Abstract
Paraneoplastic glomerulonephritis is a rare complication of malignancy that is frequently mistaken for idiopathic glomerulonephritis. Failure to recognize paraneoplastic glomerulonephritis can subject patients to ineffective and potentially harmful therapy. Pathology of paraneoplastic glomerulonephritis varies between different types of malignancies. This Review describes the association of glomerulonephritis with both solid tumors and hematological malignancies The pathogenetic mechanisms of many glomerular lesions seem to relate to altered immune responses in the presence of a malignancy Studies in the Buffalo/Mna rat model of spontaneous thymoma and nephrotic syndrome indicate that polarization of the immune response toward a T-helper-2 (TH2) profile has an important role in the development of thymoma-associated glomerular lesions. Furthermore, overexpression of the TH2 cytokine interleukin 13 in transgenic rats induces minimal change disease. Such findings from experimental studies might facilitate the identification of biomarkers that can distinguish paraneoplastic glomerulonephritis from idiopathic and other secondary glomerulonephritides. This Review describes potential pathogenetic mechanisms for paraneoplastic glomerulonephritides associated with different malignancies and highlights the need for a multidisciplinary approach to the management of patients with paraneoplastic glomerulonephritis.
Introduction
Paraneoplastic glomerulonephritides are glomerular lesions that are not directly related to tumor burden, invasion, or metastasis, but rather are induced by products from tumor cells.1 The first series of paraneoplastic glomerulonephritis was published over 40 years ago by Lee et al.2 Since then, solid tumor-associated membranous nephropathy and Hodgkin lymphoma-associated minimal change disease (MCD) have become recognized as ‘classic’ paraneoplastic glomerulonephritides. Other glomerulonephritides, however, including focal segmental glomerulosclerosis (FSGS), membranoproliferative glomerulonephritis, IgA nephropathy, and rapidly progressive glomerulonephritis (RPGN), are also associated with malignancy. Treatment of paraneoplastic glomerulonephritis is quite different from treatment of idiopathic glomerulonephritis; therefore, recognition of paraneoplastic glomerulonephritis is clinically important. In general, a diagnosis of paraneoplastic glomerulonephritis should be considered if the glomerulonephritis occurs in the presence of malignancy, remits after ablation of malignancy, and recurs in association with the recurrence of malignancy3. However, in reality, the diagnosis of paraneoplastic glomerulonephritis could be difficult due to delayed diagnosis of malignancy, presence of other secondary causes of glomerulonephritis, and rare occurrence of certain paraneoplastic glomerulonephritis after ablation of malignancy.3
This Review aims to provide a reference for the diagnosis and treatment of paraneoplastic glomerulonephritis for the practicing physician and to explore potential pathophysiological mechanisms of paraneoplastic glomerulonephritis with an emphasis on immunological mechanisms. The pathogenesis of each type of paraneoplastic glomerulonephritis is closely related to nature of its respective neoplasm. Therefore, glomerular lesions associated with solid tumors and hematological malignancies are discussed separately. Glomerular lesions caused by plasma cell dyscrasias such as amyloidosis, light chain deposition disease, and other immunoglobulin-related glomerular lesions are induced by monoclonal immunoglobulins or their light or heavy chains. Theoretically, these lesions comprise paraneoplastic syndromes; however, these disease entities are well established, and are not traditionally considered to be paraneoplastic glomerulonephritides.1
Solid tumors
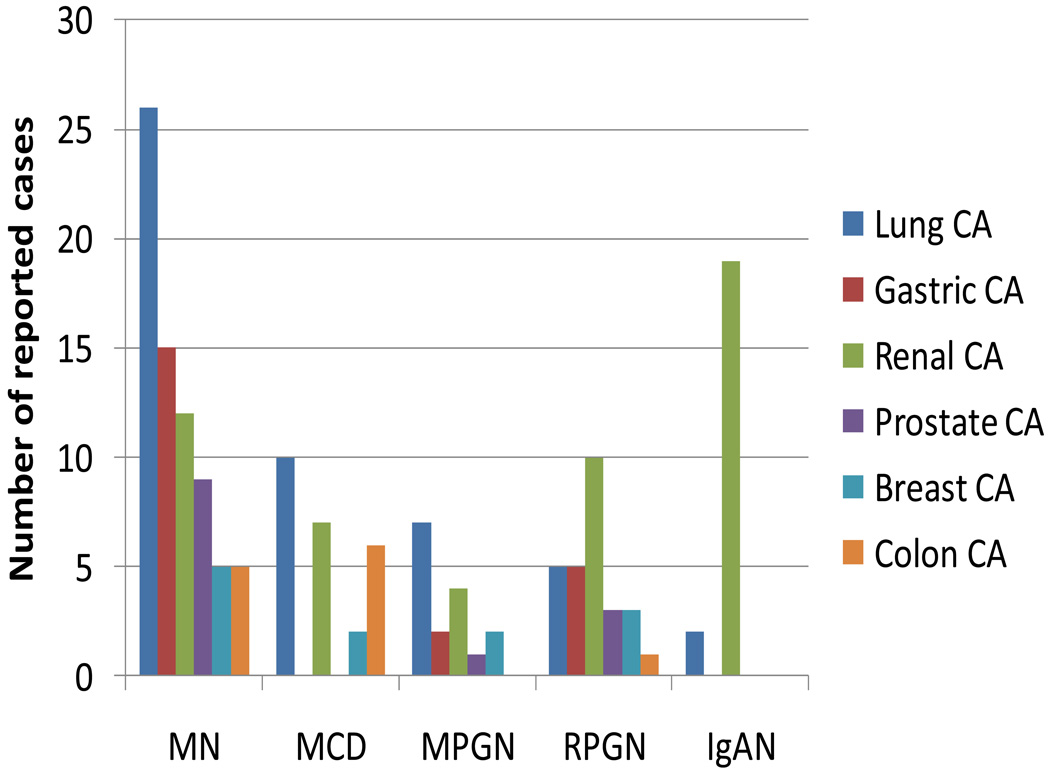
Reported cases of paraneoplastic glomerulonephritis associated with common solid tumors based on data published by Bacchetta et al.3 and on case reports published over the past 2 years are shown in Figure 1. 4–7 Membranous nephropathy is in general the most commonly reported paraneoplastic glomerulonephritis; MCD, membranoproliferative glomerulonephritis, RPGN and IgA nephropathy have been reported less frequently. Of note, this Figure and subsequent Figures are based on reported cases of paraneoplastic glomerulonephritis and by no means reflect the epidemiology of paraneoplastic glomerulonephritis.
Figure 1.
Paraneoplastic glomerulonephritis associated with solid tumors. Data are taken from several sources.3–7 and are presented as number of reported cases of specific types of paraneoplastic glomerulonephritides associated with each type of cancer. Specific numbers of lung cancer types for each glomerulonephritis type are: for membranous nephropathy: non-small cell lung cancer (NSCLC): 15, small cell lung cancer (SCLC):6, carcinoid: 2, mesothelioma: 2, lymphoepithelioma: 1; for MCD: NSCLC: 8, SCLC: 1, mesothelioma: 1; for MPGN: NSCLC: 4, SCLC:1; carcinoid: 2; for RPGN: NSCLC: 4, SCLC: 1; for IgAN: NSCLC: 1, SCLC: 1. Specific numbers of renal cancer types for each glomerulonephritis type are: for membranous nephropathy: renal cell carcinoma (RCC): 11, justaglomerular cell tumor: 1; for MCD: RCC: 6, oncocytoma:1; for MPGN: RCC: 3, Wilm’s tumor:1; for IgAN: RCC: 18, oncocytoma: 1. Abbreviations: IgAN: IgA nephropathy; MCD: minimal change disease; MN: membranous nephropathy; MPGN: membranoproliferative glomerulonephritis; RPGN: rapidly progressive glomerulonephritis.
Membranous nephropathy
In a 1999 review, Ronco1 noted that paraneoplastic membranous nephropathy was characterized by a marked male preponderance, age >50 years, and full-blown nephrotic syndrome that the neoplasm and membranous nephropathy manifested themselves within 12 months of each other. These characteristics were confirmed in a 2006 retrospective study by Lefaucheur et al.8 To date, this study represents the largest series of patients with cancer-associated membranous nephropathy. It is based on a cohort of 240 patients with biopsy proven membranous nephropathy; the prevalence of cancer in this cohort is 10%. Two major risk factors separated paraneoplastic membranous nephropathy from idiopathic membranous nephropathy: age >65 years and smoking >20 pack-years. All patients with paraneoplastic membranous nephropathy had active cancer. In most patients with paraneoplastic membranous nephropathy, the diagnosis of cancer and membranous nephropathy was made within 1 year; however, only half of these patients had symptoms of cancer at the time of membranous nephropathy diagnosis. The vast majority of tumors were carcinomas, which were most frequently localized to the lung (eight patients) and prostate (five patients). Interestingly, complete remission of nephrotic syndrome was observed in six of 12 patients whose tumor was in remission, but not in any patient whose tumor was not in remission. These findings support a causal relationship between cancer and membranous nephropathy.
The pathology of membranous nephropathy is characterized by subepithelial deposits of immune complex. Tumor antigens, including carcinoembryonic antigen, prostate specific antigen, and melanoma antigens, have been reported in association with cancer-associated membranous nephropathy; however, whether these antigens directly cause membranous nephropathy has not been established.3 One autopsy report that used immunofluorescence labeling reported glomerular deposits in 10–27% of kidneys from patients with a malignancy.9 The incidence of glomerular deposits is even higher if assessed by electron microscopy.10 Of note, however, the deposits identified by these two studies were found in the mesangial9or subendothelial10 space, which is in contrast to those found in membranous nephropathy. It is possible that tumor antigens per se are not enough to cause paraneoplastic membranous nephropathy and enhanced immune reactions triggered by the cancer itself may be required in the development of membranous nephropathy. Lefaucheur et al.8 reported that paraneoplastic membranous nephropathy is characterized by an increased number of inflammatory cells in glomeruli compared with that seen in idiopathic membranous nephropathy. Furthermore, IgG1 and IgG2 subtypes are markedly more prominent in the kidneys of patients with paraneoplastic membranous nephropathy than in those with idiopathic membranous nephropathy, whereas levels of IgG4 are not different.11
Human IgG1 and IgG2 are T-helper (TH)-1 cell-related isotypes (IL-12 and interferon-driven), whereas IgG4 is a TH2 cell-related isotype (IL-4 and IL-13-driven).12 TH1-predominant responses seem to be strongly associated with proliferative and crescentic forms of glomerulonephritis, which result in severe renal injury, whereas TH2-type responses are associated with membranous patterns of injury.13 In paraneoplastic membranous nephropathy, both TH1 and TH2 cytokines may be activated by tumor antigens or other stimulants, resulting in the unique pattern of IgG subtype and increased numbers of inflammatory cells. More studies are needed to confirm this hypothesis, and more importantly, to establish whether increased numbers of inflammatory cells and IgG1 and IgG2 staining are diagnostic for paraneoplastic membranous nephropathy and justify an intensive investigation for malignancy.
MCD
MCD has been reported in association with lung, colon, and renal cancers (Figure 1). Similar to membranous nephropathy, ablation of the tumor frequently achieves remission of MCD; therefore, factors produced by cancer cells may contribute to the pathogenesis of paraneoplastic MCD. Vascular endothelial growth factor (VEGF) is one potential candidate because of its ability to increase glomerular permeability. Taniguchi et al.14 reported a patient with rectal adenocarcinoma associated with MCD in whom levels of VEGF were elevated. After tumor resection, proteinuria disappeared and VEGF levels decreased to normal. The role of VEGF in the pathogenesis of MCD and FSGS is further supported by the findings that overexpression of VEGF in podocytes induces collapsing FSGS in experimental animal models15 and that serum VEGF levels are elevated in children with nephrotic syndrome.16 Whether MCD is indeed a consequence of VEGF overexpression in cancer cells remains to be determined.
Membranoproliferative glomerulonephritis
Membranoproliferative glomerulonephritis has been reported in association with solid tumors, such as lung, renal and gastric cancers, albeit rarely (Figure 1).3 In these patients, tests for hepatitis C virus (HCV) and cryoglobulin were negative,17–19 which led one group of researchers to propose that paraneoplastic membranoproliferative glomerulonephritis is an immune-complex disease that is induced by the combination of tumor antigen formation and the inability of the host to effectively clear antigens.17 Tumor removal has been shown to induce remission of membranoproliferative glomerulonephritis in some cases.17, 20 Interestingly, one case of membranoproliferative glomerulonephritis developed after surgical removal of a bronchial carcinoid tumor, but responded to prednisone therapy.18 Prednisone was also effective for paraneoplastic membranoproliferative glomerulonephritis without ablation of tumors in two reported cases with metastatic prostate cancers. 19 Therefore, the primary treatment goal for membranoproliferative glomerulonephritis associated with solid tumors is tumor ablation. In instances where this goal is not possible, a trial of prednisone treatment to control nephrotic syndrome seems reasonable.
Rapidly progressive glomerulonephritis
RPGN has been reported in association with malignancies, in particular renal cell carcinoma, gastric, and lung cancers. In 1984, Biava et al.21 reported seven cases of RPGN associated with a coexisting non-renal malignancy (six carcinomas and one lymphoma). Although this study was reported before the discovery of anti-neutrophil cytoplasmic antibodies (ANCAs), an assessment of renal pathology revealed pauci-immune crescentic glomerulonephritis, consistent with vasculitis. Patients with ANCA-associated vasculitis have a higher risk of malignancy than general population;22, 23 however many of these malignancies may relate to the immunosuppressive therapy given for treatment of vasculitis.24
The association of ANCA-associated vasculitis with solid tumors has been sporadically reported.22, 23, 25–28 These reports demonstrate that ANCA-associated vasculitis can occur concurrently with cancer, but cancers are frequently diagnosed after the vasculitis. The pathogenetic mechanisms by which neoplasms lead to the development of ANCA-associated vasculitis and RPGN are largely unknown. Moreover, only a few case reports describe the response of paraneoplastic RPGN to treatment. Administration of prednisone and cyclophosphamide—agents that are typically given for ANCA-associated vasculitis—to patients who had undergone tumor removal resulted in partial or complete remission of vasculitis in most patients.27 Whether vasculitis may respond to tumor ablation alone has not been tested; however, two patients who had untreatable cancers achieved remission of vasculitis in response to immunosuppressive therapy.25, 26 For patients who will have tumor removed, if there are no contraindications, it is reasonable to treat paraneoplastic RPGN with steroid and cyclophosphamide immediately, to prevent irreversible damage in the kidney.
IgA nephropathy
The association between IgA nephropathy and solid tumors of the respiratory tract, buccal mucosa and nasopharynx was first reported by Mustonen et al.29 in 1984. Subsequent reports of paraneoplastic IgA nephropathy mainly described patients with renal cell carcinoma (Figure 1),7,30 The paraneoplastic nature of IgA nephropathy is supported by the finding that IgA nephropathy resolved after tumor removal and evidence of IgA staining within the renal cell carcinoma.7 Interestingly, a diagnosis of renal cell carcinoma was made in two patients because of the rapid growth of renal lesions in response to corticosteroid therapy for IgA nephropathy.30 Tumor ablation seems to be the treatment of choice for paraneoplastic IgA nephropathy, whereas corticosteroid therapy may promote tumor growth. Henoch–Schönlein purpura (HSP), a systemic vasculitis that occurs in association with IgA nephropathy, has also been reported in association with solid tumors, most commonly lung cancers.31 The information on treatment of paraneoplastic HSP is limited.31
Thrombotic microangiopathy
Thrombotic microangiopathy is a multisystemic disorder that leads to thrombocytopenia, microangiopathic hemolytic anemia and ischemic manifestations owing to platelet agglutination. Damage to the central nervous system and kidney can also manifest. Strictly speaking, thrombotic microangiopathy is not considered to be a form of glomerulonephritis; however, discussion of thrombotic microangiopathy is included in this Review because the renal manifestations of thrombotic microangiopathy, such as hematuria and proteinuria, are similar to those that occur with glomerulonephritis. Thrombotic microangiopathy broadly includes thrombocytopenic thrombotic purpura (TTP) and hemolytic uremic syndrome (HUS); however, the discovery of ADAMTS13 has led to the classification of thrombotic microangiopathy being better defined.32 Thrombotic microangiopathy has been observed in patients with mucin-producing carcinomas, predominantly gastric, lung and breast cancers. At the time of thrombotic microangiopathy diagnosis, the malignancy can either be widely disseminated or isolated to the bone marrow.33 In patients with cancer-associated thrombotic microangiopathy, ADAMTS13 activity is not severely impaired. In addition, they responded poorly to plasma exchange therapy.34 The pathogenesis of cancer-associated thrombotic microangiopathy therefore clearly differs from TTP, in which ADAMTS13 activity is generally below 5% of normal and response rate to plasma exchange therapy is high.32
Cancer-associated thrombotic microangiopathy is most likely caused by microvascular tumor emboli and as such, thrombotic microangiopathy is not strictly speaking a paraneoplastic syndrome because it is directly related to tumor metastasis. The striking differences between cancer-associated thrombotic microangiopathy and TTP were illustrated by Francis et al.,35 who examined a series of 10 patients with ‘cancer-associated thrombotic microangiopathy’ from the Oklahoma TTP/HUS registry. These patients fulfilled the clinical criteria for TTP and received plasma exchange therapy, but unfortunately died soon after the initial diagnosis of TTP. Malignancy was diagnosed by bone marrow biopsy in six patients and on autopsy in two. Overall, cancer-associated thrombotic microangiopathy seems to carry a poor prognosis. Diagnosis is frequently difficult and may require a bone marrow biopsy. The lack of response to plasma exchange therapy may be an important hint for investigating malignancy as a cause of thrombotic microangiopathy. Plasma exchange therapy is not recommended once a diagnosis of cancer-associated thrombotic microangiopathy has been made.35
Hematological malignancies
Hematological malignancies are heterogeneous diseases of different cell lineages. In this Review, lymphoid, myeloid, and thymus malignancies are discussed separately because the patterns and pathogenetic mechanisms of these neoplasms are different. Of note, although thymoma-associated paraneoplastic glomerulonephritis is extremely rare, it serves as an unique disease model for investigating T-cell dysregulation as a potential pathogenetic mechanism for glomerular lesions and deserves separate discussion.
Lymphoid malignancies
Paraneoplastic glomerulonephritides are well known to occur in association with chronic lymphoid neoplasms, but rarely occur in patients with acute lymphocytic leukemia.36 In addition to being associated with Hodgkin lymphoma, non-Hodgkin lymphoma, and chronic lymphocytic leukemia,36,37 specific types of glomerulonephritis have been reported in association with rare lymphoid malignancies, such as cutaneous T-cell lymphoma (which includes mycosis fungoides and the leukemic variant, Sézary syndrome),38, 39 and hairy cell leukemia (a chronic mature B cell lymphoproliferative disease, in which B cells possess abundant cytoplasms with micro-filamentous ‘hairy’ projections).40,41
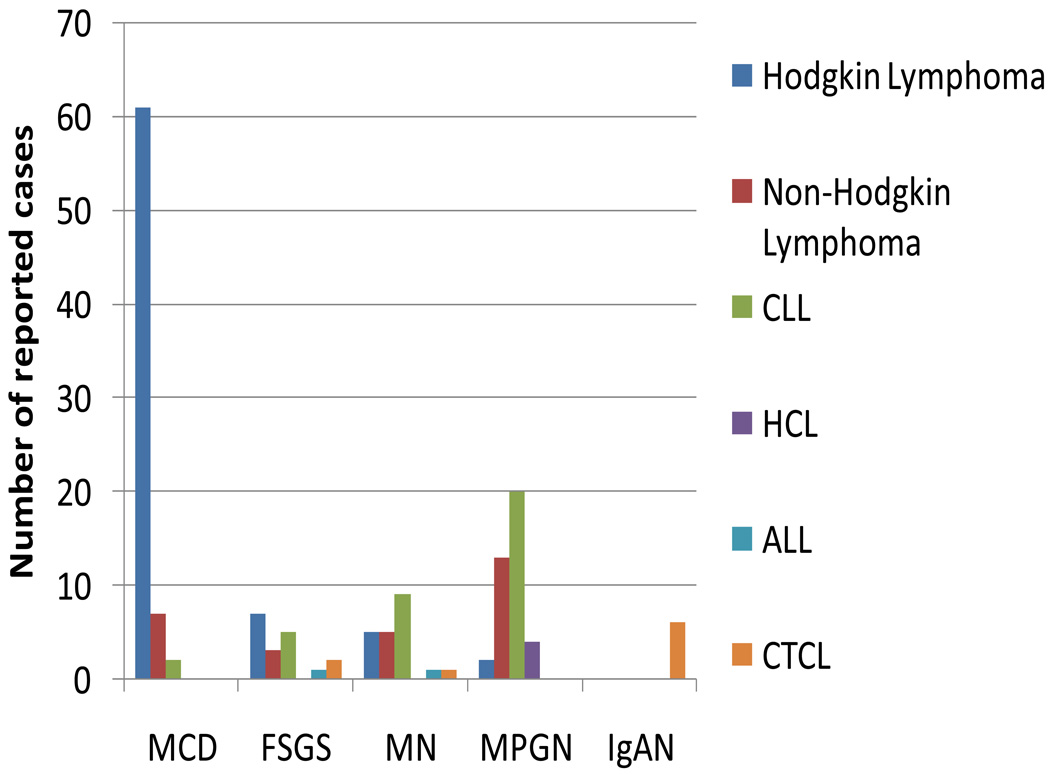
MCD is the most common paraneoplastic glomerulonephritis associated with Hodgkin lymphoma, followed by FSGS, whereas membranoproliferative glomerulonephritis and membranous nephropathy are more commonly associated with chronic lymphocytic leukemia, hairy cell leukemia, and non-Hodgkin lymphoma (Figure 2). IgA nephropathy has only been reported in association with cutaneous T-cell lymphoma. These patterns of disease are interesting from an immunological viewpoint because Hodgkin lymphoma is predominantly a disease of the T-cell lineage, whereas chronic lymphocytic leukemia, hairy cell leukemia and most non-Hodgkin lymphomas are B-cell-lineage diseases.
Figure 2.
Paraneoplastic glomerulonephritis associated with lymphoid malignancies. Data are taken from several sources.36,37,93–96 Paraneoplastic glomerulonephritis associated with HCL,40,41 97 and CTCL38,39,98–101 are also included. Numbers are reported cases of specific types of paraneoplastic glomerulonephritis associated with each malignancy. Abbreviations: ALL: acute lymphocytic leukemia, CLL: chronic lymphocytic leukemia; CTCL, cutaneous T-cell lymphoma; FSGS, focal segmental glomerulosclerosis; HCL, hairy cell leukemia; IgAN: IgA nephropathy; MCD: minimal change disease; MN: membranous nephropathy; MPGN: membranoproliferative glomerulonephritis.
MCD and FSGS
MCD occurs in about 1% of patients with Hodgkin lymphoma and the occurrence of FSGS is about one-tenth that of MCD.36,37 Audard et al.37 evaluated a cohort of 21 adult patients with MCD and classic Hodgkin lymphoma in a retrospective study. MCD was diagnosed in close proximity to Hodgkin lymphoma or its relapse in about half these patients. Both Hodgkin lymphoma and MCD responded to chemotherapy. Hodgkin lymphoma was diagnosed many months after MCD in five patients. MCD in these five patients was resistant to steroid and ciclosporin therapy, and only responded to chemotherapy. Similarly, excellent responses to chemotherapy were observed in patients with Hodgkin lymphoma-associated FSGS.36 A poor response of MCD to steroid or ciclosporin therapy therefore seems to be indicative of occult Hodgkin lymphoma.
A further clinical observation made by Audard et al.37 was that 71% of patients with Hodgkin lymphoma and MCD had systemic symptoms (that is, fever, weight loss, and night sweats), and 90% had positive laboratory parameters for inflammatory syndrome (as assessed by C-reactive protein level, sedimentation rate, and fibrinogen level).37 Increased cytokine levels, particularly levels of TH2 cytokines, could be responsible for this inflammatory response since Hodgkin lymphoma is associated with an expansion of T cells polarized towards a TH2-like phenotype.42 Furthermore, IL-13, a TH2-type cytokine, and its receptor are expressed constitutively in Reed–Sternberg cells in patients with Hodgkin lymphoma.43 A 2007 study demonstrated that overexpression of IL-13 in rats induces a MCD-like nephropathy characterized by proteinuria, hypoalbuminemia, hypercholesterolemia, and the fusion of podocyte foot processes.44 Further studies are needed to verify whether IL-13 is the plasma vascular permeability factor that causes MCD and/or FSGS in patients with Hodgkin lymphoma.
Membranoproliferative glomerulonephritis
The incidence of nephrotic syndrome in patients with chronic lymphocytic leukemia is <1%, with membranoproliferative glomerulonephritis being the most common pathology.45 Membranoproliferative glomerulonephritis in the setting of chronic lymphocytic leukemia, hairy cell leukemia and B cell non-Hodgkin lymphoma might be caused by monoclonal immunoglobulin, which is secreted by B-cell clones. In some cases, membranoproliferative glomerulonephritis is associated with mixed cryoglobulinemia, mainly comprising types I and II, which involves a monoclonal immunoglobulin.46 In past 2 decades, HCV infection has been implicated in the development of B cell non-Hodgkin lymphoma. In a series of 16 patients with HCV infection complicated by type II mixed cryoglobulinemia and membranoproliferative glomerulonephritis, examination of bone marrow biopsy samples revealed low grade B cell non-Hodgkin lymphoma in 68% of patients.47 The high incidence of subclinical lymphoma suggests that type II mixed cryoglobulinemia is a precancer condition and that the expansion of clonal B cells may eventually progress to B-cell lymphoma. This sequence of events may also occur in patients with non-HCV mixed cryoglobulinemia. In a series of 20 patients with non-HCV associated type II mixed cryoglobulinemia and membranoproliferative glomerulonephritis, four patients developed B cell lymphoma; two of these had a relapse of membranoproliferative glomerulonephritis.48
The assumption that membranoproliferative glomerulonephritis with type II mixed cryoglobulinemia may represent a paraneoplastic glomerulonephritis owing to occult B-cell lymphoma has considerable clinical implications. The response rate of HCV-associated type II mixed cryoglobulinemia to combined treatment with interferon and ribavirin is about 60%.49 Patients who failed to respond to interferon and ribavirin, however, responded well to rituximab, an anti-B cell (CD20) monoclonal antibody, which is used to treat B-cell malignancies.50 It is possible that patients who responded to rituximab had paraneoplastic membranoproliferative glomerulonephritis associated with subclinical B-cell lymphoma, which was induced by HCV infection.
Membranous nephropathy
Membranous nephropathy is less common than membranoproliferative glomerulonephritis in relation to lymphoid malignancies. In one series of glomerulonephritis associated with chronic lymphocytic leukemia and non-Hodgkin lymphoma, the ratio of membranoproliferative glomerulonephritis to membranous nephropathy was 8:1.51 Features atypical of membranous nephropathy, including segmental mesangial proliferation, subepithelial deposits of fibrillary material, and monoclonal IgG kappa light chain deposits, have been reported in disease associated with chronic lymphocytic leukemia and non-Hodgkin lymphoma.51,52 Some of these findings suggest that similar to membranoproliferative glomerulonephritis, monoclonal proteins produced by B cells may be involved in pathogenesis of paraneoplastic membranous nephropathy. Of note, paraneoplastic membranous nephropathy frequently responds to treatment of the underlying malignancy.
IgA nephropathy
IgA nephropathy has been reported in patients with cutaneous T-cell lymphoma, and seems to have a relatively stable clinical course.39 As in patients with Hodgkin lymphoma, TH2 polarization has been observed in patients with cutaneous T-cell lymphoma.53 Whether skin involvement of the malignancy has any role in the pathogenesis of IgA nephropathy is unclear.
Myeloid malignancies
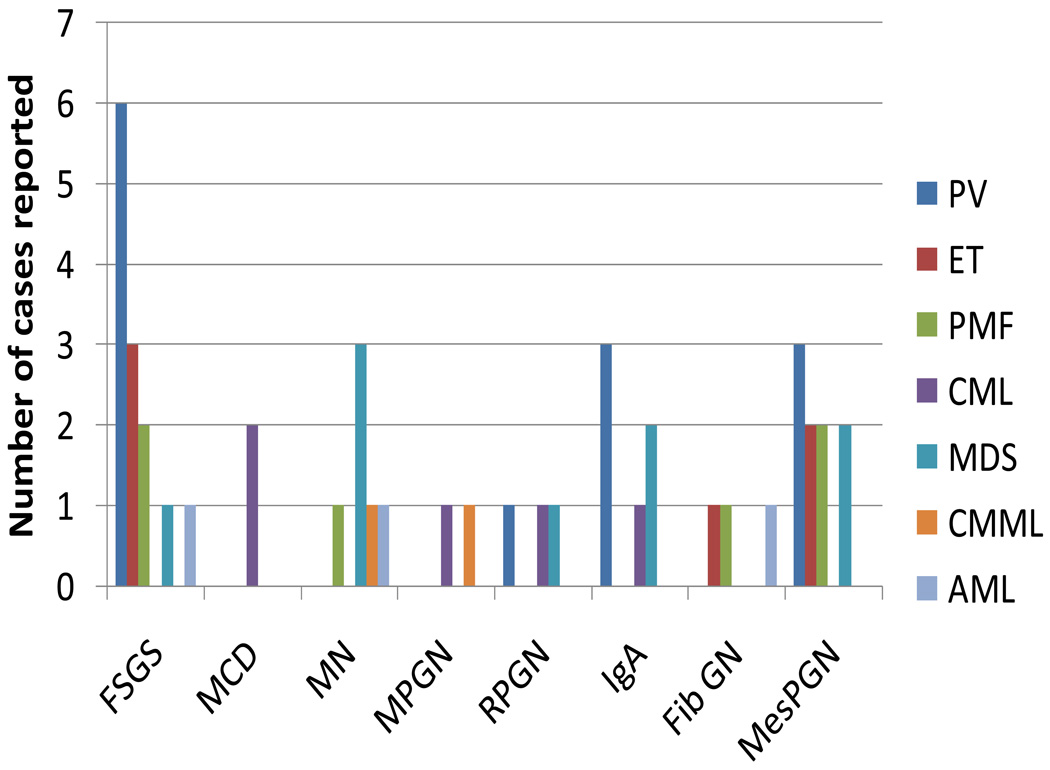
Chronic myeloid malignancies are a highly heterogeneous group of disorders and can occur in association with a variety of glomerulonephritides. However, paraneoplastic glomerulonephritides have been reported infrequently in patients with myeloid neoplasms. In particular, acute myelogenous leukemia, like acute lymphocytic leukemia, has been rarely reported in association with glomerulonephritides (Figure 3).54–56 Elucidation of the molecular pathogenetic mechanisms of several myeloid neoplasms has led to the reclassification of chronic myeloid neoplasms into three categories: myeloproliferative neoplasms, myelodysplastic syndromes and myelodysplastic-myeloproliferative disorders (Table 1).57,58
Figure 3.
Paraneoplastic glomerulonephritis associated with myeloid malignancies. Data are taken from several sources54–56,59–61,64–72,74–77,79,102–114 and are presented as numbers of specific types of paraneoplastic glomerulonephritis associated with different myeloid malignancies. Abbreviations: AML, acute myeloid leukemia; CML, chronic myelogenous leukemia; CMML, chronic myelomonocytic leukemia; ET, essential thrombocythemia; FibGN: fibrillary glomerulonephritis; FSGS, focal segmental glomerulosclerosis; IgAN: IgA nephropathy; MDS, myelodysplastic syndrome; MesPGN: mesangial proliferative glomerulonephritis; MCD, minimal change disease; MN, membranous nephropathy; MPGN: membranoproliferative glomerulonephritis; PMF, primary myelofibrosis; PV, polycythemia vera; RPGN, rapidly progressive glomerulonephritis.
Table 1.
The 2008 WHO classification of chronic myeloid neoplasms
| Category | Characteristics | Diseases |
|---|---|---|
| Myeloproliferative neoplasms | Effective maturation of RBC, WBC, and platelets |
Chronic myelogenous leukemia, polycythemia vera, essential thrombocythemia, primary myelofibrosis, chronic neutrophilic leukemia, chronic eosinophilic leukemia, mastocytosis, unclassifiable myeloproliferative neoplasm |
| Myelodysplastic syndrome | Ineffective maturation of RBC, WBC or platelets with dysplastic features |
Refractory cytopenia with unilineage dysplasia, refractory anemia with ring sideroblasts, refractory cytopenia with multilineage dysplasia, refractory anemia with excess sideroblasts, myelodysplastic syndrome with isolated deletion of chromosome 5q, unclassifiable myelodysplastic syndrome, refractory cytopenia of childhood |
| Myelodysplastic–myeloproliferative disorders |
Hybrid dysplastic and proliferative features |
Chronic myelomonocytic leukemia, juvenile myelomonocytic leukemia, atypical chronic myeloid leukemia, unclassifiable myelodysplastic– myeloproliferative, neoplasm |
Among the myeloproliferative neoplasms, polycythemia vera, essential thrombocythemia, primary myelofibrosis are associated with FSGS and mesangial proliferative glomerulonephritis.59–61 The prevalence of glomerulonephritis in patients with polycythemia vera, essential thrombocythemia and primary myelofibrosis is about 3.6%.60 The Val617Phe mutation in JAK2, which causes abnormal cell proliferation, is present in more than 90% of patients with polycythemia vera and in nearly half of those with essential thrombocythemia or primary myelofibrosis.57 Patients with this mutation have abnormal megakaryocytes, in terms of both number and function. High platelet counts have been proposed as a risk factor for FSGS. Plasma and urine levels of platelet-derived growth factor (PDGF) are elevated in patients with myeloproliferative neoplasms62 and PDGF has been shown to enhance mesangial proliferation and fibrosis.63 Indeed, in one case of FSGS associated with essential thrombocythemia, nephrotic syndrome occurred after a sudden rise in platelet count following a splenectomy.64 Interestingly, although thrombocytosis also occurs in chronic myelogenous leukemia, FSGS has not been reported to be associated with chronic myelogenous leukemia, and plasma PDGF levels in patients with chronic myelogenous leukemia were normal.62 Treatment with myelosuppressive agents or regular phlebotomies for myeloproliferative neoplasms had achieved partial remission of FSGS in some cases.60, 65, 66.
Chronic myelogenous leukemia is the most common myeloproliferative neoplasm, but is rarely associated with glomerulonephritis. All individuals that have chronic myelogenous leukemia carry the Philadelphia chromosome, which encodes the BCR–ABL1 fusion protein that dysregulates tyrosine kinase function.57 Chronic myelogenous leukemia has an indolent clinical course, which has led to the suggestion that glomerulonephritis may occur independently of the malignancy. In support of this theory, immunosuppressive therapy was effective in two cases of MCD and one of RPGN without any effect on the chronic myelogenous leukemia. 67–69 On the other hand, one case of chronic myelogenous leukemia-associated membranoproliferative glomerulonephritis reportedly responded to the tyrosine kinase inhibitor imatinib, suggesting that chronic myelogenous leukemia may cause or exacerbate membranoproliferative glomerulonephritis.70
The clinical presentations of myelodysplastic syndrome range from indolent conditions with a near-normal life expectancy to forms approaching the severity of acute myelogenous leukemia.58 The prevalence of paraneoplastic glomerulonephritis in patients with myelodysplastic syndrome and chronic myelomonocytic leukemia (CMML), a myelodysplastic/myeloproliferative disorder , is 2% and 27%, respectively, as reported in a small series of 114 patients with myelodysplastic syndrome and 11 patients with CMML.71 Myelodysplastic syndrome is associated with autoimmunity and approximately 10% of patients with myelodysplastic syndrome have clinical autoimmune disorders.72 The transformation of normal bone marrow stem cells into dysplastic cells has been suggested to induce an autoimmune T-cell response with the bone marrow as the target organ. This autoimmune attack results in a chronic overproduction of cytokines, such as TNF.73 These cytokines may have an important role in the development of paraneoplastic glomerulonephritis associated with myelodysplastic syndrome.
A variety of glomerulonephritides have been reported to be associated with myelodysplastic syndrome (Figure 3), and in general, their response to immunosuppressive treatment is good .74–76 Only one case of membranous nephropathy and one of membranoproliferative glomerulonephritis have been reported in association with CMML; possibly because kidney biopsy is frequently contraindicated in these patients.77 Monocytes may be involved in pathogenesis of paraneoplastic glomerulonephritis since monocyte counts are higher in patients with nephrotic range proteinuria than in those without.71. Of note, severe monocytosis causes marked lysozymuria, which may present as nephrotic range proteinuria.78 This pseudonephrotic syndrome should be excluded before a diagnosis of paraneoplastic glomerulonephritis is made. Chemotherapy effectively ameliorated paraneoplastic glomerulonephritis in three patients,71,77 and a 2008 case report demonstrated mycophenolate treatment to be effective for CMML-associated membranous nephropathy.79
Thymoma
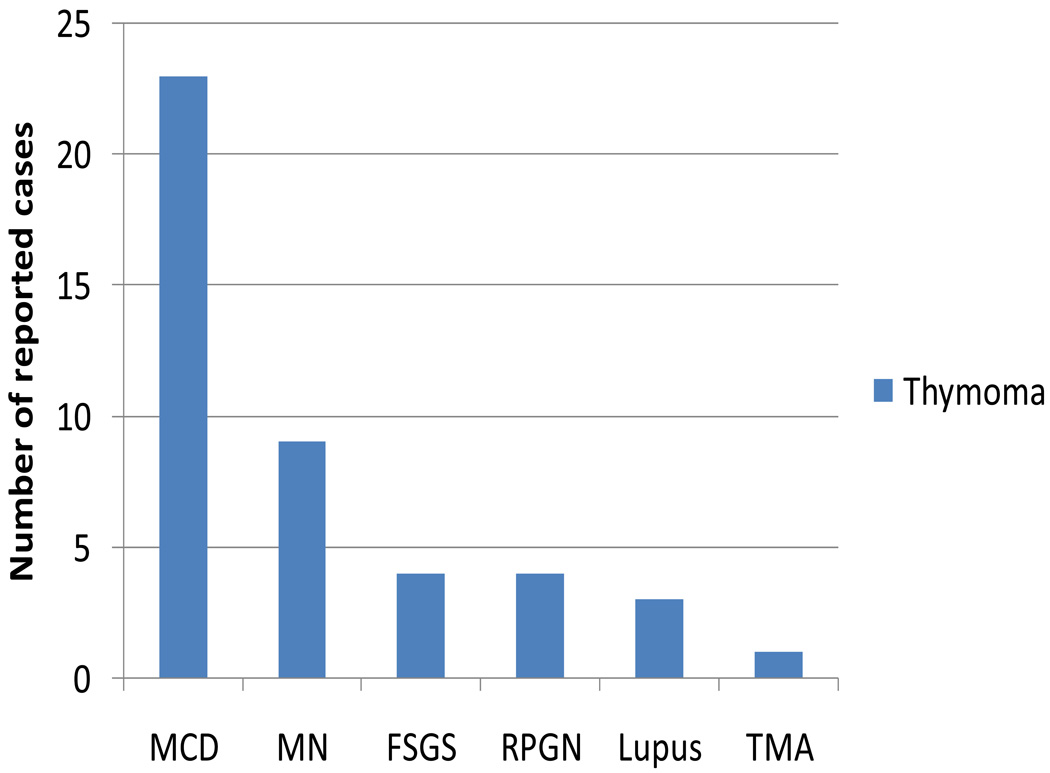
Thymoma is a rare malignancy that is unique because of its association with a variety of autoimmune diseases, including myasthenia gravis, systemic lupus erythematosus, pure red cell aplasia, and pemphigus vulgaris. This association implies that severe T-cell dysregulation occurs in patients with thymoma—a feature that may be implicated in the pathogenesis of thymoma-associated paraneoplastic glomerulonephritis. The prevalence of paraneoplastic glomerular diseases in patients with thymoma is about 2%, higher than that for Hodgkin lymphoma. MCD is the most common paraneoplastic glomerulonephritis associated with thymoma, followed by membranous nephropathy, FSGS, RPGN, and lupus nephritis (Figure 4). In 2005, Karras et al.80 reported the largest series of paraneoplastic glomerulonephritis associated with thymoma. Interestingly, they observed that thymoma-associated membranous nephropathy seems to have a distinct clinical presentation from thymoma-associated MCD. Histologically, thymoma can be divided into epithelial predominant or lymphocyte predominant tumors. Membranous nephropathy tends to be associated with epithelial predominant thymoma, is always diagnosed with either newly diagnosed or recurrent thymoma, and typically resolves after tumor ablation. MCD tends to be associated with lymphocyte-predominant thymoma, is frequently diagnosed after tumor removal, and responds relatively well to steroid therapy (with a combined complete and partial remission rate of 60%). Thymoma-associated membranous nephropathy seems to have a similar pathogenetic mechanism to that of solid tumor-associated membranous nephropathy, whereas thymoma-associated MCD could be associated with persistent T-cell dysfunction after thymoma removal.80
Figure 4.
Paraneoplastic glomerulonephritis associated with thymoma. Data are from several sources80,115–118 and are presented as number of reported cases of specific types of paraneoplastic glomerulonephritis associated with thymoma. Abbreviations: FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MN, membranous nephropathy; RPGN: rapidly progressive glomerulonephritis; TMA, thrombotic microangiopathy.
Over the past 2 decades, the Buffalo/Mna rat has been used as an experimental model of combined thymoma, myasthenia and glomerulopathy. Thymoma in this model is characterized by primary hyperplasia of cortical epithelial cells and a large number of proliferating lymphocytes, which have a normal distribution of CD5+, CD4+ and CD8+ cells.81 Nephrotic syndrome appears at 1 month of age and renal pathology initially shows MCD-like glomerular disease followed by the late development of FSGS. Thymectomy either in infancy82 or adulthood83 does not prevent or attenuate the nephrotic syndrome. Renal macrophage activation, T-cell infiltration, and TH2 polarization precede the development of nephrotic syndrome in this model.83 These findings are consistent with the hypothesis proposed by Shalhoub over 35 years ago that MCD is caused by T-cell dysfunction.84 Corticosteroids or other immunosuppressive drugs such as cyclophosphamide and ciclosporin are, however, ineffective for treating this disease.83 In 2009, Le Berre et al. found that induction of T-regulatory (TREG) cells attenuated nephrotic syndrome in Buffalo/Mna rats. Induction of TREG cells also reduced renal macrophage and T-cell infiltration and suppressed renal messenger (m)RNA expression of the TH2-type cytokines IL10 and IL13 but did not affect mRNA levels of the TH1-type cytokine TNF.85 These studies confirm the importance of TH2 polarization in thymoma-associated nephrotic syndrome, and suggest that induction of TREG cells could be a new potential therapeutic strategy for thymoma-associated MCD and FSGS.
Management
The most difficult issue in the management paraneoplastic glomerulonephritis is the clinical recognition of this syndrome. The consequence of delayed diagnosis could be serious because patients may be subjected to potentially harmful treatment. In patients who present with glomerulonephritis and pre-existing neoplasm, it is important to rule out glomerular lesions induced by cancer treatment. Bone marrow transplantation is known to cause membranous nephropathy via graft-versus-host disease.86 A variety of glomerular diseases are induced by anti-cancer agents (Table 2).87 Thrombotic microangiopathy is the most common lesion caused by chemotherapy agents. Mitomycin C is associated with a 2–10% risk of thrombotic microangiopathy; this risk increases considerably for cumulative doses of ≥ 40 mg/m2.88 Newer agents known to induce thrombotic microangiopathy include gemcitabine and anti-VEGF agents. Gemcitabine-induced thrombotic microangiopathy is dose-related and usually reversible if diagnosed early. Similar to other causes of drug-induced thrombotic microangiopathy, plasma exchange therapy is not helpful in this setting.89 Thrombotic microangiopathy induced by anti-VEGF agents is mainly restricted to renal presentation, that is, characterized by proteinuria, hypertension and acute kidney injury. Microangiopathic hemolysis and thrombocytopenia are rarely reported.90 Lastly, high dose pamidronate91and interferon α92 have been reported to cause collapsing FSGS and FSGS, respectively.
Table 2.
Glomerular lesions caused by cancer treatments
| Glomerular lesions | Cancer treatment agents |
|---|---|
| Thrombotic microangiopathy | Mitomycin C, gemcitabine, anti-VEGF agents |
| Collapsing focal segmental glomerulosclerosis | Pamidronate |
| Focal segmental glomerulosclerosis | Interferon α |
The recognition of paraneoplastic glomerulonephritis before the detection of malignancy requires a high index of suspicion. Routine age-appropriate screening for malignancy should be performed in patients with glomerulonephritis, including fecal occult blood testing in stool and colonoscopy in patients over 50 years of age (or if indicated for other reasons), mammography in women over 40 years of age, and prostate-specific-antigen testing in men over the age of 50 years. Patients with a history of smoking should be assessed by chest radiography or possibly chest CT to exclude lung cancer. Investigation for Hodgkin lymphoma is warranted for patients with MCD, particularly those who have systemic symptoms or who are resistant to traditional MCD treatment. Initial studies for patients with membranoproliferative glomerulonephritis should include assessment of HCV status, and testing for cryoglobulins and monoclonal immunoglobulins. Bone marrow biopsy is indicated if any hint of occult lymphoma or leukemia is present.
A multidisciplinary approach involving nephrologists, oncologists and other care givers is necessary for treating both malignancy and paraneoplastic glomerulonephritis. Symptomatic treatment for nephrotic syndrome in paraneoplastic glomerulonephritis is the same as in non-cancer patients. Specific treatment for cancer-associated glomerular lesions (Table 3) is often different from that used for idiopathic glomerulonephritis. These recommendations are mainly based on case series and reports as reviewed in previous sections. After successful treatment, long term follow up with renal function tests, urinalysis, and spot urine protein:creatinine ratio is important for patients with paraneoplastic glomerulonephritis. For specific glomerulonephritides, serology testing, for example for cryoglobulin, monoclonal immunoglobulin, and ANCA, should be performed. Recurrence of any glomerular lesion should prompt a work up for cancer relapse. Likewise, any recurrence of malignancy should alert physicians to look for recurrence of paraneoplastic glomerulonephritis.
Table 3.
Treatment for paraneoplastic glomerulonephritis according to type of malignancy
| Malignancy | Treatment for paraneoplastic glomerulonephritis |
|---|---|
| Solid tumors | Tumor ablation, additional immunosuppression for RPGN |
| Lymphoid malignancies | Ablation of malignancies |
| Myeloid malignancies | |
| CML | Immunosuppression for MCD and RPGN |
| PV, ET, PMF | Myelosuppression, phlebotomy |
| MDS | Immunosuppression |
| CMML | Ablation of malignancies |
| Thymoma | |
| Epithelial predominant | Tumor ablation |
| Lymphocyte predominant | Immunosuppression |
Abbreviations: CML, chronic myelogenous leukemia; CMML, chronic myelomonocytic leukemia; ET, essential thrombocythemia; MCD, minimal change disease; MDS, myelodysplastic syndrome; PMF, primary myelofibrosis; PV, polycythemia vera; RPGN, rapidly progressive glomerulonephritis.
Conclusions
Paraneoplastic glomerulonephritis, a rare secondary cause of glomerulonephritis and a complication of cancer, remains a challenge to both nephrologists and oncologists. Altered immune responses seem to play a have role in the pathogenesis of paraneoplastic glomerulonephritis. This notion is supported by studies in Buffalo/Mna rats, in which TH2 polarization induces thymoma-associated MCD and FSGS.83, 85 Recognition of paraneoplastic glomerulonephritis and subsequent detection of an undiagnosed malignancy could be life-saving. The treatment of paraneoplastic glomerulonephritis is targeted at the cause—the neoplasm—and requires a multidisciplinary approach to monitor both the cancer and the glomerular lesions. Studies to identify diagnostic biomarkers of paraneoplastic glomerulonephritis in blood, urine, or kidney biopsy samples by use of proteomic or other approaches are critically needed to facilitate the early diagnosis of this disease.
Acknowledgments
The authors’ research is currently supported by NIH grant R01DK082718.
Footnotes
Competing interests
The authors declare no competing interests.
Review criteria
Articles discussed in this Review were found by searching PubMed using the search terms: “paraneoplastic glomerulonephritis”, “malignancy”, “cancer”, “lymphoma”, “thymoma”, “myelodysplastic syndrome”, “myeloproliferative neoplasms”, “membranous nephropathy”, “minimal change disease”, “focal segmental glomerulosclerosis”, “IgA nephropathy”, “membranoproliferative glomerulonephritis”, “rapidly progressive glomerulonephritis”, “thrombotic microangiopathy”, “hemolytic uremic syndrome”, “thrombotic thrombocytopenic purpura”, “chemotherapy”, and “anticancer drugs”. Articles were restricted to those published in English, but no date restrictions were placed on the search. All selected papers were full-text papers, and the reference lists of identified papers were searched for further leads, particularly in the area of related animal models.
Contributions
Both authors contributed equally to researching data for the article, discussing content, writing, and reviewing/editing the article before submission.
References
- 1.Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int. 1999;56:355–377. doi: 10.1046/j.1523-1755.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee JC, Yamauchi H, Hopper J., Jr The association of cancer and the nephrotic syndrome. Ann Intern Med. 1966;64:41–51. doi: 10.7326/0003-4819-64-1-41. [DOI] [PubMed] [Google Scholar]
- 3.Bacchetta J, Juillard L, Cochat P, Droz JP. Paraneoplastic glomerular diseases and malignancies. Crit Rev Oncol Hematol. 2009;70:39–58. doi: 10.1016/j.critrevonc.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Chiu KC, et al. Paraneoplastic polymyositis associated with crescentic glomerulonephritis. Ren Fail. 2008;30:939–942. doi: 10.1080/08860220802353918. [DOI] [PubMed] [Google Scholar]
- 5.Nunez S, et al. Association between scleroderma, renal cell carcinoma and membranous nephropathy. Clin Nephrol. 2009;71:63–68. doi: 10.5414/cnp71063. [DOI] [PubMed] [Google Scholar]
- 6.Arenas MD, et al. Nephrotic syndrome as paraneoplastic manifestation of a primary pulmonary lymphoepithelioma-like carcinoma. Clin Nephrol. 2009;72:206–210. doi: 10.5414/cnp72206. [DOI] [PubMed] [Google Scholar]
- 7.Mimura I, Tojo A, Kinugasa S, Uozaki H, Fujita T. Renal cell carcinoma in association with IgA nephropathy in the elderly. Am J Med Sci. 2009;338:431–432. doi: 10.1097/MAJ.0b013e3181ae1b12. [DOI] [PubMed] [Google Scholar]
- 8.Lefaucheur C, et al. Membranous nephropathy and cancer: Epidemiologic evidence and determinants of high-risk cancer association. Kidney Int. 2006;70:1510–1517. doi: 10.1038/sj.ki.5001790. [DOI] [PubMed] [Google Scholar]
- 9.Beaufils H, Jouanneau C, Chomette G. Kidney and cancer: results of immunofluorescence microscopy. Nephron. 1985;40:303–308. doi: 10.1159/000183483. [DOI] [PubMed] [Google Scholar]
- 10.Pascal RR, Iannaccone PM, Rollwagen FM, Harding TA, Bennett SJ. Electron microscopy and immunofluorescence of glomerular immune complex deposits in cancer patients. Cancer Res. 1976;36:43–47. [PubMed] [Google Scholar]
- 11.Ohtani H, et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant. 2004;19:574–579. doi: 10.1093/ndt/gfg616. [DOI] [PubMed] [Google Scholar]
- 12.Holdsworth SR, Kitching AR, Tipping PG. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999;55:1198–1216. doi: 10.1046/j.1523-1755.1999.00369.x. [DOI] [PubMed] [Google Scholar]
- 13.Tipping PG, Kitching AR. Glomerulonephritis, Th1 and Th2: what's new? Clin Exp Immunol. 2005;142:207–215. doi: 10.1111/j.1365-2249.2005.02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi K, et al. Rectal cancer with paraneoplastic nephropathy: association of vascular endothelial growth factor. Dig Surg. 2004;21:455–457. doi: 10.1159/000083474. [DOI] [PubMed] [Google Scholar]
- 15.Eremina V, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheong HI, et al. Circulating VEGF and TGF-beta1 in children with idiopathic nephrotic syndrome. J Nephrol. 2001;14:263–269. [PubMed] [Google Scholar]
- 17.Ahmed M, Solangi K, Abbi R, Adler S. Nephrotic syndrome, renal failure, and renal malignancy: an unusual tumor-associated glomerulonephritis. J Am Soc Nephrol. 1997;8:848–852. doi: 10.1681/ASN.V85848. [DOI] [PubMed] [Google Scholar]
- 18.Sartelet H, et al. Membranoproliferative glomerulonephritis (MPGN) and pulmonary carcinoid tumour. Nephrol Dial Transplant. 1997;12:2405–2406. doi: 10.1093/ndt/12.11.2405. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed MS, Wong CF, Abraham KA. Membrano-proliferative glomerulonephritis associated with metastatic prostate carcinoma--should immunosuppressive therapy be considered? Nephrol Dial Transplant. 2008;23:777. doi: 10.1093/ndt/gfm681. [DOI] [PubMed] [Google Scholar]
- 20.Reshi AR, Mir SA, Gangoo AA, Shah S, Banday K. Nephrotic syndrome associated with transitional cell carcinoma of urinary bladder. Scand J Urol Nephrol. 1997;31:295–296. doi: 10.3109/00365599709070351. [DOI] [PubMed] [Google Scholar]
- 21.Biava CG, Gonwa TA, Naughton JL, Hopper J., Jr Crescentic glomerulonephritis associated with nonrenal malignancies. Am J Nephrol. 1984;4:208–214. doi: 10.1159/000166810. [DOI] [PubMed] [Google Scholar]
- 22.Tatsis E, Reinhold-Keller E, Steindorf K, Feller AC, Gross WL. Wegener's granulomatosis associated with renal cell carcinoma. Arthritis Rheum. 1999;42:751–756. doi: 10.1002/1529-0131(199904)42:4<751::AID-ANR19>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 23.Pankhurst T, Savage CO, Gordon C, Harper L. Malignancy is increased in ANCA-associated vasculitis. Rheumatology (Oxford) 2004;43:1532–1535. doi: 10.1093/rheumatology/keh374. [DOI] [PubMed] [Google Scholar]
- 24.Faurschou M, et al. Malignancies in Wegener's granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol. 2008;35:100–105. [PubMed] [Google Scholar]
- 25.Edgar JD, Rooney DP, McNamee P, McNeill TA. An association between ANCA positive renal disease and malignancy. Clin Nephrol. 1993;40:22–25. [PubMed] [Google Scholar]
- 26.Hruby Z, Bronowicz A, Rabczynski J, Kopec W, Szewczyk Z. A case of severe anti-neutrophil cytoplasmic antibody (ANCA)-positive crescentic glomerulonephritis and asymptomatic gastric cancer. Int Urol Nephrol. 1994;26:579–586. doi: 10.1007/BF02767663. [DOI] [PubMed] [Google Scholar]
- 27.Baschinsky DY, Baker PB, Niemann TH, Wilmer WA. Pauci-immune ANCA-positive crescentic glomerulonephritis associated with metastatic adenocarcinoma of the lung. Am J Kidney Dis. 2000;36:E24. doi: 10.1053/ajkd.2000.17727. [DOI] [PubMed] [Google Scholar]
- 28.Diez-Porres L, et al. ANCA-associated vasculitis as paraneoplastic syndrome with colon cancer: a case report. Lupus. 2005;14:632–634. doi: 10.1191/0961203305lu2153cr. [DOI] [PubMed] [Google Scholar]
- 29.Mustonen J, Pasternack A, Helin H. IgA mesangial nephropathy in neoplastic diseases. Contrib Nephrol. 1984;40:283–291. doi: 10.1159/000409763. [DOI] [PubMed] [Google Scholar]
- 30.Magyarlaki T, et al. Renal cell carcinoma and paraneoplastic IgA nephropathy. Nephron. 1999;82:127–130. doi: 10.1159/000045388. [DOI] [PubMed] [Google Scholar]
- 31.Pertuiset E, et al. Adult Henoch-Schonlein purpura associated with malignancy. Semin Arthritis Rheum. 2000;29:360–367. doi: 10.1053/sarh.2000.6988. [DOI] [PubMed] [Google Scholar]
- 32.Tsai HM. Thrombotic thrombocytopenic purpura: a thrombotic disorder caused by ADAMTS13 deficiency. Hematol Oncol Clin North Am. 2007;21:609–632. doi: 10.1016/j.hoc.2007.06.003. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werner TL, Agarwal N, Carney HM, Rodgers GM. Management of cancer-associated thrombotic microangiopathy: what is the right approach? Am J Hematol. 2007;82:295–298. doi: 10.1002/ajh.20783. [DOI] [PubMed] [Google Scholar]
- 34.Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103:4043–4049. doi: 10.1182/blood-2003-11-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francis KK, Kalyanam N, Terrell DR, Vesely SK, George JN. Disseminated malignancy misdiagnosed as thrombotic thrombocytopenic purpura: A report of 10 patients and a systematic review of published cases. Oncologist. 2007;12:11–19. doi: 10.1634/theoncologist.12-1-11. [DOI] [PubMed] [Google Scholar]
- 36.Mallouk A, Pham PT, Pham PC. Concurrent FSGS and Hodgkin's lymphoma: case report and literature review on the link between nephrotic glomerulopathies and hematological malignancies. Clin Exp Nephrol. 2006;10:284–289. doi: 10.1007/s10157-006-0437-4. [DOI] [PubMed] [Google Scholar]
- 37.Audard V, et al. Minimal change nephrotic syndrome and classical Hodgkin's lymphoma: report of 21 cases and review of the literature. Kidney Int. 2006;69:2251–2260. doi: 10.1038/sj.ki.5000341. [DOI] [PubMed] [Google Scholar]
- 38.Ramirez G, Stinson JB, Zawada ET, Moatamed F. IgA nephritis associated with mycosis fungoides. Report of two cases. Arch Intern Med. 1981;141:1287–1291. [PubMed] [Google Scholar]
- 39.Bajel A, et al. IgA nephropathy associated with cutaneous T cell lymphoma. Leuk Lymphoma. 2009;50:2083–2085. doi: 10.3109/10428190903288472. [DOI] [PubMed] [Google Scholar]
- 40.Douglas MF, Schwartz MM, Sharon Z. Cryoglobulinemia and immune-mediated glomerulonephritis in association with hairy cell leukemia. Am J Kidney Dis. 1985;6:181–184. doi: 10.1016/s0272-6386(85)80024-x. [DOI] [PubMed] [Google Scholar]
- 41.Abboud I, et al. A paraneoplastic membranoproliferative glomerulonephritis with isolated C3 deposits associated with hairy cell leukaemia. Nephrol Dial Transplant. 2010;25:2026–2028. doi: 10.1093/ndt/gfq153. [DOI] [PubMed] [Google Scholar]
- 42.Kuppers R, Schwering I, Brauninger A, Rajewsky K, Hansmann ML. Biology of Hodgkin's lymphoma. Ann Oncol. 2002;13 Suppl 1:11–18. doi: 10.1093/annonc/13.s1.11. [DOI] [PubMed] [Google Scholar]
- 43.Ohshima K, et al. Interleukin-13 and interleukin-13 receptor in Hodgkin's disease: possible autocrine mechanism and involvement in fibrosis. Histopathology. 2001;38:368–375. doi: 10.1046/j.1365-2559.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 44.Lai KW, et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol. 2007;18:1476–1485. doi: 10.1681/ASN.2006070710. [DOI] [PubMed] [Google Scholar]
- 45.Da'as N, et al. Kidney involvement and renal manifestations in non-Hodgkin's lymphoma and lymphocytic leukemia: a retrospective study in 700 patients. Eur J Haematol. 2001;67:158–164. doi: 10.1034/j.1600-0609.2001.5790493.x. [DOI] [PubMed] [Google Scholar]
- 46.Favre G, et al. Membranoproliferative Glomerulonephritis, Chronic Lymphocytic Leukemia, and Cryoglobulinemia. Am J Kidney Dis. 2009 doi: 10.1053/j.ajkd.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 47.Mazzaro C, et al. Hepatitis C virus risk: a hepatitis C virus related syndrome. J Intern Med. 2000;247:535–545. doi: 10.1046/j.1365-2796.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- 48.Matignon M, et al. Clinical and morphologic spectrum of renal involvement in patients with mixed cryoglobulinemia without evidence of hepatitis C virus infection. Medicine (Baltimore) 2009;88:341–348. doi: 10.1097/MD.0b013e3181c1750f. [DOI] [PubMed] [Google Scholar]
- 49.Saadoun D, Resche-Rigon M, Thibault V, Piette JC, Cacoub P. Antiviral therapy for hepatitis C virus--associated mixed cryoglobulinemia vasculitis: a long-term followup study. Arthritis Rheum. 2006;54:3696–3706. doi: 10.1002/art.22168. [DOI] [PubMed] [Google Scholar]
- 50.Cacoub P, Delluc A, Saadoun D, Landau DA, Sene D. Anti-CD20 monoclonal antibody (rituximab) treatment for cryoglobulinemic vasculitis: where do we stand? Ann Rheum Dis. 2008;67:283–287. doi: 10.1136/ard.2006.065565. [DOI] [PubMed] [Google Scholar]
- 51.Moulin B, et al. Glomerulonephritis in chronic lymphocytic leukemia and related B-cell lymphomas. Kidney Int. 1992;42:127–135. doi: 10.1038/ki.1992.270. [DOI] [PubMed] [Google Scholar]
- 52.Evans DJ, Macanovic M, Dunn MJ, Pusey CD. Membranous glomerulonephritis associated with follicular B-cell lymphoma and subepithelial deposition of IgG1-kappa paraprotein. Nephron Clin Pract. 2003;93:c112–c118. doi: 10.1159/000069548. [DOI] [PubMed] [Google Scholar]
- 53.Papadavid E, et al. The relevance of peripheral blood T-helper 1 and 2 cytokine pattern in the evaluation of patients with mycosis fungoides and Sezary syndrome. Br J Dermatol. 2003;148:709–718. doi: 10.1046/j.1365-2133.2003.05224.x. [DOI] [PubMed] [Google Scholar]
- 54.Dosa S, et al. Acute myelomonocytic leukemia associated with nephrotic syndrome. A case report with immunological studies. Nephron. 1983;34:125–129. doi: 10.1159/000182994. [DOI] [PubMed] [Google Scholar]
- 55.Levi I, Dinour D, Ben-Bassat I, Raanani P. Acute myeloid leukemia associated with nephrotic syndrome: case report and literature review. Leuk Lymphoma. 2002;43:1133–1136. doi: 10.1080/10428190290021443. [DOI] [PubMed] [Google Scholar]
- 56.Sahiner S, Ayli MD, Yuksel C, Onec K, Abayli E. Membranous nephropathy associated with acute myeloid leukemia. Transplant Proc. 2004;36:2618–2619. doi: 10.1016/j.transproceed.2004.09.082. [DOI] [PubMed] [Google Scholar]
- 57.Klco JM, Vij R, Kreisel FH, Hassan A, Frater JL. Molecular pathology of myeloproliferative neoplasms. Am J Clin Pathol. 2010;133:602–615. doi: 10.1309/AJCPPPZ1WFVGNE4A. [DOI] [PubMed] [Google Scholar]
- 58.Komrokji RS, Zhang L, Bennett JM. Myelodysplastic syndromes classification and risk stratification. Hematol Oncol Clin North Am. 24:443–457. doi: 10.1016/j.hoc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Plomley RF, et al. Polycythemia vera and glomerulonephritis. Aust N Z J Med. 1983;13:125–129. doi: 10.1111/j.1445-5994.1983.tb02666.x. [DOI] [PubMed] [Google Scholar]
- 60.Au WY, Chan KW, Lui SL, Lam CC, Kwong YL. Focal segmental glomerulosclerosis and mesangial sclerosis associated with myeloproliferative disorders. Am J Kidney Dis. 1999;34:889–893. doi: 10.1016/S0272-6386(99)70047-8. [DOI] [PubMed] [Google Scholar]
- 61.Kaygusuz I, et al. Focal segmental glomerulosclerosis associated with idiopathic myelofibrosis. Ren Fail. 2010;32:273–276. doi: 10.3109/08860220903573286. [DOI] [PubMed] [Google Scholar]
- 62.Gersuk GM, Carmel R, Pattengale PK. Platelet-derived growth factor concentrations in platelet-poor plasma and urine from patients with myeloproliferative disorders. Blood. 1989;74:2330–2334. [PubMed] [Google Scholar]
- 63.Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- 64.Haraguchi K, et al. Focal segmental glomerulosclerosis associated with essential thrombocythemia. Clin Exp Nephrol. 2006;10:74–77. doi: 10.1007/s10157-005-0391-6. [DOI] [PubMed] [Google Scholar]
- 65.Kosch M, et al. Focal sclerosis with tip lesions secondary to polycythaemia vera. Nephrol Dial Transplant. 2000;15:1710–1711. doi: 10.1093/ndt/15.10.1710-a. [DOI] [PubMed] [Google Scholar]
- 66.Okuyama S, et al. Focal segmental glomerulosclerosis associated with polycythemia vera: report of a case and review of the literature. Clin Nephrol. 2007;68:412–415. doi: 10.5414/cnp68412. [DOI] [PubMed] [Google Scholar]
- 67.Sudholt BA, Heironimus JD. Chronic myelogenous leukemia with nephrotic syndrome. Arch Intern Med. 1983;143:168–169. [PubMed] [Google Scholar]
- 68.Gago ES, Luno E, Quinones L, Jonte F, Alvarez J. Chronic myelogenous leukemia and glomerulonephritis: report of a new case. Am J Med. 1986;81:1121–1122. doi: 10.1016/0002-9343(86)90433-x. [DOI] [PubMed] [Google Scholar]
- 69.Majdan M, et al. Chronic myelogenous leukaemia associated with rapidly progressive glomerulonephritis. Nephrol Dial Transplant. 1994;9:562–563. doi: 10.1093/ndt/9.5.562. [DOI] [PubMed] [Google Scholar]
- 70.Dwyer JP, et al. Chronic myeloid leukemia-associated membranoproliferative glomerulonephritis that responded to imatinib mesylate therapy. Clin Nephrol. 2007;67:176–181. doi: 10.5414/cnp67176. [DOI] [PubMed] [Google Scholar]
- 71.Saitoh T, et al. Myelodysplastic syndromes with nephrotic syndrome. Am J Hematol. 1999;60:200–204. doi: 10.1002/(sici)1096-8652(199903)60:3<200::aid-ajh6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 72.Enright H, et al. Paraneoplastic autoimmune phenomena in patients with myelodysplastic syndromes: response to immunosuppressive therapy. Br J Haematol. 1995;91:403–408. doi: 10.1111/j.1365-2141.1995.tb05310.x. [DOI] [PubMed] [Google Scholar]
- 73.Voulgarelis M, Giannouli S, Ritis K, Tzioufas AG. Myelodysplasia-associated autoimmunity: clinical and pathophysiologic concepts. Eur J Clin Invest. 2004;34:690–700. doi: 10.1111/j.1365-2362.2004.01417.x. [DOI] [PubMed] [Google Scholar]
- 74.Paydas S, Tuncer I, Zorludemir S, Gonlusen G. A case with membranous glomerulonephritis and myelodysplastic syndrome. Nephron. 1992;62:231–232. doi: 10.1159/000187040. [DOI] [PubMed] [Google Scholar]
- 75.Bogdanovic R, et al. Glomerular involvement in myelodysplastic syndromes. Pediatr Nephrol. 2001;16:1053–1057. doi: 10.1007/s004670100025. [DOI] [PubMed] [Google Scholar]
- 76.Nishida M, et al. Focal segmental glomerulosclerosis in a girl with myelodysplastic syndrome. Nephrol Dial Transplant. 2004;19:2639–2641. doi: 10.1093/ndt/gfh345. [DOI] [PubMed] [Google Scholar]
- 77.Morschhauser F, et al. Glomerular injury in chronic myelomonocytic leukemia. Leuk Lymphoma. 1995;18:479–483. doi: 10.3109/10428199509059648. [DOI] [PubMed] [Google Scholar]
- 78.Mok CC, Tam SC, Kwong YL. Pseudonephrotic syndrome caused by lysozymuria. Ann Intern Med. 1994;121:818. doi: 10.7326/0003-4819-121-10-199411150-00020. [DOI] [PubMed] [Google Scholar]
- 79.Enriquez R, et al. Severe renal complications in chronic myelomonocytic leukemia. J Nephrol. 2008;21:609–614. [PubMed] [Google Scholar]
- 80.Karras A, de Montpreville V, Fakhouri F, Grunfeld JP, Lesavre P. Renal and thymic pathology in thymoma-associated nephropathy: report of 21 cases and review of the literature. Nephrol Dial Transplant. 2005;20:1075–1082. doi: 10.1093/ndt/gfh615. [DOI] [PubMed] [Google Scholar]
- 81.Hirokawa K, et al. Age-related hyperplasia of the thymus and T-cell system in the Buffalo ratImmunological and immunohistological studies Virchows Arch B Cell Pathol Incl Mol Pathol 19905938–47. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura T, et al. The effect of thymectomy on the development of nephropathy in spontaneous thymoma rats of the BUF/Mna strain. Clin Exp Immunol. 1988;71:350–352. [PMC free article] [PubMed] [Google Scholar]
- 83.Le Berre L, et al. Renal macrophage activation and Th2 polarization precedes the development of nephrotic syndrome in Buffalo/Mna rats. Kidney Int. 2005;68:2079–2090. doi: 10.1111/j.1523-1755.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 84.Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 85.Le Berre L, et al. Induction of T regulatory cells attenuates idiopathic nephrotic syndrome. J Am Soc Nephrol. 2009;20:57–67. doi: 10.1681/ASN.2007111244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Otani M, Shimojo H, Shiozawa S, Shigematsu H. Renal involvement in bone marrow transplantation. Nephrology (Carlton) 2005;10:530–536. doi: 10.1111/j.1440-1797.2005.00478.x. [DOI] [PubMed] [Google Scholar]
- 87.Sahni V, Choudhury D, Ahmed Z. Chemotherapy-associated renal dysfunction. Nat Rev Nephrol. 2009;5:450–462. doi: 10.1038/nrneph.2009.97. [DOI] [PubMed] [Google Scholar]
- 88.Lesesne JB, et al. Cancer-associated hemolytic-uremic syndrome: analysis of 85 cases from a national registry. J Clin Oncol. 1989;7:781–789. doi: 10.1200/JCO.1989.7.6.781. [DOI] [PubMed] [Google Scholar]
- 89.Humphreys BD, et al. Gemcitabine-associated thrombotic microangiopathy. Cancer. 2004;100:2664–2670. doi: 10.1002/cncr.20290. [DOI] [PubMed] [Google Scholar]
- 90.Gurevich F, Perazella MA. Renal effects of anti-angiogenesis therapy: update for the internist. Am J Med. 2009;122:322–328. doi: 10.1016/j.amjmed.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 91.Markowitz GS, et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol. 2001;12:1164–1172. doi: 10.1681/ASN.V1261164. [DOI] [PubMed] [Google Scholar]
- 92.Shah M, et al. Interferon-alpha-associated focal segmental glomerulosclerosis with massive proteinuria in patients with chronic myeloid leukemia following high dose chemotherapy. Cancer. 1998;83:1938–1946. [PubMed] [Google Scholar]
- 93.Mutluay R, et al. Membranoproliferative glomerulonephritis and light-chain nephropathy in association with chronic lymphocytic leukemia. Clin Nephrol. 2008;70:527–531. doi: 10.5414/cnp70527. [DOI] [PubMed] [Google Scholar]
- 94.Vilayur E, Trevillian P, Walsh M. Monoclonal gammopathy and glomerulopathy associated with chronic lymphocytic leukemia. Nat Clin Pract Nephrol. 2009;5:54–58. doi: 10.1038/ncpneph0989. [DOI] [PubMed] [Google Scholar]
- 95.Yang AH, et al. The clinicopathological implications of endothelial tubuloreticular inclusions found in glomeruli having histopathology of idiopathic membranous nephropathy. Nephrol Dial Transplant. 2009;24:3419–3425. doi: 10.1093/ndt/gfp288. [DOI] [PubMed] [Google Scholar]
- 96.Alshayeb H, Wall B. Non Hodgkin's lymphoma associated membranoproliferative glomerulonephritis: rare case of long term remission with chemotherapy: a case report. Cases J. 2009;2:7201. doi: 10.4076/1757-1626-2-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herrman J, Gabriel F. Membranoproliferative glomerulonephritis in a patient with hairy-cell leukemia treated with alpha-II interferon. N Engl J Med. 1987;316:112–113. doi: 10.1056/NEJM198701083160216. [DOI] [PubMed] [Google Scholar]
- 98.Moe SM, Baron JM, Coventry S, Dolan C, Umans JG. Glomerular disease and urinary Sezary cells in cutaneous T-cell lymphomas. Am J Kidney Dis. 1993;21:545–547. doi: 10.1016/s0272-6386(12)80402-1. [DOI] [PubMed] [Google Scholar]
- 99.Navarro-Antolin J, et al. Rapidly progressive fibrillary glomerulonephritis and cutaneous T-cell lymphoma. Nephron. 1996;73:107–108. doi: 10.1159/000189016. [DOI] [PubMed] [Google Scholar]
- 100.Cather JC, Jackow C, Yegge J, Hagemeister F, Duvic M. Mycosis fungoides with focal segmental glomerular sclerosis and nephrotic syndrome. J Am Acad Dermatol. 1998;38:301–305. doi: 10.1016/s0190-9622(98)70569-9. [DOI] [PubMed] [Google Scholar]
- 101.Sato M, Sohara M, Kitamura Y, Hatamochi A, Yamazaki S. Ichthyosiform mycosis fungoides: report of a case associated with IgA nephropathy. Dermatology. 2005;210:324–328. doi: 10.1159/000084759. [DOI] [PubMed] [Google Scholar]
- 102.Springate JE, Brecher M, Brentjens J, Feld LG. Glomerulonephritis and chronic myelogenous leukemia. Child Nephrol Urol. 1988;9:298–300. [PubMed] [Google Scholar]
- 103.Kanauchi M, Dohi K, Shiiki H, Fujii Y, Ishikawa H. Henoch-Schonlein purpura nephritis associated with polycythemia vera. Intern Med. 1994;33:36–40. doi: 10.2169/internalmedicine.33.36. [DOI] [PubMed] [Google Scholar]
- 104.Sharma RK, et al. Focal segmental glomerulosclerosis in a patient with polycythemia rubra vera. Nephron. 1995;69:361. doi: 10.1159/000188499. [DOI] [PubMed] [Google Scholar]
- 105.Kasuno K, et al. IgA nephropathy associated with polycythaemia vera: accelerated course. Nephrol Dial Transplant. 1997;12:212–215. doi: 10.1093/ndt/12.1.212. [DOI] [PubMed] [Google Scholar]
- 106.Oymak O, et al. Polycythemia vera presenting with rapidly progressive glomerulonephritis and pyoderma gangrenosum. Nephron. 2000;86:346–347. doi: 10.1159/000045793. [DOI] [PubMed] [Google Scholar]
- 107.Chung J, Park PG, Song KI. IgA nephropathy in a patient with polycythemia vera. Clinical manifestation of chronic renal failure and heavy proteinuria. Am J Nephrol. 2002;22:397–401. doi: 10.1159/000065236. [DOI] [PubMed] [Google Scholar]
- 108.Asaba K, et al. Fibrillary glomerulonephritis associated with essential thrombocytosis. Clin Exp Nephrol. 2003;7:296–300. doi: 10.1007/s10157-003-0250-2. [DOI] [PubMed] [Google Scholar]
- 109.Perazella MA, Buller GK. Nephrotic syndrome associated with agnogenic myeloid metaplasia. Am J Nephrol. 1994;14:223–225. doi: 10.1159/000168720. [DOI] [PubMed] [Google Scholar]
- 110.Liu TT, Chen JB, Chen WJ, Kuo CY, Lee CT. Idiopathic myelofibrosis associated with renal extramedullary hematopoiesis and nephrotic syndrome: case report. Chang Gung Med J. 2000;23:169–174. [PubMed] [Google Scholar]
- 111.Pamuk ON, et al. Nephrotic syndrome associated with agnogenic myeloid metaplasia. Leuk Lymphoma. 2002;43:661–663. doi: 10.1080/10428190290012245. [DOI] [PubMed] [Google Scholar]
- 112.Kornblihtt LI, et al. Primary myelofibrosis in a patient who developed primary biliary cirrhosis, autoimmune hemolytic anemia and fibrillary glomerulonephritis. Ann Hematol. 2008;87:1019–1020. doi: 10.1007/s00277-008-0516-6. [DOI] [PubMed] [Google Scholar]
- 113.Komatsuda A, et al. Crescentic glomerulonephritis accompanied by myeloperoxidase-antineutrophil cytoplasmic antibodies in a patient having myelodysplastic syndrome with trisomy 7. Am J Kidney Dis. 1998;31:336–340. doi: 10.1053/ajkd.1998.v31.pm9469507. [DOI] [PubMed] [Google Scholar]
- 114.Apostolou T, et al. Atheroembolic renal disease and membranous nephropathy, in a patient with myelodysplastic syndrome, eosinophilia, and trisomy 8. Nephrol Dial Transplant. 2002;17:1336–1338. doi: 10.1093/ndt/17.7.1336. [DOI] [PubMed] [Google Scholar]
- 115.Lee HC, Cheng YF, Chuang FR, Chen JB, Hsu KT. Minimal change nephrotic syndrome associated with malignant thymoma: case report and literature review. Chang Gung Med J. 2001;24:576–581. [PubMed] [Google Scholar]
- 116.Parambil JG, Keogh KA, Fervenza FC, Ryu JH. Microscopic polyangiitis associated with thymoma, exacerbating after thymectomy. Am J Kidney Dis. 2006;48:827–831. doi: 10.1053/j.ajkd.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 117.Chung S, et al. Simultaneous and sustained remission of intractable myasthenia gravis and focal segmental glomerulosclerosis with tacrolimus treatment. Clin Nephrol. 2008;70:59–61. doi: 10.5414/cnp70059. [DOI] [PubMed] [Google Scholar]
- 118.Prasad KP, Alahakoon DG, Vidanagama U. Case of generalised myasthenia gravis with membranous nephropathy. Ceylon Med J. 2008;53:25–26. doi: 10.4038/cmj.v53i1.224. [DOI] [PubMed] [Google Scholar]