Abstract
The contribution of plasminogen (Plg)/plasmin, which have claimed to be the main fibrinolytic regulators in the bone metabolism, remains unclear. This study evaluated how the absence of Plg affects the function of osteoblast (OB) and osteoclast (OC). There was a larger population of pre-OCs in bone marrow-derived cells from the Plg−/− mice than the population of that from the WT mice. In addition, the absence of Plg suppressed the expression of osteoprotegerin in OBs. Moreover, an exogenous plasmin clearly induced the osteoprotegerin expression in Plg−/− OBs. The osteoclastogenesis of RAW264.7 mouse monocyte/macrophage lineage cells in co-culture with OBs from the Plg−/− mice was significantly accelerated in comparison with that in co-culture with OBs from the WT mice. Intriguingly, the accelerated OC differentiation of RAW264.7 cells co-cultured with Plg−/− OBs was clearly suppressed by the treatment of an exogenous plasmin. Consequently, Plg−/− mice display decreased bone mineral density. These findings could eventually lead to the development of new clinical therapies for bone disease caused by a disorder of the fibrinolytic system.
Keywords: Bone, Cell Differentiation, Plasmin, Plasminogen, Plasminogen Regulation, Osteoblasts, Osteoclasts
Introduction
The fibrinolytic system contains plasminogen (Plg),2 a proenzyme, which is converted to the active serine protease plasmin, a main component of the fibrinolytic system, through the action of a tissue-type plasminogen activator (tPA) or urokinase-type PA (uPA). The inhibition of the system may occur through the neutralization of the plasminogen activators or plasmin, and this neutralization is achieved mainly by the plasminogen activator inhibitor-1 (PAI-1) or α2-antiplasmin (α2AP), respectively. PAI-1, the primary endogenous inhibitor of tPA or uPA, plays an important role in inhibiting arterial clot lysis (1). α2AP rapidly inactivates plasmin, resulting in the formation of a stable inactive complex, plasmin-α2AP (2). Apart from the removal of fibrin, the fibrinolytic system also plays a pivotal role in such phenomena as embryogenesis, proliferation, migration, wound healing, fibrosis, and tumorigenesis (3–9).
It is suggested that fibrinolytic factors such as tPA, uPA, uPA receptor, and PAI-1 are involved in bone metabolism as follows. The absence of tPA and uPA enhanced OB differentiation and formation of a mineralized bone matrix and increased bone formation and bone mass (10). The absence of PAI-1 protects against trabecular bone loss induced by estrogen deficiency, suggesting a site-specific role for PAI-1 in bone turnover (11). In addition, uPA receptor-lacking mice displayed increased bone mineral density (BMD), increased osteogenic potential of OBs, decreased OC formation, and cytoskeletal reorganization in mature OCs (12). However, the physiological roles of fibrinolytic main regulators such as Plg/plasmin in bone metabolism are not precisely understood.
The receptor activator of NF-κB (RANK), its ligand RANKL, and OPG control OC function (13, 14). RANK activated by RANKL has proven to be absolutely required for OC development (15). RANKL is neutralized by OPG that specifically binds to RANKL. OPG is expressed in many tissues apart from OBs, including heart, kidney, liver, spleen, and bone marrow (13). However, molecular mechanisms of OPG expression remain to be elucidated. We herein report the crucial role of fibrinolytic main regulators Plg/plasmin in bone metabolism especially on the point of view of how the regulators affect the ability of pre-OCs in bone marrow to differentiate into OCs, OBs to induce OC differentiation, and OBs to mineralize extracellular matrix.
MATERIALS AND METHODS
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Animals
The Plg-deficient (Plg−/−) mice (16) were kindly provided by Prof. D. Collen (University of Leuven, Belgium).
Wild type (WT) and Plg−/− mice littermates were housed in groups of two to five in filter-top cages with a fixed 12-h light, 12-h dark cycle. The body weights of mice were measured weekly.
Reagents
Plasmin, aprotinin, α2AP, ϵ-aminocaproic acid, and other chemical substances were obtained from Sigma.
Cell Culture
Bone marrow cells, RAW264.7 mouse monocyte/macrophage lineage cells (American Type Culture Collection), and primary OBs were maintained in α-minimal essential medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) and 1% penicillin/streptomycin (Invitrogen) at 37 °C in a humidified atmosphere of 5% CO2, 95% air. Primary OBs derived from mice calvaria were obtained as described previously (17).
OC Differentiation Assay
Bone marrow-derived cells that include a population of pre-OCs were obtained from tibia of 5–7-week-old adult mice. Mouse bone marrow cells were cultured for 3 days with RANKL (100 ng/ml) and M-CSF (100 ng/ml) in 48-well plates. In other experiments, RAW264.7 cells were co-cultured with OBs from the Plg+/+ and Plg−/− mice for 3 days in the absence or presence of interleukin-1β (IL-1β) (5 ng/ml) or prostaglandin E2 (PGE2) (1 μm) in 48-well plates. Cells were then fixed and stained for tartrate-resistant acid phosphatase (TRAP; a marker enzyme of OCs) as described previously (17). TRAP-positive multinucleated cells containing three or more nuclei were counted as OCs, under microscopic examination.
Bone Resorption Assay
To estimate bone resorption activity of differentiated OCs from bone marrow cells of the Plg+/+ and Plg−/− mice, the cells were stimulated with RANKL (100 ng/ml) and M-CSF (100 ng/ml) for 7 days on the BioCoatTM OsteologicTM multiple test slides, which consisted of submicron synthetic calcium phosphate thin film coated onto various culture vessels (BD Biosciences). The nonresorbed area of calcium phosphate film was then visualized by using a method of von Kossa staining, as follows. After fixation of the cells in the culture with 5% glutaraldehyde, the calcium phosphate film was treated with 5% silver nitrate for 30 min. Then the staining was developed with 5% sodium carbonate in 25% formalin. The stained film in each well was photographed under light microscopy, and then the image was inverted to yield the negative image; the black image represents the resorbed area in the calcium phosphate film.
Bone Histology
Bone histomorphometry of tibia in 5-week-old male Plg+/+ and Plg−/− mice was performed. Each tibia was removed and fixed in 4% paraformaldehyde for 2 days and then demineralized with 10% EDTA for 14 days before embedding in paraffin. Paraffin-embedded tissue was serially sectioned at a distance of 4–7 μm. Then the sections were stained with hematoxylin and eosin (H&E) and TRAP by using a TRAP kit (Sigma). For the quantitative evaluation of the intensity of TRAP staining of bone marrow tissue in decalcified sections of tibia from the Plg+/+ and Plg−/− mice, the TRAP-stained images obtained from separate fields on the specimens (n = 6) were analyzed by using ImageJ.
Measurement of Bone Mineral Density
BMD was measured as described by Kanazawa et al. (18) and Nishiwaki et al. (19). BMD of the proximal tibia of the Plg+/+ and Plg−/− mice at the indicated time was evaluated by using peripheral quantitative computed tomography with a fixed x-ray fan beam of 50-μm spot size, at 1 mA and 50 kV (LaTheta LCT-100S; Aloka, Tokyo, Japan).
RNA Isolation and Quantitative RT-PCR
Total RNA was extracted as described previously (6). First strand cDNA was synthesized from total RNA by using the PrimeScript RT reagent kit (Takara). Quantitative RT-PCR (qRT-PCR) was performed on the IQ5 real time PCR detection system (Bio-Rad) with SYBR Green technology on cDNA generated from the reverse transcription of purified RNA. The two-step PCRs were performed at 92 °C for 1 s and 60 °C for 10 s. OPG mRNA expression was normalized against GAPDH mRNA expression using the comparative cycle threshold method. We used the following primer sequence: OPG, 5′-CAATGGCTGGCTTGGTTTCATAG-3′ and 5′-CTGAACCAGACATGACAGCTGGA-3′; GAPDH, 5′-TGTGTCCGTCGTGGATCTGA-3′ and 5′-TTGCTGTTGAAGTCGCAGGAG-3′.
Western Blot Analysis
We performed a Western blot analysis for detection of OPG, phospho-ERK1/2, phospho-p38 MAPK, ERK1/2, and p38 MAPK as described previously (20). We detected OPG, phospho-ERK1/2, phospho-p38 MAPK, ERK1/2, and p38 MAPK by incubation with a polyclonal OPG antibody (rabbit IgG, from GeneTex Inc.), anti-phospho-ERK1/2 antibody (Cell Signaling Technology, Danvers, MA), anti-phospho-p38 MAPK antibody (Cell Signaling Technology, Danvers, MA), anti-ERK1/2 antibody (Cell Signaling Technology, Danvers, MA), and anti-p38 MAPK antibody (Cell Signaling Technology, Danvers, MA).
Measurement of Alkaline Phosphatase Activity
We measured alkaline phosphatase (ALP) activity as described previously (20). Primarily cultured OBs were cultured for 14 day with differentiation media (media supplemented with 10 mm β-glycerophosphate and 10 nm dexamethasone and 50 μg/ml ascorbic acid) in 6-well plates. After 14 days, cells were then washed, and proteins in cells were extracted with a lysis buffer (10 mm Tris-HCl, pH 7.5, 0.1% Triton X-100). ALP activity was determined using p-nitrophenyl phosphate (Sigma) as a substrate.
Statistical Analysis
All data are expressed as mean ± S.E. The significance of the effect of each treatment (p < 0.05) was determined by analysis of variance followed by the Student's Newman-Keuls test.
RESULTS
Histological and Radiological Evaluation of the Status of Endochondral Ossification in Plg-deficient Mice
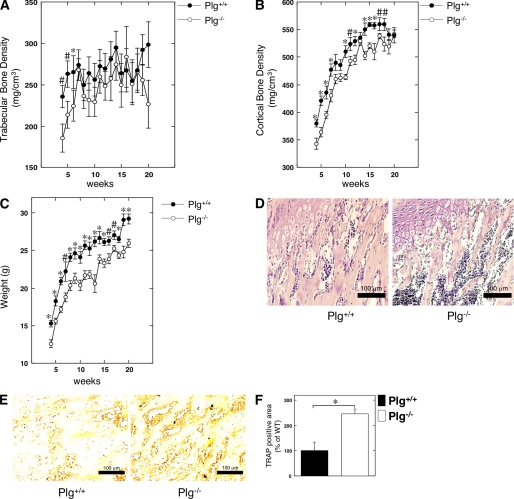
The BMDs in the Plg+/+ and Plg−/− mice at 4–20 weeks were radiologically assessed using peripheral quantitative computed tomography. Intriguingly, the trabecular BMD in tibia from the Plg−/− mice was significantly lower than that from the Plg+/+ mice at 4–6 weeks after birth (Fig. 1A). In addition, the cortical BMD in tibia from the Plg−/− mice was significantly lower than that from the Plg+/+ mice at 4–18 weeks after birth (Fig. 1B). The decrease of cortical BMD seemed to parallel that of the body weight decrease in the Plg−/− mice at 4–18 weeks after birth (Fig. 1, B and C). Next, the status of endochondral ossification in tibia from the Plg+/+ and Plg−/− was histologically compared to clarify the effect of the fibrinolytic system in bone metabolism. As shown in Fig. 1D, H&E staining of a decalcified section of tibia from the 5-week-old mice showed that the layer of chondrocytes and trabecular bone formation in the medullary cavities were observed in both Plg+/+ and Plg−/− mice. The TRAP staining of the decalcified section of the tibias from the 5-week-old mice revealed that the area of TRAP-positive bone marrow tissue in the tibias from the Plg−/− mice was significantly larger than that of the tissue from the Plg+/+ mice (Fig. 1E). In addition, the intensity of the TRAP staining on the decalcified sections of bone marrow tissue in the Plg+/+ and Plg−/− mice was quantitatively evaluated as described under “Materials and Methods.” As shown in Fig. 1F, the intensity of TRAP staining on decalcified sections of bone marrow tissue in tibias from the Plg−/− mice was much stronger than in those from the Plg+/+ mice.
FIGURE 1.
Bone histomorphometry and bone mineral density in Plg-deficient mice. A, trabecular BMD in the proximal tibia of male Plg+/+ and Plg−/− mice was obtained from pQCT measurement (n = 13). B, cortical BMD in the proximal tibia of the Plg+/+ and Plg−/− mice was obtained from pQCT measurement (n = 13). C, growth curves of the Plg+/+ and Plg−/− mice (n = 13). D and E, bone histomorphometry of tibia in 5-week-old male Plg+/+ and Plg−/− mice (D, H&E; E, TRAP). D, layer of chondrocytes and trabecular bone formation in the medullary cavities were observed in both Plg+/+ and Plg−/− mice. E, TRAP-positive area in the bone marrow tissue of the tibias from Plg−/− mice was much larger than that in the tissue specimens obtained from Plg+/+ mice. F, intensity of TRAP staining on the decalcified sections of bone marrow tissue in the Plg+/+ and Plg−/− mice was quantitatively evaluated as described under “Materials and Methods” (n = 6). The intensity of TRAP staining on the sections from the Plg−/− mice was much stronger than that of sections from Plg+/+ mice. The data represent the mean ± S.E. *, p < 0.01; #, p < 0.05.
Effect of the Plg Deficiency on the Osteoclastogenesis of Bone Marrow-derived Cells
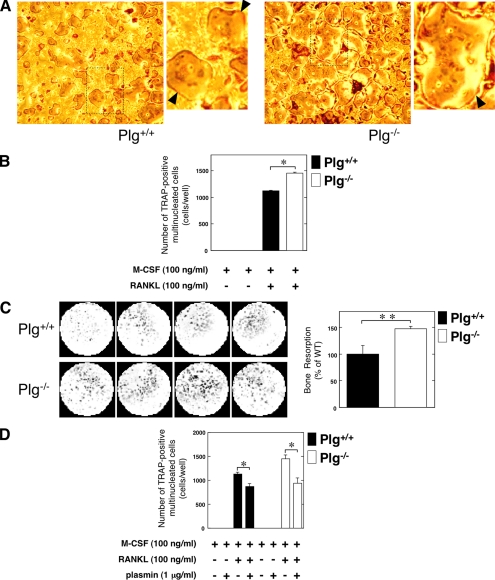
We evaluated how the fibronolytic system affects OC differentiation and function. The pre-OC population in bone marrow-derived cells from the Plg+/+ and Plg−/− mice were evaluated after stimulation with RANKL and M-CSF, respectively. As shown in Fig. 2A, many TRAP-positive multinucleated OCs were observed in bone marrow cell cultures derived from the Plg−/− mice tibia. Therefore, an up-regulation of the TRAP-positive cell number in the Plg−/− mice-derived bone marrow cells was observed (Fig. 2B). In addition, the bone resorption activity of OCs differentiated from bone marrow-derived cells was compared in the Plg+/+ and Plg−/− mice. There was an up-regulation of the bone resorption activity of Plg−/− mice-derived bone marrow cells (Fig. 2C). Intriguingly, plasmin significantly inhibited the M-CSF- and RANKL-induced OC differentiation of bone marrow cells derived from the Plg−/− and Plg+/+ mice (Fig. 2D).
FIGURE 2.
Effect of Plg deficiency on osteoclastogenesis and the OC function. A, bone marrow cells from the Plg+/+ and Plg−/− mice were cultured for 3 days in the absence or presence of RANKL (100 ng/ml) and M-CSF (100 ng/ml). Mature OCs were identified as TRAP-positive multinucleated cells. The magnified image of boxed area was showed on the right of the original image. The arrowheads indicate osteoclasts. B, number of TRAP-positive multinucleated cells in A was determined from three different cultures. C, bone resorption activity of OCs differentiated from bone marrow-derived cells obtained from the Plg+/+ and Plg−/− mice was compared. Bone marrow-derived cells from the Plg+/+ and Plg −/− mice were cultured on the BioCoatTM OsteologicTM multiple test slides, which consisted of submicron synthetic calcium phosphate thin film coated onto various culture vessels, for 7 days in the presence of RANKL (100 ng/ml) and M-CSF (100 ng/ml) (n = 4). Next, the resorbed areas of the calcium phosphate film were visualized as described under “Materials and Methods.” The histogram, right panel, shows quantitative representations of bone resorption obtained from densitometry analysis. The densitometry results were expressed as the mean density. D, bone marrow cells from the Plg+/+ and Plg−/− mice were cultured for 3 days in the presence of M-CSF (100 ng/ml). Some cells were cultured in the presence or absence of RANKL (100 ng/ml) or plasmin (1 μg/ml) as indicated. The number of multinucleated TRAP-positive cells was determined from four different cultures. The data represent the mean ± S.E. *, p < 0.01; **, p < 0.05.
Plasmin Induced the OPG Expression in OBs
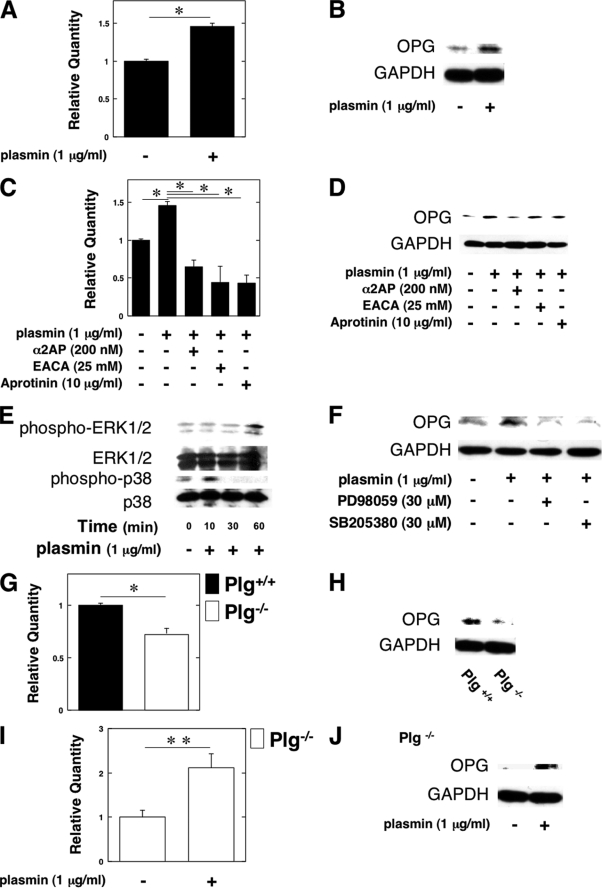
To clarify how plasmin suppresses osteoclastogenesis in vivo, we examined whether plasmin up-regulates the expression of OPG in OBs from the WT mice in vitro by qRT-PCR and a Western blot analysis. Plasmin clearly induced OPG expression in OBs from the WT mice (Fig. 3, A and B). In addition, the effect of various plasmin inhibitors (α2AP; serine protease inhibitor, aprotinin; lysine analog, ϵ-aminocaproic acid) on plasmin-induced OPG expression was investigated. These plasmin inhibitors clearly abrogated the plasmin-induced OPG expression (Fig. 3, C and D).
FIGURE 3.
Plasmin induced the OPG expression in OBs. A–D, OBs from the WT mice were cultured for 24 h in either the absence or presence of plasmin (1 μg/ml). Plasmin-induced expression of OPG gene in OBs from the WT mice was evaluated by qRT-PCR (A) or a Western blot analysis (B). C and D, some cultures were further treated with plasmin inhibitors as follows: α2AP (200 nm), ϵ-aminocaproic acid (25 mm), and aprotinin (10 μg/ml). The expression of OPG mRNA in OBs from the WT mice was then measured by qRT-PCR (C) or a Western blot analysis (D). E, OBs from the WT mice were stimulated with 1 μg/ml plasmin for the indicated periods. Phosphorylation of ERK1/2 and p38 MAPK was evaluated by a Western blot analysis using antibodies to ERK1/2 and p38 MAPK. F, OBs from the WT mice were pretreated with 30 μm PD98059 or 30 μm SB203580 for 60 min and then stimulated with 1 μg/ml plasmin for 24 h. The expression of OPG in OBs from the WT mice was evaluated by a Western blot analysis. G and H, OPG expression in OBs from the Plg+/+ and Plg−/− mice was evaluated by qRT-PCR (G) or a Western blot analysis (H). I and J, OBs from the Plg−/− mice were cultured for 24 h in the absence or presence of plasmin (1 μg/ml). The OPG expression in OBs from the Plg−/− mice was evaluated by qRT-PCR (I) or a Western blot analysis (J). The data represent the mean of three individual experiments ± S.E. *, p < 0.01; **, p < 0.05.
In addition, we examined the plasmin-stimulated phosphorylation of ERK1/2 and p38 MAPK to determine whether plasmin activates ERK1/2 and p38 MAPK in OBs. Plasmin activated ERK1/2 and p38 MAPK in OBs (Fig. 3E). We also examined whether the ERK1/2 and p38 MAPK pathways are associated with the plasmin-induced expression of OPG in OBs by using the inhibitor of MEK and p38 MAPK (PD98059 and SB203580). PD98059 and SB203580 attenuated plasmin-induced expression of OPG in OBs (Fig. 3F). These data suggest that plasmin induces OPG expression through the ERK1/2 and p38 MAPK pathways.
Moreover, qRT-PCR and a Western blot analysis revealed that the expression of OPG was suppressed in OBs from the Plg−/− mice (Fig. 3, G and H), thus suggesting that the absence of plasmin may result in the acceleration of osteoclastogenesis of pre-OCs in accordance with the depletion of OPG synthesis in OBs. There was no difference in the status of RANKL mRNA expression in OBs from the Plg+/+ and Plg−/− mice (data not shown). Moreover, plasmin induced OPG expression in Plg−/− OBs (Fig. 3, I and J).
Effects of Plg Deficiency on the Ability of OBs to Induce Osteoclastogenesis of RAW264.7 Mouse Monocyte/Macrophage Lineage Cells
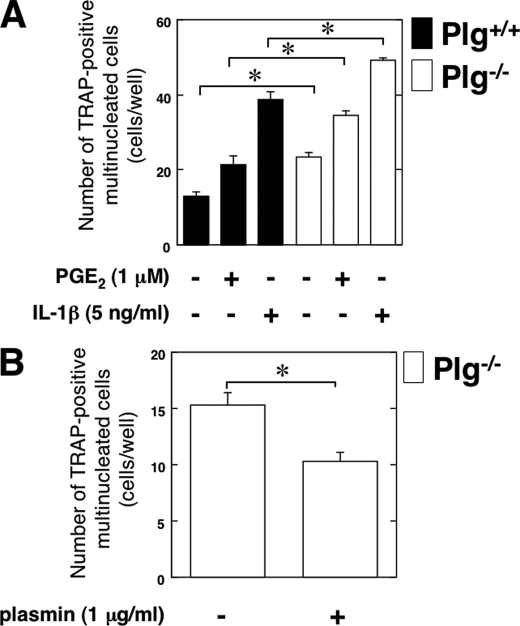
The status of OC differentiation of RAW264.7 mouse monocyte/macrophage lineage cells in co-culture with Plg−/− OBs was examined to clarify how Plg deficiency affects OB function for osteoclastogenesis. The ability of Plg−/− OBs to induce OC differentiation of pre-OC RAW264.7 cells was compared with Plg+/+ OBs. The OBs were co-cultured with RAW264.7 cells under stimulation with the inflammatory mediators interleukin 1-β (IL-1β) or prostaglandin E2 (PGE2). Inflammatory mediators induce RANKL expression on OBs (21). The inflammatory mediator-induced RANKL expression on OBs was expected to induce the osteoclastogenesis of the co-cultured RAW264.7 cells. As shown in Fig. 4A, IL-1β or PGE2 increased the number of TRAP-positive multinucleated cells co-cultured with OBs. Intriguingly, the number of TRAP-positive multinucleated cells co-cultured with Plg−/− OBs lacking OPG expression was significantly higher than that co-cultured with Plg+/+ OBs with or without IL-1β or PGE2. In addition, the number of TRAP-positive multinucleated cells co-cultured with Plg−/− OBs was decreased by plasmin (Fig. 4B).
FIGURE 4.
Effects of Plg deficiency on the ability of OBs to induce osteoclastogenesis of RAW264.7 cells. A, RAW264.7 cells and OBs from the Plg+/+ and Plg−/− mice were co-cultured for 3 days in the absence or presence of IL-1β or PGE2. B, RAW264.7 cells and OBs from the Plg−/− mice were co-cultured for 3 days in the absence or presence of plasmin. Mature OCs were identified as multinucleated TRAP-positive cells. The number of multinucleated TRAP-positive cells was determined from six different cultures. The data represent the mean ± S.E. *, p < 0.01.
Effect of Plg Deficiency on the ALP Activity in OBs
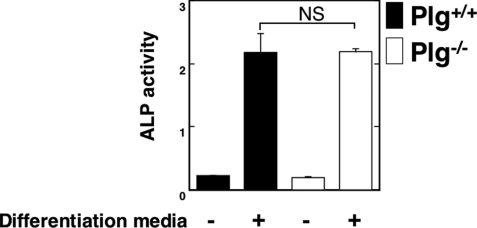
The ALP activity in Plg−/− OBs was compared with Plg+/+ OBs under stimulation with OB differentiation media as described under “Materials and Methods.” The absence of Plg did not affect the ALP activity in undifferentiated and differentiated OBs (Fig. 5).
FIGURE 5.
Effect of Plg deficiency on the ALP activity in OBs. ALP activity in OBs from the Plg+/+ and Plg−/− mice was evaluated (n = 4). The data represent the mean ± S.E. NS, not significant.
Rescue of the Down-regulated BMD in Plg-deficient Mice by the Injection of Plasmin
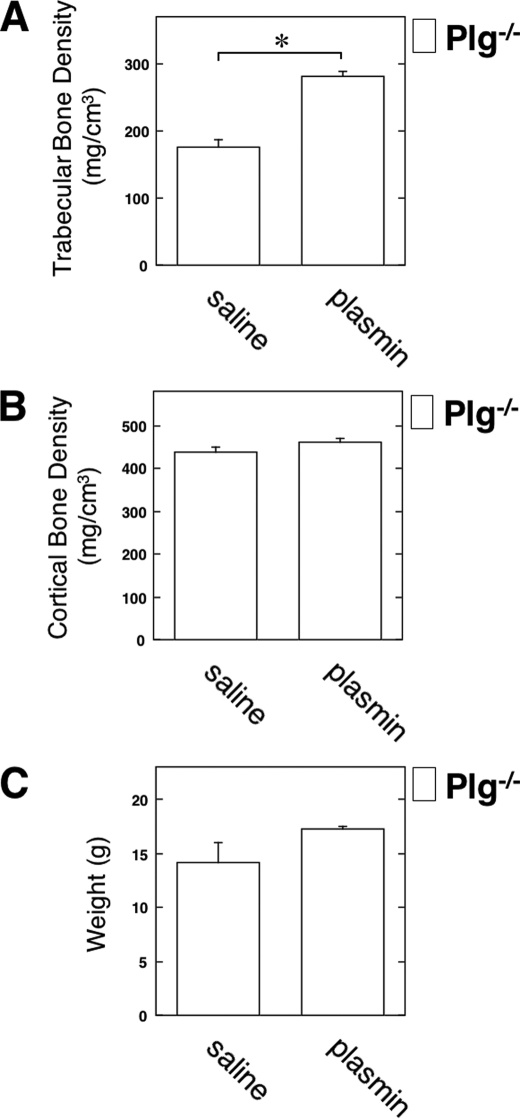
To clarify the effect of exogenous plasmin on bone formation in vivo, we evaluated the status of the BMD in the Plg−/− mice with or without plasmin injection. The plasmin injection clearly increased the trabecular BMD in the Plg−/− mice (Fig. 6A). However, the plasmin injection did not affect the cortical BMD and the weight in the Plg−/− mice (Fig. 6, B and C).
FIGURE 6.
Rescue of the down-regulated BMD in Plg-deficient mice by the injection of plasmin. Intraperitoneal injection with saline or plasmin (1 mg/kg) in the 5-week-old male Plg−/− mice was carried out weekly for up to 3 weeks. Then the trabecular BMD (A), the cortical BMD (B), and the weight (C) in the male Plg−/− mice were measured by pQCT (n = 3). The data represent the mean ± S.E. *, p < 0.01.
DISCUSSION
Fibrinolytic factors have been suggested to play an important role in bone metabolism. PAs and PAI-1 are involved in bone resorption by OCs (22, 23). However, the role of Plg/plasmin in bone metabolism was not precisely understood. This study showed that Plg/plasmin plays an important role in bone metabolism by regulating the function of both OBs and OCs.
The trabecular BMD in the tibias from the Plg−/− mice was significantly lower than that from the Plg+/+ mice at 4–6 weeks after birth (Fig. 1A). In contrast, the cortical BMD in the tibias from the Plg−/− mice was significantly lower than that from the Plg+/+ mice at 4–18 weeks after birth (Fig. 1B). Therefore, the decrease in the trabecular BMD in Plg−/− mice seemed to be transient; however, the decrease in the cortical BMD in the mice was consistently observed from the juvenile growth period to adulthood. In addition, TRAP staining of decalcified sections of tibias from the 5-we-old mice revealed that the intensity of TRAP staining of bone marrow tissue in the tibias from the Plg−/− mice was significantly stronger than that from the Plg+/+ mice (Fig. 1, E and F). Thus, the histoenzymatic assessment indicated that the OC differentiation in bone marrow tissue of the Plg−/− mice might be more vigorously induced than that in the Plg+/+ mice.
The binding of RANKL to its receptor RANK triggers intricate and distinct signaling cascades that control lineage commitment and osteoclast activation (13). OPG inhibits osteoclast formation and bone resorption by blocking RANKL/RANK interactions (14). This study showed that plasmin increased the OPG expression in WT OBs (Fig. 3, A–D). Moreover, the expression level of OPG was decreased in Plg−/− OBs compared with Plg+/+ OBs (Fig. 3, G and H), suggesting that absence of plasmin may result in an acceleration of OB-mediated osteoclastogenesis of pre-OCs in accordance with the depletion of OPG expression in OBs. In fact, the number of TRAP-positive multinucleated RAW264.7 cells co-cultured with Plg−/− OBs was significantly higher than that of the cells co-cultured with Plg+/+ OBs (Fig. 4A). Intriguingly, plasmin significantly inhibited the M-CSF- and RANKL-induced OC differentiation of bone marrow cells derived from the Plg+/+ and Plg−/− (Fig. 2D), suggesting that plasmin might attenuate osteoclastogenesis by its direct effects on pre-OCs. In addition, there was a larger population of pre-OCs in bone marrow-derived cells from the Plg−/− mice in comparison with the Plg+/+ mice (Fig. 2, A–C). The level of ALP activity in Plg−/− OBs was similar to that in Plg+/+ OBs (Fig. 5), thus suggesting that the bone-mineralizing activity of OBs in the Plg−/− mice might be comparable with that in the Plg+/+ mice. Consequently, the Plg−/− mice display decreased bone mineral density in accordance with the enhanced ability of OBs to induce osteoclastogenesis of pre-OCs, the loss of the direct and suppressive effect of plasmin on pre-OCs differentiating into mature OCs, and the increased pre-OC population in bone marrow cells. In fact, the injection of plasmin into the Plg−/− mice clearly rescued the diminished trabecular BMD during the juvenile growth period (Fig. 6).
Plasmin activates a latent transforming growth factor β (TGF-β) (24, 25) trapped in extracellular matrix to induce an OPG expression in extracellular matrix-harbored OBs. The accelerated expression of OPG on OBs might result in the suppression of the OB-mediated osteoclastogenesis. It is under investigation by us whether deficiency of activated TGF-β causes decreased bone mineral density and decreased body weight in Plg−/− mice. However, plasmin directly activates various intracellular signaling through annexin A2 in macrophage (26). Plasmin activates macrophages via the annexin A2 heterotetramer composed of annexin A2 and S100A10 with subsequent stimulation of Janus kinase JAK1/TYK2 signaling. JAK1/TYK2 leads to STAT3 activation, Akt-dependent nuclear factor-κB (NF-κB) activation, and phosphorylation of ERK1/2 and p38 MAPK. Furthermore, inhibitors of JAK, p38 MAPK, and NF-κB revealed that these signaling pathways are indispensable for the plasmin-mediated tumor necrosis factor-α and IL-6 induction in the cells. In addition, angiostatin, a fragment of plasmin(ogen), is a ligand and an antagonist for integrin α9β1 (27). Angiostatin, representing the kringle domains of plasmin, alone did not induce the migration of Chinese hamster ovary (CHO) cells, but simultaneous activation of the G protein-coupled protease-activated receptor-1 with an agonist peptide induced the migration on angiostatin. These facts suggest that plasmin directly stimulates various cell lineages without an indirect cell stimulation through an activation of some growth factors such as TGF-β. We showed that plasmin activated ERK1/2 and p38 MAPK, and the inhibition of ERK1/2 and p38 MAPK attenuated plasmin-induced OPG expression (Fig. 3, E and F). In addition, plasmin activated JNK, but the inhibition of JNK did not attenuate plasmin-induced OPG expression (data not shown). These data suggest that plasmin induces OPG expression through the ERK1/2 and p38 MAPK pathways. However, the time lag between the activation of p38 MAPK and ERK1/2 after plasmin stimulation in OBs might depend on the hierarchy of ERK1/2 and p38 MAPK in the plasmin-induced signal transduction. The ERK1/2 might be the downstream target of p38 MAPK directly activated by plasmin in OBs. Further investigations would be required to clarify the details.
These results strongly suggest that the plasmin activity regulates both OB and OC functions and then plays an important role in bone metabolism. These findings may provide new insights into the development of clinical therapies for the prevention of bone loss-related disorders.
This work was supported by Grant-in-aid for Young Scientists B:21790097 (to Y. K.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan Society for the Promotion of Science, and Grant-in-aid for Strategic Medical Science Research Center from the Ministry of Education, Culture, Sports, Science and Technology of Japan, 2010-2014.
- Plg
- plasminogen
- TRAP
- tartrate-resistant acid phosphatase
- OC
- osteoclast
- OB
- osteoblast
- OPG
- osteoprotegerin
- BMD
- bone mineral density
- PGE2
- prostaglandin E2
- RANK
- receptor activator of NF-κB
- RANKL
- RANK ligand
- tPA
- tissue-type plasminogen activator
- uPA
- urokinase-type PA
- PAI-1
- plasminogen activator inhibitor-1
- M-CSF
- macrophage colony-stimulating factor
- ALP
- alkaline phosphatase
- qRT
- quantitative RT.
REFERENCES
- 1. Braaten J. V., Handt S., Jerome W. G., Kirkpatrick J., Lewis J. C., Hantgan R. R. (1993) Blood 81, 1290–1299 [PubMed] [Google Scholar]
- 2. Lijnen H. R., De Cock F., Van Hoef B., Schlott B., Collen D. (1994) Eur. J. Biochem. 224, 143–149 [DOI] [PubMed] [Google Scholar]
- 3. Carmeliet P., Collen D. (1996) Semin. Thromb. Hemost. 22, 525–542 [DOI] [PubMed] [Google Scholar]
- 4. Matsuno H., Ishisaki A., Nakajima K., Okada K., Ueshima S., Matsuo O., Kozawa O. (2003) Blood 102, 3621–3628 [DOI] [PubMed] [Google Scholar]
- 5. Kanno Y., Kuroki A., Minamida M., Kaneiwa A., Okada K., Tomogane K., Takeuchi K., Ueshima S., Matsuo O., Matsuno H. (2008) Thromb. Res. 123, 336–341 [DOI] [PubMed] [Google Scholar]
- 6. Kanno Y., Hirade K., Ishisaki A., Nakajima K., Suga H., Into T., Matsushita K., Okada K., Matsuo O., Matsuno H. (2006) J. Thromb. Haemost. 4, 1602–1610 [DOI] [PubMed] [Google Scholar]
- 7. Kanno Y., Kuroki A., Okada K., Tomogane K., Ueshima S., Matsuo O., Matsuno H. (2007) J. Thromb. Haemost. 5, 2266–2273 [DOI] [PubMed] [Google Scholar]
- 8. Kanno Y., Kaneiwa A., Minamida M., Kanno M., Tomogane K., Takeuchi K., Okada K., Ueshima S., Matsuo O., Matsuno H. (2008) J. Invest. Dermatol. 128, 2792–2797 [DOI] [PubMed] [Google Scholar]
- 9. Kanno Y., Kawashita E., Minamida M., Kaneiwa A., Okada K., Ueshima S., Matsuo O., Matsuno H. (2010) Am. J. Pathol. 176, 238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daci E., Everts V., Torrekens S., Van Herck E., Tigchelaar-Gutterr W., Bouillon R., Carmeliet G. (2003) J. Bone Miner. Res. 18, 1167–1176 [DOI] [PubMed] [Google Scholar]
- 11. Daci E., Verstuyf A., Moermans K., Bouillon R., Carmeliet G. (2000) J. Bone Miner. Res. 15, 1510–1516 [DOI] [PubMed] [Google Scholar]
- 12. Furlan F., Galbiati C., Jorgensen N. R., Jensen J. E., Mrak E., Rubinacci A., Talotta F., Verde P., Blasi F. (2007) J. Bone Miner. Res. 22, 1387–1396 [DOI] [PubMed] [Google Scholar]
- 13. Wada T., Nakashima T., Hiroshi N., Penninger J. M. (2006) Trends Mol. Med. 12, 17–25 [DOI] [PubMed] [Google Scholar]
- 14. Hofbauer L. C., Heufelder A. E. (2001) J. Mol. Med. 79, 243–253 [DOI] [PubMed] [Google Scholar]
- 15. Li J., Sarosi I., Yan X. Q., Morony S., Capparelli C., Tan H. L., McCabe S., Elliott R., Scully S., Van G., Kaufman S., Juan S. C., Sun Y., Tarpley J., Martin L., Christensen K., McCabe J., Kostenuik P., Hsu H., Fletcher F., Dunstan C. R., Lacey D. L., Boyle W. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ploplis V. A., Carmeliet P., Vazirzadeh S., Van Vlaenderen I., Moons L., Plow E. F., Collen D. (1995) Circulation 92, 2585–2593 [DOI] [PubMed] [Google Scholar]
- 17. Suda T., Jimi E., Nakamura I., Takahashi N. (1997) Methods Enzymol. 282, 223–235 [DOI] [PubMed] [Google Scholar]
- 18. Kanazawa S., Ota S., Sekine C., Tada T., Otsuka T., Okamoto T., Sønderstrup G., Peterlin B. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 14465–14470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishiwaki T., Yamaguchi T., Zhao C., Amano H., Hankenson K. D., Bornstein P., Toyama Y., Matsuo K. (2006) J. Bone Miner. Res. 21, 596–604 [DOI] [PubMed] [Google Scholar]
- 20. Kanno Y., Into T., Lowenstein C. J., Matsushita K. (2008) Cardiovasc. Res. 77, 221–230 [DOI] [PubMed] [Google Scholar]
- 21. Nakashima T., Kobayashi Y., Yamasaki S., Kawakami A., Eguchi K., Sasaki H., Sakai H. (2000) Biochem. Biophys. Res. Commun. 275, 768–775 [DOI] [PubMed] [Google Scholar]
- 22. Daci E., Udagawa N., Martin T. J., Bouillon R., Carmeliet G. (1999) J. Bone Miner. Res. 14, 946–952 [DOI] [PubMed] [Google Scholar]
- 23. Everts V., Daci E., Tigchelaar-Gutter W., Hoeben K. A., Torrekens S., Carmeliet G., Beertsen W. (2008) Bone 43, 915–920 [DOI] [PubMed] [Google Scholar]
- 24. Thirunavukkarasu K., Miles R. R., Halladay D. L., Yang X., Galvin R. J., Chandrasekhar S., Martin T. J., Onyia J. E. (2001) J. Biol. Chem. 276, 36241–36250 [DOI] [PubMed] [Google Scholar]
- 25. Lyons R. M., Gentry L. E., Purchio A. F., Moses H. L. (1990) J. Cell Biol. 110, 1361–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Q., Laumonnier Y., Syrovets T., Simmet T. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 1383–1389 [DOI] [PubMed] [Google Scholar]
- 27. Majumdar M., Tarui T., Shi B., Akakura N., Ruf W., Takada Y. (2004) J. Biol. Chem. 279, 37528–37534 [DOI] [PubMed] [Google Scholar]