Abstract
Regulated mRNA decay is essential for eukaryotic survival but the mechanisms for regulating global decay and coordinating it with growth, nutrient, and environmental cues are not known. Here we show that a signal transduction pathway containing the Pkh1/Pkh2 protein kinases and one of their effector kinases, Pkc1, is required for and regulates global mRNA decay at the deadenylation step in Saccharomyces cerevisiae. Additionally, many stresses disrupt protein synthesis and release mRNAs from polysomes for incorporation into P-bodies for degradation or storage. We find that the Pkh1/2-Pkc1 pathway is also required for stress-induced P-body assembly. Control of mRNA decay and P-body assembly by the Pkh-Pkc1 pathway only occurs in nutrient-poor medium, suggesting a novel role for these processes in evolution. Our identification of a signaling pathway for regulating global mRNA decay and P-body assembly provides a means to coordinate mRNA decay with other cellular processes essential for growth and long-term survival. Mammals may use similar regulatory mechanisms because components of the decay apparatus and signaling pathways are conserved.
Keywords: MRNA, P-body, Protein Kinase C (PKC), Protein Synthesis, Signal Transduction, Deadenylation, mRNA Decay
Introduction
Messenger RNA decay is vital for control of eukaryotic gene expression and large-scale analyses suggest that as many as half of all changes in mRNA levels during stress are due to mRNA decay (1, 2). Progress in understanding the mechanisms of mRNA decay is notable, yet we still do not understand how global or basal decay is regulated or how decay and processing body (P-body)3 formation are coupled to stresses and nutrients. In this work we identify a signal transduction pathway that regulates global mRNA decay and P-body formation during nutrient limitation.
A well studied route of mRNA decay in eukaryotes, termed deadenylation-dependent decay, begins by shortening or deadenylation of the 3′ polyadenosine (poly(A)) tail, a rate-limiting step in mRNA decay (reviewed in Refs. 3–5). Deadenylation in mammals is also the initiating event in other mRNA decay pathways involving AU-rich elements, destabilizing elements in coding regions, nonsense codons, and micro-RNAs (3–8).
Once a poly(A) tail is shortened, further degradation proceeds by removal of the 5′ N7-methylguanosine (m7G) cap from the mRNA (called “decapping”) followed by 5′-to-3′ exonuclease digestion by XrnI/KemI protein. Many mRNAs are degraded by this pathway in Saccharomyces cerevisiae, which has played key roles in elucidating the mechanisms of eukaryotic mRNA decay (9). Alternatively, a deadenylated transcript can be degraded in the 3′-to-5′ direction by the exosome (6, 7, 10).
Deadenylation of most mRNAs in S. cerevisiae begins with removal of ∼20 bases from the poly(A) tail by the Pan2-Pan3 deadenylase (11), followed by the Ccr4-Pop2-Not (12) deadenylase (9, 13), which shortens the tail to around 10 bases at which point decapping occurs (14). Mammals also use this two-step deadenylation mechanism, which is likely to be conserved in most eukaryotes (15).
Decapping begins by binding of the Lsm1–7 complex to the partially deadenylated mRNA, followed by recruitment of the Dcp1/Dcp2 decapping enzyme whose activity is stimulated by the Dhh1 and Pat1 proteins (7, 16). Decapping and 5′-to-3′ exonucleolytic degradation can occur on cytoplasmic foci termed P-bodies (10, 17, 18) or can occur independently of P-bodies (19–22). Recently deadenylation, decapping, and 5′-to-3′ decay have been shown to also occur on translating mRNAs in yeast cells (23).
To survive nutrient depletion and other stresses, eukaryotes have evolved complex coping mechanisms including changes in the rate of global mRNA decay. Mammals respond to UV-B radiation, hydrogen peroxide, heat and high osmolarity by reducing the rate of mRNA decay at the deadenylation step (24). S. cerevisiae cells also reduce the rate of deadenylation in response to these stresses and to glucose withdrawal, a severe nutritional stress (25, 26). Additionally, many stresses inhibit translation initiation and the mRNAs released from polysomes are sequestered into P-bodies (3, 4, 7, 16, 27). P-bodies not only mediate mRNA decay, but studies in S. cerevisiae show that P-bodies repress translation and store mRNAs for reuse (28). P-bodies or related structures called stress granules appear to perform similar functions in mammals (3, 4, 7, 29).
We are interested in identifying new processes controlled by the Pkh1 and Pkh2 protein kinases in S. cerevisiae because of their roles in regulating growth and survival in response to nutritional cues and environmental stresses and because they are homologs of mammalian phosphoinositide-dependent protein kinase 1 (PDK1) (30, 31). Pkh1/2 typically control cellular processes by regulating AGC-type protein kinases including Pkc1, Ypk1, and Ypk2, orthologs of mammalian serum- and glucocorticoid-inducible protein kinase 1 and Sch9, an ortholog of mammalian Akts/PKBs and S6 kinases (32, 33). Mammalian PDK1 also controls AGC-type protein kinases involved in many cellular processes (34).
By using synthetic genetic array (SGA) analysis (35) to find new cellular processes controlled by Pkh1/2, we show that the Pkh1/2-Pkc1 pathway is required for and regulates the basal rate of mRNA decay at the deadenylation step as well as the formation of P-bodies induced by stresses. Unexpectedly, global deadenylation and mRNA decay along with P-body assembly only require Pkh1/2-Pkc1 pathway activity when cells are growing on nutrient-poor medium, establishing a connection between nutrient availability and mRNA decay.
EXPERIMENTAL PROCEDURES
Strains and Media
Yeast strains and plasmids used in these studies are described under supplemental Tables S1 and 2. Cells were grown in YPD (1% yeast extract, 2% peptone, 2% glucose) or synthetic medium containing 2 or 4% glucose (SD) or 2% galactose and 1% sucrose (SG) plus 0.34% yeast nitrogen base (Difco), 1% ammonium sulfate, 30 mg/liter of adenine sulfate, tryptophan, and tyrosine, and 20 mg/liter each of histidine, leucine, lysine, methionine, and uracil. Solid medium contained 2% agar. A nutrient-enriched version of SD medium contained all 20 amino acids, adenine, uracil, inositol, and para-aminobenzoic acid (36).
Analysis of mRNA Decay, Deadenylation, P-bodies, and Protein Synthesis
Experiments were based on published procedures (36–38). To shut-off transcription of reporter genes, cells were grown to an A600 nm of 0.3–0.4 in SG medium and GAL gene expression was repressed by centrifuging cells and suspending in SD (4% glucose) medium. Where indicated in the text, transcription was also shut-off by using thiolutin (4 or 8 μg/ml). RNA was isolated as previously described (36). A total of 20 μg of RNA from each time point was separated by electrophoresis on a 1.4% agarose gel or 6% denaturing PAGE gel. Northern analysis was performed by using 32P-labeled 5′-end oligonucleotides (5′-AATTCCCCCCCCCCCCCCCCCCA-3′, 5′-GCCCAATGCTGGTTTAGAGACGATGATAGCATTTTCTAGCTCAGCATCAGTGATCTTAGGG-3′ and 5′-CCATACCTCTACCACCGGGGTGCTTTCTGTGCTTACCG-3′ as the probes to detect MFA2pG, GAL1-L, and CYH2 mRNAs, respectively. Blots were stripped and reprobed using a 32P-labeled 5′-end oligonucleotides (5′-GTCTAGCCGCGAGGAAGG-3′) complementary to the SCR1 RNA, which served as a loading control for each lane. For experiments involving a temperature shift, cells were grown at 25 °C, switched to 39 °C, and transcription was shut-off after 30, 60, or 120 min as indicated in the figures. Radioactive signals on Northern blots were quantified by using a PhosphorImager and mRNA half-lives were calculated from mRNA signals normalized to the SCR1 signal.
The role of Pkh1/2 and Pkc1 in P-body assembly was examined in the strains listed in the figure legends. Cells grown to an A600 nm of 0.3–0.4 in SD-Leu-Ura medium at 25 °C were switched to 39 °C for various times before inducing P-body formation by glucose starvation, which involved washing cells once by centrifugation and suspending in S medium lacking glucose for 10 min followed immediately by fluorescent microscopy. To induce hypotonic stress, cells were grown in SD-Leu-Ura medium, washed with and then suspended in water for 10 min, and examined by fluorescent microscopy. To induce hypertonic stress, cells were washed with and suspended in medium containing 1 m KCl for 15 min. Microscopy was performed with a Nikon ECLIPSE E600 equipped with a PLAN APO ×100, 1.40 oil immersion objective. Room temperature samples were photographed with a SPOT RT 9.0 Monochrome-6 camera using the MetaMorph (version 6.3.0) acquisition software. Images were processed by using Adobe Photoshop (7.0).
Protein synthesis was measured using a slightly modified version of a published procedure (39). Cells were grown at 25 °C to an A600 nm of 0.4 in SD medium, shifted to 39 °C, and at 0, 10, 30, and 60 min, triplicate 1-ml samples were incubated for 5 min at 39 °C with 2 μl of a radioactive solution containing [35S]methionine and [35S]cysteine (EXPRESS35S35S Protein Labeling Mix, NEG0700, 1150 Ci/mmol, New England Biolabs). Protein synthesis was stopped by adding 0.5 ml of 20% cold TCA to each reaction and further sample processing was as described previously (39).
Miscellaneous
SGA analyses were performed as previously described (40). Thiolutin (Enzo Life Science International) was dissolved in dimethyl sulfoxide at 1 mg/ml and used at a concentration of 4 or 8 μg/ml.
RESULTS
Synthetic Genetic Array Links Pkh1/2 to mRNA Decay
To use the SGA method as a way to find new processes regulated by Pkh1/2 we constructed a query strain, RCD587 (pkh1ts::URA3 pkh2::NAT), carrying a temperature-sensitive pkh1 allele and a pkh2 deletion allele, because deletion of both genes is lethal (30). Crossing this strain to the set of 4,700 viable yeast deletion mutants (35) produced 140 slow-growing haploid triple mutant strains (pkh1ts::URA3 pkh2::NAT geneX::KAN). This group contained strains mutated in genes with known roles in processes controlled by Pkh1/2 thereby validating the screen. For example, Pkh1/2 phosphorylate Pkc1, which regulates cell wall biosynthesis (31) via the cell integrity pathway (41–44). Our screen identified genes including ROM2, SLT2, SMI1, and BCK1 encoding components of this pathway and FKS1, GAS1, GAS4, GAS5, LAS21, YUR1, RIM21, and OST6 that encode proteins necessary for cell wall functions.
A novel and unanticipated synthetic slow growing strain carried the dhh1Δ mutation. Dhh1 is a DEXD/H-box helicase that stimulates mRNA decapping, represses translation, and functions in P-body formation (27, 45–47). A weaker synthetic slow growth interaction occurred with the KEM1/XRN1 gene, responsible for the 5′ to 3′ degradation of decapped mRNAs (48). These data suggested that Pkh1/2 have a role in mRNA decay.
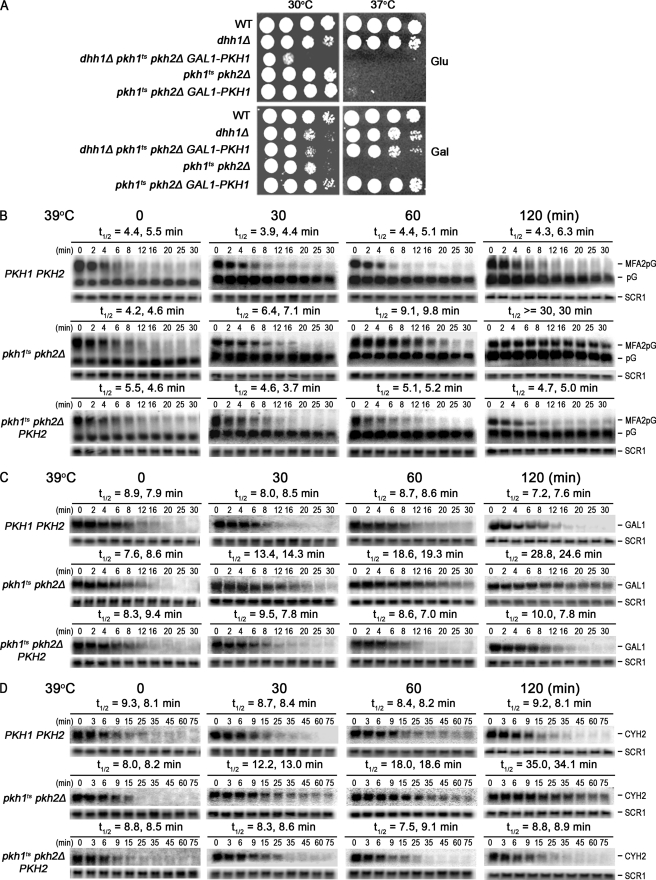
To verify the DHH1 interaction, the pkh1ts pkh2Δ query strain (referred to as pkhts) was transformed with a CEN vector carrying PKH1 under control of the GAL1 promoter to make PKH1 expression galactose-inducible and glucose-repressible. DHH1 was deleted in these cells to produce pkh1ts pkh2Δ dhh1::KAN (pGAL1-PKH1) cells whose growth was more impaired at 30 °C than either parent or wild-type cells when GAL1-PKH1 expression was repressed by glucose (Fig. 1A, compare the 30 °C Glu and Gal panels), thus, validating the slow-growth phenotype found in the SGA assay.
FIGURE 1.
Pkh1/2 regulate mRNA decay. A, to verify the SGA data suggesting that Pkh1/2 play roles in mRNA decay, growth of parental dhh1::KAN and pkh2::NAT pkh1D398G::ura3 cells was compared with triple mutant dhh1::KAN pkh2::NAT pkh1D398G::ura3 cells transformed with a CEN vector carrying PKH1 controlled by the galactose-inducible and glucose-repressible GAL1 promoter. Cells were grown to stationary phase in YPD medium, diluted 10-fold, spotted onto YPD (Glu) or YPGal (Gal) plates, and incubated at 30 or 37 °C, the restrictive temperature for strains carrying the temperature-sensitive pkh1tsD398G allele (31). Strains are from top to bottom: Y7092/YCplac33GAL, RCD936/YCplac33GAL, RCD937/YCplac33GAL-PKH1, RCD856/YCplac33GAL, and RCD856/YCplac33GAL-PKH1. B, the rate of MFA2pG mRNA decay was measured in WT (Y7092/pRP485/pRS315), pkh1ts pkh2Δ (RCD856/pRP485/pRS315), and pkh1ts pkh2Δ cells carrying PKH2 (RCD856/pRP485/pRS315-PKH2). Cells were grown to early log phase at 25 °C, switched to 39 °C for 30, 60, or 120 min and transcription of the MFA2pG gene was shut-off by suspending cells in medium containing glucose (4% final concentration) and thiolutin (4 μg/ml final concentration) at time 0. RNA extracted at the indicated times was analyzed on agarose gels by Northern blotting. Similar procedures were used to measure the rate of decay of the endogenous GAL1-L (C) and CYH2 mRNAs (D) except that transcription of CYH2 was shut-off by using only thiolutin (8 μg/ml). Rates of decay (t½) calculated from two experiments are shown above the panels. SCR1 RNA was used as a loading control and pG at the right of figure indicates the position of the 3′ degradation intermediate. Half-life values are shown for two experiments.
Pkh1/2 Regulate mRNA Decay
To determine whether Pkh1/2 regulates mRNA decay, we measured the half-life (t½) of the MFA2pG reporter mRNA by Northern blotting (49). In these and subsequent experiments, transcription of the plasmid-borne GAL1-MFA2pG gene was activated by growing cells in the presence of galactose and shutoff by adding glucose to the culture medium. In pkhts mutant cells (pkh1ts pkh2Δ) grown at 25 °C the MFA2pG mRNA had a average t½ of 4.4 min, similar to the average t½ of 4.9 min in wild-type cells (Fig. 1B, 0 min time point). In pkhts cells the rate of decay became slower the longer the cells were at the restrictive temperature of 39 °C so that after 30 min of incubation the t½ was 6.7 min and after 120 min it was >30 min (Fig. 1B). In contrast, the t½ did not change in wild-type cells over this time course. The longer t½ in pkhts cells is due to reduced Pkh activity because the wild-type t½ is nearly restored when the PKH2 gene is returned to mutant cells on a single-copy plasmid (Fig. 1B). These results show that Pkh1/2 regulate MFA2pG mRNA decay.
To determine whether the behavior of the MFA2pG mRNA in pkhts cells is representative of global mRNA decay, two endogenous mRNAs were examined. The GAL1 mRNA had an average t½ of 8.4 min in wild-type cells at time 0 and this value did not change during 2 h of incubation at the restrictive temperature (Fig. 1C). In pkhts cells the average t½ was 8 min at time 0 but slowed to 13.9 min following 30 min incubation at 39 °C and continued to decrease to about 26 min after 2 h. The same trends were seen with the CYH2 mRNA with the t½ remaining relatively constant in wild-type cells (average of about 8.5 min) and becoming longer in pkhts cells during the 2-h incubation at 39 °C (Fig. 1D). For both of these endogenous mRNAs the slowing of the decay rate in pkhts cells was prevented by supplying PKH2. The t½ values in wild-type cells are similar to published values (9, 50). We conclude from these data that global mRNA decay requires functional Pkh1/2.
Pkh1/2 Regulate the Deadenylation Step in mRNA Decay
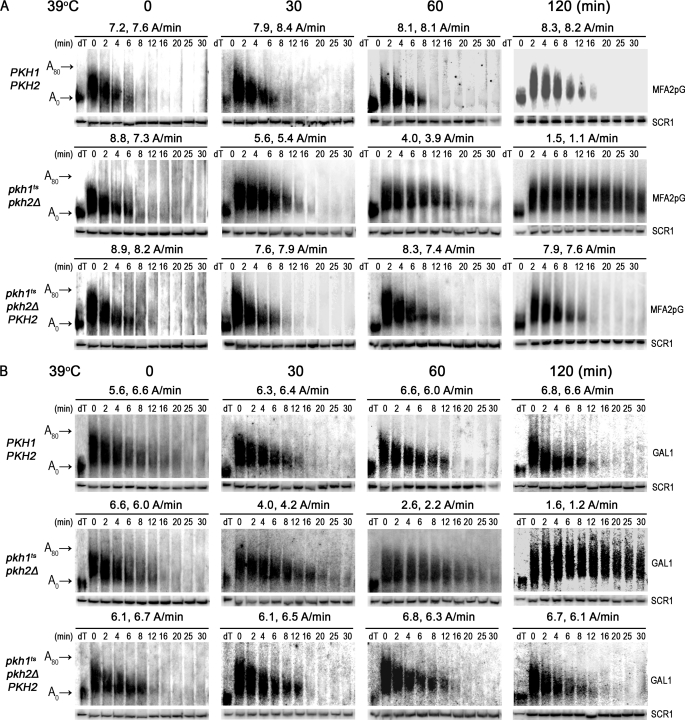
Deadenylation and decapping are highly regulated steps in mRNA decay (3, 5, 7, 51, 52). To determine whether Pkh1/2 regulate either step we analyzed a portion of the RNA samples used to generate the data shown in Fig. 1 by using high-resolution polyacrylamide gel electrophoresis (PAGE) (9, 53). Under these conditions most of the poly(A) tail on MFA2pG mRNA in wild-type cells was hydrolyzed within 8 min or at the rate of about 8 adenosines (A)/min, a rate that remained constant over 2 h (Fig. 2A). Mutant pkhts cells had a similar rate of deadenylation at time 0, but after 30 min at 39 °C the rate dropped to 5.5 A/min and continued to drop over the course of the experiment to a rate of 1.3 A after 2 h. Returning PKH2 to mutant cells restored a normal rate of deadenylation. Similar affects on the rate of deadenylation of endogenous GAL1 mRNA were observed (Fig. 2B). We conclude from these data that Pkh1/2 are required for and regulate the deadenylation step in mRNA decay.
FIGURE 2.
Pkh1/2 regulate the deadenylation step of mRNA decay. To measure the rate of deadenylation of the MFA2pG (A) and GAL1-L (B) mRNAs, portions of the RNA samples used for the Northern blots shown in Fig. 1 were also examined by PAGE. Lanes labeled dT show the mobility of mRNA without a poly(A) as indicated at the left of the figure (A0) or the position of an mRNA with an intact poly(A) tail (A80). Rates of deadenylation in A/min are shown for 2 experiments.
Pkc1 Controls mRNA Decay and Deadenylation
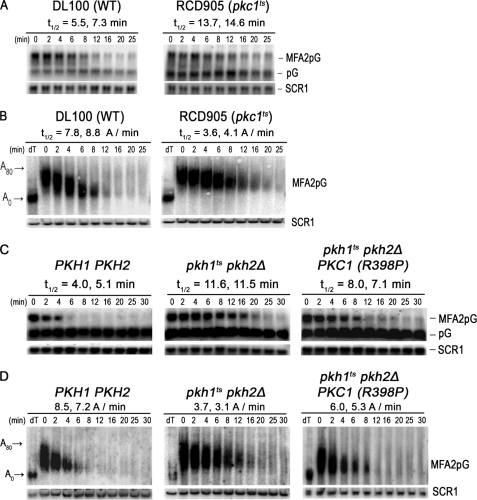
Because most functions of Pkh1/2 are mediated by downstream protein kinases Pkc1, Ypk1/2, and Sch9, we determined if any of these kinases control mRNA decay. Analysis of sch9Δ and ypk1ts ypk2Δ mutant cells indicated that Sch9 and Ypk1/2 are not required for mRNA decay (supplemental Fig. S1). Because PKC1 is an essential gene, we examined mRNA decay in a strain with a temperature-sensitive pkc1 allele (54) using procedures similar to those used with pkhts cells except that pkc1ts cells were incubated at the restrictive temperature of 39 °C for 1 h only before mRNA decay was examined. This shorter incubation time was necessary to avoid cell lysis, which occurs at longer incubation times because the Pkc1 pathway is the master regulator of cell wall maintenance and repair (55). We verified experimentally that less than 20% of the cells lysed after 60 min at 39 °C.
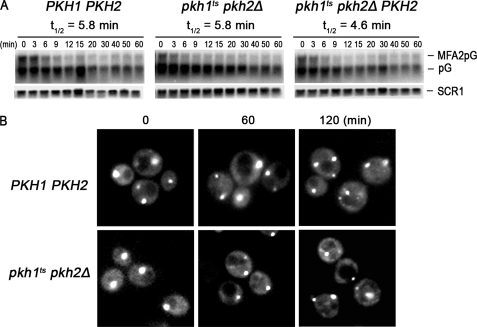
The half-life of MFA2pG mRNA in pkc1ts cells shifted to the restrictive temperature was 14.2 min, or about 2-fold slower than the 6.4-min half-life observed in wild-type cells (Fig. 3A). The affect of Pkc1 on deadenylation was analyzed by subjecting a portion of RNA to denaturing polyacrylamide gel electrophoresis, which revealed that the reduced rate of decay was due to impaired deadenylation in pkc1ts cells and not to impaired decapping or hydrolysis of the mRNA body in the 5′ to 3′ direction by the XrnI exonuclease (Fig. 3B). For example, in wild-type cells the poly(A) tails were degraded at an average rate of 8.3 A/min, whereas in pkc1ts cells they were degraded at an average rate of 3.8 A/min.
FIGURE 3.
Pkc1 controls mRNA decay and deadenylation. A, to determine whether Pkc1 controls MFA2pG mRNA decay, the rate of decay was examined in cells with wild-type PKC1 (DL100/pRP485-2) or a pkc1ts allele (RCD905/pRP485-2). Cells were grown at 39 °C for 60 min before transcriptional shut-off using glucose (4%) and thiolutin (4 μg/ml). RNA was extracted at the indicated time and analyzed on agarose gels. B, a portion of the RNAs shown in panel A was subjected to PAGE to examine the rate of deadenylation. RNA analyses were done as described in the legend to Figs. 1 and 2. C, to demonstrate that Pkc1 is the downstream effector of Pkh1/2 responsible for regulating mRNA decay and deadenylation, we compared MFA2pG mRNA decay in pkhts cells (RCD856/pRP485/pRS315-PKC1R398P) transformed with a plasmid expressing an allele of PKC1 encoding a constitutively active kinase to WT (Y7092/pRP485/pRS315) and pkhts cells transformed with just the vector (RCD856/pRP485/pRS315). The procedures for growing cells, incubating them for 1 h at the restrictive temperature, and analyzing mRNA decay were done exactly as described in the legend for Fig. 1B. D, portions of the RNA samples used for the Northern blots shown in panel C were also examined by PAGE to measure rates of deadenylation. Lanes labeled dT show the mobility of mRNA without a poly(A) as indicated at the left of the figure (A0) or the position of an mRNA with an intact poly(A) tail (A80).
Our data are consistent with Pkc1 acting downstream of Pkh1/2 to regulate deadenylation. But to directly verify this hypothesis we sought to show that a constitutively active PKC1 allele (54) would bypass impaired deadenylation in pkhts mutant cells because such an allele would not require active Pkh. In pkhts mutant cells grown for 1 h at a restrictive temperature the t½ of MFA2pG mRNA was 11.5 min or a little over twice as long as the 4.5 min t½ observed in wild-type cells (Fig. 3C). Mutant cells carrying the constitutive PKC1 allele on a CEN vector had a t½ for MFA2pG mRNA of 7.5 min, showing that the rate of decay was partially restored to the wild-type rate. Likewise, the constitutive PKC1 allele partially restored the rate of deadenylation (Fig. 3D). Partial restoration of the decay and deadenylation rates is expected because cells have both wild-type and constitutive Pkc1 activity. Based upon our data for mRNA decay and deadenylation in pkc1ts cells we conclude that Pkc1 activity is required for and regulates the deadenylation step of the mRNA decay pathway.
The Pkh1/2-Pkc1 Pathway Regulates P-body Formation
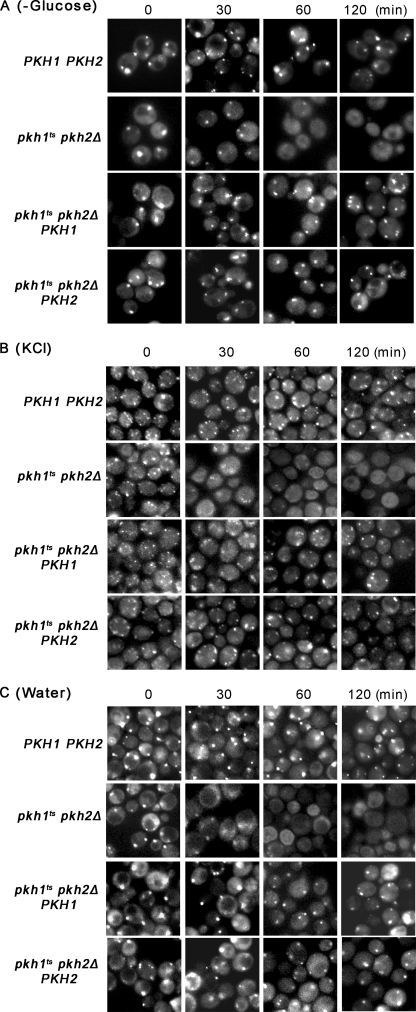
We determined if the Pkh1/2-Pkc1 pathway controls P-body assembly because the mechanism for controlling their assembly is not known. The Dcp2 subunit of the decapping enzyme, having the green fluorescent protein (GFP) fused to its C terminus, served as a visual reporter for P-body formation (17). The pkhts cells were grown at 25 °C, switched to 39 °C for various times, starved 10 min for glucose to induce P-body formation, and examined by fluorescent microscopy. Before the temperature shift (time 0), one or two large P-bodies appeared in wild-type, pkhts, and pkhts cells carrying the PKH1 or PKH2 genes on a CEN vector (Fig. 4A). After 30 min at 39 °C fewer P-bodies formed in pkhts than in wild-type cells and they were smaller sized. At the 60- and 120-min time points the difference in P-body size and number between pkhts and wild-type or pkhts mutant cells carrying the PKH1 or PKH2 gene became even more pronounced.
FIGURE 4.
Pkh1/2 control P-body assembly. To determine whether Pkh1/2 control P-body assembly, wild-type cells (WT) (Y7092/pRP1175/pRS316), pkh1ts pkh2Δ (RCD856/pRP1175/pRS316), and pkh1ts pkh2Δ cells carrying PKH1 (RCD856/pRP1175/pRS316-PKH1) or PKH2 (RCD856/pRP1175/pRS316-PKH2) were grown to an A600 of 0.3 in SD-Ura-Leu at 25 °C. Cultures were switched to 39 °C and incubated for the times indicated above each panel and then P-body assembly was examined after starving cells for glucose (A), stressing with 1 m KCl (B) or stressing with water (C). P-bodies were visualized by fluorescent microscopy using Dcp2 carrying a C-terminal GFP tag. Experiments were performed three times.
P-bodies can be induced also by hypertonic stress, imposed by suspending cells in medium containing 1 m KCl, and hypotonic stress, imposed by suspending cells in water (25). These two stress conditions had the same affect on P-body assembly as did glucose starvation: after 30 min of stress fewer P-bodies and of reduced size formed in pkhts mutant cells compared with wild-type or mutant cells carrying the PKH1 or PKH2 genes (Fig. 4, B and C).
Control experiments were performed for P-body formation data presented in Fig. 4. First, assembly of P-bodies after a shift to 39 °C required glucose starvation, because without it there were few if any P-bodies in any of the strains examined (supplemental Fig. S2A). This experiment also shows that stressing cells by shifting to 39 °C does not induce P-bodies. Next, the decrease in P-body assembly in pkhts mutant cells shifted to 39 °C was not due to a reduction in the concentration of the Dcp2-GFP reporter protein because immunoblotting showed that the protein level remained constant (supplemental Fig. S2B). We conclude from these data that Pkh1/2 are required for and regulate P-body assembly induced by nutritional and environmental stresses.
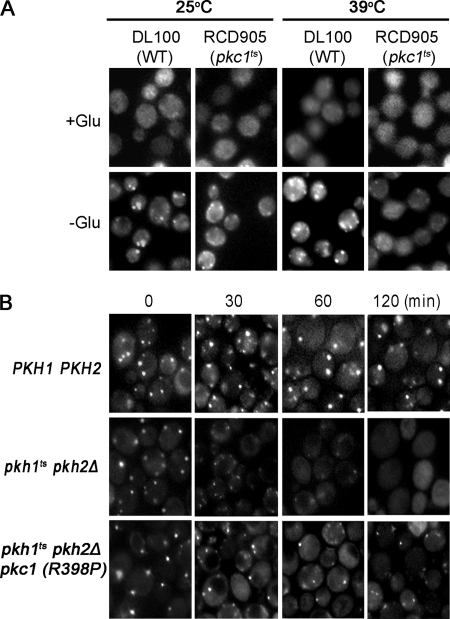
To determine whether Pkc1 plays a role in P-body assembly, pkc1ts cells carrying the Dcp2-GFP reporter protein were examined by florescence microscopy. Wild-type and pkc1ts cells grown at 25 °C to early log phase and not starved for glucose have very few P-bodies but when starved for glucose for 10 min P-bodies form in most cells (Fig. 5A). Following growth for 1 h at 39 °C to inactivate the temperature-sensitive Pkc1, wild-type and pkc1ts cells have very few P-bodies when not starved for glucose, indicating that the temperature shift is not stressful enough to induce P-body assembly. However, P-bodies do form in wild-type cells starved for glucose, whereas in pkc1ts cells the number and size of P-bodies is very much reduced compared with wild-type cells or to mutant cells grown at 25 °C (Fig. 5). These data demonstrate that Pkc1 activity is required for P-body assembly. We also demonstrated that Pkc1 controls P-body assembly by showing that a constrictively active PKC1 allele (R398P) carried on a CEN vector partially restores P-body assembly in pkhts cells subjected to glucose starvation (Fig. 5B).
FIGURE 5.
Pkc1 controls P-body assembly. A, to determine whether Pkc1 controls P-body assembly, wild-type cells (DL100/pRP1175) and pkh1ts (RCD905/pRP1175) mutant cells were grown to early log phase at 25 °C, treated for 10 min with synthetic medium lacking (−Glu) or containing glucose (+Glu) and then examined by fluorescence microscopy. Another sample of cells was shifted to 39 °C for 60 min and then P-bodies were induced or not induced by glucose starvation, and cells were examined by fluorescent microscopy. B, to demonstrate that Pkc1 works downstream of Pkh1/2 to regulate P-body formation induced by glucose starvation, P-body assembly was examined in pkh1ts pkh2Δ (RCD856/pRP1175/pRS315-PKC1R398P) producing a constitutively active version of Pkc1.
Deadenylation was suggested recently to be a prerequisite for P-body formation in cultured mammalian cells (51) and our data imply that this is true in yeast as well. To examine this possibility further, we studied ccr4Δ pan2Δ cells because they lack both yeast deadenylases and mRNA deadenylation is blocked (9). P-body formation induced by glucose withdrawal is normal in ccr4Δ cells (56), but as far as we are aware, similar studies have not been performed in pan2Δ or ccr4Δ pan2Δ mutant cells. We found that glucose starvation induces P-body formation in ccr4Δ pan2Δ cells nearly as well as in wild-type cells or in the single ccr4Δ or pan2Δ mutants (supplemental Fig. S3). We conclude that deadenylation is not a requirement for P-body formation, at least not under the experimental conditions examined.
Pkc1 Does Not Control Deadenylation by Down-regulating Protein Synthesis
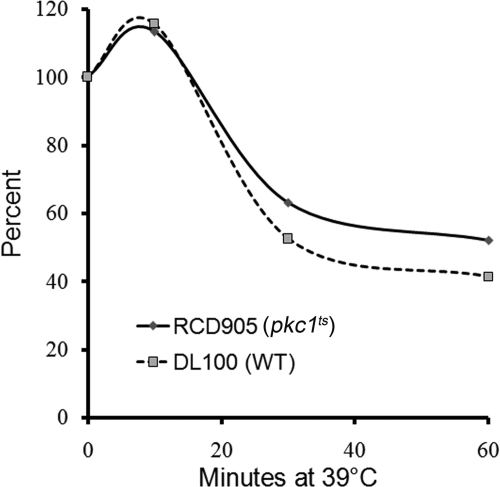
Many stresses depress protein synthesis and deadenylation in tandem as, for example, when yeast cells are starved for glucose, subjected to very high temperatures, or changes in osmotic pressure (26). To determine whether Pkc1 controls deadenylation by this general stress response or by another mechanism that is specific for deadenylation and does not involve protein synthesis, we measured the rate of protein synthesis at various times after shifting pkc1ts mutant cells to a restrictive temperature and compared the results to wild-type cells. The rate of protein synthesis in pkc1ts mutant cells was nearly identical to that in wild-type cells and both increased slightly after 10 min at the restrictive temperature (Fig. 6). Thereafter, the rate of synthesis decreased to 50–60% of the starting value after about 30 min of incubation and remained at this level. These results are clearly different from the 80–90% drop in the rate of protein synthesis that occurs immediately after cells are starved for glucose (39). Our protein synthesis results for pkc1ts cells are very similar to published data for pkhts cells, consistent with Pkc1 working downstream of Pkh1/2 to regulate deadenylation (57). We conclude from these data that the Pkh1/2-Pkc1 pathway controls deadenylation by a unique mechanism that does not involve down-regulating protein synthesis.
FIGURE 6.
Pkc1 does not control deadenylation by down-regulating protein synthesis. The rate of protein synthesis was measured in wild-type DL100 and pkh1ts (RCD905) mutant cells following a shift to 39 °C. Each time point represents the average of three values, which differed from each other by less than 10%.
Nutrients Influence mRNA Decay and P-body Assembly
All of the experiments presented thus far were done using cells grown in defined medium with a small number of supplemental amino acids and bases. On the other hand, most studies on mRNA decay and P-body formation in S. cerevisiae have used cells grown in defined medium enriched with many nutrients including all 20 natural occurring amino acids, adenine, uracil, inositol, and para-aminobenzoic acid (36). We attempted to show that the Pkh1/2-Pkc1 pathway regulates mRNA and P-body formation in this enriched medium, but repeatedly found that growing pkhts cells at 39 °C did not inhibit mRNA decay (Fig. 7A) or P-body formation (Fig. 7B). These data show that the Pkh1/2-Pkc1 pathway only controls mRNA decay and P-body formation when at least some nutrients are absent from culture medium, whereas in nutrient-rich conditions the pathway is bypassed and no longer controls either process. Preliminary experiments indicate that no single nutrient is influencing mRNA decay; rather it seems that a combination of nutrients is responsible.
FIGURE 7.
Nutrients affect mRNA decay and P-body assembly. The rate of MFA2pG mRNA decay (A) was measured as described in the legend to Fig. 1 and P-body assembly induced by glucose starvation (B) was examined as described in the legend to Fig. 3. For both experiments cells were grown in nutrient-enriched SD medium.
DISCUSSION
Our data show that global deadenylation in unstressed cells and P-body assembly induced by nutritional and environmental stresses require and are thus controlled by the Pkh1/2-Pkc1 signaling pathway.
A potential concern about our data is that effects of a non-permissive temperature on growth or transition through the cell cycle in pkh1ts mutant cells are the root cause of the defects in mRNA decay and P-body formation, rather than a direct effect of deficiency in pkh1 and pkh2. There are several reasons why this is not very likely. First, Pkh1/2 have no known direct effect on the cell cycle nor are they regulated by the cell cycle. The terminal phenotype of pkh1ts pkh2Δ mutant cells at a non-permissive temperature is lysis, which does not occur at any particular step in the cell cycle, although budding cells are more susceptible to lysis. Mutant pkhts cells lyse because inactivation of downstream protein kinases including Pkc1, the master controller of cell wall metabolism, and Ypk1/2 rather than Pkh1/2 directly control cell wall metabolism.
Second, pkc1 deletion and pkc1ts mutant cells arrest at the small-bud stage of the cell cycle but this is believed to occur because of lysis at the bud tip where the wall is most fragile (44, 55). Because pkc1 mutant cells proliferate when osmotic support is provided in the culture medium, Pkc1 is not essential for growth or cell cycle progression (55, 58). Therefore, it seems very unlikely that mRNA decay and P-body formation are impaired in pkc1ts mutant cells at a restrictive temperature because of a block in the cell cycle. Third, it also seems very unlikely that a constitutively active Pkc1 protein, without need of Pkh1/2, restores mRNA decay (Fig. 3C) and P-body formation (Fig. 4) in pkhts mutant cells by a mechanism involving the cell cycle. The mechanism most likely involves Pkc1 acting directly on proteins required for mRNA decay and P-body formation, some of which are known to interact with Pkc1.
Fourth, the three temperature-sensitive mutant strains (pkh1ts pkh2Δ, pkc1ts, and ypk1ts ypk2Δ) we studied share some phenotypes including cell lysis. In contrast to this shared phenotype, only the pkh1ts and pkc1ts mutants impair mRNA decay and P-body formation, whereas the ypk1ts ypk2Δ mutant does not. These data argue that the Pkc1 branch, not the Ypk1/2 branch, of the pathways downstream of Pkh1/2 specifically controls mRNA decay and P-body formation and does so in a way that is independent of the cell cycle.
Fifth, other data do not support a connection between the cell cycle and mRNA decay or P-body formation. Although a temperature shift does tend to slow growth transiently and synchronize cells for a short time, it has been shown that shifting cells from 25 to 37 or 38 °C has no effect on mRNA decay (26) or P-body formation (25).
Sixth, there is no known correlation between effects on growth or the cell cycle and mRNA decay. In fact, some temperature-sensitive mutants show a faster rate of mRNA decay than do wild-type cells, which is the opposite result of what we find with pkh1ts pkh2Δ and pkc1ts mutant cells (53). In addition, there is no known connection between growth or the cell cycle and P-body formation.
Finally, our finding that protein synthesis is not inhibited in pkc1ts cells at the restrictive temperature (Fig. 6) while both mRNA decay and P-body formation are inhibited (Figs. 3 and 5) shows that only select processes are impaired when Pkc1 is inactivated. These data also show that turning off the Pkh1/2-Pkc1 pathway produces a unique phenotype: impairment of mRNA decay without blocking protein synthesis. This is in contrast to glucose starvation and other stresses that block both mRNA decay and protein synthesis.
A concern about specificity is also raised by the length of time required at a restrictive temperature for pkhts or pkc1ts cells to show impaired mRNA decay and P-body assembly. Cellular processes controlled by Pkh1/2 and Pkc1 have been identified mostly by examining cells carrying a temperature-sensitive mutant allele and these studies reveal a range of times between the shift to a restrictive temperature and process impairment. Cell lysis, reflecting synthesis and repair of the cell wall, is a classic phenotype of pkc1ts cells that occurs slowly and after 1 or 2 h at a restrictive temperature about 20 or 80%, respectively, of cells lysed (55, 58). Because of the Pkh1/2 work upstream of Pkc1, pkhts cells should lyse even more slowly after shifting to a restrictive temperature than pkc1ts cells and this is the case, because few cells have lysed after 1 h and only 20% after 2 h (31). Thus, the time frame we see for impairment of mRNA decay and P-body formation is similar to that for cell wall synthesis and repair, supporting the conclusion that Pkh1/2-Pkc1 act in a specific manner to promote mRNA decay and P-body formation.
Clues linking Pkc1 to mRNA decay have existed for a decade, but their significance has been uncertain. Our results establish the significance of some of these clues and add impetus for evaluating others. Pkc1 was found to interact with the poly(A)-binding protein Pab1 (59), implying a role for Pkc1 in controlling a function of Pab1, possibly its role in deadenylation (60–62). A large-scale screen found that multiple copies of the PKC1 gene partially suppressed some phenotypes of pop2Δ, dhh1Δ, and mpt5/puf5Δ cells (63). Suppression of pop2Δ phenotypes implies a role for Pkc1 in deadenylation, whereas suppression of dhh1Δ phenotypes could have several potential roles in mRNA decay or P-body assembly as discussed below. Mpt5/Puf5 binds RNAs and has multiple functions, but its role in message-specific decay most likely involves Pkc1 (64, 65). Another clue was uncovered during studies of cells with a temperature-sensitive eIF5A protein, recently shown to promote translation elongation (66). At a restrictive temperature, growth and mRNA decay were impaired (67) and growth could be restored by multiple copies of PKC1 or PAB1 (67). Restoration of mRNA decay was not examined, but based upon our results it seems likely that it was at least partially restored. The observation that inhibiting translational elongation in an eIF5A mutant also inhibits mRNA decay suggests coupling of the two processes and recent data showing decay occurring on translating polysomes support this idea (23).
Based upon published data there are several ways the Pkh1/2-Pkc1 pathway could control deadenylation. Pkc1 could promote deadenylation by directly phosphorylating Pab1 because they physically interact. However, protein kinases do not generally bind tightly to substrates so such binding might position Pkc1 to phosphorylate a protein(s) that interacts with Pab1. Other ways to regulate deadenylation would be for Pkc1 to phosphorylate the Ccr4-Pop2-Not deadenylase complex, with or without affecting Pab1, or to phosphorylate an unidentified component of translating polysomes, which has a role in deadenylation. Less direct ways for Pkc1 to regulate deadenylation cannot be excluded at this time.
Results from both in vitro and in vivo assays implicate Pab1 in regulating deadenylation catalyzed by the Ccr4-Pop2-Not deadenylase (60, 61, 67, 68). Likewise, mammalian PABPC1, the counterpart of yeast Pab1, interacts with both the Pan2/Pan3 and the Ccr4-Caf1-Not deadenylases to control deadenylation and these interactions are mediated by the transcription termination factor eRF3, Pan3, and TOB, an anti-proliferative factor (62). These authors suggested that PABPC1 is “a regulatory platform for mRNA deadenylation.” Similar interactions involving eRF3 and Pan3 are found in yeast (69), suggesting that Pab1/PABPC1 are conserved players in the mechanism(s) for regulating deadenylation.
Others have also shown that the TOB/BTG2 protein stimulates deadenylation by interacting with PABPC1 and the Ccr4-Caf1-Not deadenylase (70, 71). Although there is no apparent yeast homolog of TOB, the yeast Puf and the fly and yeast Smaug proteins (72–74) may perform analogous functions by binding to the 3′ untranslated region of specific mRNAs followed by stimulation of deadenylation. For example, Mpt5/Puf5 bound to an mRNA interacts with the Ccr4-Pop2(Caf1) component of the Ccr4-Pop2(Caf1)-Not deadenylase, thereby stimulating deadenylation of the target transcript and repressing its translation (65). Other studies in yeast indicate that Ccr4-Pop2-Not recruitment to poly(A) tails is also a potential point for controlling deadenylation (61, 75, 76).
Another mechanistic question raised by our results is how does the Pkh1/2-Pkc1 pathway regulate P-body assembly. Current models envision that translational repression induced by nutrient deficiency or environmental stresses lead to P-body assembly because mRNAs exit polysomes and enter new protein complexes termed repressed mRNPs. Repressed mRNPs then associate with two protein subcomplexes in yeast that promote self-assembly into P-bodies (21): one subcomplex contains Edc3, Dcp1, Dcp2, and Dhh1 and the other contains Pat1, Xrn1, and Lsm1–7 (3, 4, 52). The Pkh1/2-Pkc1 pathway might affect P-body assembly by controlling formation of repressed mRNPs or by controlling formation of one or both of the two protein subcomplexes on the repressed mRNP.
Dhh1 and Pat1 promote translational repression during glucose starvation (27) and either or both of these proteins could be targeted by the Pkh1/2-Pkc1 pathway. Our SGA data favor Pat1 because only deletion of the DHH1 gene (Fig. 1), which leaves Pat1 functional, and not deletion of the PAT1 gene (data not shown), which leaves Dhh1 functional, produces synthetic slow growth in combination with the pkh1ts pkh2Δ mutations. Also, data showing that multiple copies of PKC1 suppress the temperature-sensitive growth defect of dhh1Δ cells are consistent with Pkc1 affecting the Pat1 protein. Previous studies also suggest that Pat1 plays a role in P-body assembly because unstressed, log phase, and glucose-starved pat1 mutant cells have a reduced number of P-bodies (56).
Our finding that increasing the nutrient content of culture medium overrides the dependence of mRNA deadenylation and P-body formation on the Pkh1/2-Pkc1 pathway likely played valuable roles during evolution. The underlying mechanism is unclear but identification of the nutrients responsible for the override should provide clues. Preliminary experiments indicate that no individual amino acid or other supplemental nutrient can override the Pkh1/2-Pkc1 dependence, but further work is needed to identify which nutrient combinations activate the override mechanism.
It has been suggested recently that deadenylation is a prerequisite for P-body formation in mammals (51). Our data (Figs. 4, 5, and supplemental S3) show that this is only true in yeast cells when deadenylation is prevented by inhibiting the Pkh1/2-Pkc1 pathway and not when deadenylation is impaired by mutation of both deadenylase genes (ccr4Δ panΔ) (9, 26, 77). Because the mode of blocking deadenylation determines whether P-bodies form, it seems prudent to wait until the mechanism of P-body induction is better defined before deciding if deadenylation is a prerequisite for their formation in yeast.
Homologs of the yeast Pkh1/2-Pkc1 pathway are likely to play roles in mRNA decay and P-body assembly in other organisms because most of the general deadenylation-dependent mRNA decay and P-body components are conserved from yeasts to man (3, 4, 52, 78). Furthermore, like Pkh1/2 in yeast, mammalian PDK1 mediates growth and stress signaling by controlling members of the AGC protein kinase family including PKB/Akts and some species of PKC (79), any one of which could regulate deadenylation or P-body formation. Regulation of mRNA decay in mammals could be more complex than in yeast because of many more deadenylase complexes (5).
So why does the Pkh1/2-Pkc1 pathway regulate mRNA decay and P-body assembly? It may provide a way to monitor nutrients and environmental stress and integrate these with processes necessary for growth and survival. Pkc1, Sch9, and Ypk1/2 control a diverse array of cellular processes ranging from growth and cell division to stress resistance and longevity (32), to which we can now add mRNA decay and P-body assembly. Pkh1/2 act to coordinate the processes controlled by the downstream kinases and to integrate these outputs with ones controlled by other signaling pathways vital for growth, stress resistance, and survival.
Supplementary Material
Acknowledgments
We thank Dr. Jeff Coller and his students for teaching us the art of measuring mRNA decay and deadenylation and Dr. Roy Parker for providing yeast strains and plasmids.
This work was supported, in whole or in part, by National Institutes of Health Grant GM41302 and core facilities Grant P20-RR020171 from the National Center for Research Resources (to R. C. D.) and grants from the Canadian Institutes of Health and Research, Genome Canada, and the Ontario Genomics Institute (to C. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 and Tables S1–S2.
- P-bodies
- processing bodies
- pkhts
- cells with the pkh1ts::URA and pkh2::NAT alleles
- ypkts
- cells with the ypk1-1ts::HIS3 ypk2-Δ1::TRP1 alleles
- SGA
- synthetic genetic array
- PDK1
- phosphoinositide-dependent protein kinase 1.
REFERENCES
- 1. Fan J., Yang X., Wang W., Wood W. H., 3rd, Becker K. G., Gorospe M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10611–10616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheadle C., Fan J., Cho-Chung Y. S., Werner T., Ray J., Do L., Gorospe M., Becker K. G. (2005) BMC Genomics 6, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parker R., Sheth U. (2007) Mol. Cell 25, 635–646 [DOI] [PubMed] [Google Scholar]
- 4. Eulalio A., Behm-Ansmant I., Izaurralde E. (2007) Nat. Rev. Mol. Cell Biol. 8, 9–22 [DOI] [PubMed] [Google Scholar]
- 5. Goldstrohm A. C., Wickens M. (2008) Nat. Rev. Mol. Cell Biol. 9, 337–344 [DOI] [PubMed] [Google Scholar]
- 6. He F., Li X., Spatrick P., Casillo R., Dong S., Jacobson A. (2003) Mol. Cell 12, 1439–1452 [DOI] [PubMed] [Google Scholar]
- 7. Garneau N. L., Wilusz J., Wilusz C. J. (2007) Nat. Rev. Mol. Cell Biol. 8, 113–126 [DOI] [PubMed] [Google Scholar]
- 8. Shyu A. B., Wilkinson M. F., van Hoof A. (2008) EMBO J. 27, 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tucker M., Valencia-Sanchez M. A., Staples R. R., Chen J., Denis C. L., Parker R. (2001) Cell 104, 377–386 [DOI] [PubMed] [Google Scholar]
- 10. Coller J., Parker R. (2004) Annu. Rev. Biochem. 73, 861–890 [DOI] [PubMed] [Google Scholar]
- 11. Brown C. E., Sachs A. B. (1998) Mol. Cell. Biol. 18, 6548–6559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai Y., Salvadore C., Chiang Y. C., Collart M. A., Liu H. Y., Denis C. L. (1999) Mol. Cell. Biol. 19, 6642–6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daugeron M. C., Mauxion F., Séraphin B. (2001) Nucleic Acids Res. 29, 2448–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Denis C. L., Chen J. (2003) Prog. Nucleic Acid Res. Mol. Biol. 73, 221–250 [DOI] [PubMed] [Google Scholar]
- 15. Yamashita A., Chang T. C., Yamashita Y., Zhu W., Zhong Z., Chen C. Y., Shyu A. B. (2005) Nat. Struct. Mol. Biol. 12, 1054–1063 [DOI] [PubMed] [Google Scholar]
- 16. Fillman C., Lykke-Andersen J. (2005) Curr. Opin. Cell Biol. 17, 326–331 [DOI] [PubMed] [Google Scholar]
- 17. Sheth U., Parker R. (2003) Science 300, 805–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson P., Kedersha N. (2006) J. Cell Biol. 172, 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chu C. Y., Rana T. M. (2006) PLoS Biol. 4, e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoecklin G., Mayo T., Anderson P. (2006) EMBO Rep. 7, 72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Decker C. J., Teixeira D., Parker R. (2007) J. Cell Biol. 179, 437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eulalio A., Behm-Ansmant I., Schweizer D., Izaurralde E. (2007) Mol. Cell. Biol. 27, 3970–3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu W., Sweet T. J., Chamnongpol S., Baker K. E., Coller J. (2009) Nature 461, 225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gowrishankar G., Winzen R., Dittrich-Breiholz O., Redich N., Kracht M., Holtmann H. (2006) Biol. Chem. 387, 323–327 [DOI] [PubMed] [Google Scholar]
- 25. Teixeira D., Sheth U., Valencia-Sanchez M. A., Brengues M., Parker R. (2005) RNA 11, 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hilgers V., Teixeira D., Parker R. (2006) RNA 12, 1835–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coller J., Parker R. (2005) Cell 122, 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brengues M., Teixeira D., Parker R. (2005) Science 310, 486–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson P., Kedersha N. (2008) Trends Biochem. Sci. 33, 141–150 [DOI] [PubMed] [Google Scholar]
- 30. Casamayor A., Torrance P. D., Kobayashi T., Thorner J., Alessi D. R. (1999) Curr. Biol. 9, 186–197 [DOI] [PubMed] [Google Scholar]
- 31. Inagaki M., Schmelzle T., Yamaguchi K., Irie K., Hall M. N., Matsumoto K. (1999) Mol. Cell. Biol. 19, 8344–8352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dickson R. C., Sumanasekera C., Lester R. L. (2006) Prog. Lipid Res. 45, 447–465 [DOI] [PubMed] [Google Scholar]
- 33. Urban J., Soulard A., Huber A., Lippman S., Mukhopadhyay D., Deloche O., Wanke V., Anrather D., Ammerer G., Riezman H., Broach J. R., De Virgilio C., Hall M. N., Loewith R. (2007) Mol. Cell 26, 663–674 [DOI] [PubMed] [Google Scholar]
- 34. Mora A., Komander D., van Aalten D. M., Alessi D. R. (2004) Semin. Cell Dev. Biol. 15, 161–170 [DOI] [PubMed] [Google Scholar]
- 35. Tong A. H., Lesage G., Bader G. D., Ding H., Xu H., Xin X., Young J., Berriz G. F., Brost R. L., Chang M., Chen Y., Cheng X., Chua G., Friesen H., Goldberg D. S., Haynes J., Humphries C., He G., Hussein S., Ke L., Krogan N., Li Z., Levinson J. N., Lu H., Ménard P., Munyana C., Parsons A. B., Ryan O., Tonikian R., Roberts T., Sdicu A. M., Shapiro J., Sheikh B., Suter B., Wong S. L., Zhang L. V., Zhu H., Burd C. G., Munro S., Sander C., Rine J., Greenblatt J., Peter M., Bretscher A., Bell G., Roth F. P., Brown G. W., Andrews B., Bussey H., Boone C. (2004) Science 303, 808–813 [DOI] [PubMed] [Google Scholar]
- 36. Coller J. (2008) Methods Enzymol. 448, 267–284 [DOI] [PubMed] [Google Scholar]
- 37. Passos D. O., Parker R. (2008) Methods Enzymol. 448, 409–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nissan T., Parker R. (2008) Methods Enzymol. 448, 507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ashe M. P., De Long S. K., Sachs A. B. (2000) Mol. Biol. Cell 11, 833–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tong A. H., Boone C. (2006) Methods Mol. Biol. 313, 171–192 [DOI] [PubMed] [Google Scholar]
- 41. Jung U. S., Levin D. E. (1999) Mol. Microbiol. 34, 1049–1057 [DOI] [PubMed] [Google Scholar]
- 42. Roberts C. J., Nelson B., Marton M. J., Stoughton R., Meyer M. R., Bennett H. A., He Y. D., Dai H., Walker W. L., Hughes T. R., Tyers M., Boone C., Friend S. H. (2000) Science 287, 873–880 [DOI] [PubMed] [Google Scholar]
- 43. Lagorce A., Hauser N. C., Labourdette D., Rodriguez C., Martin-Yken H., Arroyo J., Hoheisel J. D., François J. (2003) J. Biol. Chem. 278, 20345–20357 [DOI] [PubMed] [Google Scholar]
- 44. Levin D. E. (2005) Microbiol. Mol. Biol. Rev. 69, 262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coller J. M., Tucker M., Sheth U., Valencia-Sanchez M. A., Parker R. (2001) RNA 7, 1717–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fischer N., Weis K. (2002) EMBO J. 21, 2788–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tseng-Rogenski S. S., Chong J. L., Thomas C. B., Enomoto S., Berman J., Chang T. H. (2003) Nucleic Acids Res. 31, 4995–5002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parker R., Song H. (2004) Nat. Struct. Mol. Biol. 11, 121–127 [DOI] [PubMed] [Google Scholar]
- 49. Decker C. J., Parker R. (1993) Genes Dev. 7, 1632–1643 [DOI] [PubMed] [Google Scholar]
- 50. Zuk D., Jacobson A. (1998) EMBO J. 17, 2914–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zheng D., Ezzeddine N., Chen C. Y., Zhu W., He X., Shyu A. B. (2008) J. Cell Biol. 182, 89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Franks T. M., Lykke-Andersen J. (2008) Mol. Cell 32, 605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schwartz D. C., Parker R. (1999) Mol. Cell. Biol. 19, 5247–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Drgonová J., Drgon T., Roh D. H., Cabib E. (1999) J. Cell Biol. 146, 373–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Levin D. E., Bartlett-Heubusch E. (1992) J. Cell Biol. 116, 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Teixeira D., Parker R. (2007) Mol. Biol. Cell 18, 2274–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meier K. D., Deloche O., Kajiwara K., Funato K., Riezman H. (2006) Mol. Biol. Cell 17, 1164–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paravicini G., Cooper M., Friedli L., Smith D. J., Carpentier J. L., Klig L. S., Payton M. A. (1992) Mol. Cell. Biol. 12, 4896–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mangus D. A., Amrani N., Jacobson A. (1998) Mol. Cell. Biol. 18, 7383–7396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Simón E., Séraphin B. (2007) Nucleic Acids Res. 35, 6017–6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yao G., Chiang Y. C., Zhang C., Lee D. J., Laue T. M., Denis C. L. (2007) Mol. Cell. Biol. 27, 6243–6253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Funakoshi Y., Doi Y., Hosoda N., Uchida N., Osawa M., Shimada I., Tsujimoto M., Suzuki T., Katada T., Hoshino S. (2007) Genes Dev. 21, 3135–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hata H., Mitsui H., Liu H., Bai Y., Denis C. L., Shimizu Y., Sakai A. (1998) Genetics 148, 571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Seay D., Hook B., Evans K., Wickens M. (2006) RNA 12, 1594–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goldstrohm A. C., Seay D. J., Hook B. A., Wickens M. (2007) J. Biol. Chem. 282, 109–114 [DOI] [PubMed] [Google Scholar]
- 66. Saini P., Eyler D. E., Green R., Dever T. E. (2009) Nature 459, 118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Valentini S. R., Casolari J. M., Oliveira C. C., Silver P. A., McBride A. E. (2002) Genetics 160, 393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mangus D. A., Evans M. C., Agrin N. S., Smith M., Gongidi P., Jacobson A. (2004) Mol. Cell. Biol. 24, 5521–5533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hosoda N., Kobayashi T., Uchida N., Funakoshi Y., Kikuchi Y., Hoshino S., Katada T. (2003) J. Biol. Chem. 278, 38287–38291 [DOI] [PubMed] [Google Scholar]
- 70. Mauxion F., Faux C., Séraphin B. (2008) EMBO J. 27, 1039–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ezzeddine N., Chang T. C., Zhu W., Yamashita A., Chen C. Y., Zhong Z., Yamashita Y., Zheng D., Shyu A. B. (2007) Mol. Cell. Biol. 27, 7791–7801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Semotok J. L., Cooperstock R. L., Pinder B. D., Vari H. K., Lipshitz H. D., Smibert C. A. (2005) Curr. Biol. 15, 284–294 [DOI] [PubMed] [Google Scholar]
- 73. Zaessinger S., Busseau I., Simonelig M. (2006) Development 133, 4573–4583 [DOI] [PubMed] [Google Scholar]
- 74. Rendl L. M., Bieman M. A., Smibert C. A. (2008) RNA 14, 1328–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Goldstrohm A. C., Hook B. A., Wickens M. (2008) Methods Enzymol. 448, 77–106 [DOI] [PubMed] [Google Scholar]
- 76. Ohn T., Chiang Y. C., Lee D. J., Yao G., Zhang C., Denis C. L. (2007) Nucleic Acids Res. 35, 3002–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tucker M., Staples R. R., Valencia-Sanchez M. A., Muhlrad D., Parker R. (2002) EMBO J. 21, 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kulkarni M., Ozgur S., Stoecklin G. (2010) Biochem. Soc. Trans. 38, 242–251 [DOI] [PubMed] [Google Scholar]
- 79. Peifer C., Alessi D. R. (2008) Chem. Med. Chem. 3, 1810–1838 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.