Abstract
Homotypic vacuole fusion occurs by sequential priming, docking and fusion reactions. Priming frees the HOPS complex (Vps 11, 16, 18, 33, 39 and 41) to activate Ypt7p for docking. Here we explore the roles of the GDP and GTP states of Ypt7p using Gdi1p (which extracts Ypt7:GDP), Gyp7p (a GTPase-activating protein for Ypt7p:GTP), GTPγS or GppNHp (non-hydrolyzable nucleotides), and mutant forms of Ypt7p that favor either GTP or GDP states. GDP-bound Ypt7p on isolated vacuoles can be extracted by Gdi1p, although only the GTP-bound state allows docking. Ypt7p is converted to the GTP-bound state after priming and stably associates with HOPS. Gyp7p can cause Ypt7p to hydrolyze bound GTP to GDP, driving HOPS release and accelerating Gdi1p-mediated release of Ypt7p. Ypt7p extraction does not inhibit the Ca2+-triggered cascade that leads to fusion. However, in the absence of Ypt7p, fusion is still sensitive to GTPγS and GppNHp, indicating that there is a second specific GTPase that regulates the calcium flux and hence fusion. Thus, two GTPases sequentially govern vacuole docking and fusion.
Keywords: GTPase/HOPS/vacuole docking/vacuole fusion/Ypt7p
Introduction
Intracellular vesicular traffic employs mechanisms that are highly conserved among all organelles and organisms. Significant progress has been made in identifying proteins that catalyze and regulate trafficking (Jahn and Sudhof, 1999; Guo et al., 2000). At least three classes of proteins—SNAREs, the Ypt/Rab GTPases and tethering factors—act together to specify the docking and fusion of transport vesicles at appropriate sites. Ypt/Rab proteins regulate initial vesicle tethering through association with large oligomeric complexes of tethering factors and Ypt/Rab effectors (Sacher et al., 1998; Guo et al., 1999; McBride et al., 1999; Price et al., 2000). Whether directly or indirectly, Ypt/Rab proteins appear to modulate the interaction of membrane SNARE proteins, which stably dock vesicles (Lian et al., 1994; Ungermann et al., 2000) at their target membrane. The final stage of fusion, which results in the mixing of membrane and aqueous components, is triggered by a flux of Ca2+ (Lupashin et al., 1996; Coorssen et al., 1998; Peters and Mayer, 1998; Brose et al., 2000).
Most of the factors that are required in other vesicular trafficking pathways have functional counterparts in the homotypic fusion of yeast vacuoles. The colorimetric assay of in vitro vacuole fusion (Haas, 1995), combined with advanced yeast genetics, makes this a favorable model for study. Kinetic dissection of vacuole fusion has defined three sequential stages of the reaction: priming, docking and fusion. Priming is driven by the ATPase action of Sec18p (NSF), which releases Sec17p (α-SNAP) from vacuoles and dissociates pre-existing cis-SNARE complexes, a prerequisite for the subsequent formation of trans-SNARE complexes between docked vacuoles (Mayer et al., 1996; Ungermann et al., 1998). Prior to the formation of trans-SNARE pairs, the Ypt/Rab GTPase Ypt7p (Wichmann et al., 1992) associates with effector proteins of the HOPS (homotypic fusion and vacuole protein sorting) complex (Vps 11, 16, 18, 33, 39 and 41) to tether vacuoles reversibly (Price et al., 2000; Seals et al., 2000). All of the HOPS subunits have also been found in an independent screen for vacuole morphology mutants (Vam 1, 9, 8, 5, 6 and 2, respectively) (Wada et al., 1992), and the altered vacuole morphology is readily understood as deriving from a defect in vacuole homotypic fusion. Rab-mediated tethering leads to the formation of trans-SNARE pairs, yielding stably docked vacuoles. Finally, fusion occurs in a reaction that requires Ca2+, calmodulin and protein phosphatase 1 (Peters and Mayer, 1998; Peters et al., 1999).
Eleven yeast Ypt/Rab proteins are known to regulate vesicle–organelle docking at unique intracellular sites (Lazar et al., 1997). Ypt/Rab proteins bind and hydrolyze GTP, and thus cycle between active (GTP-bound) and inactive (GDP-bound) states while membrane bound (Rybin et al., 1996; this study). GTP hydrolysis also renders them extractable by Gdi1p, which is thought to aid in recycling to target membranes (Wilson et al., 1996). Ypt/Rab proteins have a slow intrinsic rate of GTP hydrolysis, which is enhanced by GTPase-activating proteins (GAPs) (Albert et al., 1999). Guanine nucleotide exchange factors (GEFs), localized to specific membranes, exchange GDP for GTP on Ypt/Rab proteins and thereby catalyze their activation (Soldati et al., 1994; Ullrich et al., 1994).
The functional nucleotide state of Ypt7p at each step of vacuole fusion has not been defined. For example, the fusion reaction remains sensitive to Gdi1p as long as it is sensitive to Ypt7p antibodies (Mayer and Wickner, 1997), suggesting that Ypt7p might function in the GDP-bound state. In contrast, the HOPS complex, which is an effector for Ypt7p, will stably bind to recombinant glutathione S-transferase–Ypt7:GTP but not to the GDP-bound or nucleotide-free forms of the recombinant protein (Price et al., 2000; Seals et al., 2000). A component of the HOPS complex, Vps39p, functions as a GEF and in two-hybrid analysis shows a preference for binding to the nucleotide-free or GDP-bound state of Ypt7p (Wurmser et al., 2000).
To address the functional state of Ypt7p in situ we have employed Gdi1p (which can extract the GDP-bound form of Ypt7p), Gyp7p (which activates Ypt7p for GTP hydrolysis), GTPγS and GppNHp (which lock Ypt7p in the GTP-bound state), and mutations in Ypt7p that favor the GTP- or GDP-bound states. Now we report that Ypt7p:GTP is the active form that stably binds the HOPS complex during docking. Ypt7p is no longer needed after docking as it can then be extracted from vacuoles by the combined action of Gyp7p and Gdi1p without the loss of subsequent fusion. Nevertheless, the fusion reaction remains sensitive to GTPγS or GppNHp, indicating that a second GTPase is required for the late fusion stage of the reaction. This GTPase functions in conjunction with the BAPTA-sensitive calcium flux from the vacuole lumen.
Results
GTP-bound Ypt7p is functional
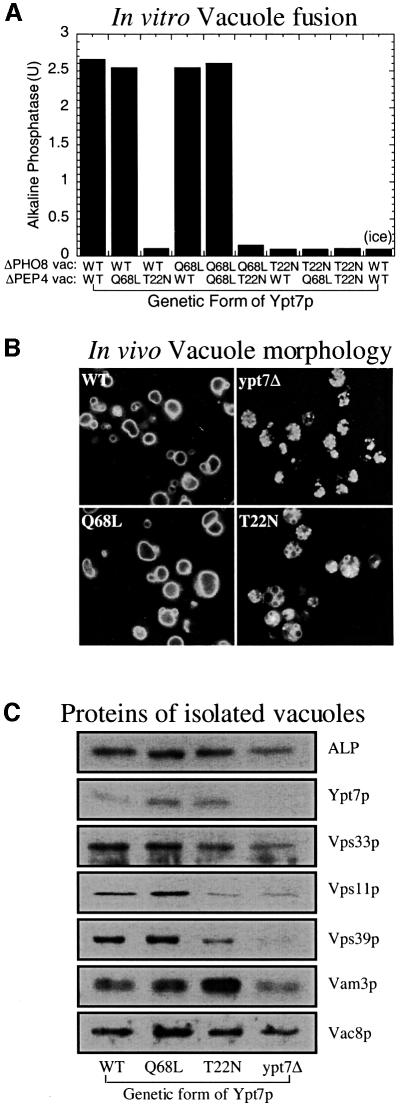
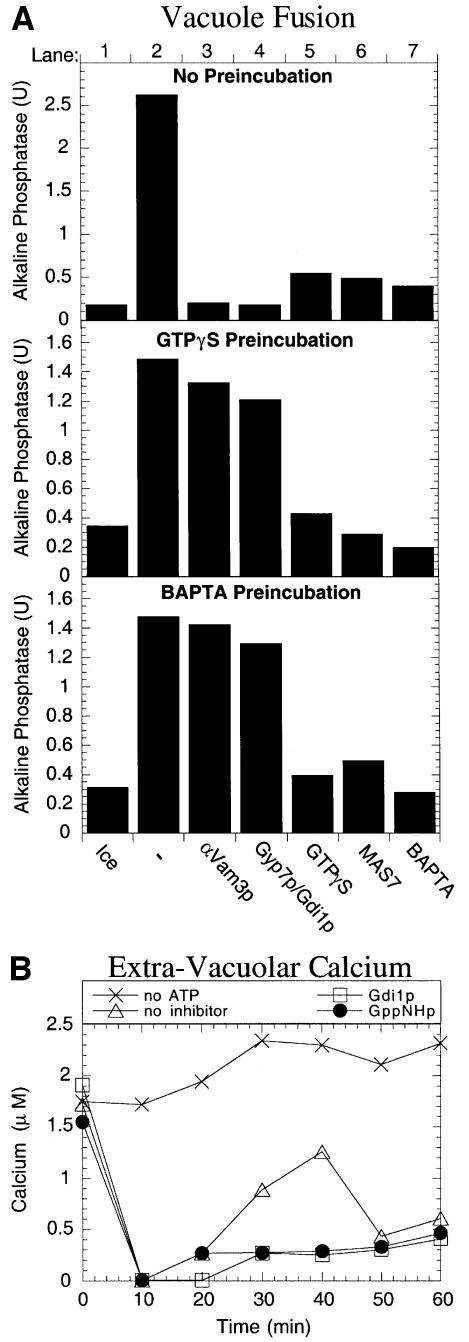
To identify the active form of Ypt7p, homotypic vacuole fusion reactions were performed with vacuoles containing Ypt7p that is wild type or mutant with amino acid substitutions that favor the GTP-bound state [Ypt7(Q68L)p] or GDP-bound state [Ypt7(T22N)p] (Stenmark et al., 1994; Wada et al., 1996). Vacuoles containing Ypt7(Q68L)p fuse as well as wild-type vacuoles (Figure 1A), while vacuoles containing Ypt7(T22N)p cannot support fusion even when only one vacuole partner contains the mutant protein (Figure 1A). Consistent with fusion assays, the strain containing Ypt7(Q68L)p has normal vacuole morphology and Ypt7(T22N)p causes vacuole fragmentation similar to that seen in the null mutant (Figure 1B). These mutations do not destabilize Ypt7p (Figure 1C), but affect its associations, as seen by the modest reduction in levels of HOPS complex subunits Vps39p, Vps11p and Vps33p, and enhanced levels of Vam3p in vacuoles from the T22N mutant (Figure 1C). These studies suggest that not only is Ypt7p required on both vacuole fusion partners (Haas et al., 1995), but that each Ypt7p is only active in its GTP-associated state.
Fig. 1. Only GTP-bound Ypt7p is functional. (A) In vitro fusion of vacuoles containing either wild-type Ypt7p (WT) or mutant forms favoring the GTP- (Q68L) or GDP- (T22N) bound state. Standard fusion reactions (see Materials and methods; 90 min at 27°C) were assayed for alkaline phosphatase. One reaction with wild-type vacuoles was kept on ice for 90 min (ice). (B) In vivo vacuole staining with FM4-64 of BJ3505 strains with wild-type (WT) or GTP-favoring (Q68L) Ypt7p, GDP-favoring Ypt7p (T22N), or the null mutant (ypt7Δ). (C) Protein composition of vacuoles. Vacuolar protein (10 µg) from these strains was analyzed by SDS–PAGE and immunoblotting.
Enhanced Ypt7p GTPase activity blocks vacuole docking
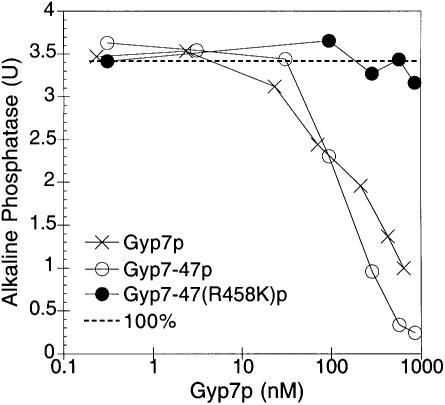
To determine independently the importance of the GTP or GDP nucleotide state of Ypt7p, we stimulated the GTP to GDP conversion in our in vitro fusion assay by adding purified Gyp7p, a GAP of Ypt7p (Vollmer et al., 1999). His6-Gyp7p has been purified from yeast and shown to activate the intrinsic GTPase activity of recombinant Ypt7p by several orders of magnitude (Albert et al., 1999). An N-terminally truncated GAP, Gyp7-47p, contains the catalytic domain and is somewhat more active than the full-length GAP, whereas the mutant Gyp7-47(R458K)p with an amino acid substitution of the conserved catalytic arginine residue is virtually inactive (Albert et al., 1999). As shown in Figure 2, the addition of ∼1 µM Gyp7p or Gyp7-47p inhibits fusion, whereas the addition of the inactive GAP, Gyp7-47(R458K)p, does not affect fusion. Thus, the acceleration of Ypt7p GTP hydrolyzing activity in situ on the intact organelle can effectively block fusion.
Fig. 2. Gyp7p blocks vacuole fusion. Fusion reactions containing the indicated concentrations of Gyp7p, Gyp7-47p or Gyp7-47(R458K)p were incubated for 90 min at 27°C and assayed for alkaline phosphatase activity.
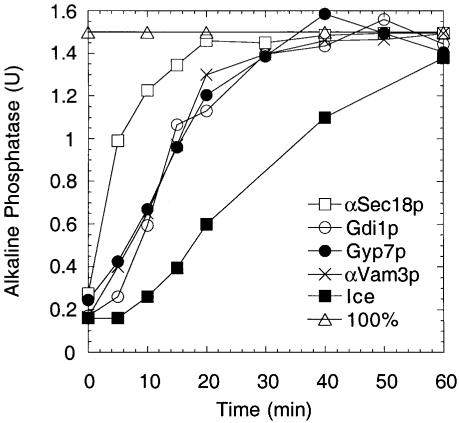
To further establish the stage inhibited by Gyp7p, we added it at various times to vacuole fusion reactions. The fusion reaction becomes resistant to Gyp7p addition with the same kinetics as seen for the docking inhibitors Gdi1p and anti-Vam3p antibodies, suggesting that Gyp7p blocks until docking is complete (Figure 3) and hence that Ypt7p is needed in its GTP-bound form to catalyze docking.
Fig. 3. Fusion reactions become resistant to Gyp7p at docking. Fusion reactions (270 µl) were incubated at 27°C. At the indicated times, aliquots (30 µl) were removed and added to tubes on ice or containing buffer, anti-Sec18p antibodies, anti-Vam3p antibodies, Gdi1p or Gyp7p. Reactions were incubated for 90 min (total) and then assayed for alkaline phosphatase.
GTPγ S locks Ypt7p in an active state that is no longer GAP sensitive
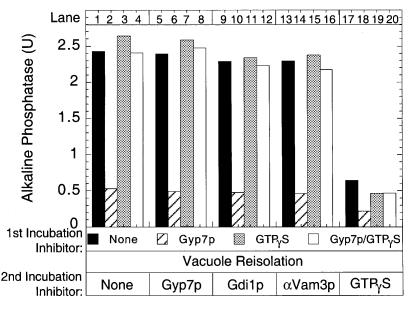
By employing the non-hydrolyzable GTPγS, the inhibitory effect of Gyp7p can unambiguously be assigned to its role in promoting GTP hydrolysis. Fusion reactions containing GTPγS, Gyp7p or both were incubated for 30 min, which is normally enough time to allow the completion of docking. Unbound inhibitors were removed by vacuole re-isolation and the vacuoles were resuspended in reaction buffer with no inhibitors or with either Gyp7p, Gdi1p, anti-Vam3p antibodies or GTPγS (Figure 4). Reactions pre-incubated with Gyp7p do not recover fusion activity (Figure 4, compare black and hatched bars), which suggests that Gyp7p blocks docking and that its action cannot be reversed by simply re-isolating the vacuoles. However, reactions pre-incubated with GTPγS (lane 3) or both GTPγS and Gyp7p (lane 4) completely recover activity. This activity is not diminished by the presence of docking stage inhibitors during the second incubation (lanes 7 and 8, 11 and 12, 15 and 16), and thus GTPγS can bypass the inhibition of docking by Gyp7p. GTPγS has also been shown to be a potent inhibitor of the fusion stage of the reaction (Mayer et al., 1996) and its addition to these docked vacuoles still blocks the completion of fusion (lanes 17–20). The inhibitory effect of Gyp7p is thus specific for its promotion of GTP hydrolysis during the docking reaction.
Fig. 4. Gyp7p inhibition is mediated via GTP hydrolysis. Fusion reactions (150 µl) were incubated at 27°C with Gyp7p, GTPγS, or both Gyp7p and GTPγS. After 30 min, vacuoles were re-isolated and aliquots added to reaction buffer with no inhibitor or with Gyp7p, Gdi1p, anti-Vam3p antibodies or GTPγS, and incubated for 60 min at 27°C before being assayed for alkaline phosphatase.
Gyp7p stimulates HOPS release without Ypt7p release
The HOPS complex binds to Ypt7p to initiate docking (Price et al., 2000; Seals et al., 2000). Removal of Ypt7p by Gdi1p treatment results in a priming-dependent release of the HOPS complex. To address whether the nucleotide-bound state of Ypt7p is critical for continued HOPS association, vacuoles were primed with ATP and treated with Gyp7-47p, the catalytic domain of a GAP that is highly active on Ypt7p (Albert et al., 1999). Primed vacuoles do not normally release a significant portion of Vps39p (Figure 5A) or other components of the HOPS complex (data not shown). Treatment with Gdi1p efficiently causes the release of Vps39p, Ypt7p (Figure 5A) and other known HOPS complex components (data not shown). However, Gyp7-47p treatment causes the release of Vps39p (Figure 5A) and other HOPS components (data not shown) without Ypt7p extraction. The inactive GAP Gyp7-47(R458K)p does not cause HOPS release (Figure 5A), showing that release is specifically due to GAP activity. Since HOPS is released and docking is blocked by either removing Ypt7p (Gdi1p extraction) or by accelerating the Ypt7p GTPase activity and accumulating its GDP-bound form, HOPS is only stably associated with GTP-bound Ypt7p.
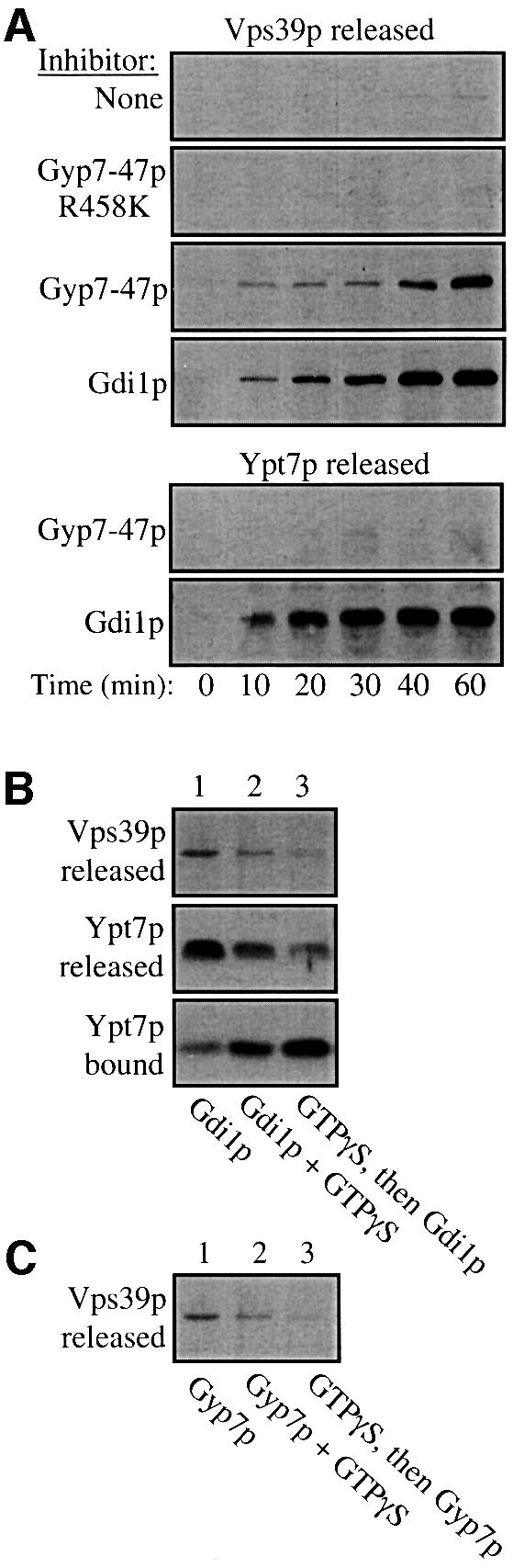
Fig. 5. Gyp7p induces HOPS release by promoting GTP hydrolysis. (A) Release of HOPS and Ypt7p. Fusion reactions (180 µl) containing 36 µg of only BJ3505 vacuoles were incubated in the presence of reaction buffer with no inhibitor or with Gyp7-47(R458K)p, Gyp7-47p or Gdi1p at 27°C. At the indicated times, 30 µl were removed from each reaction and the vacuoles were collected by centrifugation (16 000 g for 5 min at 4°C). Supernatants (15 µl) were analyzed for the release of Vsp39p (top panel) and Ypt7p (bottom panel) by immunoblotting. (B) GTPγS reduces the Gdi1p-stimulated release of Ypt7p and HOPS. Fusion reactions containing only BJ3505 vacuoles were incubated with Gdi1p (lane 1), Gdi1p and GTPγS (lane 2), or GTPγS (lane 3) for 30 min at 27°C. Reactions containing Gdi1p or Gdi1p and GTPγS (lanes 1 and 2) were placed on ice while the reaction containing GTPγS only (lane 3) was given Gdi1p and incubated for an additional 30 min. The samples were centrifuged at 16 000g for 5 min and 15 µl of the supernatant were analyzed by immunoblotting for Vps39p release (top panel) and Ypt7p release (middle panel). An equal portion of the vacuolar pellet was analyzed for Ypt7p (bottom panel). (C) GTPγS reduces the Gyp7p-stimulated release of HOPS. The same experiment as described in (B) was performed, except that Gyp7p was substituted for Gdi1p. Ypt7p is not released by Gyp7p and therefore was not analyzed.
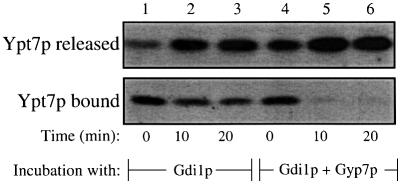
To determine the specificity of Gdi1p action, vacuoles were incubated in the presence of excess GTPγS. GTPγS may exchange into the Ypt7p nucleotide-binding site and thereby block the action of either Gdi1p or Gyp7p. This should allow HOPS to stably associate with Ypt7p and be resistant to release. Whereas Gdi1p induces the release of Ypt7p, and hence Vps39p, from vacuoles (Figure 5B, lane 1), GTPγS inhibits these release reactions (lane 2), and pre-incubation with GTPγS prior to Gdi1p addition (lane 3) blocks release more thoroughly. These data clearly show that the release of Ypt7 (and hence of HOPS) by Gdi1p is restricted to its GDP form. Similarly, pre-incubation with GTPγS prior to Gyp7p addition almost completely blocks Vps39p release (Figure 5C). These results demonstrate that HOPS associates stably with the GTP-bound form of vacuolar Ypt7p and is resistant to release by Gdi1p or Gyp7p when this Rab is in its active, GTP-bound state.
Gyp7p accelerates Gdi1p-induced Ypt7p release
Gdi1p can extract some Ypt7p from vacuoles at 0°C (Figure 6, lane 1), and further extraction occurs at 27°C (lanes 2 and 3). Although Gyp7p alone does not extract Ypt7p (Figure 5A), it dramatically enhances the extraction by Gdi1p (Figure 6, lanes 4–6 as compared with lanes 1–3). The simplest explanation of these findings is that the Ypt7p that is resistant to extraction by Gdi1p alone is associated with GTP, and that Gyp7p promotes GTP hydrolysis and thereby renders the resulting Ypt7p:GDP susceptible to Gdi1p extraction.
Fig. 6. Gyp7p enhances Gdi1p-mediated Ypt7p release. Fusion reactions (90 µl) containing 18 µg of BJ3505 vacuoles only were incubated with Gdi1p or with Gdi1p and Gyp7p at 27°C. At the indicated times, 30 µl aliquots were removed, centrifuged at 16 000 g for 5 min, and equal portions of the supernatant and vacuole pellet fractions were analyzed by immunoblotting for Ypt7p.
A second GTPase for vacuole fusion
Although GTPγS inhibits the late-stage (fusion) reaction, it was possible that this reflected a requirement for GTP hydrolysis by Ypt7p for the fusion reaction to proceed. To address this question directly, we exploited the fact that a mixture of Gyp7p and Gdi1p will efficiently extract Ypt7p (Figure 6). Vacuoles were incubated for 25 min with the calcium chelator BAPTA to reversibly arrest the reaction at the docking stage, and a portion was mixed with Gdi1p + Gyp7p. The extraction of Ypt7p by Gdi1p + Gyp7p is as efficient in BAPTA-arrested vacuoles (Figure 7A, lanes 3 and 4) as for vacuoles that were not exposed to the chelator (lanes 1 and 2). The two populations of vacuoles incubated for 30 min with BAPTA, one bearing normal Ypt7p (Figure 7B, top panel) and the other without Ypt7p due to extraction by Gyp7p + Gdi1p during the last 5 min of this incubation (Figure 7B, bottom panel), were re-isolated to remove the BAPTA block and allowed to fuse. Fusion in this second incubation did not need Ypt7p (Figure 7B, top versus bottom panel, lane 2) and was insensitive to all docking inhibitors (lanes 3–7), but remained fully sensitive to GppNHp (lane 8) and GTPγS (lane 9) even when Ypt7p had been removed by the prior Gyp7p + Gdi1p-mediated extraction. Vacuole fusion exhibits comparable sensitivities to GTPγS and GppNHp, two non-hydrolyzable GTP analogs, but is not affected by the addition of hydrolyzable GTP (Figure 7C). These data suggest that GTP hydrolysis by GTPase(s) other than Ypt7p is needed after the docking stage of the reaction.
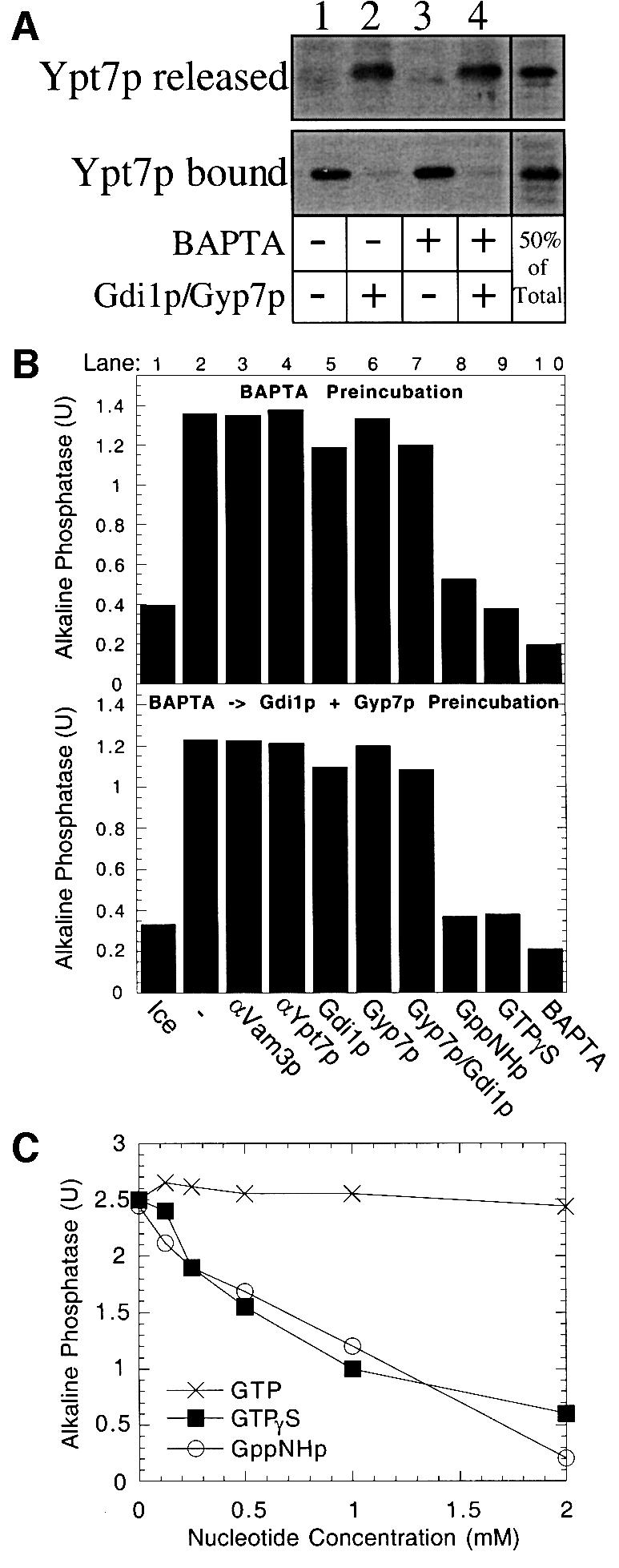
Fig. 7. Docked vacuoles no longer require Ypt7p yet remain sensitive to GTPγS. (A) Ypt7p release. Fusion reactions (60 µl) containing only BJ3505 (12 µg) vacuoles were incubated at 27°C in the presence or absence of 5 mM BAPTA. After 25 min, 30 µl aliquots were added to tubes containing reaction buffer or Gdi1p and Gyp7p, and incubated for an additional 5 min. Samples were centrifuged at 16 000 g for 5 min and the supernatant (Ypt7p released) and pellet (Ypt7p bound) fractions were analyzed by immunoblotting. (B) Fusion reactions (270 µl) were incubated at 27°C for 25 min in the presence of 2.5 mM BAPTA to allow docking without fusion. Reactions then received reaction buffer (top panel) or Gdi1p and Gyp7p (bottom panel) and were incubated at 27°C for an additional 5 min. Vacuoles were re-isolated and aliquots added to tubes containing reaction buffer on ice or reaction buffer with either no inhibitor, anti-Vam3p antibodies, anti-Ypt7p antibodies, GTPγS, BAPTA, Gdi1p, Gyp7p, or both Gdi1p and Gyp7p. Reactions were supplemented with LMA1 and calmodulin (5 µg/ml and150 µg/ml, respectively) and incubated (27°C, 90 min) before assaying for alkaline phosphatase activity. (C) Non-hydrolyzable GTP analogs inhibit vacuole fusion. Two-fold serial dilutions of Mg-GTP,Mg-GTPγS or Mg-GppNHp were added to fusion reactions. Alkaline phosphatase activity was measured after 90 min at 27°C.
The late-stage GTPase and calcium requirements are functionally linked
Although inhibition by the calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) is reversible, the subsequent fusion is still sensitive to GTPγS (Figure 7B, lane 9). To examine more closely whether GTPγS and BAPTA inhibit distinct stages of the reaction, vacuoles were pre-incubated with either GTPγS or BAPTA for 45 min. Whereas the overall reaction is blocked by BAPTA or GTPγS, by MAS7, a peptide inhibitor of heterotrimeric G-proteins (Haas et al., 1994), or by the docking inhibitors Gdi1p + Gyp7p or anti-Vam3p antibodies (Figure 8A, lanes 3–7, top panel), GTPγS and BAPTA are reversible inhibitors (lanes 1 and 2, middle and bottom panels). Vacuoles had completed docking during pre-incubation with GTPγS or BAPTA, as they had acquired resistance to anti-Vam3p antibodies or to Gdi1p + Gyp7p (lanes 3 and 4, middle and bottom panels). However, fusion remained sensitive to BAPTA in vacuoles that had been blocked by GTPγS (lane 7, middle panel); likewise, fusion remained sensitive to GTPγS in vacuoles that had been blocked by BAPTA (lane 5, bottom panel). Fusion also remained senstive to MAS7 after either treatment (lane 6, middle and bottom panels). During the course of a normal fusion reaction, a flux of Ca2+ is transiently released from the vacuole lumen in a docking-dependent manner (Peters and Mayer, 1998). This calcium flux is necessary for membrane fusion. Addition of the non-hydrolyzable nucleotide GppNHp blocks the release of Ca2+ during vacuole fusion reactions as effectively as the docking-stage inhibitor Gdi1p (Figure 8B). Taken together, these results show that the requirement for Ca2+ and for the second GTPase are inseparable during the final stages of vacuole fusion, and suggest that this GTPase may regulate the release of Ca2+ from the vacuole lumen.
Fig. 8. The molecular requirements for the late-stage GTPase activity and Ca2+ are functionally linked. (A) Reactions blocked for fusion by either the calcium chelator BAPTA or GTPγS are still sensitive to the other inhibitor. Fusion reactions (120 µl) were incubated at 27°C for 45 min in the presence of 2 mM Mg-GTPγS (middle panel) or 2.5 mM BAPTA (bottom panel) to allow docking without fusion. One set of reactions was not pre-treated (top panel). Vacuoles were re-isolated and aliquots added to tubes containing reaction buffer on ice or reaction buffer with either no inhibitor, anti-Vam3p antibodies, Gdi1p + Gyp7p, 2 mM GTPγS, 50 µM MAS7 or 2.5 mM BAPTA. Reactions were supplemented with LMA1 and calmodulin (5 µg/ml and 150 µg/ml, respectively) and incubated (27°C, 90 min) before assaying for alkaline phosphatase activity. (B) Non-hydrolyzable GTP analogs and G-protein ligands inhibit the release of Ca2+ during vacuole fusion reactions. Fusion reactions (700 µl) were incubated at 27°C in the presence of Gdi1p, 2 mM Mg-GppNHp or no inhibitor. A control reaction did not contain an energy source. At the indicated times, 100 µl samples were removed, centrifuged at 9 000 g for 5 min, and 90 µl of the supernatant were assayed for Ca2+ (see Material and methods).
Discussion
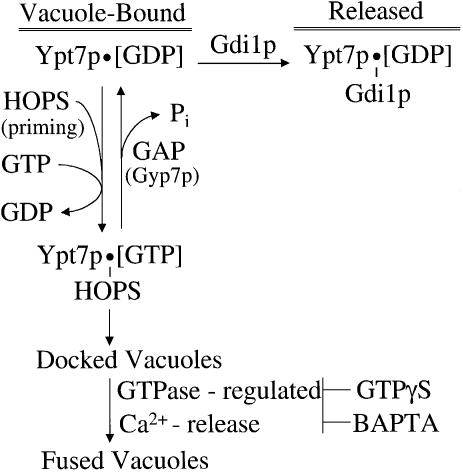
These studies suggest a working model for the regulation of vacuole fusion by the sequential action of two GTPases (Figure 9). Ypt7p is largely in its GDP form on isolated vacuoles, as it is extractable by Gdi1p (Price et al., 2000; Ungermann et al., 2000; Figures 5 and 6). Priming releases the HOPS complex from SNARE associations (Price et al., 2000; Seals et al., 2000) and activates it for binding to Ypt7p, first to catalyze the exchange of bound GDP for GTP and then to stably associate with Ypt7p:GTP. This exchange of GDP for GTP is crucial for stable HOPS association with Ypt7p on the intact vacuole, as the addition of excess Gyp7p promotes both HOPS release (Figure 5A) and Gdi1p-induced Ypt7p release (Figure 6), unless the Ypt7p is locked in its triphosphate form by GTPγS. The HOPS–Ypt7p:GTP, associated with Vam7p (Ungermann et al., 2000), promotes a reversible tethering reaction between vacuoles (Ungermann et al., 1998), which is a prerequisite for trans-SNARE pairing. Docking triggers a GTPase-regulated calcium flux from the vacuole, activating a complex of calmodulin and protein phosphatase 1 to regulate downstream steps that lead to fusion (Peters and Mayer, 1998; Peters et al., 1999).
Fig. 9. A working model for the regulation of vacuole docking and fusion by the sequential chain of two GTPases. See Discussion.
A second, unidentified GTPase appears to be required for the fusion stage of the reaction. The fusion reaction becomes resistant to Ypt7p antibodies or Gdi1p during docking, whereas GTPγS is a late-stage inhibitor (Mayer et al., 1996). Although this kinetic disparity might have suggested that vacuole fusion requires a second GTPase, it could also have reflected a need for Ypt7p to hydrolyze its bound GTP to permit fusion. Furthermore, Ypt7p normally remains bound to the vacuole throughout the reaction, would not be expected to be Gdi1p sensitive in its GTP-bound state during docking, and might have adopted a conformation that is resistant to antibody. However, we now show (Figure 7) that Ypt7p can be extracted from docked vacuoles by the combined action of Gyp7p and Gdi1p without impairing the subsequent fusion reaction. Nevertheless, the fusion of docked vacuoles after the removal of Ypt7p remains fully sensitive to GTPγS and GppNHp. While vesicle budding and the fusion of these vesicles at target organelles can require separate GTPases (Barlowe, 1997), there is little precedent for docking and fusion being regulated by separate GTPases. Endosome fusion in a mammalian cell-free system may involve Arf GTPases in addition to Rab 5 (Lenhard et al., 1992; Rybin et al., 1996). We have also shown that the requirements for Ca2+ and a GTPase during the final stages of vacuole fusion cannot be separated (Figure 8A). Normally, Ca2+ is transiently released from the vacuole to trigger membrane fusion. This release can be inhibited by GppNHp (Figure 8B), suggesting a role for a GTPase in regulating calcium release. The molecular identification of the GTPase(s) required for the late step of vacuole fusion will be essential for further functional studies. The yeast genome contains a limited number of GTPases; available antibodies and yeast strains with defined deletions may facilitate this search.
Several GAPs of yeast are known, and their substrate specificities on recombinant Ypt proteins show substantial overlap (Strom et al., 1993; Du et al., 1998; Albert and Gallwitz, 1999, 2000; Albert et al., 1999). This redundancy presumably accounts for the absence of a phenotype when their genes are deleted. An actual effect on the function of the organelle-bound Ypt/Rab protein has not yet been shown. Our current studies establish that Gyp7p (full-length or catalytic domain) can act on Ypt7p in the intact vacuole and thereby regulate vacuole homotypic fusion. Multiple rounds of fusion, which are not measured by our assay, may require GTP hydrolysis by Ypt7p, releasing HOPS to join in the formation of a new 65S complex (Price et al., 2000; Seals et al., 2000). We suggest that Gyp7p may normally participate in activating Ypt7p for GTP hydrolysis as part of this ‘recycling’ pathway.
Ypt7p has a central role in docking and can only fulfill that role after priming releases HOPS and Vam7p, allowing them to associate with Ypt7p and catalyze GDP:GTP exchange. Further studies are needed to establish which other proteins, if any, are in complex with Ypt7:GTP, HOPS and Vam7p, and whether copies of this complex on apposing vacuoles bind directly to each other to mediate tethering or whether they support tethering in a more indirect manner. Ypt7p-mediated tethering leads to trans-pairing of SNAREs, but again it is unclear whether additional proteins and subreactions mediate this connection in the pathway. Once docking is completed, Ypt7p has fulfilled its function and can be extracted by the successive action of Gyp7p and Gdi1p without impairing the subsequent fusion step (Figure 7).
Materials and methods
Strains and media
Yeast strains used in this study are given in Table I. For vacuole isolation, yeast strains were grown in YPD media (20 g/l of dextrose, 20 g/l of peptone, 10 g/l of yeast extract) to an OD600 of ∼1. For the production of GAP proteins, BJ2168 was transformed with the plasmids pYES2T-GYP7-His6, pYES2T-GYP7-47-His6 and pYES2T-GYP7-47(R458K)-His6 (Albert et al., 1999). Transformants were grown to an OD600 of ∼5 in SD-ura medium (Bio101) containing 2% galactose.
Table I. Yeast strains used in this study.
| Strain | Genotype |
|---|---|
| BJ3505 | Mata, lys2-208, trp1-Δ101, ura3-52, his3-Δ200, gal2, can, prb1-Δ1.6R, pep4::HIS3 |
| DKY6281 | Mata, lys2-801, trp1-Δ901, ura3-52, his3-Δ200, leu2-3,112, suc2-Δ9, pho8::TRP1 |
| BJ3505(Q68L) | Mata, lys2-208, trp1-Δ101, ura3-52, his3-Δ200, gal2, can, prb1-Δ1.6R, pep4::HIS3, ypt7::YPT7(Q68L) |
| DKY6281(Q68L) | Mata, lys2-801, trp1-Δ901, ura3-52, his3-Δ200, leu2-3,112, suc2-D9, pho8::TRP1, ypt7::YPT7(Q68L) |
| BJ3505(T22N) | Mata, lys2-208, trp1-Δ101, ura3-52, his3-Δ200, gal2, can, prb1-Δ1.6R, pep4::HIS3, ypt7::URA3, ypt7::YPT7(T22N) |
| DKY6281(T22N) | Mata, lys2-801, trp1-Δ901, ura3-52, his3-Δ200, leu2-3,112, suc2-D9, pho8::TRP1, ypt7::YPT7(T22N) |
| BJ3505-7 | Mata, lys2-208, trp1-Δ101, ura3-52, his3-Δ200, gal2, can, prb1-Δ1.6R, pep4::HIS3, ypt7::URA3 |
| BJ2168 | Mata, leu2-3,112, trp1-Δ101, ura3-52, prb1-1122, pep4-3, pcr1-407 |
Protein and antibody purification
Gdi1p, His6-Sec18p, LMA1 and calmodulin were purified from Escherichia coli as previously described (Garrett et al., 1994; Haas and Wickner, 1996; Xu et al., 1997; Peters and Mayer, 1998). The C-terminal His6-tagged GAP proteins Gyp7p, Gyp7-47p and Gyp7-47(R458K)p were purified from yeast BJ2168 harboring galactose-inducible overexpression plasmids (Albert et al., 1999). Analysis of GAP proteins by SDS–PAGE and densitometry showed that >85% purity was achieved for Gyp7-47p and Gyp7-47(R458K)p, and >70% purity for full-length Gyp7p after Ni2+-NTA (Qiagen) and MonoQ ion exchange (Pharmacia) chromatography. Antibodies against Sec18p, Ypt7p and Vam3p were previously described (Ungermann et al., 1998).
Biochemical reagents
All biochemical reagents were dissolved or dialyzed in PS buffer (20 mM PIPES–KOH pH 6.8, 200 mM sorbitol). Protein concentrations were determined by Bradford assay (Bradford, 1976). Inhibitors were added at the following final concentrations: affinity-purified Sec18p antibodies (50 µg/ml); affinity-purified Ypt7p antibodies (50 µg/ml); anti-Vam3p IgG (250 µg/ml); Gdi1p (60 µg/ml); Gyp7p (30 µg/ml); Gyp7-47p (15 µg/ml); Gyp7-47(R458K)p (15 µg/ml); Mg-GTPγS (2 mM); Mg-GppNHp (2 mM); BAPTA (2.5 mM); MAS7 (50 µM). FM4-64 (Molecular Probes) staining of vacuoles was performed as previously described (Vida and Emr, 1995) and photographs were taken on a Zeiss fluorescence microscope using Tmax 400 film (Kodak). Calcium concentrations of fusion reaction supernatants were measured by adding 10 µl of 25 µM Calcium-Green II (Molecular Probes) to 90 µl of centrifuged supernatant and 400 µl of water, and comparing the emission spectrum (Hitachi F-3010 Fluorimeter) to a standard curve of Ca2+-EGTA buffered solutions with known calcium concentrations (Molecular Probes).
Vacuole fusion
Vacuoles were isolated as described by Haas (1995). Standard fusion reactions contain 3 µg (each) of vacuoles isolated from BJ3505 and DKY6281 strains in 30 µl of reaction buffer (20 mM PIPES–KOH pH 6.8, 200 mM sorbitol, 125 mM KCl, 5 mM MgCl2, 10 µM coenzyme A, 1 mM Mg-ATP, 40 mM creatine phosphate, 0.5 mg/ml creatine kinase, 6.6 ng/ml leupeptin, 16.6 ng/ml pepstatin, 16.6 µM o-phenanthroline, 3.3 µM Pefabloc SC). Reactions that required vacuole re-isolation were diluted 5-fold with ice-cold PS buffer and centrifuged (11 000 g for 4 min at 4°C).
Acknowledgments
Acknowledgements
We are indebted to Dietrich Scheglmann for providing YPT7 mutant strains. We thank A.Merz, D.Seals and T.Yahr for critical assessment of this manuscript, and N.Margolis, S.Oldfield and N.Thorngren for expert technical assistance. This work was supported by grants from the National Institute of General Medical Sciences to W.W. and from the Deutsche Forschungsgemeinschaft to D.G.
References
- Albert S. and Gallwitz,D. (1999) Two new members of a family of Ypt/Rab GTPase activating proteins. Promiscuity of substrate recognition. J. Biol. Chem., 274, 33186–33189. [DOI] [PubMed] [Google Scholar]
- Albert S. and Gallwitz,D. (2000) Msb4p, a protein involved in Cdc42p-dependent organization of the actin cytoskeleton, is a Ypt/Rab-specific GAP. Biol. Chem., 381, 453–456. [DOI] [PubMed] [Google Scholar]
- Albert S., Will,E. and Gallwitz,D. (1999) Identification of the catalytic domains and their functionally critical arginine residues of two yeast GTPase-activating proteins specific for Ypt/Rab transport GTPases. EMBO J., 18, 5216–5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. (1997) Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J. Cell Biol., 139, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem., 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Brose N., Rosenmund,C. and Rettig,J. (2000) Regulation of transmitter release by unc-13 and its homologues. Curr. Opin. Neurobiol., 10, 303–311. [DOI] [PubMed] [Google Scholar]
- Coorssen J.R., Blank,P.S., Tahara,M. and Zimmerberg,J. (1998) Biochemical and functional studies of cortical vesicle fusion: the SNARE complex and Ca2+ sensitivity. J. Cell Biol., 143, 1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L.L., Collins,R.N. and Novick,P.J. (1998) Identification of a Sec4p GTPase-activating protein (GAP) as a novel member of a Rab GAP family. J. Biol. Chem., 273, 3253–3256. [DOI] [PubMed] [Google Scholar]
- Garrett M.D., Zahner,J.E., Cheney,C.M. and Novick,P.J. (1994) GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J., 13, 1718–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Roth,D., Walch-Solimena,C. and Novick,P. (1999) The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J., 18, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Sacher,M., Barrowman,J., Ferro-Novick,S. and Novick,P. (2000) Protein complexes in transport vesicle targeting. Trends Cell Biol., 10, 251–255. [DOI] [PubMed] [Google Scholar]
- Haas A. (1995) A quantitative assay to measure homotypic fusion in vitro. Methods Cell Sci., 17, 283–294. [Google Scholar]
- Haas A. and Wickner,W. (1996) Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF). EMBO J., 15, 3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Haas A., Conradt,B. and Wickner,W. (1994) G-protein ligands inhibit in vitro reactions of vacuole inheritance. J. Cell Biol., 126, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A., Scheglmann,D., Lazar,T., Gallwitz,D. and Wickner,W. (1995) The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J., 14, 5258–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R. and Sudhof,T.C. (1999) Membrane fusion and exocytosis. Annu. Rev. Biochem., 68, 863–911. [DOI] [PubMed] [Google Scholar]
- Lazar T., Gotte,M. and Gallwitz,D. (1997) Vesicular transport: how many Ypt/Rab-GTPases make a eukaryotic cell? Trends Biochem. Sci., 22, 468–472. [DOI] [PubMed] [Google Scholar]
- Lenhard J.M., Kahn,R.A. and Stahl,P.D. (1992) Evidence for ADP-ribosylation factor (ARF) as a regulator of in vitro endosome–endosome fusion. J. Biol. Chem., 267, 13047–13052. [PubMed] [Google Scholar]
- Lian J.P., Stone,S., Jiang,Y., Lyons,P. and Ferro-Novick,S. (1994) Ypt1p implicated in v-SNARE activation. Nature, 372, 698–701. [DOI] [PubMed] [Google Scholar]
- Lupashin V.V., Hamamoto,S. and Schekman,R.W. (1996) Biochemical requirements for the targeting and fusion of ER-derived transport vesicles with purified yeast Golgi membranes. J. Cell Biol., 132, 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Wickner,W. (1997) Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF). J. Cell Biol., 136, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Wickner,W. and Haas,A. (1996) Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell, 85, 83–94. [DOI] [PubMed] [Google Scholar]
- McBride H.M., Rybin,V., Murphy,C., Giner,A., Teasdale,R. and Zerial,M. (1999) Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell, 98, 377–386. [DOI] [PubMed] [Google Scholar]
- Peters C. and Mayer,A. (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature, 396, 575–580. [DOI] [PubMed] [Google Scholar]
- Peters C., Andrews,P.D., Stark,M.J., Cesaro-Tadic,S., Glatz,A., Podtelejnikov,A., Mann,M. and Mayer,A. (1999) Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science, 285, 1084–1087. [DOI] [PubMed] [Google Scholar]
- Price A., Seals,D., Wickner,W. and Ungermann,C. (2000) The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J. Cell Biol., 148, 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybin V., Ullrich,O., Rubino,M., Alexandrov,K., Simon,I., Seabra,M.C., Goody,R. and Zerial,M. (1996) GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature, 383, 266–269. [DOI] [PubMed] [Google Scholar]
- Sacher M. et al. (1998) TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J., 17, 2494–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals D.F., Eitzen,G., Margolis,N., Wickner,W.T. and Price,A. (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl Acad. Sci. USA, 97, 9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati T., Shapiro,A.D., Svejstrup,A.B. and Pfeffer,S.R. (1994) Membrane targeting of the small GTPase Rab9 is accompanied by nucleotide exchange. Nature, 369, 76–78. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Parton R.G., Steele-Mortimer,O., Lutcke,A., Gruenberg,J. and Zerial,M. (1994) Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J., 13, 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom M., Vollmer,P., Tan,T.J. and Gallwitz,D. (1993) A yeast GTPase-activating protein that interacts specifically with a member of the Ypt/Rab family. Nature, 361, 736–739. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Horiuchi,H., Bucci,C. and Zerial,M. (1994) Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature, 368, 157–160. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Sato,K. and Wickner,W. (1998) Defining the functions of trans-SNARE pairs. Nature, 396, 543–548. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Price,A. and Wickner,W. (2000) A new role for a SNARE protein as a regulator of the Ypt7/Rab-dependent stage of docking. Proc. Natl Acad. Sci. USA, 97, 8889–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida T.A. and Emr,S.D. (1995) A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol., 128, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer P., Will,E., Scheglmann,D., Strom,M. and Gallwitz,D. (1999) Primary structure and biochemical characterization of yeast GTPase-activating proteins with substrate preference for the transport GTPase Ypt7p. Eur. J. Biochem., 260, 284–290. [DOI] [PubMed] [Google Scholar]
- Wada Y., Ohsumi,Y. and Anraku,Y. (1992) Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. J. Biol. Chem., 267, 18665–18670. [PubMed] [Google Scholar]
- Wada Y., Ohsumi,Y. and Anraku,Y. (1996) Mutational analysis of Vam4/Ypt7p function in the vacuolar biogenesis and morphogenesis in the yeast, Saccharomyces cerevisiae. Protoplasma, 191, 126–135. [Google Scholar]
- Wichmann H., Hengst,L. and Gallwitz D. (1992) Endocytosis in yeast: evidence for the involvement of a small GTP-binding protein (Ypt7p). Cell, 71, 1131–1142. [DOI] [PubMed] [Google Scholar]
- Wilson A.L., Erdman,R.A. and Maltese,W.A. (1996) Association of Rab1B with GDP-dissociation inhibitor (GDI) is required for recycling but not initial membrane targeting of the Rab protein. J. Biol. Chem., 271, 10932–10940. [DOI] [PubMed] [Google Scholar]
- Wurmser A.E., Sato,T.K. and Emr,S.D. (2000) New component of the vacuolar Class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking/fusion. J. Cell Biol., 151,551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Mayer,A., Muller,E. and Wickner,W. (1997) A heterodimer of thioredoxin and I(B)2 cooperates with Sec18p (NSF) to promote yeast vacuole inheritance. J. Cell Biol., 136, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]