Abstract
Copper is a naturally occurring element found as a component of many minerals. It is an essential nutrient that is normally present in a wide variety of tissues. In humans, ingestion of large quantities of copper salts may cause gastrointestinal, hepatic, and renal effects with symptoms such as severe abdominal pain, vomiting, diarrhea, hemolysis, hepatic necrosis, hematuria, proteinuria, hypotension, tachycardia, convulsions, coma, and death. The chronic toxicity of copper has been characterized in patients with Wilson’s disease, a genetic disorder causing copper accumulation in tissues. Although the clinical manifestations of Wilson’s disease (cirrhosis of the liver, hemolytic anemia, neurologic abnormalities, and corneal opacities) are known, the cellular and molecular events associated with copper toxicity are poorly understood. In the present study, we used human liver carcinoma (HepG2) cells as a model to study the cytotoxicity, and the potential mechanisms of copper-induced toxicity and carcinogenesis. We hypothesized that copper-induction of stress genes may play a role in the cellular and molecular events leading to toxicity and tumor formation in liver cells. To test this hypothesis, we performed the MTT-assay for cell viability, the CAT-Tox(L) assay for gene induction, to assess the transcriptional activation of stress genes. Data obtained from the MTT assay indicated a strong dose-response relationship with respect to copper toxicity. Upon 48 h of exposure, the chemical dose required to cause 50% reduction in cell viability (LD50) was computed to be 220.5 ± 23.8 μg/mL copper sulfate. The CAT-Tox (L) assay showed statistically significant inductions (p < 0.05) of a significant number of stress genes including c-fos, HMTIIA, HSP70, GRP78, RARE, GADD153, and RARE. These data support previous research indicating that copper overload is hepatotoxic. The CAT-Tox data on the other hand indicate that copper overload induces proteotoxic effects (HMTIIA, HSP70, GRP78), inflammatory reactions/oxidative stress (c-fos), and growth arrest and DNA damage (p53, GADD153). The induction of RARE points to its potential involvement in growth and development.
INTRODUCTION
Copper is an essential nutrient that is incorporated into a number of metalloenzymes involved in hemoglobin formation, carbohydrate metabolism, catecholamine biosynthesis, and cross-linking of collagen, elastin, and hair keratin. The copper-dependent enzymes function to reduce molecular oxygen. Some of these enzymes are cytochrome c oxidase, superoxide dismutase, ferroxidases, monoamine oxidase, and dopamine β-monooxygenase. The ability of copper to cycle between an oxidized state, Cu(II), and reduced state, Cu(I), is used by cuproenzymes involved in redox reactions. However, it is this property of copper that also makes it potentially toxic because the transitions between Cu(II) and Cu(I) can result in the generation of superoxide and hydroxyl radicals [1]
In the United States, the median intake of copper from food is 0.93–1.3 mg/day for adults (0.013–0.019 mg Cu/kg/day using a 70-kg reference body weight). A recommended dietary allowance (RDA) of 0.9 mg/day (0.013 mg/kg/day) has recently been established [3]. Studies with laboratory animals have shown copper to be of moderate acute toxicity. The available animal and human data indicate that respiratory, gastrointestinal, hematological, hepatic, endocrine, and ocular effects occur after exposure to large amounts of copper. Liver damage has been reported in individuals ingesting lethal doses of copper sulfate. Liver effects have also been observed in individuals diagnosed with Wilson’s disease, Indian childhood cirrhosis, or idiopathic copper toxicosis.
In humans, copper-induced hepatic damage is dependent on several factors including genetics, age, and copper intake. Copper is readily absorbed from the stomach and small intestine. After nutritional requirements are met, there are several mechanisms that prevent copper overload. Excess copper absorbed into gastrointestinal mucosal cells is bound to the metal binding protein metallothionein. This bound copper is excreted when the cell is sloughed off. Copper that eludes the intestinal barrier can be stored in the liver or incorporated into bile and excreted in the feces.
Although copper homeostasis plays an important role in the prevention of copper toxicity, exposure to excessive levels of copper can result in a number of adverse health effects including liver and kidney damage, anemia, immunotoxicity, and developmental toxicity. Many of these effects are consistent with oxidative damage to membranes or macromolecules. With the exception of several defined syndromes—Wilson’s disease, Indian childhood cirrhosis, and iodopathic copper toxicosis—liver effects are rarely reported in humans, although this has not been extensively investigated [9]. Using the human liver carcinoma cell line as a model, this research was designed to determine the cytotoxicity and to assess the transcriptional activation of stress-related genes associated with copper sulfate exposure.
MATERIALS AND METHODS
Chemicals
Copper sulfate, CuSO4, CAS No. 7758-98-7 with a purity of 99.0% was purchased from Chem Service Inc. in West Chester, Pennsylvania. Dulbecco’s Modified Eagle’s Minimal Essential Medium (DMEM), Lot No. 1016511 was purchased from Life Technologies in Grand Island, New York.
Cell Culture
Human liver carcinoma (HepG2) cells, and thirteen recombinant constructs generated by creating stable transfectants of different mammalian promoter–chloramphenicol acetyltransferase (CAT)-gene fusions were obtained from Xenometrix, Inc. in Boulder, Colorado. For each construct, a unique stress gene promoter or response element was fused to the CAT reporter gene. In the laboratory, cells were stored in liquid nitrogen until use. Next, they were processed and maintained following routine standard protocol implemented in our laboratory [3].
Gene Profile and Cytotoxicity Assays
The seeded plates were incubated for 24 h at 37°C in a 5% incubator, followed by a replacement of the old medium by a fresh one containing the appropriate amount of copper sulfate. For quality assurance/quality control purposes, positive control plates were also made using known inducers including 3-methyl cholathrene (3MC-10 μM) for cytochrome P450 1A1 - CYP1A1, cyclic AMP response element - CRE, 45-kDa growth arrest and DNA damage - GADD45, 53-kDa tumor suppressor protein - p53RE, and xenobiotic response element - XRE; methyl methane sulfonate (MMS-100 μg/mL) for glutathione-s-transferase Ya subunit - GSTYa, metallothionein - HMTIIA, proto-oncogene - cfos, 70-kDa heat shock protein - HSP70, NF-kB response element - NFk-BRE, 153-kDa growth arrest and DNA damage - GADD153, and 78-kDa glucose-regulated protein - GRP78; and all-trans retinoic acid (RA-10 μM) for retinoic acid response element - RARE. Chemical exposures involved polypropylene 96-well microtiter plates for the purpose of chemical dilutions. A constant volume of 20 μL was transferred from each well of the chemical dilution plate to the plate containing the cells to give each cell line five chemical doses and a zero control dose, each in triplicate. After chemical exposure, the cells were re-incubated for 48 hours at 37°C and 5% CO2. Following the incubation period, the total protein was measured by the Bradford method, at 600 nm using a microtiter plate reader. A standard sandwich ELISA was performed, and in the final step, a horseradish peroxidase catalyzed a color change reaction that was measured at 405 nm [4]. The HepG2 parental cell line was dosed in the same manner as the recombinant cell lines and was used to perform the MTT [3-(4,5-dimetylthiazol-2-yl)-2,5- diphenyltetrazolium bromide]-based cell viability assay using a microtiter plate reader with the wavelength set at 550 nm [5].
Statistical Analysis
Fold inductions of the CAT-gene for each recombinant cell line at each copper sulfate concentration were calculated using the CAT-Tox computer software based on the optical density readings at 600 nm and 405 nm. The software also converted the 550 nm readings to cell viability percentages. Standard deviations were determined and the Student’s t-test values were computed to determine if there were significant differences in cell viability and gene expression in treated cells compared to the control cells. Graphs were made to illustrate the dose-response relationship with respect to cytotoxicity and gene expression.
RESULTS AND DISCUSSION
Cytotoxicity Assay
The cytotoxic effect of copper sulfate on human liver carcinoma cells (HepG2) is shown in Figure 1. This figure denotes a strong dose-response relationship with respect to copper sulfate toxicity to HepG2 cells. The correlation coefficient was computed to be. The percentages of cell viability were 100.0 ± 0.0%, 70.8 ± 9.0, 68.8 ± 4.6, 13.1 ± 12.9, 5.1 ± 7.2 and 3.8 ± 2.2% at 0, 62.5, 125, 250, 500 and 1,000 μg/mL of copper sulfate, respectively. There was therefore a gradual decrease in cell viability with increasing doses of copper sulfate. Upon 48 h of exposure the dose (LD50) required to produce 50% reduction in the viability of HepG2 cells was computed to be 220.5 + 23.8 μg/mL.
Figure 1.
Cytotoxicity of copper sulfate to HepG2 cells
This study demonstrated that copper sulfate is acutely toxic to human liver carcinoma cells. The toxic potency was dose dependent. Several studies in our laboratory have also shown that other metal-containing compounds display some degrees of toxicity to HepG2 cells. The 48 h-LD50 values of 6.1±0.8, 8.55±0.58 and 49.0±18 μg/mL have been reported from our laboratory for cadmium chloride, arsenic trioxide and lead nitrate, respectively [3, 6,7]; indicating that these compounds appear to be more toxic to HepG2 cells than copper sulfate. The order of decreasing toxicity is cadmium chloride>arsenic trioxide> lead nitrate>copper sulfate.
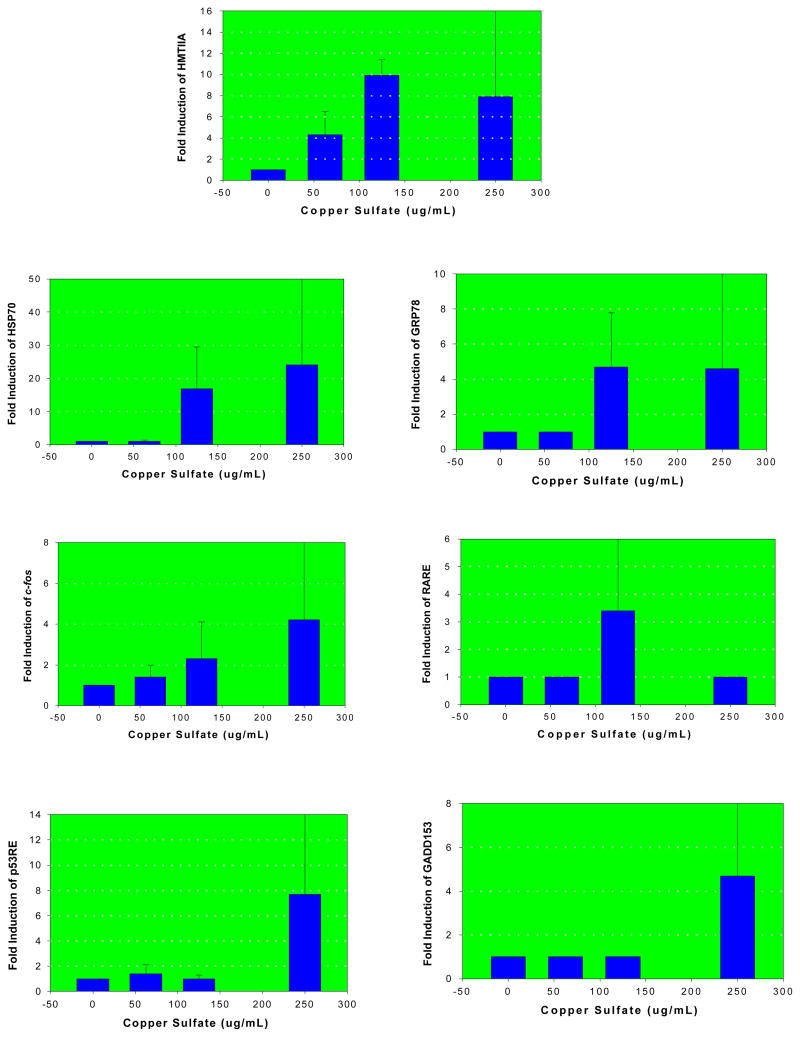
Gene Profile Assay
Seven (HMTIIA, cfos, HSP70, p53RE, GADD153, RARE and GRP78) out of the thirteen constructs evaluated showed a statistically significant level (p<0.05) of induction/activation associated with copper sulfate exposure compared to the negative control. Figure 2 illustrates the specific fold-induction of these genes as a function of copper sulfate concentration. The induction of HMTIIA, c-fos and HSP70 genes was concentration-dependent. The fold inductions were 4.1, 10 and 8 for HMTIIA; 1, 17 and 24 for HSP70; 1.5, 2 and 4.2 for c-fos, 1, 4.5 and 4.5 for GRP78, 1, 3.4 and 1 for RARE, 1.4, 1 and 7.8 for p53RE and 1, 1 and 4.4 for GADD153 in 62.5, 125 and 250 μg/mL copper sulfate, respectively. No significant fold inductions (p>0.05) were recorded for the cytochrome P450 1A1-CYP 1A1, glutathione-S-transferase Ya subunit-GSTYa, xenobiotic response element–XRE, nuclear factor kappa (B site) response element-NFkBRE, 45-kDa growth arrest and DNA damage-GADD45, and cyclic AMP response element. The roles played by these important genes in cell biology have been subject of several investigations [3, 4, 6–8].
Figure 2.
Fold inductions of HMTIIA, HSP70, GRP78, cfos, RARE, p53RE, and GADD153 in human liver carcinoma cells exposed to copper sulfate
Induction of metallothionein (HMTIIA) gene by copper sulfate was highly significant. Metallothioneins (MT) are low molecular weight cysteine rich, heavy metal binding proteins. Metal binding to MT serves as a protective mechanism that reduces the toxicity of metals to cells. Hence, MT is known to be involved in the cellular defense mechanism against metal toxicity [9]. Activation of the 70-kDa heat shock protein (HSP70) and the 78-kDa glucose regulated protein (GRP78) is indicative of the potential proteotoxic effect of copper sulfate. The gene responsible for HSP70 is expressed in response to a wide range of physiological and chemically induced stress conditions. Protein perturbations either by direct protein damage or disruption of nascent chain elongation or folding are the necessary conditions needed to up-regulate HSP70 transcription [10]. GRP48 is a major endoplasmic reticulum protein that functions as a chaperone. Its up-regulation has been associated with proteins that are malfolded because of mutagenesis, under-glycosylation, or other stress conditions [11].
The potential for oxidative damage induced by copper sulfate is evidenced by the induction of the c-fos gene. This gene is part of the AP-1 transcription factor, and is induced as a result of oxidative stress and inflammatory reactions from a variety of environmental compound including DNA damaging agents [11]. The induction of the tumor suppressor protein (p53) response element and the 153-kDa growth arrest and DNA damage (GADD153) gene by copper sulfate at the 250 ug/ml concentration, is indicative of its potential to induce DNA damage and cell cycle arrest at higher level of exposure. P53 is involved in the transcription of genes that negatively control cell growth and/or invasion through the p53 response element [12]. GADD153 activation as a result of DNA damage may be associated with alterations in DNA sequence, as well as conformational changes in its helical structure [13]. The activation of the retinoic acid response element (RARE) by copper sulfate is indicative of its potential influence on growth and development. RARE has been reported to be activated by retinoid analogs that are involved in cellular growth and differentiation [14].
Acknowledgments
This research was supported in part by a grant from the National Institutes of Health (Grant N0.1G12RR13459), through the NIH-RCMI Center for Environmental Health, and in part by a grant from the U.S. Department of the Army (Cooperative Agreement No. W912H2-04-2-0002) through the CMCM Program at Jackson State University. The authors thank Dr. Abdul Mohamed and Dr. Richard Price for their technical support in this research.
References
- 1.Camakaris J, Voskoboinik I, Mercer JF. Molecular mechanisms of copper homeostasis. Biochem Biophys Res Commun. 1999;261(2):225–232. doi: 10.1006/bbrc.1999.1073. [DOI] [PubMed] [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Copper. Atlanta, GA: 2002. [PubMed] [Google Scholar]
- 3.Tchounwou PB, Yedjou CG, Dorsey WC. Arsenic trioxide–induced transcriptional activation of stress genes and expression of related proteins in human liver carcinoma cells. Cellular and Molecular Biology. 2003;49(7):1071–1079. [PubMed] [Google Scholar]
- 4.Todd MD, Lee MJ, Williams JL, Nalenzny JM, Gee P, Benjamin MB, Farr SB. The CAT-Tox Assay: A sensitive and specific measure of stress induced transcription in transformed human liver cells. Fundament Appl Toxicol. 1995;28:118–128. doi: 10.1006/faat.1995.1153. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Applications to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 6.Tchounwou PB, Ishaque AB, Schneider J. Cytotoxicity and transcriptional activation of stress genes in human liver carcinoma cells exposed to cadmium chloride. Molecular and Cellular Biochemistry. 2001;222:21–28. [PubMed] [Google Scholar]
- 7.Tchounwou PB, Yedjou CG, Foxx DN, Ishaque AB, Shen E. Lead-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma cells. Molecular and Cellular Biochemistry. 2004;255:161–170. doi: 10.1023/b:mcbi.0000007272.46923.12. [DOI] [PubMed] [Google Scholar]
- 8.Cherian MG, Howell SB, Imura N, Klassen CD, Koropatnick J, Lazo JS, Waalkes MP. Contemporary issues in toxicology: role of metallothionein in carcinogenesis. Toxicol Appl Pharmacol. 1994;126:1–5. doi: 10.1006/taap.1994.1083. [DOI] [PubMed] [Google Scholar]
- 9.Morimoto RI, Sarge KD, Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem. 1992;267:2987–3990. [PubMed] [Google Scholar]
- 10.Tully DB, Collins BJ, Overstreet JD, Smith CS, Dinse GE, Mumtaz MM, Chappin RE. Effects of arsenic, cadmium, chromium and lead on gene expression regulated by a battery of 13 different promoters in recombinant HepG2 cells. Toxicol Appl Pharmacol. 2000;168:79–90. doi: 10.1006/taap.2000.9014. [DOI] [PubMed] [Google Scholar]
- 11.Morimoto RI, Tissieres A, Georgopoulos C, editors. Stress Proteins in Biology and Medicine. Cold Spring Harbor Laboratory Press; New York: 1990. [DOI] [PubMed] [Google Scholar]
- 12.Katsan MB, Onyekwere O, Sidransy D, Vogelstaein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 13.Luethy JD, Holbrook NJ. The pathway regulating GADD 153 induction in response to DNA damage is independent of protein kinase C and tyrosine kinases. Cancer Res. 1994;54:1902–1906. [PubMed] [Google Scholar]
- 14.Allenby G, Bosque MT, Saunders M, Kramer S, Speck J. Retinoid acid receptors and retinoid X receptors: Interactions with endogenous retinoid acids. Proc Natl Acad Sci. 1993;90:30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]