Abstract
Growth and differentiation of postnatal hair follicles are controlled by reciprocal interactions between the dermal papilla and the surrounding epidermal hair precursors. The molecular nature of these interactions is largely unknown, but they are likely to involve several families of signaling molecules, including Fgfs, Wnts and Bmps. To analyze the function of Bmp signaling in postnatal hair development, we have generated transgenic mice expressing the Bmp inhibitor, Noggin, under the control of the proximal Msx2 promoter, which drives expression in proliferating hair matrix cells and differentiating hair precursor cells. Differentiation of the hair shaft but not the inner root sheath is severely impaired in Msx2–Noggin transgenic mice. In addition to hair keratins, the expression of several transcription factors implicated in hair development, including Foxn1 and Hoxc13, is severely reduced in the transgenic hair follicles. Proliferating cells, which are normally restricted to the hair matrix surrounding the dermal papilla, are found in the precortex and hair shaft region. These results identify Bmps as key regulators of the genetic program controlling hair shaft differentiation in postnatal hair follicles.
Keywords: Bmps/hair follicle/hair shaft differentiation/Noggin
Introduction
Mammalian hair development is controlled by a series of reciprocal epithelial–mesenchymal interactions. The first visible sign of hair formation is the hair placode, a thickening of the embryonic ectoderm induced by the underlying mesoderm. A signal from the hair placode then causes the mesenchymal cells to condense. A second mesodermal signal in turn induces proliferation in the ectodermal placode, which starts to grow down into the mesenchyme. Eventually, the epithelial cells surround the mesodermal condensation, which forms the dermal papilla, a permanent structure at the base of the follicle thought to control growth and differentiation of the hair (Hardy, 1992).
Once the basic structure of the hair follicle is established, differentiation of the concentric layers of keratinocytes begins. The peripheral layer forms the outer root sheath (ORS), which is continuous with the basal layer of the epidermis. Within the hair follicle, two new layers, the inner root sheath (IRS) and the hair shaft, develop from proliferating precursor cells in the matrix region surrounding the dermal papilla. As cells move distally, they stop dividing and differentiate according to their medio-lateral position within the follicle. Cells positioned next to the ORS form the IRS while centrally located precursors give rise to the hair shaft, consisting of the outer cuticle, the cortex and the central medulla.
Recent years have seen substantial progress in the identification of regulators of early hair and feather development, two initially very similar processes. Members of the Tnf, Fgf and Wnt families of signaling molecules are candidate inducers of epithelial appendages, while Bmps seem to regulate the spacing between them (Widelitz et al., 1996; Gat et al., 1998; Noramly and Morgan, 1998; Headon and Overbeek, 1999). Shh signaling induces epithelial proliferation and is required for the initial downgrowth of the hair follicle (Oro et al., 1997; St-Jacques et al., 1998; Chiang et al., 1999). Although many of these molecules continue to be expressed in the postnatal hair follicle, their function at later stages is less well understood. Proper hair formation requires not only that the hair shaft and IRS are specified correctly, but also that proliferation and differentiation are coordinated along the proximo-distal axis. The proliferation of hair matrix cells is thought to be controlled in part by Fgf7, which is expressed in the dermal papilla and is a potent inducer of keratinocyte proliferation in vitro (Finch et al., 1989). Wnt signaling seems to participate in the induction of hair shaft differentiation. The pathway is specifically activated in precortex cells at the base of the hair shaft, and binding sites for the transcription factor Lef1, which mediates transcriptional responses to Wnt signaling, are found in the promoter regions of many hair keratin genes (Zhou et al., 1995; DasGupta and Fuchs, 1999). Finally, Bmp signaling has been implicated in the regulation of both proliferation and differentiation in the hair follicle. Several Bmp family members are expressed in the postnatal hair follicle. The two closely related genes, Bmp2 and Bmp4, are both activated in hair shaft precursors, while expression of Bmp4 is also found in the dermal papilla (Wilson et al., 1999). Ectopic expression of Bmp4 in the ORS inhibits proliferation in the hair matrix and activates hair keratin genes in the ORS (Blessing et al., 1993). Expression of Bmp7 has been reported in the IRS and ORS and the dermal papilla (Takahashi and Ikeda, 1996). Bmp8a and b are transcribed specifically in the IRS during the first postnatal hair cycle (Zhao and Hogan, 1996).
The identification of antagonists that bind Bmps extracellularly and prevent them from interacting with their Bmp receptors has provided tools to analyze the role of Bmp signaling in hair development. To inhibit Bmp signaling specifically in the postnatal hair follicle we have generated transgenic mice expressing Noggin, a known inhibitor of Bmp2, 4 and 7, under the control of 439 bp of the mouse Msx2 promoter (Liu et al., 1994; Zimmerman et al., 1996). This promoter drives transgene expression in the hair bulb, including hair matrix cells and early differentiating precursors of the IRS and hair shaft. Transgenic mice show a range of hair abnormalities: from bare patches in the coat up to complete loss of all external hairs, including vibrissae. Hair follicles are formed normally, but differentiation of hair shaft cells is severely impaired. Expression of both hair keratins and a number of transcriptional regulators implicated in the control of hair differentiation is reduced or absent. In addition, proliferation is no longer restricted to the hair matrix, but is also found in the precortex and distal hair shaft region. Our results show that Bmps are a key component of the signaling network controlling hair development and are required to induce the genetic program regulating hair shaft differentiation in the anagen hair follicle.
Results
Msx2–Noggin transgenic mice lack all hair types including vibrissae
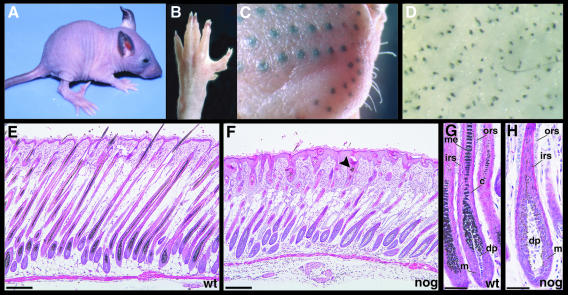
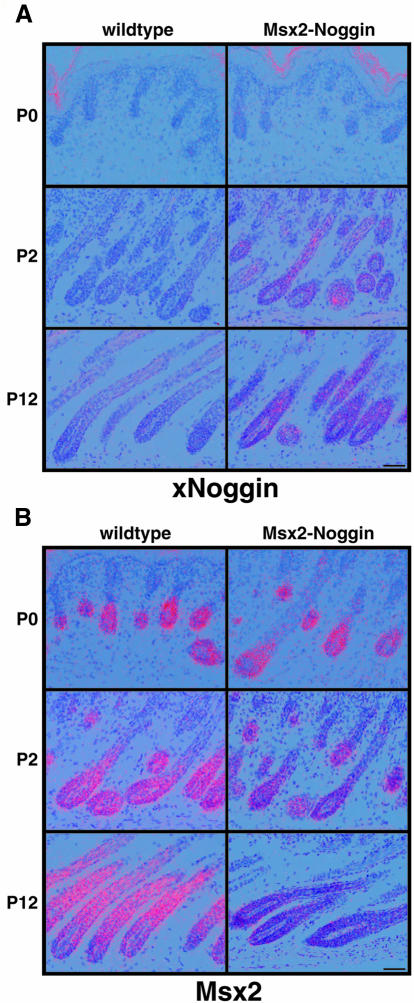
We have generated transgenic mice expressing Xenopus Noggin under the control of 439 bp of the mouse Msx2 promoter. This promoter fragment has previously been reported to drive expression exclusively in the apical ectodermal ridge of the limb buds of E11.5 mouse embryos (Liu et al., 1994). In agreement with this observation and with known functions of Bmp signaling in limb development, we find limb abnormalities in the Msx2–Noggin transgenic animals, which will be reported separately (Figure 1B). In addition, the transgenic animals show a loss of body hairs and vibrissae. The hair defects observed in seven independent transgenic lines vary from bare patches up to the loss of >80% of the body hair and vibrissae. One transgenic line that has almost no external hairs and completely lacks vibrissae was chosen for further analysis (Figure 1A). Despite the lack of external hair, vibrissae pads are formed in their normal pattern and melanin is deposited in the dorsal skin, where hairs should normally have emerged (Figure 1C and D). Sections of the dorsal skin of 12-day-old pups show that approximately normal numbers of hair follicles are formed in Msx2–Noggin mice compared with wild-type littermates. However, the transgenic follicles have a wavy appearance and do not form a proper hair shaft. Although they show distinct anterior–posterior polarity, with a posteriorly located sebaceous gland, deeper portions of the follicles are often misaligned (Figure 1E and F). The transgenic dermal papillae often appear less compact, the hair shaft shows no distinct cortex and medulla, and melanin production seems to be reduced (Figure 1G and H).
Fig. 1. Msx2–Noggin transgenic mice form hair follicles but lack external hairs. (A) Three-week-old Msx2–Noggin transgenic mouse of line #14. This line shows only mild limb abnormalities, with forelimbs often appearing normal and hindlimbs having an abnormal spreading of the first digit, small anterior outgrowths and malformed claws (B, left hind paw, 6 weeks). Vibrissae pads form in a normal pattern and can be recognized by melanin deposits (C, 6 weeks). Similarly, melanin-containing material is seen in the dorsal back skin (D, 6 weeks). Hematoxylin–eosin-stained sections of wild-type (E and G) and transgenic (F and H) P12 dorsal skin with hair follicles in anagen of the first hair cycle. Hair follicles in the transgenic animals are wavy, and deposits of keratinous material (arrowhead) are present beneath the epidermis. Dermal papillae in the transgenic follicles have a loose appearance and no cortex or medulla can be recognized in the hair shaft region. c, cortex; dp, dermal papilla; irs, inner root sheath; m, matrix; me, medulla; ors, outer root sheath. Scale bars: E, F, 50 µm; G, H, 25 µm.
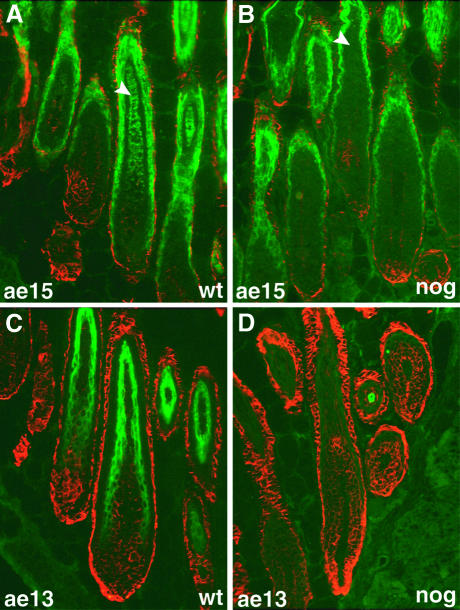
To analyze the abnormal hair follicles in more detail, we used immunohistochemistry to identify the three main layers of the anagen hair follicle: the hair shaft, the IRS and the ORS. Three well characterized antibodies were used: MK5, recognizing keratin 5 expressed in the ORS and basal layer of the epidermis; AE15, specific for trychohyalin found in characteristic granules of the IRS and medulla; and AE13, detecting acidic hair keratins expressed in the cortex and cuticle of the hair shaft (Lynch et al., 1986; O’Guin et al., 1992). Keratin 5 in the ORS and trychohyalin in the IRS are expressed normally in the transgenic hair follicles. In contrast, trychohyalin expression in the central region of the hair follicles, corresponding to cells of the hair shaft medulla, is only seen in wild-type skin (Figure 2A and B). Expression of acidic hair keratins is also missing in the transgenic follicles, suggesting that hair shaft differentiation is severely impaired (Figure 2C and D). Although both the IRS and hair shaft cells are derived from proliferating cells in the matrix region, interference with Bmp signaling seems to inhibit the formation of the hair shaft selectively.
Fig. 2. Expression of keratin 5, trychohyalin and acidic hair keratins in transgenic and wild-type hair follicles. Frozen sections were prepared from wild-type (A and C) and transgenic (B and D) P12 dorsal skin and assayed by indirect immunofluorescence staining for the expression of keratin 5 (MK5, red) in the ORS, and trychohyalin (AE15, green) in the IRS and medulla (A and B), or acidic hair keratins (AE13, green) in the cortex and cuticle (C and D). Keratin 5 expression in the ORS is normal in Msx2–Noggin hair follicles. Trychohyalin is present in the IRS, but absent from the center of the transgenic hair follicles, where the medulla should have formed (arrowhead in B). Expression of acidic hair keratins in the cortex and cuticle is completely lost in the transgenic hair follicles (D).
Expression of the Msx2–Noggin transgene in relation to Bmp2 and 4
To analyze where in the hair follicle ectopic Noggin interferes with Bmp signaling we determined Noggin transgene expression in relation to that of Bmp2 and Bmp4, encoding the two Bmp family members that Noggin binds to with high affinity (Zimmerman et al., 1996).
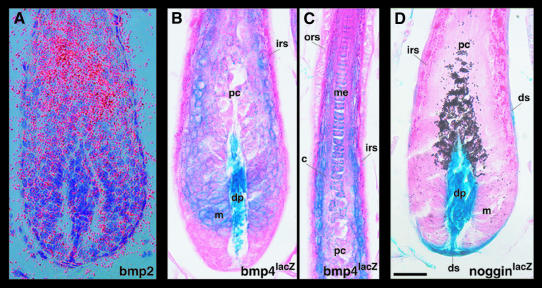
Bmp2 is expressed specifically in the precortex of the anagen hair follicle (Figure 3A). To look at the expression of Noggin and Bmp4 in the hair follicle we took advantage of lacZ knock-in alleles of these two genes (Brunet et al., 1998; Lawson et al., 1999). The highest Bmp4 expression, as monitored by β-galactosidase activity, is found in the dermal papilla. In differentiating keratinocytes, Bmp4 shows a complementary expression pattern to Bmp2. Bmp4 is most strongly expressed in the distal hair matrix region, in the medulla and in hair shaft cells adjacent to the IRS, which possibly represent precursors of the cuticle. Cells in the precortex express only low levels of Bmp4. Distally, β-galactosidase activity was detected in cells resembling the companion layer (Figure 6C). The lower third of the hair bulb, where progenitors of the IRS are located, is completely devoid of Bmp4lacZ expression (Figure 3B and C). NogginlacZ is co-expressed with Bmp4 in the dermal papilla, but in addition lacZ staining extends into the cells of the dermal sheath located at the base of the hair follicle and surrounding the hair bulb (Figure 3D).
Fig. 3. Expression of Bmp2, Bmp4 and Noggin in anagen hair follicles. A 35S-labeled antisense riboprobe for Bmp2 was hybridized to sections of dorsal skin of 12-day-old wild-type mice (A). Dorsal skin of albino Bmp4lacZ (P4) and pigmented NogginlacZ (P12) heterozygous mice was processed for β-galactosidase activity, sectioned and counterstained with eosin (B–D). c, cortex; dp, dermal papilla; ds, dermal sheath; irs, inner root sheath; m, matrix; me, medulla; ors, outer root sheath; pc, precortex. Scale bar: 25 µm.
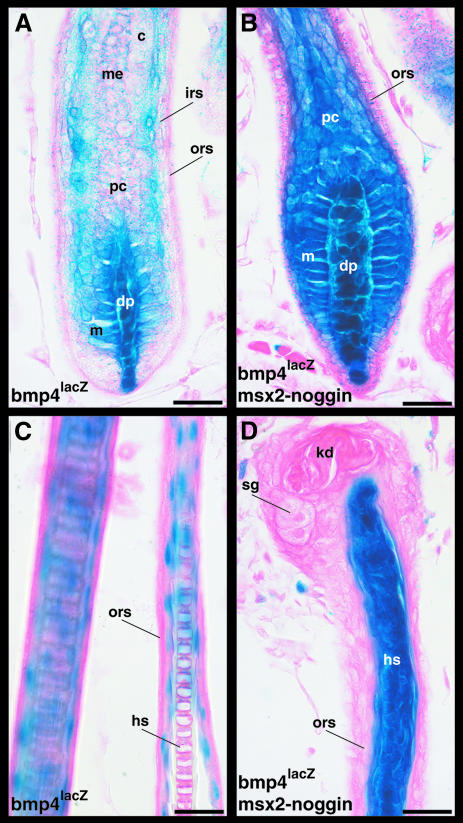
Fig. 6. Negative feedback control of Bmp4 expression in epithelial cells. Dorsal skin samples of P12 albino Msx2–Noggin transgenic and non-transgenic mice carrying the Bmp4lacZ allele were processed for β-galactosidase activity, sectioned and counterstained with eosin. Bmp4lacZ expression is greatly enhanced and expanded distally in the Msx2–Noggin transgenic (B and D) compared with non-transgenic (A and C) hair follicles. Note the lack of lacZ-negative cells at the unusually narrow base and in the IRS region of the transgenic hair follicles (B). The lacZ-positive cells in distal wild-type hair follicles resemble cells of the companion layer (C). Because of the strong β-galactosidase staining in the distal hair shaft, it is difficult to determine whether this layer is present in transgenic hair follicles or not (D). c, cortex; dp, dermal papilla; hs, hair shaft; irs, inner root sheath; kd, keratinous deposit; m, matrix; me, medulla; ors, outer root sheath; pc, precortex; sg, sebaceous gland. Scale bar: 25 µm.
Expression of the Msx2–Noggin transgene is first detected at low levels in hair follicles of P2 pups and does not increase significantly until day 12. Expression is seen in epithelial cells of the hair bulb and in the precortex and hair shaft region, while the ORS and dermal papilla appear negative (Figure 4A). These data show that the Msx2–Noggin transgene is co-expressed with Bmp2 and 4 in precursor cells of the hair shaft, the structure mostly affected in the transgenic animals, and is therefore likely to inhibit Bmp signaling in this region.
Fig. 4. Expression of the Msx2–Noggin transgene and the endogenous Msx2 gene in wild-type and transgenic postnatal hair follicles. Sections of dorsal skin of newborn, P2 and P12 mice were probed with 35S-labeled antisense riboprobes for Xenopus Noggin (A) and mouse Mxs2 (B). With the onset of detectable Noggin transgene expression at P2, expression of Msx2 is reduced in transgenic compared with wild-type hair follicles. Scale bar: 50 µm.
This hypothesis is supported by the analysis of endogenous Msx2 gene expression in the transgenic hair follicles. Like the Msx2–Noggin transgene, Msx2 itself is expressed in the hair matrix and in differentiating precursors of the hair shaft and IRS (Figure 4B) (Jiang et al., 1999). We see a strong downregulation of Msx2 expression in the transgenic follicles, suggesting that Bmp signaling normally controls endogenous Msx2 gene expression in hair follicles. This could explain the low expression level of the Msx2–Noggin transgene, which might negatively regulate its own transcription.
Bmp signaling controls expression of transcriptional regulators of hair differentiation
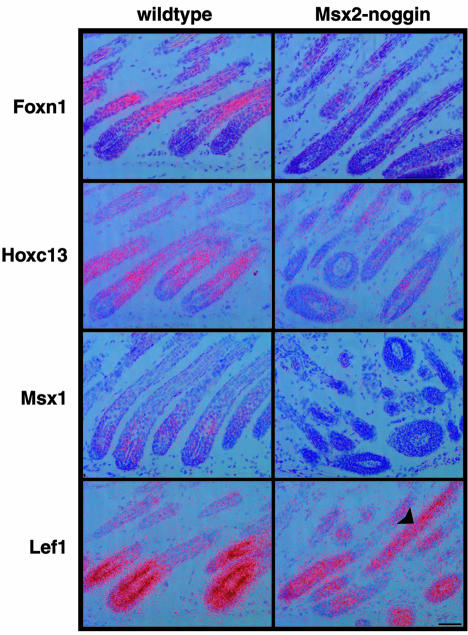
The loss of keratin and trychohyalin expression in the hair shaft region of transgenic follicles suggests an important function for Bmp signaling in the control of hair shaft differentiation, but leaves open the question of where in the genetic network Bmps act. Bmp signaling might directly control the expression of terminal differentiation markers like keratins, or act further upstream in the regulatory hierarchy. A number of transcriptional regulators have been implicated in the control of hair follicle development. Among these, Hoxc13 and the forkhead transcription factor Foxn1 (formerly Whn, Winged helix nude) are of interest for this study because loss of their function severely impairs hair shaft differentiation without interfering with hair follicle formation (Nehls et al., 1994; Godwin and Capecchi, 1998). Within the normal anagen hair follicle, Foxn1 is expressed in non-proliferating precursor cells of the hair shaft and IRS, but in the transgenic follicles expression is completely lost (Figure 5) (Lee et al., 1999). Similarly, Hoxc13 expression in differentiating precursor cells in anagen hair follicles is strongly reduced in transgenic animals (Figure 5). Msx1 and 2, encoding two closely related homeobox transcription factors, are co-expressed in the developing hair follicle and have redundant functions in the induction of hair follicle formation, which is severely impaired in their absence (Satokata et al., 2000). We find that expression of Msx1, like that of Msx2, is strongly reduced in the hair bulb of transgenic hair follicles (Figure 5). The HMG box transcription factor Lef1 is thought to regulate keratin genes directly and has been implicated as a possible Bmp target in tooth and hair development (Kratochwil et al., 1996; Botchkarev et al., 1999). Lef1 mRNA is expressed in precursors of the hair shaft in both the hair matrix and the precortex (DasGupta and Fuchs, 1999). In hair follicles of the Msx2–Noggin mutant mice we see only a minor reduction of Lef1 expression in the hair bulb, but the expression extends further distally than normal (Figure 5).
Fig. 5. Key regulators of hair formation are downregulated in Msx2–Noggin transgenic mice. Expression of Foxn1, Hoxc13, Msx1 and Lef1 in wild-type and transgenic hair follicles of P12 dorsal skin was analyzed by radioactive section in situ hybridization. Expression of Foxn1, Hoxc13 and Msx1 is strongly reduced in the transgenic hair follicles, while Lef1 mRNA levels are almost normal but extend into the hair shaft (arrowhead). Scale bar: 50 µm.
These results place Bmp signaling upstream of a number of transcriptional regulators implicated in the control of hair development. The strong reduction in the expression of Msx1, Msx2, Foxn1 and Hoxc13 suggests that they are part of a genetic program activated by Bmps. In contrast, Lef1 expression is maintained independently of Bmp signaling. However, the distal expansion of the Lef1 expression domain in transgenic hair follicles raises the possibility that Bmps directly or indirectly inhibit Lef1 transcription during normal differentiation.
Expression of Bmp4 in the hair matrix is inhibited by Bmp signaling
The dermal papilla is thought to be the source of signals regulating growth and differentiation of the hair matrix cells. Bmp4 expressed in the dermal papilla might therefore be the primary signal inducing differentiation and Bmp4 expression in the surrounding keratinocytes. To determine whether Bmp4 expression in the hair matrix requires Bmp signaling we analyzed the expression of the Bmp4lacZ allele in mice carrying the Msx2–Noggin transgene. Surprisingly, expression of Bmp4lacZ is greatly increased in the presence of ectopic Noggin, suggesting that the endogenous Bmp4 gene is normally repressed by Bmp signaling (Figure 6A and B). Laterally, intense β-galactosidase staining extends all the way up to the ORS in the transgenic hair follicles, in contrast to wild-type follicles where Bmp4lacZ expression is absent in the IRS. The proximal Bmp4lacZ-negative cell population present at the base of the wild-type hair follicles is reduced or absent in most transgenic follicles (Figure 6A and B). In addition, expression of Bmp4lacZ is expanded distally along the entire hair shaft (Figure 6C and D).
These results show that epithelial expression of Bmp4 is normally repressed rather than activated by Bmp signaling. This argues against any model in which Bmp4 produced in the dermal papilla is required for activation of Bmp4 expression in the differentiating keratinocytes. Instead, this negative autoregulatory feedback loop is likely to buffer Bmp activity in the epithelial cells. Negative autoregulation of Bmp4 expression is not restricted to hair keratinocytes, but has also been observed in the perichondrium during chondrogenesis and it may therefore stabilize Bmp activity in additional developmental processes (Pathi et al., 1999).
Proximal restriction of proliferation is lost in Noggin transgenic hair follicles
Proliferation and differentiation in the anagen hair follicle have to be coordinated to ensure the growth of a structurally intact hair. Bmp4 suppresses proliferation in the hair matrix when expressed ectopically in the ORS (Blessing et al., 1993). Therefore, we used BrdU labeling to study the proliferation of precursor cells in the hair bulb of wild-type and Noggin transgenic anagen hair follicles.
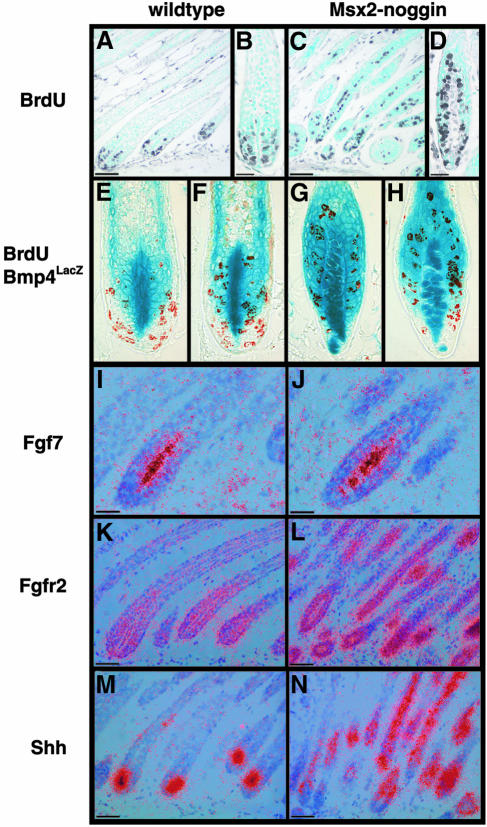
In wild-type mice, proliferating cells are confined to the proximal half of the hair bulb (Figure 7A and B). In contrast, no such restriction is seen in transgenic follicles, where proliferating cells are found throughout the hair bulb, in the precortex and farther distal in the hair shaft (Figure 7C and D). Interestingly, the total number of proliferating cells within the P12 transgenic hair bulbs including the precortex does not increase significantly compared with the number of cycling cells in the wild-type hair matrix [32.5 ± 4.6 in wild-type versus 39.0 ± 9.3 in transgenic hair follicles (n = 29), not counting cells in the distal hair shaft] and there are actually fewer proliferating cells at the base of the transgenic follicles than normal.
Fig. 7. Altered proliferation in Msx2–Noggin transgenic hair follicles. Sections of dorsal skin of P12 wild-type and transgenic animals injected with BrdU 4 h before being sacrificed were analyzed for BrdU incorporation by immunohistochemistry. Skin samples from animals carrying the Bmp4lacZ allele were also assayed for β-galactosidase activity. Proliferating cells in wild-type follicles are restricted to the hair matrix (A and B), but are distributed throughout the hair bulb, the precortex and the hair shaft in the transgenic skin (C and D). Bmp4lacZ expression and proliferation overlap in the distal hair matrix of normal hair follicles (E and F). Proliferating cells are found throughout the lacZ-positive hair bulb of transgenic hair follicles (G). LacZ-negative cells at the base of transgenic hair follicles show little proliferation compared with lacZ-negative proximal cells in normal follicles (compare H with E and F). Expression of Fgf7, Fgfr2 and Shh was determined by radioactive in situ hybridization. No change is seen in the expression of Fgf7 in the dermal papilla (I and J), while Fgfr2 expression in the transgenic hair follicles is increased and extends further into the hair shaft than normal (K and L). Shh expression, which is normally restricted to a small group of cells in the anterior or posterior hair matrix, is found throughout the distal hair bulb and in the hair shaft region of transgenic follicles (M and N). Scale bars: A, C, K–M, 50 µm; B, D, I, J, 25 µm.
To determine whether there is a correlation between proliferation and Bmp4 expression we performed BrdU labeling in wild-type and Noggin transgenic animals carrying the Bmp4lacZ allele, and simultaneously stained for β-galactosidase expression and BrdU incorporation. The Bmp4-negative cell population at the base of the hair follicle always contains a high percentage of proliferating cells. A considerable number of BrdU-positive cells can also be found among the Bmp4-positive hair matrix cells, but the extent of proliferation in this cell population shows a greater variability (Figure 7E and F). In the transgenic hair follicles, most proliferating cells express Bmp4. In the rare case that a significant population of Bmp4-negative cells is present proximally, proliferation in these cells seems rather limited (Figure 7G and H).
In summary, our data show that Bmp signaling causes the complete inhibition of proliferation in the precortex, but is compatible with extensive proliferation in the matrix region.
Influence of Bmp signaling on the expression of known growth factors for keratinocytes
The dermal papilla is thought to be the source of growth promoting signals for the surrounding hair matrix cells. Part of this activity may be mediated by Fgf7, a potent inducer of keratinocyte proliferation (Finch et al., 1989). To see whether the altered proliferation in the transgenic hair follicles correlates with changes in the Fgf growth signal, we determined the expression of Fgf7 in anagen hair follicles, but found no difference between wild-type and transgenic mice (Figure 7I and J). Similarly, expression of Fgf10, the only other Fgf known to be expressed in the dermal papilla, is unchanged (data not shown) (Hamada et al., 1999). The receptor for Fgf7 and 10, Fgfr2, is expressed in the hair matrix and its expression has been reported to be activated by Bmp signaling in embryonic avian skin during feather bud formation (Rosenquist and Martin, 1996; Noramly and Morgan, 1998). The level of Fgfr2 mRNA is elevated in the transgenic hair follicles and the expression extends farther distal into the hair shaft than normal (Figure 7K and L). This suggests that Bmp signaling negatively regulates Fgfr2 expression in the mouse hair follicle, opposite to its function in the chicken epidermis (Noramly and Morgan, 1998).
Another signaling molecule implicated in the control of proliferation in the hair follicle is Shh, which is required for the downgrowth of the hair placode into the underlying mesenchyme (St-Jacques et al., 1998; Chiang et al., 1999). Shh expression is normally found at the tip of the growing hair placode and becomes restricted to a small cell population in the anterior or posterior hair matrix in the mature anagen hair follicle (Figure 7M) (Gat et al., 1998). In Noggin transgenic follicles, Shh is expressed throughout the distal hair bulb and in the hair shaft region corresponding to the domain of ectopic cell proliferation (Figure 7N).
In conclusion, the analysis of growth factor expression in the transgenic hair follicles suggests that alterations in Fgf signaling are not responsible for the loss of proximal hair matrix cells or ectopic distal proliferation, while expanded Shh expression may induce proliferation in the precortex and hair shaft region.
Discussion
A number of studies in mouse and chicken have identified Bmps as negative regulators of embryonic hair follicle and feather formation. In contrast, this work suggests a positive function for Bmp signaling in postnatal hair development. Using the Bmp inhibitor Noggin we have shown that Bmp signaling is required to induce differentiation of hair shaft progenitors, which is coupled to the loss of their proliferative capacity and thereby restricts proliferation to the hair matrix.
Bmp4 expression as a marker of hair shaft progenitors in the hair matrix
It is generally assumed that lineage commitment of progenitor cells in the hair matrix is position dependent, but the timing, spatial organization and factors involved in this process remain largely unknown. While the layers of the hair follicle are eventually organized in a medio-lateral fashion, they probably originate from cells in different proximo-distal positions along the boundary with the dermal papilla (Epstein and Maibach, 1969). According to this model, precursors of the outer layers, belonging to the IRS, are located near the base of the dermal papilla, while precursors of the central hair shaft occupy more distal positions. Epithelial expression of Bmp4 in the hair bulb is restricted to the distal matrix, while IRS cells and proximal matrix cells are Bmp4 negative. Bmp4-positive and -negative hair matrix cells therefore coincide with the putative hair shaft and IRS precursor domains, respectively. To our knowledge, Bmp4 expression is the first marker that links the medio-lateral to the putative proximal–distal lineage border.
Functions of Bmp signaling in hair shaft and IRS differentiation
Inhibition of Bmp signaling prevents the induction of hair shaft differentiation, as shown by the absence of trychohyalin in the medulla and acidic hair keratins in the cortex and cuticle of Noggin transgenic hair follicles. In addition, the expression of a number of transcriptional regulators of keratinocyte differentiation, like Hoxc13, Foxn1, Msx1 and 2, is strongly reduced, suggesting that Bmps act upstream of a complex genetic program rather than directly activating terminal hair shaft differentiation markers. Expression of Lef1, which has also been implicated in hair shaft differentiation, does not seem to require Bmp signaling, suggesting that Bmps do not control the entire hair shaft differentiation program. The transcription factor Lef1, which mediates nuclear responses to Wnt signaling, is activated in the precortex, where it is thought to control the transcription of hair shaft-specific genes directly (Zhou et al., 1995; DasGupta and Fuchs, 1999). This suggests that Lef1 plays a positive role in hair formation and cooperates with Bmps to induce hair shaft differentiation. This contrasts with the antagonistic interaction between Bmp signaling and Lef1 during early stages of hair follicle development, where the inhibitory activity of Bmps is thought to be mediated in part by their repression of Lef1 expression (Botchkarev et al., 1999).
Cells in the precortex and hair shaft of transgenic hair follicles continue to proliferate and to express genes normally downregulated during differentiation, like Lef1, Fgfr2 and Bmp4, suggesting that these cells remain hair shaft precursors. Since transgenic Noggin expression may not completely suppress Bmp activity, we cannot tell whether hair shaft progenitors are independent of Bmp signaling or whether they require a low level of Bmp activity, insufficient to induce differentiation. Previous studies have shown that Bmp4 overexpression in the ORS both inhibits proliferation and induces ectopic expression of hair keratins, suggesting that an increase in Bmp activity in the precortex is normally sufficient to inhibit proliferation and induce differentiation (Blessing et al., 1993).
In contrast to the hair shaft, the IRS seems to differentiate normally in the Msx2–Noggin transgenic hair follicles, although Bmp4-negative IRS cells are missing. This absence may be caused by an expansion of the normal Bmp4 expression domain into the IRS, which would leave the origin of the IRS cells unchanged. However, the narrow base of the transgenic hair follicles suggests a loss of the proximal Bmp4-negative cells and consequently their putative descendants in the IRS. In this case, the outer layer of the hair shaft precursors would be respecified to form the IRS, suggesting that it is the final medio-lateral position of the precursors rather than their origin that determines the lineage decision. Fate mapping within the hair follicle will be required to distinguish between these two possibilities.
Control of proliferation in the anagen hair follicle
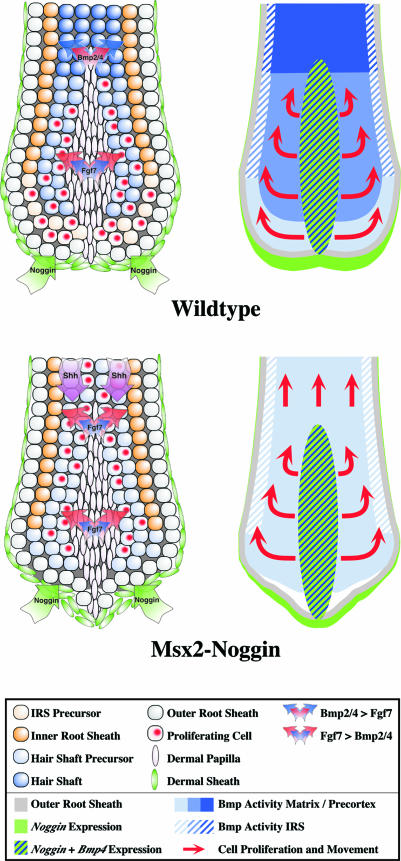
In the normal anagen hair follicle, proliferating cells are only found in the hair matrix. Upon suppression of Bmp activity by ectopic Noggin, cycling cells also appear in the precortex and hair shaft, suggesting that differentiation signals play a key role in restricting proliferation to the hair matrix (Figure 7). Our results suggest two mechanisms by which Bmps may prevent distal proliferation. One is the restriction of growth factor expression to the hair matrix, as seen for Shh, expression of which spreads distally in the absence of Bmp-induced differentiation (Figure 7M and N). A mitogenic function for Shh in the hair shaft region is supported by earlier findings that Shh is required for the downgrowth of the hair placode into the dermis (St-Jacques et al., 1998; Chiang et al., 1999). The second mechanism is the direct antagonism between growth and differentiation signals. Fgf7 is a potent mitogen for keratinocytes, but neither the expression of Fgf7 in the dermal papilla nor that of its receptor, Fgfr2, in the surrounding hair matrix and precortex is significantly affected by Bmp signaling (Finch et al., 1989) (Figure 7I–K). This implies that it is the relative local strength of proliferation and differentiation signals that determines the behavior of cells in the hair matrix and precortex. Growth signals, including Fgfs, prevail in the hair matrix, while differentiation signals are stronger in the precortex, where an increase in Bmp signaling may be sufficient to tip the balance toward differentiation (Figure 8).
Fig. 8. Model for the role of Bmp2/4, Fgf7 and Noggin in the regulation of proliferation and differentiation of keratinocytes in wild-type and transgenic hair follicles. The two representations of the hair bulb predict the prevailing influence of growth or differentiation factors (left) and the distribution of Bmp activity (right). In the left representations, only the prevailing signaling activity is printed in the arrows. In the right representations, the intensity of the blue color indicates the level of Bmp activity (low, medium or high). The red arrows symbolize the presence of proliferating cells and their movement. According to the model, high Bmp activity in the precortex of wild-type hair follicles suppresses proliferation and induces differentiation, while moderate Bmp activity in the distal hair matrix is compatible with proliferation induced by Fgf7 from the dermal papilla. Both the lack of epithelial Bmp4 expression and Noggin released from the proximal dermal sheath are likely to create a zone of low Bmp activity at the base of the hair follicle. In the transgenic hair follicle, ectopic Noggin inhibits Bmp signaling in the distal hair matrix and precortex, preventing hair shaft differentiation. Consequently, Shh expression is not localized to the hair matrix and activates ectopic proliferation in the precortex and hair shaft region. The proximal Bmp4-negative cell population is absent and the IRS in the transgenic hair follicle is possibly derived from lateral hair shaft precursor cells located adjacent to the ORS. Further details of the model are described in the text.
The proximal, highly proliferative Bmp4-negative cell population is missing in transgenic hair follicles, raising the possibility that Bmp signaling is directly or indirectly involved in promoting survival and/or proliferation of proximal hair matrix cells. Interestingly, Foxn1, which is strongly downregulated in the transgenic hair follicles, has been shown to control a feedback signal in the epidermis, where it induces proliferation in the basal layer when overexpressed in the adjacent suprabasal cells (Prowse et al., 1999). Foxn1 may therefore similarly upregulate the production of growth or survival factors for proximal matrix cells in differentiating hair keratinocytes.
Model for three zones of Bmp activity in the anagen hair follicle
Previous transgenic experiments, and the present study, show that Bmp signaling suppresses proliferation in the hair matrix and is required for the induction of hair shaft differentiation in the precortex (Blessing et al., 1993). These findings suggest that Bmp activity is low among the proliferating cells in the proximal half of the hair bulb and high in differentiating cells in the precortex. How do the complex expression patterns of Bmp2, Bmp4 and Noggin contribute to the establishment of this Bmp activity gradient?
The highest Bmp4 expression is found in the dermal papilla, but it shows no apparent proximo-distal gradation. Similarly, Noggin is expressed evenly throughout the dermal papilla and cannot account by itself for the formation of a Bmp activity gradient. Graded Bmp activity could be generated if inactive Bmp–Noggin complexes are released from the dermal papilla and are then activated specifically in the precortex by a tolloid-like protease. A sea urchin metalloprotease capable of antagonizing Noggin function has recently been identified (Wardle et al., 1999). Alternatively, Noggin might simply reduce the overall amount of active Bmp4 released from the dermal papilla, thereby generating a moderate, but evenly distributed Bmp signal. In this case, the epithelial expression of Bmps together with Noggin released from the proximal dermal sheath is likely to generate the putative Bmp activity gradient.
Outside the dermal papilla, Noggin is normally expressed in the proximal dermal sheath, Bmp4 in hair shaft precursors in the distal hair matrix and Bmp2 in differentiating hair shaft cells in the precortex. We hypothesize that these sources of Bmps and Noggin, together with Bmp4 released from the dermal papilla, create three zones of Bmp activity, which allow proliferation proximally and induce differentiation distally (Figure 8).
According to this model, Noggin produced in the proximal dermal sheath inhibits Bmp4 released from the dermal papilla and distal hair matrix cells to create a proximal zone of low Bmp activity. The importance of a signal from the proximal dermal sheath for the growth of hair follicles has been shown in hair reconstitution experiments in which the dermal papilla surrounded by ORS cells has to contact dermal sheath cells to induce the formation of a new hair follicle (Jahoda and Reynolds, 1996). Noggin could be part of this signal. In the distal hair matrix, Bmp activity is buffered by the inhibition of epithelial Bmp4 expression by Bmp signaling. The existence of such a feedback control is suggested by the increased Bmp4lacZ expression in Noggin transgenic hair follicles (Figure 6). Hair shaft precursors in this region are therefore likely to receive an evenly distributed Bmp signal, which we think is moderate since it still allows extensive proliferation. In the precortex, locally expressed Bmp2, together with Bmp4 released from the dermal papilla, could generate a high level of Bmp activity that cannot be balanced by the feedback reduction of Bmp4 expression, leading to an increase in Bmp signaling that triggers differentiation. The sharp fall in Bmp4lacZ expression in the precortex is consistent with high Bmp activity in this region.
Targets of Bmp signaling in the hair follicle
The expression of four genes, Foxn1, Hoxc13, Msx1 and Msx2, is strongly reduced or absent in Msx2–Noggin transgenic hair follicles, suggesting that they lie in a genetic pathway directly controlled by Bmp signaling. Can the loss of any of these genes account for the phenotype of the Msx2–Noggin transgenic mice?
Foxn1 mutant nude mice develop a normal number of hair follicles, but show incomplete differentiation of the hair shafts, which form a discernible hair cortex and medulla but rarely penetrate the skin. Although Foxn1, like Bmps, is thought to influence both the proliferation and differentiation of hair keratinocytes, the nude hair phenotype is much weaker than that of the Msx2–Noggin transgenic mice (Kopf-Maier et al., 1990; Brissette et al., 1996). The loss of Foxn1 expression in the Noggin transgenic hair follicles may therefore contribute to the observed phenotype, but does not fully explain it.
Hoxc13 mutant mice develop hair follicles with hair shafts that do not protrude through the skin (Godwin and Capecchi, 1998). The morphological abnormalities and the molecular nature of the defect in Hoxc13 mutant hair follicles have not been characterized extensively, so it is difficult to say how they compare to the Msx2–Noggin phenotype. Nevertheless, the co-expression of Bmp4 and Hoxc13 is very striking. It is not only seen in the hair follicle, but extends to other keratinized structures like the nails and the filiform papillae of the tongue (our unpublished observation), suggesting that Bmps and Hoxc13 form part of a more general genetic program directing ‘hard’ keratin expression.
Msx1 and Msx2 have been proposed as Bmp target genes in a number of tissues, suggesting that they mediate a common rather than a tissue-specific function of Bmp signaling (Vainio et al., 1993; Furuta et al., 1997). Overexpression of Msx2 in the hair matrix reduces proliferation and induces premature differentiation, consistent with it mediating part of the growth regulatory functions of Bmps (Jiang et al., 1999). Gene inactivation of Msx1 and 2 has shown that they function redundantly during hair development (Satokata et al., 2000). The requirement of Msx function for hair follicle induction raises the possibility that Bmp activity is already necessary at early stages of hair follicle development to maintain expression of Msx1 and 2.
None of the Bmp-regulated transcription factors identified here clearly mediates the entire function of Bmp signaling in the hair follicle revealed by this study. This implies that it is the coordinated regulation of multiple factors by Bmps that controls hair shaft differentiation rather than the activation of a single key regulator.
Materials and methods
Generation of Msx2–Noggin transgenic mice
The Msx2 transgene was constructed by cloning 439 bp of the Msx2 promoter described in Liu et al. (1994) in front of the rabbit β-globin second intron and poly(A) signal, and inserting the xNoggin coding sequence from pNogginΔ5′ (Smith and Harland, 1992) after the intron. The transgene was released from the vector backbone and injected into one-cell (B6D2)F2 embryos. Transgenic founders usually showed a weaker phenotype than their offspring and were verified by PCR using an xNoggin-specific primer pair (5′ GCCAACATTATCTGCACATC, 3′ CTACTTTCACATAGCGAGGC). To facilitate the molecular analyses of the skin sections, line #14 was crossed into the CD1 albino background. Owing to low fertility of female transgenic animals, the line was maintained by backcrossing transgenic males with CD1 females. The phenotype of the transgenic animals did not change in the different genetic backgrounds.
Immunohistochemistry
Frozen sections (10 µm) of wild-type and transgenic P12 dorsal anterior skin were fixed for 30 min at room temperature in 4% paraformaldehyde and analyzed by indirect immunofluorescence. The primary antibodies used were rabbit anti-keratin 5 (MK5; BabCo, Richmond, CA) and the mouse monoclonal antibodies anti-trychohyalin (AE15; O’Guin et al., 1992) and anti-acidic hair keratin (AE13; Lynch et al., 1986). Cy3-coupled donkey anti-rabbit IgG and fluorescein-coupled donkey anti-mouse IgG secondary antibodies (Jackson Laboratory, West Grove, PA) were used. Slides were mounted with antifade (Biomeda, Foster City, CA) and analyzed using a Zeiss Axioplan 2 microscope.
In situ hybridization
Dorsal anterior skin samples were fixed overnight in 4% paraformaldehyde, embedded in paraffin and sectioned at 5 µm. 35S-labeled in situ probes were generated from the following templates using the transcription kit from Roche Molecular Biochemicals (Indianapolis, IN): pNogginΔ5′ (Smith and Harland, 1992); pBSwhn containing 650 bp (1223–1873) of the Foxn1 cDNA (H.Kulessa, unpublished); pTZ19λ2.2 (Msx1) (Hill et al., 1989); pHox8 (Msx2) (Monaghan et al., 1991); pKGFr-1 containing a cDNA fragment corresponding to amino acids 548–703 of the Fgfr2 (gift of Dr A.McMahon, Harvard University, Boston, MA); pKGF (Fgf7) (Mason et al., 1994); pGL1-3′ (Lef1) (Travis et al., 1991); pmBmp-2a (Bmp2) (Lyons et al., 1989).
β-galactosidase assay
Dorsal anterior skin of Bmp4lacZ and NogginlacZ heterozygous mice was fixed for 2 h at 4°C in 4% paraformaldehyde, washed twice in phosphate-buffered saline (PBS) and incubated for 20 h at 37°C in the dark in X-Gal staining solution containing 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, 1 mg/ml X-Gal and 0.02% NP-40 in PBS. After staining, the tissues were washed twice in PBS and fixed overnight in 4% paraformaldehyde at 4°C. The skin biopsies were dehydrated using isopropanol as the final step instead of xylene to avoid leaching of the blue precipitate, embedded in paraffin, and sectioned at 7 µm.
BrdU labeling
Mice were injected with 50 µg of BrdU and 10 µg of FdU per gram body weight in PBS and killed 4 h later. Dorsal anterior skin samples were fixed overnight in 4% paraformaldehyde, embedded in paraffin and sectioned at 5 µm. The sections were digested with 20 µg/ml proteinase K for 15 min at 37°C and blocked by incubation with 10% sheep serum for 30 min at room temperature. BrdU incorporation was detected by immunostaining using the cell proliferation kit from Amersham (Amersham UK) as recommended by the manufacturer. BrdU-positive nuclei in the hair bulb region of longitudinally sectioned follicles from four sibling pairs were counted. The misalignment of the transgenic hair follicles led to a greater variability in the plane of section through the hair bulb, causing the high standard variation.
For the simultaneous analyses of BrdU incorporation and Bmp4lacZ expression, skin samples of BrdU-injected mice were treated as described above for the β-galactosidase assay, but sectioned at 5 µm. Sections were blocked with 10% goat serum, and a Cy3-coupled goat anti-mouse IgG secondary antibody (Jackson Laboratory, West Grove, PA) was used for the immunodetection of BrdU.
Acknowledgments
Acknowledgements
We thank Lorene Batts for excellent technical assistance. Dr S.K.Dey, UK Kansas City, kindly provided the NogginlacZ skin for Figure 3. We thank Dr R.Harland, UC Berkeley, for subsequently providing the NogginlacZ mice to us. Antibodies AE13 and AE15 were a gift from Dr T.T.Sun, NYU New York. A.R.Godwin, UK Kansas City, provided the Hoxc13 plasmid. H.K. was supported by an EMBO Longterm Fellowship and is now an HHMI Associate. B.L.M.H. is a HHMI Investigator.
References
- Blessing M., Nanney,L.B., King,L.E., Jones,C.M. and Hogan,B.L. (1993) Transgenic mice as a model to study the role of TGF-β-related molecules in hair follicles. Genes Dev., 7, 204–215. [DOI] [PubMed] [Google Scholar]
- Botchkarev V.A. et al. (1999) Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nature Cell Biol., 1, 158–164. [DOI] [PubMed] [Google Scholar]
- Brissette J.L., Li,J., Kamimura,J., Lee,D. and Dotto,G.P. (1996) The product of the mouse nude locus, Whn, regulates the balance between epithelial cell growth and differentiation. Genes Dev., 10, 2212–2221. [DOI] [PubMed] [Google Scholar]
- Brunet L.J., McMahon,J.A., McMahon,A.P. and Harland,R.M. (1998) Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science, 280, 1455–1457. [DOI] [PubMed] [Google Scholar]
- Chiang C. et al. (1999) Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev. Biol., 205, 1–9. [DOI] [PubMed] [Google Scholar]
- DasGupta R. and Fuchs,E. (1999) Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development, 126, 4557–4568. [DOI] [PubMed] [Google Scholar]
- Epstein W. and Maibach,H. (1969) Cell proliferation and movement in human hair bulbs. Adv. Biol. Skin, 9, 83–97. [Google Scholar]
- Finch P.W., Rubin,J.S., Miki,T., Ron,D. and Aaronson,S.A. (1989) Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science, 245, 752–755. [DOI] [PubMed] [Google Scholar]
- Furuta Y., Piston,D.W. and Hogan,B.L. (1997) Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development, 124, 2203–2212. [DOI] [PubMed] [Google Scholar]
- Gat U., DasGupta,R., Degenstein,L. and Fuchs,E. (1998) De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell, 95, 605–614. [DOI] [PubMed] [Google Scholar]
- Godwin A.R. and Capecchi,M.R. (1998) Hoxc13 mutant mice lack external hair. Genes Dev., 12, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K., Ozawa,K., Itami,S. and Yoshikawa,K. (1999) Human fibroblast growth factor 10 expression in dermal papilla cells, outer root sheath cells and keratinocytes. Exp. Dermatol., 8, 347–349. [PubMed] [Google Scholar]
- Hardy M.H. (1992) The secret life of the hair follicle. Trends Genet., 8, 55–61. [DOI] [PubMed] [Google Scholar]
- Headon D.J. and Overbeek,P.A. (1999) Involvement of a novel Tnf receptor homologue in hair follicle induction. Nature Genet., 22, 370–374. [DOI] [PubMed] [Google Scholar]
- Hill R.E., Jones,P.F., Rees,A.R., Sime,C.M., Justice,M.J., Copeland,N.G., Jenkins,N.A., Graham,E. and Davidson,D.R. (1989) A new family of mouse homeo box-containing genes: molecular structure, chromosomal location, and developmental expression of Hox-7.1. Genes Dev., 3, 26–37. [DOI] [PubMed] [Google Scholar]
- Jahoda C.A. and Reynolds,A.J. (1996) Dermal–epidermal interactions. Adult follicle-derived cell populations and hair growth. Dermatol. Clin., 14, 573–583. [DOI] [PubMed] [Google Scholar]
- Jiang T.X., Liu,Y.H., Widelitz,R.B., Kundu,R.K., Maxson,R.E. and Chuong,C.M. (1999) Epidermal dysplasia and abnormal hair follicles in transgenic mice overexpressing homeobox gene MSX-2. J. Invest. Dermatol., 113, 230–237. [DOI] [PubMed] [Google Scholar]
- Kopf-Maier P., Mboneko,V.F. and Merker,H.J. (1990) Nude mice are not hairless. A morphological study. Acta Anat. (Basel), 139, 178–190. [DOI] [PubMed] [Google Scholar]
- Kratochwil K., Dull,M., Farinas,I., Galceran,J. and Grosschedl,R. (1996) Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev., 10, 1382–1394. [DOI] [PubMed] [Google Scholar]
- Lawson K.A., Dunn,N.R., Roelen,B.A., Zeinstra,L.M., Davis,A.M., Wright,C.V., Korving,J.P. and Hogan,B.L. (1999) Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev., 13, 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Prowse,D.M. and Brissette,J.L. (1999) Association between mouse nude gene expression and the initiation of epithelial terminal differentiation. Dev. Biol., 208, 362–374. [DOI] [PubMed] [Google Scholar]
- Liu Y.H., Ma,L., Wu,L.Y., Luo,W., Kundu,R., Sangiorgi,F., Snead,M.L. and Maxson,R. (1994) Regulation of the Msx2 homeobox gene during mouse embryogenesis: a transgene with 439 bp of 5′ flanking sequence is expressed exclusively in the apical ectodermal ridge of the developing limb. Mech. Dev., 48, 187–197. [DOI] [PubMed] [Google Scholar]
- Lynch M.H., O’Guin,W.M., Hardy,C., Mak,L. and Sun,T.T. (1986) Acidic and basic hair/nail (‘hard’) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to ‘soft’ keratins. J. Cell Biol., 103, 2593–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons K.M., Pelton,R.W. and Hogan,B.L. (1989) Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-β-like genes coordinately regulate aspects of embryonic development. Genes Dev., 3, 1657–1668. [DOI] [PubMed] [Google Scholar]
- Mason I.J., Fuller-Pace,F., Smith,R. and Dickson,C. (1994) FGF-7 (keratinocyte growth factor) expression during mouse development suggests roles in myogenesis, forebrain regionalisation and epithelial–mesenchymal interactions. Mech. Dev., 45, 15–30. [DOI] [PubMed] [Google Scholar]
- Monaghan A.P., Davidson,D.R., Sime,C., Graham,E., Baldock,R., Bhattacharya,S.S. and Hill,R.E. (1991) The Msh-like homeobox genes define domains in the developing vertebrate eye. Development, 112, 1053–1061. [DOI] [PubMed] [Google Scholar]
- Nehls M., Pfeifer,D., Schorpp,M., Hedrich,H. and Boehm,T. (1994) New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature, 372, 103–107. [DOI] [PubMed] [Google Scholar]
- Noramly S. and Morgan,B.A. (1998) BMPs mediate lateral inhibition at successive stages in feather tract development. Development, 125, 3775–3787. [DOI] [PubMed] [Google Scholar]
- O’Guin W.M., Sun,T.T. and Manabe,M. (1992) Interaction of trichohyalin with intermediate filaments: three immunologically defined stages of trichohyalin maturation. J. Invest. Dermatol., 98, 24–32. [DOI] [PubMed] [Google Scholar]
- Oro A.E., Higgins,K.M., Hu,Z., Bonifas,J.M., Epstein,E.H.,Jr and Scott,M.P. (1997) Basal cell carcinomas in mice overexpressing sonic hedgehog. Science, 276, 817–821. [DOI] [PubMed] [Google Scholar]
- Pathi S., Rutenberg,J.B., Johnson,R.L. and Vortkamp,A. (1999) Interaction of Ihh and BMP/Noggin signaling during cartilage differentiation. Dev. Biol., 209, 239–253. [DOI] [PubMed] [Google Scholar]
- Prowse D.M., Lee,D., Weiner,L., Jiang,N., Magro,C.M., Baden,H.P. and Brissette,J.L. (1999) Ectopic expression of the nude gene induces hyperproliferation and defects in differentiation: implications for the self-renewal of cutaneous epithelia. Dev. Biol., 212, 54–67. [DOI] [PubMed] [Google Scholar]
- Rosenquist T.A. and Martin,G.R. (1996) Fibroblast growth factor signalling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev. Dyn., 205, 379–386. [DOI] [PubMed] [Google Scholar]
- Satokata I. et al. (2000) Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nature Genet., 24, 391–395. [DOI] [PubMed] [Google Scholar]
- Smith W.C. and Harland,R.M. (1992) Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell, 70, 829–840. [DOI] [PubMed] [Google Scholar]
- St-Jacques B. et al. (1998) Sonic hedgehog signaling is essential for hair development. Curr. Biol., 8, 1058–1068. [DOI] [PubMed] [Google Scholar]
- Takahashi H. and Ikeda,T. (1996) Transcripts for two members of the transforming growth factor-β superfamily BMP-3 and BMP-7 are expressed in developing rat embryos. Dev. Dyn., 207, 439–449. [DOI] [PubMed] [Google Scholar]
- Travis A., Amsterdam,A., Belanger,C. and Grosschedl,R. (1991) LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor α enhancer function. Genes Dev., 5, 880–894. [DOI] [PubMed] [Google Scholar]
- Vainio S., Karavanova,I., Jowett,A. and Thesleff,I. (1993) Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell, 75, 45–58. [PubMed] [Google Scholar]
- Wardle F.C., Angerer,L.M., Angerer,R.C. and Dale,L. (1999) Regulation of BMP signaling by the BMP1/TLD-related metalloprotease, SpAN. Dev. Biol., 206, 63–72. [DOI] [PubMed] [Google Scholar]
- Widelitz R.B., Jiang,T.X., Noveen,A., Chen,C.W. and Chuong,C.M. (1996) FGF induces new feather buds from developing avian skin. J. Invest. Dermatol., 107, 797–803. [DOI] [PubMed] [Google Scholar]
- Wilson N., Hynd,P.I. and Powell,B.C. (1999) The role of BMP-2 and BMP-4 in follicle initiation and the murine hair cycle. Exp. Dermatol., 8, 367–368. [PubMed] [Google Scholar]
- Zhao G.Q. and Hogan,B.L. (1996) Evidence that mouse Bmp8a (Op2) and Bmp8b are duplicated genes that play a role in spermatogenesis and placental development. Mech. Dev., 57, 159–168. [DOI] [PubMed] [Google Scholar]
- Zhou P., Byrne,C., Jacobs,J. and Fuchs,E. (1995) Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev., 9, 700–713. [DOI] [PubMed] [Google Scholar]
- Zimmerman L.B., De Jesus-Escobar,J.M. and Harland,R.M. (1996) The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell, 86, 599–606. [DOI] [PubMed] [Google Scholar]