Abstract
Objective
Individuals with semantic dementia (SD) have impaired autobiographical memory (AM), but the extent of the impairment has been controversial. According to one report (Westmacott et al., 2001), patient performance was better when visual cues were used instead of verbal cues; however, the visual cues used in that study (family photographs) provided more retrieval support than do the word cues that are typically used in AM studies. In the present study, we sought to disentangle the effects of retrieval support and cue modality.
Method
We cued AMs of 5 SD patients and 5 controls with words, simple pictures, and odors. Memories were elicited from childhood, early adulthood, and recent adulthood; they were scored for level of detail and episodic specificity.
Results
The patients were impaired across all time periods and stimulus modalities. Within the patient group, words and pictures were equally effective as cues (Friedman test; χ2 = 0.25, p = 0.61), whereas odors were less effective than both words and pictures (for words vs. odors, χ2 = 7.83, p = 0.005; for pictures vs. odors, χ2 = 6.18, p = 0.01). There was no evidence of a temporal gradient in either group (for SD patients, χ2 = 0.24, p = 0.89; for controls, χ2 < 2.07, p = 0.35).
Conclusions
Once the effect of retrieval support is equated across stimulus modalities, there is no evidence for an advantage of visual cues over verbal cues. The greater impairment for olfactory cues presumably reflects degeneration of anterior temporal regions that support olfactory memory.
Keywords: AUTOBIOGRAPHICAL MEMORY, ODORS, SEMANTIC DEMENTIA
Semantic dementia (SD) is a progressive neurological disorder characterized by temporal lobe atrophy and the gradual decline of semantic memory (Snowden, Goulding, & Neary, 1989). Individuals with SD also have impaired autobiographical event memory (AM), but the extent of this impairment has been controversial. Some investigations have found that older memories tend to be impoverished while those from more recent periods tend to be intact (Graham et al., 2003; Moss et al., 2003; Nestor et al., 2002; Piolino et al., 2003; Snowden, Griffiths, & Neary, 1996), which is the reverse of the Ribot gradient seen in Alzheimer’s disease (e. g. Piolino et al., 2003). Others have found that AM drops off precipitously for events beyond the last few years, suggesting that a step function better describes the impairment (Graham & Hodges, 1997; Hou, Miller, & Kramer, 2005). Under some testing conditions, the impairment is ungraded (Ivaniou et al., 2006) or entirely absent (McKinnon et al., 2006; Moss et al., 2003; Westmacott et al., 2001). Data on the temporal gradient in SD have been used to refine neuropsychological models of memory (Moscovitch et al., 2005), so this issue is of theoretical importance.
Several factors could account for the differences across studies. First, some reports suggest that patients with mild SD have a graded impairment while those with moderate SD show a step function instead (Ivaniou et al., 2006, Matuszewski et al., 2009), meaning that the results of a particular investigation may depend on the severity of the disorder in the patient or patients tested. Moreover, in mild SD, additional retrieval support (i.e., detailed cues) can elevate performance to normal or near-normal levels (McKinnon et al., 2006; Moss et al., 2003), perhaps because retrieval support helps patients overcome strategic-retrieval deficits secondary to frontal pathology (Moss et al., 2003; Nestor et al., 2002).
The choice of test modality can also influence the pattern of AM impairment. Most AM tests are essentially verbal; in the Galton-Crovitz test, for example, memories are cued with single keywords such as HORSE. SD patients have particular difficulty with verbal material, however, and it would be unsurprising to find that a patient cannot generate a detailed memory to the keyword HORSE if he or she no longer knows what horses are. Moreover, SD patients can relearn words (Snowden & Neary, 2002), and these words might be disproportionately linked to recent experiences (Moss et al., 2003). Therefore, the apparent temporal gradient found in cue-word studies might reflect the degradation and reacquisition of semantic knowledge rather than the integrity of AM.
Two studies tried to circumvent these problems by using visual stimuli. Westmacott and colleagues (2001) cued AMs of an SD patient with family photographs. The patient seemed to perform well (there was no normal control group, but performance was at 85% or higher) and no temporal gradient was found. Graham and colleagues (2003), however, tested a patient using family photographs and found that he scored about 50% during the remote period. They did not test the recent period and therefore could not assess the temporal gradient.
Although these studies suggested that visual cues might facilitate recall in SD, they did not completely resolve the question. The main limitation arises from fundamental differences between the visual and verbal cues that have been used to test AM in SD. The nouns used in Galton-Crovitz tasks are generally high-frequency and easily imageable, but not personally relevant. Family photographs, by contrast, are more personally relevant than a noun, and they contain additional cues (such as the people involved) that might help patients construct appropriate stories. Accordingly, existing studies suffer from a confound between cue modality and retrieval support.
Other nonverbal cues have not been investigated. Odors, for example, can evoke autobiographical memories (Rubin, Groth, & Goldsmith, 1984), particularly memories that are older (Willander & Larson, 2006), more emotional (Herz & Cupchik, 1995; Rubin et al., 1984), and more detailed (Chu & Downes, 2002); additionally, odor-evoked memories have not been thought about or talked about as frequently (Rubin et al., 1984; Willander & Larsson, 2006). While odor identification is impaired in SD (Luzzi et al., 2007), an odor can trigger a memory even if a participant cannot identify it (Rubin et al., 1984). Thus, odors tend to elicit the very memories that SD patients find hardest to retrieve.
We therefore tested a group of SD patients and controls with a Galton-Crovitz paradigm that used words, simple pictures, and odors as cues. We hypothesized that pictures and words would be equally effective, while odors would be superior; we also predicted a temporal gradient with superior recall for recent memories.
Method
Participants
Patients were recruited from the Memory and Aging Center at the University of California, San Francisco. Study procedures were reviewed and approved by the Institutional Review Board of the University of California, Los Angeles, and the Committee on Human Research of the University of California, San Francisco. All participants provided informed consent. Each patient received a standardized evaluation that included a history (from both the patient and a secondary source), general and neurological examinations, and a neuropsychological battery. The battery consisted of the Clinical Dementia Rating Scale (Morris, 1993), the Mini Mental Status Exam (Folstein et al., 1975), backwards digit span (Wechsler, 1997), the California Verbal Learning Test (Delis et al., 2000), the 15-item Boston Naming Test (Kaplan et al., 1983), phonemic and semantic fluency (Benton & Hamsher, 1976), and modified versions of the Trail Making Test and the Rey-Osterreith Figure (Lezak, 1995). Patients underwent neurological examination, neuropsychological testing, and brain MRI scans (see Figure 1 for a representative scan) and were categorized as control or FTLD. FTLD subjects met criteria of Neary and colleagues (1998) for frontotemporal dementia (also called behavioral variant [bvFTD]) or semantic dementia (SD). For this study, patients were only included if they were diagnosed with SD. Table 1 presents demographics and neuropsychological test results. Normal control subjects (n=5) were recruited from the community or from the patients’ family members. They had no neurological complaints, normal neurological and neuropsychological examinations, and clinical dementia rating (CDR; Morris, 1993) scores of 0 or Mini Mental Status Exam (Folstein et al., 1976) scores above 24. They were matched to the patients on age, gender, and education; there were no statistically significant differences in these variables between the two groups (for age, t(8) = 0.88, p = 0.40; for education, t(8) = 0.95, p = 0.37).
Figure 1.
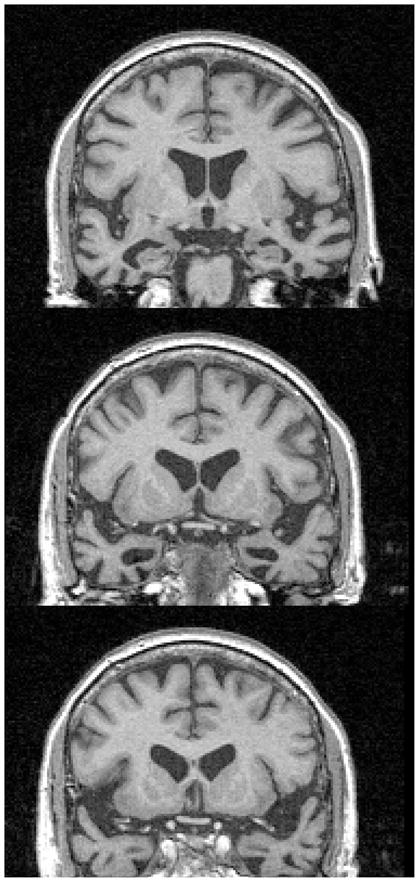
MRI scan of a representative patient (P01).
Table 1.
Demographic and neuropsychological information for the 5 SD patients.
| P01 | P02 | P03 | P04 | P05 | Mean | SD | |
|---|---|---|---|---|---|---|---|
| Age | 65 | 59 | 55 | 66 | 61 | 61.2 | 4.5 |
| Sex | F | F | F | F | M | ||
| Education (years) | 13 | 13 | 14 | 16 | 16 | 14.4 | 1.5 |
| Alcohol Sniff Test (cm) | 8.5 | 9.5 | 11.5 | 7.5 | 30 | 13.4 | 9.4 |
| UPSIT—verbal (# correct) | 11 | 5 | 12 | 12 | 4 | 8.8 | 4.0 |
| UPSIT—visual (# correct) | 11 | 5 | 11 | 7 | 7 | 8.2 | 2.7 |
| Spontaneous Naming (# correct) | 0 | 0 | 6 | 1 | 3 | 2.0 | 2.5 |
| CDR | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0.6 | 0.2 |
| MMSE (max=30) | 26 | 30 | 27 | 27 | 28 | 27.6 | 1.5 |
| CVLT Trial 4 | 5 | 7 | 7 | 5 | 5 | 5.8 | 1.1 |
| CVLT 30″ free recall | 1 | 6 | 4 | 1 | 1 | 2.6 | 2.3 |
| CVLT 10′ free recall | 0 | 7 | 4 | 1 | 0 | 2.4 | 3.0 |
| CVLT 10′ recognition | 5 | 9 | 5 | 6 | 6 | 6.2 | 1.6 |
| 15-Item BNT | 2 | 10 | 4 | 6 | 3 | 5.0 | 3.2 |
| Design fluency | 7 | n/a | 12 | 10 | 12 | 10.3 | 2.4 |
| FAS average | 8 | 11 | 9 | 4 | 3 | 7.0 | 3.4 |
| Animals | 2 | 11 | 11 | 3 | 3 | 6.0 | 4.6 |
| Modified Rey | 13 | 10 | 13 | 15 | 16 | 13.4 | 2.3 |
| Stroop | 38 | 56 | 37 | 33 | 25 | 37.8 | 11.4 |
| Modified Trails (sec) | 35 | 35 | 20 | 21 | 52 | 32.6 | 13.0 |
| Modified Trails (errors) | 0 | 1 | 0 | 0 | 0 | 0.2 | 0.4 |
| Reverse digit span | 4 | 5 | 4 | 5 | 5 | 4.6 | 0.5 |
Note. UPSIT = University of Pennsylvania Smell Identification Test; CDR = Clinical Dementia Rating Scale; MMSE = Mini Mental Status Exam; CVLT = California Verbal Learning Test; BNT = Boston Naming Test; Modified Rey = Modified Rey-Osterreith Complex Figure Test; n/a = not administered; SD = standard deviation.
Olfactory Tests
We assessed basic olfactory function with the Alcohol Sniff Test (Davidson & Murphy, 1997). In this test, participants are asked to close their eyes and breathe normally; the experimenter then opens an alcohol pad, holds it at the level of the participant’s chest, and raises it towards the participant with each inhalation. The experimenter records the distance in centimeters at which the participant detects the odor. The test is repeated and the mean distance in centimeters is taken as the olfactory threshold.
Odor identification was assessed using the University of Pennsylvania Smell Identification Test (UPSIT; Sensonics, Haddon Heights, NJ), a four-alternative forced-choice scratch-and-sniff test. The original version uses verbal response options; given the verbal difficulties in SD, however, we created visual response options for half the questions. We also administered a spontaneous naming test of 16 odors.
Autobiographical Memory
The AM test employed the Galton-Crovitz design. The word and odor cues were a subset of those that had been used in previous studies (Rubin et al., 1984; Rubin, Schrauf, & Greenberg, 2003), and the picture cues were a subset of the Snodgrass and Vanderwart (1980) set of line drawings (see Appendix 1 for a list). Stimuli were selected so that the frequency, familiarity, and imageability of the verbal referents were equated across stimulus modalities (Table 2; Wilson, 1988), since previous work has shown that these factors have a significant effect on performance (Lambon Ralph et al., 1998; Jefferies et al., 2009). Statistical analyses provided no evidence for a significant difference in these factors across groups (for frequency, F = 0.31, p = 0.74; for familiarity, F = 0.00; p = 0.9995; for imageability, F = 0.00, p = 0.996).
Appendix 1.
Stimuli used in the Galton-Crovitz autobiographical memory test. For the odors, the names given in parentheses are the most common verbal referents (when different from the actual name of the stimulus).
| Words | Pictures | Odors |
|---|---|---|
| Candy | Plane | Coffee |
| Doctor | Apple | Baby powder (powder) |
| Dress | Bicycle | Suntan lotion (lotion) |
| Child | Book | Cologne (perfume) |
| Wine | Car | Popcorn (corn) |
| Fire | Cat | Mothballs (bleach) |
| Ocean | Dog | Onion |
| Dirt | Fish | Cedar (wood) |
| Flower | Kite | Mint (toothpaste) |
| Water | Leaf | Cinnamon (spice) |
| Paper | Mouse | Chocolate |
| Mountain | Boat | Soap |
Table 2.
Familiarity, imageability, and frequency of the verbal referents of the stimuli.
| Familiarity | Imageability | Frequency | |
|---|---|---|---|
| Words | 563.3 (472–641) | 607.4 (547–634) | 73.8 (14–213) |
| Pictures | 572.9 (481–643) | 615.3 (556–638) | 68.8 (1–274) |
| Odors | 563.3 (518–625) | 595.0 (479–632) | 66.9 (4–297) |
Note. All values are expressed as mean (range). Values for familiarity, imageability, and frequency were obtained from Wilson (1988).
Words and pictures were presented individually on paper; olfactory cues were presented in opaque glass jars and refreshed as necessary. The same set of stimuli was used for all participants. As in previous studies (Hou et al., 2005), the task was broken down into three time periods: childhood (roughly ages 0–17), young adulthood (18–35), and recent life (age 36 to the present). Given the age of our participants, this produced three periods of approximately equal length. For each period, most participants were given four cues from each modality (owing to time constraints, two patients and one control were given three of each cue type per period), and cues were randomly assigned to periods. Within each period, stimuli were blocked by modality (e. g. 4 words followed by 4 pictures followed by 4 odors). The presentation of modality blocks was counterbalanced with the constraint that participants could not receive two blocks of the same modality in a row.
On each trial, the interviewer presented the participant with a cue and asked him or her to generate a memory of a specific event from one of the periods. The interviewer provided general cues (“Can you tell me anything more?”) but did not prompt participants with specific events. Participants estimated their age at the original event, and the interviewer encouraged them to select a different memory if they recalled an event from the incorrect period. Responses were audio or videotaped, with the exception of one patient and one control whose responses were transcribed by hand during testing. The memory-generation task was not timed; participants were allowed to continue until they finished describing the memory (or declared that they were unable to think of a memory associated with the cue).
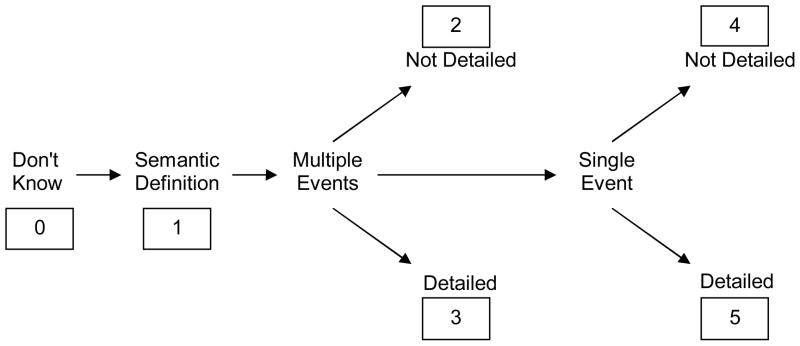
Two raters scored each transcript using the method of Graham and Hodges (1997 Graham and Hodges (2003). These raters were blinded to participant group,1 and the transcripts were redacted in such a way that the raters could not identify the cue modality and time period that were used to elicit the memory. This scoring method uses an ordinal rating scale from 0 to 5 and scores memories both for detail and episodic specificity (Figure 2). A trial is assigned a score of 0 if the participant generates no memory at all, and it is assigned a score of 1 if the participant gives a basic semantic definition of the stimulus (“People have them as pets” for DOG; for confidentiality reasons, these examples are all fictitious). Memories for multiple events receive a 2 if they are not detailed (“We have adopted many dogs”) and a 3 if they are more detailed (“Every summer we bring our Rottweiler to the lake, and he loves playing fetch in the water. He loved it so much that even when he was shivering cold, he would still drop the ball at our feet over and over again.”) Memories for single events received a 4 if they were not detailed (“I remember when we went to the pound and adopted our Labrador retriever”) and a 5 if they were more detailed (“One day, our dog ran away. I had opened the door to take out the garbage, and I left it open for a moment to go back and grab something else, and she just ran right out. I was so upset that I couldn’t even go to work; I just spent the whole day walking around the neighborhood looking for her. Finally I found her in a park where we often took her to play. I was so relieved even though I was in trouble at work because I was so upset that I’d forgotten to call them and tell them I wasn’t coming in.”).
Figure 2.
Diagram of autobiographical memory rating scale (redrawn from Graham and Hodges, 1997, with permission).
Results
Olfactory tests
All participants had olfactory thresholds above the impaired level. On the UPSIT, all controls were within normal limits; all patients were in the chance or severely impaired range. The patients performed significantly worse than the controls (F(1, 8) = 29.93, p = 0.0006). There were no differences between the verbal-response and the visual-response portions of the test for either group (for the patients, t = −0.34, p = 0.74; for the controls, t = 0.11, p = 0.91). Spontaneous odor naming was also severely impaired in the patient group (t(8) = 3.02, p = 0.016).
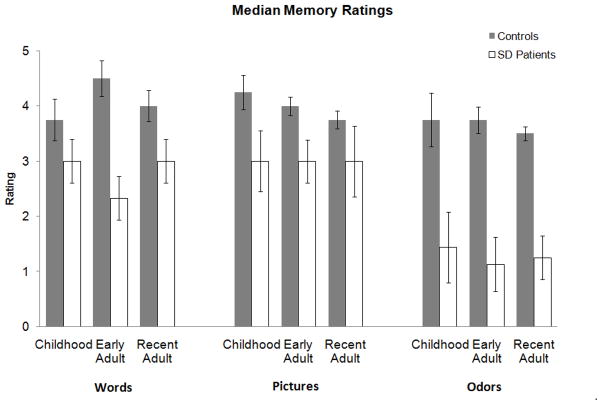
Autobiographical memory (Figure 2; Table 3)
Table 3.
Distribution of memory ratings broken down by patient group and stimulus modality.
| 0 | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Words | ||||||
| Patients | 0.07 | 0.06 | 0.30 | 0.32 | 0.19 | 0.07 |
| Controls | 0.00 | 0.02 | 0.07 | 0.19 | 0.42 | 0.30 |
| Pictures | ||||||
| Patients | 0.09 | 0.04 | 0.25 | 0.32 | 0.23 | 0.08 |
| Controls | 0.00 | 0.00 | 0.07 | 0.28 | 0.30 | 0.35 |
| Odors | ||||||
| Patients | 0.76 | 0.06 | 0.06 | 0.11 | 0.02 | 0.00 |
| Controls | 0.00 | 0.02 | 0.14 | 0.33 | 0.26 | 0.25 |
Note. All values are expressed as ratios. Values in each row do not always add up to 1.00 because of rounding.
Since the memory ratings are ordinal, we analyzed the data using nonparametric methods. Wilcoxon rank-sum tests showed that, overall, patient memories received lower ratings than those of controls (z = −6.84, p < 0.0001). Further Wilcoxon rank-sum tests analyses showed that this effect held true across all periods (for childhood, z = −3.43, p = 0.0006; for young adulthood, z = −4.18, p < 0.0001; for recent life, z = −3.95, p < 0.0001); it also held across all cue modalities (for words, z = −3.91, p < 0.0001; for pictures, z = −3.77, p = 0.0002; for odors, z = −4.66, p < 0.0001).
We then examined the effects of cue modality and period within each participant group. For controls, Friedman analyses provided no evidence for an effect of cue modality (χ2 = 3.81, p = 0.15). For SD patients, however, the effect of cue modality was significant (χ2 = 26.87, p < 0.0001). Further comparisons showed that word and picture cues were equally effective (χ2 = 0.25, p = 0.62), and both were more effective than odor cues (for words vs. odors, χ2 = 20.96, p < 0.0001; for pictures vs. odors, χ2 = 19.14, p < 0.0001). We found no evidence for an effect of period within either group (for SD patients, χ2 = 0.24, p = 0.89; for controls, χ2 < 2.07, p = 0.35).
These analyses included three patients who were unable to generate any memories to odors. To avoid floor effects, we reran the relevant analyses with their data excluded. The pattern of results did not change. Overall, Wilcoxon rank-sum tests showed that patient memories received lower ratings than control memories (z = −4.80, p = 0.0001), and the same held for odor-cued memories specifically (z = −3.37, p = 0.0008). Within the patient group, Friedman tests showed that odors were still less effective than words or pictures (for words vs. odors, χ2 = 7.83, p = 0.005; for pictures vs. odors, χ2 = 6.18, p = 0.01).
The previous analyses included all of the memories that participants provided; however, not all of these memories are actually AMs. Memories rated 4 or 5 cover specific events, while memories rated 2 or 3 cover merged events and those rated 0 or 1 are not autobiographical at all. We therefore collapsed memories rated 4 or 5 into a single category and examined the occurrence of these memories across periods, cue modalities, and groups (see Piolino and colleagues (2003) for a similar approach). Wilcoxon rank-sum tests showed that controls generated more memories of specific events than patients did (z = −6.47, p < 0.0001). This effect held true for all cue modalities (for words, z = −4.00, p < 0.0001; for pictures, z = −3.26, p = 0.0011; for odors, z = −4.57, p < 0.0001). It also held true for all periods (for childhood, z = −3.10, p = 0.002; for young adulthood, z = −4.15, p < 0.0001; for recent life, z = −3.81, p = 0.0001).
Discussion
We cued AMs of SD patients and controls with words, odors, and pictures using a Galton-Crovitz paradigm; the memories were scored using Graham and colleagues’ (2003) method. Relative to controls, patients were impaired across all cue types and stimulus modalities. There was no evidence of a temporal gradient. This pattern of results was still observed when analysis was confined to memories for specific events.
In healthy adults, odors are potent cues of AM (Rubin, Groth, & Goldsmith, 1984). In the SD patients, however, odors were almost entirely ineffective: they rarely elicited memories of specific events, and they failed to elicit any memories whatsoever in three patients. Even when these patients were excluded, odors were still less effective than cues in other modalities. The disproportionate impairment of odor-cued memories presumably reflects the degeneration of olfactory regions in the anterior temporal lobe (Eslinger, Damasio, & Van Hoesen, 1982; Mummery et al., 2000) and their connections to medial temporal regions that subserve AM (Moscovitch et al., 2005).
Pictures and words, by contrast, were equally effective cues of AM in the patient group, and performance was impaired in both conditions. This finding contrasts with Westmacott and colleagues’ (2001) finding of relatively intact performance when visual cues were used. We suggest that their results do not indicate an advantage for visual cues per se; rather, they might indicate that their visual cues (family photographs) provided more retrieval support than the single words and phrases that are typically used as verbal cues. In accordance with this view, additional retrieval support in the verbal domain can raise patients’ performance to near-normal levels, at least when the disorder is mild (McKinnon et al., 2006; Moss et al., 2003).
Regardless of cue type, SD patients’ memories showed no temporal gradient or step function. This result was unexpected: most studies have shown that recent AMs are better preserved than older AMs, especially when (as here) the cues provide little retrieval support (Moss et al., 2003) and the disorder is mild (Ivaniou et al., 2006, Matuszewski et al., 2009). While our use of only three periods may have prevented us from detecting a gradient, other studies using the same number of periods did find temporal gradients (Hou et al., 2005).
These conclusions are tempered by several limitations. First, we only examined cues that provided low levels of retrieval support, so we do not know if visual cues might be advantageous at higher levels of support. Second, we did not directly examine autobiographical facts or personal semantic memories (Hou et al., 2005; McKinnon et al., 2006), though the patients’ ability to remember merged events suggests that such memories are partially spared. Third, we were only able to test 5 participants with SD; given the variability across patients, it is possible that we did not have enough power to detect a temporal gradient. Fourth, though we did our best to equate frequency, imageability, and familiarity across stimulus types, differences may remain, especially given the substantial individual differences in odor identification abilities. Further investigations along these lines will clarify the nature of the disorder in SD and provide additional insight into the neural bases of AM.
Figure 3.
Median ratings of memories for three time periods cued by words (left), pictures (center), and odors (right). Bars indicate the standard error of the median (calculated as the standard error of the mean multiplied by 1.25).
Acknowledgments
National Institutes of Health (NINDS R01 NS050915, NIA P50 AG03006, NIA P01 AG019724, NIMH F32 MH074264); State of California (DHS 04-35516); Alzheimer’s Disease Research Center of California (03-75271 DHS/ADP/ARCC); Larry L. Hillblom Foundation; John Douglas French Foundation for Alzheimer’s Research; Koret Foundation; McBean Family Foundation
Footnotes
As a practical matter, the SD patients have noticeable and characteristic language difficulties, and therefore the raters are sometimes able to realize who is who. We do not believe that this is a critical issue, however, because the raters were blinded to study hypotheses as well as the modality and period from which each memory came.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
References
- Benton AL, Hamsher K. Multilingual aphasia examination. Iowa City, IA: University of Iowa; 1976. [Google Scholar]
- Chu S, Downes JJ. Proust nose best: odors are better cues of autobiographical memory. Memory & Cognition. 2002;30:511–518. doi: 10.3758/bf03194952. [DOI] [PubMed] [Google Scholar]
- Davidson TM, Murphy C. Rapid clinical evaluation of anosmia: The alcohol sniff test. Archives of Otolaryngology, Head, & Neck Surgery. 1997;123:591–594. doi: 10.1001/archotol.1997.01900060033005. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Eslinger PJ, Damasio AR, Van Hoesen GW. Olfactory dysfunction in man. Brain & Cognition. 1982;1:259–285. doi: 10.1016/0278-2626(82)90028-8. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Graham KS, Hodges JR. Differentiating the roles of the hippocampal complex and the neocortex in long-term memory storage: Evidence from the study of semantic dementia and Alzheimer’s disease. Neuropsychology. 1997;11:77–89. doi: 10.1037//0894-4105.11.1.77. [DOI] [PubMed] [Google Scholar]
- Graham KS, Kropelnicki A, Goldman WP, Hodges JR. Two further investigations of autobiographical memory in semantic dementia. Cortex. 2003;39:729–750. doi: 10.1016/s0010-9452(08)70861-x. [DOI] [PubMed] [Google Scholar]
- Herz RS, Cupchik GC. The emotional distinctiveness of odor-evoked memories. Chemical Senses. 1995;2:517–528. doi: 10.1093/chemse/20.5.517. [DOI] [PubMed] [Google Scholar]
- Hou CE, Miller BL, Kramer JH. Patterns of autobiographical memory loss in dementia. International Journal of Geriatric Psychiatry. 2005;20:809–815. doi: 10.1002/gps.1361. [DOI] [PubMed] [Google Scholar]
- Ivaniou A, Cooper JM, Shanks MF, Venneri A. Patterns of impairment in autobiographical memory in the degenerative dementias constrain models of memory. Neuropsychologia. 2006;44:1936–1955. doi: 10.1016/j.neuropsychologia.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Jones RW, Lambon Ralph MA. Comprehension of concrete and abstract words in semantic dementia. Neuropsychology. 2009;23:492–499. doi: 10.1037/a0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Graham KS, Ellis AW, Hodges JR. Naming in semantic dementia—what matters? Neuropsychologia. 1998;36:775–784. doi: 10.1016/s0028-3932(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. Oxford University Press; New York: 1995. [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Matuszewski V, Piolino P, Belliard S, de la Sayette V, Laisney M, Lalevée C, et al. Patterns of autobiographical memory impairment according to disease severity in semantic dementia. Cortex. 2009;45:456–472. doi: 10.1016/j.cortex.2007.11.006. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Black SE, Miller B, Moscovitch M, Levine B. Autobiographical memory in semantic dementia: Implications for theories of limbic-neocortical interaction in remote memory. Neuropsychologia. 2006;44:2421–2429. doi: 10.1016/j.neuropsychologia.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, et al. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. Journal of Anatomy. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HE, Kopelman MD, Cappelletti M, de Mornay Davies P, Jaldow E. Lost for word or loss of memories? Autobiographical memory in semantic dementia. Cognitive Neuropsychology. 2003;20:703–732. doi: 10.1080/02643290242000916. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47:36–45. [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham KS, Bozeat S, Simons JS, Hodges JR. Memory consolidation and the hippocampus: further evidence from studies of autobiographical memory in semantic dementia and frontal variant frontotemporal dementia. Neuropsychologia. 2002;40:633–654. doi: 10.1016/s0028-3932(01)00155-5. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Belliard S, Matuszewski V, Lalevée C, de la Sayette V, et al. Autobiographical memory and autonoetic consciousness: triple dissociation in neurodegenerative diseases. Brain. 2003;126:2203–2219. doi: 10.1093/brain/awg222. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Groth E, Goldsmith DJ. Olfactory cuing of autobiographical memory. American Journal of Psychology. 1984;97:493–507. [PubMed] [Google Scholar]
- Rubin DC, Schrauf RW, Greenberg DL. Belief and recollection of autobiographical memories. Memory & Cognition. 2003;31:887–901. doi: 10.3758/bf03196443. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: A form of circumscribed cerebral atrophy. Behavioural Neurology. 1989;2:167–182. [Google Scholar]
- Snowden JS, Griffiths HL, Neary D. Semantic-episodic memory interactions in semantic dementia: Implications for retrograde memory function. Cognitive Neuropsychology. 1996;13:1101–1137. [Google Scholar]
- Snowden JS, Neary D. Relearning of verbal labels in semantic dementia. Neuropsychologia. 2002;40:1715–1728. doi: 10.1016/s0028-3932(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Westmacott R, Leach L, Freedman M, Moscovitch M. Different patterns of autobiographical memory loss in semantic dementia and medial temporal lobe amnesia: a challenge to consolidation theory. Neurocase. 2001;7:37–55. doi: 10.1093/neucas/7.1.37. [DOI] [PubMed] [Google Scholar]
- Willander J, Larsson M. Smell your way back to childhood: Autobiographical odor memory. Psychonomic Bulletin & Review. 2006;13:240–244. doi: 10.3758/bf03193837. [DOI] [PubMed] [Google Scholar]
- Wilson MD. The MRC Psycholinguistic Database: Machine Readable Dictionary, Version 2. Behavioural Research Methods, Instruments and Computers. 1988;20:6–11. Available from University of Western Australia Web site http://www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm.