memory (max=50) | Semantic Fluency (number of words) | |
|---|---|---|---|---|
| NC (n=16) | 61.9 (3.7) 37 to 90 |
11.8 (.9) 6 to 19 |
27.1 (1.6) 16 to 42 |
53.6 (1.8) 43 to 67 |
| HIV (n=26) | 94.1 (13.7) 41 to 373 |
9.8 (.7) 3 to 18 |
21.9 (1.6) 10 to 36 |
44.6 (2.0) 26 to 65 |
| ALC (n=28) | 88.6 (9.4) 31 to 213 |
10.2 (.5) 5 to 16 |
24.4 (1.5) 11 to 37 |
47.6 (2.4) 27 to 79 |
| HIV+ALC (n=32) | 101.3 (8.0) 47 to 240 |
8.4 (.6) 2 to 16 |
20.3 (1.5) 3 to 34 |
44.7 (1.6) 27 to 64 |
Table 3.
Correlations between Presidents Test scores and ancillary cognitive scores
| Free Recall: Candidates | Recognition: Candidate Pairs | Recognition: Election Date | Sequencing: Candidates | |
|---|---|---|---|---|
| HIV Group (n=26) | ||||
| Trails B | −.38 | −.42 | −.46 | −.51* |
| Letter-Number Sequencing | .62* | .53* | .42 | .52* |
| Logical Memory | .64* | .46 | .57* | .44 |
| Semantic Fluency | .62* | .47 | .61* | .67* |
| Free Recall: Candidates | Recognition: Candidate Pairs | Recognition: Election Date | Sequencing: Candidates | |
| ALC Group (n=28) | ||||
| Trails B | −.46 | .71* | −.17 | −.48 |
| Letter-Number Sequencing | .59* | .59* | .44 | .68* |
| Logical Memory | .55* | .57* | .26 | .57* |
| Semantic Fluency | .59* | .47 | .48* | .39 |
| Free Recall: Candidates | Recognition: Candidate Pairs | Recognition: Election Date | Sequencing: Candidates | |
| HIV+ALC Group (n=32) | ||||
| Trails B | −.21 | −.07 | .19 | −.13 |
| Letter-Number Sequencing | .27 | .17 | .17 | .27 |
| Logical Memory | .22 | .13 | .02 | .10 |
| Semantic Fluency | .57* | .46* | .20 | .33 |
p<.01
Multiple regression analyses examined the unique contribution of significant ancillary test scores to President Test scores in the HIV and ALC groups. In the HIV group, free recall and recognition remote memory scores were predicted by Logical Memory scores (Table 4). Remote recognition memory scores in the HIV group were additionally predicted by Semantic Fluency scores as were sequencing scores on the remote memory test. In the ALC group, free remote memory recall score was predicted by Semantic Fluency, remote recognition score was predicted by Trails B score, and sequencing score was predicted by Letter-Number Sequencing score. Separate multiple regression analyses (Table 5) were conducted to assess whether these selective relationships between Presidents Test scores and ancillary test scores held after taking years of education into account. Although education contributed independently to President Test scores, the unique contributions of the ancillary test scores to free recall, recognition, and sequencing scores within each group were independent of education.
Table 4.
Multiple Regressions: Predicting President Test scores in the HIV and ALC groups
| HIV Group Dependent Measure | Predictors | Beta-Coefficient | p-value |
|---|---|---|---|
| Free Recall | Letter-Number Seq | .20 | .301 |
| Logical Memory | .41 * | .018 | |
| Semantic Fluency | .33 | .073 | |
| Recognition: Election Dates | Logical Memory | .40 * | .021 |
| Semantic Fluency | .46 ** | .009 | |
| Sequencing | Letter-Number Seq | .01 | .976 |
| Semantic Fluency | .56 * | .017 | |
| Trails B | −.25 | .220 | |
| ALC Group Dependent Measure | Predictors | Beta-Coefficient | p-value |
| Free Recall | Letter-Number Seq | .18 | .373 |
| Logical Memory | .30 | .126 | |
| Semantic Fluency | .44 * | .012 | |
| Recognition: Candidate Pairs | Letter-Number Seq | .22 | .250 |
| Logical Memory | .12 | .550 | |
| Trails B | −.53 ** | .006 | |
| Sequencing | Letter-Number Seq | .50 * | .018 |
| Logical Memory | .25 | .220 | |
p<.01;
p<.05
Table 5.
Multiple Regressions: Years of education as a predictor of President Test scores
| HIV Group Dependent Measure | Predictors | Beta-Coefficient | p-value |
|---|---|---|---|
| Free Recall | Years of Education | .43 * | .014 |
| Semantic Fluency | .43 * | .014 | |
| Recognition: Election Dates | Years of Education | .18 | .336 |
| Logical Memory | .33 | .069 | |
| Semantic Fluency | .39 * | .032 | |
| Sequencing | Years of Education | .42 * | .014 |
| Semantic Fluency | .46 ** | .009 | |
| ALC Group Dependent Measure | Predictors | Beta-Coefficient | p-value |
| Free Recall | Years of Education | .53 ** | .002 |
| Semantic Fluency | .35 * | .024 | |
| Recognition: Candidate Pairs | Years of Education | .42 ** | .003 |
| Trails B | −.57 *** | .001 | |
| Sequencing | Years of Education | .47 ** | .003 |
| Letter-Number Sequencing | .43 ** | .007 | |
| HIV+ALC Group Dependent Measure | Predictors | Beta-Coefficient | p-value |
| Free Recall | Years of Education | .43 ** | .003 |
| Semantic Fluency | .50 *** | .001 | |
| Recognition: Candidate Pairs | Years of Education | .47 ** | .003 |
| Semantic Fluency | .38 * | .014 | |
p<.01;
p<.05
Disease-Related Variables and President Test Scores
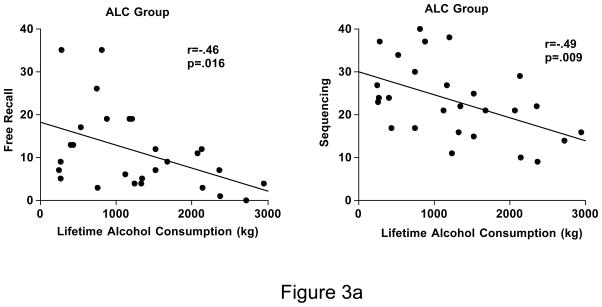
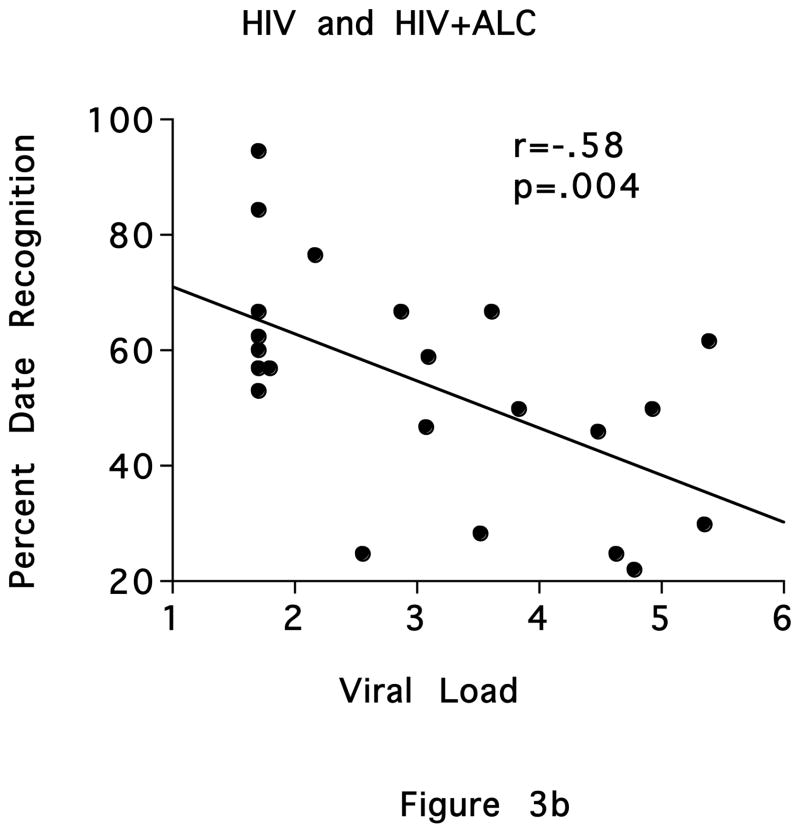
In the ALC group, lower free recall (r=−.46, p=.016) and sequencing (r=−.49, p=.009) scores were significantly related to greater lifetime alcohol consumption (Figure 3a). Scores on the Presidents Test were not related to CD4 cell count in the HIV or HIV+ALC groups. In the 23 individuals who had detectable viral load in these groups, poorer recognition of dates correlated significantly with higher viral load (r=−.58, p=.004) (Figure 3b).
Figure 3.
Figure 3a. Scatterplots depicting the relationships between free recall and sequencing of presidential candidates and estimated amount of lifetime alcohol consumed in the ALC group.
Figure 3b. Scatterplot depicting the relationship between recognition of election year and viral load in the HIV and HIV+ALC groups.
Within the combined HIV-infected group (HIV and HIV+ALC), individuals who were diagnosed with AIDS at any point during their illness (n=13) did not score lower on total free recall, recognition, or sequencing of presidential candidates than those without an AIDS diagnosis. However, ANCOVAs controlling for education indicated that HIV-infected individuals (HIV and HIV+ALC) who were diagnosed with Hepatitis C in their lifetime (n=19) were significantly more impaired in free recall (F(1,49)=5.39, p=.025) and marginally more impaired in sequencing of presidential candidates (F(1,49)=3.34, p=.074) than HIV-infected individuals without Hepatitis C.
Almost one-third of the subjects in the single diagnosis groups were diagnosed with Hepatitis C; neither age nor education differed between individuals with and without Hepatitis C in these groups. In the ALC group, individuals with Hepatitis C (n=6) scored lower in free recall (Mann-Whitney Z=2.2, p=.028) and recognition of election dates (Z=2.13, p=.033) than ALC individuals without Hepatitis C. In the HIV group, individuals with Hepatitis C (n=7) had lower free recall (Z=2.27, p=.023) and sequencing scores (Z=2.93, p=.003) than HIV individuals without Hepatitis C.
Depression Symptomatology and Presidents Test Scores
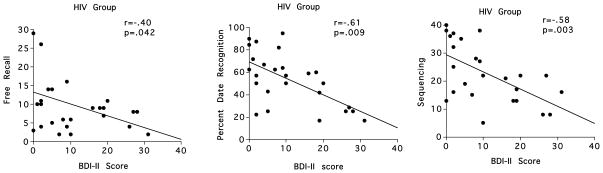
In the HIV group higher scores on the BDI-II significantly correlated with lower scores on free recall (r=−.40, p=.042), date recognition (r=−.61, p=.0009), and sequencing (r=−.58, p=.003) (Figure 4). By contrast, BDI-II scores were not significantly correlated with remote semantic memory scores in the ALC group.
Figure 4.
Scatterplots depicting the relationships between free recall, election date recognition, and sequencing and BDI-II scores in the HIV group.
Post-hoc multiple regression analyses rerun to include BDI-II score indicated that BDI-II score was a unique predictor of date recognition (p=.006) scores, after accounting for the contribution of years of education, Logical Memory, and Semantic Fluency scores, and was a modest predictor for sequencing score (p=.055) after accounting for years of education and Semantic Fluency in the HIV group. By contrast, BDI-II score was not a unique predictor of free recall score.
Discussion
In support of our initial hypothesis, the HIV+ALC group showed impairments in selective remote semantic memory processes compared with healthy adults. The deficits were limited to sequencing and free recall of semantic information, leaving recognition intact. Despite the difference in years of education between the comorbid and healthy group, education alone did not fully account for these group differences. Although the HIV and ALC groups consistently scored below the normal comparison group, differences among these groups were not statistically significant.
Selective disease related variables were associated with remote semantic memory processes. Specifically, in the alcohol group greater lifetime alcohol consumption was predictive of poorer free recall and sequencing of remote semantic information. Age did not account for this relationship; age was not related to amount of lifetime alcohol consumption in the chronic alcohol group. In the HIV and the HIV+ALC groups, viral loads were related to date recognition, a task thought to require intact frontally based neural systems, and individuals with Hepatitis C showed greater impairment on free recall and sequencing scores than HIV-infected individuals not comorbid for Hepatitis C. In addition, individuals with chronic alcoholism and Hepatitis C had poorer free recall and date recognition performance than individuals with chronic alcoholism who did not have Hepatitis C. Taken together, these results reveal a potential fragility of components of remote memory in alcoholism and HIV infection that reach a threshold of detection when compounded with other secondary conditions, notably Hepatitis C.
Tests of remote memory using time-limited memoranda can track points of robust and impaired memory consolidation in amnesic patients. Individuals with amnesia of a known onset typically exhibit a stepped impairment, such that memories antecedent to the amnesic event are relatively intact but those afterwards are lost (cf., Kopelman, 1989). In our study, temporal gradients were not observed in any alcoholic or HIV-infected group. There were no notable differences between recall and recognition for historical names from the last 20 years (1988–2004) compared with the previous 20 years (1964–1988). These results suggest that memory for past historical names and events within one’s lifetime was not affected by duration of the memory enhancing consolidation processes. In addition, the pattern of performance in the HIV+ALC group, recognition significantly better than recall with no temporal gradient, is similar to the pattern of remote semantic memory performance of individuals with subcortical/frontostriatal dysfunction and provides behavioral support of frontally-based pathology in HIV infection and chronic alcoholism as observed in in vivo imaging (HIV: Chang et al., 1997; Pfefferbaum et al., 2009) (ALC: Pfefferbaum et al., 1997) and neuropathological studies (HIV: Navia et al., 1986) (ALC: Harper, 1998; Kril and Harper, 1989).
In support of our secondary hypothesis, component processes predicting remote semantic memory differed among the clinical groups. In the comorbid group, ability to recall and recognize remote semantic information was selectively associated with semantic fluency. By contrast, remote semantic memory scores in the alcohol and HIV groups were predicted by multiple mnemonic and nonmnemonic processes, and processes which were largely idiosyncratic for each group. Thus, although the alcohol and HIV groups did not differ from each other in remote semantic memory performance, they appeared to invoke different cognitive processes and possibly different neural systems to perform these tasks (cf., Fama et al., 2004b).
Education was related to aspects of recall and recognition of semantic information in the clinical groups, with more years of education associated with higher scores. This relationship serves as a reminder that when interpreting cognitive tests it is important to assess the potential influence of developmental and experiential variables to performance. Despite the educational differences between the normal comparison group and the clinical groups, the ability to recognize names of historical figures did not differ among groups, with and without education as a statistical covariate. It was only on sequencing and free recall of this information that differences between the comorbid and normal comparison groups were observed. This pattern of results suggests that information about presidential candidates and elections has been learned and retained over time in individuals with HIV infection, chronic alcoholism, or both, but that ability to retrieve and work with this information is affected. This hypothesized retrieval deficit, as opposed to an actual loss of semantic information, is consistent with reports of other neurological conditions affecting structures of the basal ganglia and associated frontostriatal neural pathways (Cummings, 1993; Heindel et al., 1989; Huber et al., 1989).
Limitations of this study include the sensitivity of the remote memory measure used and the differences between the normal comparison group and the clinical groups in years of education. Although the Presidents Test has been shown to document remote memory deficits in neurodegenerative disorders (e.g., AD, PD, KS) it may not be the most appropriate measure to document remote memory impairments in younger cohorts. In addition, although we statistically controlled for the influence of education on remote memory performance it is always preferable to have the normal comparison group with education range matching the patient group.
In conclusion, this study provides behavioral evidence of the untoward effects associated with the comorbidity of HIV infection and chronic alcoholism and exacerbated by Hepatitis C infection on selective component processes of remote memory. This study also extends the behavioral evidence, observed via remote memory processes, for frontally based system compromise associated with HIV infection and chronic alcoholism. Clinically, the remote memory impairments observed in the comorbid group can have implications for personal and professional activities (Gorman et al., 2009; Woods et al., 2009). Ability to store, retain, retrieve, and manipulate previously learned information is imperative for successful implementation and completion of daily tasks including management of personal health care, finances, and long-term tasks and projects.
Figure 1.
Number correct for free recall, recognition (individual candidates, pairs of candidates, election year), and sequencing of elected and defeated presidential candidates from 1928–2004 for each subject group.
Acknowledgments
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism AA017347, AA005965, and AA017168.
References
- Albert MS, Butters N, Brandt J. Patterns of remote memory in amnesic and demented patients. Arch Neurol. 1981;38:495–500. doi: 10.1001/archneur.1981.00510080057008. [DOI] [PubMed] [Google Scholar]
- Albert MS, Butters N, Levin J. Temporal gradients in the retrograde amnesia of patients with alcoholic Korsakoff’s disease. Arch Neurol. 1979;36:211–216. doi: 10.1001/archneur.1979.00500400065010. [DOI] [PubMed] [Google Scholar]
- Bartok JA, Martin EM, Pitrak DL, Novak RM, Pursell KJ, Mullane KM, Harrow M. Working memory deficits in HIV-seropositive drug users. J Int Neuropsychol Soc. 1997;3:451–456. [PubMed] [Google Scholar]
- Beatty WW, Salmon DP, Butters N, Heindel WC, Granholm EL. Retrograde amnesia in patients with Alzheimer’s disease or Huntington’s disease. Neurobiol Aging. 1988;9:181–186. doi: 10.1016/s0197-4580(88)80048-4. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Brandt J, Benedict R. Assessment of retrograde amnesia: Findings with a new public events procedure. Neuropsychology. 1993;7:217–227. [Google Scholar]
- Bright P, Buckman J, Fradera A, Yoshimasu H, Colchester AC, Kopelman MD. Retrograde amnesia in patients with hippocampal, medial temporal, temporal lobe, or frontal pathology. Learn Mem. 2006;13:545–557. doi: 10.1101/lm.265906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo JM, Courtney MG, Melrose RJ, Stern CE. Putamen hypertrophy in nondemented patients with human immunodeficiency virus infection and cognitive compromise. Arch Neurol. 2007;64:1275–1280. doi: 10.1001/archneur.64.9.1275. [DOI] [PubMed] [Google Scholar]
- Cermak L. The episodic-semantic distinction in amnesia. In: Squire LR, Butters N, editors. Neuropsychology of Memory. Guilford Press; New York: 1984. pp. 55–62. [Google Scholar]
- Cermak LS. Models of memory loss in Korsakoff and alcoholic patients. In: Parsons OA, Butters N, Nathan PE, editors. Neuropsychology of Alcoholism. Guilford Press; New York: 1987. pp. 207–226. [Google Scholar]
- Chang L, Ernst T, Tornatore C, Aronow H, Melchor R, Walot I, Singer E, Cornford M. Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology. 1997;48:836–845. doi: 10.1212/wnl.48.4.836. [DOI] [PubMed] [Google Scholar]
- Conigliaro J, Justice AC, Gordon AJ, Bryant K. Role of alcohol in determining human immunodeficiency virus (HIV)-relevant outcomes: A conceptual model to guide the implementation of evidence-based interventions into practice. Med Care. 2006;44:S1–S6. doi: 10.1097/01.mlr.0000223659.36369.cf. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Fama R, Marsh L, Sullivan EV. Dissociation of remote and anterograde memory impairment and neural correlates in alcoholic Korsakoff syndrome. J Int Neuropsychol Soc. 2004a;10:427–441. doi: 10.1017/S135561770410310X. [DOI] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: Contributions from explicit memory, executive function, and age. Alcohol Clin Exp Res. 2004b;28:1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Nichols BN, Pfefferbaum A, Sullivan EV. Working and episodic memory in HIV infection, alcoholism, and their comorbidity: baseline and 1-year follow-up examinations. Alcohol Clin Exp Res. 2009;33:1815–1824. doi: 10.1111/j.1530-0277.2009.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Sullivan EV, Shear PK, Cahn D, Marsh L, Lim KO, Yesavage JA, Tinklenberg JR, Pfefferbaum A. Structural brain correlates of verbal and nonverbal fluency measures in Alzheimer’s disease. Neuropsychology. 2000a;14:29–40. [PubMed] [Google Scholar]
- Fama R, Sullivan EV, Shear PK, Stein M, Yesavage JA, Tinklenberg JR, Pfefferbaum A. Extent, pattern, and correlates of remote memory impairment in Alzheimer’s disease and Parkinson’s disease. Neuropsychology. 2000b;14:265–276. doi: 10.1037//0894-4105.14.2.265. [DOI] [PubMed] [Google Scholar]
- Farinpour R, Martin EM, Seidenberg M, Pitrak DL, Pursell KJ, Mullane KM, Novak RM, Harrow M. Verbal working memory in HIV-seropositive drug users. J Int Neuropsychol Soc. 2000;6:548–555. doi: 10.1017/s1355617700655042. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1998. [Google Scholar]
- Gabrieli JD. Cognitive neuroscience of human memory. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol Rev. 2009;19:186–203. doi: 10.1007/s11065-009-9095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JE, Saveanu RV, Bornstein RA. The effect of previous alcohol abuse on cognitive function in HIV infection. Am J Psychiatry. 2004;161:249–254. doi: 10.1176/appi.ajp.161.2.249. [DOI] [PubMed] [Google Scholar]
- Hamsher K, Roberts RJ. Memory for recent U.S. presidents in patients with cerebral disease. J Clin Exp Neuropsychol. 1985;7:1–13. doi: 10.1080/01688638508401238. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57:101–110. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Heaton R, Grant I, Matthews C. In: Neuropsychological Assessment of Neuropsychiatric Disorders. Grant I, Adams K, editors. Oxford University Press; New York: 1986. pp. 110–120. [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, Wolfson T, Velin R, Marcotte TD, Hesselink JR, Jernigan TL, Chandler J, Wallace M, Abramson I. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer’s, Huntington’s, and Parkinson’s disease patients. J Neurosci. 1989;9:582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SJ, Shuttleworth EC, Freidenberg DL. Neuropsychological differences between the dementias of Alzheimer’s disease and Parkinson’s disease. Arch Neurol. 1989;46:1287–1291. doi: 10.1001/archneur.1989.00520480029015. [DOI] [PubMed] [Google Scholar]
- Karnofsky DA. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. Columbia University Press; New York: 1949. pp. 191–205. [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Remote and autobiographical memory, temporal context memory and frontal atrophy in Korsakoff and Alzheimer patients. Neuropsychologia. 1989;27:437–460. doi: 10.1016/0028-3932(89)90050-x. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Frontal dysfunction and memory deficits in the alcoholic Korsakoff Syndrome and Alzheimer-type dementia. Brain. 1991;114:117–137. [PubMed] [Google Scholar]
- Kopelman MD. The neuropsychology of remote memory. In: Squire L, Gainotti G, editors. Handbook of Neuropsychology. Vol. 3. Elsevier; New York: 1993. pp. 215–238. [Google Scholar]
- Kopelman MD, Bright P, Fulker H, Hinton N, Morrison A, Verfaellie M. Remote semantic memory in patients with Korsakoff’s syndrome and herpes encephalitis. Neuropsychology. 2009;23:144–157. doi: 10.1037/a0014447. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of the alcoholic brain. Acta Neuropathologica. 1989;79:200–204. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. Oxford University Press; New York: 2004. [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangels JA, Gershberg FB, Shimamura AP, Knight RT. Impaired retrieval from remote memory in patients with frontal lobe damage. Neuropsychology. 1996;10:32–41. [Google Scholar]
- Marslen-Wilson WD, Teuber HL. Memory for remote events in anterograde amnesia: recognition of public figures from newsphotographs. Neuropsychologia. 1975;13:353–364. doi: 10.1016/0028-3932(75)90013-5. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Rains N, Grbesic S, Pursell K, Nunnally G, Bechara A. Delayed nonmatch-to-sample performance in HIV-seropositive and HIV-seronegative polydrug abusers. Neuropsychology. 2003;17:283–288. doi: 10.1037/0894-4105.17.2.283. [DOI] [PubMed] [Google Scholar]
- Martin EM, Sullivan TS, Reed RA, Fletcher TA, Pitrak DL, Weddington W, Harrow M. Auditory working memory in HIV-1 infection. J Int Neuropsychol Soc. 2001;7:20–26. doi: 10.1017/s1355617701711022. [DOI] [PubMed] [Google Scholar]
- Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addict Biol. 2003;8:33–37. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART) Nelson Publishing Company; Windsor, Canada: 1982. [Google Scholar]
- Newcombe F. Missile Wounds of the Brain. Oxford University Press; London: 1969. [Google Scholar]
- Nixon SJ, Kujawski A, Parsons OA, Yohman JR. Semantic (verbal) and figural memory impairment in alcoholics. J Clin Exp Neuropsychol. 1987;9:311–322. doi: 10.1080/01688638708405053. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Learning and memory deficits in detoxified alcoholics. NIDA Res Monogr. 1990;101:136–155. [PubMed] [Google Scholar]
- Peavy G, Jacobs D, Salmon DP, Butters N, Delis DC, Taylor M, Massman P, Stout JC, Heindel WC, Kirson D, Atkinson JH, Chandler JL, Grant I. Verbal memory performance of patients with human immunodeficiency virus infection: evidence of subcortical dysfunction. The HNRC Group. J Clin Exp Neuropsychol. 1994;16:508–523. doi: 10.1080/01688639408402662. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV. Frontostriatal fiber bundle compromise in HIV infection without dementia. AIDS. 2009;23:1977–1985. doi: 10.1097/QAD.0b013e32832e77fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Piolino P, Lamidey V, Desgranges B, Eustache F. The semantic and episodic subcomponents of famous person knowledge: dissociation in healthy subjects. Neuropsychology. 2007;21:122–135. doi: 10.1037/0894-4105.21.1.122. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Aupée AM, Chételat G, Mézenge F, Beaunieux H, de la Sayette V, Viader F, Baron JC, Eustache F, Desgranges B. Morphological and glucose metabolism abnormalities in alcoholic Korsakoff’s syndrome: group comparisons and individual analyses. PLoS One. 2009;4:e7748. doi: 10.1371/journal.pone.0007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- Roberts RJ, Hamsher KD, Bayless JD, Lee GP. Presidents Test performance in varieties of diffuse and unilateral cerebral disease. J Clin Exp Neuropsychol. 1990;12:195–208. doi: 10.1080/01688639008400967. [DOI] [PubMed] [Google Scholar]
- Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, Weiner MW. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11:70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek JR, Johnson SA, White DA, Salmon DP, Taylor KI, Delapena JH, Paulsen JS, Heaton RK, Grant I. Retrograde amnesia in dementia: comparison of HIV-associated dementia, Alzheimer’s disease, and Huntington’s disease. Neuropsychology. 2004;18:692–699. doi: 10.1037/0894-4105.18.4.692. [DOI] [PubMed] [Google Scholar]
- Sagar HJ, Cohen NJ, Sullivan EV, Corkin S, Growdon JH. Remote memory function in Alzheimer’s disease and Parkinson’s disease. Brain. 1988;111:185–206. doi: 10.1093/brain/111.1.185. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Sanders HI, Warrington EK. Memory for remote events in amnesic patients. Brain. 1971;94:661–668. doi: 10.1093/brain/94.4.661. [DOI] [PubMed] [Google Scholar]
- Sassoon SA, Fama R, Rosenbloom MJ, O’Reilly A, Pfefferbaum A, Sullivan EV. Component cognitive and motor processes of the digit symbol test: differential deficits in alcoholism, HIV infection, and their comorbidity. Alcohol Clin Exp Res. 2007;31:1315–1324. doi: 10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Benson DF. The temporal pattern of retrograde amnesia in Korsakoff’s disease. Neurology. 1974;24:527–530. doi: 10.1212/wnl.24.6.527. [DOI] [PubMed] [Google Scholar]
- Skinner HA. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Addiction Research Foundation; Toronto, Canada: 1982. [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices: The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Squire LR, Cohen NJ. Remote memory, retrograde amnesia and the neuropsychology of memory. In: Cermak LS, editor. Human Memory and Amnesia. Lawrence Erlbaum; Hillsdale, NJ: 1982. pp. 275–303. [Google Scholar]
- Squire LR, Cohen NJ. Human memory and amnesia. In: Lynch G, McGaugh JL, Weinberger NM, editors. Psychobiology of Learning and Memory. Guilford Press; New York: 1984. [Google Scholar]
- Squire LR, Zola SM. Amnesia, memory and brain systems. Philos Trans R Soc Lond B Biol Sci. 1997;352:1663–1673. doi: 10.1098/rstb.1997.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stout JC, Salmon DP, Butters N, Taylor M, Peavy G, Heindel WC, Delis DC, Ryan L, Atkinson JH, Chandler JL, Grant I, Velin RA, Oldfield EC, Wallace MR, Malone J, McCutchan JA, Spector SA, Thal L, Heaton RK, Hesselink J, Jernigan T, Wiley CA, Olshen R, Abramson I, Dupont R, Patterson T, Zisook S, Jeste D, Sieberg H, Weinrich JD. Decline in working memory associated with HIV infection. Psychol Med. 1995;25:1221–1232. doi: 10.1017/s0033291700033195. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2009;44:155–165. doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tross S, Price R, Navia B, Thaler H, Gold J, Hirsch DA, Sidtis J. Neuropsychological characterization of the AIDS dementia complex: a preliminary report. AIDS. 1988;2:81–88. doi: 10.1097/00002030-198804000-00002. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG. Functional brain imaging studies of cortical mechanisms for memory. Science. 1995;270:769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Reiss L, Roth HL. Knowledge of New English vocabulary in amnesia: an examination of premorbidly acquired semantic memory. J Int Neuropsychol Soc. 1995;1:443–453. doi: 10.1017/s1355617700000540. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Sanders HI. The fate of old memories. Q J Exp Psychol. 1971;23:432–442. [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Revised. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Westmacott R, Moscovitch M. The contribution of autobiographical significance to semantic memory. Mem Cognit. 2003;31:761–774. doi: 10.3758/bf03196114. [DOI] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]