Abstract
OBJECTIVES
To assess the relationship between rate of change in muscle strength and all-cause mortality.
DESIGN
A prospective observational study of the causes and course of physical disability.
SETTING
Twelve contiguous ZIP code areas in Baltimore, Maryland.
PARTICIPANTS
Three hundred and seven community-dwelling women aged 70–79 years at study baseline.
MEASUREMENTS
The outcome is all-cause mortality (1994–2009); predictors include up to seven repeated measurements of handgrip, knee extension, and hip flexion strength, with a median follow-up time of 9 years. Demographic factors, body mass index, smoking status, number of chronic diseases, depressive symptoms, physical activity, Interlukin-6, and albumin were assessed at baseline and included as confounders. The associations between declining muscle strength and mortality were assessed using a joint longitudinal and survival model..
RESULTS
Grip and hip strength declined an average of 1.10 and 1.31 kg per year between age 70 and 75and 0.50 and 0.39 kg/year thereafter, respectively; knee strength declined at a constant rate of 0.57 kg/year. Faster rates of decline in grip and hip strength, but not knee strength, independently predicted of mortality after accounting for their baseline levels and potential confounders (Hazard Ratio (HR)=1.33 (95% confidence interval (CI)=1.06–1.67), 1.14 (CI=0.91–1.41), and 2.62 (CI=1.43–4.78) for every 0.5 standard deviation increase in rate of decline in grip, knee, and hip strength, respectively.
CONCLUSION
Monitoring the rate of decline in grip and hip flexion strength in addition to the absolute levels may greatly improve the identification of women most at risk of dying.
Keywords: handgrip strength, hip strength, knee strength, mortality, older women
INTRODUCTION
Age-related decline in muscle strength has been attributed to the loss of muscle mass and muscle quality referred to as sarcopenia. Such changes begin in midlife1–3 and often result in significant functional and clinical consequences including increased risk of falling, physical disability, and frailty in older adults4–7. Several studies have also shown that reduced muscle strength is associated with all-cause mortality8–14; and muscle strength as an indicator of muscle quality is a more important predictor of mortality risk than muscle mass13, 14.
Epidemiologic research to date aimed at characterizing age-related changes in muscle strength and their effects on adverse health outcomes has primarily relied on cross-sectional measurements of strength. From a single measurement, it is impossible to distinguish whether or not the measurement represents an individual’s usual “normal” level or an already compromised level relative to his/her peak strength in the past. The magnitude and nature of the change in muscle strength can only be determined in a longitudinal setting. The data on longitudinal changes in muscle strength are limited, most of which consist of measurements at two different points in time15.
Both upper (e.g. grip) and lower extremity (e.g. knee extension) strength have been associated with mortality risk. While lower-extremity strength is deemed to be of great importance in daily functioning in real life, therefore is commonly measured in clinical trials16, grip strength is typically favored over knee or hip strength in epidemiological studies due to its ease of measurement, higher reliability, and high correlation with other strength measures9. However, strength may decline at different rates, contemporaneously or not, for different muscle groups due to factors such as “local” diseases affecting specific musculoskeletal regions, differences in amount of muscle tissue/muscle mass, absolute strength at baseline, and frequency and intensity of use. To our knowledge, neither the correlations among individual-level changes in grip, knee, and hip strength over time nor their relative predictive ability for all-cause mortality has been examined in older adults.
Using data from the Women’s Health and Aging Study (WHAS) II with up to 7 measurements of muscle strength over 10 years, this study aims to characterize (i) individual as well as population average trajectories of age- and aging-related changes in grip, knee, and hip strength over time; (ii) the interrelationships among individual rates of change in grip, knee, and hip strength over time; (iii) the effects of absolute strength and rate of change in strength on all-cause mortality; and (iv) potential mediating effects of health characteristics on the strength-mortality association.
METHODS
Study Population
WHAS II is a prospective cohort study of 436 women age 70–79 years who were recruited from an age-stratified sample of Medicare beneficiaries, in 12 contiguous zip codes of Baltimore City and County, Maryland. Study eligibility criteria included either no functional difficulty or self-reported difficulty in 1 of 4 domains of physical function: mobility, upper extremity, high functioning, and self-care tasks and Mini-Mental Status Exam scores of 24 or higher16; thus, participants were representative of the two-thirds highest functioning women 70–79 years who were living in the community. Interviews and physical examinations were conducted at baseline, beginning in 1994, and at six follow-up examinations (approximately 18 months apart except for the interval between the third and the fourth exam, which was, on average, 3 years). A median follow-up time of 10 years resulted between 1994 and 2008. The study was approved by the Johns Hopkins University Institutional Review Board. The analytic sample for this study consisted of 307 women who had baseline data available on all covariates and had at least three measurements on grip, knee, and hip strength prior to death, at study dropout, or by the end of the follow-up period. Compared to the study sample, the 91 women who were excluded from the analysis due to having two or fewer measurements of grip, knee, and hip strength were older and had a higher proportion of African Americans, lower levels of education, higher prevalence of obesity, lower levels of physical activity, and higher levels of IL-6. There was no significant difference in disease burden, smoking status, albumin levels, grip, knee, or hip strength between the two groups at baseline. The 38 women who were excluded due to missing blood draws (n=37) or body mass index (BMI) value (n=1) at baseline on average had greater hip strength, but were otherwise comparable to the study sample (Table 1). As a sensitivity analysis, we rerun the analyses by including the 36 of the 38 women with complete information on age, race, education, and BMI. The results were similar to those of the minimally adjusted model reported in Table 3.
Table 1.
Summary of demographic and health characteristics of WHAS II samples at baseline (total n=436).
| Characteristic | Analytic Sample (n=307) | Excluded Due to Missing Strength Measurements (n=91)* | Excluded Due to Missing Covariates (n=38)† |
|---|---|---|---|
| Mean (SD) or %‡ | Mean (SD) or % | Mean (SD) or % | |
| Age (years) | 73.6 (2.8) | 74.7 (2.6) | 74.2 (2.8) |
| Race | |||
| Caucasian | 83.7% | 72.5% | 79.0% |
| African American | 16.3% | 27.5% | 18.4% |
| Other | 0% | 1.1% | 2.6% |
| Years of education | 12.9 (3.2) | 11.4 (3.6) | 12.2 (3.3) |
| Number of diseases§ | 1.5 (1.0) | 1.6 (1.1) | 1.6 (1.1) |
| Body Mass Index (kg/m2): | |||
| <18.5 | 3.6% | 3.3% | 0% |
| ≥18.5 & <25 | 38.4% | 25.3% | 36.8% |
| ≥25 & <30 | 39.4% | 31.9% | 36.8% |
| ≥30 | 18.6% | 38.5% | 23.7% |
| Physical Activity | |||
| Inactive | 5.9% | 14.2% | 2.6% |
| Insufficient | 33.9% | 44.0% | 26.3% |
| Recommended | 60.3% | 39.6% | 68.4% |
| Smoking Status | |||
| Never Smoker | 52.7% | 55.0% | 65.8% |
| Former Smoker | 38.1% | 27.5% | 26.3% |
| Current Smoker | 9.1% | 15.4% | 5.3% |
| GDS¶ ≥ 10 | 7.5% | 12.1% | 5.3% |
| Albumin (g/dL) | 4.28 (0.27) | 4.23 (0.29) | - |
| Interlukin-6 (pg/mL) | 2.96 (2.20–3.86) | 3.60 (2.60–5.44) | - |
| Grip Strength (kg) | 23.5 (5.0) | 23.0 (4.6) | 22.9 (4.5) |
| Knee strength (kg) | 21.8 (5.9) | 21.1 (5.1) | 23.3 (4.8) |
| Hip strength (kg) | 18.7 (6.7) | 19.5 (6.7) | 21.8 (6.7) |
The subset of those who had 2 or fewer measurements of grip, knee, and hip strength
The subset of those who had missing demographic, health characteristics, or blood draws at baseline.
Percentages may not add up to 1 due to missing data.
Presence of “definite” diseases including angina pectoris/myocardial infarction, congestive heart failure, peripheral artery disease, hip fractures, osteoarthritis of the hip, knee, and/or hand, Parkinson’s, rheumatoid arthritis, osteoarthritis, stroke, pulmonary diseases, diabetes mellitus, cancer, spinal stenosis, and disc disease.
Geriatric Depression Scale (GDS)
Table 3.
Associations of knee and hip strength at age 70 and rate of decline over time with total mortality in WHAS II (n=307).
| Minimally Adjusted Model* | Fully Adjusted Model† | ||||||
|---|---|---|---|---|---|---|---|
| HR‡ | 95% CI | p-value | HR | 95% CI | p-value | ||
| Strength at Age 70 (kg)§ | Grip | 1.42 | 1.18, 1.70 | <0.01 | 1.34 | 1.13, 1.59 | <0.01 |
| Knee | 1.36 | 1.11, 1.67 | <0.01 | 1.35 | 1.10, 1.66 | <0.01 | |
| Hip | 3.77 | 2.15, 6.61 | <0.01 | 3.11 | 1.75, 5.50 | <0.01 | |
| Rate of decline¶ | Grip | 1.41 | 1.13, 1.77 | <0.01 | 1.33 | 1.06, 1.67 | 0.01 |
| Knee | 1.17 | 0.92, 1.48 | 0.20 | 1.14 | 0.91, 1.41 | 0.26 | |
| Hip | 3.17 | 1.76, 5.72 | <0.01 | 2.62 | 1.43, 4.78 | <0.01 | |
adjusted for age, race, education, and BMI
adjusted for age, race, education, BMI, number of diseases, smoking status, depressive symptoms, physical activity, albumin, and interlukin-6.
hazard ratio (HR)
Numbers presented are HR estimates are for 0.5 standard deviation (SD) unit decrease in grip (1.9 kg), knee (2.3 kg), and hip (2.7 kg) strength at age 70.
Numbers presented are HR estimates for 0.5 SD unit increase in annual rate of decline in grip (0.07 kg/year), knee (0.08 kg/year), and hip (0.14 kg/year) strength.
Measures of Muscle Strength
Isometric grip strength was measured in kilograms using a JAMAR hand dynamometer (Model #PC 5030J1, J.A. Preston Co., Jackson, MI). The test was performed three times on each hand with the participant in the sitting position with the arm to be tested pressing against her side at a right angle. Study participants were instructed to grab the metal handles of the dynamometer and squeeze as hard as they can. The maximum measurement in the non-dominant hand was used in the analyses. The non-dominant hand was selected because it is less subject than the dominant hand is to the variable “non-whole body” influences of trauma, repetitive use syndromes, or strengthening from daily activities.
Maximal isometric strength of the hip flexion and knee extensor muscles was measured using a hand held dynamometer (Nicholas Manual Muscle Tester: Model #01160, Lafayette Instrument, Inc., Lafayette, IN)18. The tests were performed twice on each side while the participant was in a sitting position with her knee extended 75° from the horizontal position for knee strength test, and with her hips and knees flexed at 90° for hip strength test. During testing, participants were coached to gradually increase the force to the greatest possible level while the tester was opposing. Strength is expressed as kilograms of force the examiner had to apply to break the isometric contraction. The maximum of left and right hip and knee strength was used in the analyses.
Grip and knee strength were assessed at baseline and at each follow-up exam for a maximum of seven measurements per study participant; hip strength was assessed at each of the first five follow-up exams for a maximum of six measurements per study participant. Using the results of the three trials of the handgrip test and the two trials of the hip and knee tests, conducted in sequence by the same examiner with only a few seconds in between trials, we calculated intra-rater reliability coefficient, averaging 0.90, 0.89, and 0.86 across exams for grip, knee, and hip strength tests, respectively.
Total Mortality
Data on all-cause mortality were obtained through follow-up interviews with proxies, obituaries, and matching with the National Death Index, with the most recent update completed on January 28, 2009.
Covariates
To assess the independent effect of muscle strength decline on total mortality, we included age, race, education, and BMI as well as the following covariates in multivariate adjusted analyses.
Chronic diseases
Presence or absence of 14 major chronic diseases and conditions at baseline (angina pectoris/myocardial infarction, congestive heart failure, peripheral artery disease, hip fractures, osteoarthritis of the hip, knee, and/or hand, Parkinson’s, rheumatoid arthritis, osteoarthritis, stroke, pulmonary diseases, diabetes mellitus, cancer, spinal stenosis, and disc disease) was adjudicated by physicians based on pre-defined criteria19. The number of “definite” conditions, out of 14, was used as a summary measure of disease burden.
Health habits
Smoking status was classified based on self-report into: current smoker, former smoker, and never smoker. Physical activity was assessed through self-report of total minutes per week spent in six moderate intensity activities (i.e., walking for exercise, heavy household chores, heavy outdoor work, regular exercise, dancing, and bowling) using a modified version of the Minnesota Leisure Time Physical Activity Questionnaire4. Women were classified as being “Inactive”, or having “Insufficient” or “Recommended” level of physical activity if they reported 0, 0–149, or ≥150 min/wk of activity, respectively. The cutoff of 150 min/wk corresponds with CDC/ACSM recommendations of ≥30 minutes of moderate intensity physical activity on most (5) days of the week20.
Mental health
Depressive symptomatology was assessed using the 30-item Geriatric Depression Scale. Women with scores of 10 or above were considered to have high depressive symptoms.
Biomarkers of inflammation and malnutrition
Blood samples were collected from 391 (90%) women at baseline by venipuncture between 9 a.m. and 2 p.m. in a non-fasting state, processed, and stored at −70C until analysis. Plasma interlukin-6 (IL-6) was measured in duplicate by ELISA (High Sensitivity kit, R&D Systems, Inc., Minneapolis, MN) from frozen specimens and the average of the two measures was used. The lower detection limit was 0.1 pg/ml, and the interassay coefficient of variation was 7%. Albumin was measured with dye-binding bromocresol green.
Data analysis
To describe change in grip, knee, and hip strength over time, for each strength measure, we plotted a random sample of 10 individual trajectories of strength within each decile group of strength at study baseline. The population average trajectories of changes in strength were examined using smooth splines with four degrees of freedom for identification of potential nonlinear trends. Knee strength declined at a steady, linear rate over 10 years. However, rates of decline (i.e., slopes) differed before and after age 75 for grip and hip strength. We used linear random effects growth curve models (REGCM) to assess population average rates of change in strength over time (i.e. fixed effects) by including age centered at 70 in the models as a time-dependent covariate. To account for the nonlinear time trends in changes of grip and hip strength, a two-piece linear spline was used in the REGCM with one knot fixed at age 75 (see details in Appendix). The knot was identified via profile likelihood approach. We also included race, education, BMI, physical activity, and IL-6 that were predictive of early dropout in the model as covariates to minimize the impact of non-ignorable missing data21. To account for between-person heterogeneity in terms of individual deviation from the population mean trajectory, intercept (i.e. strength at age 70) and age slope (for knee) or age slope before age 75 (for grip and hip) were modeled as random effects with an unstructured variance-covariance matrix.
Next, we compared rates of decline across muscle groups by analyzing the three strength measures jointly in a multivariate REGCM (see details in Appendix). Measure-specific strength at age 70 and rates of decline were included as population means and as random effects, to model individual differences. This multivariate modeling approach allowed us directly to compare and test mean differences in standardized rates of decline across muscle groups.
To assess the effects of baseline as well as change in grip, knee, and hip strength over time on the risk of mortality, we first estimated individual specific strength at age 70 and rate of change in strength from the REGCM with only intercept and age slope in the model as fixed and random effects. We then plotted the Kaplan Meier Survival curves by tertiles of strength at age 70 and median split of rate of change in strength. The tertile or median cutoffs were selected to achieve a reasonable balance between adequate number of survival events in each strength category for valid representation of the data and minimal loss of power due to categorization.
Finally, we performed joint analysis of the repeated measurements of strength and time-to-death by explicitly modeling the dependency between the time trends of strength change and the survival time via a mixed effects model (see details in Appendix)22. In the joint model, log survival time was entered as the response variable and individual-specific strength at age 70 and individual-specific age slope estimated from the REGCMwere entered as main effects; baseline age centered at 70, race, education, BMI, smoking status, number of diseases, GDS, physical activity level, IL-6, and albumin were also included as potential confounders. To assess the degree to which change in muscle strength improved the prediction of mortality above and beyond baseline level, we calculated the Maddala’s likelihood-based R2M statistic23–24, which approximates the coefficient of determination (R2) as in linear regression, therefore can be interpreted as explained variation for the dependent variable (i.e. survival time). Analyses were conducted in SAS (version 9.1) and SPLUS (version 2000; Insightful Inc., Seattle, WA).
RESULTS
Table 1 summarizes the demographic and health characteristics of our study sample at baseline. The mean grip strength in the non-dominant hand was 23.5kg (standard deviation (SD) = 5.0 kg); the mean knee and hip strength was 21.8 kg (SD=5.9) and 18.7 kg (SD=6.7), respectively, at baseline.
More than seventy percent of the 307 women in this study had 5 or more measurements of grip and knee, and hip strength, with a median follow-up time of 9.4, 9.3, 9.0 years for grip, knee, and hip strength, respectively, before study dropout or death. Attrition due to study withdrawal was minimal over the first three exams (8.7%, n=38 at Exam 2; 2%, n=8 at Exam 3), and increased at Exams 4 (14%, n=55), 5 (11%, n=37), 6 (17%, n=51), and 7 (21%, n=52). Twenty-nine percent of the 307 women died during the study, with an incidence rate of 24.6 per 1000 person-years.
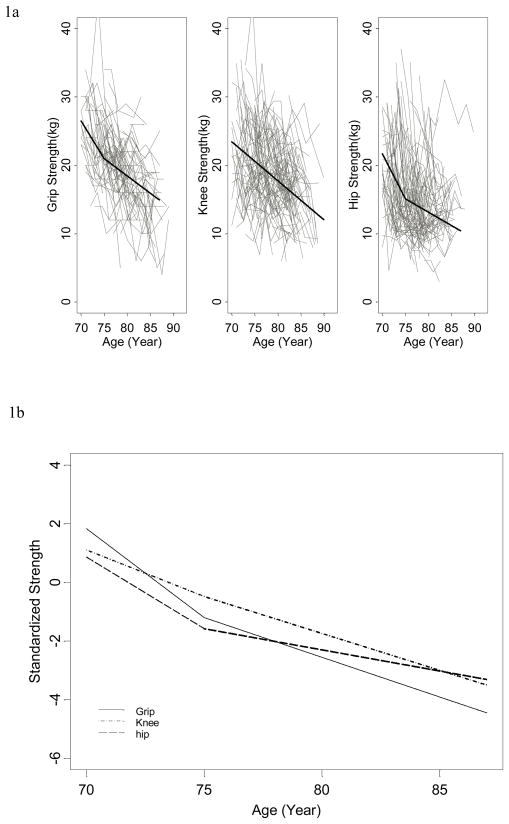
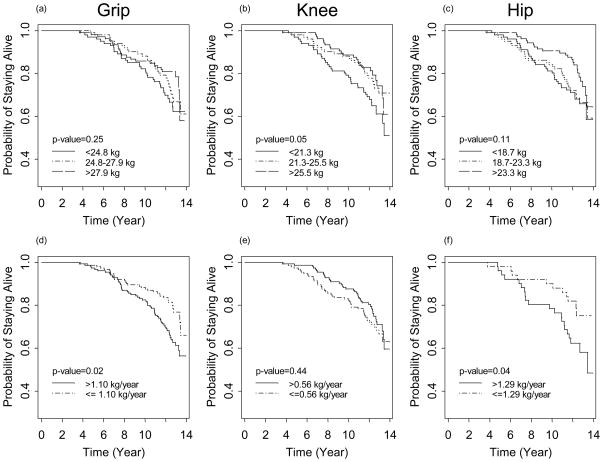
As shown in Table 2, there was in general a declining trend in mean grip, knee, and hip strength over time. Figure 1a displays a representative sample of individual trajectories of change in grip, knee, and hip strength with age. Note that the absolute values and the rate of change in strength appear to be quite heterogeneous among different study participants and the average trends denoted by thick lines underestimated the individual-level changes. The random effects model estimated that the mean grip, knee, and hip strength was 26.5, 23.4, and 21.7 kg at age 70, respectively; grip strength declined an average of 1.10 kg per year (range: 0.59 – 1.83) between age 70 and 75 (p<0.01) and 0.50 kg/year (range: −0.01 – 1.23) thereafter; knee strength declined at a constant rate of 0.57 kg/year (range: 0.15 – 1.00); hip strength declined an average of 1.31 kg per year (range: 0.59 – 2.17) between age 70 and 75 (p<0.01) and 0.39 kg/year (range: −0.33 – 1.25) thereafter. Moreover, regardless of baseline value of strength, all study participants experienced a significant decline in grip, knee, and hip strength between age 70 and 75. Figure 1b compares the standardized estimates of average rates of decline in grip, knee, and hip strength after adjusting for race, education, BMI, IL-6 and physical activity. Grip strength declined at the fastest rate between age 70 and 75, followed by hip strength. While knee strength shows slowest but steady decline initially, the rate of decline for grip and hip slowed down significantly after age 75, with the decline being much more attenuated in hip relative to grip and knee after age 75.
Table 2.
Sample mean and standard deviation of grip, knee, and hip strength by study visit in WHAS II.
| Grip Strength (kg) | Knee Strength (kg) | Hip Strength (kg) | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Baseline | 306 | 23.5 (5.0) | 302 | 21.8 (5.9) | 302 | 18.7 (6.7) |
| Year 1.5 | 292 | 22.3 (4.6) | 286 | 20.0 (5.5) | 285 | 15.8 (5.8) |
| Year 3 | 295 | 18.5 (3.9) | 284 | 19.1 (4.8) | 283 | 12.9 (4.6) |
| Year 6 | 244 | 18.7 (4.6) | 244 | 19.1 (5.0) | 241 | 12.3 (4.3) |
| Year 7.5 | 204 | 17.4 (4.7) | 229 | 16.7 (4.8) | 231 | 12.9 (4.4) |
| Year 9 | 206 | 17.1 (4.9) | 202 | 15.4 (4.8) | 204 | 12.5 (4.4) |
| Year 10.5 | 122 | 17.7 (4.2) | 123 | 15.6 (4.8) | - | - |
Figure 1.
Figure 1a. Trajectories of grip, knee, hip strength over time in Women’s Health and Aging Study (n=307), with thin lines represent individual trajectories and thick lines represent population-average trends estimated from linear random effects models.
Figure 1b. Estimated population-average trajectories of change in grip, knee, and hip strength over time among Caucasian women with 12 years of education, 18.5≤BMI<25, IL-6<2.5 pg/mL (i.e. bottom tertile), and >150 min/wk physical activity in WHAS II. The estimates of strength plotted on the Y-axis were standardized to facilitate comparisons across muscle groups.
Having observed the evidence of uniform decline in all three muscle groups at a population mean level, it would be interesting to see whether the changes occurred simultaneously across muscle groups within a person. We found that the individual estimates of grip, knee, and hip strength at age 70 were positively correlated, with the correlation between knee and hip being the strongest (Pearson’s correlation r=0.67). Although there was positive correlation in rate of decline between hip and knee (r=0.51) within a person, the correlation between grip and knee (r=0.10) or between grip and hip (r=0.17) was much weaker.
Next we examined whether absolute strength and change in strength over time are associated with all-cause mortality. Mortality risk increased in a step-wise fashion as absolute levels of grip, knee, and hip strength decreased (Figure 2(a)–(c)); however, the crude associations were not statistically significant, possibly due to weakened power as a result of categorization of the absolute strength. Figure 2(d)–(f) compares Kaplan-Meier survival curves of faster vs. slower decliners (i.e. below vs. above median rate of decline). Due to the fact that very few women in the bottom (or upper) tertile of knee or hip strength at age 70 experienced rate of decline greater (or less) than 0.56 kg/year in knee strength or 1.29 kg/year in hip strength, the comparison was limited to women in middle tertile groups of strength at age 70. As shown in Figures 2(d)–(f), the groups of women experiencing faster decline in grip and/or hip strength had significantly elevated risk of total mortality for all (p<0.05); there was no significant effect of rate of decline in knee strength on total mortality (p=0.44).
Figure 2.
Unadjusted Kaplan-Meier survival curves by tertiles of the estimated strength at age 70 (2a-c) and median split of the estimated annual rate of decline in strength (2d-f), with the latter restricted to the subset of women in the middle tertile of strength at age 70. Quoted p-values were based on log-rank tests of the differences among the survival curves.
The joint longitudinal and survival analyses showed that higher grip, knee, and hip strength at age 70 were significantly associated with lower risk of mortality after adjusting for age, race, education, and BMI at study baseline. Specifically, the risk of mortality was 1.4 (95% confidence interval (CI)=1.2–1.7, p<0.01), 1.4 (CI=1.1–1.7, p<0.01), and 3.8 (CI=2.2–6.6, p<0.01) times higher for every 0.5 SD decrease in grip (i.e. 1.9 kg), knee (i.e. 2.3 kg), and hip (i.e. 2.7 kg) strength at 70, respectively. Furthermore, the risk of mortality was 1.4 (CI=1.1–1.8, p<0.01) and 3.2 (CI=1.8–5.7, p<0.01) times higher for every 0.5 SD increase in the rate of decline in grip (=0.07 kg/year) and hip strength (=0.14 kg/year), respectively; and the associations were independent of grip and hip strength at age 70. The results remained essentially unchanged after further adjustment for disease burden, physical activity, GDS, smoking, albumin, and IL-6 (Table 3). For hip and grip strength, including change in muscle strength as a predictor in addition to baseline level explained an extra 20% of total variance in survival time compare to the model with the baseline level alone; there was no meaningful gain in percent variance explained by including change in knee strength (<2%). Older age, greater number of chronic diseases, and higher levels of IL-6 were also independently associated with higher risk of total mortality.
DISCUSSION
The study provides the first longitudinal evidence linking aging-associated loss of skeletal muscle strength with all-cause mortality over 10 years in initially high functioning older women living in a community. Specifically, we found that rate of change in grip and hip flexion strength was independent of absolute strength in predicting mortality, and the associations are independent of age, disease burden, life style, nutritional status, inflammation, and mental well-being. In a previous analysis using data from a sample of moderately to severely disabled women aged 65 and older, it was shown that grip strength was associated with all-cause mortality and cardiovascular mortality after adjusting for demographic and health characteristics similar to those used in our study9. The fact that we arrived at the same conclusion using the least disabled women strengthens the argument that there may be an independent pathway other than disability linking strength and mortality that is not clearly understood. As declines in strength are a central component of frailty, and predictor of development of frailty itself 6, frailty may be in the causal pathway. Our study also confirms the findings from earlier studies, in that the association between strength and mortality cannot be fully explained by muscle mass. Although BMI is rather a crude measure of muscle mass, and separate measures of lean and fat mass of the upper and lower extremities as well as the whole body were not available in WHAS, Newman et al. found little difference in the strength-mortality association regardless of whether BMI or measures of lean and fat mass or muscle and fat areas are used13. Therefore, it is unlikely that the selection of measures of body composition could meaningfully alter the independent effect of strength on mortality.
An argument could be made that the reason rate of strength decline was a strong predictor of mortality is that declining strength may be part of the dying process such that the overall rate of decline might have been overly influenced by measurements taken closer to the time of death. Among the 88 women who died in the study, the time between the last measurement and death ranged from 2 weeks to 9.2 years with a median of 2.3 years; 19 (22%) women died within one year of the last measurement of strength. As a sensitivity analysis, we rerun the analyses by excluding these 19 women; the results remained unchanged. Therefore, the significant effect of declining strength on mortality was unlikely driven by declining strength close to death.
The fact that lower and declining hip strength were more strongly associated with mortality than lower and declining grip strength suggests that the former may be more strongly influenced by or contribute to mobility or catastrophic events such as falls that account for significant mortality. In fact, both experimental and observational studies have shown that reduced muscle strength, especially of the lower limbs, is one of the most important risk factors for falls7, 25. In our study, we found that absolute knee extension strength, but not loss of knee strength was predictive of mortality. However, it may be premature to conclude that the rate of change in knee strength is not an important clinical target. Future studies are needed to validate these findings.
Consistent with findings from earlier studies, rate of decline in strength varies across people10, 15, 26, and our analyses have shown that such heterogeneity persisted even after accounting for age and health characteristics. We also found initial evidence that population-average strength declined at different rates in different muscle groups, with grip strength having the fastest rate of decline. In addition, we found that the decline was, on average, slower in grip and hip after age 75 than it was between age 70–75, suggesting that strength plateaus may occur at old age. The slowing rate of decline may indicate important thresholds beyond which external and/or internal compensatory mechanisms may be activated in an attempt to restore homeostasis, albeit at a suboptimal level. For example, some people may compensate for underlying strength-loss-associated functional decrements by adapting to a modified daily routine (e.g. cutting back on physical activities) in order to maintain basic level of performance in real life, thereby retard subsequent decline. To investigate whether the slow-down predominantly existed among women survivors over the course of the study, we analyzed trajectories of change in grip and hip strength stratified by survival status. While the rate difference remained statistically significant and of comparable magnitude in grip strength among the deceased, it was no longer statistically significant in hip strength despite a 45% reduction in average rate of decline after age 75 (results not shown). More research is warranted to assess the generalizability of these findings.
In this study, we confirmed the findings of earlier studies that cross-sectional measurements of grip, knee, and hip strength are highly correlated. While women experiencing faster rate of decline in hip strength were also more likely to exhibit faster rate of decline in knee strength and vice versa, the association between grip and knee or between grip and hip however appears to be much weaker. Such discordance in the rate of strength change between upper and lower body muscle groups warrants further investigation. One hypothesis is that body composition may have a greater impact on lower body strength changes than upper body strength changes possibly due to disproportionate body mass overload on the lower body. For example, individuals may become less mobile due to obesity, exacerbating declines in strength, perhaps more so in the lower extremities than the upper extremities. Testing of this hypothesis is beyond the scope of this work.
Strengths of our study include its prospective design, long-term follow-up, and its initially high functioning women. In addition, the statistical methodology employed in this study greatly facilitated the inference concerning the effects of individual trends in strength change (i.e. a time-dependent covariate) on all-cause mortality while simultaneously accounting for measurement error in the repeated measurements of strength and mortality-dependent study dropout. One limitation of the study is missing data – as in any epidemiological study with a long-term follow-up, particularly those involving older adults. The varying degrees of missing data on strength among study participants were arguably informative (i.e. non-ignorable missing21) rather than random. The degree to which the missing data may bias a given analysis depends on the analytic method used and the degree to which those with observed data represent those in the population targeted by sampling27. By including indicators of health risks (i.e. race, education, BMI, level of physical activity, and inflammation) that were associated with study drop-out in the longitudinal models as covariates, we would hope to achieve a reasonable approximation to the missing at random assumption21, thereby providing at least partial protection against biased inference that would otherwise be induced by informative dropout unaccounted for by mortality risk. On the other hand, given there was an overall decline in grip, knee, and hip strength over time, selective loss of the oldest and most cognitively and physically impaired participants likely resulted in conservative estimates of true decline and its effect on total mortality. However, the magnitude of such selection bias is hard to quantify without additional modeling assumptions that can neither be confirmed nor refuted based on the observed data.
An overwhelming body of observational and experimental evidence suggests that aging-related decline in muscle strength is reversible through progressive resistance strength training (PRT) even in the very old16. A recent Cochrane review of 73 trials on this topic found a moderate-to-large beneficial effect of PRT on lower-limb extensor muscle strength, with a mean difference of 0.84 SD (95% CI=0.67–1.00) over 8–12 weeks between PRT and non-PRT groups16. While the leg extensor group of muscles was the most frequently evaluated in the PRT studies16, grip strength as a prognostic nutritional parameter was often assessed in the nutritional intervention studies28–30. In a trial of 80 malnourished adults in middle and old age with benign digestive disease, Norman et al. reported a 0.48 SD improvement in grip strength after a three-month treatment with protein and energy rich supplements compared to 0.1 SD among the controls29. An 8-week Oral nutrition supplementation given to 136 older adults aged 75 and older following hospital discharge was also found to be associated with 0.39 SD increase in grip strength compared to 0.12 SD in non-supplemented controls28. The fact that we found an average decline of about 0.2 SD per year in grip and hip strength between age 70 and 75 give us a great sense of hope and optimism for the future of exercise and nutritional therapies.
In summary, muscle strength is an important marker and a potential cause of mortality risk in old women. Monitoring the rate of decline in grip and hip flexion strength in addition to the absolute levels may greatly improve the identification of women most at risk of dying. It is also important to note that due to observed limited heterogeneity in the rate of decline among women with extreme high or low strength at age 70, the results from our study may be most relevant for women with values at the middle of the strength distribution where individual differences in rate of change could be most predictive of future mortality. Despite the benefits of PRT and nutritional intervention for preventing, delaying, and reversing muscle strength decline in older adults, we do not know whether improved muscle strength translates into improved performance of daily tasks31–33, as the latter may involves a number of other factors (e.g., home environment, psychological, social support) that may be independent of or interact with muscle strength. Our findings do raise the question of whether there are critical points in the decline in muscle strength where interventions would be most effective and should be targeted for intervention.
Acknowledgments
Sponsor’s Role: Had no role in the design, methods, subject recruitment, data collections, analysis, or preparation of paper.
This study was supported by the Johns Hopkins Older Americans Independence Center under contracts P30-AG02133 and R01-AG11703, R01-AG023701 from the National Institute on Aging, National Institute of Health. Dr. Guralnik was supported in part by the Intramural Research Program, National Institute on Aging, NIH.
Appendix
To assess the effects of baseline as well as change in grip, knee, and hip strength over time on the risk of mortality, we performed joint analysis of the repeated measurements of strength and time-to-death by explicitly modeling the dependency between the time trends of strength change and the survival time via a nonlinear mixed effects model34. Specifically, the joint model consists of two submodels: linear random effects growth curve model (REGCM) for the longitudinal process of change in strength (termed “longitudinal submodel”) and accelerated failure time model (AFT) for the survival process (termed “survival submodel”). Suppose that the ith person had grip strength measurements yi1, …, yini over ni successive exams (i=1, …, n), the REGCM characterizes the trajectory of grip strength as:
| [1] |
where tij is the time in years of person i’s jth evaluation since age 70 (j=1, …, ni); β0 + β1tij is the population mean trajectory by which grip strength changes over time (i.e. fixed effects), and bi0 + bi1tij is the deviation of person i’s trajectory from the mean trajectory (i.e. random effects), such that β0 + bi0 and β1 + bi1 can be interpreted as the individual-level grip strength at age 70 and the annual rate of decline in grip strength, respectively, for person i. Model [1] typically assumes that (bi0, bi1, εij) are normally distributed with mean 0 and the residual errors ε are independent of (bi0, bi1). The REGCM differs from the conventional mean effects regression in that it describes the extent to which individual baseline and rate of change vary about the population mean (i.e. between-person heterogeneity) via the random effects. The degree and significance of the heterogeneity can be assessed based on the variances of bi0 and bi1. To account for the nonlinear time trends in changes of grip and hip strength, a two-piece linear spline was used in the REGCM with one knot fixed at age 75:
| [2] |
where, I(tij-5) is a binary indicator (=1 if tij>5, 0 otherwise); bi0 and bi1 are the random intercept and random slope before age 75, respectively. β1 and β1+β2 are the rates of change in strength before and after age 75, respectively. We were unable to estimate random effect for age slope after age 75, which could either mean that there was no additional significant between-person variability in age slope after age 75 after controlling for the between-person heterogeneity before age 75, or the sample size and/or the cluster size (i.e., number of repeated measurements within a subject) are too small to reliably estimate.
The validity of the REGCM relies on the assumption of data missing at random21, we included race, education, BMI, physical activity, and IL-6 that were predictive of early dropout in the model as covariates in models [1] and [2] to minimize the impact of non-ignorable missing data, a statistical adjustment technique commonly used in the missing data literature. Since the main interest of our study is to assess the independent association between change in muscle strength and mortality, the covariates adjusted in the longitudinal submodel hence bear little scientific interest.
The survival submodel is specified as
| [3] |
where the survival time (i.e. time to death since study baseline), st, was assumed to follow a Weibull distribution (k,μi(st)) with shape parameter k and mean survival time μi(st) for person i. The fact that the relationship between log(−log(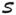 (st)), where
(st)), where  (st) is the Kaplan Meier estimate of the survival function, and log(st) can be approximated by a straight line supports the validity of the Weibull distribution assumption. In [3], zi denotes the confounders (e.g. age at study baseline) of the association between strength and mortality; bi0 and bi1, respectively, are individual-specific absolute strength at age 70 and rate of decline estimated from the longitudinal submodel [1] for knee strength and submodel [2] for grip and hip strength, with corresponding regression coefficients γ1 and γ2 representing the effects of individual trajectory of strength on log survival time. For the ease of model interpretation, we converted the regression coefficients γ=(γ1,γ2) estimated from the model [3] using the formula exp(−kγ) to permit the usual hazard ratio interpretation as in the conventional Cox proportional hazards model. The parameters for the two submodels were estimated jointly via maximum likelihood using SAS PROC NLMIXED as detailed by Guo and Carlin34.
(st) is the Kaplan Meier estimate of the survival function, and log(st) can be approximated by a straight line supports the validity of the Weibull distribution assumption. In [3], zi denotes the confounders (e.g. age at study baseline) of the association between strength and mortality; bi0 and bi1, respectively, are individual-specific absolute strength at age 70 and rate of decline estimated from the longitudinal submodel [1] for knee strength and submodel [2] for grip and hip strength, with corresponding regression coefficients γ1 and γ2 representing the effects of individual trajectory of strength on log survival time. For the ease of model interpretation, we converted the regression coefficients γ=(γ1,γ2) estimated from the model [3] using the formula exp(−kγ) to permit the usual hazard ratio interpretation as in the conventional Cox proportional hazards model. The parameters for the two submodels were estimated jointly via maximum likelihood using SAS PROC NLMIXED as detailed by Guo and Carlin34.
Goodness-of-fit criteria including log likelihood ratio, the Akaike Information Criterion (AIC), and the Bayesian Information criterion (BIC) were used for model selection. To assess global goodness-of-fit of the joint model, we visually compared the observed to the model-based estimates of population average trajectories of strength and time-to-death. The close agreement between the observed and the fitted estimates indicated that the joint longitudinal and survival model fit the data reasonably well (results not shown).
To compare the rates of decline in grip, hip, and knee strength, we extended the univariate REGCM [1] to a multivariate REGCM as follows:
| [4] |
where, Iknee, and Ihip are binary indicators with a value of 1 if yij is a measurement of the type of strength denoted by the subscript of I and 0 otherwise; β01 represents population average grip strength at age 70 and β02 (β03) is the difference between population mean grip strength and knee (hip) strength at age 70; β11 is population average rate of change in grip strength and β12 (β13) is the difference between the population average rate of change in grip and that of knee (hip); (bi01, bi02, bi03) and (bi11, bi12, bi13) denote random effects that follow a multivariate normal distribution with an unstructured variance-covariance matrix. The nonlinear age effect on changes of grip and hip strength was accommodated by including in [4] a linear spline for age as in Model [2].
Footnotes
Preliminary results of this study were presented at the Gerontological Society of America 62nd Annual Scientific Meeting in Atlanta, Georgia on Nov. 19, 2009.
Conflict of Interest: None.
Author Contributions: Qian-Li Xue, PhD: content, concept and design, analysis and interpretation of the data, drafting of the manuscript, statistical expertise. Brock A. Beamer, MD: content, concept and design, obtaining funding, interpretation of the data, critical revision for important intellectual content. Paulo HM Chaves, MD, PhD: content, concept and design, interpretation of the data, critical revision for important intellectual content. Jack M. Guralnik, MD, PhD: content, concept and design, interpretation of the data, critical revision for important intellectual content, supervision. Linda P. Fried, MD, MHS: content, concept and design, obtaining funding, acquisition of subjects and data, supervision, critical revision for important intellectual content.
References
- 1.Nair KS. Muscle Protein-Turnover - Methodological Issues and the Effect of Aging. J Gerontol A Biol Sci Med Sci. 1995;50:107–112. doi: 10.1093/gerona/50a.special_issue.107. [DOI] [PubMed] [Google Scholar]
- 2.Viitasalo JT, Era P, Leskinen AL, et al. Muscular Strength Profiles and Anthropometry in Random Samples of Men Aged 31–35, 51–55 and 71–75 Years. Ergonomics. 1985;28:1563–1574. [Google Scholar]
- 3.Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997;83:1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 4.Rantanen T, Guralnik JM, Sakari-Rantala R, et al. Disability, physical activity, and muscle strength in older women: The Women’s Health and Aging Study. Arch Phys Med Rehabil. 1999;80:130–135. doi: 10.1016/s0003-9993(99)90109-0. [DOI] [PubMed] [Google Scholar]
- 5.Visser M, Deeg DJ, Lips P, et al. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–386. doi: 10.1111/j.1532-5415.2000.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 6.Xue QL, Bandeen-Roche K, Varadhan R, et al. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63:984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 7.Moreland JD, Richardson JA, Goldsmith CH, et al. Muscle weakness and falls in older adults: A systematic review and meta-analysis. J Am Geriatr Soc. 2004;52:1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 8.Rantanen T, Harris T, Leveille SG, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55:M168–173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 9.Rantanen T, Volpato S, Ferrucci L, et al. Handgrip strength and cause-specific and total mortality in older disabled women: Exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 10.Metter EJ, Talbot LA, Schrager M, et al. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 11.Laukkanen P, Heikkinen E, Kauppinen M. Muscle strength and mobility as predictors of survival in 75–84-year-old people. Age Ageing. 1995;24:468–473. doi: 10.1093/ageing/24.6.468. [DOI] [PubMed] [Google Scholar]
- 12.Al Snih S, Markides KS, Ray L, et al. Handgrip strength and mortality in older Mexican Americans. J Am Geriatr Soc. 2002;50:1250–1256. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- 13.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the Health, Aging and Body Composition Study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 14.Cesari M, Pahor M, Lauretani F, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64:377–384. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: Cross-sectional and longitudinal perspectives. J Gerontol. 1990;45:M82–88. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- 16.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database of Systematic Reviews. 2009:277. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried LP, Bandeen-Roche K, Chaves PHM, et al. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M43–M52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 18.Rantanen T, Guralnik JM, Izmirlian G, et al. Association of muscle strength with maximum walking speed in disabled older women. Am J Phys Med Rehabil. 1998;77:299–305. doi: 10.1097/00002060-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Guralnik J, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. (NIH publication no. 95–4009) [Google Scholar]
- 20.United States Department of Health and Human Services. Healthy People 2010. U.S. DHHS; 2000. [Google Scholar]
- 21.Rubin DB. Inference and Missing Data. Biometrika. 1976;63:581–592. [Google Scholar]
- 22.Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics. 2000;1:465–480. doi: 10.1093/biostatistics/1.4.465. [DOI] [PubMed] [Google Scholar]
- 23.Maddala GS. Limited –dependent and Qualitative Variables in Econometrics. Cambridge, U.K.: Cambridge University Press; 1983. [Google Scholar]
- 24.Schemper M, Stare J. Explained variation in survival analysis. Stat Med. 1996;15:1999–2012. doi: 10.1002/(SICI)1097-0258(19961015)15:19<1999::AID-SIM353>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 25.Pijnappels M, van der Burg PJ, Reeves ND, et al. Identification of elderly fallers by muscle strength measures. Eur J Appl Physiol. 2008;102:585–592. doi: 10.1007/s00421-007-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purser JL, Pieper CF, Poole C, et al. Trajectories of leg strength and gait speed among sedentary older adults: longitudinal pattern of dose response. J Gerontol A Biol Sci Med Sci. 2003;58:M1125–1134. doi: 10.1093/gerona/58.12.m1125. [DOI] [PubMed] [Google Scholar]
- 27.Little RJA, Rubin DB. Statistical Analysis with Missing Date. New York: Wiley; 1987. [Google Scholar]
- 28.Price R, Daly F, Pennington CR, et al. Nutritional supplementation of very old people at hospital discharge increases muscle strength: A randomized controlled trial. Gerontology. 2005;51:179–185. doi: 10.1159/000083991. [DOI] [PubMed] [Google Scholar]
- 29.Norman K, Kirchner H, Freudenreich M, et al. Three month intervention with protein and energy rich supplements improve muscle function and quality of life in malnourished patients with non-neoplastic gastrointestinal disease – A randomized controlled trial. Clin Nutr. 2008;27:48–56. doi: 10.1016/j.clnu.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Alvares-da-Silva MR, Reverbel DS. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–117. doi: 10.1016/j.nut.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Keysor JJ. Does late-life physical activity or exercise prevent or minimize disablement? A critical review of the scientific evidence. Am J Prev Med. 2003;25:129–136. doi: 10.1016/s0749-3797(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 32.Miszko TA, Cress ME, Slade JM, et al. Effect of strength and power training on physical function in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2003;58:171–175. doi: 10.1093/gerona/58.2.m171. [DOI] [PubMed] [Google Scholar]
- 33.de Vreede PL, Samson MM, van Meeteren NL, et al. Functional-task exercise versus resistance strength exercise to improve daily function in older women: A randomized, controlled trial. J Am Geriatr Soc. 2005;53:2–10. doi: 10.1111/j.1532-5415.2005.53003.x. [DOI] [PubMed] [Google Scholar]
- 34.Guo X, Carlin BP. Separate and joint modeling of longitudinal and event time data using standard computer packages. Am Stat. 2004;58:16–24. [Google Scholar]