Abstract
Background
Impulsive behavior in humans predicts the onset of drinking during adolescence and alcohol use disorders (AUDs) in adulthood. It is also possible, however, that heavy drinking may increase impulsive behavior by affecting the development of brain areas that support behavioral control or through other associated mechanisms. This study examined whether drinking heavily during adolescence is related to changes in impulsive behavior with a specific focus on how the association differs across individuals, contingent on the developmental course of their impulsiveness.
Method
Data came from a sample of boys (N=503) who were followed annually from approximate age 8 to age 18 and again at approximate age 24/25. Heavy drinking was defined as experiencing a blood alcohol concentration (BAC) level of .08% or higher. At each assessment, the parent and child each reported whether the child was impulsive.
Results
First, group-based trajectory analysis was used to identify four groups differing in the level and slopes of their trajectories of impulsive behavior from age 9 to age 17: low (13.9%), early adolescence-limited (18.7%), moderate (60.8%) and high (6.6%). These trajectory groups differed in their prevalence of any heavy drinking, peak BACs, and rates of alcohol dependence in adolescence and AUD in early adulthood, with the less impulsive groups being lower on these measures than the more impulsive groups. Heavy drinking was then entered into the model as a time-varying covariate; this measure was lagged so that the results represent change in impulsive behavior the year following heavy drinking. Among boys on the moderate trajectory, those who drank heavily were rated as significantly more impulsive the following year compared to those who did not drink heavily.
Conclusions
The association between heavy drinking and impulsive behavior may depend on earlier levels of impulsive behavior with those who are moderately impulsive appearing to be at greatest risk for increased impulsive behavior following heavy drinking. Further research is needed to clarify this association.
Keywords: impulsivity, impulsive behavior, heavy drinking, adolescence, trajectories
Childhood and adolescent impulsive behavior consistently has been found to predict early-onset drinking and alcohol use disorders (AUD) in adolescence and adulthood (Dawe and Loxton, 2004; Gullo and Dawe, 2008; Verdejo-Garcia et al., 2008). Although the direction of the association is debatable, recent studies suggest that impulsive behavior generally precedes the onset of heavy drinking (Verdejo-Garcia et al., 2008). This predominant temporal pattern may simply be due in part to general developmental trends; that is, impulsive behavior tends to manifest during childhood, while heavy drinking usually starts later. It is generally known that acute alcohol causes increases in impulsive behavior across species, but less is known about the effects of chronic alcohol use on impulsivity in animals or humans (Dick et al., 2010). While there is some evidence linking chronic alcohol use with decreased behavioral self-control in late-stage addiction (e.g., Koob and LeMoal, 2009), virtually nothing is known about the developmental course of alcohol use and impulsive behavior earlier in the life span and prior to the onset of AUD (Dick et al.,2010;Verdejo-Garcia et al., 2008). The aim of this study was to provide an initial examination of the prospective associations between heavy drinking episodes and changes in impulsive behavior among a sample of males followed from childhood (before heavy drinking started) through late adolescence. We focused specifically on how these associations might differ for boys who were already on different trajectories of impulsive behavior.
Impulsive Behavior and Alcohol Use
Moeller and colleagues (2001, p. 1784) defined impulsivity as “a predisposition toward rapid, unplanned reactions to internal or external stimuli without regard to the negative consequences of these reactions…” In recent studies, impulsivity has been described as multi-faceted and it has been assessed by many different self-report questionnaires as well as laboratory paradigms (Dawe and Loxton, 2004; Dick et al., 2010). In this initial paper, we study impulsive behavior as defined by the parent and the child.
Adolescence is a period of development characterized by high levels of impulsive behavior. Adolescence is also the period in which drinking is initiated for most adolescents, with a substantial proportion of adolescents engaging in heavy drinking behavior during this period. Crews and colleagues (2007) proposed that heavy alcohol use in adolescence can lead to alterations in brain structure that reduce behavioral (impulse) control, which could promote further heavy drinking. This hypothesis has been supported by several animal studies (e.g., Clark et al., 2008; Crews et al., 2000; Crews et al., 2007; Ernst et al., 2005; Nasrallah et al., 2009; Pascual et al., 2009). For example, Nasrallah et al. (2009) found that early adolescent alcohol use led to increased risky decision making, an indicator of altered behavioral control.
Correlational studies have found higher rates of impulsive behavior in samples with substance use disorders compared to non-using controls (Verdejo-Garcia et al., 2008). In addition, heavy drinking in adolescence has been linked to impairment of executive cognitive functioning (ECF), which governs impulse control (Giancola et al., 2001; Moss et al., 1994; Squeglia et al., 2009). Longitudinal studies have found deteriorations in visuospatial functioning and attention (two indicators of ECF) in adolescents with AUD who continued heavy drinking and reported hangovers or withdrawal symptoms compared to those with AUD who stopped drinking (Tapert and Brown, 1999; Tapert et al., 2002). A recent study examined changes in neurocognitive functioning among adolescents as they transitioned from nondrinking to drinking (Squeglia et al., 2009). The findings indicated that initiating moderately heavy drinking and experiencing hangovers adversely influenced neurocognitive functioning. The authors suggested that neurocognitive deficits linked to heavy drinking during this critical developmental period may lead to direct and indirect changes in neuromaturational course, and these effects could extend into adulthood.
In summary, alcohol-induced disruption of adolescent brain development (Crews et al., 2007) may be associated with reduced ECF, which can result in heightened impulsive behavior. In turn, heightened impulsive behavior may lead to further heavy drinking and thereby increase negative alcohol use consequences and the risk for addiction. Therefore, it is important to better understand the associations between heavy drinking and impulsive behavior during adolescent development. Most previous research has examined the hypothesis that impulsivity may increase risk for heavy drinking with generally positive support (Verdejo-Garcia et al., 2008). We extend the literature by examining the possibility that heavy drinking may also increase impulsive behavior. We note that these two hypotheses are not mutually exclusive; rather, bi-directional effects may lead to a cycle of impulsive behavior increasing the likelihood of heavy drinking, which further increases impulsive tendencies. Finally, we acknowledge that the association of heavy drinking and impulsive behavior is almost certainly influenced by common factors such as temperament, family and peer characteristics, biological vulnerabilities, and educational achievement. In this initial study, however, we focused on the time-lagged association between heavy drinking and impulsive behavior to provide a foundation for the future examination of more complex and integrative models.
Current Study
This prospective human study examined the association between heavy drinking and impulsive behavior during adolescence, taking into account earlier differences in impulsive behavior. The aim was to examine whether heavy drinking in one year was associated with changes in impulsive behavior trajectories in the following year. Although several factors (e.g., personality, delinquency, temperament, biological characteristics, and educational achievement) may be related to both heavy drinking and impulsive behavior, focusing on these two constructs alone and teasing apart the nature of their relationship will enhance our understanding of how they are related and fill a gap in the literature on how heavy drinking during adolescence, rather than chronic heavy drinking evidenced by individuals with AUD, is related to impulsive behavior.
The sample was comprised solely of males. This sample was informative for this initial study because boys, compared to girls, exhibit higher average levels of impulsive behavior (e.g., Nolen-Hoeksema, 2004) and drink more heavily during adolescence (Johnston et al., 2009). Although it is possible that there may be gender differences in associations between heavy drinking and impulsive behavior (Squeglia et al., 2009; Giedd et al., 2006), we were particularly interested in how the association between heavy drinking and subsequent impulsive behavior might differ across males contingent on their level and trajectory of impulsive behavior during adolescence. We expected that, overall, boys would increase their levels of impulsive behavior following their exposure to heavy drinking. We predicted that adolescents with very high levels of impulsive behavior would be relatively less amenable to change in response to the effects of heavy drinking because their levels of impulsive behavior were already quite high without much room to increase. Therefore, we expected to see stronger effects of heavy drinking among those adolescents with more moderate levels of impulsive behavior. In order to test these hypotheses, we used group-based trajectory analysis to define typical impulsive behavior trajectories and then examined whether heavy drinking was associated with deflections from (i.e., increases or decreases off of) these typical trajectories.
Methods
Design and Sample
We analyzed longitudinal data (N=503) from the Pittsburgh Youth Study (PYS; Loeber et al., 2008), a study of the development of substance use, delinquency and mental health problems among inner-city males. In 1987, a random sample of first grade students from the City of Pittsburgh public schools was screened. Initially 85% of the target sample was screened and the 15% nonparticipation rate did not result in sample bias in achievement test results and racial distribution, the only two variables that could be compared from school records (Loeber et al., 2008). After the initial screening, the 30% who screened at highest risk for antisocial behavior (based on parent, teacher, and participant information) were included in the sample for follow-up, together with 30% randomly selected from the remainder for a total sample of 503 boys.
At the first assessment, the boys averaged 7 years of age. The first eight assessments were conducted half-yearly and combined to create an annual measure, and the rest were conducted yearly through average age 20. A further follow-up assessment was conducted approximately five years later when the boys averaged 24-25 years of age. Participation rates averaged above 90% across yearly assessments and were 85% at the age 24-25 follow-up.
A little more than half (55.9%) of the sample was African American, and almost all the rest (41.4%) was White, reflecting the racial composition of the City of Pittsburgh public schools when the study began. The rest of the sample was mixed or other. Over 90% lived with their biological mother at the initial assessment. More than one-third of the boys' families received public assistance or food stamps. (For greater detail, see Loeber et al., 2008.)
Measures
At each wave, boys who drank in the last year were asked the typical quantity per occasion (6-point scale ranging from 0 to 6 or more drinks) separately for beer, wine and hard liquor. They also reported their body weight at each assessment. Using a modified Widmark (1932/1981) formula, we calculated the typical blood alcohol concentration (BAC) each year for each type of beverage and then selected the highest BAC at each assessment. The National Institute on Alcohol Abuse and Alcoholism (2004) has designated a BAC of .08% or higher as an indicator of excessive drinking. Therefore, each year that a boy achieved a BAC of at least .08% (allowing for one hour of burnoff) was counted as heavy drinking. The heavy drinking variable was dichotomized and coded 1 in all periods that the boy reached a BAC of .08% or above and 0 in all other periods. None of the boys had drunk heavily before age 9.
The Child Behavior Checklist (CBCL;Achenbach, 1991) completed by the parent/primary caretaker and the Youth Self-Report (YSR; Achenbach, 1991) completed by the participant were used to measure impulsive behavior. Both instruments contained a single item, “is impulsive or acts without thinking,” with an ordinal response set of: 0 (“not true”), 1 (“somewhat or sometimes true”), and 2 (“very true or often true”). We took the maximum score from the parent or child report at each age from ages 9 to 17, as supported by research on inter-informant agreement and the “best-estimate” approach (e.g., Baillargeon et al., 2001; Cantwell et al., 1997). This best estimate approach is often used in studies that have both parent and child reports because it provides a more sensitive estimate of traits and symptoms compared to using only one informant.
At approximate age 18 and again at the age 24/25 follow up, AUD was assessed using the Diagnostic Interview Schedule (DIS; Robins & Helzer, 1988). The DIS has demonstrated reliability and construct validity in previous investigations (see Malgady et al., 1992). At approximate age 18, a trained interviewer administered the paper version of Diagnostic Interview Schedule for DSM-III-R diagnoses (DIS-III-R; Robins and Helzer, 1988). At approximate age 24/25, participants were administered the computerized version of the DIS for DSM-IV diagnosis (DIS-IV; Erdman et al., 1992). For this study, we created two dichotomized variables at each DIS administration. One consisted of having met (coded 1) or not met (coded 0) the lifetime criteria for alcohol abuse or (probable or definite) dependence (AUD) and the second was having met (coded 1) or not met (coded 0) the lifetime criteria for probable or definite alcohol dependence (DEP).
Analyses
Group-based trajectory analysis (Nagin, 1999, 2005; Nagin and Tremblay, 2001) using the SAS PROC TRAJ macro (Jones et al., 2001) was used to define trajectory groups and examine predictors of deflections off those trajectories.1 This approach was chosen because it allowed us to examine how initial episodes of heavy drinking were associated with changes in impulsive behavior. Unlike other approaches, it did not require us to simply predict from one particular age to the next; instead, it allowed us to examine several typical impulsive behavior trajectories over time, and examine changes from those trajectories following heavy drinking, at whatever age it occurred. In addition, this method allowed us to examine potential differences in these associations by trajectory groups that reflect differences in level of impulsive behavior. As noted above, we did not expect youth with very high levels of impulsive behavior to evidence substantial changes in this behavior following heavy drinking, and we expected the largest changes in those with moderate levels of impulsive behavior. Finally, this analytic approach was more appropriate for our ordinal measure of impulsive behavior compared to other methods that require continuous variables (e.g., autoregressive models). Thus, although there are advantages and disadvantages to different analytic approaches, this was considered to be the most appropriate analytic method to address our questions.
First, trajectories of impulsive behavior were identified using data from ages 9 through 17. We began with a two-group model, then added trajectory groups one at a time until the best-fitting model of distinctive trajectories was determined (we tested up to a seven-group model). Procedures outlined in Nagin (2005) were used to select the model that best fit the data. Specifically, we examined fit statistics (Bayesian Information Criterion [BIC]), considered the size of the trajectory groups (i.e., we eliminated models with trajectory groups comprised of less than 5% of the sample because this potentially indicates overextraction of groups and the resulting groups would subsequently have had low power to detect group differences), examined the average posterior probabilities (pp) of assignment to the most likely class (above the .7 minimum threshold), and inspected how well the trajectories were distinguished from each other. In these initial analyses, intercept and linear, quadratic, and cubic slope terms were included to model the trajectories. Once the best-fitting number of trajectories was determined, we examined the significance of each slope term and pared down the insignificant slope terms, using BIC values as a guide, until the best-fitting model was obtained (Nagin, 2005). As will be shown in Table 1 (row 1, standard deviations), there was considerable variability within each group in terms of impulsive behavior at each age. This indicates that within the trajectory group, it was possible to predict deflections off of the mean trajectories. It should be noted, however, that our ability to detect negative deflections off the low group and positive deflections off the high group may have been compromised by the restricted range of our measure. The resulting impulsive behavior trajectory groups were compared in terms of drinking patterns and problems using chi square analysis and analysis of variance (ANOVA).
Table 1. Group Differences in Impulsive Behavior, Adolescent Drinking, and Late Adolescent and Young Adult Alcohol Use Disorders.
| Trajectory Group | ||||
|---|---|---|---|---|
| Low | Adolescence-Limited | Moderate | High | |
| Mean impulsive behavior score ages 9-17 | 0.17 (SD=0.37) | 0.49 (SD=0.46) | 0.88 (SD=0.55) | 1.55 (SD=0.54) |
| Percent drinking heavily by age 17 | 31.4% | 31.9% | 41.6% | 57.6% |
| Mean peak BAC by age 17 | .05% | .06% | .08% | .10% |
| Mean age of onset of heavy drinking among boys who drank heavily by age 17 | 14.7 (SD=1.7) | 14.4 (SD=1.9) | 14.6 (SD=1.9) | 14.8 (SD=1.1) |
| Mean age of onset of drinking among boys who drank heavily by age 17 | 13.1 (SD=3.2) | 11.9 (SD=2.7) | 11.8 (SD=3.1) | 10.8 (SD=3.3) |
| % AUD at approx. age 18 | 6.6% | 9.6% | 15.1% | 20.7% |
| % AUD at approx. age 24/25 | 26.2% | 37.7% | 45.2% | 41.9% |
| % DEP at approx. age 18 | 0.00% | 1.20% | 4.38% | 10.34% |
| % DEP at approx. age 24/25 | 6.56% | 9.52% | 11.07% | 13.79% |
AL=Adolescence-Limited; Mod=Moderate; BAC=Blood Alcohol Concentration; AUD=Alcohol Use Disorder (abuse or dependence); DEP=Alcohol Dependence (probable or definite); approx.=approximate; SD= standard deviation.
After the overall trajectories of impulsive behavior were defined, the model was re-estimated with heavy drinking included as a time-varying pre-lagged predictor. Although this type of analysis has the potential to reassign participants to different groups, 92% of the participants were assigned to the same group in both models. Using this approach, we could examine whether, for example, if heavy drinking occurred at age 11, it was associated with a significant deflection off the impulsive behavior trajectory at age 12. All years from ages 8 to 16 were examined for possible heavy drinking, and, thus, each individual's pattern of heavy drinking over time was allowed to vary. In each year that a boy drank heavily he received a code of “1” and in each year that he did not drink heavily he received a code of “0.”. For example, if a boy began drinking heavily at one age and continued to drink heavily during each subsequent year, he would have heavy drinking codes of “1” for every year starting from the year in which he first initiated heavy drinking. In contrast, if a boy drank heavily during one year but never again during the period under study, he would have a code of “1” only during the year he drank heavily, but “0”s for all other years.
This analytic approach accounts for prior levels of impulsive behavior by grouping boys based on impulsive behavior trajectories beginning in childhood. This also accounts for external influences on impulsive behavior (for example, the influence of genetic predispositions to impulsive behavior and lack of parental monitoring should be “wrapped into” the assignment to trajectory groups). Therefore, the significance of the covariate (heavy drinking) represents a measure of association between heavy drinking and impulsive behavior, above and beyond trajectory group membership or early influences of other variables.
Parameter estimates and their associated significance levels provided specific information about how heavy drinking was related to changes in impulsive behavior for boys within each trajectory group. These effects can be thought of as deflections (increases or decreases) from the boy's main trajectory. These findings would not be specific to a particular age; instead, they would indicate that at any age, a boy in that trajectory group who had drunk heavily during that year would be likely to have higher levels of impulsive behavior the following year than a boy who had not experienced that event during that year.
All available data were used in the analyses. Proc Traj uses maximum likelihood estimation to deal with missing data in forming trajectories (Jones et al., 2001). Fifteen participants had all missing data on heavy drinking and therefore their data did not contribute to the models examining the effect of heavy drinking on impulsive behavior. Those 15 cases did not differ significantly from other participants on risk status assessed at screening (χ2=0.73, df=1, p>.05).
Results
Trajectories of Impulsive Behavior
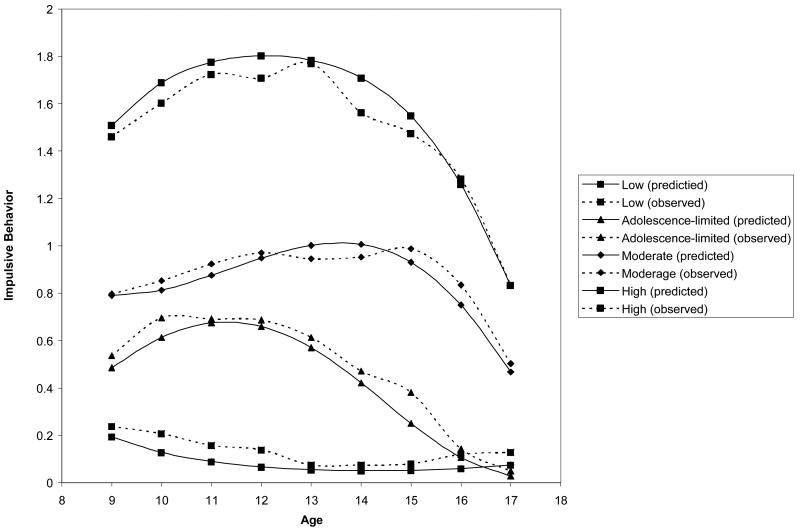
Based on the model selection procedure described above, the four-group model fit best.2 The four different impulsive behavior trajectory groups identified from ages 9 to 17 were: 1) low (n=70, 13.9%, pp=.90), 2) early adolescence-limited (n=94, 18.7%, pp=.78), 3) moderate (n=306, 60.8%, pp=.88), and 4) high (n=33, 6.6%, pp=.88) (see Figure 1). The low, early adolescence-limited, and high groups were best characterized with linear and quadratic terms, whereas the moderate group required a cubic term as well. The low group displayed very low levels of impulsive behavior throughout late childhood and adolescence. The early adolescence-limited group displayed some impulsive behavior during late childhood and early adolescence but then declined rapidly. The moderate group displayed relatively high levels of impulsive behavior through middle adolescence and then declined. Finally, the high group was highly impulsive in childhood and adolescence but began to decline somewhat in later adolescence.
Figure 1.
Impulsive behavior trajectories from ages 9 through 17.
Table 1 shows means and standard deviations of impulsive behavior for the four groups, as well as mean and percentage differences among the four groups on drinking patterns during adolescence and AUD in late adolescence and young adulthood. As expected, the overall impulsive behavior mean was lowest in the low impulsive behavior group, next lowest in the adolescence-limited group, higher in the moderate group, and highest in the high group. In addition, the standard deviation for impulsive behavior was lowest for the low impulsive behavior group, who maintained a relatively stable level of impulsive behavior throughout adolescence. The standard deviations were similar across the other three groups especially for the moderate and high groups. Thus, despite large differences in levels of impulsive behavior, the latter three groups displayed similar levels of variation. The two middle groups showed a range in scores from 0 to 2 at most ages. However, the low group had a restricted range (0-1) at all ages except age 15 and the high group had a restricted range (1-2) between ages 9 and 13 (the range data at each age are not shown in the table but are available from the first author upon request).
Analyses of variance and chi square analyses were conducted to compare the four trajectory groups on drinking variables. Rates of heavy drinking at some point during adolescence (by age 17) differed across the trajectory groups, with the highest rates found in the high group and the lowest rates found in the low and early adolescence-limited groups (χ2=9.22, df=3, p<.05). Average peak BAC between ages 8 and 17 differed significantly across groups (F(487,3)=4.30, p<.01). Specifically, the moderate and high groups reported significantly higher peak BACs than the low and early adolescence-limited groups. Among those who had engaged in heavy drinking by age 17 (n=192), there were no significant differences among groups in their age of first heavy drinking (F(3,188)=.25, p>.05) or age at first drink (F(3,161)=1.86, p>.05). Because of small and/or empty cells, it was inadvisable to compare the statistical significance of differences in AUD or DEP between the four groups, especially at age 18. However, descriptively, the AUD and DEP percentages increased linearly across groups. In addition, when chi square analyses were conducted combining the moderate and high groups vs. the low and early adolescence-limited groups, the former, compared to the latter, two groups were significantly more likely to meet the criteria for lifetime DEP at age 18 (χ2=8.02, df=1, p<.05) and AUD at age 25 (χ2=5.79, df=1, p<.05). Overall these results suggest that the four impulsive behavior trajectories differed in their alcohol use behaviors; especially the high and moderate groups differed from the low and early adolescence-limited groups.
Effects of Heavy Drinking on Changes in Impulsive Behavior
When we added heavy drinking as a lagged time-varying variable to the model, heavy drinking was associated with a positive deflection off the moderate trajectory (parameter estimate=.21, SE=.08, p<.05). Heavy drinking was not associated with significant deflections off of the other three trajectories (low: parameter estimate=.33, SE=.26; early adolescence-limited: parameter estimate=.27, SE=.25; high: parameter estimate=-.15, SE=.29; all p-values >.05). Thus, heavy drinking was associated with an increase in impulsive behavior in the following year among boys who were moderately high in impulsive behavior during adolescence. Note, however, that the standard errors were considerably larger for the other three groups than the moderate group. Therefore, the fact that only the moderate group showed a significant effect of heavy drinking may reflect a power issue (i.e., smaller number of boys in each of these groups compared to the moderate group) rather than substantive differences among groups.
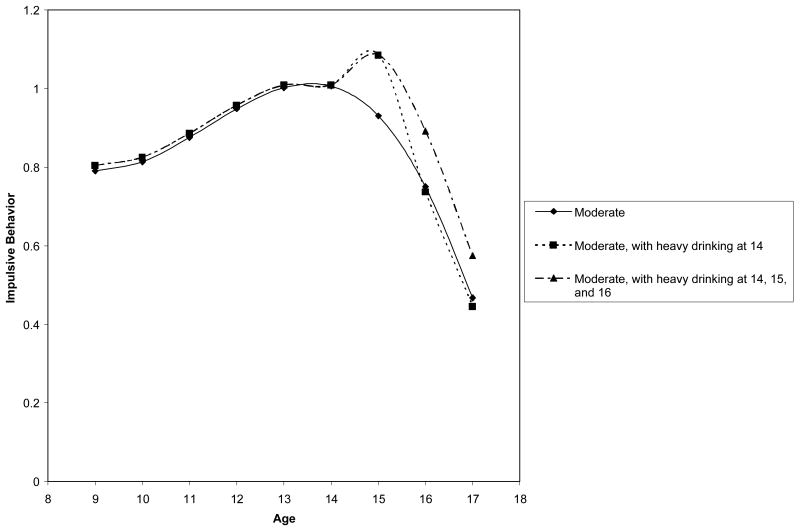
The effect of heavy drinking for the moderate group is illustrated in Figure 2, which represents prototypical plots of the model-predicted impulsive behavior levels of boys in the moderate trajectory group who (1) first drank heavily at 14 but never again during the period under study, and (2) first drank heavily at 14 and continued to drink heavily every year during the period under study. The results of these analyses (along with the prototypical plot) show that boys in the moderate group who first drank heavily at age 14, compared to those who did not drink heavily by age 14, showed higher levels of impulsive behavior at age 15. In addition, those boys who first drank heavily at age 14 and continued to drink heavily at ages 15 and 16 maintained their increased levels of impulsive behavior throughout adolescence. The analyses did not directly test what happened to boys who first drink heavily at a particular age and then did not drink heavily again. However, the model-predicted plots of boys exhibiting this pattern suggest that those boys who did not continue to drink heavily returned to levels of impulsive behavior comparable to those boys who had not drunk heavily.
Figure 2.
Prototypical plot of: (1) the model-predicted levels of impulsive behavior for a hypothetical boy in the moderate trajectory group who experienced his first occasion of heavy drinking at age 14 but did not continue to drink heavily at 15 or 16; and (2) the model-predicted levels of impulsive behavior for a hypothetical boy in the moderate trajectory group who experienced his first occasion of heavy drinking at age 14 and continued to drink heavily at ages 15 and 16 (along with the overall model-predicted trajectory for comparison).
Discussion
This study extended previous research on the association between impulsive behavior and heavy drinking by prospectively examining this association from childhood to young adulthood, and examining the potential effect of heavy drinking on impulsive behavior. As expected, based on earlier studies (Dawe and Loxton, 2004; Gullo and Dawe, 2008; Verdejo-Garcia et al., 2008), we found that lower, compared to higher, levels of impulsive behavior in early adolescence were associated with less heavy drinking, lower peak BACs, and lower rates of alcohol dependence in adolescence, as well as lower likelihoods of AUD in young adulthood.
This study further tested whether heavy drinking in adolescence was associated with change in adolescents' levels of impulsive behavior in the following year. We found that for boys who were on a moderate trajectory of impulsive behavior, heavy drinking was associated with increased levels of impulsive behavior during the following year. In contrast, for boys who were relatively low in impulsive behavior (i.e., the low and early adolescence-limited trajectories groups) and boys who were very high in impulsive behavior, there was no significant association between heavy drinking and changes in impulsive behavior. The findings for the moderate group, which was comprised of over half (61%) of all boys, were consistent with our hypothesis and support the notion that heavy drinking is associated with increased impulsive behavior. Furthermore, given the potential lack of power to detect changes in the other groups due to smaller sample sizes and the somewhat restricted range of our measure (especially in the high and low groups), it is possible that this effect generalizes to all male adolescents. The effect could be due to brain alterations (Crews et al., 2007; Ernst et al., 2009). It is also possible that psychosocial mechanisms contribute to this finding; for example, boys who are only moderately impulsive who begin drinking heavily may be experiencing a “downward spiral” of functioning (perhaps engaging in delinquent behavior, reducing their engagement with school, etc.); these changes could be associated with less thinking before acting. Future research should test for potential mediating effects.
Boys in the high impulsive behavior group did not change their levels of impulsive behavior after drinking heavily. We had predicted that we would not see much change in this group given their relatively stable high levels of impulsive behavior. The lack of an effect on the high impulsive behavior group could be due to a ceiling effect; that is, these boys were so impulsive already that they could not be rated as more impulsive on our measure, which further limits power. Similarly, boys in the low and early adolescence-limited groups, who were relatively low in impulsive behavior during the years of alcohol initiation, also did not change their levels of impulsive behavior, at least significantly as detected by these analyses. Perhaps their executive cognitive reserve was sufficiently high (as indicated by their very low levels of impulsive behavior), that any impairment due to heavy drinking would not be very noticeable. Overall, the risk was greatest for those boys who were relatively impulsive but not at the highest levels.
This study did not directly examine what happened after a boy first engaged in heavy drinking but then did not drink heavily during the subsequent year. However, the model-predicted levels of impulsive behavior for such boys in the moderate group suggested a rebound effect after stopping heavy drinking. This finding is consistent with evidence for neural plasticity and cognitive recovery following cessation or reduction of heavy drinking in adults, while resumed drinking is associated with further brain damage and cognitive deficit (Bartsch et al., 2007; Bates et al., 2005; Pfefferbaum et al., 1995). Research by Tapert and colleagues (Tapert and Brown, 1999; Tapert et al., 2002) on adolescents with AUDs also supports the possibility of rebound effects on attention.
In this study we operationalized heavy drinking by typical quantity consumed each year. It might be necessary, however, to also include a measure of frequency to account for the extent and persistence of heavy drinking, which we did not do in this study. In addition, this study used a single item measure of impulsive behavior, without demonstrated reliability and validity. However, the fact that we found a linear association from the low to the high trajectory group for most measures of drinking, to some extent, validates the impulsive behavior trajectory groups and, thereby, the measure of impulsive behavior. Nevertheless, these results need to be interpreted cautiously and require replications using a better measure of impulsivity.
Previous research has found that impulsivity is multi-faceted. Dawe and colleagues (Dawe and Loxton, 2004; Gullo and Dawe, 2008) identified at least two different components, one representing rash, spontaneous or disinhibited behavior including high sensation seeking and another involving reward sensitivity. Furthermore, recent research indicates that even the disposition to rash behavior can be described by five different dispositions that are only moderately related: positive urgency, negative urgency, lack of planning, lack of perseverance, and sensation seeking (Dick et al., 2010). How our participants and their caretakers defined impulsive behavior is not known and it could mean different things to different people. Nevertheless, previous research indicates that the various types of impulsivity identified in the literature all relate to drinking behavior (Verdejo-Garcia et al., 2008). They may, however, be related differently to different measures of drinking behavior. For example, “urgency traits predict problem drinking, whereas sensation seeking predicts frequency of drinking” (Dick et al., 2010, p. 219). Therefore, it would be useful in future research to determine if heavy drinking during adolescence affects various dimensions of impulsivity differently.
Another limitation of this study was that it focused only on boys. Squeglia et al. (2009) found stronger brain effects of early adolescent heavy drinking for girls than for boys. Furthermore, neuropsychological changes in the developing adolescent brain take place sooner for girls than boys (Giedd et al., 2006). Therefore, the findings from this study should be replicated among female adolescents. Furthermore, we did not control for other demographic factors, such as race. However, chi-square analysis indicated no significant association between race and impulsive behavior trajectory group membership (χ2 =1.23, df=3, p=.75), and a supplemental analysis adjusting for the effect of race yielded an identical pattern of significant findings. In addition, this study assessed the associations between heavy drinking in one year and changes in impulsive behavior in the following year. We chose this method to ensure that the change in impulsive behavior was occurring after the heavy drinking occasion (i.e., so they were not occurring in the same year), but this time frame may have been less than ideal. It would have been helpful to be able to tell exactly when the change in impulsive behavior was occurring relative to the heavy drinking episode. Furthermore, during adolescence the brain (especially the frontal lobes that control ECF) is still in a significant state of maturation and this issue should be considered when interpreting the findings from this study. Also, other factors, such as head trauma, could potentially affect changes in the brain and affect these findings. We did not have measures of ECF or head injuries and thus these factors were not examined in this study. It is also conceivable that other factors that have an onset at the same time as heavy drinking (e.g., peer influences) could affect ratings of impulsive behavior and therefore our results. Such factors should be considered in future research.
With these caveats in mind, the findings of this initial study of the prospective effects of heavy drinking on impulsive behavior during adolescence may have important implications for prevention and future research. The existing research suggests that impulsive behavior in adolescence may be related to a relatively underdeveloped orbitofrontal cortex (Squeglia et al., 2009). As Squeglia and colleagues (2009) suggested, neurocognitive changes that occur during adolescence may be a mechanism through which individuals are placed at greater risk for heavy drinking and AUD (see also Gullo and Dawe, 2008). Thus, more research is needed to examine neurocognitive functioning during adolescence and its relationship to heavy drinking, especially among youths who differ in ECF. Our findings suggest that these associations may differ for boys with different levels of impulsive behavior and specifically that they may be strongest for the majority of boys who are moderately high in impulsive behavior during middle adolescence. Therefore, preventive interventions may have to be designed differently for boys with varying levels of impulsive behavior. Future research will be needed to clarify the mechanisms behind the associations that we found and to further explore adolescent behavior and neurobiology in the context of the developing brain (Crews et al., 2007).
Acknowledgments
This research was supported, in part, by grants from the National Institute on Alcohol Abuse and Alcoholism (ARRA R01 AA016798; P50 AA011605; K02 AA00325), National Institute on Drug Abuse (K01 DA022456; R01 DA411018), the National Institute of Mental Health (P30 MH079920; R01 MH73941), the Office of Juvenile Justice and Delinquency Prevention (96-MU-FX-0012; OJJDP 2005-JK-FX-0001); and the Department of Health of the Commonwealth of the State of Pennsylvania. Points of view in this document are those of the authors and do not necessarily represent the official position or policies of the U.S. Department of Justice.
Footnotes
Proc Traj assumes homogeneity within classes. There are advantages and disadvantages to this approach, compared to approaches that allow for within-class variation. Models that allow for within-class variation tend to fit well with fewer trajectories. However, due to this variation, sometimes individuals who are assigned to one trajectory group may actually have trajectories that are more similar to those of another group (in level, slope, or both) for part of the time period studied, obscuring the meaning of membership in a particular trajectory. For a full discussion of the pros and cons of these two approaches, see Nagin (2005).
The BIC for the four-group model was -4592.37. This compared to -4693.13, - 4610.55, -4583.39, -4583.67, -4583.28, and -4582.80, for the two-group, three-group, five-group, six-group and seven-group models, respectively. Although the absolute BIC value for the four-group model was larger than the five-group model, the difference between the two was less than 10 (Wasserman, 2000) and the BIC curve began to flatten at the four-group model. In addition, for the five-group model one of the groups contained only 4.4% of the sample. Therefore, we chose the four-group model as the best fitting model.
References
- Achenbach TM. Integrative Guide to the 1991 CBCL/4-18, YSR, and TRF Profiles. Burlington, VT: University of Vermont, Department of Psycology; 1991. [Google Scholar]
- Baillargeon RH, Boulerice B, Tremblay RE, Zoccolillo M, Vitaro F, Kohen DE. Modeling interinformant agreement in the absence of a “gold standard”. J Child Psychol Psychiatry. 2001;42:463–473. [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Bates ME, Voelbel GT, Buckman JF, Labouvie EW, Barry D. Short-term neuropsychological recovery in clients with substance use disorders. Alcoholism: Clin Exp Res. 2005:29367–377. doi: 10.1097/01.alc.0000156131.88125.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell DP, Lewinsohn PM, Rohde P, Seeley JR. Correspondence between adolescent report and parent report of psychiatric diagnostic data. J Am Acad Child Adolesc Psychiatry. 1997;36:610–619. doi: 10.1097/00004583-199705000-00011. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res. 2008;32:375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, III, Knapp DJ. Binge ethanol causes differential brain damage in young adolescent rats compared to adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O'Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alchol use disorders. Addict Bio. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman HP, Klei MH, Greist JH, Skare SS, Justead JJ, Robins LN, Helzer JE, Goldring E, Hamburger M, Miller JP. A comparison of two computer-administered versions of the NIMH Diagnostic Interview Schedule. J Psychiatr Res. 1992;26:85–95. doi: 10.1016/0022-3956(92)90019-k. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Shoal GD, Mezzich AC. Constructive thinking, executive functioning, antisocial behavior, and drug use involvement in adolescent females with a substance use disorder. Exper Clin Psychopharm. 2001;9:215–227. doi: 10.1037//1064-1297.9.2.215. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, et al. Puberty-related influences on brain development. Molec Cell Endocrin. 2006:254–255. 154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gullo MJ, Dawe S. Impulsivity and adolescent substance use: Rashly dismissed as “all-bad”? Neurosci Biobehav Rev. 2008;32:1507–1518. doi: 10.1016/j.neubiorev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975-2008: Vol I, Secondary school students (No 09-7402) Bethesda, MD: National Institute on Drug Abuse; 2009. [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociology Methods Research. 2001;29:374–393. [Google Scholar]
- Koob GF, LeMoal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Loeber R, Farrington D, Stouthamer-Loeber M, White HR. Violence and Serious Theft: Developmental Course and Origins from Childhood to Adulthood. New York: Routledge Press; 2008. [Google Scholar]
- Malgady RG, Rogler LH, Tyron WW. Issues of validity in the diagnostic interview. J Psychiatr Res. 1992;26:59–67. doi: 10.1016/0022-3956(92)90016-h. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE. A neuropsychologic profile of adolescent alcoholics. Alcohol Clin Exp Res. 1994;18:159–163. doi: 10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: A semi-parametric group-based approach. Psychol Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-Based Modeling of Development. Harvard University Press; Cambridge, MA: 2005. [Google Scholar]
- Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: A group-based method. Psychol Methods. 2001;6:18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TWH, Bernstein IL. Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(41):17600–17604. doi: 10.1073/pnas.0906629106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA council approves definition of binge drinking. NIAAA Newsletter. 2004:3. Retrieved from http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf.
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism: Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE. The Diagnostic Interview Schedule: Its development, evaluation, and use. Soc Psych Pysch Epid. 1988;23:6–16. doi: 10.1007/BF01788437. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: Four-year outcomes. J Int Neuropsychol Soc. 1999;5:481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Wasserman L. Bayesian model selection and model averaging. J Math Psychol. 2000;44:92–107. doi: 10.1006/jmps.1999.1278. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence- a unique target of ethanol effects. In: Dahl RE, Spear LP, editors. Adolescent Brain Development: Vulnerabilities and Opportunities. Academy of Sciences; New York: 2004. pp. 206–220. [DOI] [PubMed] [Google Scholar]
- Widmark E. Principles and Applications of Mediocolegal Alcohol Determination (Die theoretischen Grundlagen und die praktische Verwendbarkeit der gerichtlich-medizinischen Alkoholbestimmung—German) Biomedical Publications; Foster City, California: 1932/1981. [Google Scholar]