Abstract
Components of the ERK cascade are recruited to genes, but it remains unknown how they are regulated at these sites. The RNA-binding protein heterogeneous nuclear ribonucleoprotein (hnRNP) K interacts with kinases and is found along genes including the mitogen-inducible early response gene EGR-1. Here, we used chromatin immunoprecipitations to study co-recruitment of hnRNP K and ERK cascade activity along the EGR-1 gene. These measurements revealed that the spatiotemporal binding patterns of ERK cascade transducers (GRB2, SOS, B-Raf, MEK, and ERK) at the EGR-1 locus resemble both hnRNP K and RNA polymerase II (Pol II). Inhibition of EGR-1 transcription with either serum-responsive factor knockdown or 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole altered recruitment of all of the above ERK cascade components along this locus that mirrored the changes in Pol II and hnRNP K profiles. siRNA knockdown of hnRNP K decreased the levels of active MEK and ERK at the EGR-1, changes associated with decreased levels of elongating pre-mRNA and less efficient splicing. The hnRNP K dependence and pattern of ERK cascade activation at the c-MYC locus were different from at EGR-1. Ribonucleoprotein immunoprecipitations revealed that hnRNP K was associated with the EGR-1 but not c-MYC mRNAs. These data suggest a model where Pol II transcription-driven recruitment of hnRNP K along the EGR-1 locus compartmentalizes activation of the ERK cascade at these genes, events that regulate synthesis of mature mRNA.
Keywords: Gene Regulation, Gene Transcription, MAP Kinases (MAPKs), RNA Polymerase II, RNA Splicing, ERK, hnRNP K
Introduction
Localized spatiotemporal activation of kinases is an important mode to regulate kinase specificity (1). It has been known for more than 20 years that kinases can translocate to the nucleus (2–4), but it was not until very recently that multiple kinases were discovered to be directly recruited to genes (5–10). In fact, recent observations suggest that activation of entire canonical kinase cascades is recapitulated along gene loci (11). Compartmentalization of signaling cascades within discrete genomic regions would serve to control specificity of kinase action (12–14). In addition, direct recruitment of multiple components of kinase cascades to a given locus may reflect local information processing microcircuits.
hnRNP K is an ancient RNA-binding protein initially identified as a component of the heterogeneous nuclear ribonucleoprotein (hnRNP)3 complex (15, 16). The K protein has been implicated to regulate many processes including transcription (17, 18), mRNA splicing (19), and stability (20). K protein in addition to RNA-binding domains contains protein-interacting modules that, on one hand, bind kinases (21–24) and, on the other hand, associate with transcription (18, 25), RNA processing (26), and translation (27, 28) factors. Moreover, the K protein-mediated interactions are regulated by signaling cascades including those initiated by mitogens (29, 30). These observations are consistent with K protein acting as a docking platform within microcircuits where it serves to integrate signaling cascades by facilitating cross-talk between kinases and factors at sites of RNA-directed processes (22, 31, 32).
The extracellular signal-regulated kinases p44 (ERK1) and p42 (ERK2) belong the family of MAPKs (33). They are activated by a variety of mitogenic ligands that bind to surface receptor tyrosine kinases (RTKs) (13, 34). ERKs are among critical terminal effectors of kinase cascades that regulate virtually every intracellular response. Nuclear translocation of ERK1 and ERK2 is an accepted mode of their action (3–4, 35). Importantly, most of the components of the ERK1/2 signaling module have been found bound to genes in ChIP assays (11, 36, 37). ERKs target a large repertoire of nuclear protein substrates; thus compartmentalization at genomic sites explains one way by which their specificity is controlled in the intracellular environment. Still, it is not known how they are recruited and regulated along genes.
Early growth response-1 (EGR-1, two exons, 3,824-bp-long locus) is an immediate early gene that encodes a zinc finger transcription factor (38). EGR-1 is rapidly and transiently expressed in many cell types following mitogenic stimulation, which then targets genes involved in signal transduction and gene expression (39). hnRNP K is recruited to inducible gene loci including EGR-1. Intriguingly, ERK1/2 is bound to EGR-1 in a pattern that resembles K protein (36). Moreover, hnRNP K binds (40), regulates (41), and is a substrate (20) of ERK1/2 and other components of the ERK cascade. These previously published studies may reflect compartmentalized hnRNP K-controlled bursts of ERK activity in the vicinity of inducibly transcribed genes. Here, we explored the role and interaction of hnRNP K with the ERK1/2 cascade along inducible genes following mitogenic stimulation.
EXPERIMENTAL PROCEDURES
Cells
HCT116 WT human colon carcinoma cells lines were grown in plastic six-well plates in McCoy's medium supplemented with 10% FBS (42).
ChIP Assay
Chromatin cross-linking and cell harvesting was done as described before (36, 43). Chromatin was sheared in a Bioruptor (Diagenode, Philadelphia, PA) (0.5-ml tubes) using the protocol 30-s on-off cycles for 15 min at high intensity. Chromatin immunoprecipitation assays were done using the matrix ChIP platform as described previously (36, 43).
Ribonucleoprotein Immunoprecipitation (RIP) Assays
HCT116 WT cells were grown in plastic six-well culture plates to 50–60% confluence and then rendered quiescent by lowering FBS concentration to 0.5%. The cells were treated 24 h later with warmed (37 °C) McCoy's medium supplemented with 10% FBS for the given time points. The cells were harvested, then washed once with ice-cold PBS, and stored at −80 °C. At the day of assay, the cells were lysed in 100 μl of immunoprecipitation buffer (150 mm NaCl, 5 mm EDTA, 1% Triton X-100, 0.5% Nonidet P-40, 50 mm Tris-HCl, pH 7.5) containing the protease (Sigma; P5726), phosphatase (Sigma; P2850 and P8340), and ribonuclease (Sigma; R2520) (20 units/ml) inhibitors. Next, the lysates were treated with ultrasounds in Bioruptor (Diagenode) using 30-s on-off cycles for 15 min at medium intensity, and supernatants were cleared by centrifugation at 10,000 × g/4 °C.
Ribonucleoprotein immunoprecipitation assays were performed similarly to the matrix ChIP protocol (36) with minor changes. Briefly, in-lab prepared polypropylene 96-well plates were incubated overnight at room temperature with 0.2 mg of protein A in 100 μl of PBS/well. After a wash (200 μl PBS/well), the well walls were blocked with 100 μl of blocking buffer (30 min, room temperature) containing ribonuclease inhibitor (5 units/ml). The wells were cleared and incubated with 0.25 μg of hnRNP K 54 antibody diluted in 50 μl of blocking buffer/well (60 min, room temperature). Samples (50 μl of lysate) were added to wells, and plates were floated in ultrasonic water bath (60 min, 4 °C) to accelerate ribonucleoprotein-antibody binding. The wells were washed three times with 100 μl of immunoprecipitation buffer and once with 100 μl of TE buffer (10 mm Tris-HCl, 1 mm EDTA, pH 7.0). The wells were incubated with 50 μl of RNA extraction solution (QuickExtractTM RNA extraction kit; Epicenter) for 3 min at room temperature, followed by DNase I treatment performed according to the RNA extraction kit protocol. RNA samples were either stored at −80 °C or were used promptly for cDNA synthesis.
Pre-mRNA Assays
The cells were transfected with either hnRNP K siRNA or noncomplementary (NC) siRNA at final concentration of 22 nm using Lipofectamine RNAiMAX. 24 h after transfection, the cells were switched to low serum medium, and 24 h after quiescence, the cells were treated with 10% FBS for 0, 5, 15, 30, 60, and 180 min. Total RNA was extracted from cells using TRIzol® Plus RNA purification kit (Invitrogen) followed by on-column PureLinkTM DNase (Invitrogen) treatment. One μg of total RNA and random hexamers were used in cDNA synthesis. Levels of specific pre-mRNA or mature mRNAs were assessed by real time PCR using primer pairs amplifying intron-exon or exon-exon junction, respectively (see Table 2).
TABLE 2.
PCR primer list
Coordinates of PCR products refer to the NCBI36/hg18 human genome sequence.
| Human EGR-1, −10 kb chr5:137,818,912–137,819,274 | |
| Forward | GCCACATTCAGTTCTCGCTTT |
| Reverse | TTCTCCTTTCCCTGGCAACA |
| Human EGR-1, Promoter chr5:137,828,955–137,829,077 | |
| Forward | AATAAATCATAAGGTCCTGCCATATTAGGGCTTa |
| Reverse | AATAAATCATAATGGGATCTCTCGCGACTCCa |
| Human EGR-1, Ex1 chr5:137,829,326–137,829,446 | |
| Forward | AATAAATCATAAAGCTCTCCAGCCTGCTCGTa |
| Reverse | AATAAATCATAAGGTAGTTGTCCATGGTGGGCa |
| Human EGR-1, Ex2 chr5:137,830,366–137,830,532 | |
| Forward | TGAACAACGAGAAGGTGCTG |
| Reverse | GGTCATGCTCACTAGGCCAC |
| Human EGR-1, Ex2.3 chr5:137,832,569–137,832,720 | |
| Forward | GCTGAGCTGAGCTTCGGTTC |
| Reverse | TCGCCGCCTACTCAGTAGGTA |
| Human EGR-1, +1 kb chr5:137,833,607–137,833,734 | |
| Forward | CAAGCCAAGAATCCTTCCAG |
| Reverse | CCGGATGGGAACTTTAGACA |
| Human EGR-1, +5.5 kb chr5:137,834,463–137,834,613 | |
| Forward | GGCCCAATTCTTTCTTGTGC |
| Reverse | TTTTGCCAGGAGGCTCACAG |
| Human EGR-1, Ex1-Ex2 cDNA | |
| Forward | CAGCACCTTCAACCCTCAG |
| Reverse | AGCGGCCAGTATAGGTGATG |
| Human EGR-1, Ex1-Intron1 chr5:137,829,598–137,829,760 | |
| Forward | CAGCACCTTCAACCCTCAG |
| Reverse | GAAACCCGGCTCTCATTCTA |
| Human β-actin Ex4-Ex5 cDNA | |
| Forward | AGAGCTACGAGCTGCCTGAC |
| Reverse | AAGGTAGTTTCGTGGATGCC |
| Human c-MYC, Ex3 chr8:128,822,473–128,822,545 | |
| Forward | CCTCACAACCTTGGCTGAGT |
| Reverse | CCAAAGTCCAATTTGAGGCA |
| Human c-MYC, Ex1-Ex2 cDNA | |
| Forward | CACCGAGTCGTAGTCGAGGT |
| Reverse | GCTGCTTAGACGCTGGATTT |
| Human c-MYC, Ex1-Intron1 chr8:128,818,006–128,818,075 | |
| Forward | TGCAGCTGCTTAGACGCTGGA |
| Reverse | AGGCAAGTGGACTTCGGTGCT |
| Human Nr4A3, Ex8 chr9:101,668,741–101,668,834 | |
| Forward | TAAAGCAGGTGATTCCTCCC |
| Reverse | CCATTAACAGCTGGTGGCTT |
| Human Nr4A3, Ex3-Ex4 cDNA | |
| Forward | CATCGGTTTCGACGTCTCTT |
| Reverse | ACTACGGCGTGCGAACCT |
| Human Nr4A3, Ex3-Intron4 chr9:101,631,079–101,631,199 | |
| Forward | TGCAAGGGCTTTTTCAAGGT |
| Reverse | ACAGCTCTCAAACCGGGAAGA |
| Human hnRNPK, Ex17 chr9:85,773,503–85,773,590 | |
| Forward | TTCCCAACCCACCCCCAAACT |
| Reverse | TGCCTATGTCCAACAAGAGATGCAC |
| Human hnRNPK, Ex1-Ex3 cDNA | |
| Forward | AATGCCAGTGTTTCAGTCCC |
| Reverse | AGGCCCTCTTCCAAGGTAGG |
| Human hnRNPK, Ex1-Intron1 chr9:85,785,169–85,785,262 | |
| Forward | CGCAGCCTTTCAGGGAGCCC |
| Reverse | TGTCCCACTTGTTCGCGGCC |
| Human GAPDH, Prom-Ex1 chr12:6,513,840–6,513,950 | |
| Forward | ACCTTGGGCTGGGACTGGCT |
| Reverse | AGAGAGCGAAGCGGGAGGCT |
| Human GAPDH, Ex2-Ex4 cDNA | |
| Forward | GAAGATGGTGATGGGATTTC |
| Reverse | GAGGTGAAGGTCGGAGTC |
a Primers containing flaps (83).
RESULTS
Inducible Co-recruitment of hnRNP K, Pol II, and the ERK Cascade Components along the Human EGR-1 Locus
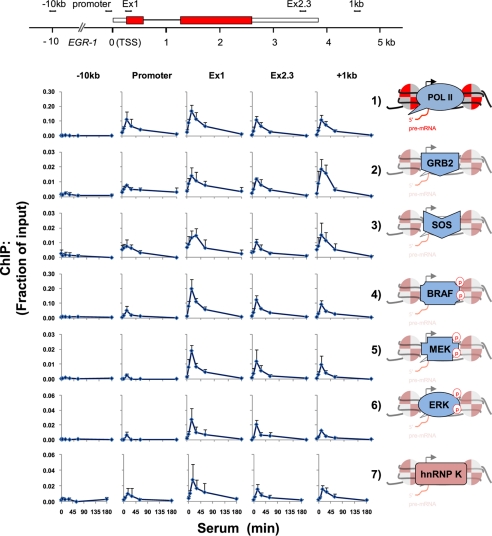
We have previously found that mitogen-activated expression of the EGR-1 gene is associated with recruitment of ERK1/2 to this locus along with its nuclear target hnRNP K (36, 41, 44). We used a panel of antibodies to hnRNP K, Pol II, and the components of the ERK cascade (1) in matrix ChIP assays (36) to examine their spatiotemporal binding profiles along the EGR-1 gene in the serum-treated HCT116 WT human colon carcinoma cell line (42). The results of each series of ChIP measurements are depicted in a graph grid format where each graph shows the density of an epitope (y-axis, fraction of input chromatin DNA) as a function of time following serum addition (x-axis, min).
In response to serum treatment, the Pol II level transiently increased along EGR-1 with a peak at 15 min and returning to base line by 3 h (Tables 1 and 2, Fig. 1, row 1, and supplemental Fig. S1). In agreement with other studies (45, 46), Pol II levels were highest at the transcription start site and decreased along the gene. At +1 kb downstream from the poly(A) site, Pol II kinetics was similar to that seen at the end of the EGR-1 gene. The Pol II signal was weak at the −10 kb intergenic site, suggestive of low or no transcription. The adapter protein GRB2 contains SH2 domains that bind to the phosphotyrosine residues in the activated RTKs (47, 48). GRB2 also interacts directly with hnRNP K (49) and like hnRNP K is present in the nucleus. As shown in Fig. 1 (row 2) and supplemental Fig. S1, GRB2 is transiently recruited all along the EGR-1 locus, with peak levels seen at 15 min following serum stimulation and returning to base line by 3 h. It has been shown that 0.5–1.5 kb downstream of the poly(A) sites, Pol II pauses, allowing the recruitment of mRNA processing factors (45, 46). Interestingly, the highest inducible levels of GRB2 were present at +1 kb downstream from the EGR-1 poly(A) signal. There was little or no binding of GRB2 at an intergenic site −10 kb upstream from the transcription start site.
TABLE 1.
Antibodies list
| Antibody | Catalog number | Source | Manufacturer | Peptide immunogen |
|---|---|---|---|---|
| Pol II CTD (4H8) | sc-47701 | Mouse monoclonal | Santa Cruz | CTD |
| hnRNP K | Rabbit polyclonal | Ref. 32 | Carboxyl terminus | |
| GRB2 | sc-255 | Rabbit polyclonal | Santa Cruz | Carboxyl terminus |
| SOS1 + SOS2 | S15530 | Rabbit polyclonal | Transduction Lab | Mouse Sos1 amino acids 1–109 mouse Sos2 amino acids 1095–1297 |
| B-Raf (C-19) | sc-166 | Rabbit polyclonal | Santa Cruz | Carboxyl terminus |
| p-B-Raf | sc-28006r | Rabbit polyclonal | Santa Cruz | Thr(P)-598/Ser(P)-601 |
| MEK1/2 | M17030 | Rabbit polyclonal | BD Transduction | |
| MEK1 +MEK2 | ab70613 | Rabbit polyclonal | Abcam | MEK Ser-218/222 and MEK2 Ser-222/226 |
| pMEK1/2(S217/221) | 9121 | Rabbit polyclonal | Cell Signaling | Phospho-MEK1 (Ser(P)-217/221) |
| ERK1(K-23) | sc-94 | Rabbit polyclonal | Santa Cruz | subdomain XI |
| pERK1/2 | 9101 | Rabbit polyclonal | Cell Signaling | Thr(P)-202/Tyr(P)-204 |
| pElk-1(S383) | 9181 | Rabbit polyclonal | Cell Signaling | Ser(P)-383 |
| SRF(G-20) | sc-335 | Rabbit polyclonal | Santa Cruz | Carboxyl terminus |
| Pol II (N20) | sc-899 | Rabbit polyclonal | Santa Cruz | Amino terminus |
| H3 | ab1791 | Rabbit polyclonal | Abcam | Carboxyl terminus of human histone H3 |
| H3K4me3 | 07-473 | Rabbit polyclonal | Millipore | Peptide corresponding to residues surrounding and including trimethylated Lys-4 of histone H3 |
| H3K27me3 | 07-449 | Rabbit polyclonal | Millipore | Peptide containing the sequence AR(me3K)SAP |
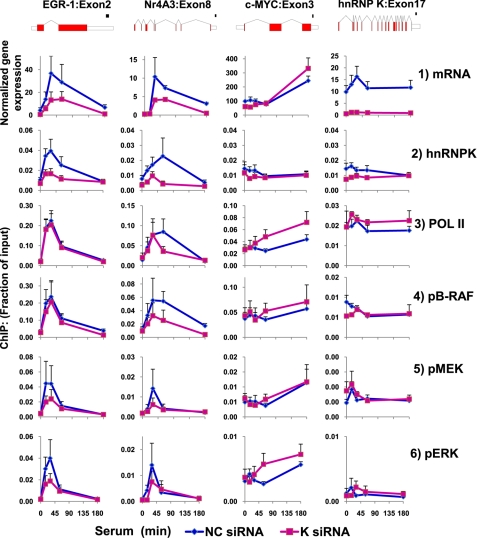
FIGURE 1.
Time course binding profile of ERK cascade components, hnRNP K, and Pol II along the serum-induced EGR-1 gene. Matrix ChIP analysis (36) of sheared chromatin from a time course of serum-treated (10% FBS for 0, 5, 15, 30, 60, and 180 min) HCT116 WT human colon carcinoma cells was done using in-lab coated protein A polypropylene 96-well plates. The antibodies used are listed in Table 1. Real time PCR was done using primers to the regions shown in the diagram of the EGR-1 gene (the two exons are shown as boxes). The primers are listed in Table 2. The ChIP results are expressed as fractions of input DNA. The graphs represent the means ± S.E. from six experiments. The RNA Pol II, ERK signaling components, and hnRNP K are shown to the right of the time course graphs. Protein phosphorylation is shown by a red letter p in a red circle. A more detailed profile is shown in supplemental Fig. S1.
Immediately downstream of GRB2 is the guanine nucleotide exchange factor SOS, which binds GRB2 via its SH3 domain. When the GRB2-SOS complex docks to phosphorylated RTKs, SOS becomes activated (47). The serum-induced pattern of SOS recruitment to EGR-1 was remarkably similar to that observed for GRB2 (Fig. 1, rows 2 and 3, and supplemental Fig. S1). This suggests that GRB2 and SOS are recruited to the gene as a complex. Activated SOS promotes the removal of GDP from Ras, which then binds GTP and becomes active. Induced Ras activates the Raf family of protein kinases. Raf-1 (C-Raf) can form heterodimers with B-Raf, a key process that regulates their activity and involves a number of other proteins (50–52). There are reports that Ras is present in the nucleus (53). However, using different antibodies in matrix ChIP assays, we were not able to detect Ras along the EGR-1 locus. Raf-1 is found in the nucleus (54). Activation of B-Raf kinase requires phosphorylation of the Thr-598/Ser-601 residues (52, 55). A screen using a panel of anti-Raf antibodies detected total and phosphorylated (Thr(P)-598/Ser(P)-601) B-Raf (Fig. 1, row 4, and supplemental Fig. S1, rows 4 and 5) but not the Raf-1 enzyme along the EGR-1 gene. There was a serum-induced binding profile of B-Raf along the EGR-1 gene, and the profile of the total B-Raf along the EGR-1 gene and at +1 kb closely resembled that of GRB2-SOS (supplemental Fig. S1, rows 2 and 3). There was little or no binding of B-Raf at the −10 kb upstream intergenic site. Raf kinases phosphorylate and activate MEK1/2 (33). Mitogenic stimulation can induce translocation of MEK1/2 to the nucleus (4, 56). Serum treatment caused recruitment of MEK1/2 along the EGR-1 gene and at the +1 kb downstream site; both total and phosphorylated forms were detected (Fig. 1, row 5, and supplemental Fig. S1, rows 6 and 7). No MEK was detected at the −10-kb intergenic site. MEK phosphorylates and activates ERK1/2 (4, 50). There was an inducible binding of ERK along the EGR-1 gene and at +1 kb but not at the −10 kb intergenic site (Fig. 1, row 6, and supplemental Fig. S1, rows 8 and 9). The transcription factor Elk is known to regulate EGR-1 expression (57, 58). ERK phosphorylates Elk at serine 383, pElk (59). We found pElk at the EGR-1 promoter and along the gene, but surprisingly much higher levels of pElk were present at the +1 kb downstream site (supplemental Fig. S1, row 11). In contrast to the induced changes at the ERG-1 locus, the levels of ERK components at the highly expressed GAPDH housekeeping gene were constitutively high, matched levels of mRNA and Pol II, and were not induced by serum (supplemental Fig. S2). This observation is similar to measurements made in livers (11). These results suggest that at least a pool of the active ERK components is preassembled in the nucleus. In sum, in agreement with a previous study in insulin-treated hepatocytes (11), the above ChIP assays revealed that nearly the entire activated ERK signaling apparatus (GRB2, SOS, B-Raf, MEK, and ERK) and its target transcription factor Elk is found along the EGR-1 locus in serum-treated colon carcinoma cells (Fig. 1 and supplemental Fig. S1). Also, we obtained similar results with EGF-treated cells, suggesting that the direct recruitment of ERK cascade components to the EGR-1 gene (data not shown) is a common feature for RTK induction of this gene.
The recruitment of the ERK cascade components along the transcribed EGR-1 locus resembled the profiles of Pol II and hnRNP K (Fig. 1, rows 1 and 7, and supplemental Fig. S1, rows 1 and 10). This similarity suggests that the interaction of the ERK signaling module with the EGR-1 locus depends on transcription of this gene. This possibility was tested next.
Transcription-dependent Recruitment of the ERK Cascade Components along the EGR-1 Locus
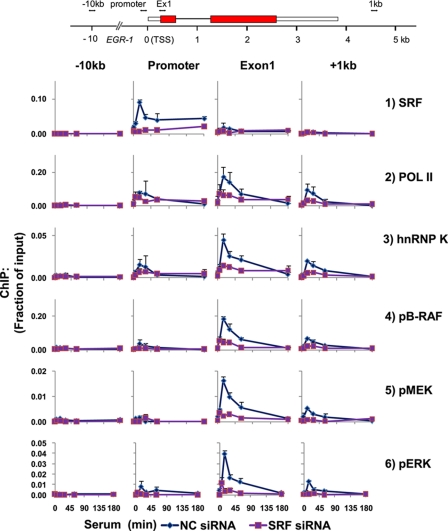
Serum-responsive factor (SRF) initiates EGR-1 transcription through its binding to several sites within the EGR-1 promoter (60). We used siRNA SRF knockdown to test the effects of blocking Pol II initiation on the recruitment of the ERK cascade components to the EGR-1 locus. In cells transfected with NC siRNA, there was constitutive and inducible recruitment of SRF to the EGR-1 promoter, and little or no SRF binding was detected along the transcribed and intergenic regions (Fig. 2, row 1, and supplemental Fig. S3, row 1). In sharp contrast, the use of siRNA specific to SRF decreased constitutive and inducible SRF levels at the promoter. As expected, the SRF knockdown decreased serum-inducible Pol II recruitment along the EGR-1 gene. In siRNA NC and SRF-transfected cells, the kinetics of hnRNP K recruitment paralleled the Pol II profile (Fig. 2, rows 2 and 3, and supplemental Fig. S3, rows 2 and 3), suggesting that inducible hnRNP K binding along the EGR-1 locus is dependent on EGR-1 transcription and thus may involve binding to the nascent transcript (61).
FIGURE 2.
siRNA SRF knockdown inhibits serum-induced co-recruitment of Pol II, hnRNP K, and ERK signaling cassette along the EGR-1 locus. The cells were transfected using Lipofectamine RNAiMAX with either SRF siRNA or NC siRNA. 24 h after transfection, the cells were switched to low serum medium, and 24 h after quiescence, the cells were treated with 10% FBS for 0, 5, 15, 30, 60, and 180 min. Matrix ChIP data are presented as fractions of input DNA (means ± S.E., n = 3 experiments). A more detailed profile is shown in supplemental Fig. S3.
SRF knockdown inhibited activation and recruitment of B-Raf, MEK1/2, and ERK1 all along the gene and at the +1 kb site (Fig. 2 and supplemental Fig. S3) as well as recruitment of GRB2 and SOS1/2 (supplemental Fig. S3, rows 4 and 5). Taken together, the SRF knockdown experiments suggest that the recruitment of ERK cascade components and hnRNP K to the EGR-1 locus is driven by transcribing Pol II.
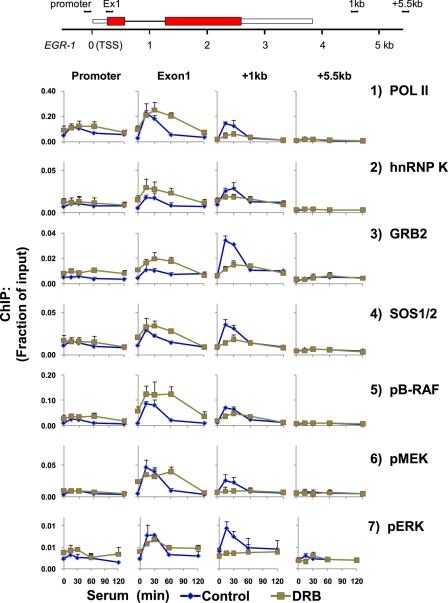
The adenosine analog 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) inhibits the Pol II Ser-2 carboxyl-terminal domain kinase, CDK9, a component of the positive transcription elongation factor (P-TEF-b) (62–64). We used DRB to explore the role of Pol II elongation on the recruitment of ERK cascade components to the EGR-1 gene (Fig. 3 and supplemental Fig. S4). At exon 1 (∼200 bp downstream of transcription start site), DRB increased Pol II constitutive levels and spread out the duration of the inducible peak. For example, without DRB the Pol II peak was detected 15 min after serum treatment and was back to base line at 60 min, whereas with DRB pretreatment, the Pol II levels at 60 min after serum treatment were nearly as high as the levels seen 15–30 min after serum treatment. Further downstream along the gene, the DRB pretreatment decreased the amplitude and flattened the serum-induced Pol II signal, and the effect was most pronounced at the site +1 kb 3′ to the poly(A) signal (Fig. 3, row 1, and supplemental Fig. S4, row 1).
FIGURE 3.
DRB-inhibited Pol II elongation decreases serum-induced levels of hnRNP K and ERK signaling cassette along EGR-1 regions distal to the transcription start site. HCT116 WT cells grown to 40–60% confluence were made quiescent overnight by lowering FBS concentration in the medium from 10 to 0.5%. After 24 h, the cells were pretreated with DRB (50 μm dissolved in dimethyl sulfoxide) in 0.5% FBS medium for 6 h. Dimethyl sulfoxide alone was used as control. The cells were switched to 10% FBS medium for 0, 15, 30, 60, and 120 min, at which times they were cross-linked, and chromatin was sheared. Matrix ChIP data are presented as fraction of input DNA (means ± S.E., n = 3 experiments). A more detailed profile is shown in supplemental Fig. S4.
The DRB effects on all of the ERK cascade components and hnRNP K mirrored the altered Pol II elongation profile along the EGR-1 gene and at +1 kb downstream of poly(A) (supplemental Fig. S4). Thus, the DRB experiment provides further evidence that recruitment of hnRNP K and the ERK cascade transducers to the EGR-1 locus is linked to Pol II transcription. hnRNP K regulates the ERK pathway component activity (29, 40) and could be involved in the recruitment/activation of this cascade along the EGR-1 locus. This possibility was tested next.
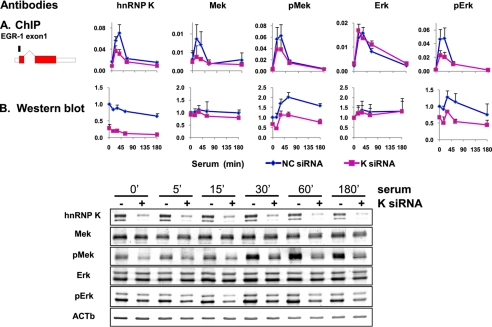
siRNA hnRNP K Knockdown Inhibits the Serum-induced MEK Recruitment and ERK Activation along the EGR-1 Locus
Transfection of cells with hnRNP K siRNA decreased the total levels of hnRNP K protein in cell lysates (Fig. 4B) as well as decreased the constitutive and inducible level of K protein along the EGR-1 locus (Fig. 4A). The siRNA K protein knockdown inhibited inducible levels of both the total and the active Ser(P)-217/Ser(P)-221 form of MEK1/2 at the EGR-1 gene (Fig. 4A). This suggests that the MEK1/2 recruitment to the EGR-1 gene is K protein-dependent. K protein siRNA had little or no effect on the kinetics of the total ERK1 recruitment but inhibited the inducible Thr(P)-202/Tyr(P)-204 pERK1/2 signal. This suggests that hnRNP K regulates ERK1 activity but not its recruitment along the EGR-1 locus. Because Pol II density is also not altered at the EGR-1 gene by siRNA hnRNP K knockdown, the inducibly bound ERK could directly interact with the Pol II (e.g. carboxyl-terminal domain). The dissociation of MEK1/2 binding from ERK1 recruitment uncovered by the hnRNP K knockdown experiment suggests that the two enzymes are recruited to the EGR-1 locus independent of each other.
FIGURE 4.
The patterns of MEK1/2 and ERK1/2 phosphorylation and the effects of hnRNP K at the EGR-1 gene (A, ChIP) compared with whole cells (B, Western blots) are different. The cells were transfected with either hnRNP K siRNA or NC siRNA in a presence of Lipofectamine RNAiMAX. 24 h after transfection, the cells were switched to low serum medium, and 24 h after quiescence, the cells were treated with 10% FBS for 0, 5, 15, 30, 60, and 180 min. A, matrix ChIP assay was done using antibodies to either hnRNP K, total MEK1/2 (Mek), phospho-MEK1/2 (pMek), total ERK1 (Erk), or phospho-ERK1/2 (pErk). ChIP results are shown for exon 1 of the EGR-1 gene (means ± S.E., n = 3 experiments). B, cells were harvested, and lysates were resolved by SDS-PAGE and electrotransferred to PVDF membrane. Blotted proteins were assessed by Western blot analysis using the same antibodies as in A. Protein band intensities were assessed by densitometry using OptiQuant image analysis software (Packard). The data were normalized for β-actin input and expressed as fold change of 0′ time point. The data represent the means ± S.E. (n = 2 experiments).
According to the traditional view, ERK1 and ERK2 are activated in the cytosol and then move to sites of their action, including the nucleus. hnRNP K modulates MEK/ERK activity (41) and is abundant in the cytoplasm where it could regulate MEK activation of ERK prior to their nuclear translocation. To explore this possibility, we compared time course measurement of MEK and ERK phosphorylation in cell lysates (Western blots; Fig. 4B) with the pattern of their binding along the EGR-1 gene (ChIP assays; Fig. 4A). Compared with their inducible levels along the EGR-1 gene, the serum induction of pMEK1/2 and pERK1/2 assayed in cell lysates had low amplitude, was slower, and lasted longer. hnRNP K knockdown had no effect on the total cell MEK1/2 and ERK1 protein levels. The comparative analysis between kinase ChIP assays and Western blots revealed significant profile differences, suggesting local control of ERK by MEK activities along the EGR-1 locus. In fact, the time course of kinase binding along the EGR-1 looked much more like Pol II density profile (Figs. 1–3) than the Western blot pattern (Fig. 4B), suggesting that in situ control is linked to elongating Pol II. hnRNP K is known to interact with ERK (20) and is co-recruited with ERK cascade to the EGR-1 gene (Figs. 1–4), and its siRNA knockdown decreases recruitment of MEK1/2 (Fig. 4A). These observations suggest that the in situ activation of ERK involves hnRNP K. The model of in situ regulation is consistent with the observation that the spatiotemporal profiles of active B-Raf, MEK1/2, and ERK1/2 at two other immediate early genes, NR4A3 (eight exons, 45,037-bp-long gene) (65) and c-MYC (three exons, 5,364-bp-long gene) (66), are different from that at EGR-1 but nonetheless also resemble Pol II density (Fig. 5, row 3) and mRNA (Fig. 5, row 1) profiles of these two serum-induced genes. Remarkably, none of these profiles resembled the kinetics seen in whole cell lysates (Fig. 4). The local control may represent recruitment of already active components to the gene or de novo in situ activation. Compared with EGR-1 activation, the profile was more strikingly different at the c-MYC locus where the highest levels of Pol II and kinases were observed at 3 h following serum stimulation, at a time point when the levels at EGR-1 return to base line (Fig. 5). Moreover, although at the EGR-1 and NR4A3 (nuclear receptor subfamily 4, group A, member 3) genes the profiles of kinase recruitment were similar to K protein, at the c-MYC gene there was serum-induced kinase recruitment with little or no change in the levels of K protein. This observation suggests that at the c-MYC gene ERK cascade and hnRNP K protein are recruited independent of each other and unlike EGR-1 and NR4A3 genes may not involve RNA binding (see below and Fig. 7).
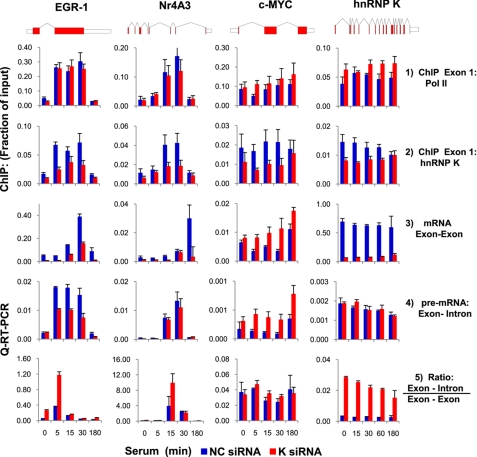
FIGURE 5.
siRNA hnRNP K knockdown inhibits serum-induced mRNA levels and co-recruitment of ERK kinase cascade along the EGR-1 and NR4A3 but not c-MYC locus. The cells were transfected with either hnRNP K siRNA or NC siRNA using Lipofectamine RNAiMAX. 24 h after transfection, the cells were switches to low serum medium, and 24 h after quiescence, the cells were treated with 10% FBS for 0, 15, 30, 60, and 180 min. Total RNA was extracted from cells as described previously (85). RT reaction was carried out using random hexamers. The levels of specific mRNAs were assessed by real time RT-PCR using gene-specific primer pairs to EGR-1, NR4A3, c-MYC, and hnRNP K genes mRNAs (Table 2; means ± S.E., n = 3 experiments). Chromatin was extracted and sheared. Matrix ChIP data are presented as fractions of input DNA (means ± S.E., n = 3 experiments).
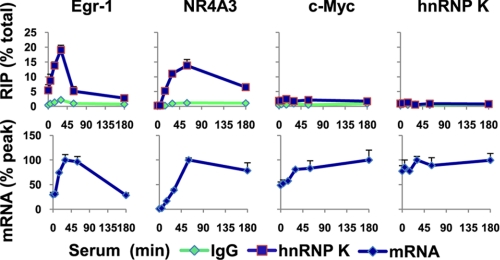
FIGURE 7.
hnRNP K binds EGR-1 and Nr4A3 but neither c-MYC nor its own mRNA. Top row, cell lysates from a time course of serum-treated (10% FBS for 0, 5, 15, 30, 60, and 180 min) HCT116 WT human colon carcinoma cells were prepared, and microplate-based RIP was done using antibody to hnRNP K protein (see “Experimental Procedures”). RIP levels were assessed by real time RT-PCR using gene-specific primer pairs to EGR-1, NR4A3, c-MYC, and hnRNP K cDNAs. The data are expressed as percentages of average total mRNA levels (means ± S.E., n = 3 experiments). Bottom row, total RNA was extracted from cells as described previously (85). RT reaction was carried out using random hexamers. The levels of specific mRNAs were assessed by quantitative RT-PCR using gene-specific primer pairs to EGR-1, NR4A3, c-MYC, and hnRNP K genes mRNAs (Table 2). The results are expressed as percentages of peak levels (means ± S.E., n = 3 experiments).
The siRNA hnRNP K Knockdown Inhibits Serum-induced EGR-1 Expression
We also used the siRNA knockdown to explore the role of K protein in the serum-induced EGR-1 expression (Fig. 5). In cells transfected with hnRNP K siRNA, the constitutive and inducible EGR-1 mRNA levels were lower compared with cells transfected with NC siRNA (Fig. 5, row 1). Interestingly, siRNA K protein did not alter the Pol II occupancy at the EGR-1 gene (Fig. 5, row 3). siRNA K protein also inhibited serum-induced NR4A3 mRNA expression with little or no change in Pol II density at this locus. siRNA K protein knockdown decreased the level of serum-induced ERK kinase cascade at the NR4A3 gene. In sharp contrast to EGR-1, siRNA K protein knockdown appears to enhance serum-induced c-MYC mRNA as well as Pol II and active ERK1/2 levels without clear changes in hnRNP K density at this locus. hnRNP K knockdown also increased Pol II levels at its own locus (17 exons, 12,571-bp-long gene) without altering kinase levels. It should be noted that although siRNA markedly decreased levels of hnRNP K in cell lysates as a whole (Fig. 4) and the serum-inducible K protein at the EGR-1 and NR4A3 genes (Fig. 5), the reduction K protein levels at the c-MYC and hnRNP K genes were smaller (Fig. 5, row 2). This observation may reflect the fact that a pool of hnRNP K resides within some nucleoprotein compartments (67) that are more resistant to 24–48 h of siRNA K protein knockdown. Finally, siRNA to hnRNP K decreased serum-induced pB-Raf at the Nr4A3 but not at the EGR-1 locus (Fig. 5, row 4). Taken together, these results suggest that kinase cascade microcircuits are not the same for all inducible genes.
hnRNP K Regulates EGR-1 Pre-mRNA Elongation and Processing
siRNA K protein knockdown inhibited serum-induced EGR-1 mRNA expression with little or no change in Pol II occupancy at this locus (Table 3 and Figs. 5 and 6, rows 1–3). This could mean that K protein regulates expression of EGR-1 post-transcriptionally. However, ChIP assays along transcribed regions are measures of Pol II density and not necessarily rates of elongation. Quantitative RT-PCR analysis of unspliced transcripts has been used as surrogate to estimate rates for elongation (68). We used this approach to analyze serum-induced synthesis of unspliced pre-mRNA levels as a measure of elongation rates. This experiment showed that serum-induced unspliced EGR-1 pre-mRNA (exon 1-intron 1) was reduced by K protein knockdown (Fig. 6, row 4), suggesting that hnRNP K stimulates EGR-1 transcription elongation. Whereas the effects of K protein knockdown along the Nr4A3 pre-mRNA (exon3-intron4) were less pronounced, at the c-MYC (Myc proto-oncogene protein) locus, the levels of pre-mRNA (exon 1-intron 1) fragments were increased. Finally, K protein knockdown had no effect on its own pre-mRNA levels.
TABLE 3.
siRNA sequences
| siRNA | Sequence |
|---|---|
| SRF1 | 5′-GCCAGUGAGACAGGCCAUGUGUAUA-3′ |
| 5′-UAUACACAUGGCCUGUCUCACUGGC-3′ | |
| SRF2 | 5′-CCAGAGAAUGAGUGCCACUGGCUUU-3′ |
| 5′-AAAGCCAGUGGCACUCAUUCUCUGG-3′ | |
| hnRNP K | 5′-AAUAUUAAGGCUCUCCGUACA*UU*-3′ |
| 5′-UGUACGGAGAGCCUUAAUAUU*UU*-3′ |
FIGURE 6.
siRNA hnRNP K decreases elongation and splicing rates of EGR-1 and NR4A3 but c-MYC pre-mRNA. The cells were transfected with either hnRNP K siRNA or NC siRNA (see “Experimental Procedures” and Table 3) made quiescent and treated with 10% FBS for 0, 5, 15, 30, 60, and 180 min. Total RNA was extracted, DNAse-treated, and used in RT reaction. The levels of specific mature mRNAs and pre-mRNA were assessed by quantitative (Q) RT-PCR using primer pairs amplifying exon-exon and exon-intron junction, respectively, of EGR-1, NR4A3, c-MYC, and hnRNP K genes (Table 2). The results were normalized for β-actin mRNA (means ± S.E., n = 3 experiments).
All of the steps of RNA processing appear to be linked to Pol II elongation (68–70). Both hnRNP K (19, 61) and RAS-RAF-MEK-ERK signaling cascade (71) have been shown to regulate pre-mRNA splicing. We calculated the ratio of unspliced to spliced RNA as a measure of splicing efficiency. In cells with K protein knockdown, the ratio was higher for the EGR-1 and Nr4A3 pre-mRNA but not for c-MYC pre-mRNA (Fig. 6, row 5). These experiments suggest that hnRNP K protein regulates EGR-1 gene transcription elongation as well as pre-mRNA splicing. These studies also underscore the heterogeneity of K protein effects on different genes.
hnRNP K Is Associated with EGR-1 and NR4A3 but Not c-MYC and hnRNP K Transcripts
hnRNP K binds RNA directly and is associated with many transcripts in vivo (72). We adapted the microplate matrix ChIP platform for RIP assays (“Experimental Procedures”) and used it to test whether hnRNP K binds to the EGR-1 and NR4A3 mRNAs in the whole cell lysates. The RIP assays showed that hnRNP K associates with the EGR-1 and NR4A3 but not c-MYC and hnRNP K mRNAs (Fig. 7). Taken together, these results suggest that hnRNP K regulates EGR-1 and NR4A3 mRNA levels co-transcriptionally through direct binding to this transcript. Thus, RNA binding may allow hnRNP K to bridge ERK cascade to the EGR-1 and NR4A3 genes through K recruitment to the elongating pre-mRNAs.
DISCUSSION
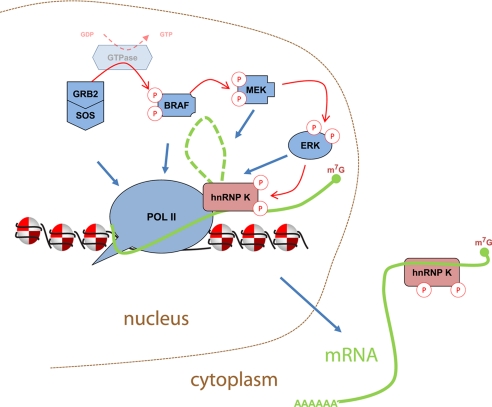
The discovery that terminal kinases such as ERK have hundreds of protein substrates has fueled great interest to understand mechanisms that control their specificity (13, 33). Thus far, several elements have been identified that account for kinase specificity including amino acid consensus sequence, subcellular localization or compartmentalization, duration and magnitude of the signals, cross-talk with other pathways, and scaffolds that modulate their activity (1, 12, 13). Below we discuss the elements of ERK specificity revealed in the current study and suggest a model for hnRNP-K-compartmentalized in situ control of ERK kinase cascade at the EGR-1 gene, which in turn regulates K protein-dependent mRNA synthesis and processing (Fig. 8).
FIGURE 8.
hnRNP K-compartmentalized model of ERK activation at target loci. Mitogen-inducible Pol II-mediated EGR-1 transcription allows recruitment of GRB2, SOS, and active B-Raf, MEK, and ERK along the gene (Figs. 1–3). Binding/activation of MEK and MEK-catalyzed activation of ERK (Fig. 4) at EGR-1 depends on binding of hnRNP K to elongating Pol II-pre-mRNA (Fig. 7). In turn, activated ERK phosphorylates EGR-1 RNA-bound hnRNP K, which maintains pre-mRNA elongation, splicing, and stability and drives export of the ribonucleoprotein complex to the cytoplasm (29).
Compartmentalization of the ERK Cascade Signaling Module at Inducible Genes
It has been previously demonstrated that nearly all of the components of the ERK pathway translocate to the nucleus (73–76). This and another recent study from our laboratory (11) provide evidence that in fact most of the ERK cascade components, from GRB2 down to ERK, are inducibly recruited along an inducible gene (Figs. 1–3). These signal transducers were not found in the intergenic regions flanking the EGR-1, indicating that the ERK activation cascade is compartmentalized to discrete sites, restricting their action to only relevant gene locus-associated substrates along the Pol II transcribed region.
Timing of the ERK Cascade Induction along Inducible Genes
Under the conditions of the present study, the serum-induced levels of active MEK/ERK assessed by Western blot analysis in whole cell lysates (Fig. 4B) were relatively low. In sharp contrast, the induced ChIP levels of all the ERK cascade components at the EGR-1 and the Nr4A3 loci were robust and short-lived. Moreover, the temporal profiles of ERK cascade at EGR-1 and Nr4A3 were very different from the one at the c-MYC and hnRNP K (Fig. 5) or the GAPDH genes (11). The levels of active MEK/ERK seen by Western blot analysis represent the sum of MEK/ERK activities in different subcellular compartments/sites. Thus, the local burst of ERK activities occurring at different time points after mitogenic stimulation, as illustrated by the different profiles at EGR-1 and c-MYC (Fig. 5), would not be as readily detectable in bulk cell fractions.
The short burst of localized activity (e.g. at the EGR-1 locus) would ensure that ERK will phosphorylate only those substrates that are present at the correct time in the immediate vicinity of the compartmentalized signaling cassette. The sharp peaks of activity will also provide a means for more precise cross-talk timing with other signaling cascades, further increasing specificity (52). In the cases studied here, the activation of the ERK cascade components is linked to the elongating Pol II along inducible genes. The tight coupling to Pol II seems like an ideal way for these signal transducers to specifically regulate in a timely fashion serum-induced factors involved in co-transcriptional RNA processing (Fig. 8). In fact, a recent study suggests that synchronized activation of ERK cascade components along genes appears to be important in maintaining effective transcription (11).
Activation of ERK at Inducible Gene Loci
The unexpected kinetic differences between MEK-ERK activities measured in whole cell lysates compared with those measured at the specific genomic sites (Fig. 4) are not consistent with the traditional view of cytoplasmic ERK activation. Thus, these observations raise questions about the pathways that activate ERK at these genes in the absence of comparable changes in the cell as whole. Most of the ERK cascade components (77) as well as RTKs (78–80) have previously been found in the nucleus, and mechanisms of their nuclear translocation are beginning to be defined (77). These factors could be preassembled in the nucleus so that induction of EGR-1 and Nr4A3 may not require serum-induced nuclear translocation of these components. We have measured high constitutive levels of ERK components at the GAPDH gene (supplemental Fig. S2) (11). We have also found high constitutive levels of insulin receptor at insulin-responsive genes without detectable insulin (11). EGFR is also bound to genes and exhibits transcriptional activity (76). Thus, RTKs could also be constitutively preassembled in the nucleus and serve to initiate activation of the ERK cascade module at inducible gene loci. Because we did not detect RAS, Ran, known to be chromatin-associated and to interact with Shc (81), could be an alternative small GTPase that could link nuclear RTKs to Grb2/SOS. We have found that a specific MEK inhibitor, U0126, blocks inducible recruitment of all components, GRB2, SOS, B-Raf, MEK, and ERK, to the EGR-1 gene without any effects on histone marks (supplemental Fig. S5, rows 8 and 9). This observation suggests the presence of a different upstream activation ERK cascade module that indirectly controls the ERK signaling microcircuit at the EGR-1 gene. For example, a cytoplasmic ERK activation module could drive nuclear translocation, binding, and activation of SRF, Elk (Fig. 2 and supplemental Fig. S3), and/or other transcription factors to the EGR-1 locus initiating Pol II recruitment/elongation. In turn, hnRNP K binding activates the ERK microcircuit, a process that may involve all of the cascade components including chromatin-bound RTKs.
hnRNP K acts as a docking platform at sites of RNA-directed processes (22, 31, 82). hnRNP K exists in complexes with ERK1/2 and regulates their activities (40, 82). We show that in response to serum treatment, hnRNP K recruitment along the EGR-1 gene is linked to Pol II transcription (Figs. 1–4). The hnRNP K knockdown inhibited MEK1/2 and ERK1/2 activities along the EGR-1 and NR4A3 genes (Fig. 5). This observation is consistent with a model where K protein is a functional scaffold for the ERK cascade along these loci.
ERK Targets along Inducible Genes
There could be several targets of the K protein-mediated discrete activation and recruitment of ERK1/2 along the EGR-1 and NR4A3 genes. Inhibition of MEK-ERK activity by hnRNP K knockdown had little or no effect on Pol II occupancy along the EGR-1 and NR4A3 genes (Fig. 5), suggesting that processivity was not altered. Pol II processivity can be uncoupled from the rate of elongation (84). Moreover, pre-mRNA processing is linked to Pol II elongation. Consistent with this view, we provide evidence that K protein regulates both the rate of elongation and splicing (Fig. 6). Thus, ERK cascade targets could include elongation and/or splicing factors.
ERK1/2 phosphorylates Ser-284 and Ser-353 of hnRNP K (29). Thus, after hnRNP K-mediated ERK activation along the EGR-1 gene, the K protein itself may become ERK substrate regulating the splicing activity of K. ERK-mediated phosphorylation also drives K protein export from the nucleus (29). ERK-mediated phosphorylation of hnRNP K increases stability of K protein-bound transcripts (20) and also drives K protein export from the nucleus (29). Thus, the ERK-catalyzed phosphorylation can also play a role in hnRNP K-bound EGR-1 mRNA stability and transport to the cytoplasm (Fig. 8).
In sum, we obtained evidence that nearly the full complement of the ERK cascade components is recruited along the inducible genes coupled to Pol II elongation. Binding of hnRNP K that accompanies these signal transducers plays a role in MEK1/2 recruitment and ERK1/2 activation along the inducible EGR-1 and NR4A3 loci. This study illustrates for the first time how a spatiotemporally restricted chain of events starting with Pol II recruitment followed by K protein binding to inducible loci, presumably to nascent transcript, controls kinases, which in turn regulate rate of mRNA synthesis and processing. In this regard, compartmentalized intracellular bursts of activation of kinase signaling cascades at different time points following mitogenic activation could be a more ubiquitous phenomenon than previously recognized.
Supplementary Material
Acknowledgment
We thank Steve Flanagin (University of Washington) for developing GraphGrid and other software tools for matrix ChIP data analysis.
This work was supported, in whole or in part, by National Institutes of Health Grants R37-DK45978 and RO-1 GM45134 (to K. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- hnRNP
- heterogeneous nuclear ribonucleoprotein
- EGR-1
- Early growth response-1
- DRB
- 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole
- RIP
- ribonucleoprotein immunoprecipitation
- RTK
- tyrosine kinase receptor
- SRF
- serum-responsive factor
- Pol II
- polymerase II
- NC
- noncomplementary.
REFERENCES
- 1. Shaul Y. D., Seger R. (2007) Biochim. Biophys. Acta 1773, 1213–1226 [DOI] [PubMed] [Google Scholar]
- 2. Nigg E. A., Hilz H., Eppenberger H. M., Dutly F. (1985) EMBO J. 4, 2801–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seth A., Gonzalez F. A., Gupta S., Raden D. L., Davis R. J. (1992) J. Biol. Chem. 267, 24796–24804 [PubMed] [Google Scholar]
- 4. Seger R., Krebs E. G. (1995) FASEB J. 9, 726–735 [PubMed] [Google Scholar]
- 5. Chow C. W., Davis R. J. (2006) Cell 127, 887–890 [DOI] [PubMed] [Google Scholar]
- 6. Bungard D., Fuerth B. J., Zeng P. Y., Faubert B., Maas N. L., Viollet B., Carling D., Thompson C. B., Jones R. G., Berger S. L. (2010) Science 329, 1201–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vicent G. P., Ballaré C., Nacht A. S., Clausell J., Subtil-Rodríguez A., Quiles I., Jordan A., Beato M. (2006) Mol. Cell 24, 367–381 [DOI] [PubMed] [Google Scholar]
- 8. Simone C., Forcales S. V., Hill D. A., Imbalzano A. N., Latella L., Puri P. L. (2004) Nat. Genet. 36, 738–743 [DOI] [PubMed] [Google Scholar]
- 9. Edmunds J. W., Mahadevan L. C. (2006) Science 313, 449–451 [DOI] [PubMed] [Google Scholar]
- 10. Pokholok D. K., Zeitlinger J., Hannett N. M., Reynolds D. B., Young R. A. (2006) Science 313, 533–536 [DOI] [PubMed] [Google Scholar]
- 11. Nelson J. D., LeBoeuf R. C., Bomsztyk K. (2011) Diabetes 60, 127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy L. O., Blenis J. (2006) Trends Biochem. Sci. 31, 268–275 [DOI] [PubMed] [Google Scholar]
- 13. McKay M. M., Morrison D. K. (2007) Oncogene 26, 3113–3121 [DOI] [PubMed] [Google Scholar]
- 14. Wong W., Scott J. D. (2004) Nat. Rev. Mol. Cell Biol. 5, 959–970 [DOI] [PubMed] [Google Scholar]
- 15. Matunis M. J., Michael W. M., Dreyfuss G. (1992) Mol. Cell. Biol. 12, 164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swanson M. S., Dreyfuss G. (1988) Mol. Cell. Biol. 8, 2237–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei C. C., Zhang S. L., Chen Y. W., Guo D. F., Ingelfinger J. R., Bomsztyk K., Chan J. S. (2006) J. Biol. Chem. 281, 25344–25355 [DOI] [PubMed] [Google Scholar]
- 18. Michelotti E. F., Michelotti G. A., Aronsohn A. I., Levens D. (1996) Mol. Cell. Biol. 16, 2350–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Expert-Bezançon A., Le Caer J. P., Marie J. (2002) J. Biol. Chem. 277, 16614–16623 [DOI] [PubMed] [Google Scholar]
- 20. Chen L. C., Liu H. P., Li H. P., Hsueh C., Yu J. S., Liang C. L., Chang Y. S. (2009) Oncogene 28, 1904–1915 [DOI] [PubMed] [Google Scholar]
- 21. Weng Z., Thomas S. M., Rickles R. J., Taylor J. A., Brauer A. W., Seidel-Dugan C., Michael W. M., Dreyfuss G., Brugge J. S. (1994) Mol. Cell. Biol. 14, 4509–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adolph D., Flach N., Mueller K., Ostareck D. H., Ostareck-Lederer A. (2007) Mol. Cell. Biol. 27, 1758–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ostrowski J., Schullery D. S., Denisenko O. N., Higaki Y., Watts J., Aebersold R., Stempka L., Gschwendt M., Bomsztyk K. (2000) J. Biol. Chem. 275, 3619–3628 [DOI] [PubMed] [Google Scholar]
- 24. Schullery D. S., Ostrowski J., Denisenko O. N., Stempka L., Shnyreva M., Suzuki H., Gschwendt M., Bomsztyk K. (1999) J. Biol. Chem. 274, 15101–15109 [DOI] [PubMed] [Google Scholar]
- 25. Denisenko O. N., O'Neill B., Ostrowski J., Van Seuningen I., Bomsztyk K. (1996) J. Biol. Chem. 271, 27701–27706 [DOI] [PubMed] [Google Scholar]
- 26. Shnyreva M., Schullery D. S., Suzuki H., Higaki Y., Bomsztyk K. (2000) J. Biol. Chem. 275, 15498–15503 [DOI] [PubMed] [Google Scholar]
- 27. Skalweit A., Doller A., Huth A., Kähne T., Persson P. B., Thiele B. J. (2003) Circ. Res. 92, 419–427 [DOI] [PubMed] [Google Scholar]
- 28. Yano M., Okano H. J., Okano H. (2005) J. Biol. Chem. 280, 12690–12699 [DOI] [PubMed] [Google Scholar]
- 29. Habelhah H., Shah K., Huang L., Ostareck-Lederer A., Burlingame A. L., Shokat K. M., Hentze M. W., Ronai Z. (2001) Nat. Cell Biol. 3, 325–330 [DOI] [PubMed] [Google Scholar]
- 30. Ostrowski J., Kawata Y., Schullery D. S., Denisenko O. N., Higaki Y., Abrass C. K., Bomsztyk K. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 9044–9049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bomsztyk K., Denisenko O., Ostrowski J. (2004) BioEssays 26, 629–638 [DOI] [PubMed] [Google Scholar]
- 32. Van Seuningen I., Ostrowski J., Bustelo X. R., Sleath P. R., Bomsztyk K. (1995) J. Biol. Chem. 270, 26976–26985 [DOI] [PubMed] [Google Scholar]
- 33. Yao Z., Seger R. (2009) Biofactors 35, 407–416 [DOI] [PubMed] [Google Scholar]
- 34. Ishibe S., Joly D., Liu Z. X., Cantley L. G. (2004) Mol. Cell 16, 257–267 [DOI] [PubMed] [Google Scholar]
- 35. Turjanski A. G., Vaqué J. P., Gutkind J. S. (2007) Oncogene 26, 3240–3253 [DOI] [PubMed] [Google Scholar]
- 36. Flanagin S., Nelson J. D., Castner D. G., Denisenko O., Bomsztyk K. (2008) Nucleic Acids Res. 36, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lawrence M. C., McGlynn K., Shao C., Duan L., Naziruddin B., Levy M. F., Cobb M. H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13315–13320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sukhatme V. P. (1990) J. Am. Soc. Nephrol. 1, 859–866 [DOI] [PubMed] [Google Scholar]
- 39. Kubosaki A., Tomaru Y., Tagami M., Arner E., Miura H., Suzuki T., Suzuki M., Suzuki H., Hayashizaki Y. (2009) Genome Biol. 10, R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laury-Kleintop L. D., Tresini M., Hammond O. (2005) J. Cell. Biochem. 95, 1042–1056 [DOI] [PubMed] [Google Scholar]
- 41. Chang J. W., Koike T., Iwashima M. (2009) Int. Immunol. 21, 1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fukuda R., Hirota K., Fan F., Jung Y. D., Ellis L. M., Semenza G. L. (2002) J. Biol. Chem. 277, 38205–38211 [DOI] [PubMed] [Google Scholar]
- 43. Naito M., Zager R. A., Bomsztyk K. (2009) J. Am. Soc. Nephrol. 20, 1787–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lewis T. S., Hunt J. B., Aveline L. D., Jonscher K. R., Louie D. F., Yeh J. M., Nahreini T. S., Resing K. A., Ahn N. G. (2000) Mol. Cell 6, 1343–1354 [DOI] [PubMed] [Google Scholar]
- 45. Glover-Cutter K., Kim S., Espinosa J., Bentley D. L. (2008) Nat. Struct. Mol. Biol. 15, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rahl P. B., Lin C. Y., Seila A. C., Flynn R. A., McCuine S., Burge C. B., Sharp P. A., Young R. A. (2010) Cell 141, 432–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. (1993) Nature 363, 45–51 [DOI] [PubMed] [Google Scholar]
- 48. Schlessinger J. (2000) Cell 103, 211–225 [DOI] [PubMed] [Google Scholar]
- 49. Mikula M., Dzwonek A., Karczmarski J., Rubel T., Dadlez M., Wyrwicz L. S., Bomsztyk K., Ostrowski J. (2006) Proteomics 6, 2395–2406 [DOI] [PubMed] [Google Scholar]
- 50. Dhillon A. S., Hagan S., Rath O., Kolch W. (2007) Oncogene 26, 3279–3290 [DOI] [PubMed] [Google Scholar]
- 51. Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. (1992) Nature 358, 417–421 [DOI] [PubMed] [Google Scholar]
- 52. Kyriakis J. M. (2007) Biochim. Biophys. Acta 1773, 1238–1247 [DOI] [PubMed] [Google Scholar]
- 53. Kocher H. M., Senkus R., Al-Nawab M., Hendry B. M. (2005) Nephrol. Dial. Transplant. 20, 886–891 [DOI] [PubMed] [Google Scholar]
- 54. Reiterer G., Chen L., Tassef R., Varner J. D., Chen C. Y., Yen A. (2010) Cell Cycle 9, 3297–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang B. H., Guan K. L. (2000) EMBO J. 19, 5429–5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim S. J., Kahn C. R. (1997) Biochem. J. 323, 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang G., Balamotis M. A., Stevens J. L., Yamaguchi Y., Handa H., Berk A. J. (2005) Mol. Cell 17, 683–694 [DOI] [PubMed] [Google Scholar]
- 58. Hasan R. N., Schafer A. I. (2008) Circ. Res. 102, 42–50 [DOI] [PubMed] [Google Scholar]
- 59. Janknecht R., Hunter T. (1997) EMBO J. 16, 1620–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu S. Q., Minami T., Donovan D. J., Aird W. C. (2002) Blood 100, 4454–4461 [DOI] [PubMed] [Google Scholar]
- 61. Revil T., Pelletier J., Toutant J., Cloutier A., Chabot B. (2009) J. Biol. Chem. 284, 21458–21467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boehm A. K., Saunders A., Werner J., Lis J. T. (2003) Mol. Cell. Biol. 23, 7628–7637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mikula M., Hanusek K., Paziewska A., Dzwonek A., Rubel T., Bomsztyk K., Ostrowski J. (2010) BMC Mol. Biol. 11, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yankulov K., Yamashita K., Roy R., Egly J. M., Bentley D. L. (1995) J. Biol. Chem. 270, 23922–23925 [DOI] [PubMed] [Google Scholar]
- 65. Pearen M. A., Muscat G. E. (2010) Mol. Endocrinol. 24, 1891–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Notari M., Neviani P., Santhanam R., Blaser B. W., Chang J. S., Galietta A., Willis A. E., Roy D. C., Caligiuri M. A., Marcucci G., Perrotti D. (2006) Blood 107, 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Matunis M. J., Xing J., Dreyfuss G. (1994) Nucleic Acids Res. 22, 1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Singh J., Padgett R. A. (2009) Nat. Struct. Mol. Biol. 16, 1128–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Perales R., Bentley D. (2009) Mol. Cell 36, 178–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shilatifard A., Conaway R. C., Conaway J. W. (2003) Annu. Rev. Biochem. [DOI] [PubMed] [Google Scholar]
- 71. Weg-Remers S., Ponta H., Herrlich P., König H. (2001) EMBO J. 20, 4194–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Klimek-Tomczak K., Wyrwicz L. S., Jain S., Bomsztyk K., Ostrowski J. (2004) J. Mol. Biol. 342, 1131–1141 [DOI] [PubMed] [Google Scholar]
- 73. Oláh Z., Komoly S., Nagashima N., Joó F., Rapp U. R., Anderson W. B. (1991) Exp. Brain Res. 84, 403–410 [DOI] [PubMed] [Google Scholar]
- 74. Romero F., Ramos-Morales F., Domínguez A., Rios R. M., Schweighoffer F., Tocqué B., Pintor-Toro J. A., Fischer S., Tortolero M. (1998) J. Biol. Chem. 273, 7776–7781 [DOI] [PubMed] [Google Scholar]
- 75. Carpenter G. (2003) Curr. Opin. Cell Biol. 15, 143–148 [DOI] [PubMed] [Google Scholar]
- 76. Lin S. Y., Makino K., Xia W., Matin A., Wen Y., Kwong K. Y., Bourguignon L., Hung M. C. (2001) Nat. Cell Biol. 3, 802–808 [DOI] [PubMed] [Google Scholar]
- 77. Zehorai E., Yao Z., Plotnikov A., Seger R. (2010) Mol. Cell. Endocrinol. 314, 213–220 [DOI] [PubMed] [Google Scholar]
- 78. Hsu S. C., Hung M. C. (2007) J. Biol. Chem. 282, 10432–10440 [DOI] [PubMed] [Google Scholar]
- 79. Aleksic T., Chitnis M. M., Perestenko O. V., Gao S., Thomas P. H., Turner G. D., Protheroe A. S., Howarth M., Macaulay V. M. (2010) Cancer Res. 70, 6412–6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Podlecki D. A., Smith R. M., Kao M., Tsai P., Huecksteadt T., Brandenburg D., Lasher R. S., Jarett L., Olefsky J. M. (1987) J. Biol. Chem. 262, 3362–3368 [PubMed] [Google Scholar]
- 81. George R., Chan H. L., Ahmed Z., Suen K. M., Stevens C. N., Levitt J. A., Suhling K., Timms J., Ladbury J. E. (2009) Cell Mol. Life Sci. 66, 711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wolf D., Witte V., Clark P., Blume K., Lichtenheld M. G., Baur A. S. (2008) Cell Host Microbe 4, 398–408 [DOI] [PubMed] [Google Scholar]
- 83. Afonina I., Ankoudinova I., Mills A., Lokhov S., Huynh P., Mahoney W. (2007) BioTechniques 43, 770–774 [DOI] [PubMed] [Google Scholar]
- 84. Mason P. B., Struhl K. (2005) Mol. Cell 17, 831–840 [DOI] [PubMed] [Google Scholar]
- 85. Ostrowski J., Kawata Y., Schullery D. S., Denisenko O. N., Bomsztyk K. (2003) Nucleic Acids Res. 31, 3954–3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.