Abstract
Reducing the mammalian target of rapamycin (mTOR) activity increases lifespan and health span in a variety of organisms. Alterations in protein homeostasis and mTOR activity and signaling have been reported in several neurodegenerative disorders, including Alzheimer disease (AD); however, the causes of such deregulations remain elusive. Here, we show that mTOR activity and signaling are increased in cell lines stably transfected with mutant amyloid precursor protein (APP) and in brains of 3xTg-AD mice, an animal model of AD. In addition, we show that in the 3xTg-AD mice, mTOR activity can be reduced to wild type levels by genetically preventing Aβ accumulation. Similarly, intrahippocampal injections of an anti-Aβ antibody reduced Aβ levels and normalized mTOR activity, indicating that high Aβ levels are necessary for mTOR hyperactivity in 3xTg-AD mice. We also show that the intrahippocampal injection of naturally secreted Aβ is sufficient to increase mTOR signaling in the brains of wild type mice. The mechanism behind the Aβ-induced mTOR hyperactivity is mediated by the proline-rich Akt substrate 40 (PRAS40) as we show that the activation of PRAS40 plays a key role in the Aβ-induced mTOR hyperactivity. Taken together, our data show that Aβ accumulation, which has been suggested to be the culprit of AD pathogenesis, causes mTOR hyperactivity by regulating PRAS40 phosphorylation. These data further indicate that the mTOR pathway is one of the pathways by which Aβ exerts its toxicity and further support the idea that reducing mTOR signaling in AD may be a valid therapeutic approach.
Keywords: Aging, Alzheimers Disease, mTOR, Tau, Transgenic, 3xTg-AD, AD, Abeta, Plaques, Tangles
Introduction
Amyloid plaques and neurofibrillary tangles are hallmark neuropathological lesions of Alzheimer disease (AD),3 the most common form of neurodegenerative disorder (1). Neurofibrillary tangles are intracellular inclusions formed of hyperphosphorylated Tau (2–4). Plaques are extracellular inclusions mainly formed of a small peptide called amyloid-β (Aβ) (5, 6). Clinically, AD is characterized by profound memory loss and cognitive dysfunction (7). Growing evidence is converging on soluble Aβ as a mediator of early cognitive decline in AD (8, 9). Although the molecular mechanisms underlying Aβ-induced cognitive decline remain elusive, soluble Aβ oligomers have been shown to alter signal transduction pathways that are key for learning and memory, suggesting that alterations in such pathways may underlie the onset of cognitive decline in AD (10).
The mammalian target of rapamycin (mTOR) is a conserved Ser/Thr kinase that forms two multiprotein complexes known as mTOR complex (mTORC) 1 and 2 (11). mTORC1 controls protein homeostasis; its activity is inhibited by rapamycin, and it contains mTOR, raptor, proline-rich Akt substrate 40 kDa (PRAS40), and mLT8. mTORC2, which is insensitive to rapamycin, controls cellular shape by modulating actin function and contains mTOR, rictor, mLST8, and hSIN (11, 12). In mTORC1, raptor binds to mTOR substrates and is necessary for mTOR activity (13). PRAS40 is another mTOR regulatory protein, which inhibits mTOR by directly binding to it (14, 15). When PRAS40 is phosphorylated at Thr-246, it detaches from mTOR, thereby facilitating mTOR activity (14, 15). Several mTOR functions are executed via its downstream targets, namely the 70-kDa ribosomal protein S6 kinase I (p70S6K), which is directly activated by mTOR through phosphorylation at the Thr-389 site (16–19), and the eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), an inhibitor or protein translation whose function is reduced when phosphorylated by mTOR (11). Notably, the steady-state levels of p70S6K phosphorylated at Thr-389 are often used as an indirect measurement of mTOR output as they greatly correlate with mTOR activity (16, 18, 19).
mTOR activity is physiologically regulated by extracellular stimuli (e.g. insulin/insulin-like growth factor, cell energy status, nutrients, and stress) via different signaling transduction pathways (11), some of which are modulated by Aβ (20–24). Indeed, evidence from AD brains shows that mTOR signaling is selectively increased in neurons predicted to develop neurofibrillary tangles and that such an increase correlates with Tau phosphorylation (25–28). This evidence has led to the hypothesis that the chronic increase in mTOR function occurring during aging may facilitate the development of Tau pathology (27).
By regulating both protein synthesis and degradation, mTOR plays a key role in controlling protein homeostasis and hence brain function; indeed, mTOR activity has been directly linked to learning and memory (29–32). Specifically, although mTOR activity is necessary for learning and memory, mTOR hyperactivity is detrimental (33). For example, complete inhibition of mTOR by rapamycin has detrimental effects on long term memory facilitation and consolidation in gerbils and Aplysia californica (31, 32). In contrast, low concentrations of rapamycin improve memory deficits associated with cannabinoid consumption and rescue learning deficits in a mouse model of tuberous sclerosis, which is characterized by hyperactive mTOR (29, 30). In summary, there seems to be an optimal window for mTOR activity that is most beneficial for learning and memory, and alterations that lead to an increase or decrease in mTOR signaling outside such an optimal window may have detrimental effects on learning and memory (34–36).
Recent reports show that pharmacologically or genetically reducing mTOR signaling significantly increases lifespan and health span in mice (37). The role of mTOR signaling in aging has also been clearly shown in lower organisms (38–42). We recently showed that pharmacologically reducing mTOR signaling in the brains of 3xTg-AD mice, an animal model of AD, rescues the Aβ-induced cognitive decline (43). This improvement in learning and memory was linked to an autophagy-mediated reduction in Aβ and Tau pathology (43). Notably, these data were also independently confirmed in another mouse model of AD (44). Considering the role of mTOR in aging and learning and memory, elucidating the molecular pathways leading to mTOR deregulation in AD, which is the objective of this study, may lead to a better understanding of the disease pathogenesis.
EXPERIMENTAL PROCEDURES
Mice and Surgical Procedures
The derivation and characterization of the 3xTg-AD and the APP/Tau mice are described elsewhere (45, 46). Mice were group-housed and kept on a 12-h light:12-h dark schedule. All mice were given ad libitum access to food and water. All animal procedures were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and all appropriate measures were taken to minimize pain and discomfort in experimental animals. Mice were anesthetized with avertin (1.3% tribromoethanol, 0.8% amyl alcohol, given 0.6 ml/25 g of body weight) and placed in a stereotactic apparatus. Mice received single intrahippocampal injections of either 6E10 (2 μg) or conditioned medium prepared as described below. Solutions were injected through a 33-gauge injector attached to a 5-μl Hamilton syringe. The coordinates, with respect to bregma, were −0.34 mm posterior, −2.2 mm lateral, and −1 mm ventral to the skull. Injections occurred over 5 min, after which the cannula was left in place for an additional 5 min to allow for diffusion. Animals were kept on a warming pad until they had fully recovered from anesthesia. To prevent damage to the scalp sutures, mice were kept in individual cages until they were sacrificed for tissue processing.
Antibodies, mTOR Activity, and Inhibitors
With the exception of the anti-APP 6E10 antibody (Chemicon, Temecula, CA), all the other antibodies used here were purchased from Cell Signaling, Beverly, MA. The Akt inhibitor VII, TAT-Akt-in was purchased from Calbiochem. The PIM-1 inhibitor 1 was purchased from Tocris Bioscience (Ellisville, MO). mTOR activity was measured using the K-LISATM mTOR activity kit (EMD Chemicals, Gibbstown, NJ) following the manufacturer's protocol.
Conditioned Medium
7PA2 cells were grown in DMEM with 10% fetal bovine serum at 37 °C with humidified environment (5% CO2) in 75-cm2 cell culture flasks until 90% confluent. At this time, the medium was replaced with 7 ml of serum-free DMEM for 18 h. Conditioned medium was then centrifuged to remove debris and frozen at −80 °C. The medium was concentrated using Amicon Ultra-15 centrifuge filters (Millipore, Billerica, MA) with a 3000 molecular weight cut-off.
Protein Extraction, Western Blot, Immunohistochemistry, and ELISA
Mice were transcardially perfused with PBS. Subsequently, one-half brain was dropped-fixed in 4% paraformaldehyde in PBS for 48 h and then transferred to 0.02% sodium azide in PBS until slicing. The other half was frozen in dry ice for biochemical analysis. Frozen brains were homogenized in a solution of tissue protein extraction reagent (T-PER, Pierce) containing 0.7 mg/ml pepstatin A supplemented with a Complete mini protease inhibitor tablet (Roche Applied Science) and phosphatase inhibitors (Invitrogen).
For Western blot, proteins were resolved by 10% Bis-Tris SDS-PAGE (Invitrogen) under reducing conditions and transferred to a nitrocellulose membrane as described in Ref. 47. Densitometric analysis was conducted using the ImageJ software from the National Institutes of Health. The protein levels reported in the figures were obtained as a ratio between the band intensity for the protein of interest and the band intensity of β-actin, used as loading control. Aβ40 and Aβ42 levels were measured using a sandwich ELISA protocol as described in Ref. 48.
For immunohistochemical analysis, 30-μm-thick sections were obtained using a vibratome slicing system (Leica Microsystems, Wetzlar, Germany), and sections were stored at 4 °C in 0.02% sodium azide in PBS. Sections were processed as described in Ref. 49.
Statistical Analyses
Statistical analyses were conducted using multifactor analysis of variance followed by post hoc Bonferroni test to determine individual differences among groups. Student's t test was used when suitable.
RESULTS
mTOR signaling is increased in selected neurons of AD brains (25, 26, 28, 50–52). Considering the major role of mTOR in regulating protein homeostasis (11), unveiling the molecular pathways leading to its deregulation in AD may lead to a better understanding of the disease pathogenesis. Commonly, mTOR activity is indirectly determined by measuring the steady-state levels of phosphorylated p70S6K and 4E-BP1, two downstream targets of mTOR (11, 19). Using Western blot analysis, we measured the levels of p70S6K and 4E-BP1 in the brains of 3xTg-AD mice as a function of age. Two-month-old 3xTg-AD mice have no detectable neuropathological alterations in the brain (45). At this age, we found that the levels of p70S6K and 4E-BP1 were similar between 3xTg-AD mice and age- and gender-matched non-transgenic (NonTg) mice (n = 6/genotype; Fig. 1, A–C). As the 3xTg-AD mice age, they gradually accumulate Aβ and Tau pathology in their brains. Specifically, 6-month-old mice are characterized by intraneuronal accumulation of soluble Aβ (45, 53). Tau pathology (e.g. somatodendritic Tau accumulation and hyperphosphorylation) starts to become apparent at this age, although to a smaller degree than intraneuronal Aβ (45, 48). Intraneuronal Aβ accumulation and Tau pathology are both significantly higher at 12 months of age when compared with 6-month-old mice (45). We found that the ratio of the steady-state levels of p70S6K phosphorylated at Thr-389 over the levels of total p70S6K was significantly increased in the brains of 6- and 12-month-old 3xTg-AD mice when compared with age-matched NonTg mice (Fig. 1, D, E, G, and H). Similarly, we found that at both ages, the ratio of 4E-BP1 phosphorylated at Ser-65 over total 4E-BP1 was also increased in the 3xTg-AD mice when compared with age- and gender-matched NonTg mice (Fig. 1, D, F, G, and I). Notably, previous reports clearly show that mTOR directly phosphorylates p70S6K and 4E-BP1 at Thr-389 and Ser-65, respectively (16–19). To directly assess mTOR activity in the brains of the 3xTg-AD mice, we measured mTOR enzymatic activity using an ELISA system (see “Experimental Procedures”). Consistent with the Western blot data, we found no changes in mTOR activity in pre-pathological 2-month-old mice (Fig. 1J); in contrast, we found a significant increase at 6 and 12 months of age when compared with age- and gender-matched NonTg mice (Fig. 1J). These results clearly indicate that as in AD brains, mTOR is hyperactive in the 3xTg-AD mice, and such an increase in mTOR activity mirrors the progression of Aβ and Tau pathology previously established in this mouse model of AD.
FIGURE 1.
mTOR signaling and activity are increased in 3xTg-AD mice. A, representative Western blots of protein extracted from the brains of 2-month-old 3xTg-AD and NonTg mice (n = 6/genotype) and probed with the indicated antibodies. B and C, quantitative analyses of the blots show that the p70S6K phosphorylation at Thr-389 (indicated as the ratio of total over phosphorylated levels) and 4E-BP1 phosphorylation at Ser-65 (indicated as the ratio of total over phosphorylated levels) were not significantly different between 3xTg-AD and NonTg mice. D, representative Western blots of protein extracted from the brains of 6-month-old 3xTg-AD and NonTg mice (n = 6/genotype) and probed with the indicated antibodies. E and F, quantitative analyses of the blots show that the p70S6K phosphorylation at Thr-389 (indicated as the ratio of total over phosphorylated levels) and 4E-BP1 phosphorylation at Ser-65 (indicated as the ratio of total over phosphorylated levels) were significantly higher in the brains of the 3xTg-AD mice when compared with NonTg mice (n = 6/genotype). G, representative Western blots of protein extracted from the brains of 12-month-old 3xTg-AD and NonTg mice (n = 6/genotype) and probed with the indicated antibodies. H and I, quantitative analyses of the blots show that the p70S6K phosphorylation at Thr-389 (indicated as the ratio of total over phosphorylated levels) and 4E-BP1 phosphorylation at Ser-65 (indicated as the ratio of total over phosphorylated levels) were significantly higher in the brains of the 3xTg-AD mice when compared with NonTg mice (n = 6/genotype). J, mTOR enzymatic activity was significantly increased in the brains of 6- and 12-month old 3xTg-AD mice when compared with age- and gender-matched NonTg mice. In contrast, no differences were detected at 2 months of age (n = 6/genotype/time point). Data are presented as means ± S.E. *, p < 0.05. **, p < 0.01.
Previously, we showed that Aβ causes mTOR hyperactivity in Chinese hamster ovary (CHO) cells stably transfected with a cDNA encoding APP751 containing the V717F familial AD mutation (known as 7PA2 cells (54)). To determine whether Aβ accumulation also causes mTOR hyperactivity in vivo, we first used a genetic approach to prevent Aβ accumulation in the brains of the 3xTg-AD mice. We previously reported the generation of the APP/Tau mice, which were obtained by breeding the 3xTg-AD mice to NonTg mice (45). In doing so, we replaced the mutant PS1 alleles with its wild type counterpart. Six-month-old APP/Tau mice have virtually no Aβ deposits in the CA1 pyramidal neurons (Fig. 2, A and B) and in other brain regions (45). We next sought to determine the effect of preventing Aβ accumulation on mTOR signaling and activity. Western blot experiments indicate that phosphorylation of p70S6K at Thr-389 (reported as the ratio of phosphorylated over total protein levels) was significantly reduced in the APP/Tau mice (Fig. 2, C and D), indicating a reduction of mTOR activity. Indeed, we found that mTOR enzymatic activity was similar between APP/Tau and NonTg mice (Fig. 2E). Because 6-month-old APP/Tau mice do not show any Aβ accumulation, despite no changes in APP and Tau expression levels with respect to 3xTg-AD mice (45), these data highlight a strong correlation between Aβ levels and mTOR hyperactivity.
FIGURE 2.
Genetically preventing Aβ accumulation in 3xTg-AD mice reduces mTOR activity. A and B, representative microphotographs showing CA1 pyramidal neurons of 6-month-old 3xTg-AD and APP/Tau mice (n = 6/genotype). Sections were stained with an anti-Aβ antibody. C, representative Western blots of protein extracted from the brains of 6-month-old 3xTg-AD and APP/Tau mice (n = 6/genotype) and probed with the indicated antibodies. D, quantitative analyses of the blots show that p70S6K phosphorylation at Thr-389 (indicated as the ratio of total over phosphorylated levels) was significantly reduced in the APP/Tau mice when compared with 3xTg-AD mice. E, mTOR enzymatic activity was significantly reduced in the brains of 6-month-old APP/Tau mice when compared with age-matched 3xTg-AD mice. Notably, mTOR activity was not statistically significant between NonTg and APP/Tau mice, suggesting that high Aβ levels are necessary to induce mTOR hyperactivity in the 3xTg-AD mice. (n = 6/genotype). Data are presented as means ± S.E. *, p < 0.05.
Recently, it was shown that presenilin 1 (PS1) modulates autophagy induction (55), which is negatively regulated by mTOR (11), suggesting a possible interaction between PS1 and mTOR. We thus sought to determine whether the effects on mTOR in the APP/Tau mice were thoroughly mediated by a reduction in Aβ levels or were simply due to the lack of mutant PS1. To address this question, we stereotaxically injected 2 μg of the anti-Aβ antibody 6E10 into the left hippocampi of 6-month-old 3xTg-AD mice. We and others have shown that a single intrahippocampal injection of anti-Aβ antibodies is sufficient to remove Aβ deposits (49, 56, 57). Three days after injections, we measured Aβ levels by sandwich ELISA in the injected hippocampi and used the contralateral uninjected hippocampi as internal controls. Consistent with previous reports, we found that a single intrahippocampal injection of 6E10 was sufficient to significantly reduce Aβ levels (Fig. 3A). To determine the effects of immunologically reducing Aβ on mTOR signaling, we measured the steady-state levels of total and phosphorylated p70S6K by Western blot. We found that reducing Aβ levels led to a significant reduction in p70S6K phosphorylation (Fig. 3, B and C). Additionally, we found that mTOR enzymatic activity was significantly lower in the injected hippocampi when compared with the contralateral uninjected hippocampi (Fig. 3D), strongly suggesting that in 3xTg-AD mice, high levels of Aβ are necessary to induce mTOR hyperactivity and that the mTOR hyperactivity is not simply due to the to the effects of mutant PS1 on autophagy.
FIGURE 3.
Lowering Aβ levels reduces mTOR activity. 6E10 was stereotaxically injected into the left hippocampi of 6-month-old mice (n = 6). The right uninjected hippocampi were used as internal controls. A, sandwich ELISA measurements show that Aβ levels were significantly reduced in the ipsilateral (Ipsi) hippocampi (receiving 6E10) when compared with the contralateral (Contra) uninjected hippocampi (n = 6). B, representative Western blots of protein extracted from the left hippocampi of 6-month-old 3xTg-AD injected with 6E10 (ipsilateral) and the right hippocampi used as internal control (contralateral; n = 6) and probed with the indicated antibodies. C, quantitative analyses of the blots show that reducing Aβ levels with 6E10 lowered p70S6K phosphorylation at Thr-389 (indicated as the ratio of total over phosphorylated levels) in the ipsilateral hippocampi (receiving the anti-Aβ antibody). No changes were detected in the contralateral hippocampi. D, mTOR enzymatic activity was significantly reduced in the hippocampi of 6-month-old 3xTg-AD mice receiving 6E10 when compared with contralateral uninjected hippocampi. (n = 6/group). Data are presented as means ± S.E. *, p < 0.05. **, p < 0.01.
To further understand the relation between Aβ and mTOR and to determine whether Aβ is sufficient to increase mTOR signaling in vivo, we took advantage of the 7PA2 cell line. The 7PA2 cells secrete Aβ oligomers into the culture medium (54), which have been shown to impair several neuronal functions, including long term potentiation and learning and memory (58–60). Conditioned medium (CM) from 7PA2 and untransfected CHO cells was obtained as described under “Experimental Procedures” and concentrated using Amicon Ultra-15 centrifuge filters. To determine the effects of naturally secreted Aβ on mTOR, we injected 7PA2 CM into the left hippocampi of NonTg mice. The right, uninjected hippocampi were used as internal controls. Mice were sacrificed 24 (n = 4) and 72 (n = 10) h after injection, and their hippocampi were processed for biochemical analysis. Six additional NonTg mice were injected with concentrated conditioned medium obtained from untransfected CHO cells (n = 6). We found that mTOR signaling (as determined by the levels of phosphorylated p70S6K) and activity were unaltered between ipsilateral and contralateral hippocampi, 24 h after Aβ injection (data not shown). In contrast, we found a significant increase in p70S6K phosphorylation and in mTOR activity in the hippocampi of mice injected with 7PA2 CM and sacrificed 72 h after injections, when compared with mice injected with CHO CM (Fig. 4, A–C). Notably, mice receiving CHO CM underwent the same amount of stress when compared with mice injected with 7PA2 CM, suggesting that the effects on mTOR could not simply be due to an increase in stress in these mice. To confirm that the effect on mTOR was due to Aβ, concentrated 7PA2 CM was immunodepleted with 6E10 prior to the intrahippocampal injections. Six mice were injected with the immunodepleted medium and sacrificed 72 h later. We found that immunodepleted 7PA2 CM had no effects on the p70S6K phosphorylation and mTOR enzymatic activity (Fig. 4, A–C). Taken together, our data suggest that high Aβ levels are both sufficient and necessary to increase mTOR activity in the brains of 3xTg-AD mice.
FIGURE 4.

Soluble Aβ is sufficient to increase mTOR activity. A, representative Western blots of protein extracted from the hippocampi of 2-month-old NonTg mice stereotaxically injected with concentrated CM from CHO or 7PA2 cells (n = 6/group). Six additional mice were injected with 7PA2 CM that were precleared of Aβ with 6E10. B, quantitative analyses of the blots indicate that injection of CHO CM or precleared 7PA2 CM did not significantly change the phosphorylation of p70S6K at Thr-389 (indicated as the ratio of total over phosphorylated levels). In contrast, injections of 7PA2 CM (highly enriched in soluble Aβ oligomers) significantly increased p70S6K phosphorylation at Thr-389. C, mTOR enzymatic activity was significantly increased in the hippocampi of 2-month-old NonTg mice injected with 7PA2 CM when compared with the hippocampi of mice injected with CHO CM or precleared 7PA2 CM (n = 6/group). Data are presented as means ± S.E. *, p < 0.05.
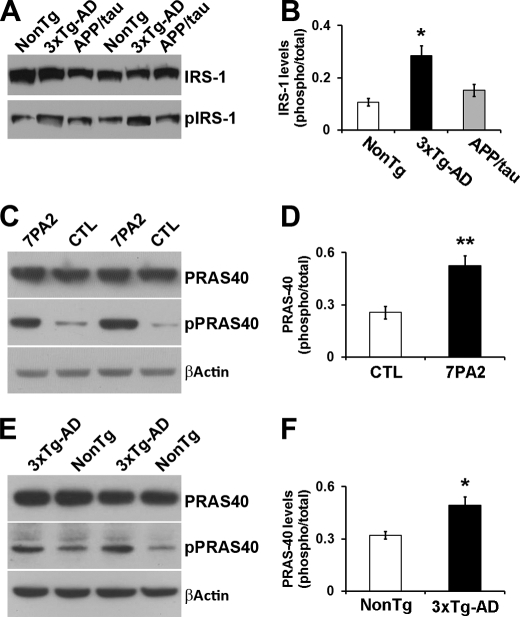
Physiologically, mTOR activity is regulated by several proteins, including the PRAS40, which inhibits mTOR by directly binding to it (14, 15). Specifically, insulin signaling facilitates the Akt-mediated phosphorylation of PRAS40 at Thr-246. Once phosphorylated, PRAS40 detaches from mTOR, thereby facilitating mTOR activity (14, 15). To determine whether the insulin/PRAS40 pathway is involved in Aβ-induced mTOR hyperactivity, we first measured the levels of the insulin receptor substrate 1 (IRS1) phosphorylated at Ser-616 (herein referred to phospho-IRS1). We found that the levels of phospho-IRS1 are increased in the brains of the 3xTg-AD mice but not in the APP/Tau mice when compared with NonTg mice (Fig. 5, A and B). This is consistent with results showing that Aβ oligomers increase phospho-IRS1 levels in primary neurons (61). Considering this and other evidence indicating that Aβ oligomers alter brain insulin signaling (61–63), we next tested whether PRAS40 phosphorylation plays a role in the Aβ-induced deregulation in mTOR activity. We first measured the levels of total and phosphorylated PRAS40 in 7PA2 cells and found that PRAS40 phosphorylation at Thr-246 was significantly higher in 7PA2 cells (Fig. 5, C and D). To determine whether these alterations were also present in vivo, we measured PRAS40 levels in the brains of 6-month-old mice. Consistent with the data from 7PA2 cells, we found that PRAS40 phosphorylation was significantly higher in 3xTg-AD mice when compared with age- and gender-matched NonTg mice (n = 6/genotype; Fig. 5, E and F).
FIGURE 5.
Phosphorylation of PRAS40 is significantly increased in the hippocampi of 3xTg-AD mice. A, representative Western blots of protein extracted from 6-month-old NonTg, 3xTg-AD, and APP/Tau mice and probed with the indicated antibodies (n = 6/genotype). B, quantitative analysis of the blots indicates that the ratio pIRS1/IRS1 was significantly increased in 3xTg-AD mice when compared with NonTg mice and APP/Tau mice. No difference was detected between the NonTg and APP/Tau mice. C, representative Western blots of protein extracted from 7PA2 and control (CTL) CHO cells and probed with the indicated antibodies (n = 6/group). D, quantitative analyses of the blots indicate that phosphorylation of PRAS40 at Thr-246 (indicated as the ratio of total over phosphorylated levels) was significantly increased in the 7PA2 cells when compared with control cells. E, representative Western blots of protein extracted from the hippocampi of 6-month-old 3xTg-AD and NonTg mice and probed with the indicated antibodies (n = 6/group). F, quantitative analyses of the blots indicate that phosphorylation of PRAS40 at Thr-246 (indicated as the ratio of total over phosphorylated levels) was significantly increased in the hippocampi of the 3xTg-AD mice when compared with NonTg mice. Data are presented as means ± S.E. *, p < 0.05. **, p < 0.01.
The data presented suggest that the Aβ-induced alteration in mTOR activity in the 7PA2 cells and in the 3xTg-AD mice may be mediated by PRAS40 phosphorylation, which is physiologically phosphorylated by Akt and by the protein kinase PIM-1 (64, 65). To better understand the role of PRAS40 in the Aβ-mediated mTOR hyperactivity, we injected concentrated 7PA2 CM into the left hippocampi of NonTg mice in the presence or absence of the Akt inhibitor VII, TAT-Akt-in and the PIM-1 inhibitor 1 (n = 6/group). The right uninjected hippocampi were used as internal controls. Additionally, mice were also injected with CHO CM. Three days after the injections, we measured the steady-state levels of total and phosphorylated PRAS40. We found that PRAS40 phosphorylation at Thr-246 was significantly higher in the hippocampi of mice injected with 7PA2 CM when compared with mice injected with CHO CM (Fig. 6, A and B). However, 7PA2 CM injected with the Akt inhibitor VII, TAT-Akt-in failed to increase PRAS40 phosphorylation (Fig. 6, A and B). Similarly, 7PA2 CM injected in the presence of the PIM-1 inhibitor 1 was not sufficient to increase PRAS40 phosphorylation (Fig. 6, A and B). Most important, although mTOR enzymatic activity was significantly higher in the hippocampi receiving 7PA2 CM when compared with the hippocampi injected with CHO CM, we found that 7PA2 CM did not significantly increase mTOR signaling in the presence of the Akt inhibitor VII, TAT-Akt-in or the PIM-1 inhibitor 1.
FIGURE 6.
PRAS40 phosphorylation plays a key role in the Aβ-mediated increase in mTOR activity. A, representative Western blots of protein extracted from the hippocampi of 2-month-old NonTg mice stereotaxically injected with concentrated CM from CHO and 7PA2 cells in the presence or absence of the Akt inhibitor VII, TAT-Akt-in (Akt inh) or the PIM-1 inhibitor 1 (PIM-1 inh) (n = 6/group). B, quantitative analyses of the blots indicate that injection of 7PA2 CM significantly increased PRAS40 phosphorylation (indicated as the ratio of total over phosphorylated levels). However, when 7PA2 CM was injected with the Akt or PIM-1 inhibitors, PRAS40 phosphorylation levels were significantly reduced when compared with baseline levels (mice injected with CHO CM), indicating that the inhibitor was able to block PRAS40 in vivo. C, mTOR enzymatic activity was significantly increased in the hippocampi of 2-month-old NonTg mice injected with 7PA2 CM when compared with the hippocampi of mice injected with CHO CM, 7PA2 CM + Akt inhibitor VII, TAT-Akt-in, and 7PA2 CM + PIM-1 inhibitor 1 (n = 6/group), suggesting that PRAS40 phosphorylation is necessary for the Aβ-induced increase in mTOR activity. D, representative Western blots of protein extracted from the hippocampus of 6-month-old mice and probed with the indicated antibodies. E, quantitative analyses of the blots indicate that the phosphorylation levels of TSC-1 were similar between the three groups of mice. Data are presented as means ± S.E. *, p < 0.05. **, p < 0.01.
Inhibition of AKT leads to reduced PRAS40 phosphorylation and reduced mTOR activity. AKT can also regulate mTOR activity by phosphorylating tuberous sclerosis protein 1 (TSC-1). To determine whether the AKT effects on mTOR could also be due to this pathway, we assessed how the levels of TSC-1 phosphorylation change in relation to Aβ levels. We found that in NonTg, 3xTg-AD, and APP/Tau mice, phosphorylation of TSC-1 was not statistically significant, strongly suggesting that the effects of Aβ on mTOR are not mediated by the AKT/TSC-1 pathways. Although we cannot exclude that inhibition of Akt and PIM-1 may block the Aβ-induced mTOR hyperactivity by different pathways, taken together, the data presented here strongly indicate a primary role for PRAS40 phosphorylation in the Aβ-mediated mTOR hyperactivity.
DISCUSSION
Strong evidence from yeast, Drosophila, and worms consistently shows that reducing mTOR activity extends lifespan (38–42). Recently, these data have been replicated in mammals, with reports that pharmacologically reducing mTOR with rapamycin increases lifespan in mice (37). Further supporting the role of mTOR signaling in aging, deletion of the S6K1 gene, which encodes for the p70S6K protein, increases lifespan and health span in mice (66). Although the effects of increasing mTOR activity and signaling on lifespan remain to be established, evidence shows that hyperactive hippocampal mTOR signaling leads to deficits in long term potentiation and learning and memory (29, 43). Thus, it is tempting to speculate that chronically higher mTOR activity may have detrimental effects on lifespan and health span. Here, we provide compelling evidence that soluble Aβ increases mTOR activity. We first show that mTOR is hyperactive in the brains of the 3xTg-AD mice and that mTOR activity can be brought to baseline levels by reducing or preventing Aβ accumulation, indicating that high Aβ levels are necessary for mTOR hyperactivity in the 3xTg-AD mice. We further show that soluble Aβ oligomers are sufficient to cause mTOR hyperactivity in NonTg mice. Considering that mTOR activity increases as a function of age (67), the presence of high Aβ levels may exacerbate the age-dependent increase in mTOR activity and the associated cognitive decline. Along these lines, high levels of mTOR signaling have been reported in AD brains (25, 27, 50–52). Thus, together these data suggest that one way by which Aβ can exert its toxicity is by increasing mTOR activity.
A series of in vitro studies has shown that Aβ increases PI3K/Akt pathway (20–23), which is one of the pathways that regulate mTOR signaling (11). Here, we extend those data and report the novel finding that naturally secreted soluble Aβ increases mTOR activity and signaling in vivo. We further identify PRAS40 as a molecular link between Aβ accumulation and mTOR hyperactivity. Toward this end, when PRAS40 is phosphorylated by Akt or PIM-1 at Thr-246, it does not bind to mTOR and hence release its inhibitory effects on mTOR activity (14, 15, 65). Our data strongly support the conclusion that PRAS40 phosphorylation plays a key role in the Aβ-induced mTOR hyperactivity. Thus, taken together, these data indicate that Aβ accumulation increases the activity of the PI3K/Akt pathways, leading to PRAS40 phosphorylation and mTOR hyperactivity. This hypothesis is consistent with data showing that insulin signaling, which is altered by Aβ oligomers (61–63), increases mTOR activity by increasing Akt-mediated phosphorylation of PRAS40 (14, 15). Notably, we showed that in 6-month-old 3xTg-AD mice, Aβ increases the levels of phospho-IRS1, which is consistent with previous reports from mice and human brains (61, 68). When IRS1 is phosphorylated at Ser-616, its activity is reduced. Nevertheless, it has previously been shown that AKT activity can occur even in low IRS1 activity. Further studies are necessary to determine whether the increase in phospho-IRS1 levels is directly caused by Aβ or whether it occurs as a consequence of mTOR hyperactivity. Toward this end, a negative feedback loop in which mTOR phosphorylates IRS1 at Ser-616 has been identified (69).
mTOR is a negative regulator of autophagy, a conserved intracellular system designed for the degradation of long lived proteins and organelles in lysosomes (70–72). Although the role of autophagy in AD is not well understood and contradicting reports have been published, it has been suggested that autophagy can facilitate Aβ and Tau turnover (e.g. (43, 73, 74). Thus, we propose that Aβ accumulation increases mTOR signaling, which in turn further facilitates the buildup of Aβ and Tau, thereby exacerbating cognitive decline. The data presented here showing that Aβ accumulation increases mTOR signaling, and our previous results showing that decreasing mTOR signaling in the brains of the 3xTg-AD mice decreases Aβ and Tau levels by increasing autophagy induction strongly support this view.
We have previously shown that Aβ accumulation facilitates Tau pathology and that this can occur via different mechanisms (45, 49, 75–77). Here, we propose that Aβ may also facilitate Tau accumulation by increasing mTOR activity. Indeed, a direct link between Tau and mTOR has been proposed by different laboratories. For example, PI3K/mTOR signaling regulates Tau phosphorylation (24), and mTOR activation enhances Tau-induced neurodegeneration in a Drosophila model of tauopathies (78). Further strengthening the mTOR/Tau link are the data resulting from investigations of AD brains showing that mTOR signaling is selectively increased in neurons predicted to develop neurofibrillary tangles and that such an increase correlates with Tau phosphorylation (25–28). This evidence has led to the hypothesis that the chronic increase in mTOR function occurring during aging may facilitate the development of Tau pathology (27).
Although the role of Aβ in the onset of cognitive decline in AD is widely accepted, the molecular mechanisms underlying the Aβ-induced cognitive deficits remain elusive. Considering the reports showing that mTOR hyperactivity has detrimental effects on learning and memory (29, 43) and the data presented here, it is plausible that one way by which Aβ accumulation causes memory deficits is by increasing mTOR signaling. Toward this end, we have previously shown that pharmacologically reducing mTOR activity with rapamycin in the 3xTg-AD mice to NonTg levels rescued early cognitive deficits and decreased Aβ and Tau levels (43). Notably, these data were independently replicated in another mouse model of AD (44) and indicate that rapamycin may have valid therapeutic properties to ameliorate AD pathology. It should be pointed out, however, that there is growing evidence that rapamycin may also have effects independent of mTOR (79–82). For example, rapamycin suppresses mTOR-dependent translation of some classes of mRNAs but not others (83). Additionally, rapamycin binds to L-type voltage-dependent Ca2+ channels, and it is thought that this binding may mediate some of the neuroprotective properties of rapamycin (84). The data presented here suggest that an alternative way to reduce mTOR signaling is by modulating PRAS40 phosphorylation.
This work was supported, in whole or in part, by National Institutes of Health Grants K99/R00 AG29729-4 (to S. O.) and RC2AG036613 (to S. O., Sub-Principal Investigator) from the NIA.
- AD
- Alzheimer disease
- Aβ
- amyloid-β
- APP
- amyloid precursor protein
- mTOR
- mammalian target of rapamycin
- mTORC
- mTOR complex
- PRAS40
- proline-rich Akt substrate 40
- NonTg
- non-transgenic
- CM
- conditioned medium
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Querfurth H. W., LaFerla F. M. (2010) N. Engl. J. Med. 362, 329–344 [DOI] [PubMed] [Google Scholar]
- 2. Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 4913–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ihara Y., Nukina N., Miura R., Ogawara M. (1986) J. Biochem. 99, 1807–1810 [DOI] [PubMed] [Google Scholar]
- 4. Kosik K. S., Joachim C. L., Selkoe D. J. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 4044–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glenner G. G., Wong C. W. (1984) Biochem. Biophys. Res. Commun. 120, 885–890 [DOI] [PubMed] [Google Scholar]
- 6. Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 4245–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welsh K. A., Butters N., Hughes J. P., Mohs R. C., Heyman A. (1992) Arch. Neurol. 49, 448–452 [DOI] [PubMed] [Google Scholar]
- 8. Glabe C. C. (2005) Subcell. Biochem. 38, 167–177 [DOI] [PubMed] [Google Scholar]
- 9. Klein W. L. (2002) Neurochem. Int. 41, 345–352 [DOI] [PubMed] [Google Scholar]
- 10. Palop J. J., Mucke L. (2010) Nat. Neurosci. 13, 812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wullschleger S., Loewith R., Hall M. N. (2006) Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 12. Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J. L., Bonenfant D., Oppliger W., Jenoe P., Hall M. N. (2002) Mol. Cell 10, 457–468 [DOI] [PubMed] [Google Scholar]
- 13. Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2002) Cell 110, 163–175 [DOI] [PubMed] [Google Scholar]
- 14. Sancak Y., Thoreen C. C., Peterson T. R., Lindquist R. A., Kang S. A., Spooner E., Carr S. A., Sabatini D. M. (2007) Mol. Cell 25, 903–915 [DOI] [PubMed] [Google Scholar]
- 15. Wang L., Harris T. E., Roth R. A., Lawrence J. C., Jr. (2007) J. Biol. Chem. 282, 20036–20044 [DOI] [PubMed] [Google Scholar]
- 16. Das F., Ghosh-Choudhury N., Mahimainathan L., Venkatesan B., Feliers D., Riley D. J., Kasinath B. S., Choudhury G. G. (2008) Cell. Signal. 20, 409–423 [DOI] [PubMed] [Google Scholar]
- 17. Guertin D. A., Sabatini D. M. (2007) Cancer Cell 12, 9–22 [DOI] [PubMed] [Google Scholar]
- 18. Hay N. (2005) Cancer Cell 8, 179–183 [DOI] [PubMed] [Google Scholar]
- 19. Hay N., Sonenberg N. (2004) Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 20. Bhaskar K., Miller M., Chludzinski A., Herrup K., Zagorski M., Lamb B. T. (2009) Mol. Neurodegener. 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ito S., Kimura K., Haneda M., Ishida Y., Sawada M., Isobe K. (2007) Exp. Gerontol. 42, 532–537 [DOI] [PubMed] [Google Scholar]
- 22. Ito S., Sawada M., Haneda M., Ishida Y., Isobe K. (2006) Neurosci. Res. 56, 294–299 [DOI] [PubMed] [Google Scholar]
- 23. Martín D., Salinas M., López-Valdaliso R., Serrano E., Recuero M., Cuadrado A. (2001) J. Neurochem. 78, 1000–1008 [DOI] [PubMed] [Google Scholar]
- 24. Meske V., Albert F., Ohm T. G. (2008) J. Biol. Chem. 283, 100–109 [DOI] [PubMed] [Google Scholar]
- 25. An W. L., Cowburn R. F., Li L., Braak H., Alafuzoff I., Iqbal K., Iqbal I. G., Winblad B., Pei J. J. (2003) Am. J. Pathol. 163, 591–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pei J. J., Björkdahl C., Zhang H., Zhou X., Winblad B. (2008) J. Alzheimers Dis. 14, 385–392 [DOI] [PubMed] [Google Scholar]
- 27. Pei J. J., Hugon J. (2008) J. Cell Mol. Med. 12, 2525–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Griffin R. J., Moloney A., Kelliher M., Johnston J. A., Ravid R., Dockery P., O'Connor R., O'Neill C. (2005) J. Neurochem. 93, 105–117 [DOI] [PubMed] [Google Scholar]
- 29. Ehninger D., Han S., Shilyansky C., Zhou Y., Li W., Kwiatkowski D. J., Ramesh V., Silva A. J. (2008) Nat. Med. 14, 843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Puighermanal E., Marsicano G., Busquets-Garcia A., Lutz B., Maldonado R., Ozaita A. (2009) Nat. Neurosci. 12, 1152–1158 [DOI] [PubMed] [Google Scholar]
- 31. Casadio A., Martin K. C., Giustetto M., Zhu H., Chen M., Bartsch D., Bailey C. H., Kandel E. R. (1999) Cell 99, 221–237 [DOI] [PubMed] [Google Scholar]
- 32. Tischmeyer W., Schicknick H., Kraus M., Seidenbecher C. I., Staak S., Scheich H., Gundelfinger E. D. (2003) Eur. J. Neurosci. 18, 942–950 [DOI] [PubMed] [Google Scholar]
- 33. Bolduc F. V., Bell K., Cox H., Broadie K. S., Tully T. (2008) Nat. Neurosci. 11, 1143–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cao R., Li A., Cho H. Y. (2009) J. Neurosci. 29, 12372–12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Inoki K., Corradetti M. N., Guan K. L. (2005) Nat. Genet. 37, 19–24 [DOI] [PubMed] [Google Scholar]
- 36. Zeng L. H., Rensing N. R., Wong M. (2009) J. Neurosci. 29, 6964–6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M., Flurkey K., Nadon N. L., Wilkinson J. E., Frenkel K., Carter C. S., Pahor M., Javors M. A., Fernandez E., Miller R. A. (2009) Nature 460, 392–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jia K., Chen D., Riddle D. L. (2004) Development 131, 3897–3906 [DOI] [PubMed] [Google Scholar]
- 39. Kaeberlein M., Powers R. W., 3rd, Steffen K. K., Westman E. A., Hu D., Dang N., Kerr E. O., Kirkland K. T., Fields S., Kennedy B. K. (2005) Science 310, 1193–1196 [DOI] [PubMed] [Google Scholar]
- 40. Kapahi P., Zid B. M., Harper T., Koslover D., Sapin V., Benzer S. (2004) Curr. Biol. 14, 885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Powers R. W., 3rd, Kaeberlein M., Caldwell S. D., Kennedy B. K., Fields S. (2006) Genes Dev. 20, 174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vellai T., Takacs-Vellai K., Zhang Y., Kovacs A. L., Orosz L., Müller F. (2003) Nature 426, 620. [DOI] [PubMed] [Google Scholar]
- 43. Caccamo A., Majumder S., Richardson A., Strong R., Oddo S. (2010) J. Biol. Chem. 285, 13107–13120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spilman P., Podlutskaya N., Hart M. J., Debnath J., Gorostiza O., Bredesen D., Richardson A., Strong R., Galvan V. (2010) PloS One 5, e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oddo S., Caccamo A., Tseng B., Cheng D., Vasilevko V., Cribbs D. H., LaFerla F. M. (2008) J. Neurosci. 28, 12163–12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oddo S., Caccamo A., Shepherd J. D., Murphy M. P., Golde T. E., Kayed R., Metherate R., Mattson M. P., Akbari Y., LaFerla F. M. (2003) Neuron 39, 409–421 [DOI] [PubMed] [Google Scholar]
- 47. Caccamo A., Majumder S., Deng J. J., Bai Y., Thornton F. B., Oddo S. (2009) J. Biol. Chem. 284, 27416–27424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oddo S., Caccamo A., Cheng D., Jouleh B., Torp R., LaFerla F. M. (2007) J. Neurochem. 102, 1053–1063 [DOI] [PubMed] [Google Scholar]
- 49. Oddo S., Billings L., Kesslak J. P., Cribbs D. H., LaFerla F. M. (2004) Neuron 43, 321–332 [DOI] [PubMed] [Google Scholar]
- 50. Chang R. C., Wong A. K., Ng H. K., Hugon J. (2002) Neuroreport 13, 2429–2432 [DOI] [PubMed] [Google Scholar]
- 51. Onuki R., Bando Y., Suyama E., Katayama T., Kawasaki H., Baba T., Tohyama M., Taira K. (2004) EMBO J. 23, 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peel A. L., Bredesen D. E. (2003) Neurobiol. Dis. 14, 52–62 [DOI] [PubMed] [Google Scholar]
- 53. Oddo S., Caccamo A., Tran L., Lambert M. P., Glabe C. G., Klein W. L., LaFerla F. M. (2006) J. Biol. Chem. 281, 1599–1604 [DOI] [PubMed] [Google Scholar]
- 54. Koo E. H., Squazzo S. L. (1994) J. Biol. Chem. 269, 17386–17389 [PubMed] [Google Scholar]
- 55. Lee J. H., Yu W. H., Kumar A., Lee S., Mohan P. S., Peterhoff C. M., Wolfe D. M., Martinez-Vicente M., Massey A. C., Sovak G., Uchiyama Y., Westaway D., Cuervo A. M., Nixon R. A. (2010) Cell 141, 1146–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oddo S., Caccamo A., Smith I. F., Green K. N., LaFerla F. M. (2006) Am. J. Pathol. 168, 184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wilcock D. M., DiCarlo G., Henderson D., Jackson J., Clarke K., Ugen K. E., Gordon M. N., Morgan D. (2003) J. Neurosci. 23, 3745–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cleary J. P., Walsh D. M., Hofmeister J. J., Shankar G. M., Kuskowski M. A., Selkoe D. J., Ashe K. H. (2005) Nat. Neurosci. 8, 79–84 [DOI] [PubMed] [Google Scholar]
- 59. Podlisny M. B., Ostaszewski B. L., Squazzo S. L., Koo E. H., Rydell R. E., Teplow D. B., Selkoe D. J. (1995) J. Biol. Chem. 270, 9564–9570 [DOI] [PubMed] [Google Scholar]
- 60. Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]
- 61. Ma Q. L., Yang F., Rosario E. R., Ubeda O. J., Beech W., Gant D. J., Chen P. P., Hudspeth B., Chen C., Zhao Y., Vinters H. V., Frautschy S. A., Cole G. M. (2009) J. Neurosci. 29, 9078–9089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cohen E., Paulsson J. F., Blinder P., Burstyn-Cohen T., Du D., Estepa G., Adame A., Pham H. M., Holzenberger M., Kelly J. W., Masliah E., Dillin A. (2009) Cell 139, 1157–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao W. Q., De Felice F. G., Fernandez S., Chen H., Lambert M. P., Quon M. J., Krafft G. A., Klein W. L. (2008) FASEB J. 22, 246–260 [DOI] [PubMed] [Google Scholar]
- 64. Nascimento E. B., Snel M., Guigas B., van der Zon G. C., Kriek J., Maassen J. A., Jazet I. M., Diamant M., Ouwens D. M. (2010) Cell. Signal. 22, 961–967 [DOI] [PubMed] [Google Scholar]
- 65. Zhang F., Beharry Z. M., Harris T. E., Lilly M. B., Smith C. D., Mahajan S., Kraft A. S. (2009) Cancer Biol. Ther. 8, 846–853 [DOI] [PubMed] [Google Scholar]
- 66. Selman C., Tullet J. M., Wieser D., Irvine E., Lingard S. J., Choudhury A. I., Claret M., Al-Qassab H., Carmignac D., Ramadani F., Woods A., Robinson I. C., Schuster E., Batterham R. L., Kozma S. C., Thomas G., Carling D., Okkenhaug K., Thornton J. M., Partridge L., Gems D., Withers D. J. (2009) Science 326, 140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Martinez-Vicente M., Cuervo A. M. (2007) Lancet Neurol. 6, 352–361 [DOI] [PubMed] [Google Scholar]
- 68. Moloney A. M., Griffin R. J., Timmons S., O'Connor R., Ravid R., O'Neill C. (2010) Neurobiol. Aging 31, 224–243 [DOI] [PubMed] [Google Scholar]
- 69. Easton J. B., Kurmasheva R. T., Houghton P. J. (2006) Cancer Cell 9, 153–155 [DOI] [PubMed] [Google Scholar]
- 70. Cuervo A. M. (2004) Mol. Cell. Biochem. 263, 55–72 [DOI] [PubMed] [Google Scholar]
- 71. Klionsky D. J., Emr S. D. (2000) Science 290, 1717–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jung C. H., Jun C. B., Ro S. H., Kim Y. M., Otto N. M., Cao J., Kundu M., Kim D. H. (2009) Mol. Biol. Cell 20, 1992–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Boland B., Kumar A., Lee S., Platt F. M., Wegiel J., Yu W. H., Nixon R. A. (2008) J. Neurosci. 28, 6926–6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pickford F., Masliah E., Britschgi M., Lucin K., Narasimhan R., Jaeger P. A., Small S., Spencer B., Rockenstein E., Levine B., Wyss-Coray T. (2008) J. Clin. Invest. 118, 2190–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oddo S. (2008) J. Cell. Mol. Med. 12, 363–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Oddo S., Caccamo A., Cheng D., LaFerla F. M. (2009) Brain Pathol. 19, 421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oddo S., Vasilevko V., Caccamo A., Kitazawa M., Cribbs D. H., LaFerla F. M. (2006) J. Biol. Chem. 281, 39413–39423 [DOI] [PubMed] [Google Scholar]
- 78. Khurana V., Lu Y., Steinhilb M. L., Oldham S., Shulman J. M., Feany M. B. (2006) Curr. Biol. 16, 230–241 [DOI] [PubMed] [Google Scholar]
- 79. Malagelada C., Jin Z. H., Jackson-Lewis V., Przedborski S., Greene L. A. (2010) J. Neurosci. 30, 1166–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Choo A. Y., Blenis J. (2009) Cell Cycle 8, 567–572 [DOI] [PubMed] [Google Scholar]
- 81. Thoreen C. C., Kang S. A., Chang J. W., Liu Q., Zhang J., Gao Y., Reichling L. J., Sim T., Sabatini D. M., Gray N. S. (2009) J. Biol. Chem. 284, 8023–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thoreen C. C., Sabatini D. M. (2009) Autophagy 5, 725–726 [DOI] [PubMed] [Google Scholar]
- 83. Choo A. Y., Yoon S. O., Kim S. G., Roux P. P., Blenis J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 17414–17419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ruan B., Pong K., Jow F., Bowlby M., Crozier R. A., Liu D., Liang S., Chen Y., Mercado M. L., Feng X., Bennett F., von Schack D., McDonald L., Zaleska M. M., Wood A., Reinhart P. H., Magolda R. L., Skotnicki J., Pangalos M. N., Koehn F. E., Carter G. T., Abou-Gharbia M., Graziani E. I. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]