Abstract
Priapism is a debilitating disease for which there is at present no clinically accepted pharmacologic intervention. It has been estimated that priapism lasting more than 24 hours in patients is associated with a 44–90% rate of erectile dysfunction (ED). In this investigation we determined in two animal models of priapism (opiorpin-induced priapism in the rat and priapism in a mouse model of sickle cell disease) if there is evidence for an increase in markers of oxidative stress in corporal tissue. In both animal models we demonstrate that priapism results in increased levels of lipid peroxidation, glutathione S-transferase activity, and oxidatively damaged proteins in corporal tissue. Using Western blot analysis we demonstrated there is up regulation of the ubiquitination ligase proteins, Nedd-4 and Mdm-2, and the lysososomal autophage protein, LC3. The anti-apoptotic protein, Bcl-2, was also up regulated. Overall, we demonstrate that priapism is associated with increased oxidative stress in corporal tissue and the activation of protein degradation pathways. Since oxidative stress is known to mediate the development of ED resulting from several etiologies (for example ED resulting from diabetes and aging) we suggest that damage to erectile tissue resulting from priapism might be prevented by treatments targeting oxidative stress.
Keywords: Priapism, sickle cell disease, oxidative stress, Nedd-4, Mdm-2, LC3, Bcl-2
Introduction
Priapism is a persistent, often painful, penile erection that continues hours beyond, or is unrelated to, sexual stimulation 1. It can lead to progressive fibrosis of the erectile tissue and ultimately result in erectile dysfunction (ED) 2. It has been estimated that priapism lasting more than 24 hours is associated with a 44–90% rate of ED 3, 4. Priapism can be associated with the use of pharmacological agents, particularly antipsychotics 5, recreational drugs, hematological disorders, metabolic disorders, trauma, tumors, neurological disorders and bites from spiders and scorpions 6. Men with sickle cell disease are particularly prone to priapism, where its incidence is about 40% 7, 8. We have demonstrated that in animal models over-expression of the genes encoding opiorphins (rat Vcsa1 or human hSMR3B) is associated with the development of priapism, and that there is up-regulation of opiorphins in the corpora of sickle cell mice prior to the development of priapism 9–11.
Recently there has been progress in understanding the molecular events leading to priapism. Endothelial nitric oxide synthase knock-out (eNOS−/−) mice exhibit priapic-like, excessive erectile tendencies 12. Later work demonstrated that in the corpora of the eNOS−/− mice there was reduced phospodiesterase-5 (PDE-5) expression 13. Following sexual stimulation, resulting in increased release of neuronal nitric oxide (NO), large amounts of cyclic guanosine monophosphate (cGMP) were produced because of the lowered levels of PDE-5, resulting in excessive corporal smooth muscle tissue relaxation. It has also been reported that mice lacking adenosine deaminase (ADA), an enzyme necessary for the breakdown of adenosine, display unexpected priapic activity 14. Treatment of these mice with ADA enzyme therapy corrected the priapic-like activity suggesting that it was dependent on elevated adenosine levels. Further genetic and pharmacologic evidence demonstrated that A2B adenosine receptor-mediated (A2BR-mediated) cyclic adenosine monophosphate (cAMP) and cGMP induction was required for elevated adenosine-induced prolonged penile erection. In sickle cell mice there was also elevated adenosine levels and A2BR activation associated with the development of priapism. Our group recently demonstrated that over-expression of opiorphins in corporal tissue can result in priapism through a mechanism which involves activation of polyamine synthesis 9.
Men with sickle cell disease are at greater risk for the development of priapism 7, 8. Sickle cell mouse models have suggested that oxidative stress, resulting from hemolysis is a major component for the development of vasculopathies 15–17. Hemolysis results in the release of hemoglobin into the plasma where it can react and deplete levels of NO, as well as release of erythrocyte arginase. Abnormally high NO levels can result in the formation of reactive oxygen species (ROS) and arginine can redirect the metabolism of endothelial L-arginine to polyamines. Potentially both these processes can result in vessel wall remodeling 18, 19.
Several novel therapies for treating or preventing priapism are being investigated, such as the use of PDE-5 inhibitors, polyethylene glycol-modified ADA or inhibitors of ornithine decarboxylase 9, 20–22. However these treatments are as yet unproven in a clinical environment, and there remains an urgent need to better understand the molecular events that result in priapism in order to facilitate the development of better treatment regimens. There is increased awareness that oxidative stress in corporal tissue is a contributing factor to the development of ED 23–28, 29. Evliyaoglu et al. 30, 31 have previously demonstrated that there are increased levels of lipid peroxides in a veno-occlusive rat model of priapism. In the present paper we describe changes in additional markers of oxidative stress in corporal tissue from two additional animal models (opiorphin induced priapism in the rat and a mouse model of sickle cell disease). Because there is potential damage to proteins as a result of oxidative stress we also investigated changes in pathways involved in protein degradation.
Materials and Methods
Animals
The animals which were used in these experiments have been described and characterized in a recent publication 9. Sprague-Dawley “retired breeder” rats (9–10 months, weighing >500 g) were obtained from Charles River Laboratories (Wilmington, MA, USA). Six animals were intracorporally injected with 100 µg pVAX-Vcsa1 or pVAX was previously described 9. Animals were invesigated one week following intracorpoal injection of plasmids. Prior to sacrifice the basal (no cavernous nerve stimulation) intracorporal pressure/blood pressure ratio was determined as previously described 10, 11, 32. Transgenic, 5 week old, sickle cell mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained in the Albert Einstein Animal Facility till the life stage to be investigated (5, 10–12 and 12–14 weeks old) was attained. Age matched C57BL/6 animals were used as controls. Six animals were investigated in each group. Immediately following sacrifice corporal tissue (in the flaccid state) was excised and stored at −70C before biochemical determintions were performed. All animal studies were approved by the Institute of Animal Studies at Albert Einstein College of Medicine.
Glutathione S-Transferase assay
To perform assays for the activity of glutathione S-transferase (GST) coporal tissue homogenates were prepared in 100mM Phosphate buffer pH-6.5 containing 2mM Ethylene diaminetetracetic acid (EDTA) at 4°C. The supernatants were separated by centrifugation using a Sorvall Centrifuge (Thermo Fisher Scientific, Waltham, MA) at 15,000×g for 30 min. The protein concentration was determined in the tissues extracts by the method of Biorad - Dc protein assay method (Biorad-laboratories, Hercules, CA). The GST assay was carried out by the method of Habig et al. 33. To 900 µl of phosphate buffer pH-6.5, 33.3 µl of 75mM reduced glutathione (Sigma, St.Louis, MO), 33.3 µl of 30mM 1 Chlroro, 2,4-Dinitrobenzene (Sigma, St.Louis, MO) in ethanol and 33.3 µl of tissue homogenates were added. The colored product formed was read at 340nm using Beckman Spectophotometer for 5 min. The blank reaction was carried out without the enzyme. The GST activity was expressed as nmoles/milligram protein.
Lipid Peroxidation
In order to determine lipid peroxidation corporal tissue homogenates were prepared in 1.15% potassium chloride. The supernatants were separated by centrifugation at 15,000×g for 30 min using an Sorvall centrifuge. The lipid peroxidation assay was carried out by the method of Ohkawa et al. (18). 1,1,3,3,- Tetramethoxy propane (Sigma, St.Louis, MO) was used as an external standard ranging from 2nM to 10nM for the standard curve. To the standards and 0.5ml of rat and mice tissue homogenates, 200 µl of 8% sodium dodecyl sulphate (SDS), 1.5 ml of 0.8% Thiobarbituric acid (Sigma, St.Louis, MO) and 20% glacial acetic acid pH-3.5 was added and boiled at 95°C for an hour. The samples were cooled and the lipid peroxides (LPO) were extracted in 15:1 ratio of n-Butnaol and Pyridine solvent (Sigma, St.Louis, MO) by centrifugation at 4000×g for 15min. The LPO levels were measured at 532 nm by Beckman Spectrophotometer. The LPO were expressed as nanomoles of malondialdehyde (MDA) formed /gram tissue.
Preparation of Protein Extracts and Western blot analysis
The expression of proteins in corporal tissue was analyzed using Western blots. Protein extractions were carried at 4°C by the method of Kanika et al. 9. Briefly, gene treated rat and transgenic sickle mice corpora tissues were homogenized using a polytron homogenizer in 50mM Tris-HCl buffer, pH-7.4, containing 10mM EDTA, 30mM sucrose and 10µl of mammalian protease inhibitor cocktail (Sigma, St.Louis, MO, USA) and the supernatants were separated by centrifugation at 15,000×g for 30 min. Protein concentrations were determined in the samples by the Biorad-Dc protein assay method.The proteins were electrophoresed on Nu PAGE® 10% Bis-Tris gels (Invitrogen, Carlsbad, CA) and then transferred to a PVDF membrane (Immun-Blot™ PVDF Membrane, Bio-Rad Laboratories, Hercules, CA) by semi-dry electrobloting for 1hr. The membranes were blocked with 5% milk in Tris Buffered saline containing 0.05% Tween-20 (TBST) for an hour, the membranes were probed with anti LC3B antibody (1:1000), anti Nedd-4 (1:5000), Mdm-2 (1:6000) (Millipore, Billerica, MA), Nitrotyrosine (NOY-7A5, Alexis Biochemicals, Farmingdale, NY), anti GAPDH (1:20,000; Abcam, Cambridge, MA), anti α-actin and Bcl-2 (1:500) antibodies (BD Biosciences, San Jose, CA) for an hour at room temperature. The bound antibodies were detected by probing with HRP-labelled anti-mouse or anti-rabbit secondary antibody (1;10000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for an hour at room temperature. Enhanced chemiluminescence was performed with Pierce® ECL Western Blotting Substrate (Pierce, Rockford, IL.) and bands were quantified by densitometry using Quantity One® software (Bio-Rad laboratories, Hercules, CA).
OxyBlot Analysis
The protein oxidation levels were determined using Oxyblot™ Protein Oxidation Detection Kit (Chemicon International, Temecula, CA) following the manufacturer’s protocol. Corporal tissue extracts were prepared in protein extraction buffer containing 50mM dithiothreitol at 4°C and the supernatants separated by centrifugation at 15,000×g for 30min. To 5 µl (15 µg) of protein extract, 5 µl of 12% SDS and 10 µl of dinitrophenylhydrazine derivatizing agents (referred to as “exp” in Figure 2C) are added and incubated at room temperature for 15min. In a control reaction (referred to as “con” in Figure 2C) proteins were prepared in the absence of Dinitrophenylhydrazine derivatizing agents. The reaction is terminated by the addition of 7.5 µl of Neutralization buffer supplied with the kit. The OxyBlot standard was loaded with 2.5 µl of SDS-sample buffer. The carbonyl derivatized samples were loaded on Nu PAGE® 10% Bis-Tris gels (Invitrogen, Carlsbad, CA) and then transferred on to a PVDF membrane (Immun-Blot™ PVDF Membrane, Bio-Rad Laboratories, Hercules, CA) by semi-dry electroblotting for 1hr. After the transfer membranes were blocked with 1% bovine serum albumin in TBST for an hour at room temperature. The membranes were probed with primary antibody (1:150) for an hour and with secondary antibody (1:300) for an hour at room temperature. Enhanced chemiluminiscence was performed with Pierce® ECL Western Blotting Substrate (Pierce, Rockford, IL) and bands were quantified by densitometry using Quantity One® software (Bio-Rad laboratories, Hercules, CA).
Fig. 2.
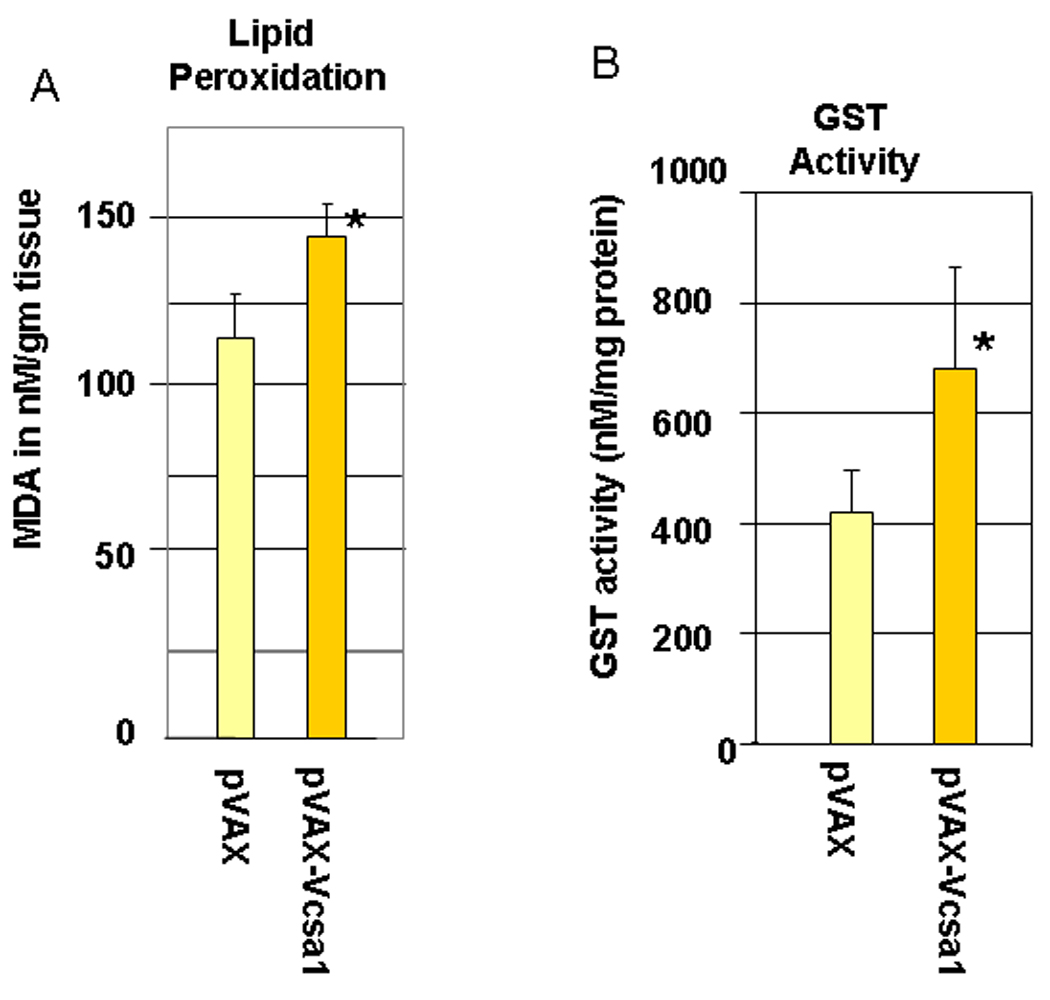
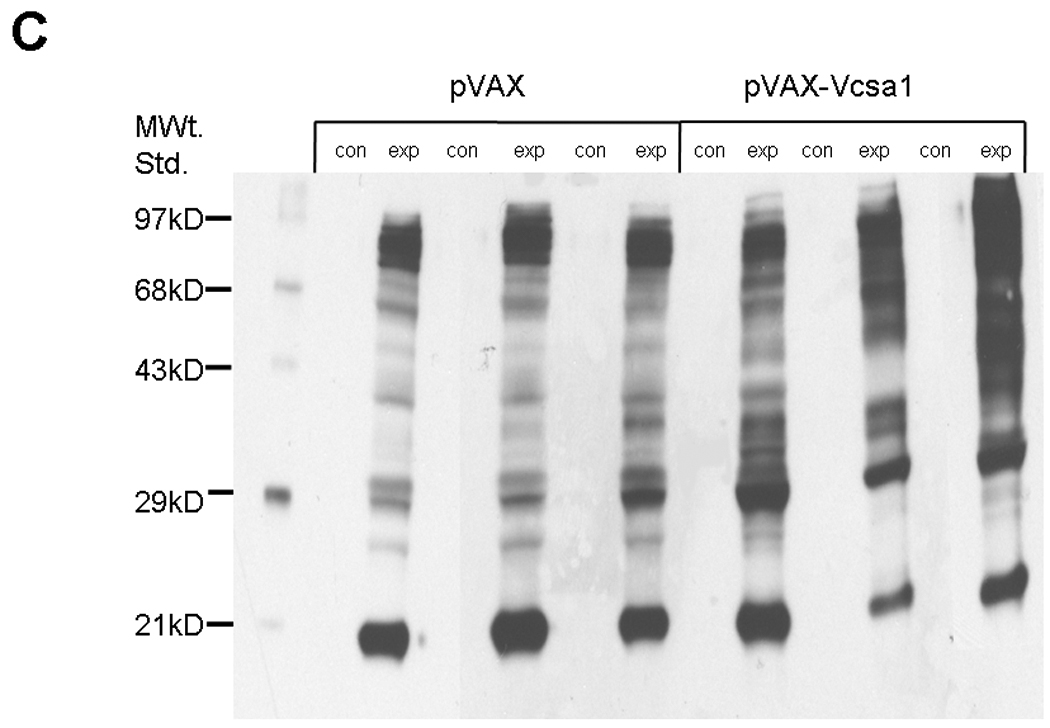
2A. Lipid peroxidation was measured by determining malondialdehyde (MDA) in coporal tissues of 6 rats with pVAX-Vcsa1 induced priapism and in 6 control animals treated with pVAX. * =significant difference from control (P<0.05). 2B. Glutathione S- transferase (GST) activity was also measured in the same animals. * =significant difference from control (P<0.05). 2C. A representative oxyblot for isolated proteins from the corpora of 3 control (pVAX-treated) and 3 opiorphin–induced (PVAX-Vcsa1 treated) priapic rats. Loading was normalized using a protein assay prior to loading. The “con” lanes are protein samples that were not derivitized with dinitrophenylhydrazine, and act as negative controls for the assay of samples “exp” that did undergo dinitrophenylhydrazine derivitization.
Statistical Analysis
The Student's t-test was used to determine significant difference in expression between experiment and control groups. A P-value < 0.05 was considered to be a significant change in expression.
Results
As previously described 9–11, 32, Sprague-Dawley rats intracorporally injected with pVAX-Vcsa1, which over-expresses the rat opiorphin homologue (sialorphin) presented evidence of a priapic-like episode one week after treatment. This evidence consists of increased basal intracorporal pressure/blood pressure ratio (ICP/BP) (Figure 1A) and visual and histological evidence of vasocongestion at necropsy (Figure 1B). The empty vector backbone, 100 µg pVAX, was used as a control and 100 µg pVAX-hSlo was used as a control for a plasmid which improves erectile function in the aging rat model without resulting in experimental priapism 34, 35.
Fig. 1.
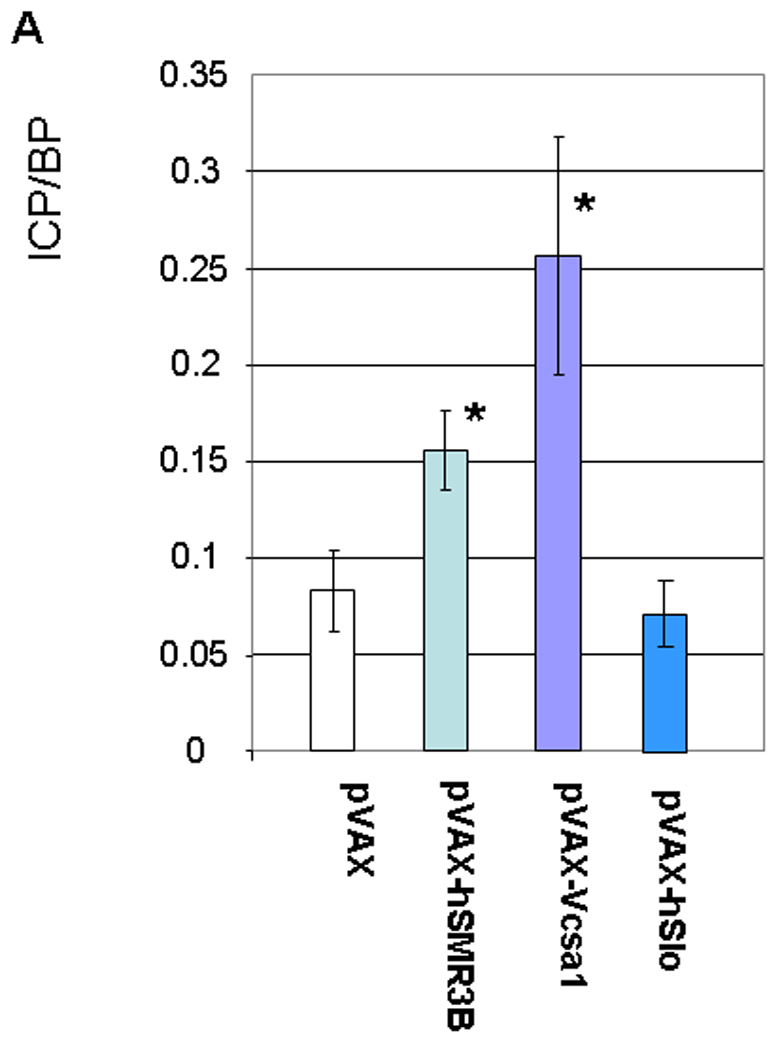
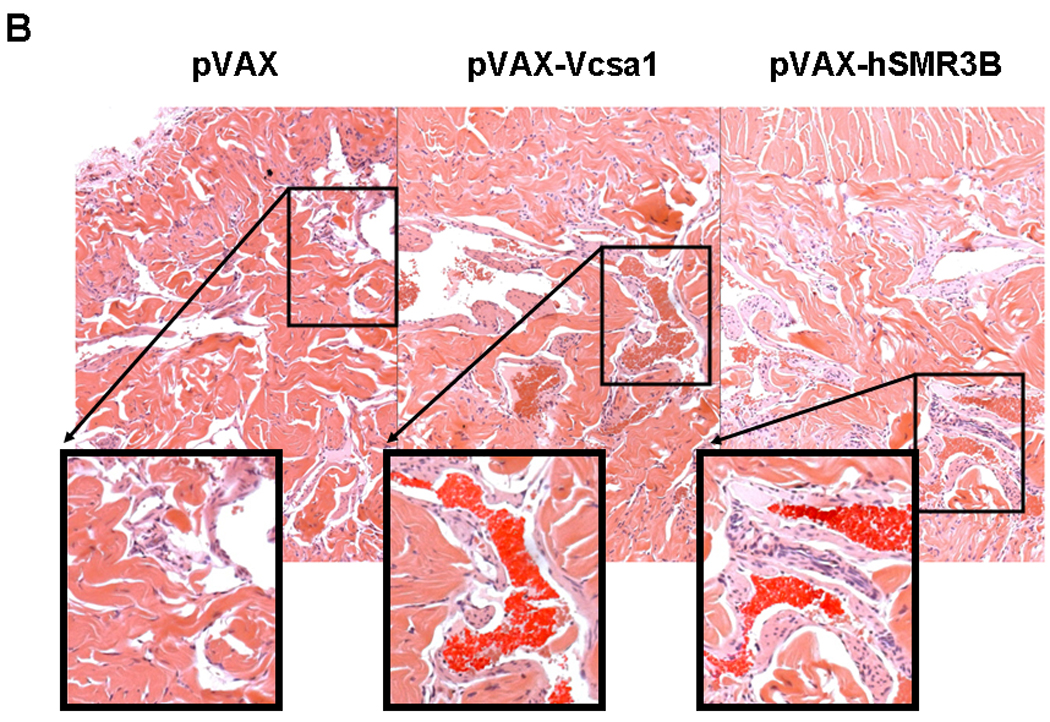
1A. (Used with permission from the American Journal of Physiology, Kanika et al. 9) Basal intacorporal pressure/blood pressure (ICP/BP) ratio one week after injection of 100 µg of plasmid (pVAX, pVAX-Vcsa1, pVAX-hSMR3B or pVAX-hSlo). The bar represents the mean basal ICP/BP from 5 animals in each group, and the error bars the standard error of the mean (SEM). * = significantly different basal ICP/BP from negative control, pVAX (Students t-test, P<0.05). 1B. Representative sections through the corpora cavernosum of rat tissue, stained with hematoxylin and eosin (HE stain). Left panel, animals treated with 100 µg pVAX (control), middle panel 100 µg pVAX-Vcsa1 and right panel 100 µg pVAX-hSMR3B. Sections of each panel are enlarged, and the presence of blood cells within the vascular spaces enhanced to demonstrate vasocongestion.
In order to determine oxidative stress levels in the corpora of animals with opiorphin induced priapism we measured the level of lipid peroxidation, GST activity and carbonyl groups introduced into proteins using an OxyBlot (Figure 2A–C). As shown in Figure 2A lipid peroxidation, as determined by the MDA levels in the corporal tissue, significantly increased (an average of 25%, P<0.05) in animals with opiorphin-induced priapism. Another indicator of oxidative stress, GST activity, was also up-regulated (by an average of 63% P<0.05) in the corpora of animals with priapism (Figure 2B).
Protein extracts from six animals of each group were also analyzed using an OxyBlot for the detection of carbonyl groups introduced into proteins by oxidative reactions. A representative blot from 3 animals for the control and priapic animals are shown in Figure 2C. Densitometric analysis of animals treated with pVAX-Vcsa1 have overall up-regulated levels of carbonyl groups (by an average of 21%, P<0.05) compared to controls, as well as a distinctly different banding pattern compared to controls. This suggests that with the onset of priapism there is not only an increase in oxidative stress, but also that a different set of proteins are targeted for nitrosylation.
In an environment where there is increased oxidative stress, there is likely to be increased oxidative damage of proteins. Therefore, using western analysis we also looked at the expression of proteins in the corpora which are involved in pathways leading to degradation of damaged proteins one week after intracorporal injection with 100 µg pVAX or pVAX-Vcsa1. The lysosomal autophage pathway for protein degradation was analyzed by comparing the ratio of short form LC3-II to long LC3-I, which can serve as a marker for autophagy (Figure 3 and Table 1). Figure 3 shows a representative blot from three animals, and densitometric analysis averaged from six animals is shown in Table 1. There is a 5.45-fold (P<0.05) increase in the level of LC3-II in the pVAX-Vcsa1 treated animals compared to the controls, and the ratio of LC3-I to LC3-II decreases from 14.6 ± 1.3 to 0.29 ± 0.34. The decrease in the ratio of LC3-I/LC3-II in the corpora of animals treated with pVAX-Vcsa1 suggests an increase in autophagic activity, potentially as a result of tissue damage resulting from priapism 36, 37.
Fig. 3.
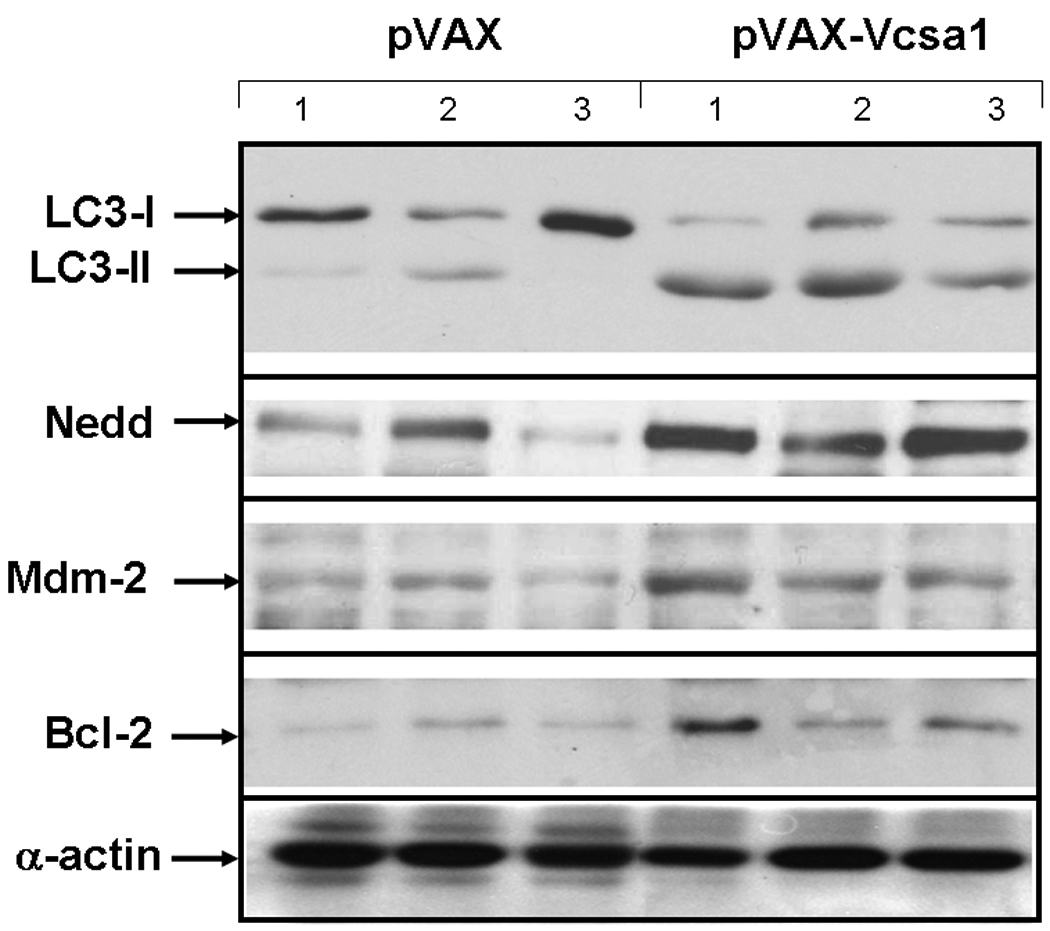
Representative blot for analysis of proteins involved in protein lysosomal autophagy (LC3), ubiquination (Nedd-4 and Mdm-2) and apoptosis (Bcl-2). Proteins were extracted from the corpora of three animals one week following intracorporal injection with 100 µg of pVAX- or pVAX-Vcsa1. Loading was normalized using a protein assay prior to loading.
Table 1. Densitometric analysis of proteins involved in protein degradation in the corpora of rats with opiorphin induced priapism.
Densitometry was performed following western blot analysis of protein extracts from the corpora of 6 rats with pVAX-Vcsa1 induced priapism or 6 control animals treated with pVAX. The fold change in expression in pVAX-Vcsa1 treated animals was compared to control (pVAX-treated) ± standard deviation.
| Protein | Fold Change Compared to Control (± standard deviation) |
|---|---|
| LC3B-I | 2.92 ± 0.32* |
| LC3B-II | 5.45 ± 0.2* |
| Nedd-4 | 2.55 ± 0.009* |
| Mdm-2 | 2.38 ± 0.2* |
| Bcl-2 | 1.45 ± 0.01* |
Significant change in the expression of protein in animals treated with plasmids expressing opiorphin compared with controls (Student's t-test, P < 0.05).
Also shown in Figure 3 and Table 1 are the expression levels of two proteins involved in ubiquitination (Nedd-4 and Mdm-2 38, 39). Both are increased in response to pVAX-Vcsa1 induced priapism. By densitometric analysis both Nedd-4 and Mdm-2 increased significantly (P<0.05) by 6.27 and 2.38-fold in the pVAX-Vcsa1 treated animals compared to the control animals (pVAX treated, Table 1). In contrast the anti-apoptotic protein, Bcl-2, is up-regulated in pVAX-Vcsa1 treated animals. Overall, our results show that pVAX-Vcsa1 induced-experimental priapism is accompanied by changes in the pathways of protein degradation in corporal tissue.
Sickle cell mice are prone to the development of a priapic-like condition 40 and we have previously investigated the biochemical changes that occur in the corpora of sickle cell mice 9. We divided the development of priapism in the sickle cell mice into three stages based on the evidence presented in Figure 4. Three life stages of the mice were studied; 1) Pre-priapic mice (approximately 5 weeks of age): sickle cell mice were not observed to have any visual evidence of priapism. Histopathology of the corpora of these mice showed no evidence of vasocongestion (Figure 4A left panel). 2) Priapic mice (approximately 10–12 weeks of age): mice were visually observed to have priapism (a prolonged erection without sexual stimulation). Histopathology of these mice showed evidence of vascongestion, but presented no evidence of necrosis of the corporal tissue (Figure 4A, middle panel). 3). Late stage priapic-mice (12–14 weeks of age): Mice at necropsy had visible signs of necrosis of corporal tissue. Histopathology of the corporal tissue from these animals had more dilated spaces with vasocongestion as well as evidence of periprostatic inflammation and mild edema associated with neutrophilia (Figure 4A, right panel). Trichrome staining of late stage-priapic mice showed a significant increase in the fibrosis scale (the ratio of collagen to smooth muscle) compared to the pre-priapic or priapic mice (Figure 4B). Age matched control animals presented no evidence of vasocongestion or inflammation.
Fig. 4.
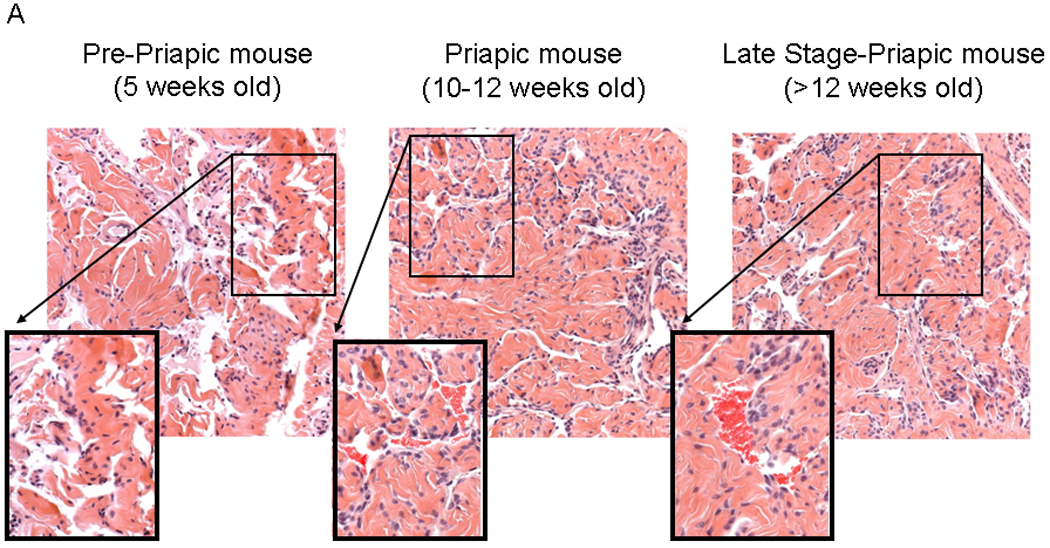
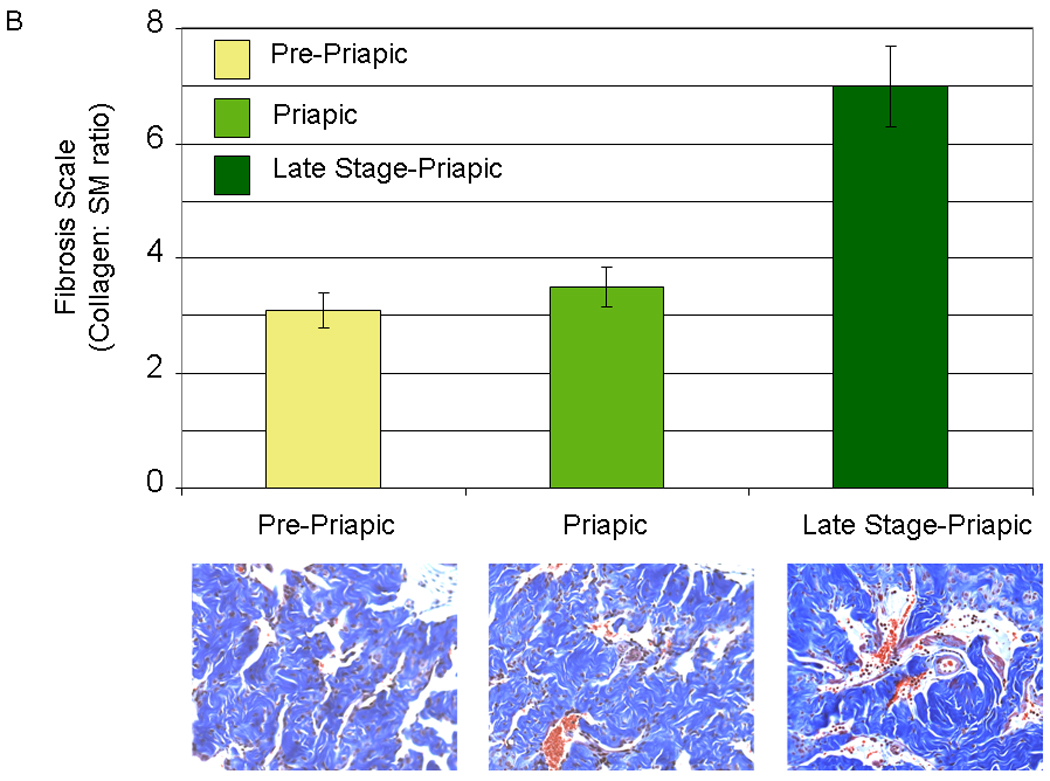
4A. (Used with permission from the American Journal of Physiology, Kanika et al. 9) Histopathology demonstrating the difference between the different life stages of the sickle cell mice. Left panel- pre-priapic mice (Pre-P, there was no evidence of priapism in animals, and histopathology showed no evidence of vascongestion). Middle panel- priapic mice (P, animals were observed to have priapism, and histopathology demonstrates vasocongestion of the corporal tissue). Right panel- late stage-priapic mice (Late stage-P, animals had obvious signs of necrosis of the corporal tissue. Histopathology demonstrated evidence of enlarged spaces with vasocongestion and inflammation of coporal tissue). Sections of each panel are enlarged, and the presence of blood cells within the vascular spaces enhanced to highlight vasocongestion. 4B (Lower Panel) Representative trichrome staining of pene sections from pre-priapic, priapic and late stage priapic sickle cell mice. (Upper Panel) Bar graph demonstrating the ratio of collagen to smooth muscle in the different groups (N=3 animals per group). The late stage-priapic animals had a significant increase in the collagen to smooth muscle ratio compared to the pre-priapic and priapic mice (P<0.05).
We have determined the state of oxidative stress and the expression of key proteins in protein degradation in sickle cell mice. In Figure 5 we show that there are increased levels of lipid peroxidation in sickle cell mice compared to control animals (Figure 5A). The experiment was performed for 3 pooled animal samples (corporal tissue) from each group in duplicate. The increase in MDA levels (by an average of 68%, P<0.05) occurs at a life stage in the sickle cell mice before there is physiological or histological evidence of priapism. In priapism animals the MDA levels are increased by average of 89% (P<0.05). Similarly, there is an increase in GST activity by an average of 16% (P<0.05, Figure 5B) in pre-priapic animals. We also investigated the effect of increased oxidative stress on corporal proteins using an OxyBlot to detect carbonylated protein residues. A representative blot is shown in Figure 5C. Densitometric analysis of the OxyBlots demonstrated that in the corpora of sickle cell mice at life stages which exhibit priapism, there is a fold-increase in the number of carbonylated residues compared to the controls (pre-priapism, 1.24 ± 0.95 fold; priapism 2.70 ± 0.6 fold, late stage-priapism 1.14 ± 0.92 fold), also indicative of oxidative stress. In addition, we also determined levels of proteins with nitrotyrosine residues in control and sickle cell mice. Nitrosylation of proteins results from reaction of nitric oxide to generate peroxynitrite radicals. A representative blot is shown in Figure 5D. Densitometric analysis of blots showed that priapic and late stage-priapic animals had significantly higher levels of nitrotyrosine proteins (1.8 ± 0.14-fold and 2.9 ± 0.08-fold) compared to non-sickle cell controls.
Fig. 5.
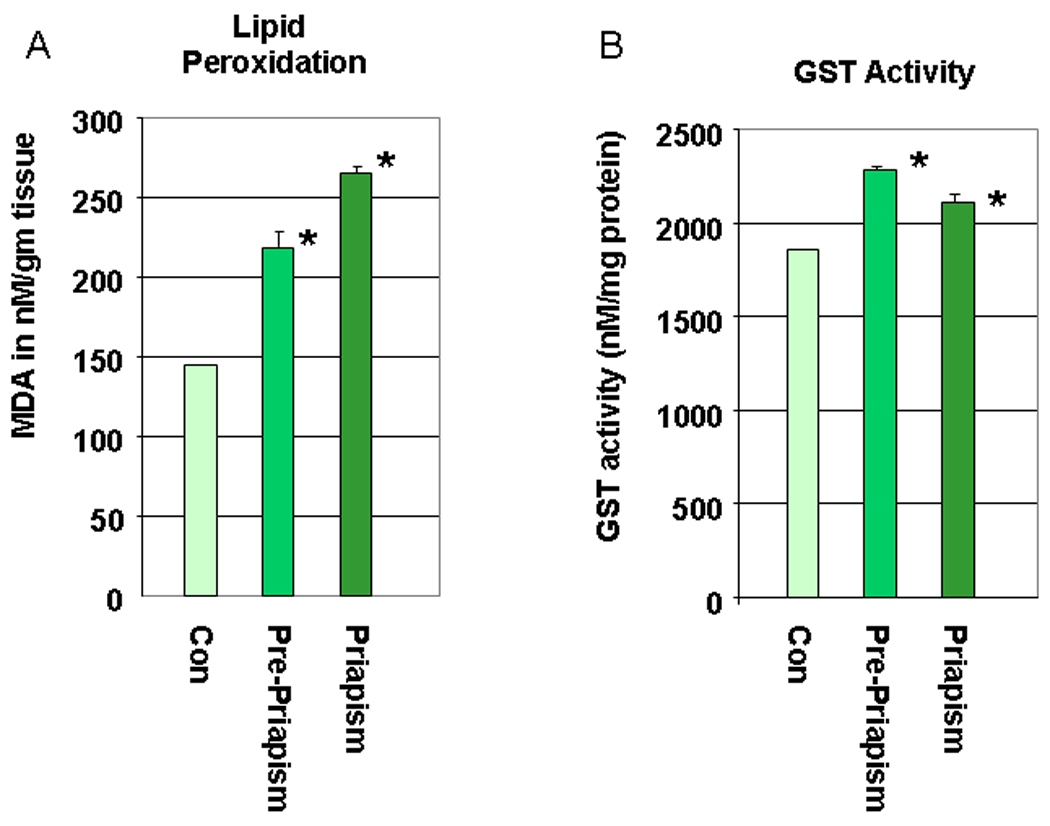
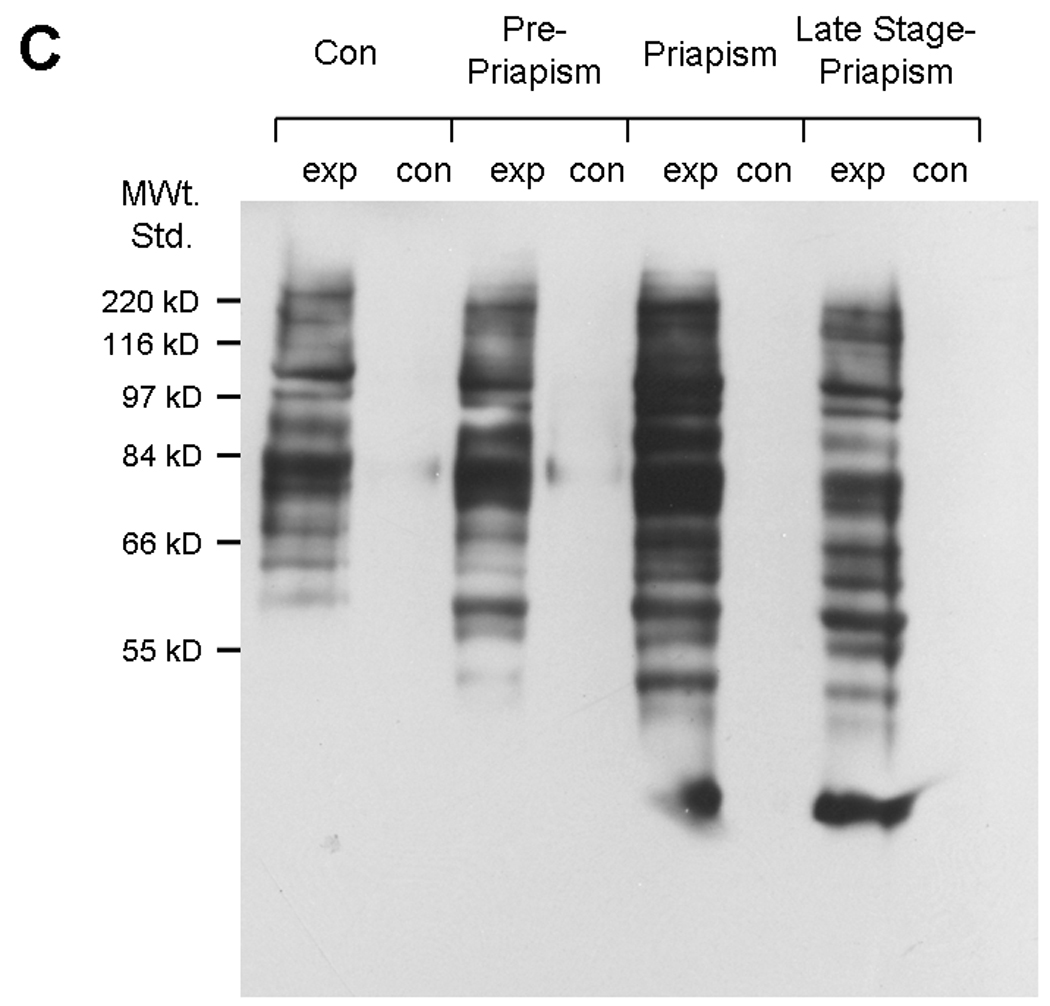
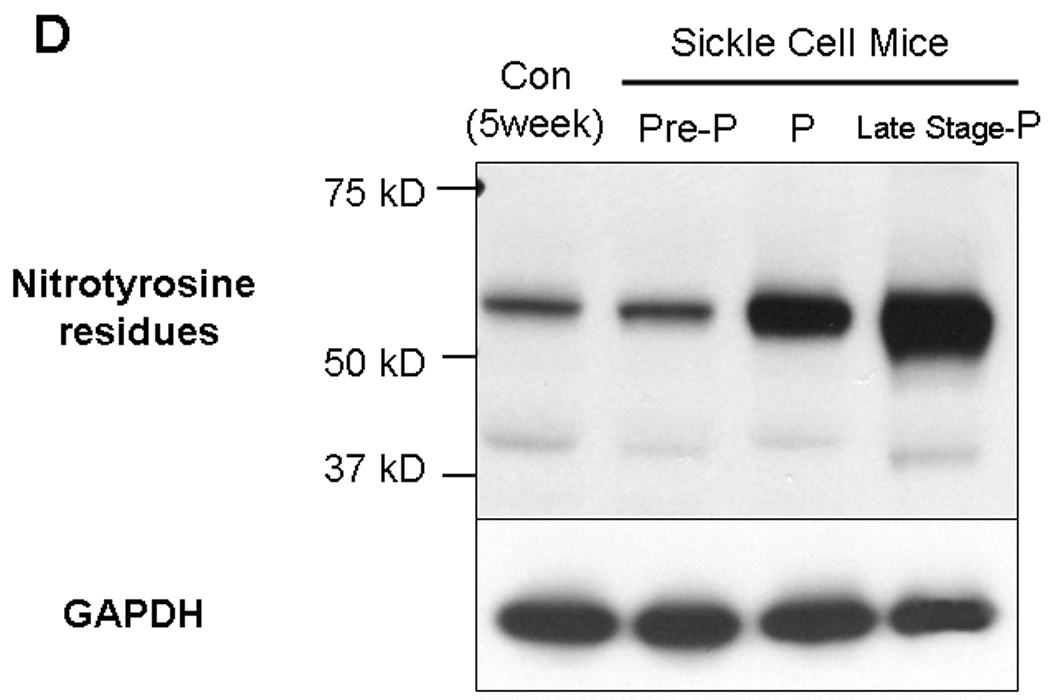
5A. Lipid peroxidation was measured by determining malondialdehyde (MDA) in coporal tissues of sickle mice at life stage before the onset of priapism (pre-priapism, with evidence of priapism and in the parent mouse line (C57BL/6). Measurements were performed on three animals in each group in duplicate. * =significant difference from control (P<0.05). 5B. Glutathione S-transferase (GST) activity was also measured in the same animals. * =significant difference from control (P<0.05). 5C. A representative oxyblot performed on protein extracts from the corpora of control mice, or sickle cell mice at different life stages. Protein loading was normalized using a protein assay prior to loading, and confirmed using ponceau staining after transfer to PVDF membranes. The “con” lanes are protein samples that were not derivitized with dinitrophenylhydrazine, and act as negative controls for the assay of samples “exp” that did undergo dinitrophenylhydrazine derivitization. 5D. Representative blot for the global analysis of nitrotyrosine residues in protein extracts from the corpora of control mice, or sickle cell mice at different life stages. Protein loading was normalized using a protein assay prior to loading, and confirmed using ponceau staining after transfer to PVDF membranes.
Using western analysis we also looked at the expression of proteins in the corpora of sickle cell mice which are involved in pathways leading to degradation of damaged proteins. As shown in Fig. 6 and Table 2 there is an increase in the expression of LC3-I (3.85 ± 0.2-fold in priapic and 4.56 ± 0.24-fold in late stage-priapic) and LC3-II (3.2 ± 0.68-fold in priapic and 1.1 ± 0.32-fold in late stage-priapic) in animals at life stages with priapism compared to controls, which may represent an increase in autophagic activity in response to corporal tissue damage from oxidative damage as a result of priapism. Pre-priapic animals did not show any significant differences in LC3-I or LC3-II expression compared to controls. Proteins involved in ubiquitination (Nedd-4 and Mdm-2, Fig. 6 and Table 2) are also increased in response to priapism in the corpora of sickle cell mice exhibiting priapism. Interestingly Nedd-4 is upregulated at a stage before there is evidence of priapism in the sickle cell mice. As with opiorphin-induced priapism, Bcl-2 is upregulated in the sickle cell mice at life stages which exhibit priapism or late stage priapism.
Fig. 6.
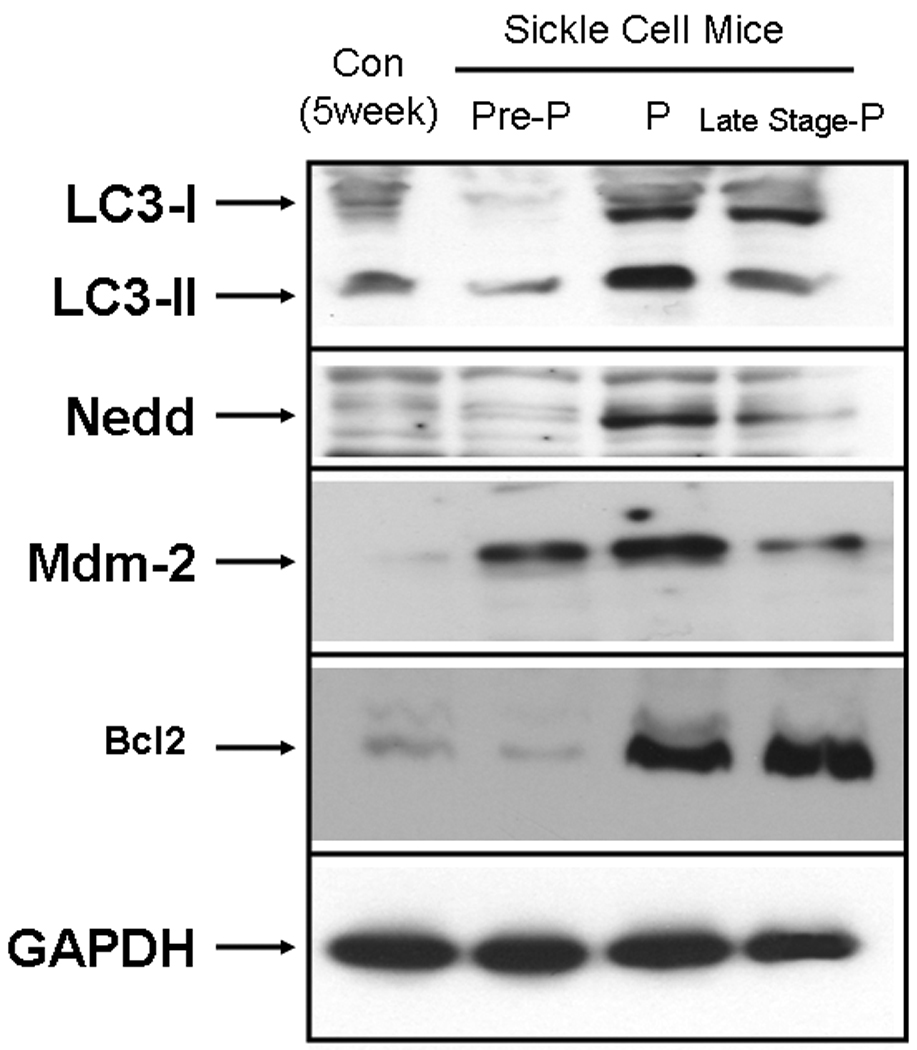
Representative blot for the global analysis of proteins involved in protein lysosomal autophagy (LC3) and ubiquination (Nedd-4 and Mdm-2). Proteins were extracted from the corpora of control or sickle cell mice at three life stages (Pre-P=pre-priapism, P=priapism and Late stage-P=late stage-priapism). Loading was normalized using a protein assay prior to loading, and equal loading confirmed using the house-keeping gene, GAPDH, glyceraldehyde-3-phopshate dehydrogenase.
Table 2. Densitometric analysis of proteins involved in protein degradation in the corpora of Sickle cell mice at different life stages.
Densitometry was performed following western blot analysis of protein extracts from the corpora of sickle cell mice at three life stages (pre-priapic, priapic or late stage priapic) and age matched (non-sickle cell mice). Fold Change in expression compared to control (non-sickle cell mice) ± standard deviation. A total of six mice were used in each group (the protein extract from two animals was pooled for analysis.
| Protein | Pre-Priapism | Priapism | Late Stage- Priapism |
|---|---|---|---|
| LC3-I | 0.2 ± 0.18 | 3.85 ± 0.2 * | 4.56 ± 0.24* |
| LC3-II | 0.59 ± 0.32 | 1.68 ± 0.5 | 1.11 ± 0.32 |
| Nedd-4 | 22.06 ± 0.32* | 32.28 ± 0.47* | 13.06 ± 0.11* |
| Mdm-2 | 1.24 ± 0.95 | 2.7 ± 1.6 | 1.14 ± 0.92 |
| Bcl-2 | 0.3 ± 0.21 | 26.61 ± 0.12 * | 39.43 ± 0.11* |
Significantly changed protein expression compared with control (Student's t-test, P < 0.05).
Discussion
We present evidence of increased oxidative stress in the corpora of two animal models of priapism (opiorphin-induced priapism and sickle cell mice). We have published several papers describing the effect of intracorporal injection of plasmids expressing opiorphins on erectile function in the rat 10, 11, 32, 41. We have shown that injecting higher amounts of these plasmids results in a priapic-like effect in the rat, evidenced by vasocongestion and an increased basal ICP/BP ratio. Although lower doses of the plasmids in these experiments resulted in improved erectile response in old animals following cavernous nerve stimulation, higher doses of the plasmids reduced this effect which we have suggested is due to cavernosal tissue damage due to the priapic-like condition 10, 11, 32. The Berkley sickle cell mouse is also a well documented animal model for priapism 40. These mice show visual evidence of priapism from 12 weeks 41, 42 (personnel communication from Claudino, M.A.) and after 12 weeks demonstrate histological evidence that there is cavernosal tissue damage 41, 43.
It is interesting to note that both etiologies resulting in a priapic-like condition have qualitatively similar effects on oxidative stress and protein degradation pathways in the corpora of the rat or mouse. There are elevated levels of lipid peroxidation, GST activity and protein oxidation in animals with priapism. These changes in markers of oxidative stress, since they occur in two very different models of priapism (sickle cell disease and gene transfer of pVAX-Vcsa1 to the corpora), are most likely due to the physiological affect of priapism on the corporal environment. However, we cannot total rule out that both models may involve common biochemical mediators. For example, sickle cell mice appear to have up-regulated expression of the mouse homologue of Vcsa1 in the penis at a pre-priapic and priapic life stage.
Our results support the observations of Evliyaoglu et al. 30, 31 who previously demonstrated that there are increased levels of lipid peroxides in a veno-occlusive rat model of priapism. Interestingly, in the corpora of late stage-priapic sickle cell mice the levels of carbonylated residues decline relative to the priapic stage (Figure 5C). There are several possible explanations for this. Animals with late stage-priapism may have higher levels of fibrosis, impaired blood flow and reduced erectile function. The overall reduction of blood flow into the penis may then result in less oxidative damage of proteins. Alternatively, the fibrotic tissue may be less susceptible to oxidative stress.
In addition we demonstrate that oxidative stress is associated with the activation of pathways involved in protein degradation. The changes in expression of proteins involved with protein degradation in the late stage-priapic mouse may explain several of the histological changes reported by Bivalacqua 43 which were documented in mice in the age group of 16–24 weeks. Mdm-2 and Nedd-4, which are both involved in the ubiquitin pathway 38, 39 are up-regulated in opiorphin and sickle cell disease induced priapism. Interestingly, in sickle cell disease Nedd-4 was upregulated at a stage in the mouse life cycle, when there is no evidence for priapism or pathology as a result of priapism. There maybe moderate changes in the biochemistry of the corpora of mice that are a response to the development of the physiological manifestation of priapism, without the mice being at a stage when there is visible evidence of priapism. If a similar pattern of up-regulation of Nedd-4 could be detected in human patients with sickle cell disease, Nedd-4 might act as a marker for individuals at risk for the development of priapism. Somewhat surprisingly, the anti-apoptotic protein Bcl-2 is up-regulated in pVAX-Vcsa1 treated animals and also in sickle cell mice with priapism. However, Bcl-2 can also act as an antioxidant, and its upregulation may represent a compensatory change in corporal cells to prevent damage and apoptosis 44, 45.
There is increased awareness that oxidative stress in corporal tissue is a contributing factor to the development of ED 23–28, 29. Our work suggests that priapism is associated with increased oxidative stress and activation of protein degradation pathways in corporal tissue. The increase in oxidative stress in the corpora of patients with priapism may be the prime determinant for why 44–90% rate of these patients subsequently suffer from ED 3, 4. However, the mechanism by which the production of reactive oxygen species (ROS) can contribute to ED is unclear. One possibility is that superoxide interacts with NO to generate peroxynitrite, reducing the available NO to participate in an erectile response 27. The peroxynitrite and superoxide have also been reported to increase the incidence of apoptosis in the endothelium 46, 47. We report for the first time that peroxynitrite levels are increased in priapic and late stage-priapic mice corporal tissue compared to normal and sickle mice corporal without priapism. However, a similar observation in the microvascular system of sickle cell mice has been reported by Kaul et al., 48, 49 who demonstrated that increased oxidative stress is associated with increased production of nitrotyrosine. Following oxidative stress, damaged proteins are degraded 50 leading to an increased level of fibrosis in corporal tissue. It has been proposed that elevated levels of fibrosis in corporal tissue can result in corporal veno-occlusive dysfunction (CVOD) 51, 52. CVOD has been suggested to play a role in the development of ED as a result of aging, diabetes and following radical prostatectomy 53–55. Recent papers have also documented sickle cell mice that have suffered from priapism, have increased fibrosis of the corporal tissue 9, 43. Therefore, the sequelae of priapism may be increased oxidative stress in the corporal tissue, followed by activation of the protein degradation pathways leading to fibrosis and ED through CVOD.
Priapism, and resultant ED, can be considered as one of several vasculopathies associated with sickle disease and includes pulmonary hypertension, cutaneous leg ulceration and stroke. Oxidative stress is also considered to be a prominent mechanism in the development of these vasculopathies 15. In sickle cell disease erythrocytes have increased concentrations of ROS compared with normal red blood cells 56, which results in changes in the composition of cell membrane lipids and abnormal erythrocyte phosphatidylserine exposure 57. Phospahtidylserine exposure induces binding of red cells to endothelial cells leading to their sequestration in peripheral blood vessels and subsequent vascular dysfunction and hemolysis. As a result of chronic hemolysis, levels of free plasma hemoglobin are increased at baseline and NO bioavailability is diminished, further contributing to vascular endothelial dysfunction. The complex interactions of hemolysis and oxidative stress, and the close relationship of these two mechanisms in sickle cell disease has led to a debate of which is the primary mechanism of vasculopathy 58.
The recognition that the generation and activity of ROS in the penis can lead to ED, has prompted investigation into the potential use of antioxidants for its treatment and prevention, particularly targeting superoxide dismutase (SOD). Bivalacqua et al. 28 demonstrated that over-expressing SOD in the corpora of diabetic rats significantly lowered superoxide anion levels and had a positive effect on erectile function. Another approach to limit super oxide production has been to treat diabetic animals with a synthetic SOD mimetic (Tempol). Animals treated with tempol showed significantly improved erectile function over controls 59. However, there are studies suggesting that administration of antioxidants following priapism are unable to restore erectile function 60. Another approach could be to prevent CVOD by protecting tissue damage 53, 54, 61. However, it is likely that these therapies will be useful to the prevent damage caused by oxidative stress with the onset of priapism, but will not be useful to recover function once the damage has occurred.
An integrative understanding of the complex mechanisms that contribute to the development of ED may identify new targets for its treatment or prevention. Although our work does not distinguish whether the observed oxidative stress in the corporal tissue in our experimental animal models is a cause or a result of priapism it adds further support for the investigation of the ability of antioxidant therapies to treat and prevent ED as a result of priapism.
Acknowledgements
This work was supported by a grant awarded by the NIH/NIDDK to Kelvin P. Davies (R01DK077665). None of the authors have a conflict of interest with the work presented in this paper.
Abbreviations
- ADA
adenosine deaminase
- A2BR
adenosine 2B receptor
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- eNOS
endothelial nitric oxide synthase
- ED
erectile dysfunction
- EDTA
ethylenediaminetetraacetic acid
- GST
glutathione S-transferase
- LPO
lipid peroxide
- MDA
malondialdehyde
- NO
nitric oxide
- PDE-5
phosphodiesterase-5
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulphate
- SOD
superoxide dismutase
- TBST
tris buffered saline containing Tween -20
References
- 1.Montague DK, Jarow J, Broderick GA, Dmochowski RR, Heaton JP, Lue TF, et al. American Urological Association guideline on the management of priapism. J Urol. 2003;170(4 Pt 1):1318–1324. doi: 10.1097/01.ju.0000087608.07371.ca. [DOI] [PubMed] [Google Scholar]
- 2.El-Bahnasawy MS, Dawood A, Farouk A. Low-flow priapism: risk factors for erectile dysfunction. BJU Int. 2002;89(3):285–290. doi: 10.1046/j.1464-4096.2001.01510.x. [DOI] [PubMed] [Google Scholar]
- 3.Pryor J, Akkus E, Alter G, Jordan G, Lebret T, Levine L, et al. Priapism. J Sex Med. 2004;1(1):116–120. doi: 10.1111/j.1743-6109.2004.10117.x. [DOI] [PubMed] [Google Scholar]
- 4.Bennett N, Mulhall J. Sickle cell disease status and outcomes of African-American men presenting with priapism. J Sex Med. 2008;5(5):1244–1250. doi: 10.1111/j.1743-6109.2008.00770.x. [DOI] [PubMed] [Google Scholar]
- 5.Sood S, James W, Bailon MJ. Priapism associated with atypical antipsychotic medications: a review. Int Clin Psychopharmacol. 2008;23(1):9–17. doi: 10.1097/YIC.0b013e3282f1c1ef. [DOI] [PubMed] [Google Scholar]
- 6.Nunes KP, Costa-Goncalves A, Lanza LF, Cortes SF, Cordeiro MN, Richardson M, et al. Tx2-6 toxin of the Phoneutria nigriventer spider potentiates rat erectile function. Toxicon. 2008;51(7):1197–1206. doi: 10.1016/j.toxicon.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowler JE, Jr, Koshy M, Strub M, Chinn SK. Priapism associated with the sickle cell hemoglobinopathies: prevalence, natural history and sequelae. J Urol. 1991;145(1):65–68. doi: 10.1016/s0022-5347(17)38248-4. [DOI] [PubMed] [Google Scholar]
- 8.Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med. 1980;140(11):1434–1437. [PubMed] [Google Scholar]
- 9.Kanika ND, Tar M, Tong Y, Kuppam DS, Melman A, Davies KP. The mechanism of opiorphin-induced experimental priapism in rats involves activation of the polyamine synthetic pathway. Am J Physiol Cell Physiol. 2009 doi: 10.1152/ajpcell.00656.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong Y, Tar M, Melman A, Davies K. The opiorphin gene (ProL1) and its homologues function in erectile physiology. BJU Int. 2008;102(6):736–740. doi: 10.1111/j.1464-410X.2008.07631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong Y, Tar M, Davelman F, Christ G, Melman A, Davies KP. Variable coding sequence protein A1 as a marker for erectile dysfunction. BJU Int. 2006;98(2):396–401. doi: 10.1111/j.1464-410X.2006.06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnett AL, Chang AG, Crone JK, Huang PL, Sezen SE. Noncholinergic penile erection in mice lacking the gene for endothelial nitric oxide synthase. J Androl. 2002;23(1):92–97. doi: 10.1002/j.1939-4640.2002.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 13.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102(5):1661–1666. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mi T, Abbasi S, Zhang H, Uray K, Chunn JL, Xia LW, et al. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest. 2008;118(4):1491–1501. doi: 10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris CR. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology Am Soc Hematol Educ Program. 2008:177–185. doi: 10.1182/asheducation-2008.1.177. [DOI] [PubMed] [Google Scholar]
- 16.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, et al. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109(7):3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34(9):906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan J, Desouza R, Westney OL, Wang R. Insights of priapism mechanism and rationale treatment for recurrent priapism. Asian J Androl. 2008;10(1):88–101. doi: 10.1111/j.1745-7262.2008.00314.x. [DOI] [PubMed] [Google Scholar]
- 21.Burnett AL. Molecular pharmacotherapeutic targeting of PDE5 for preservation of penile health. J Androl. 2008;29(1):3–14. doi: 10.2164/jandrol.107.003483. [DOI] [PubMed] [Google Scholar]
- 22.Dai Y, Zhang Y, Phatarpekar P, Mi T, Zhang H, Blackburn MR, et al. Adenosine signaling, priapism and novel therapies. J Sex Med. 2009;6(Suppl 3):292–301. doi: 10.1111/j.1743-6109.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 23.Jeremy JY, Angelini GD, Khan M, Mikhailidis DP, Morgan RJ, Thompson CS, et al. Platelets, oxidant stress and erectile dysfunction: an hypothesis. Cardiovasc Res. 2000;46(1):50–54. doi: 10.1016/s0008-6363(00)00009-2. [DOI] [PubMed] [Google Scholar]
- 24.Azadzoi KM, Schulman RN, Aviram M, Siroky MB. Oxidative stress in arteriogenic erectile dysfunction: prophylactic role of antioxidants. J Urol. 2005;174(1):386–393. doi: 10.1097/01.ju.0000161209.39959.67. [DOI] [PubMed] [Google Scholar]
- 25.Bivalacqua TJ, Armstrong JS, Biggerstaff J, Abdel-Mageed AB, Kadowitz PJ, Hellstrom WJ, et al. Gene transfer of extracellular SOD to the penis reduces O2-* and improves erectile function in aged rats. Am J Physiol Heart Circ Physiol. 2003;284(4):H1408–H1421. doi: 10.1152/ajpheart.00770.2002. [DOI] [PubMed] [Google Scholar]
- 26.Deng W, Bivalacqua TJ, Champion HC, Hellstrom WJ, Murthy SN, Kadowitz PJ. Superoxide dismutase - a target for gene therapeutic approach to reduce oxidative stress in erectile dysfunction. Methods Mol Biol. 610:213–227. doi: 10.1007/978-1-60327-029-8_13. [DOI] [PubMed] [Google Scholar]
- 27.Jones RW, Rees RW, Minhas S, Ralph D, Persad RA, Jeremy JY. Oxygen free radicals and the penis. Expert Opin Pharmacother. 2002;3(7):889–897. doi: 10.1517/14656566.3.7.889. [DOI] [PubMed] [Google Scholar]
- 28.Bivalacqua TJ, Usta MF, Kendirci M, Pradhan L, Alvarez X, Champion HC, et al. Superoxide anion production in the rat penis impairs erectile function in diabetes: influence of in vivo extracellular superoxide dismutase gene therapy. J Sex Med. 2005;2(2):187–197. doi: 10.1111/j.1743-6109.2005.20228_1.x. discussion 197-8. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal A, Nandipati KC, Sharma RK, Zippe CD, Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J Androl. 2006;27(3):335–347. doi: 10.2164/jandrol.05136. [DOI] [PubMed] [Google Scholar]
- 30.Evliyaoglu Y, Kayrin L, Kaya B. Effect of pentoxifylline on veno-occlusive priapism-induced corporeal tissue lipid peroxidation in a rat model. Urol Res. 1997;25(2):143–147. doi: 10.1007/BF01037931. [DOI] [PubMed] [Google Scholar]
- 31.Evliyaoglu Y, Kayrin L, Kaya B. Effect of allopurinol on lipid peroxidation induced in corporeal tissue by veno-occlusive priapism in a rat model. Br J Urol. 1997;80(3):476–479. doi: 10.1046/j.1464-410x.1997.00371.x. [DOI] [PubMed] [Google Scholar]
- 32.Tong Y, Tar M, Monrose V, DiSanto M, Melman A, Davies KP. hSMR3A as a marker for patients with erectile dysfunction. J Urol. 2007;178(1):338–343. doi: 10.1016/j.juro.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 34.Melman A, Biggs G, Davies K, Zhao W, Tar MT, Christ GJ. Gene transfer with a vector expressing Maxi-K from a smooth muscle-specific promoter restores erectile function in the aging rat. Gene Ther. 2008;15(5):364–370. doi: 10.1038/sj.gt.3303093. [DOI] [PubMed] [Google Scholar]
- 35.Melman A, Zhao W, Davies KP, Bakal R, Christ GJ. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J Urol. 2003;170(1):285–290. doi: 10.1097/01.ju.0000063375.12512.6e. [DOI] [PubMed] [Google Scholar]
- 36.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 37.Ezaki J, Kominami E. The intracellular location and function of proteins of neuronal ceroid lipofuscinoses. Brain Pathol. 2004;14(1):77–85. doi: 10.1111/j.1750-3639.2004.tb00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plant PJ, Correa J, Goldenberg N, Bain J, Batt J. The inositol phosphatase MTMR4 is a novel target of the ubiquitin ligase Nedd4. Biochem J. 2009;419(1):57–63. doi: 10.1042/BJ20081866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. Embo J. 2002;21(15):4037–4048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu L, Diwan B, Ward JM, Noguchi CT. Pathology of "Berkeley" sickle-cell mice includes gallstones and priapism. Blood. 2006;107(8):3414–3415. doi: 10.1182/blood-2005-11-4500. [DOI] [PubMed] [Google Scholar]
- 41.Kanika ND, Tar M, Tong Y, Kuppam DS, Melman A, Davies KP. The mechanism of opiorphin-induced experimental priapism in rats involves activation of the polyamine synthetic pathway. Am J Physiol Cell Physiol. 2009;297(4):C916–C927. doi: 10.1152/ajpcell.00656.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claudino MA, Franco-Penteado CF, Corat MA, Gimenes AP, Passos LA, Antunes E, et al. Increased cavernosal relaxations in sickle cell mice priapism are associated with alterations in the NO-cGMP signaling pathway. J Sex Med. 2009;6(8):2187–2196. doi: 10.1111/j.1743-6109.2009.01337.x. [DOI] [PubMed] [Google Scholar]
- 43.Bivalacqua TJ, Musicki B, Hsu LL, Gladwin MT, Burnett AL, Champion HC. Establishment of a Transgenic Sickle-Cell Mouse Model to Study the Pathophysiology of Priapism. J Sex Med. 2009 doi: 10.1111/j.1743-6109.2009.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 45.Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, et al. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 46.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 47.Khan MA, Thompson CS, Mumtaz FH, Mikhailidis DP, Morgan RJ, Bruckdorfer RK, et al. The effect of nitric oxide and peroxynitrite on rabbit cavernosal smooth muscle relaxation. World J Urol. 2001;19(3):220–224. doi: 10.1007/s003450000162. [DOI] [PubMed] [Google Scholar]
- 48.Kaul DK, Zhang X, Dasgupta T, Fabry ME. Arginine therapy of transgenic-knockout sickle mice improves microvascular function by reducing non-nitric oxide vasodilators, hemolysis, and oxidative stress. Am J Physiol Heart Circ Physiol. 2008;295(1):H39–H47. doi: 10.1152/ajpheart.00162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest. 2004;114(8):1136–1145. doi: 10.1172/JCI21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett. 2006;580(12):2910–2916. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez-Cadavid NF. Mechanisms of penile fibrosis. J Sex Med. 2009;6(Suppl 3):353–362. doi: 10.1111/j.1743-6109.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- 52.Melman A, Gingell JC. The epidemiology and pathophysiology of erectile dysfunction. J Urol. 1999;161(1):5–11. [PubMed] [Google Scholar]
- 53.Kovanecz I, Ferrini MG, Vernet D, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Pioglitazone prevents corporal veno-occlusive dysfunction in a rat model of type 2 diabetes mellitus. BJU Int. 2006;98(1):116–124. doi: 10.1111/j.1464-410X.2006.06268.x. [DOI] [PubMed] [Google Scholar]
- 54.Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68(2):429–435. doi: 10.1016/j.urology.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Cadavid NF, Rajfer J. Molecular pathophysiology and gene therapy of aging-related erectile dysfunction. Exp Gerontol. 2004;39(11–12):1705–1712. doi: 10.1016/j.exger.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 56.Hebbel RP, Eaton JW, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest. 1982;70(6):1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neidlinger NA, Larkin SK, Bhagat A, Victorino GP, Kuypers FA. Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction. J Biol Chem. 2006;281(2):775–781. doi: 10.1074/jbc.M505790200. [DOI] [PubMed] [Google Scholar]
- 58.Krajewski ML, Hsu LL, Gladwin MT. The proverbial chicken or the egg? Dissection of the role of cell-free hemoglobin versus reactive oxygen species in sickle cell pathophysiology. Am J Physiol Heart Circ Physiol. 2008;295(1):H4–H7. doi: 10.1152/ajpheart.00499.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawakami T, Urakami S, Hirata H, Tanaka Y, Nakajima K, Enokida H, et al. Superoxide dismutase analog (Tempol: 4-hydroxy-2, 2, 6, 6-tetramethylpiperidine 1-oxyl) treatment restores erectile function in diabetes-induced impotence. Int J Impot Res. 2009 doi: 10.1038/ijir.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muneer A, Cellek S, Ralph DJ, Minhas S. The investigation of putative agents, using an in vitro model, to prevent cavernosal smooth muscle dysfunction during low-flow priapism. BJU Int. 2008;102(8):988–992. doi: 10.1111/j.1464-410X.2008.07778.x. [DOI] [PubMed] [Google Scholar]
- 61.Ferrini MG, Kovanecz I, Sanchez S, Vernet D, Davila HH, Rajfer J, et al. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biol Reprod. 2007;76(5):915–923. doi: 10.1095/biolreprod.106.059642. [DOI] [PubMed] [Google Scholar]


