Abstract
We review the history of efforts to apply central thalamic deep brain stimulation (CT/DBS) to restore consciousness in patients in coma and vegetative state by changing the arousal state. Early experimental and clinical studies and the results of a recent single-subject human study that demonstrated both immediate behavioral facilitation and carry-over effects of CT/DBS are reviewed. We consider possible mechanisms underlying CT/DBS effects on cognitively-mediated behaviors in conscious patients in light of the anatomical connectivity and physiological specializations of the central thalamus. Immediate and carry-over effects of CT/DBS are discussed within the context of possible effects on neuronal plasticity and gene expression. We conclude that CT/DBS be studied as a therapeutic intervention to improve impaired cognitive function in severely brain-injured patients who in addition to demonstrating clinical evidence of consciousness and goal-directed behavior, retain sufficient preservation of large-scale cerebral networks within the anterior forebrain. Although available data provide evidence for proof-of-concept, very significant challenges for study design and development of CT/DBS for clinical use are identified.
OVERVIEW
Based on experimental physiology studies in the mid-twentieth century a concept of brainstem and thalamic control of forebrain arousal inspired clinical efforts to apply electrical stimulation to unconscious, severely brain-injured human subjects. Here we first review the history of clinical studies of central thalamic deep brain stimulation (CT/DBS) in patients with disorders of consciousness. The results of earlier clinical studies that examined the potential role of CT/DBS in restoring conscious awareness in patients remaining chronically unconscious in the vegetative state are reviewed as well as a recent single-subject study of CT/DBS in an awake human subject in the minimally conscious state (MCS). The implications for future study design and rationale for patient selection to test the potential use of CT/DBS are considered in light of the results of these studies. We develop a rationale to support the potential role of CT/DBS to improve impaired cognitive function in some conscious severely brain-injured patients emphasizing the anatomical and physiological specializations of the neurons within the central thalamus. Existing experimental data are reviewed with respect to observed effects of CT/DBS and limitations and future directions are considered.
REVIEW OF EARLY CLINICAL STUDIES
The pioneering experimental studies of Moruzzi and Magoun (1949) provided the first demonstration of a causal link of central thalamic electrical brain stimulation and forebrain arousal. In these studies, desynchronization patterns replaced slow waves in the EEG similar to that seen in wakeful states in response to electrical stimulation of the brainstem reticular formation and central regions of the thalamus in anesthetized cats. Three decades later, Steriade and Glenn (1982) identified a monosynaptic pathway from the midbrain reticular formation to the rostral intralaminar region of the thalamus (central lateral and paralaminar median dorsalis nuclei) suggested by the Moruzzi and Magoun studies using electroanatomical and single-unit recording methods. Single-unit recording in these cell populations linked elevations of firing rates in these neurons and wakefulness. Clinical investigators as early as the late 1960s and 1970s (McLardy et al. 1968, Hassler et al. 1969, Sturm et al. 1979) considered the potential relevance of the findings as a method for restoration of arousal and consciousness in chronically unconscious patients and carried out pilot case studies of electrical stimulation of the brainstem (tegmental midbrain), thalamus (posterior intralaminar nuclei--centromedian parafasicularis complex) and basal ganglia (globus pallidus interna). Despite eye opening and autonomic signs (increases in heart rate, blood pressure) consistent with arousal effects no reports described recovery of sustained interactive behavior nor compared behavioral assessments to DBS linked effects. Sturm et al. (1979) described brief recovery of simple command following in a single patient with focal injuries in the midbrain and thalamus following a posterior circulation stroke but the improvement disappeared after a few weeks of application.
Following on these early case reports, a multi-center study involving a total of 49 patients studied in France, Japan and the United States (Hosobuchi and Yingling 1993) applied DBS in the centromedian thalamus and cervical spinal cord to patients in the vegetative state (VS). Increases in arousal and associated physiological responses arose with deep brain stimulation in the majority of these patients with no changes in behavioral responsiveness. Although a small number of patients with traumatic brain injury (studies included anoxic, traumatic and other etiologies) were reported to have significant improvements with recovery of consistent communication these studies did not link DBS to these observed behavioral changes. Importantly, these studies clearly demonstrated that acute arousal responses alone are not dispositive of an effect on behavioral outcome, nor do they imply a role for DBS in the sustained recovery of higher integrative brain function. Patterns of arousal responses including shifts to higher frequency content ('desychronization') of the EEG simply reflect a basic and broad activation of forebrain, brainstem, and spinal cord systems (Pfaff 2005).
Having demonstrated that the appearance of arousal with DBS did not predict behavioral changes, these open label studies relied on an interpretation that the patients studied would have been unlikely to have recovered on their own. However, available data, including prospective cohort studies indicate that the results of these earlier studies are within the expectation of patterns of recovery without intervention. All the reported patients were studied well within the known timeframes for spontaneous recovery (all prior to 6 months) for VS and MCS. In the Multi-Society Task Force (1994) study, patients remaining in the vegetative state 3 months after traumatic brain injury were associated with a 35% rate recovery of consciousness at one year. Moreover 16% of these patients recovered independent function, an outcome better than any of the DBS cases reported in the series of DBS for vegetative state (Deliac et al. 1993, Yamamoto et al. 2005); additionally, roughly 20 percent of patients remaining in VS after traumatic brain injury at 6 months will emerge to MCS or higher levels (a quarter of these will still reach independence at one year). Even more problematic, however, is that the group of patients reported to have made the most considerable gains were reclassified by the investigators as MCS (Yamamoto et al., 2005). The majority (>80%) of patients in MCS, 3–6 months after injury will emerge (Lammi et al. 2005, Giacino and Kalmar 1997) some with outcomes including no disability as measured by the Disability Rating Scale. Finally, two very recent studies indicate that even waiting for patients to remain in VS or MCS for a year will not exclude significant rates of spontaneous recovery for small samples of patients. In a prospective study of 50 VS patients including anoxic, traumatic and hemorrhagic vascular injuries, 20% of patients showed spontaneous recovery of responsiveness after one year (Estraneo et al. 2010). A retrospective study including 39 patients remaining in MCS at one year found that more than half of the patients who survived emerged from MCS over two to five years after injury (Luaute et al. 2010). Thus, in light of these available statistics, no inference about the efficacy of DBS can be drawn from the fact that some VS patients (and all of the small number of MCS patients) improved over time in these earlier studies which did not link DBS to measured behavioral changes.
These early findings set severe methodological challenges for the evaluation of CT/DBS in severe brain injury. Foremost is the challenge of tracking recovery in patients with very impaired function and a likely long time course for spontaneous changes so that potential effects of any intervention (CT/DBS or pharmacological) can be disaggregated from natural history. At a minimum, blinded formal behavioral assessments are required to provide linkage of DBS to any observed behavioral changes as are blocked periods of withdrawal of stimulation. An additional and important methodological challenge set by these studies is a conceptual challenge to the approach: without evidence that arousal responses reflect a platform for behavioral gains, what is the basis for considering that DBS might be effective in restoring function in chronically unconscious patients? An alternative conceptualization is that CT/DBS would support highest level functions observed in conscious patient with fluctuating levels of behavioral interaction and cognitive capacity (Schiff, 2000, 2002a; Schiff, 2002b). The first level of recovery where such behaviors are identified is the minimally conscious state (Giacino et al. 2002).
A recent single subject study provides the first evidence that some very severely brain injured patients in MCS may benefit from central thalamic DBS. Schiff et al. (2007a) reported results of CT/DBS in a 38 year old man who had remained in MCS for 6 years following a severe traumatic brain injury. The patient had sustained a severe closed head injury associated with bilateral hemorrhages surrounding the brain, right frontal lobe contusion and deep coma. The patient had remained in vegetative state until approximately 3 months after injury when he recovered non-reflexive responsive behaviors to sensory stimulation consistent with minimally conscious state(MCS) (see (Giacino et al., 2002). For four years the patient did not advance past MCS with a best demonstrated behavioral response of inconsistent command following using eye movements; the patient could inconsistently generate saccadic eye movements in response to commands and to indicate answers by direction of eye-movement (patient demonstrated ~30% accuracy of answers to simple situational questions when able to respond at all). The patient was enrolled into the DBS study 6 years after injury and began a four-month quantitative behavioral assessment and ongoing rehabilitation therapies beginning at the time of enrollment (see Panel A, Figure 1). DBS electrodes were then placed bilaterally in the central thalami, targeting the central lateral nucleus (see Panel B, Figure 1). Over an ensuing 2 month period the electrodes remained OFF to reassess the patient’s post surgical behavioral baseline which revealed no behavioral changes associated with the placement of the electrodes. A two month period was chosen to reflect an interval twice the duration of known gene expression effects following electrode placements (Dragunow & Robertson 1988, Herrera & Robertson, 1996). Following this post-surgical OFF evaluation of DBS effects, a 5-month titration phase began. During this period, tolerance to DBS and assessment of varying stimulation parameters, including duration of stimulation was assessed. Subsequently a planned 6-month double-blind alternating crossover study to assess the impact of DBS on a series of preselected primary outcome measures was conducted.
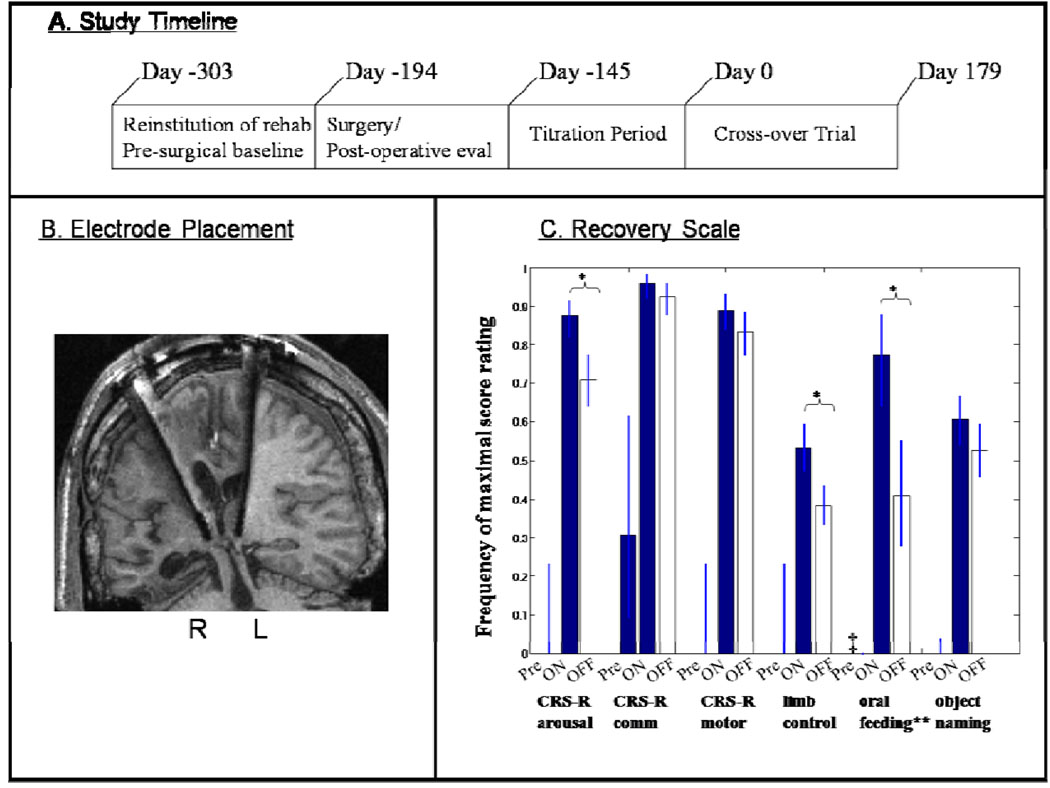
Figure 1. Summary of single subject study.
A. Study timeline. B. Electrode lead placements within the central thalamus of patient’s right ( R) and left (L) hemispheres displayed on T1 weighted MRI coronal image. C. Comparison of pre-surgical baselines and DBS ON and DBS OFF periods during a six month cross-over trial of CT/DBS in a patient with severe traumatic brain injury who remained in minimally conscious state prior to CT/DBS. Measures (marked *) showed a statistically significant dependence on electrical brain stimulation during the cross-over trial (see text). Figure elements adapted from [Schiff et al. 2007] with permission.
The patient was evaluated according to three subscales of a primary outcome measure, the Coma Recovery Scale Revised (CRS-R), a validated psychometric tool used in patients with disorders of consciousness and three tailored secondary measures developed during the titration trial (Giacino et al. 2004). Three subscales of the CRS-R which are known to reflect independent functional assessments were chosen as the primary outcome measures. Figure 1 (Panel C) organizes the results of a 6-month double-blind alternating crossover study and compares the pre-stimulation baselines of performance on each measurement to the ON and OFF periods of the cross-over study. The overall findings indicate significantly improved behavioural responsiveness in this patient as seen in the comparison of pre-stimulation frequencies of highest level behavioral response in the six categories shown. For each of these categories, with the exception of the oral feeling scale, observed improvements in the behaviors specifically reflect cognitively-mediated functions: identifying and distinguishing simultaneously presented items (working memory and sustained attention), verbal fluency and semantic retrieval, controlled sensorimotor integration and communication (see Giacino et al. 2004 for details of CRS-R testing scale). Importantly, these large differences of pre-stimulation and OFF DBS effects at the start of the cross-over phase of the trial reflect the overall impact of 5 months of exposure to DBS during the titration phase compared to ~6 months of rehabilitation efforts without concurrent DBS. In addition to the three pre-selected primary outcome measures, three additional secondary behavioral scales were studied. All of these supplementary behavioral scales were developed during the 5 month titration period when new behaviors linked to DBS were noted (see Schiff et al., 2007a) Supplementary material). All six measures (3 primary, 3 secondary) showed marked change from pre-stimulation baselines demonstrating higher level behaviors than seen prior to stimulation whether the electrodes were ON or OFF. Three measures (marked *) showed a statistically significant dependence on electrical brain stimulation during the cross-over trial as indicated by an increase in the frequency of specific cognitively mediated behaviors of attentive responsiveness measured across examination items (see Panel C, Figure 1). The highest level score for the CRS-R-arousal subscale is achieved for showing no more than 3 non-responses to an examiner’s questions across an assessment period, when responses are the top part of each subscale this improvement reflects an increase in cognitively-mediated behaviors requiring elements of executive function. Consistent ceiling performance on the CRS-R scale only appeared with exposure to DBS and remained strongly modulated during the cross-over trial. In addition to the effect on behavioral responsiveness captured the CRS-R arousal subscale, strong ON versus OFF modulation occurred for the functional limb control secondary measure which quantified purposeful movements such as combing, drinking, etc. (see description in supplementary material (Schiff et al., 2007a) and another supplementary scale that quantified recovery of oral feeding (chewing, swallowing and completing meals compared to tube feeding).
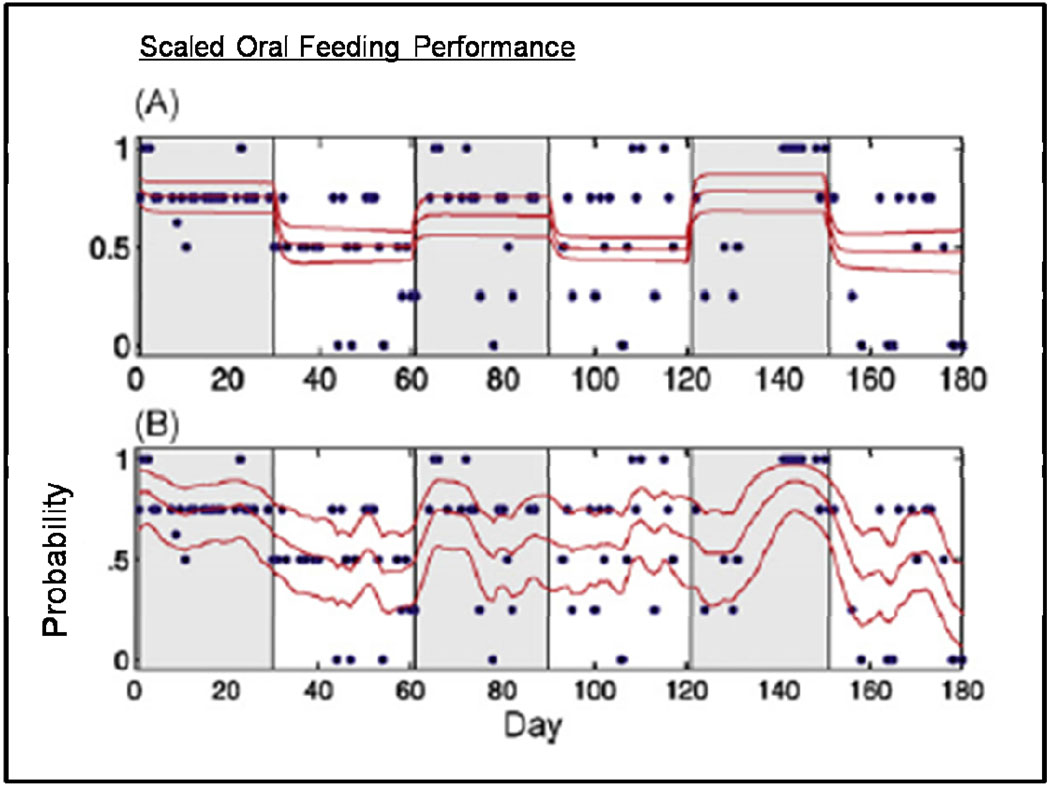
To examine CT/DBS effects in the context of this mix of anticipated time scales is challenging. Schiff et. al (2007a) examined the behavioral data shown in Figure 1 using detailed logistic regression models that tested the specific contributions of the time course of electrical stimulation against possible contributions of elapsed time (that would simply reflect the patient’s ongoing exposure to traditional rehabilitation). These analyses demonstrate statistical linkage between the observed functional improvements and recent stimulation history for both the cross-over data and effects seen during the titration phase. The logistic regression modeling for the oral feeding data provides a rigorous assessment of the statistical linkage of the CT/DBS time course and behaviors observed over the entire measurement period, but a glance at the time course of the dichotomized variable suggests that more dynamical detail might be resolvable (see Figure 2). To address this possibility directly, Smith et al. (Smith, 2009) developed a Bayesian state-space model that allows for trial-to-trial variability to be assessed as well as a full assessment of the multi-nomial behavioral data. Figure 3 shows the state-space analysis for the oral feeding data. The state space analysis demonstrates an intermediate time course for declines in the patient’s oral feeding ability during two of the DBS OFF transitions that occurred after ~2 weeks of the turning OFF of the DBS activation. During the last two weeks of the first and third DBS OFF period (Figure 2) the patient showed degrading of their ability to chew and swallow food with the appearance of periods of inability to swallow food placed in the mouth or simply remaining unarousable at feeding times (prior to DBS exposure this reflected the patient’s behavioral baseline which had required no oral feedings over 6 year post-injury phase). These observations provide evidence of a dynamic “wash-out” process and suggest further consideration of parameter adjustment such as the duty cycle (which remained at half-day for this study) as well as adjustments in trial design to limit risks of declines in function.
Figure 2. Modeling of oral feeding data.
Raw data from a 5 point multi-nomial scale for rating oral feeding behavior (Each step equal to 0.25 and ranging from 0—unable to arouse and unsafe to feed to 1—able to feed orally with no assistance) is shown as blue filled circles in Panels A and B. CT/DBS ON periods are indicated by gray shading. (A and B) Performance curves (median and 95% credible intervals) for a logistic regression (A) and state-space model (B) of the behavioral data are show. The state-space model reveals marked declines in the performance curve between days 30 and 60 and days 150 and 180; these period correspond to CT/DBS OFF periods and indicate a dynamic “wash-out” effect following cessation of the daily stimulation regime. Adapted from Smith et al. 2009 see reference for details of statistical modeling.
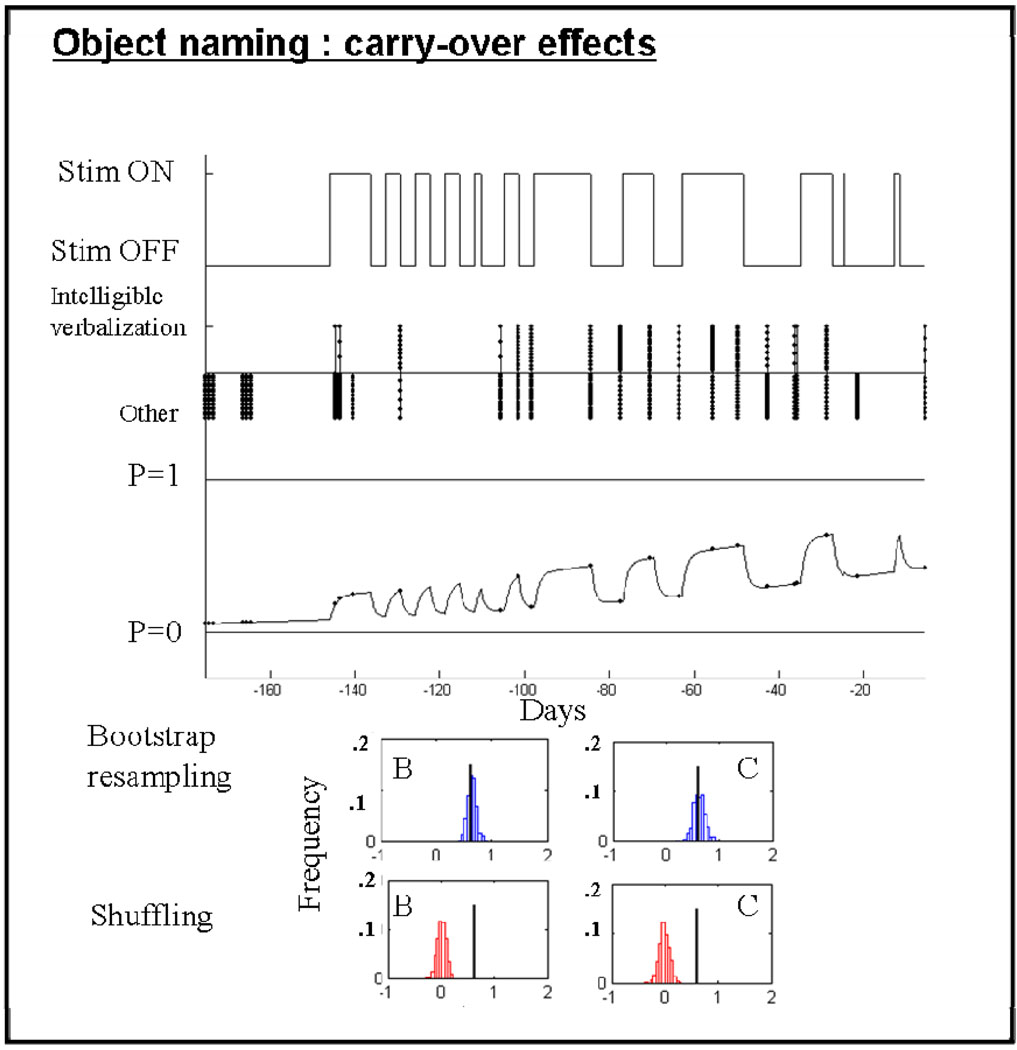
Figure 3. Object naming carry-over effects.
Figure shows the CT/DBS stimulation history, the behavioral time series for object naming as binary variable (with 1= intelligible verbal response, and 0= all other categories), and the best fitting probability model for the object naming data obtained during the titration period. Although a clear upward trend is visible (and a significant linear contribution for a variable reflecting time (‘B’, coefficient) is identified, the log likelihood is significantly improved (p<0.001) when a variable reflecting the CT/DBS stimulation-history is added (term ‘C; see Schiff et al. 2007 Supplementary Data). Data derived estimates of the coefficient values are shown as black lines, bootstrap estimates of the coefficient in blue histograms, and shuffled data estimates for the ‘B’ and ‘C’ coefficients are shown in red histograms. Neither a linear contribution or CT/DBS stimulation history contribution survive random shuffling of the behavior time series data (red histograms), but both survive 500 bootstraps. Figure elements adapted from [Schiff et al. 2007 Supplementary] with permission.
In addition to a dynamical “wash-out” of behavioral effects the marked improvements in all six measured behaviors during the cross-over trial seen when the DBS electrodes were OFF compared to the pre-stimulation baselines indicates a carry-over effect of changes that occurred after exposure to DBS during the titration period. While the study was designed to evaluate ON/OFF effects of CT/DBS during the titration period some of the behavioral data obtained during the titration period contained sufficient numbers of datapoints to develop post hoc analyses. Figure 3 shows a logistic regression model of titration testing of object naming (Supplementary Material Schiff et al. 2007). In these studies the subject was presented with visually displayed objects and asked to name them. Prior to stimulation as seen in the time line of raw data in Figure 3, no instances of intelligible and accurate verbalization (scored as 1 versus 0 for any other type of response or no response) were recorded. As the exposure to stimulation increased over time (with parallel increases in intensity of stimulation, see Schiff et al. 2007a for details) the patient regained the capacity to verbally identify the names of object accurately. The timeline of the logistic regression models shows statistical dependence on both stimulation history and a linear trend beginning at onset of the titration testing (with stimulation history providing a much larger contribution, see Schiff et al. 2007a). This modeling indicates that carry-over effects are present immediately and continue to grow roughly linearly. The presence of slow ongoing carry-over effects add a very significant further challenge to the design of CT/DBS studies (see Schiff, 2009b) and an additional caveat for the interpretation of earlier studies that lacked blocked OFF periods or formal behavioral assessments; not only do immediate arousal effects not predict future behavioral improvements but linear improvements in function may be due to either DBS or rehabilitation or time. Only rigorous study design and data analysis can distinguish the potentially separate contributions of each of these variables.
While a single-subject study, these observations collectively provide unequivocal evidence of both reproducible acute effects of DBS as well as more enduring and slowly accumulating effects. The latter suggests that biological mechanisms on multiple timescales play a role in the alteration of behavioral responses and focus attention on the potential role for mechanisms of synaptic plasticity and engagement of normal learning and memory processes (to be discussed further below). Critically the results of this study do not provide any indication of their generalizability. Taken together, with the results of earlier efforts they raise many related questions: 1) How important is a relatively high behavioral level for obtaining a meaningful and evolving response? The single-subject studied had a behavior profile at the upper end of MCS (with an average score of 19–20 on the 23 point CRS-R instrument at the start of the trial); 2) Can studies of DBS interventions prior to clear plateaus in recovery be properly designed or interpreted? 3) Can biologically based patient selection criteria be developed for DBS as opposed to syndromic behavioral criteria which are likely to have wide variance in underlying structural substrate for further recovery?
LIMITATIONS AND ETHICAL CONSIDERATIONS
The preliminary observations reviewed above suggest that CT/DBS may provide a method to artificially restore aspects of arousal regulation in some severely brain-injured patients. Several limitations can be immediately recognized. The most important caveat to be noted is that the generalizability of the single-subject results described above is completely unknown. Moreover, it is anticipated that the marked variation in patterns of structural brain damage underlying severe disability will require development of structured assessments of the integrity of cerebral systems to ultimately determine a likelihood of response to CT/DBS, rather than a diagnostic classification based on behavioral assessments (either qualitative or quantitative).
On the other hand, clinically important goals of care that would determine whether attempting CT/DBS in an investigational study is ethically proportionate in any given human subject will depend on behavioral assessments in conjunction with anatomical and physiological criteria (Schiff, 2009a). Fins (2005, 2009) has considered the goals of care for severely brain-injured human subjects in the context of the use of CT/DBS and more generally in the overall clinical context of providing diagnosis and traditional therapeutic efforts. He notes that both ethical principles and empirical studies focus attention on functional transitions that increase or preserve patient autonomy. Importantly, caregivers and family members place restoration of functional communication as clear, often first, goal of care. Other meaningful goals center on related aspects of social reintegration, such as emotional engagement and reactivity. From a theoretical point of view, restoring limited autonomy in the form of being able to express some preferences if not having capacity decisions about care is an ethical mandate. As knowledge of potential effect size and predictors response become available, clinical interventions must be calibrated against the likelihood of achieving meaningful impact on the patient’s life.
HYPOTHESIS AND RATIONALE FOR CT/DBS IN CONSCIOUS BRAIN-INJURED SUBJECTS
General considerations
In light of the above review of clinical studies, the early proposed use of CT/DBS as method to restore integrative cerebral function in the chronically unconscious brain (i.e. coma or vegetative state) is not supported by empirical data. The available data demonstrate a lower boundary where acute arousal effects of DBS are reliably elicited, stimulation is consistently applied, and no behavioral improvements are identified. It is important to consider the biological determinant of these negative outcomes and their implications for the use of CT/DBS in the severely brain-injured subject. Anatomic pathology studies demonstrate that permanent VS is associated with widespread deafferentation of the thalamus due to widespread neuronal death or disconnection following either trauma or hypoxic ischemic injury (Adams et al. 2000). Inferentially, non-responders in the early studies likely retained insufficient neuronal integrity and connectivity to re-establish large-scale cerebral networks essential for cognition and higher cognitive function (see e.g. Mesulam 1990). Presumably, sufficient thalamocortical connections to drive EEG desynchronization when electrically stimulated may be present in most such patients. In fact, theoretical models demonstrate that merely the connectivity of linked cortical pyramidal output neurons, reticular thalamic and thalamic relays neurons are sufficient to generate the variety of sleep-wake EEG patterns (Robinson et al. 2001). Consistent with this minimal requirement of underlying substrate, correlation of isolated corticothalamic circuits with preservation of desynchronized responses to sensory stimuli have been identified in chronically vegetative patients (Schiff et al. 1999b, 2002a). Taken together, the current state of knowledge makes selecting patients for CT/DBS studies on the basis of broad syndromic criteria untenable. Identifying an upper bound where measures of cerebral integrity might predict recovery above severe disability without any intervention is unavailable. While measurement of a sustained plateau in behavioral recovery (as discussed and demonstrated above) will allow for a rigorous study design, without independent consideration of what cerebral substrates are required for a meaningful response future studies are unlikely to identify selection criteria. Many MCS patients may not have sufficient recruitable cerebral resources to gain from CT/DBS; thus, efforts to match proposed mechanisms of action to the probability of response in individual subjects is required. Below we review proposed mechanisms with a view to the development of such future metrics.
Hypothetical mechanisms
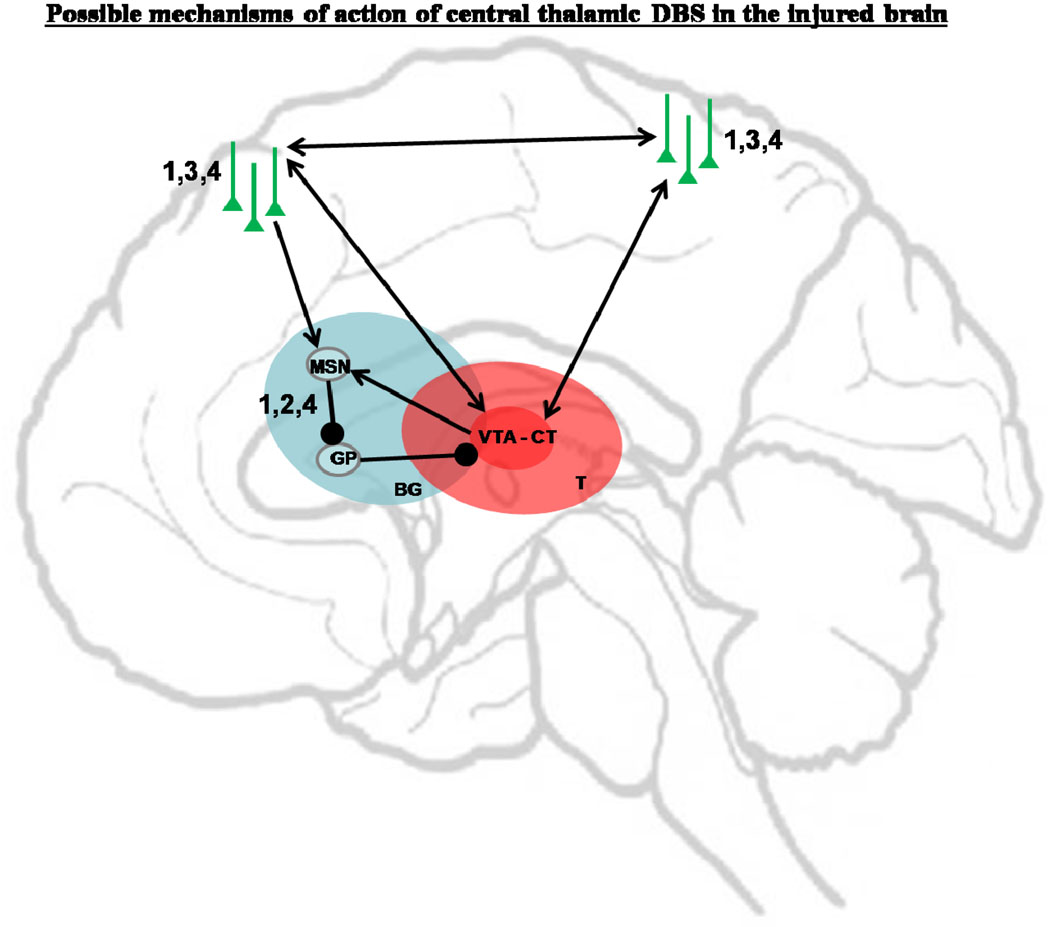
An alternative rationale for the use of CT/DBS in the severely-injured brain is focused on facilitation of behavioral responsiveness in conscious patients with prima facie evidence of integrative function and clear fluctuations in behavioral responsiveness or cognitive capacity (Schiff, 2002b; Schiff, 2002a; Schiff, 2009b). As reviewed extensively elsewhere (Schiff, 2008), the central thalamus plays a key role in arousal regulation within the wakeful state and CT/DBS may facilitate arousal regulation during behavior through several interrelated mechanisms (see Figure 4). A primary expected effect (Figure 4 #1) of CT/DBS is the eliciting of action potentials along the axons of central thalamic neurons resulting in depolarization of target neurons in the cerebral cortex (particularly regions of frontal and prefrontal cortex) and striatum through release of excitatory neurotransmitter (glutamate) at the thalamocortical and thalamostriatal synapse (Jones 2007, Smith,Y. 2008). Under physiological conditions central thalamic neurons receive a convergence of inputs from all of the neuromodulatory ‘arousal systems’ and very strong corticothalamic and mesencephalic excitatory neurotransmission. The aggregate effects of these systems set the overall levels of cerebral background synaptic activity and modulated firing rates in the central thalamus track levels of arousal, increasing during wakeful periods (Glenn and Steriade 1982). In the severely-injured brain marked reduction of background synaptic activity may be the strongest variable predicting patterns of cerebral integrative function with global reduction in cerebral metabolic rates correlating with level of behavioral state (Laureys et al., 2004). The introduction of even an artifically patterned excitatory drives to the neocortical and striatal neurons innervated by the central thalamus is likely to have significant network impact based on their broad connectivity (van der Werf et al. 2002) and strong synaptic weight as judged by the marked clinical impact of focal injuries to these neuronal populations (Schiff and Plum 2000). Increasing the level of membrane depolarization in cortical and striatal neurons can be expected to broadly increase the synaptic background activity in the severely-injured brain and may restore the normal high-frequency firing patterns observed in natural awake states which occur in depolarized state with high synaptic background activity (Steriade, 2001, Shu et al. 2003).
Figure 4. Possible mechanisms of central thalamic DBS.
(BG – basal ganglia; MSN – medium spiny neurons; GP – globus pallidus; VTA-CT: Volume of tissue activated in central thalamus; T-thalamus). 1 – depolarization of cortical and striatal neurons; 2 – depolarization of striatal neurons to allow inhibition of the pallido-thalamic projections and release of thalamocortical transmission; 3 – facilitation of cortico-cortical connections; 4 – facilitation of synaptic plasticity.
The expected depolarization of striatal neurons by CT/DBS (#2) in the severely-injured brain may have a particularly strong circuit-level impact as the medium spiny striatal neurons (MSNs) depend on high levels of corticostriatal and thalamostriatal inputs to fire action potentials (Grillner 2005). In the absence of MSN output an active inhibition of the central thalamus by pallidothalamic projections may combine with broad passive inhibition (disfacilitation) of thalamic neurons due to the relative depletion of excitatory synaptic contacts following cerebral injury. This mechanism has been suggested to play a role in partially reversible bi-hemispheric frontal and thalamic hypometabolism seen after many types of severe brain injuries (Schiff & Posner, 2007b; Schiff, 2010).
Another theoretically important mechanism of action for CT/DBS is facilitation of long-range corticocortical interactions (#3) that is proposed as one of the functional roles for central thalamic neurons (Purpura & Schiff, 1997). The wide point to point connections across the cortex originating from projection neurons in the central thalamus, have a specialized laminar specific pattern of innervation (Jones, 2001). The “matrix” neuronal types (see Jones, 2001) that likely confer the broad excitatory activation of the forebrain, selectively projects to supragranular and infragranular cortical regions driving overall increases in cortical column activity and facilitating mechanisms of long-term potentiation. These anatomical specializations are proposed to act as a coincidence detection mechanism via co-activation of the supragranular and infragranular layers (Llinas & Ribary, 1998) and has received experimental support from intracellular recording studies (Llinas et al., 2002). Finally, increasing the firing rates of cortical neurons and neurons within different subcortical nuclei driven by CT/DBS may also promote mechanisms of neuronal plasticity, learning and memory that depend on subcellular processes (#4). Experimental and clinical observations suggesting this possibility are reviewed below.
Experimental studies
Early experimental studies indicate that direct or indirect electrical stimulation of the central thalamus can facilitate behavior in alert animals with intact brains. Previous studies in primates (Fuster, 1958; Fuster & Uyeda, 1962) demonstrated enhancement of behavioral performance in a sensory-motor task following stimulation of the neurons in the mesencephalic reticular formation (MRF) which as noted above strongly innervate the CT. In a recent series of CT/DBS experiments in two alert macaca mulatta, Smith et al. (2009) showed statistical linkage of improved behavioral performance in response to a variety of selective stimulation parameters and sites within the central thalamus. Using these behavioral performance measures, Shah et al. (2009) reported on a total of 32 independent behavioral experiments in one animal where CT/DBS produced marked site-dependent differences in behavioral performances including increased performance (3/32 experiments), decreased performance (1/32), mixed effects (14/32) and no effect (14/32).These results show that CT/DBS can have complex and dynamic effects on behavior.
Perhaps more comparable to the clinical goal of improving generalized responsiveness, Shirvalkar et al. demonstrated the facilitation of untrained goal-directed rat behavior requiring object recognition memory with accompanying increases in exploratory motor behaviors following continuous unilateral electrical stimulation of the central lateral nucleus at 100Hz (Shirvalkar et al., 2006). In these studies, rats who received three days of exposure to CT/DBS at frequencies of 100Hz for thirty minutes/day showed accumulating effects of behavioral facilitation on a simple object recognition memory task. A parallel study of cerebral gene expression in rodents exposed to the same stimulation parameters following CT/DBS, revealed upregulation of memory-related immediate early genes in the anterior cingulate cortex, motor cortex and hippocampus(Shirvalkar et al., 2006). The cortical activation showed a layer specificity consistent with known patterns of CL innervations of the cortical layers (Llinas et al., 2002). Similar gene expression patterns have also been measured in rats following sleep periods occurring after induction of long-term potentiation (Ribeiro et al., 2002).
As noted above, in the single-subject study of CT/DBS in a minimally conscious patient, there is evidence that repeated exposure to CT/DBS may have a slow accumulating effect in addition to immediate effects of turning stimulation ON or OFF (Schiff et al., 2007a). These experimental findings suggest that changes in gene expression may play a key role in the observed “carry-over” effects (Figure 2) or may reflect strengthening of activated synapses or neuronal plasticity (as discussed below) and also play a role in the slow decline or wash-out” effects measured in the OFF stimulation state (Figure 3). Independent rodent studies of delayed response behaviors using an event-related and dose specific stimulation of the rostral central thalamus have found similar evidence for memory enhancement during task performance with CT/DBS (Mair & Hembrook, 2008).
CT/DBS and short-term plasticity in the neocortex
The immediate effects of CT/DBS cannot be explained by long-term changes in gene expression or engagement of normal learning and memory mechanisms via thalamocortical activation. Rather, CT/DBS appears to produce specific and immediate impact on sensorimotor integration. Experimental studies have long supported a specific integrative role for the central thalamus in several aspects of sensorimotor integration, particularly visuospatial awareness as well as coordination of ongoing multiplexing of information about sensory events used to organize motor tasks (Schlag & Schlag-Rey, 1971; Orem et al., 1973; Purpura & Schiff, 1997; Minamimoto & Kimura, 2002; Wyder et al., 2003; 2004; Tanaka, 2007). A role for the central lateral (CL) nucleus of the thalamus in visual awareness is supported by several lines of experimental evidence (reviewed in (Purpura & Schiff, 1997) including early studies in cats which demonstrated contraversive head turning and conjugate and contraversive saccadic eye movements with electrical stimulation of CL (Schlag & Schlag-Rey, 1971). Complementary studies identify contralateral visual neglect following unilateral lesions of the central lateral nucleus (Orem et al., 1973). In addition, other early studies in cats demonstrated enhanced visual evoked potentials during stimulation of the central thalamus (Hunsperger & Roman, 1976). Schlag-Rey and Schlag (Schlag-Rey & Schlag, 1984; Schlag & Schlag-Rey, 1984) first described a role for the central thalamus in primate visuo-spatial awareness. Visuomotor functions in the rostral intralaminar nuclei (primarily CL) of alert monkeys were characterized in single-unit recordings in animals performing behavioral tasks. One population of neurons ceased firing during a saccade and then rebounded with a burst of action potentials at the start of the next inter-saccadic interval. Most of these neurons demonstrated this behavior for any saccade, with the direction or amplitude of the saccade having no effect on the dynamics of the response. Other neighboring visuomotor units in the central thalamus (eye position and saccadic burst cells) were highly sensitive to the parameters of the saccade. Subsequent work by several investigators have confirmed and extended these findings (Wyder et al., 2003; 2004; Tanaka, 2007).
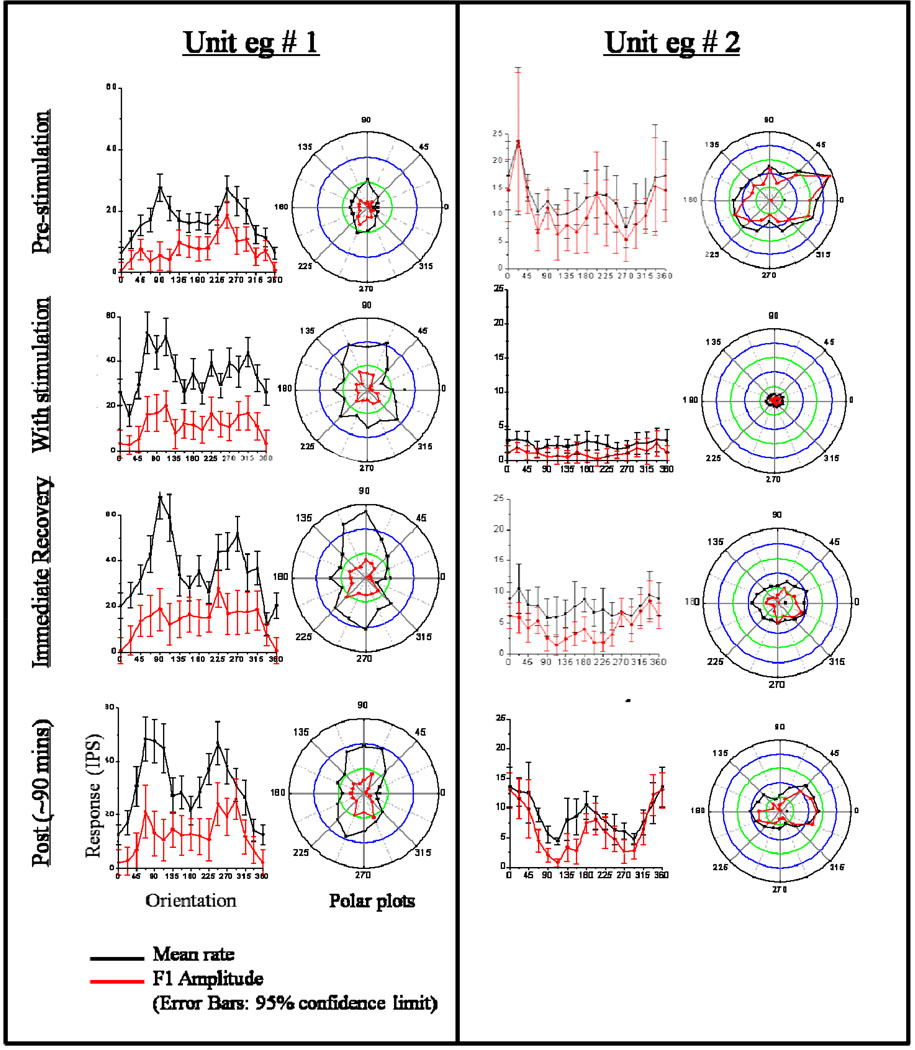
In pilot studies, the effects of CT/DBS in CL were measured using the response properties of single neurons in the primary visual cortex as an assay (Schiff et al., 1998). These experiments combined the classical experimental model of 3 sec spike-wave epilepsy using low frequency stimulation (3, 6 Hz) of the central thalamic nuclei to alter arousal and vigilance and produce ~3 sec spike and wave EEG patterns in the cortex (Hunter & Jasper, 1949) with standard quantitative characterization of response properties of single neurons in primary visual cortex (V1) in anesthetized, paralyzed macaques (Hubel & Wiesel, 1962). Measured effects of CT/DBS on orientation tuning, a hallmark property of neurons in V1, provided the assay of modulation of cortical processing. Extracellular recordings obtained during the presentation of drifting sine gratings at optimal spatial frequency, temporal frequency, and contrast were used to compute optimal receptive field properties for single visual cortical neurons. In a small number of cells studied, stimulation of the anterior/dorsal central thalamic nuclei (central lateral intralaminar and dorsal median nuclei) induced alterations of orientation tuning, as measured by mean firing rate and periodically modulated components of the neural activity (see Figure 5). In the two of three cells in which prolonged recording was maintained following stimulation, these alterations returned to baseline over 1–2 hours. Reversible changes also included decreases or increases in background firing rate and changes in direction selectivity. These observations demonstrated that a cortical response property as fundamental as orientation tuning in V1 may be modified by CT/DBS.
Figure 5. Modulation of receptive properties of macaca fasicularis primary visual cortical neurons with low frequency CT/DBS.
Left panel (Unit # 1) shows receptive field characteristics before, during and after stimulation of the central lateral nucleus. Prior to stimulation, a complex cell recorded from Layer IV of primary visual cortex shows an oriented mean response with peaks at 90 degrees and 247.5 degrees, and a directional F1 response with a peak at 247.5. Responses to the same drifting grating collected in multiple runs with each preceded by ten seconds of 3 Hz stimulation show an increased firing rate and variance of responsiveness. These changes in firing rate and pre-stimulation tuning profiles are observed to recover over the two post-stimulation tuning runs. In this example the recording may be multi-unit (because of the high firing rates in the off regions). However, the change and recovery of the orientation tuning is clear in polar plots. Right panel – Unit # 2 shows receptive field characteristics before, during and after stimulation of paralaminar median dorsalis nucleus. Prior to stimulation, the cell displayed orientation selectivity to drifting gratings with a narrowly tuned peak at 22.5 in both mean response and the first Fourier harmonic (F1). Between stimulation (10 secs of 3 Hz stimulation), the firing rate of the neuron is seen to decrease significantly although the neuron’s response remains modulated by the visual stimulation. Post simulation measurements show a slow recovery of tuning over approximately 90 minutes with recovery of a tuning profile similar to the pre-stimulation baseline. Data from the Schiff et al. (1998). For general physiological methods used to obtain single-unit recordings from the visual cortex and analytical methods used in the studies to quantitatively characterize the neuronal response see Schiff et al. (1999a).
These findings can be compared with earlier studies that combined CL stimulation with recordings from primary visual cortex that showed modulation of visual responses (Jung, 1958). However, in these early studies, responses were not characterized in terms of visual receptive fields and were recorded using non-selective stimuli, typically light flashes. Other studies using quantitative receptive field measures have shown that electrical stimulation of CL may be used to induce marked changes in the ocular dominance of single-units in primary visual cortex of the kitten (Tsumoto & Freeman, 1981). The specific alterations of receptive field properties indicate that the effect of CT/DBS is not merely an afferent block or a pure arousal effect. Alteration of receptive field properties suggests that loss of normal tuning of individual single-units may contribute to, or be a correlate of impaired awareness associated with cerebral dysfunction associated with low frequency rhythms projected by the central thalamus (cf.(Williams & Parsons-Smith, 1951). A more speculative possibility relates to other quantitative receptive field studies in the kitten that showed alteration of V1 receptive fields with body tilt (Tomko et al., 1981). The central thalamus (particularly CL) receives strong vestibular projections (Shiroyama et al., 1995) and may mediate such an effect on V1 through direct monosynaptic projections that are known to spread across the visual hemifield (Miniacchi et al., 1993). This theoretical mechanism would comport with evidence that efference copy signals related to eye movements are broadcast to cortical regions by central thalamus in support of top-down modulation cortical processing around eye-movements and attentional shifts (Purpura, 1997; Schiff, 1999b; Schiff, 2002b) as well as bottom up influences to stabilize the visual world through optokinetic reflexes which similarly pass through CL (Zee et al., 1980)
CONCLUSIONS
The concept of using CT/DBS to restore conscious interactive behavior in unconscious persons, as inspired by the original Moruzzi and Magoun (1949) experiments, is not well-supported by empirical data or theoretical rationale. In addition, natural recovery patterns after severe brain injury provide significant challenges to the evaluation of potentially significant CT/DBS effects in conscious patients improving spontaneously over time. As noted above, spontaneous recovery times are long and behavioral changes can be quite subtle such that disaggregating the effects of time and CT/DBS will be potentially very difficult as obvious CT/DBS effects (marked arousal responses both electrographic and behavioral) are known to be unlinked to outcome. Moreover, designing clinical trials to assess the impact of DBS on recovery rates will face severe challenges of statistical rigor as even patients who have reached clear plateaus in recovery may later demonstrate dynamic carry-over and washout effects once CT/DBS and behavioral rehabilitation efforts combine. Nonetheless, theoretical and empirical considerations and early proof-of-concept studies do support the concept that CT/DBS modulation of arousal regulation may have a role in aiding recovery of cognitive functions and impaired consciousness after structural brain injuries.
In our view, the road ahead will require a biological model of response probability based on consideration of the integrity of specific brain circuits and their likelihood to respond to CT/DBS matched against goals of the proposed intervention. If ultimately CT/DBS can be developed as therapeutic modality for restoring arousal regulation in the severely-injured brain, patient selection is not likely to be based on clinical syndromic criteria. The results of clinical applications of CT/DBS and experimental studies reviewed above suggest that it is most likely that observed CT/DBS effects will be best calibrated and understood on the basis of future measures of the integrity of large-scale circuits within the anterior forebrain. Anatomical and physiological considerations focus attention on cortico-striatopallidal-thalamocortical and cortico-thalamic networks supporting executive functions (i.e. working memory, sustained attention) as these systems are essential to measured CT/DBS effects on cognitively-mediated behaviors. From this point of view, the use of CT/DBS in conscious severely brain-injured subjects is distinct in theory and practice from its historical antecedents.
ACKNOWLEDGMENTS/ASSURANCES/DISCLOSURES
The data shown in Figure 5 from the Schiff et al. (1998) were recorded in experiments carried out under Weill Cornell Institutional Animal Care and Use Committee (IACUC) guidelines and done as part of NIH-NINDS grant NS02014 to Dr. Schiff. The general physiological methods used to obtain single-unit recordings from the visual cortex and analytical methods used in the studies to quantitatively characterize the neuronal response are referenced in Schiff, et al. (1999a). We acknowledge NS02014 (NDS) NS02172 (NDS) and IntElect Medical (NDS, SS) for support of our research studies. Dr. Schiff is a listed inventor on patents owned by Cornell University and licensed to IntElect Medical, Inc and acts as a scientific advisor to IntElect. IntElect Medical, Inc provided partial support for three published research articles discussed (Schiff et al. 2007, Smith et al. 2009, and Shah et al. 2009).
REFERENCES
- Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123(Pt 7):1327–1338. doi: 10.1093/brain/123.7.1327. [DOI] [PubMed] [Google Scholar]
- Bokil H, Purpura K, Schoffelen JM, Thomson D, Mitra P. Comparing spectra and coherences for groups of unequal size. J Neurosci Methods. 2007;159:337–345. doi: 10.1016/j.jneumeth.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Parent A. Striatal and cortical projections of single neurons from the central lateral thalamic nucleus in the rat. Neuroscience. 1996;72:679–687. doi: 10.1016/0306-4522(96)00001-2. [DOI] [PubMed] [Google Scholar]
- Deliac P, Richer E, Berthomieu J, Paty J, Cohadon F. Electrophysiological evolution of post-traumatic persistent vegetative states under thalamic stimulation. Neurochirurgie. 1993;39:293–303. Report on 25 observations. [PubMed] [Google Scholar]
- Dragunow M, Robertson HA. Brain injury induces c-fos protein(s) in nerve and glial-like cells in adult mammalian brain. Brain Res. 1988 Jul 12;455(2):295–299. doi: 10.1016/0006-8993(88)90088-1. [DOI] [PubMed] [Google Scholar]
- Estraneo A, Moretta P, Loreto V, Lanzillo B, Santoro L, Trojano L. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology. 2010 doi: 10.1212/WNL.0b013e3181e8e8cc. published June 16, 2010 as doi:10.1212/WNL.0b013e3181e8e8cc. [DOI] [PubMed] [Google Scholar]
- Fins JJ. The ethics of measuring and modulating consciousness: he imperative of minding time. Prog Brain Res. 2009;2009(177):371–382. doi: 10.1016/S0079-6123(09)17726-9. [DOI] [PubMed] [Google Scholar]
- Fins JJ. Clinical pragmatism and the care of brain damaged patients: toward a palliative neuroethics for disorders of consciousness. Prog. Brain Res. 2005;150:565–582. doi: 10.1016/S0079-6123(05)50040-2. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Effects of stimulation of brain stem on tachistoscopic perception. Science. 1958;127:150. doi: 10.1126/science.127.3290.150. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Uyeda AA. Facilitation of tachistoscopic performance by stimulation of midbrain tegmental points in the monkey. Exp Neurol. 1962;6:384–406. doi: 10.1016/0014-4886(62)90020-1. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, Kelly JP, Rosenberg JH, Whyte J, Zafonte RD, Zasler ND. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K. The vegetative and minimally conscious states: a comparison of clinical features and functional outcome. J. Head Trauma Rehabil. 1997;12:36–51. (1997) [Google Scholar]
- Glenn LL, Steriade M. Discharge rate and excitability of cortically projecting intralaminar thalamic neurons during waking and sleep states. J Neurosci. 1982;2:1287–1404. doi: 10.1523/JNEUROSCI.02-10-01387.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Ménard A, Saitoh K, Wikström MA. Mechanisms for selection of basic motor programs—roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hassler R, Dalle Ore G, Dieckmann G, Bricolo A, Dolce G. Behavioural and EEG arousal induced by stimulation of unspecific projection systems in a patient with post-traumatic apallic syndrome. Electroencephalogr. Clin. Neurophysiol. 1969;27:306–310. doi: 10.1016/0013-4694(69)90060-1. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y, Yingling C. The treatment of prolonged coma with neurostimulation. In: Devinsky O, Beric A, Dogali M, editors. Electrical and Magnetic Stimulation of the Brain and Spinal Cord. New York: Raven Press, Ltd.; 1993. pp. 247–252. [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsperger RW, Roman D. The integrative role of the intralaminar system of the thalamus in visual orientation and perception in the cat. Exp Brain Res. 1976;25:231–246. doi: 10.1007/BF00234015. [DOI] [PubMed] [Google Scholar]
- Hunter J, Jasper HH. Effects of thalamic stimulation in unanaesthetised animals; the arrest reaction and petit mal-like seizures, activation patterns and generalized convulsions. Electroencephalogr Clin Neurophysiol. 1949;1:305–324. doi: 10.1016/0013-4694(49)90043-7. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamus. 2nd edn. Cambridge, UK: Cambridge University Press; 2007. 2007. [Google Scholar]
- Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- Jung R. Coordination of specific and nonspecific afferent impulses at single neurons of the visual cortex. In: Jasper HH, editor. The Reticular Formation of the Brain. Little, Brown and Co; 1958. pp. 423–434. [Google Scholar]
- Kinney HC, Samuels MA. Neuropathology of the persistent vegetative state. J Neuropathol Exp Neurol. 1994;53:548–558. doi: 10.1097/00005072-199411000-00002. A review. [DOI] [PubMed] [Google Scholar]
- Lacey CJ, Bolam JP, Magill PJ. Novel and distinct operational principles of intralaminar thalamic neurons and their striatal projections. J Neurosci. 2007;27:4374–4384. doi: 10.1523/JNEUROSCI.5519-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammi MH, Smith VH, Tate RL, Taylor CM. The minimally conscious state and recovery potential: a follow-up study 2 to 5 years after traumatic brain injury. Arch. Phys. Med. Rehabil. 2005;86:746–754. doi: 10.1016/j.apmr.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci U S A. 2002;99:449–454. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Ribary U. Temporal conjunction in thalamocortical transactions. Adv Neurol. 1998;77:95–102. discussion 102–103. [PubMed] [Google Scholar]
- Luauté D, Maucort-Boulch D, Tell L, Quelard F, Sarraf T, Iwaz J, Boisson D, Fischer C. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology. 2010 doi: 10.1212/WNL.0b013e3181e8e8df. published June 16, 2010 as doi:10.1212/WNL.0b013e3181e8e8df. [DOI] [PubMed] [Google Scholar]
- Mair RG, Hembrook JR. Memory enhancement with event-related stimulation of the rostral intralaminar thalamic nuclei. J Neurosci. 2008;28:14293–14300. doi: 10.1523/JNEUROSCI.3301-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell WL, MacKinnon MA, Smith DH, McIntosh TK, Graham DI. Thalamic nuclei after human blunt head injury. J Neuropathol Exp Neurol. 2006;65:478–488. doi: 10.1097/01.jnen.0000229241.28619.75. [DOI] [PubMed] [Google Scholar]
- McLardy T, Ervin F, Mark V. Attempted inset-electrodes from traumatic coma: Neuropathological .ndings. Trans Am Neurol Assoc. 1968;93:25–30. [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990 Nov;28(5):597–613. doi: 10.1002/ana.410280502. 1990. [DOI] [PubMed] [Google Scholar]
- Minamimoto T, Kimura M. Participation of the thalamic CM-Pf complex in attentional orienting. J Neurophysiol. 2002;87:3090–3101. doi: 10.1152/jn.2002.87.6.3090. [DOI] [PubMed] [Google Scholar]
- Miniacchi D, Granato A, M S, G M. Different weights of subcortical-cortical projections upon primary sensory areas: the anterior intralaminar system. In: Miniacchi D, editor. Thalamic networks for relay and modulation. Oxford: Pergamon; 1993. pp. 209–228. [Google Scholar]
- Moruzzi G, Magoun HW. Brainstem reticular formation and activation of the EEG. Electroencephalography Clin. Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state (1) N. Engl. J. Med. 1994;30:1499–1508. doi: 10.1056/NEJM199405263302107. [DOI] [PubMed] [Google Scholar]
- Orem J, Schlag-Rey M, Schlag J. Unilateral visual neglect and thalamic intralaminar lesions in the cat. Exp Neurol. 1973;40:784–797. doi: 10.1016/0014-4886(73)90112-x. [DOI] [PubMed] [Google Scholar]
- Pfaff D. Brain Arousal and Information Processing. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Purpura KP, Schiff ND. The thalamic intralaminar nuclei: A role in visual awareness. The neuroscientist. 1997:8–15. [Google Scholar]
- Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J Neurosci. 2002;22:10914–10923. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PA, Rennie CJ, Wright JJ, Bahramali H, Gordon E, Rowe DL. Prediction of electroencephalographic spectra from neurophysiology. Phys Rev E Stat Nonlin Soft Matter Phys. 2001 Feb;63(2 Pt 1) doi: 10.1103/PhysRevE.63.021903. 021903. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND. Central thalamic deep-brain stimulation in the severely injured brain: rationale and proposed mechanisms of action. Ann N Y Acad Sci. 2009b;1157:101–116. doi: 10.1111/j.1749-6632.2008.04123.x. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Giacino JT, Fins JJ. Deep brain stimulation, neuroethics, and the minimally conscious state: moving beyond proof of principle. Arch Neurol. 2009a;66:697–702. doi: 10.1001/archneurol.2009.79. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Posner JB. Another "Awakenings". Ann Neurol. 2007b;62:5–7. doi: 10.1002/ana.21158. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, Fritz B, Eisenberg B, Biondi T, O'Connor J, Kobylarz EJ, Farris S, Machado A, McCagg C, Plum F, Fins JJ, Rezai AR. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007a;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Hudson AE, Purpura KP. Modeling wakeful unresponsiveness: characterization and microstimulation of the central thalamus. Proceedings of the Society for Neuroscience 31th Annual Meeting; San Diego, CA. 2002b. [Google Scholar]
- Schiff ND, Plum F, Rezai AR. Developing prosthetics to treat cognitive disabilities resulting from acquired brain injuries. Neurol Res. 2002a;24:116–124. doi: 10.1179/016164102101199576. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Plum F. The role of arousal and "gating" systems in the neurology of impaired consciousness. J Clin Neurophysiol. 2000;17:438–452. doi: 10.1097/00004691-200009000-00002. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Pulver M. Does vestibular stimulation activate thalamocortical mechanisms that reintegrate impaired cortical regions? Proc Biol Sci. 1999b;266:421–423. doi: 10.1098/rspb.1999.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND, Purpura KP, Victor JD. Gating of local network signals appears as stimulus-dependent activity envelopes in striate cortex. Journal of Neurophysiology. 1999a;82:2182–2196. doi: 10.1152/jn.1999.82.5.2182. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Purpura KP, Kalik SF, Victor JD. Intralaminar thalamic stimulation alters tuning properties of primary visual cortex neurons. Society for Neuroscience 28th Annual Meeting; Los Angeles, CA. 1998. [Google Scholar]
- Schlag-Rey M, Schlag J. Visuomotor functions of central thalamus in monkey. I. Unit activity related to spontaneous eye movements. J Neurophysiol. 1984;51:1149–1174. doi: 10.1152/jn.1984.51.6.1149. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Visuomotor functions of central thalamus in monkey. II. Unit activity related to visual events, targeting, and fixation. J Neurophysiol. 1984;51:1175–1195. doi: 10.1152/jn.1984.51.6.1175. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Induction of oculomotor responses from thalamic internal medullary lamina in the cat. Exp Neurol. 1971;33:498–508. doi: 10.1016/0014-4886(71)90121-x. [DOI] [PubMed] [Google Scholar]
- Shah SA, Baker JL, Ryou JW, Purpura KP, Schiff ND. Modulation of arousal regulation with central thalamic deep brain stimulation; Conf Proc IEEE Eng Med Biol Soc; 2009. pp. 3314–3317. [DOI] [PubMed] [Google Scholar]
- Shiroyama T, Kayahara T, Yasui Y, Nomura J, Nakano K. The vestibular nuclei of the rat project to the lateral part of the thalamic parafascicular nucleus (centromedian nucleus in primates) Brain Res. 1995;704:130–134. doi: 10.1016/0006-8993(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Shirvalkar P, Seth M, Schiff ND, Herrera DG. Cognitive enhancement with central thalamic electrical stimulation. Proc Natl Acad Sci U S A. 2006;103:17007–17012. doi: 10.1073/pnas.0604811103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Smith AC, Shah SA, Hudson AE, Purpura KP, Victor JD, Brown EN, Schiff ND. A Bayesian statistical analysis of behavioral facilitation associated with deep brain stimulation. J Neurosci Methods. 2009;183:267–276. doi: 10.1016/j.jneumeth.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009 Feb 16;78(2–3):60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Impact of network activities on neuronal properties in corticothalamic systems. J Neurophysiol. 2001;86:1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Glenn LL. Neocortical and caudate projections of intralaminar thalamic neurons and their synaptic excitation from midbrain reticular core. J. Neurophysiol. 1982;48:352–371. doi: 10.1152/jn.1982.48.2.352. [DOI] [PubMed] [Google Scholar]
- Sturm V, Kühner A, Schmitt HP, Assmus H, Stock G. Chronic electrical stimulation of the thalamic unspeci.c activating systemin a patient with coma due to midbrain and upper brainstem infarction. Acta Neurochir. 1979;47:235–244. doi: 10.1007/BF01406406. [DOI] [PubMed] [Google Scholar]
- Tanaka M. Spatiotemporal properties of eye position signals in the primate central thalamus. Cereb Cortex. 2007;17:1504–1515. doi: 10.1093/cercor/bhl061. [DOI] [PubMed] [Google Scholar]
- Tomko DL, Barbaro NM, Ali FN. Effect of body tilt on receptive field orientation of simple visual cortical neurons in unanesthetized cats. Exp Brain Res. 1981;43:309–314. doi: 10.1007/BF00238372. [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Freeman RD. Ocular dominance in kitten cortex: induced changes of single cells while they are recorded. Exp Brain Res. 1981;44:347–351. doi: 10.1007/BF00236575. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Brain Res. Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Williams D, Parsons-Smith G. Thalamic activity in stupor. Brain. 1951;74:377–398. doi: 10.1093/brain/74.4.377. [DOI] [PubMed] [Google Scholar]
- Wyder MT, Massoglia DP, Stanford TR. Contextual modulation of central thalamic delay-period activity: representation of visual and saccadic goals. J Neurophysiol. 2004;91:2628–2648. doi: 10.1152/jn.01221.2003. [DOI] [PubMed] [Google Scholar]
- Wyder MT, Massoglia DP, Stanford TR. Quantitative assessment of the timing and tuning of visual-related, saccade-related, and delay period activity in primate central thalamus. J Neurophysiol. 2003;90:2029–2052. doi: 10.1152/jn.00064.2003. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Katayama Y. Deep brain stimulation therapy for the vegetative state. Neuropsychol Rehabil. 2005 Jul–Sep;15(3–4):406–413. doi: 10.1080/09602010443000353. [DOI] [PubMed] [Google Scholar]
- Zee DS, Leigh RJ, Mathieu-Millaire F. Cerebellar control of ocular gaze stability. Ann Neurol. 1980;7:37–40. doi: 10.1002/ana.410070108. [DOI] [PubMed] [Google Scholar]