Abstract
Recently, mitochondrial aldehyde dehydrogenase (ALDH-2) was reported to reduce ischemic damage in an experimental myocardial infarction model. ALDH-2 activity is redox-sensitive. Therefore, we here compared effects of various electrophiles (organic nitrates, reactive fatty acid metabolites, or oxidants) on the activity of ALDH-2 with special emphasis on organic nitrate-induced inactivation of the enzyme, the biochemical correlate of nitrate tolerance. Recombinant human ALDH-2 was overexpressed in Escherichia coli; activity was determined with an HPLC-based assay, and reactive oxygen and nitrogen species formation was determined by chemiluminescence, fluorescence, protein tyrosine nitration, and diaminonaphthalene nitrosation. The organic nitrate glyceryl trinitrate caused a severe concentration-dependent decrease in enzyme activity, whereas incubation with pentaerythritol tetranitrate had only minor effects. 4-Hydroxynonenal, an oxidized prostaglandin J2, and 9- or 10-nitrooleate caused a significant inhibition of ALDH-2 activity, which was improved in the presence of Mg2+ and Ca2+. Hydrogen peroxide and NO generation caused only minor inhibition of ALDH-2 activity, whereas peroxynitrite generation or bolus additions lead to severe impairment of the enzymatic activity, which was prevented by the thioredoxin/thioredoxin reductase (Trx/TrxR) system. In the presence of glyceryl trinitrate and to a lesser extent pentaerythritol tetranitrate, ALDH-2 may be switched to a peroxynitrite synthase. Electrophiles of different nature potently regulate the enzymatic activity of ALDH-2 and thereby may influence the resistance to ischemic damage in response to myocardial infarction. The Trx/TrxR system may play an important role in this process because it not only prevents inhibition of ALDH-2 but is also inhibited by the ALDH-2 substrate 4-hydroxynonenal.
Keywords: Drug Metabolism, Nitric Oxide, Oxidative Stress, Sulfhydryl, Superoxide Ion, Mitochondrial Aldehyde Dehydrogenase, Nitro Fatty Acids, Organic Nitrates, Peroxynitrite, Thioredoxin
Introduction
Aldehyde dehydrogenases (ALDH)3 contribute to detoxification of toxic aldehydes. The mitochondrial isoform (ALDH-2) is a major sink for acetaldehyde formed from ethanol metabolism, and the East Asian variant (ALDH2*2, E504K) is responsible for alcohol intolerance in a large part of the East Asian population (1). In 2002, Chen et al. (2) identified the ALDH-2 as an organic nitrate reductase, important for the bioactivation of nitroglycerin (glyceryl trinitrate (GTN)), and thereby identified the yet unknown “enzyme receptor” for the vasodilatory action of GTN. The role of ALDH-2 for GTN bioactivation and development of nitrate tolerance was confirmed in animal experimental (3) and human studies (4). A proof at the molecular level was provided using ALDH-2−/− mice showing impaired GTN potency but normal responses to the NO donor sodium nitroprusside and isosorbide dinitrate (5). This finding was extended by demonstrating in ALDH-2−/− mice that pentaerythritol tetranitrate (PETN) and its trinitrate metabolite (PETriN) but not the di- and mononitrate metabolites (PEDN and PEMN) are bioactivated by ALDH-2 (6). Mechanistic studies on ALDH-2 inactivation revealed that reactive oxygen and nitrogen species (RONS) contribute to irreversible inactivation of the enzyme, whereas acute GTN treatment instead caused reversible inactivation (7). In the same study, dihydrolipoic acid was identified as a physiological reducing agent for inactive ALDH-2, representing an essential cofactor for organic nitrate bioactivation, whereas glutathionylation represents another inhibitory pathway. In 2008, Mayer and coworkers (8) demonstrated activation of the soluble guanylyl cyclase by GTN and purified human ALDH-2 and to a minor extent ALDH-1, a cytosolic isoform, and proposed that NO is formed and activates the soluble guanylyl cyclase. The same group demonstrated irreversible inactivation of ALDH-2 by GTN in the presence of NAD+ (9) and revealed RONS formation by purified ALDH-2 and GTN in the presence of NAD+, which was suppressed in the E268Q variant with impaired binding affinity for NAD+ (10).
Besides bioactivation of organic nitrates and detoxification of aldehydes, ALDH-2 may play a role in the prevention of ischemic heart damage. In a recent publication, Chen et al. (11) have shown that ALDH-2 activity reduces ischemic damage in an experimental MI model. These authors showed that pharmacologic (GTN, cyanamide) or genetic (protein kinase Cϵ knock-out) inhibition of ALDH-2 activity results in increased infarct area and impaired cardiac function in response to MI. These data are in good accordance with our previous observations that ALDH-2 is an important antioxidant enzyme and contributes to protection from doxorubicin- or age-induced cardiovascular complications (12, 13).
In the present study, we investigated the regulation of ALDH-2 activity by different electrophiles with special focus on the comparison of GTN and PETN. The role of the Trx/TrxR system for ALDH-2 activity was also assessed. Another aim was to identify the reactive species being formed from purified ALDH-2 in the absence and presence of GTN, suggesting that the nitrate reductase can be switched to a peroxynitrite synthase.
EXPERIMENTAL PROCEDURES
Chemicals
The chemicals used in this study are listed in the supplemental Experimental Procedures.
Overexpression and Purification of ALDH-2
Recombinant His-tagged human ALDH-2 was overexpressed in Escherichia coli strain BL21 (DE3) using the plasmid pET16B-hALDH2_wt containing a His-tagged human ALDH2_ coding region (14). The His-tagged human ALDH2 protein was purified using nickel-nitrilotriacetic acid-agarose from Qiagen (Hilden, Germany) as described by the manufacturer. The yield of the purification procedure was estimated by comparison with a BSA standard on Coomassie Blue-stained SDS-PAGE (supplemental Fig. S1).
Determination of ALDH-2 Activity
All ALDH-2 activity measurements were done at steady state rates. Conversion of 2-hydroxy-3-nitrobenzaldehyde to its benzoic acid product was followed by HPLC-based analysis by its absorbance at 340 nm as described previously (6). The oxidation of 6-methoxy-2-naphthylaldehyde (Monal 62) to the fluorescent naphthoic acid product (9) was traced by HPLC analysis (see supplemental Experimental Procedures for detailed description).
Determination of ALDH-2-derived RONS
Superoxide formation from purified ALDH-2 alone was assessed by the superoxide-specific chemiluminescence dye lucigenin (100 μm) using a chemiluminescence counter Lumat LB9507 (Berthold Technologies, Bad Wildbad, Germany). Peroxynitrite formation in the presence of ALDH-2 and GTN was determined using L-012 (100 μm) enhanced chemiluminescence using a chemiluminescence counter Lumat LB9507. We have previously shown that this luminol analog has a superior affinity for peroxynitrite, which yields the highest light emission (15, 16). Moreover, peroxynitrite-induced nitration of BSA was assessed by dot blot analysis using a specific monoclonal antibody for protein-bound 3-nitrotyrosine (1:1000, Upstate Biotech Millipore) and envisaged by a peroxidase-labeled secondary antibody (goat-anti-mouse-peroxidase-conjugated, 1:5000, Vector Laboratories) and enhanced chemiluminescence detection kit (Thermo Scientific) as described previously (17). Densitometric quantification was performed by using a high-resolution scanner (Biometra/Epson) equipped with densitometry software Gel Pro Analyzer (Media Cybernetics, Bethesda, MD). Finally, the formation of a nitrosating species from ALDH-2 and GTN was measured by N-nitrosation of diaminonaphthalene (DAN), yielding a highly fluorescent triazole using a Twinkle fluorescence plate reader (excitation, 370 nm; emission, 460 nm) as described (18).
Statistics
One-way analysis of variance statistical analysis (with Bonferroni's or Dunn's correction for comparison of multiple means) was performed where indicated. p values < 0.05 were considered significant. Data are mean ± S.E.
RESULTS
Redox Sensitivity of ALDH-2 Activity and Inhibition by Different Electrophiles
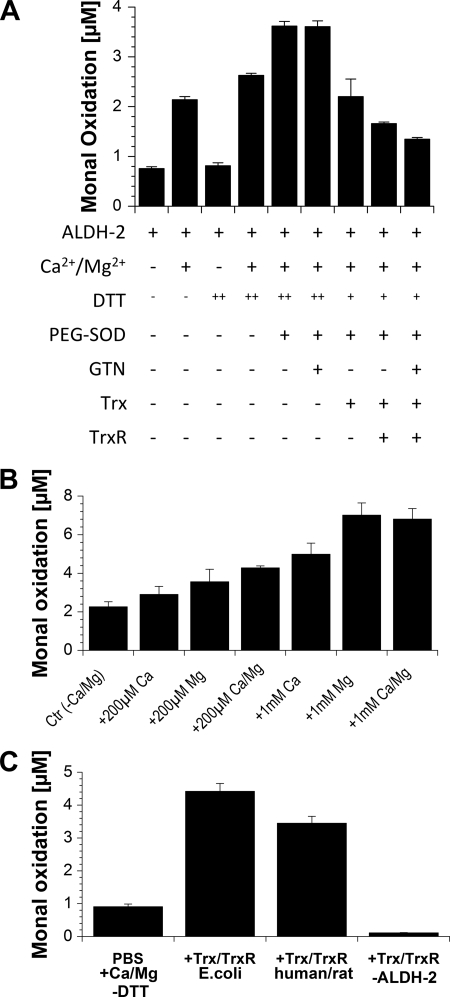
ALDH-2 activity, as measured by the conversion of Monal 62, was 2–3-fold increased in the presence of Mg2+/Ca2+ (Figs. 1 and 2). The addition of DTT had a more pronounced effect on ALDH-2 activity in the presence of Mg2+/Ca2+, and PEG-SOD further increased it under these conditions (Figs. 1 and 2). Surprisingly, PEG-SOD in the absence of DTT completely inhibited ALDH-2 activity (not shown). The Trx/TrxR system (at low concentrations) in the presence of low concentrations of DTT showed no beneficial effect on ALDH-2 activity (Fig. 1A). Additional experiments revealed that Mg2+ and Ca2+ increase the enzymatic activity with Mg2+ being the more potent one and with additive effects at lower concentrations of the ions but not at higher ones (Fig. 1B). Interestingly Zn2+ completely abolished the ALDH-2 activity (supplemental Fig. S2). High (supraphysiological) concentrations of Trx and TrxR could replace the protective effect of DTT without a significant difference between TrxR expressed in E. coli or purified from rat (Fig. 1C). Photometric measurements (based on consumption of NADPH, ΔE340) revealed that both TrxR enzymes tested reduce GSSG with high turnover, but neither cystine nor oxidized lipoic acid showed appreciable affinity for TrxR (not shown). However, the reduction of the latter two disulfides was sharply increased in the presence of Trx (not shown). When activity was measured upon longer incubation times, DTT showed a clear concentration-dependent beneficial effect on ALDH-2 activity, and preincubation of the enzyme in oxygenated buffer without DTT prior to activity measurements decreased the dehydrogenase activity (supplemental Fig. S3).
FIGURE 1.
Effect of conditions and cofactors on ALDH-2 activity. A, ALDH-2 (0.94 μg/ml) was incubated for 20 min with NAD+ (100 μm) and Monal 62 (20 μm) in PBS (Dulbecco's phosphate-buffered saline) at 37 °C. Where indicated, Ca2+/Mg2+ (each 1 mm), DTT (++, 200 μm or +, 10 μm), PEG-SOD (100 units/ml), GTN (1 μm), Trx (8 units/ml, ∼0.23 μm), and/or TrxR (46 milliunits/ml) from E. coli plus NADPH (100 μm) were coincubated. ALDH-2 activity was measured by conversion of naphthyl aldehyde substrate to its naphthoic acid product, which was detected by HPLC using fluorescence detection. B, ALDH-2 (0.94 μg/ml) was incubated for 30 min with NAD+ (200 μm), DTT (400 μm), and Monal 62 (20 μm) in PBS at 37 °C. Ca2+/Mg2+ ions were added as indicated. C, ALDH-2 (0.94 μg/ml) was incubated for 30 min with NAD+ (200 μm), NADPH (100 μm), and Monal 62 (20 μm) in PBS containing Ca2+/Mg2+ (1 mm) at 37 °C. Recombinant Trx (6.8 μm) and TrxR (690 milliunits/ml) from E. coli or human Trx (5 μm) and rat TrxR (500 milliunits/ml) were added as indicated, and as a control, ALDH-2 was omitted in one experiment. Data are mean ± S.E. of three independent experiments.
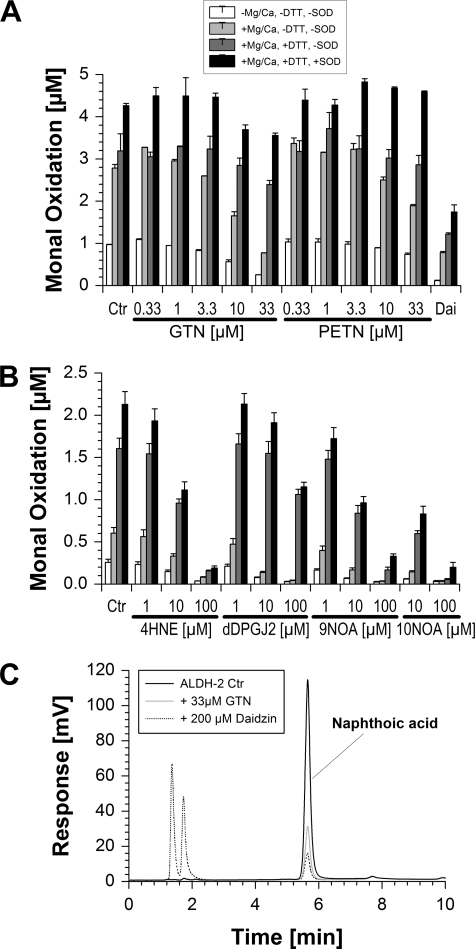
FIGURE 2.
Effects of organic nitrates and other electrophiles on ALDH-2 activity. A, ALDH-2 (0.4 μg/ml) was incubated for 45 min with NAD+ (100 μm) and Monal 62 (20 μm) in PBS (Dulbecco's phosphate-buffered saline) at 37 °C. Where indicated, Ca2+/Mg2+ (each 1 mm), DTT (200 μm), PEG-SOD (100 units/ml), GTN (0.33–33 μm), PETN (0.33–33 μm), and/or daidzin (200 μm) were coincubated. ALDH-2 activity was measured by conversion of naphthyl aldehyde substrate to its naphthoic acid product, which was detected by HPLC using fluorescence detection. Ctr, control. B, conditions as described for panel A, but inactivation of ALDH-2 (0.2 μg/ml) in response to coincubation with 4HNE (1–100 μm), dDPGJ2 (1–100 μm), 9-nitrooleic acid (9NOA, 1–100 μm), and 10-nitrooleic acid (10NOA, 10–100 μm) was studied. C, three representative chromatograms are shown for basal activity of ALDH-2 and upon inhibition by GTN (33 μm) or daidzin (200 μm). The Monal 62 oxidation product (naphthoic acid derivative) showed a retention time of 5.7 min. Data are mean ± S.E. of three independent experiments.
In the absence of Mg2+/Ca2+ and DTT, ALDH-2 activity was quite sensitively inhibited by the organic nitrate GTN (up to 70% inhibition) and to a lower extent by PETN (up to 35% inhibition) (Fig. 2A). As a control, the specific ALDH-2 inhibitor daidzin suppressed enzyme activity almost completely. Other known electrophiles such as 4HNE, dDPGJ2, 9-nitrooleic acid, and 10-nitrooleic acid showed similar effects and caused concentration-dependent inhibition of the enzyme activity (Fig. 2B). Surprisingly the reactive aldehyde MDA showed no inhibition of the ALDH-2 dehydrogenase activity at all, although the employed MDA preparation was highly active when covalent MDA binding to BSA was tested by dot blot analysis using a specific antibody for protein-bound MDA (not shown). Inhibition by GTN was efficiently suppressed in the presence of Mg2+/Ca2+ and DTT (only up to 25% inhibition) and was further improved with the on-top addition of PEG-SOD (only up to 15% inhibition) (Fig. 2A). PETN-triggered inhibition of the enzyme was even completely abolished in the presence of Mg2+/Ca2+, DTT, and PEG-SOD and almost absent upon the addition of Mg2+/Ca2+ and DTT (only up to 13% inhibition) (Fig. 2A). In contrast, the impact of DTT and PEG-SOD on ALDH-2 inhibition by all of the fatty acid metabolites was less pronounced; DTT and PEG-SOD almost failed to prevent inactivation of the enzyme at the highest employed concentration of 4HNE, dDPGJ2, 9-nitrooleic acid, and 10-nitrooleic acid, and less than 20% of ALDH-2 activity was conserved under these conditions (Fig. 2B).
The differential effect of GTN versus PETN on ALDH-2 activity was also observed in another experimental setting where a benzaldehyde substrate (3-hydroxy-3-nitronebzaldehyde) was used as well as longer incubation times. Under these conditions, even more pronounced inhibition of enzymatic activity was found (IC50 ∼0.2 μm GTN and 3 μm PETN) (supplemental Fig. S4). Antioxidants such as PEG-SOD, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO), 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), uric acid, ascorbate, or tris(carboxyethyl)phosphin had no effects on the inhibition of ALDH-2 activity by GTN (supplemental Figs. S5 and S6).
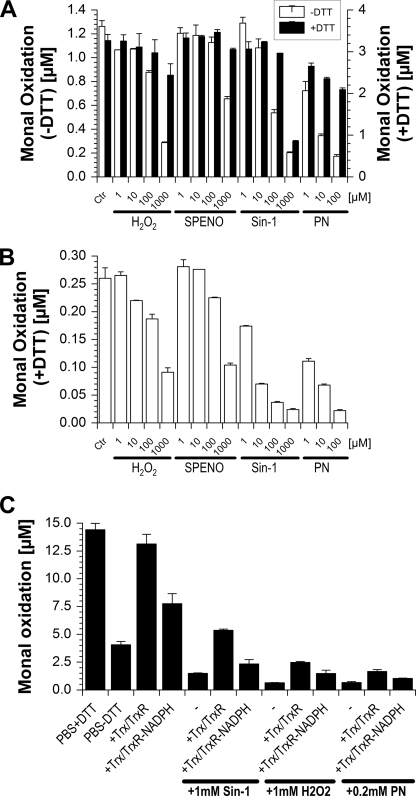
In another set of experiments, inhibitory effects of oxidants on ALDH-2 activity were tested, indicating that authentic peroxynitrite and the peroxynitrite generator Sin-1 were highly potent inhibitors of the enzymatic activity, whereas hydrogen peroxide required high concentrations and nitric oxide even in the millimolar range showed only marginal inhibitory effects (Fig. 3, A and B). DTT could effectively prevent oxidative inactivation of the enzyme activity, probably by reduction of transiently oxidized intermediates at the active site of ALDH-2 such as sulfenic acid (−SOH), whereas oxidation by the highest employed Sin-1 concentration was not reversible, either due to depletion of DTT or due to formation of higher oxidized thiol species at the enzymatic active site such as sulfonic acid (Fig. 3A). Upon long term incubation with these oxidants, reactivation of the enzymatic activity by subsequently added DTT was almost lost (Fig. 3B). The data shown in Fig. 3C again nicely demonstrate that high concentrations of Trx/TrxR may replace DTT, and in the presence of oxidants, Trx/TrxR is important for repair of inactivated ALDH-2 (observed after the addition of peroxynitrite) but also for prevention of oxidative ALDH-2 inhibition (likely observed with Sin-1 or H2O2). It should be noted that oxidants obviously cause an appreciable extent of irreversible ALDH-2 inhibition.
FIGURE 3.
Effects of electrophiles (oxidants) on ALDH-2 activity. A, conditions as described for panel A in Fig. 2, but inactivation of ALDH-2 (0.2 μg/ml) in response to coincubation (for 45 min) with hydrogen peroxide (H2O2, 1–1000 μm), the NO donor (Z)-1-[N-[3-aminopropyl]-N-[4-(3-aminopropylammonio)butyl]-amino]diazen-1-ium-1,2-diolate (SPENO, 1–1000 μm), the peroxynitrite generator 3-morpholinosydnonimine (Sin-1, 1–1000 μm), and authentic peroxynitrite (PN, 1–100 μm) was studied. Each experiment was performed in the presence or absence of DTT (200 μm). Ctr, control. B, conditions as described for panel C, but ALDH-2 was incubated for 90 min with oxidants prior to the addition of the substrate Monal 62 and DTT (200 μm) for 30 min. C, ALDH-2 (0.94 μg/ml) was incubated for 90 min with NAD+ (200 μm), with or without NADPH (400 μm), and with or without DTT (400 μm) and Monal 62 (20 μm) in PBS containing Ca2+/Mg2+ (1 mm) at 37 °C. Recombinant Trx (5 μm) from E. coli and rat TrxR (500 milliunits/ml) were added as indicated. Sin-1 and H2O2 were added after all other compounds, whereas peroxynitrite was rapidly mixed with the ALDH-2 protein before all other compounds were added. Data are mean ± S.E. of three (A and B) or two (C) independent experiments.
ALDH-2-derived Superoxide and Peroxynitrite
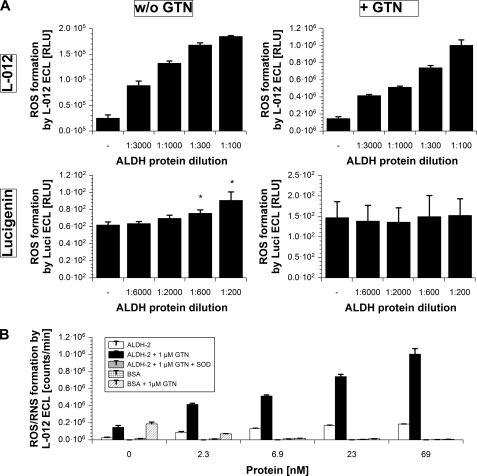
To assess whether RONS contribute to inhibition of ALDH-2 by organic nitrates, formation of RONS was detected by chemiluminescence dyes. L-012 is a luminol analog that detects peroxynitrite with the highest photon recovery followed by superoxide and finally hydrogen peroxide. Lucigenin is highly specific for superoxide but yields fewer photons. Increasing concentrations of the purified ALDH-2 caused increasing chemiluminescence signals with L-012 and lucigenin, indicating the formation of superoxide by the enzyme alone (Fig. 4A). In the presence of GTN, the L-012 chemiluminescence signal was 5–6-fold increased, whereas the one obtained with lucigenin was blunted (Fig. 4A). Because L-012 preferably detects peroxynitrite, which causes no signal with lucigenin, our observations are compatible with peroxynitrite formation from purified ALDH-2 in the presence of GTN. The L-012 data are summarized in Fig. 4B along with the following controls. The L-012 signal in the presence of ALDH-2 and GTN, probably caused by peroxynitrite formation from simultaneous fluxes of superoxide and nitric oxide, was completely abolished by the addition of PEG-SOD. In contrast to ALDH-2, neither BSA nor BSA+GTN caused an appreciable L-012 signal but even suppressed the GTN-L-012 background signal in a concentration-dependent fashion. A similar experiment was performed with a fixed concentration of ALDH-2 and increasing amounts of GTN or PETN. As expected from the inactivation data presented in Fig. 2A, GTN caused a more pronounced increase in L-012 chemiluminescence as an indicator for peroxynitrite formation, whereas PETN triggered only marginal RONS formation (supplemental Fig. S4).
FIGURE 4.
Superoxide and peroxynitrite formation from increasing concentrations of purified ALDH-2 in the presence and absence of GTN. A, ALDH-2 (6.9 μm stock based on the monomers (= 386 μg/ml) diluted 1:100–1:3000) was incubated for 5 min with NAD+ (100 μm) and either lucigenin (Luci, 100 μm) or L-012 (100 μm) in PBS (Dulbecco's phosphate-buffered saline, without DTT, Ca2+/Mg2+) at room temperature. Where indicated, 1 μm GTN was added. RONS formation was detected over 5 min and expressed as counts/min (relative light units (RLU)) at 5 min. B, conditions as described for panel A, but only the L-012 data are shown in the presence or absence of PEG-SOD (1000 units/ml). In some experiments, BSA (0–69 nm) was used instead of ALDH-2. Data are mean ± S.E. of three (L-012) or five (lucigenin) independent experiments. ROS, reactive oxygen species.
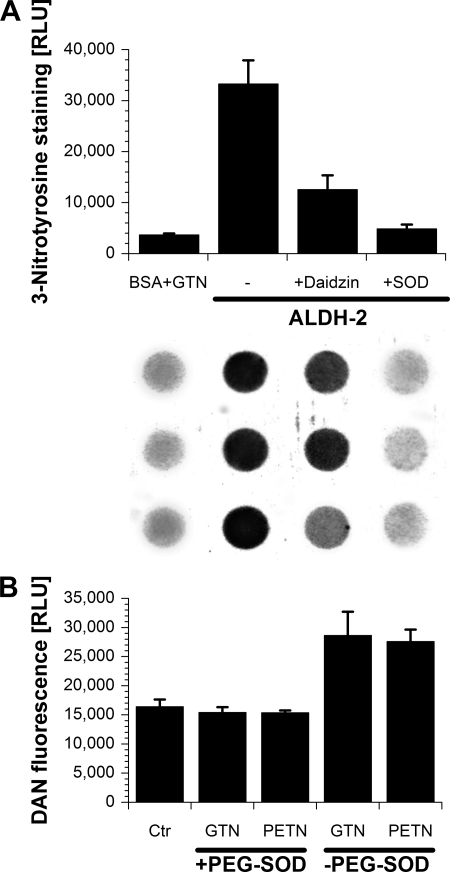
The second method for detection of ALDH-2-derived RONS formation was based on a nitration assay with BSA being the substrate for 3-nitrotyrosine formation. The addition of GTN to BSA alone had only marginal effects on protein tyrosine nitration, whereas the addition of GTN along with ALDH-2 dramatically increased the nitration signal, a footprint of peroxynitrite formation (Fig. 5A). Importantly, the nitration signal was suppressed by the specific ALDH-2 inhibitor daidzin and by removal of superoxide by PEG-SOD. Finally, we assessed the formation of a nitrosating species by ALDH-2 in the presence of GTN, which was able to mediate the N-nitrosation of DAN. The nitrosation was observed for GTN and PETN in the absence of PEG-SOD but was lost in the presence of PEG-SOD (Fig. 5B). Recently, we were able to demonstrate that nitrosation of DAN is most efficiently mediated at a nitric oxide:superoxide ratio of 3:1, yielding N2O3 as a potent nitrosating species (18). It may be speculated that ALDH-2 in the presence of GTN and PETN yields nitric oxide and some superoxide, leading to the formation of N2O3 with subsequent N-nitrosation of DAN. In the presence of PEG-SOD, the superoxide is trapped, and the remaining nitric oxide (at concentrations <10 μm) is a poor nitrosating agent.
FIGURE 5.
Peroxynitrite and nitrosating species formation from purified ALDH-2 measured by 3-nitrotyrosine staining and diaminonaphthalene-derived fluorescence. A, ALDH-2 (10 μg/ml) was incubated for 30 min with NAD+ (100 μm), BSA (1 mg/ml) in PBS (Dulbecco's phosphate-buffered saline, without DTT, Ca2+/Mg2+) at 37 °C in the absence or presence of PEG-SOD (500 units/ml), daidzin (50 μm), or GTN (10 μm). 50 μl of each sample was transferred to a nitrocellulose membrane and assayed for 3-nitrotyrosine-positive proteins. RLU, relative light units. B, ALDH-2 (10 μg/ml) was incubated for 30 min with NAD+ (100 μm) and DAN (25 μm) in PBS (Dulbecco's phosphate-buffered saline, with 200 μm DTT, without Ca2+/Mg2+) at 37 °C in the absence or presence of PEG-SOD (100 units/ml), PETN (33 μm), or GTN (33 μm). Fluorescence of the triazole product was measured. Data are mean ± S.E. of three independent experiments. Ctr, control.
DISCUSSION
With the present study, we demonstrate that ALDH-2 activity is potently regulated by electrophiles. In particular, the organic nitrate GTN, nitro-fatty acids, 4-hydroxynonenal, and peroxynitrite were highly efficient inhibitors of ALDH-2 activity. We also identified a switch of ALDH-2 from a nitric oxide synthase to a peroxynitrite synthase in the presence of GTN. Also, the purified enzyme alone may autoxidize, leading to superoxide formation.
It is well established that ALDH-2 activity relies on cysteines at its active site, one of which participates in the hydride transfer from the substrate aldehydes to the cofactor NAD+, yielding the carboxylic acid product and NADH. We have shown that oxidation of these thiols at the active site results in inhibition of ALDH-2 activity (19). Later, we identified dihydrolipoic acid as a natural cofactor of ALDH-2, providing the electrons for its reductase activity and converting organic nitrates (7) such as GTN and PETN to a vasodilating species (6). We here demonstrate that these thiols are highly susceptible to electrophilic attack of various compounds such as organic nitrates and oxidized fatty acid products but also oxidants. With the present study, we show that nitroglycerin, under certain conditions (which are not necessarily physiological), induces nitric oxide and superoxide formation from ALDH-2 with subsequent peroxynitrite formation. Based on this side reaction, nitroglycerin bioactivation, under certain conditions, may initiate a suicide catalysis, leading to inhibition of the enzymatic activity. Even in the absence of GTN, ALDH-2 may autoxidize, yielding superoxide formation and inhibition of the enzymatic activity. These observations are in good accordance with the majority of published data reporting on induction of oxidative stress and development of nitrate tolerance under chronic GTN therapy but also in response to short term treatment with this organic nitrate (20, 21). In 2004, we published the first systematic study on inactivation of ALDH-2 in isolated mitochondrial preparations by different organic nitrates identifying PETN as a highly potent organic nitrate exhibiting only minor inhibitory effects on ALDH-2 (19). PETN therapy was reported to be devoid of tolerance and oxidative stress (20, 21), which was attributed to the induction of endogenous protective pathways such as the heme oxygenase-1 system (22) as well as other cardioprotective mechanisms (23). However, in the preparations of purified ALDH-2 used here, induction of protective genes or the previously proposed “controlled uptake” and slow pharmacokinetics cannot explain the difference between GTN and PETN with respect to inhibition of ALDH-2 activity and induction of RONS. Based on the structure of PETN, it is not obvious how this compound should display direct antioxidant properties. Therefore, the only plausible possibility remains that PETN binding to ALDH-2 is different from GTN binding, preventing superoxide and peroxynitrite formation with subsequent suicide inhibition of the enzyme.
In previous reports, it was suggested that 4HNE is a reversible and less potent inhibitor of ALDH-2 as compared with the irreversible inhibitors 4-oxonon-2-enal or 4-oxonon-2-enoic acid (24). Here we observed a quite potent inhibition of purified ALDH-2 by 10 μm 4HNE, which is in accordance with previously published data (25). Because the reducing cofactor DTT did not significantly improve this inhibitory effect, we suggest irreversible inhibition of ALDH-2 by 4HNE. We could not detect any inhibitory effect of MDA on ALDH-2 activity, although our MDA preparations showed high MDA modification activity with BSA as detected by dot blot analysis using a specific antibody for MDA-modified proteins in agreement with previous data on low affinity of ALDH-2 for MDA (26) but at variance with data from the same group on complete inhibition of ALDH-2 by MDA concentrations >1 mm (27). To our best knowledge, the present study is the first one elucidating the inhibitory effects of nitro-fatty acids and the prostaglandin derivative dDPGJ2 on ALDH-2 activity. It should be noted that recent data suggest a role for ALDH-2 in preventing ischemia/reperfusion damage by priming the cell against oxidative stress via temporary challenges with carbonyl stress (28).
Oxidants such as free radicals or hydrogen peroxide represent electrophiles that easily react with sulfhydryl groups. The first systematic study on the inactivation of ALDH-2 in isolated mitochondrial preparations by oxidants was published by our group (7). In this study, peroxynitrite was identified as a highly potent inhibitor of ALDH-2 activity. Because we previously showed that GTN in vivo treatment is associated with superoxide and peroxynitrite formation (29, 30), oxidative inhibition of ALDH-2 by these reactive oxygen and nitrogen species could largely contribute to the development of nitrate tolerance and endothelial dysfunction. In addition, here we present data that ALDH-2 itself, under certain conditions, produces superoxide and peroxynitrite, which could result in suicide inhibition of the enzymatic activity. However, even more interestingly, these reactive oxygen and nitrogen species could play a role for the vasodilatory properties of GTN via intermediary formation of nitrosating species. Another new observation is that high Trx/TrxR concentrations are able to prevent inactivation of ALDH-2 and probably also catalyze the repair of oxidatively inactivated enzyme. The prevention of inactivation may be related to the fact that mammalian TrxR is a seleno-enzyme with potent RONS scavenging properties. The Trx/TrxR-conferred repair suggests that reduced Trx may enter the active site of ALDH-2 to reduce the oxidized cysteines. Based on these observations, it may be postulated that Trx/TrxR not only represents a reducing system for DTT or the physiological equivalent lipoic acid, as also previously reported (31), but also directly interacts with oxidized ALDH-2. Finally, we would like to emphasize that 4HNE is not only an inhibitor of ALDH-2 as reported herein, but 4HNE was also reported to inhibit the Trx/TrxR system (32), which opens a second possibility how 4HNE (and maybe other electrophiles) may modulate ALDH-2 activity.
It should be noted that limitations of the study are the high concentrations of Ca2+ and Mg2+ used (1 mm in some of the assays) as well as the pH of 7.4 in all experiments. These conditions do not completely reflect the conditions present in mitochondria because the pH in the matrix is thought to be pH 7.7, thereby limiting the free concentrations of Ca2+ and Mg2+ to the lower micromolar range (due to formation of insoluble phosphate salts) (33). Therefore, most assays were also performed in the absence of these ions. Moreover, considering the fact that a higher pH would shift the equilibrium from protonated sulfhydryl to deprotonated thiolate groups, this would even increase the nucleophilic character of the active site thiols in ALDH-2, making them even more susceptible to attack and inactivation by electrophiles. The purified ALDH-2 used in this study was overexpressed in E. coli, which could cause a lack of proper post-translational modifications (as compared with mammalian cells) and altered responsiveness to the electrophiles used herein. According to our observations, the cellular redox potential (simulated by the presence or absence of DTT and SOD) will largely affect the enzymatic activity of ALDH-2 as well as the reactivity of the used electrophiles. Moreover, cellular metal ion (Ca2+ and Mg2+) composition may contribute to the regulation of enzymatic activity of ALDH-2 (see supplemental Discussion).
Clinical implications are discussed below and in the supplemental Discussion. Based on a retrospective meta-analysis using the databases from two large scale postinfarction studies, Nakamura et al. (34) presented data that long term mono- and dinitrite therapy increases cardiovascular mortality. In light of the data of the present study and the new concepts introduced below, this increased mortality may be secondary to nitrate-mediated inactivation of ALDH-2. In 2008, Chen et al. (11) reported that “activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart” and vice versa that inhibition of ALDH-2 by nitroglycerin or cyanamide treatment increased infarct area in experimental MI. Therefore, inhibition of ALDH-2 by electrophiles may represent a unifying mechanism to explain increased susceptibility of cells to ischemia/reperfusion damage (e.g. in response to MI), and activation of ALDH-2 may provide an attractive concept to improve survival in response to MI (35). Very recent data on ALDH-2-dependent activation of AMP-activated kinase (36) even provide a new attractive hypothesis on an important role for ALDH-2 in controlling metabolic pathways, which could be involved in metabolic disease such as diabetes.
Supplementary Material
Acknowledgments
We thank Jörg Schreiner and Irmgard Ihrig-Biedert for expert technical assistance. We thank Henry Weiner and K. K. Ho from Purdue University (West Lafayette, IN) for providing the ALDH-2 antibody and the plasmid pT7-7-hALDH2_wt.
This work was supported by generous financial support by the Johannes Gutenberg University and University Medical Center Mainz (MAIFOR and Forschungsfonds grants to A. D.). This paper contains results that are part of the doctoral thesis of R. S.; A. D. received honoraries and research support from Actavis Deutschland GmbH, Langenfeld, Germany (manufacturer of PETN).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6 and extended Experimental Procedures and Discussion.
- ALDH-2
- mitochondrial aldehyde dehydrogenase
- DAN
- diaminonaphthalene
- dDPGJ2
- 15-deoxy-Δ12,14-prostaglandin J2
- GTN
- glyceryl trinitrate (nitroglycerin)
- 4HNE
- 4-hydroxynonenal
- L-012
- 8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4-(2H,3H)dione sodium salt
- MDA
- malondialdehyde
- PEG-SOD
- polyethylene glycolated Cu,Zn-superoxide dismutase
- PETN
- pentaerythritol tetranitrate
- RONS
- reactive oxygen and nitrogen species
- Trx
- thioredoxin
- TrxR
- thioredoxin reductase
- MI
- myocardial infarction.
REFERENCES
- 1. Goedde H. W., Agarwal D. P., Fritze G., Meier-Tackmann D., Singh S., Beckmann G., Bhatia K., Chen L. Z., Fang B., Lisker R., et al. (1992) Hum. Genet. 88, 344–346 [DOI] [PubMed] [Google Scholar]
- 2. Chen Z., Zhang J., Stamler J. S. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 8306–8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sydow K., Daiber A., Oelze M., Chen Z., August M., Wendt M., Ullrich V., Mülsch A., Schulz E., Keaney J. F., Jr., Stamler J. S., Münzel T. (2004) J. Clin. Invest. 113, 482–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mackenzie I. S., Maki-Petaja K. M., McEniery C. M., Bao Y. P., Wallace S. M., Cheriyan J., Monteith S., Brown M. J., Wilkinson I. B. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1891–1895 [DOI] [PubMed] [Google Scholar]
- 5. Chen Z., Foster M. W., Zhang J., Mao L., Rockman H. A., Kawamoto T., Kitagawa K., Nakayama K. I., Hess D. T., Stamler J. S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 12159–12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wenzel P., Hink U., Oelze M., Seeling A., Isse T., Bruns K., Steinhoff L., Brandt M., Kleschyov A. L., Schulz E., Lange K., Weiner H., Lehmann J., Lackner K. J., Kawamoto T., Munzel T., Daiber A. (2007) Brit. J. Pharmacol. 150, 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wenzel P., Hink U., Oelze M., Schuppan S., Schaeuble K., Schildknecht S., Ho K. K., Weiner H., Bachschmid M., Münzel T., Daiber A. (2007) J. Biol. Chem. 282, 792–799 [DOI] [PubMed] [Google Scholar]
- 8. Beretta M., Gruber K., Kollau A., Russwurm M., Koesling D., Goessler W., Keung W. M., Schmidt K., Mayer B. (2008) J. Biol. Chem. 283, 17873–17880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beretta M., Sottler A., Schmidt K., Mayer B., Gorren A. C. (2008) J. Biol. Chem. 283, 30735–30744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wenzl M. V., Beretta M., Gorren A. C., Zeller A., Baral P. K., Gruber K., Russwurm M., Koesling D., Schmidt K., Mayer B. (2009) J. Biol. Chem. 284, 19878–19886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen C. H., Budas G. R., Churchill E. N., Disatnik M. H., Hurley T. D., Mochly-Rosen D. (2008) Science 321, 1493–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wenzel P., Müller J., Zurmeyer S., Schuhmacher S., Schulz E., Oelze M., Pautz A., Kawamoto T., Wojnowski L., Kleinert H., Münzel T., Daiber A. (2008) Biochem. Biophys. Res. Commun. 367, 137–143 [DOI] [PubMed] [Google Scholar]
- 13. Wenzel P., Schuhmacher S., Kienhöfer J., Müller J., Hortmann M., Oelze M., Schulz E., Treiber N., Kawamoto T., Scharffetter-Kochanek K., Münzel T., Bürkle A., Bachschmid M. M., Daiber A. (2008) Cardiovasc. Res. 80, 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao Q., Weiner H., Johnston T., Crabb D. W. (1995) J. Clin. Invest. 96, 2180–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daiber A., Oelze M., August M., Wendt M., Sydow K., Wieboldt H., Kleschyov A. L., Munzel T. (2004) Free Radic. Res. 38, 259–269 [DOI] [PubMed] [Google Scholar]
- 16. Daiber A., August M., Baldus S., Wendt M., Oelze M., Sydow K., Kleschyov A. L., Munzel T. (2004) Free Radic. Biol. Med. 36, 101–111 [DOI] [PubMed] [Google Scholar]
- 17. Daiber A., Bachschmid M., Kavaklí C., Frein D., Wendt M., Ullrich V., Munzel T. (2003) Nitric Oxide 9, 44–52 [DOI] [PubMed] [Google Scholar]
- 18. Daiber A., Schildknecht S., Müller J., Kamuf J., Bachschmid M. M., Ullrich V. (2009) Free Radic. Biol. Med. 47, 458–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daiber A., Oelze M., Coldewey M., Bachschmid M., Wenzel P., Sydow K., Wendt M., Kleschyov A. L., Stalleicken D., Ullrich V., Mülsch A., Münzel T. (2004) Mol. Pharmacol. 66, 1372–1382 [DOI] [PubMed] [Google Scholar]
- 20. Münzel T., Daiber A., Mülsch A. (2005) Circ. Res. 97, 618–628 [DOI] [PubMed] [Google Scholar]
- 21. Gori T., Daiber A. (2009) Am. J. Cardiovasc. Drugs 9, 7–15 [DOI] [PubMed] [Google Scholar]
- 22. Wenzel P., Oelze M., Coldewey M., Hortmann M., Seeling A., Hink U., Mollnau H., Stalleicken D., Weiner H., Lehmann J., Li H., Förstermann U., Münzel T., Daiber A. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 1729–1735 [DOI] [PubMed] [Google Scholar]
- 23. Pautz A., Rauschkolb P., Schmidt N., Art J., Oelze M., Wenzel P., Förstermann U., Daiber A., Kleinert H. (2009) Physiol. Genomics 38, 176–185 [DOI] [PubMed] [Google Scholar]
- 24. Doorn J. A., Hurley T. D., Petersen D. R. (2006) Chem. Res. Toxicol. 19, 102–110 [DOI] [PubMed] [Google Scholar]
- 25. Florang V. R., Rees J. N., Brogden N. K., Anderson D. G., Hurley T. D., Doorn J. A. (2007) Neurotoxicology 28, 76–82 [DOI] [PubMed] [Google Scholar]
- 26. Hjelle J. J., Petersen D. R. (1983) Toxicol. Appl. Pharmacol. 70, 57–66 [DOI] [PubMed] [Google Scholar]
- 27. Hjelle J. J., Grubbs J. H., Petersen D. R. (1982) Toxicol. Lett. 14, 35–43 [DOI] [PubMed] [Google Scholar]
- 28. Endo J., Sano M., Katayama T., Hishiki T., Shinmura K., Morizane S., Matsuhashi T., Katsumata Y., Zhang Y., Ito H., Nagahata Y., Marchitti S., Nishimaki K., Wolf A. M., Nakanishi H., Hattori F., Vasiliou V., Adachi T., Ohsawa I., Taguchi R., Hirabayashi Y., Ohta S., Suematsu M., Ogawa S., Fukuda K. (2009) Circ. Res. 105, 1118–1127 [DOI] [PubMed] [Google Scholar]
- 29. Hink U., Oelze M., Kolb P., Bachschmid M., Zou M. H., Daiber A., Mollnau H., August M., Baldus S., Tsilimingas N., Walter U., Ullrich V., Münzel T. (2003) J Am Coll. Cardiol. 42, 1826–1834 [DOI] [PubMed] [Google Scholar]
- 30. Daiber A., Oelze M., Sulyok S., Coldewey M., Schulz E., Treiber N., Hink U., Mülsch A., Scharffetter-Kochanek K., Münzel T. (2005) Mol. Pharmacol. 68, 579–588 [DOI] [PubMed] [Google Scholar]
- 31. Nikitovic D., Holmgren A. (1996) J. Biol. Chem. 271, 19180–19185 [DOI] [PubMed] [Google Scholar]
- 32. Fang J., Holmgren A. (2006) J. Am. Chem. Soc. 128, 1879–1885 [DOI] [PubMed] [Google Scholar]
- 33. Chalmers S., Nicholls D. G. (2003) J. Biol. Chem. 278, 19062–19070 [DOI] [PubMed] [Google Scholar]
- 34. Nakamura Y., Moss A. J., Brown M. W., Kinoshita M., Kawai C. (1999) Am. Heart J. 138, 577–585 [DOI] [PubMed] [Google Scholar]
- 35. Budas G. R., Disatnik M. H., Mochly-Rosen D. (2009) Trends Cardiovasc. Med. 19, 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma H., Guo R., Yu L., Zhang Y., Ren J. (August 12, 2010) Eur. Heart J. 10.1093/eurheartj/ehq253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.