Abstract
Lipids are essential for the structural and functional integrity of membranes. Membrane lipids are not randomly distributed but are localized in different domains. A common characteristic of these membrane domains is their association with cholesterol. Lipid rafts and caveolae are examples of cholesterol enriched domains, which have attracted keen interest. However, two other important cholesterol domains are the exofacial and cytofacial leaflets of the plasma membrane. The two leaflets that make up the bilayer differ in their fluidity, electrical charge, lipid distribution, and active sites of certain proteins. The synaptic plasma membrane (SPM) cytofacial leaflet contains over 85% of the total SPM cholesterol as compared with the exofacial leaflet. This asymmetric distribution of cholesterol is not fixed or immobile but can be modified by different conditions in vivo: 1) chronic ethanol consumption; 2) statins; 3) aging; and 4) apoE isoform. Several potential candidates have been proposed as mechanisms involved in regulation of SPM cholesterol asymmetry: apoE, low-density-lipoprotein receptor, sterol carrier protein-2, fatty acid binding proteins, polyunsaturated fatty acids, p-glycoprotein and caveolin-1. This review examines cholesterol asymmetry in SPM, potential mechanisms of regulation and impact on membrane structure and function.
Keywords: aging, apolipoprotein E, asymmetry, caveolin, cholesterol, lipid domains
There is substantial interest in membrane lipid domains across numerous areas of biology. The roles of lipid domains in brain structure, function and neurodegeneration are certainly one of those areas as demonstrated by this special issue. Rafts and caveolae are domains that have attracted considerable attention. However, two other domains of the membrane are actually the two leaflets of the bilayer as pictured in Figure 1. There are substantial differences in the two leaflets including for example electrical charge, thickness, fluidity, and lipid distribution (Schroeder 1985;Wood et al. 2002). Lipids are asymmetrically distributed in the membrane bilayer (Fig. 1). Phosphatidylcholine (PC) and sphingomyelin (SM) are enriched in the brain exofacial or outer leaflet of the synaptic plasma membrane. There is evidence that SM is not present in the cytofacial or inner leaflet of SPM but that phosphatidylethanolamine (PE), phosphatidylserine (PS) and phosphatidylinositol (PI) are in abundance in the cytofacial leaflet. This transbilayer or asymmetric distribution of phospholipids contributes to the differences in the electrical charges of the two leaflets. The exofacial leaflet is neutral or zwitterionic and the cytofacial leaflet is more negatively charged due to the enrichment of the anionic phospholipids, PI and PS. This difference in the electrical charge of the two leaflets is associated with the accumulation of certain cationic and anionic drugs in membranes (Sweet et al. 1987). Cationic drugs acted on the cytofacial leaflet and anionic drugs affected the exofacial leaflet. Regulation of phospholipid asymmetry is thought to involve the actions of different protein translocases and transporters and that topic has been recently reviewed (Bevers and Williamson 2010). Cholesterol, a major lipid in membranes accounting for over 40 mol% of synaptic plasma membrane lipids (Wood et al. 1989a) is also asymmetrically distributed. The purpose of this review is to discuss cholesterol asymmetry in brain synaptic plasma membranes (SPM), its alteration under certain conditions, mechanisms involved in its regulation and the role of cholesterol asymmetry in membrane structure and function.
Figure 1.
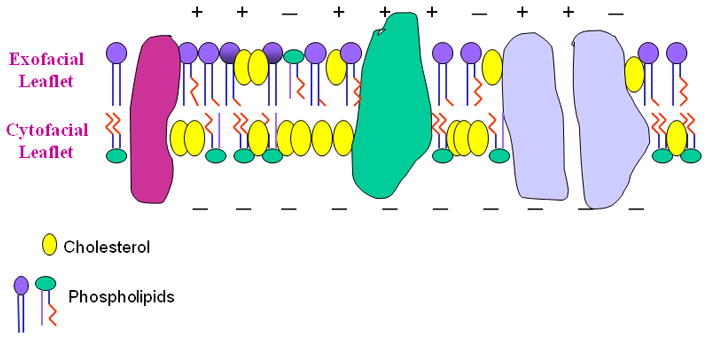
Asymmetry in Synaptic Plasma Membranes. Model of the two leaflets of the plasma membrane, showing the asymmetric distribution of cholesterol and phospholipids. Large globular structures represent proteins. Cholesterol, phosphatidylinositol, phosphatidylethanolamine, and phosphatidylserine are enriched in the cytofacial leaflet. Phosphatidylcholine and sphingomyelin are in abundance in the exofacial leaflet, and there is some phosphatidylcholine in the cytofacial leaflet. + and − denote charge properties of the two leaflets.
Cholesterol Asymmetry in Plasma Membranes
The establishment of cholesterol asymmetry in biological membranes was advanced by the earlier work of Schroeder and colleagues using quenching of the fluorescent sterol dehydroergosterol (DHE) by trinitrobenzene sulfonic acid (TNBS). A comprehensive review on DHE has been recently published (McIntosh et al. 2008) and so a detailed discussion will not be presented in the present review. Briefly, DHE is a natural fluorescent sterol found in sponge and yeast and thus does not have a bulky fluorophore attached to it as compared for example with the commonly used cholesterol analog NBD-cholesterol. DHE is structurally and functionally most similar to cholesterol as compared with other cholesterol analogs. This fluorescent sterol is used in cell culture, isolated tissue, real-time imaging in living cells and administered in vivo. A caveat to using DHE is that the commercial compound is chemically synthesized and it can contain impurities such as oxidized sterols which can perturb membrane structure and function necessitating steps to remove such contaminants (McIntosh et al. 2008).
The mouse cytofacial leaflet of SPM contains substantially more cholesterol as compared with the exofacial leaflet (Igbavboa et al. 1997;Igbavboa et al. 1996;Wood et al. 1990;Kirsch et al. 2003). The cytofacial leaflet contains approximately 85% of total SPM cholesterol. That the cytofacial leaflet contains more cholesterol than the exofacial leaflet was also observed in fibroblasts (Incerpi et al. 1992), human erythrocytes (Schroeder et al. 1991) and most recently in the plasma membrane and the endocytic recycling compartment of a Chinese hamster ovary cell line (Mondal et al. 2009). Those findings indicate that cholesterol asymmetry is a property shared by different cell types with the abundance of cholesterol contained in the cytofacial leaflet. The greater concentration of cholesterol in the cytofacial leaflet versus the exofacial leaflet is associated with the fluidity of the two leaflets. The SPM cytofacial leaflet is distinctly less fluid than the exofacial leaflet (Wood et al. 2002). The large difference in leaflet fluidity affects the ability of various molecules to partition into membranes. For example, ethanol disorders the exofacial leaflet but has little if any effect on the cytofacial leaflet (Schroeder et al. 1988;Wood et al. 1989b;Bae et al. 2005).
SPM cholesterol asymmetry is not static and it is altered by chronic ethanol consumption, statins, aging and apolipoprotein E isoform. There was approximately a two-fold increase in cholesterol in the exofacial leaflet of mice chronically administered ethanol (Wood et al. 1990). The total amount of SPM cholesterol (exofacial leaflet + cytofacial leaflet) was similar for the ethanol and control groups. Not surprisingly, the fluidity of the two leaflets in the ethanol group was altered. The exofacial leaflet became less fluid and the cytofacial leaflet became more fluid in SPM of the ethanol group. This change in fluidity was consistent with the ethanol-induced redistribution of cholesterol between the two leaflets. Chronic administration of statins (simvastatin, lovastatin, atorvastatin) altered cholesterol asymmetry in mouse SPM (Burns et al. 2006). There was an increase in exofacial leaflet cholesterol and a corresponding reduction in cytofacial leaflet cholesterol. Those results are different from the findings of an earlier study which showed that lovastatin and pravastatin but not simvastatin reduced cholesterol in the SPM exofacial leaflets of chronic drug-treated mice (Kirsch et al. 2003).
Increasing age alters SPM cholesterol asymmetry (Igbavboa et al. 1996). Mice 24–25 months of age had significantly more cholesterol in the SPM exofacial leaflet (32% cholesterol) as compared with mice 3–4 months of age (14% cholesterol). Mice 14–15 months of age also had significantly more cholesterol in the exofacial leaflet (24%) than the younger age group. In young mice, the exofacial leaflet is significantly more fluid than the cytofacial leaflet. This asymmetry in fluidity was not observed in SPM of aged mice, which may be due in part to the redistribution of cholesterol between the two leaflets. However, other factors must also contribute to the loss of differences in fluidity between the two leaflets of aged mice. Attenuation of cholesterol asymmetry has also been observed in SPM of mice expressing human apolipoprotein E4 (Hayashi et al. 2002). Both increasing age and the apoE4 allele are risk factors for Alzheimer’s disease (AD) and changes in cholesterol asymmetry may contribute to the pathophysiology associated with AD. An active area of research has been on the association between cholesterol abundance and the production of the amyloid beta-protein (Aβ) including the role of lipid rafts and this topic is reviewed elsewhere in this issue. A two-fold increase or greater of cholesterol in the exofacial leaflet observed in SPM of aged mice and mice expressing human apoE4 could certainly impact on membrane structure and function and contribute to Aβ production. What makes changes in cholesterol asymmetry observed in aged mice or mice expressing human apoE4 so notable are that major alterations can occur in the absence of total changes in SPM cholesterol abundance.
Regulation of Membrane Cholesterol Asymmetry
The abundance of cholesterol in the exofacial leaflet is strikingly less as compared with the cytofacial leaflet. An explanation for cholesterol asymmetry has not been established. There have been several potential candidates proposed as mechanisms involved in regulation of SPM cholesterol asymmetry as depicted in Figure 2. Sphingomyelin had been proposed earlier to be a factor in cholesterol asymmetry (Slotte and Bierman 1988;Porn et al. 1991). Hydrolysis of sphingomyelin in fibroblasts and Leydig tumor cells caused movement of cholesterol from the cell surface to the cell interior. Sphingomyelin accounts for approximately 2 to 4% of the total non-sterol SPM lipid and it is all contained in the exofacial leaflet (Wood et al. 1993;Rao et al. 1993). In erythrocytes, sphingomyelin is approximately 25% of total non-sterol lipid and abundance in the exofacial leaflet was between 82 and 100 % (Roelofsen 1982). Erythrocyte exofacial leaflet cholesterol is approximately 25% of total membrane cholesterol (Schroeder et al. 1991). Increasing sphingomyelin levels in the exofacial leaflet is associated with increasing cholesterol content in that leaflet. Regulation of cholesterol in the exofacial leaflet but not the cytofacial leaflet may involve interaction of cholesterol and sphingomyelin via binding, complex formation, or changes in membrane structure such as fluidity and lipid packing. In addition, sphingomyelin is a component of lipid rafts and the influence of lipid rafts on cholesterol asymmetry and vice versa are topics that have not been examined.
Figure 2.
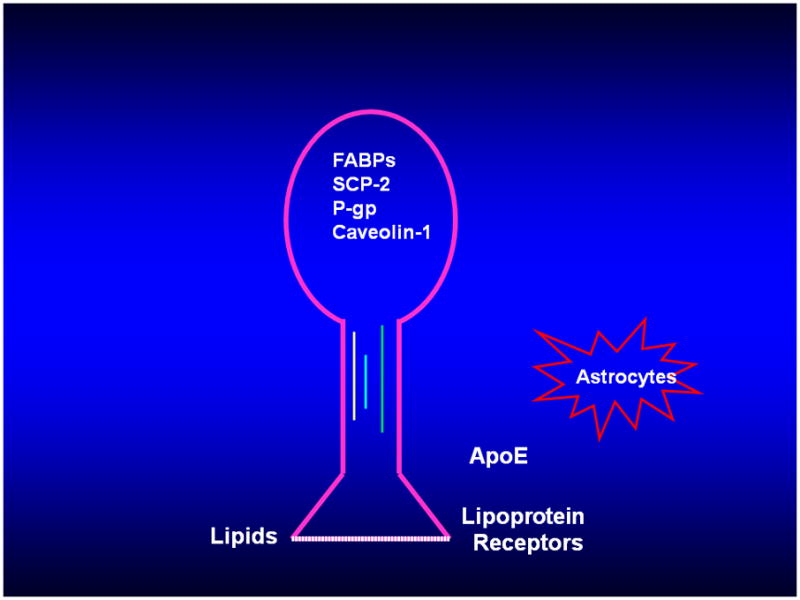
Proposed Mechanisms Involved in Regulation of Cholesterol Asymmetry in Synaptic Plasma Membranes. Neuronal model containing intracellular proteins, which may transport cholesterol to or anchor cholesterol in the two leaflets of the plasma membrane. Cholesterol may also be transported from astrocytes to the nerve terminal and taken up by members of the low density lipoprotein receptor family. Fatty acid binding proteins (FABPs), sterol carrier protein-2 (SCP-2), P-glycoprotein (P-gp).
There is evidence that both apoE and one of its receptors, the low density lipoprotein receptor (LDLR) may contribute to the maintenance of cholesterol asymmetry. SPM of mice deficient in apoE had a two-fold increase in exofacial leaflet cholesterol as compared with wild type mice (Igbavboa et al. 1997). This large difference cannot be accounted for by changes in the total amount of SPM cholesterol, which were similar in SPM of both groups. It was observed in the same study that mice deficient in the LDLR or deficient in both apoE and LDLR also showed greater abundance of cholesterol in the exofacial leaflet as compared with wild type mice. ApoE is the major cholesterol transporter in brain, it is primarily synthesized in astrocytes and it has been shown that neurons receive some of their cholesterol from astrocytes (Mauch et al. 2001). Cholesterol in neurons is unique in contrast to phospholipids because it would appear that it is not synthesized at the nerve terminal of the axon (Vance et al. 1994). The nerve terminal including the SPM may receive some astrocyte derived cholesterol, which is delivered by apoE and taken up by LDLR and other family members. This cholesterol may then be recycled to SPM. A problem with this interpretation is that the SPM of the apoE and LDLR deficient mice either singly or the double-knockout had levels of total SPM cholesterol which were similar to wildtype mice. This observation does not support a deficit in transporting cholesterol from astrocytes to the nerve terminal.
Assuming that apoE and LDLR may contribute to the maintenance of cholesterol asymmetry, the changes in the null mice did not exceed 34% of cholesterol in the exofacial leaflet. This finding would imply that additional factors are involved in regulating cholesterol asymmetry. There are data indicating that fatty acid composition may be contributors to cholesterol asymmetry in plasma membranes. Plasma membranes of L-cell fibroblasts which were fed serum enriched in unsaturated fatty acids had approximately 70% of cholesterol sequestered in the exofacial leaflet as compared with 28% in the control exofacial leaflet (Sweet and Schroeder 1988). Linking fatty acid composition with SPM cholesterol asymmetry are data showing that SPM of apoE-deficient mice was enriched in the highly unsaturated fatty acid 22:6 (n & − 3) in both the sn-1 and sn-2 positions particularly in diacyl-PE and PS (Igbavboa et al. 2002). PE and PS are in abundance in the cytofacial leaflet and an increase in the phospholipid molecular species containing 22:6 (n & − 3) may stimulate the transbilayer movement of cholesterol directly or act on a putative protein that regulates cholesterol asymmetry. For example, it was shown that fibroblasts overexpressing live fatty acid binding protein (L-FABP) had more cholesterol in the exofacial leaflet as compared to control cells (Woodford et al. 1993). L-FABP is a cytosolic protein that binds both fatty acids and cholesterol (Schroeder et al. 2008).
Two additional proteins that could play a role in maintaining membrane cholesterol asymmetry are p-glycoprotein (P-gp) and caveolin-1 (Garrigues et al. 2002;Igbavboa et al. 2009). P-gp is a member of the ATP-binding cassette transporter family of proteins having multiple functions including multidrug resistance in certain types of tumor cells (Schinkel 1997). P-gp is expressed in brain (Spector 2010). It was reported that P-gp stimulated the movement of cholesterol from the cytofacial leaflet to the exofacial leaflet in vesicles prepared from DC-3F cells overexpressing human P-gp using accessibility of cholesterol to cholesterol oxidase to determine cholesterol distribution (Garrigues et al. 2002). This translocation of cholesterol was inhibited by a P-gp inhibitor. It also was concluded in that study that the cytofacial leaflet contained more cholesterol as compared with the exofacial leaflet, which is consistent with findings in other cells types using an entirely different method (DHE fluorescence) for determining cholesterol asymmetry as discussed earlier in this review. In that paper, it was proposed that P-gp might possibly interact with caveolin-1 in increasing exofacial leaflet cholesterol. There are data showing that P-gp co-immunoprecipitates with caveolin-1 (Demeule et al. 2000). Caveolin-1 is a 22-KDa protein associated with caveolae and this protein binds cholesterol and is thought to be a key contributor to cholesterol homeostasis (Murata et al. 1995;Smart et al. 1994;Conrad et al. 1995;Uittenbogaard and Smart 2000;Pol et al. 2001;Ito et al. 2002). We have recently reported that perturbation of astrocytes by amyloid beta protein (1–42) induced movement of cholesterol and caveloin-1 from the plasma membrane to the Golgi complex (Igbavboa et al. 2009). Effects of Aβ1-42 on both cholesterol and caveolin-1 were inhibited by siRNA targeted to the caveolin-1 gene. There was also a significant reduction of cholesterol and caveolin in the Golgi complex of cells treated with only siRNA. There is evidence that caveolin may recycle lipids including cholesterol. One possibility is that cholesterol cycles in and out of the cytofacial leaflet and that caveolin may regulate cholesterol specifically in the cytofacial leaflet. Excess cholesterol in the cytofacial leaflet may be transported by caveolin or P-gp to the Golgi complex and other organelles. Caveolin may have a transbilayer effect and cycle cholesterol between the cytofacial and exofacial leaflet; similar to proteins involved in maintaining phospholipid asymmetry. A recent study found that caveolin-1 was enriched in the cytofacial leaflet and it sequestered fatty acids (Simard et al. 2010). As mentioned earlier (Sweet and Schroeder 1988), treating cells with unsaturated fatty acids altered cholesterol asymmetry and perhaps such changes could involve caveolin-1 complexing with fatty acids.
An obvious conclusion regarding regulation of cholesterol asymmetry is that a single mechanism does not appear to account for the greater abundance of cholesterol in the cytofacial leaflet as compared with the exofacial leaflet. Instead, we hypothesize that multiple mechanisms are involved which may include both proteins and lipids in regulating the transbilayer distribution of cholesterol.
Cholesterol Asymmetry and Membrane Function
It is well-established that cholesterol plays a major role in both membrane structure and protein function (Yeagle 1989;Schroeder et al. 2010;Levitan et al. 2010). How specific changes in the distribution of cholesterol in the two leaflets affect membrane function have not been extensively studied. There is some evidence that plasma membrane functions such as receptor-effector coupling, ion transporters, and translocation of proteins across the plasma membrane may be influenced by the transbilayer lipid environment including cholesterol (Schroeder et al. 2001). Export of cholesterol out of the cell to lipoprotein acceptors may be altered by changes in cholesterol asymmetry (Mondal et al. 2009). It has been reported that statin-induced redistribution of cholesterol was associated with reduced Aβ and β-CTF levels in contrast to changes in bulk cholesterol levels in brain membranes (Burns et al. 2006). SPM of chronic ethanol treated mice, which showed a doubling of cholesterol in the exofacial leaflet, were resistant to perturbation by ethanol indicative of neuronal tolerance. Changes in cholesterol asymmetry could impact on the capacity of the membrane to form domains such as lipid rafts and caveolae. Lipid and protein composition of lipid rafts from mice expressing human apoE4 differed from mice expressing human apoE3 (Igbavboa et al. 2005) and as discussed earlier apoE expressing mice had a greater percentage of SPM cholesterol in the exofacial leaflet as compared with apoE3 mice (Hayashi et al. 2002). What is not evident is whether changes in cholesterol asymmetry alters lipid rafts or in fact, lipid rafts contribute to the transbilayer distribution of cholesterol. Finally, a question, which has not been rigorously addressed, is if changes in cholesterol asymmetry are adaptive or conversely are such changes inimical to cell membrane function. The argument could be made that the changes observed in ethanol-treated mice may be adaptive i.e., reducing partitioning of ethanol into the membrane but changes in cholesterol asymmetry in SPM of aged mice or mice expressing human apoE4 which were similar to that of ethanol treated mice may not be adaptive. In the instance of the ethanol-treated mice, it is reasonable to predict that additional effects because of changes in cholesterol asymmetry would be observed which might not be adaptive. It is clear that much more research is needed to establish the functional consequences of modifying the transbilayer distribution of cholesterol in membranes.
Summary
Cholesterol is asymmetrically distributed in plasma membranes including SPM. The cytofacial leaflet contains approximately 5 to 6 fold more cholesterol than the exofacial leaflet, which has both structural and functional consequences. Cholesterol asymmetry is not static but is altered by several different conditions both in vivo and in vitro. Mechanisms regulating cholesterol asymmetry are not well-understood but the available data lead to the conclusion that multiple mechanisms may be involved. The functional consequences of changes in SPM cholesterol asymmetry include fluidity, alterations in lipid domains, lateral and transbilayer diffusion, lipid packing, and protein function. Of particular interest and the need for further research is the relationship between cholesterol asymmetry and the formation and function of lipid rafts and caveolae.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health AG-23524, AG-18357 and Department of Veterans Affairs.
Reference List
- Bae MK, Jeong DK, Park NS, Lee CH, Cho BH, Jang HO, Yun I. The effect of ethanol on the physical properties of neuronal membranes. Mol Cells. 2005;19:356–364. [PubMed] [Google Scholar]
- Bevers EM, Williamson PL. Phospholipid scramblase: An update. FEBS Lett. 2010;584:2724–2730. doi: 10.1016/j.febslet.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Burns MP, Igbavboa U, Wood WG, Duff K. Cholesterol distribution, not total levels, correlate with altered amyloid precursor protein processing in statin-treated mice. NeuroMol Med. 2006;8:319–328. doi: 10.1385/nmm:8:3:319. [DOI] [PubMed] [Google Scholar]
- Conrad PA, Smart EJ, Ying YS, Anderson RGW, Bloom GS. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J Cell Biol. 1995;131:1421–1433. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeule M, Jodoin J, Gingras D, Béliveau R. P-glycoprotein is localized in caveolae in resistant cells and in brain capillaries. FEBS Lett. 2000;466:219–224. doi: 10.1016/s0014-5793(00)01087-5. [DOI] [PubMed] [Google Scholar]
- Garrigues A, Escargueil AE, Orlowski S. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. PNAS. 2002;99:10347–10352. doi: 10.1073/pnas.162366399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Igbavboa U, Hamanaka H, Kobayashi M, Fujita SC, Wood WG, Yanagisawa K. Cholesterol is increased in the exofacial leaflet of synaptic plasma membranes of human apolipoprotein E4 knock-in mice. Neuroreport. 2002;13:383–386. doi: 10.1097/00001756-200203250-00004. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Avdulov NA, Chochina SV, Wood WG. Transbilayer distribution of cholesterol is modified in brain synaptic plasma membranes of knockout mice deficient in the low density lipoprotein receptor, apolipoprotein E, or both proteins. J Neurochem. 1997;69:1661–1667. doi: 10.1046/j.1471-4159.1997.69041661.x. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Avdulov NA, Schroeder F, Wood WG. Increasing age alters transbilayer fluidity and cholesterol asymmetry in synaptic plasma membranes of mice. J Neurochem. 1996;66:1717–1725. doi: 10.1046/j.1471-4159.1996.66041717.x. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Eckert GP, Malo TM, Studniski A, Johnson LNA, Yamamoto N, Kobayashi M, Fujita SC, Appel TR, Müller WE, Wood WG, Yanagisawa K. Murine synaptosomal lipid raft protein and lipid composition are altered by expression of human apoE3 and 4 and by increasing age. J Neurol Sci. 2005;229–230:225–232. doi: 10.1016/j.jns.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Hamilton J, Kim HY, Sun GY, Wood WG. A new role for apolipoprotein E: Modulating transport of polyunsaturated phospholipid molecular species in synaptic plasma membranes. J Neurochem. 2002;80:255–261. doi: 10.1046/j.0022-3042.2001.00688.x. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Sun GY, Weisman GA, Wood WG. Amyloid β-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes. Neuroscience. 2009;162:328–338. doi: 10.1016/j.neuroscience.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incerpi S, Jefferson JR, Wood WG, Ball WJ, Schroeder F. Na pump and plasma membrane structure in L-cell fibroblasts expressing rat liver fatty acid binding protein. Arch Biochem Biophys. 1992;298:35–42. doi: 10.1016/0003-9861(92)90090-j. [DOI] [PubMed] [Google Scholar]
- Ito JI, Nagayasu Y, Kato K, Sato R, Yokoyama S. Apolipoprotein A-I induces translocation of cholesterol, phospholipid, and caveolin-1 to cytosol in rat astrocytes. J Biol Chem. 2002;277:7929–7935. doi: 10.1074/jbc.M103878200. [DOI] [PubMed] [Google Scholar]
- Kirsch C, Eckert GP, Müller WE. Statin effects on cholesterol micro-domains in brain plasma membranes. Biochem Pharmacol. 2003;65:843–856. doi: 10.1016/s0006-2952(02)01654-4. [DOI] [PubMed] [Google Scholar]
- Levitan I, Fang Y, Rosenhouse-Dantsker A, Romanenko V. Cholesterol and ion channels. Subcellular Biochemisrty. 2010;51:509–549. doi: 10.1007/978-90-481-8622-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch DH, Nägler K, Schumacher S, Göritz C, Müller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- McIntosh AL, Atshaves BP, Huang H, Gallegos AM, Kier AB, Schroeder F. Fluorescence techniques using dehydroergosterol to study cholesterol trafficking. Lipids. 2008;43:1185–1208. doi: 10.1007/s11745-008-3194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal M, Mesmin B, Mukherjee S, Maxfield FR. Sterols are mainly in the cytoplasmic leaflet of the plasma membrane and the endocytic recycling compartment in CHO cells. Mol Biol Cell. 2009;20:581–588. doi: 10.1091/mbc.E08-07-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol A, Luetterforst R, Lindsay M, Heino S, Ikonen E, Parton RG. A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. J Cell Biol. 2001;152:1057–1070. doi: 10.1083/jcb.152.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porn MI, Tenhunen J, Slotte JP. Increased steroid hormone secretion in mouse Leydig tumor cells after induction of cholesterol translocation by sphingomyelin degradation. Biochim Biophys Acta. 1991;1093:7–12. doi: 10.1016/0167-4889(91)90131-g. [DOI] [PubMed] [Google Scholar]
- Rao AM, Igbavboa U, Semotuk M, Schroeder F, Wood WG. Kinetics and size of cholesterol lateral domains in synaptosomal membranes: Modification by sphingomyelinase and effects on membrane enzyme activity. Neurochem Int. 1993;23:45–52. doi: 10.1016/0197-0186(93)90142-r. [DOI] [PubMed] [Google Scholar]
- Roelofsen B. Phospholipases as tools to study the localization of phospholipids in biological membranes. A critical review. J Toxicol. 1982;1(1):87–197. [Google Scholar]
- Schinkel AH. The physiological function of drug-transporting P-glycoproteins. Semin Cancer Biol. 1997;8:161–170. doi: 10.1006/scbi.1997.0068. [DOI] [PubMed] [Google Scholar]
- Schroeder F. Fluorescence probes unravel asymmetric structure of membranes. Subcellular Biochemistry. 1985;11:51–100. doi: 10.1007/978-1-4899-1698-3_2. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Huang H, McIntosh AL, Atshaves BP, Martin GG, Kier AB. Caveolin, sterol carrier protein-2, membrane cholesterol-rich microdomains and intracellular cholesterol trafficking. Subcellular Biochemisrty. 2010;51:279–318. doi: 10.1007/978-90-481-8622-8_10. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Morrison WJ, Gorka C, Wood WG. Transbilayer effects of ethanol on fluidity of brain membrane leaflets. Biochim Biophys Acta. 1988;946:85–94. doi: 10.1016/0005-2736(88)90460-9. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Nemecz G, Wood WG, Morrot G, Ayraut-Jarrier M, Devaux PF. Transmembrane distribution of sterol in the human erythrocyte. Biochim Biophys Acta. 1991;1066:183–192. doi: 10.1016/0005-2736(91)90185-b. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock D, Landrock KK, Payne HR, Kier AB. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 2008;43:1–17. doi: 10.1007/s11745-007-3111-z. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Wood WG, Kier AB. Lipid domains and biological membrane function. In: Sperelakis N, editor. Cell Physiology Sourcebook, A Molecular Approach. Academic Press; San Diego, CA: 2001. pp. 81–94. [Google Scholar]
- Simard JR, Meshulam T, Pillai BK, Kirber MT, Brunaldi K, Xu S, Pilch PF, Hamilton JA. Caveolins sequester FA on the cytoplasmic leaflet of the plasma membrane, augment triglyceride formation, and protect cells from lipotoxicity. J Lipid Res. 2010;51:914–922. doi: 10.1194/jlr.M900251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte JP, Bierman EL. Depletion of plasma-membrane sphingomyelin rapidly alters the distribution of cholesterol between plasma membranes and intracellular cholesterol pools in cultured fibroblasts. Biochem J. 1988;250:653–658. doi: 10.1042/bj2500653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Conrad PA, Anderson RGW. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol. 1994;127:1185–1197. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector R. Nature and consequences of mammalian brain and CSF efflux transporters: Four decades of progress. J Neurochem. 2010;112:13–23. doi: 10.1111/j.1471-4159.2009.06451.x. [DOI] [PubMed] [Google Scholar]
- Sweet WD, Schroeder F. Polyunsaturated fatty acids alter sterol transbilayer domains in LM fibroblast plasma membranes. FEBS Lett. 1988;229:188–192. doi: 10.1016/0014-5793(88)80824-x. [DOI] [PubMed] [Google Scholar]
- Sweet WD, Wood WG, Schroeder F. Charged anesthetics selectively alter plasma membrane order. Biochemistry. 1987;26:2828–2835. doi: 10.1021/bi00384a026. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard A, Smart EJ. Palmitoylation of caveolin-1 is required for cholesterol binding, chaperone complex formation, and rapid transport of cholesterol to caveolae. J Biol Chem. 2000;275:25595–25599. doi: 10.1074/jbc.M003401200. [DOI] [PubMed] [Google Scholar]
- Vance JE, Pan D, Campenot RB, Bussiere M, Vance DE. Evidence that the major membrane lipids, except cholesterol, are made in axons of cultured rat sympathetic neurons. J Neurochem. 1994;62:329–337. doi: 10.1046/j.1471-4159.1994.62010329.x. [DOI] [PubMed] [Google Scholar]
- Wood WG, Cornwell M, Williamson LS. High performance thin-layer chromatography and densitometry of synaptic plasma membrane lipids. J Lipid Res. 1989a;30:775–779. [PubMed] [Google Scholar]
- Wood WG, Gorka C, Schroeder F. Acute and chronic effects of ethanol on transbilayer membrane domains. J Neurochem. 1989b;52:1925–1930. doi: 10.1111/j.1471-4159.1989.tb07278.x. [DOI] [PubMed] [Google Scholar]
- Wood WG, Rao AM, Igbavboa U, Semotuk M. Cholesterol exchange and lateral cholesterol pools in synaptosomal membranes of pair-fed control and chronic ethanol-treated mice. Alcohol Clin Exp Res. 1993;17:345–350. doi: 10.1111/j.1530-0277.1993.tb00773.x. [DOI] [PubMed] [Google Scholar]
- Wood WG, Schroeder F, Hogy L, Rao AM, Nemecz G. Asymmetric distribution of a fluorescent sterol in synaptic plasma membranes: Effects of chronic ethanol consumption. Biochim Biophys Acta. 1990;1025:243–246. doi: 10.1016/0005-2736(90)90103-u. [DOI] [PubMed] [Google Scholar]
- Wood WG, Schroeder F, Igbavboa U, Avdulov NA, Chochina SV. Brain membrane cholesterol domains, aging, and amyloid beta-peptides. Neurobiol Aging. 2002;23:685–694. doi: 10.1016/s0197-4580(02)00018-0. [DOI] [PubMed] [Google Scholar]
- Woodford JK, Jefferson JR, Wood WG, Hubbell T, Schroeder F. Expression of liver fatty acid binding protein alters plasma membrane lipid composition and structure in transfected L-cell fibroblasts. Biochim Biophys Acta. 1993;1145:257–265. doi: 10.1016/0005-2736(93)90297-d. [DOI] [PubMed] [Google Scholar]
- Yeagle PL. Lipid regulation of cell membrane structure and function. FASEB J. 1989;3:1833–1842. [PubMed] [Google Scholar]


