Abstract
AMP-activated protein kinase (AMPK), an evolutionarily conserved serine-threonine kinase that senses cellular energy status, is activated by stress and neurohumoral stimuli. We investigated the mechanisms by which adrenergic signaling alters AMPK activation in vivo. Brown adipose tissue (BAT) is highly enriched in sympathetic innervation, which is critical for regulation of energy homeostasis. We performed unilateral denervation of BAT in wild type (WT) mice to abolish neural input. Six days post-denervation, UCP-1 protein levels and AMPK α2 protein and activity were reduced by 45%. In β1,2,3-adrenergic receptor knock-out mice, unilateral denervation led to a 25–45% decrease in AMPK activity, protein expression, and Thr172 phosphorylation. In contrast, acute α- or β-adrenergic blockade in WT mice resulted in increased AMPK α Thr172 phosphorylation and AMPK α1 and α2 activity in BAT. But short term blockade of α-adrenergic signaling in β1,2,3-adrenergic receptor knock-out mice resulted in decreased AMPK activity in BAT, which strongly correlated with enhanced phosphorylation of AMPK on Ser485/491, a site associated with inhibition of AMPK activity. Both PKA and AKT inhibitors attenuated AMPK Ser485/491 phosphorylation resulting from α-adrenergic blockade and prevented decreases in AMPK activity. In vitro mechanistic studies in BAT explants showed that the effects of α-adrenergic blockade appeared to be secondary to inhibition of oxygen consumption. In conclusion, adrenergic pathways regulate AMPK activity in vivo acutely via alterations in Thr172 phosphorylation and chronically through changes in the α catalytic subunit protein levels. Furthermore, AMPK α Ser485/491 phosphorylation may be a novel mechanism to inhibit AMPK activity in vivo and alter its biological effects.
Keywords: Adipose Tissue, Adrenergic Receptor, AKT PKB, AMP-activated kinase (AMPK), Protein Kinase A (PKA), Tricarboxylic Acid (TCA) Cycle
Introduction
AMP-activated protein kinase (AMPK),4 an evolutionarily conserved serine threonine kinase, is a fuel-sensing enzyme complex activated by cellular stresses that increase AMP or deplete ATP including hypoxia, ischemia, glucose deprivation, uncouplers of oxidative phosphorylation, exercise, and muscle contraction (1, 2). Once activated, AMPK phosphorylates multiple downstream substrates that function to preserve the AMP:ATP ratio through inhibition of ATP-catabolizing pathways and promotion of ATP-generating pathways. Mechanisms involved in AMPK activation include 1) binding of AMP to an allosteric site on the γ subunit, which renders the holoenzyme resistant to inactivating serine phosphatases and may also have direct allosteric effects on kinase activity (1, 2) and 2) phosphorylation by upstream AMPK kinases of the α (catalytic) subunits on Thr172, which is essential for kinase activity. Recent studies employing INS-1 cells (3), hepatitis C virus harboring replicon cells (4), and isolated heart preparations (5) have demonstrated another potential regulatory mechanism; that is, AMPK may also undergo inhibitory phosphorylation on serine 485 and 491 residues of the α1 and α2 catalytic subunits, respectively. Some data suggest that upstream kinases that may mediate this phosphorylation include AKT (6) and protein kinase A (PKA) (7).
Over the last several years it has become evident that AMPK plays a major role in mediating hormonal, nutrient, and stress-related signals (1, 2). Importantly, metabolic effects of the adipocyte-secreted hormone leptin are mediated in part through alterations in AMPK activity in both the hypothalamus and peripheral tissues (8). In addition to an acute, transient direct effect of leptin to activate AMPK activity and fatty acid oxidation in muscle, there is also a sustained effect that is mediated through the hypothalamic adrenergic nervous system axis (8).
The aims of this study were to determine the cellular mechanisms by which adrenergic signaling pathways regulate AMPK activity in vivo and whether this regulation involves inhibitory phosphorylation of AMPK on Ser485/491. We studied Ser485/491 phosphorylation because the effects of phosphorylation changes at this site on AMPK activity in vivo have not been definitively reported, and this could be a novel mechanism for physiologic regulation of AMPK activity. We utilized BAT as our experimental model because the sympathetic nervous system is critical for key effects of BAT on energy homeostasis including thermogenesis (9). Three recent studies employed biochemical, molecular, and morphological approaches to convincingly demonstrate the presence of metabolically active BAT in healthy human subjects (10–12). These studies underscore the importance of investigations of the regulation of BAT function to human physiology.
Metabolic effects of the sympathetic nervous system are mediated through G-protein-coupled receptors that are broadly classified into two main subtypes: α1–2-, and β1–3-adrenergic receptors (13). Recent studies indicate a role for adrenergic receptors in regulating AMPK activity. The effects of leptin through the hypothalamus on muscle AMPK involve α-adrenergic receptors in muscle (8). The α-adrenergic agonist, phenylephrine, activates AMPK in isolated soleus muscle, C2C12 myocytes, and CHO cells (8, 14, 15), whereas the β-adrenergic agonist, isoproterenol, stimulates AMPK in isolated adipocytes (16). Norepinephrine and epinephrine, both dual α and β agonists, activate AMPK in brown adipocytes in vitro (17) and white adipocytes ex vivo (18), and in the latter, activation of AMPK was necessary for maximal activation of lipolysis (18). In contrast, epinephrine decreases palmitate-induced activation of AMPK in the perfused rat heart (19). Thus, the effect of adrenergic signaling on AMPK activity appears to be tissue-specific and may depend on the metabolic milieu.
In vivo studies demonstrated that acute exercise or epinephrine also activates AMPK in rat adipose tissue (18). Because these effects were blunted by a β-adrenergic receptor blocker, the activation of AMPK with exercise or epinephrine appeared to be mediated by β-adrenergic receptors (18). Further work is needed to understand the adrenergic regulation of AMPK in vivo under other physiologic conditions. Our study demonstrates novel mechanisms for adrenergic regulation of AMPK activity in BAT in vivo. We now show for the first time that chronic adrenergic regulation of AMPK occurs at the level of alterations in AMPK catalytic subunit levels. In contrast, acute regulation is through changes in threonine or serine phosphorylation of AMPK. Furthermore, acute regulation appears to be secondary to changes in BAT oxygen consumption. Adrenergic regulation of Ser485/491 phosphorylation of the AMPK α subunit involves upstream kinases, PKA and AKT. These data provide novel mechanistic insights into how α- and β-adrenergic signals integrate to regulate AMPK activity and expand our understanding of physiologic regulation of AMPK. Because there is currently a large amount of interest in modulating AMPK activity to treat metabolic diseases and potentially cancer, these new findings could have important therapeutic implications.
EXPERIMENTAL PROCEDURES
Materials
Phentolamine, propranolol, and isoproterenol were purchased from Sigma, AKT inhibitor (10-DEBC) was purchased from Tocris USA, and PKA inhibitor (PKA-I, 14-22) was purchased from Calbiochem.
Animals
Generation of mice lacking β-adrenergic receptors (β-AR KO) was described previously (20). β-AR KO and its wild type counterparts were bred on a C57BL/6Jx129 background. For validation of certain effects, mice on a FVB background were also employed. Mice were housed with a 14-h light/10-h dark cycle in a temperature-controlled facility with free access to water and standard laboratory chow (Purina #5008, fat content 4.5% by weight/11.9% by calories). All aspects of animal care were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
Unilateral Denervation of Brown Fat Sympathetic Nerves
On the day of the surgery mice were anesthetized by an intraperitoneal injection (0.1 ml/10g) of ketamine/xylazine (80 and 12 mg/ml, respectively) except for β-AR KO mice, for which the anesthetic dose was 75% that of the wild type. Anesthetized mice were placed on a warming pad to prevent possible hypothermia. Using a stereomicroscope, intrascapular brown adipose tissue was carefully moved outward from the surrounding muscle and white adipose tissue, thus exposing the sympathetic chain innervating brown adipose tissue (21). Five branches of the left intercostal nerve bundles were isolated and cut (denervated) without disrupting the blood circulation, and the right intercostal nerve bundle was maintained as the contralateral sham side. After completion of the procedure the skin was closed using tissue adhesive glue (Vetbond). After gain of consciousness, mice were housed individually in a clean cage for recovery, and body weight was assessed daily. 1, 2, or 6 days after recovery, animals were killed by cervical dislocation, and sham and denervated brown fat lobes were isolated and frozen at −80 °C until further analysis.
Acute Blockade of α- and β-Adrenergic Receptors
On the day of the experiment, food was withdrawn for 2 h. Mice were injected intraperitoneally with either 10 mg/kg of propranolol or 10 mg/kg phentolamine in WT mice or 8 mg/kg phentolamine in β-AR KO mice (maximum tolerated dose) or saline control. After 30 min of drug administration, animals were killed by cervical dislocation, and brown fat lobes were isolated and frozen at −80 °C until further analysis.
Preparation of Brown Fat Explants
Interscapular brown adipose tissues were obtained from 8–10-week-old mice. Tissues were washed with sterile phosphate-buffered saline and minced to a size of 1–2 mm in 6-well plates. The tissue explants were allowed to stabilize for 15 min in Krebs Henseleit buffer (1 m CaCl2, 1.2 m MgSO4, 1.2 m KH2PO4, 0.14 m KCl, 1 m HEPES in 1.2 m NaHCO3, 2.6 m NaCl, 0.01% BSA). Subsequently, explants were incubated with a pharmacological agonist or antagonist for 30 min. Media was aspirated, and explants were transferred to dinonyl phthalate oil to separate BAT from BSA. BAT was further subjected to homogenization, and measurement of AMPK and acetyl-CoA carboxylase (ACC) activity and immunoblot analysis.
Preparation of Tissue Lysates
BAT was quickly removed and snap-frozen in liquid nitrogen until processing. Tissues were homogenized in AMPK immunoprecipitating lysis buffer composed of 20 mm Tris, 50 mm NaCl, 50 mm NaF, 5 mm NaPPi, 250 mm sucrose, 1% Triton X-100, 2 mm dithiothreitol (DTT), 100 μm benzamidine, 500 μm PMSF, and 50 μg/ml each of aprotinin and leupeptin. Tissue lysates were further solubilized for 40 min at 4 °C followed by centrifugation at 14,000 × g at 4 °C for 20 min. Protein concentration in an aliquot of the supernatant was determined using the Bio-Rad DC-Protein Assay kit. Supernatants were stored at −80 °C until assays were performed.
AMPK Activity Assay
To measure AMPK activity, protein (50 μg) was immunoprecipitated with polyclonal antibodies specific to AMPK α1 (Upstate, 07-350) or α2 (Santa Cruz Biotechnology, Santa Cruz, CA, 19131) bound to protein G-Sepharose overnight. Immunoprecipitates were washed twice in immunoprecipitating lysis buffer then twice in assay buffer (240 mm HEPES and 480 mm NaCl). Kinase reactions were carried out in 40 mm HEPES (pH 7.0), 80 mm NaCl, 0.8 mm DTT, 5 mm MgCl2, 0.2 mm each of ATP and AMP, 0.1 mm SAMS peptide, and 2 μCi of [γ-32P]ATP (PerkinElmer Life Sciences) in a total volume of 50 μl. A 40-μl aliquot was spotted onto Whatman P81 paper and washed 4 times in 1% phosphoric acid. Kinase activity was calculated as [32P]ATP (nmol) incorporated/g of protein/min(22).
ACC Activity Assay
Reactions were carried out on tissue lysates (50 μg) in an assay buffer composed of 80 mm HEPES (pH 7.5), 1.7 mm DTT, 4.2 mm ATP, 1 mm NaHCO3, 8.3 mg/ml BSA, 3.3 mm MgCl2, 0.42 mm acetyl-CoA, and 6 μCi of 14CO3 (Amersham Biosciences) in the presence or absence of 2 mm citrate. Reactions were stopped by adding 1 n HCl, and samples were dried. 14CO2 radioactivity was determined with a scintillation counter. ACC activity was calculated as citrate-dependent incorporation of 14CO2 into acid-stable products (pmol/mg of protein/min) (23).
Immunoblotting
Lysates (25–50 μg of protein) were subjected to SDS-PAGE, and phosphorylation and total levels of specific proteins were measured by immunoblotting. Antibodies employed for immunoblotting included: anti-pAMPK α-Thr172 (2531), pAKT Thr308 (4056), pPKA Thr197 (4781), pAMPK β-Ser108 (4181), total AMPK β (4182), total PKA (4782), and total ACC (3662) (Cell Signaling Technology, Beverly, MA), anti-AMPK α2 (SC-19131) and UCP1 (SC-6529) (Santa Cruz Biotechnology), AMPK α1 (07-350), pACC Ser79 (07-303), pAKT Ser473 (05-669), and total AKT (05-591) (Millipore), GAPDH (3073, Imgenex), and fatty acid synthase (3844, Abcam), and CIDEA (Novus Biologicals, H00001149). Proteins were visualized with chemiluminescence (PerkinElmer). Densitometric analysis was performed employing both GeneGnome chemiluminescence imaging system and GeneTools software (Syngene, MD) and Molecular dynamics scanning densitometry. For ACC detection, membranes were incubated with streptavidin conjugated with horseradish peroxidase (RPN 1231; Amersham Biosciences) at 4 °C overnight, washed with Tris-buffered saline plus 0.4% Tween 20 (TBST) three times, and visualized by enhanced chemiluminescence (PerkinElmer).
Adenine Nucleotide Measurement
Animals were anesthetized, and BAT was isolated and subjected to freeze-clamping (24). Freeze-clamped BAT (10–20 mg wet weight) was pulverized in a mortar under liquid nitrogen and extracted with 0.6 n perchloric acid. An aliquot of the homogenate was removed for protein assay by the method of Lowry et al. (25). After neutralization and centrifugation, the supernatant was passed through a 0.45-μm filter, diadenosine pentaphosphate (100 mmol/ml) was added, and the resulting solution was passed through a 0.2-μm filter. The supernatant (200 μl) was injected into a Waters HPLC system equipped with a Waters 1525 HPLC pump and a 2487 dual wavelength (UV-visible) absorbance detector (Waters Co., Milford MA) to measure nucleotides. ATP, ADP, and AMP were separated through a C-18 reverse phase column (YMC-PackTM ODS-AQTM, 150 × 4.6 mm, particle size 3 μm, pore size 120 Å) at a flow rate of 0.8 ml/min with 50 mm ammonium dihydrogen phosphate (pH 6.0) as the mobile phase during the initial 50 min (26). Peaks were monitored by UV absorption at 254 nm and identified by comparison with the retention times of known standards. Nucleotide content was quantified using standard curves and normalized to the protein concentration in each sample.
Oxygen Consumption in Brown Fat
Interscapular brown fat was isolated and weighed. Tissue pieces of ∼10–15 mg each were minced and incubated in Krebs-Ringer bicarbonate buffer (pH 7.4; KRH, 25 mm Hepes, 5 mm pyruvate, 10 mm glucose, 10 mm fructose, 25 mm NaHCO3, and 4% fatty acid free BSA (Roche Applied Science)) at 37 °C. O2 consumption was measured polarographically (27) by a precalibrated electrode (Oxygraph, Hansatech Instruments) in an air-sealed chamber containing 500 μl of KRB buffer at 37 °C with constant agitation. After recording of the basal respiration rate, uncoupled respiration was measured by the addition of 5 mm sodium succinate (Sigma), and respiration was subsequently inhibited by the addition of 2 mm sodium cyanide (Fluka 71429) to the buffer. The final two steps inform us about the viability of the explant. Tracings were recorded, and data were computed using the digital analog program.
Statistical Analysis
Results are presented as the mean ± S.E. Differences between groups were examined for statistical significance by Student's t test or analysis of variance.
RESULTS
Denervation Decreases UCP1 Protein and AMPK Pathway Signaling
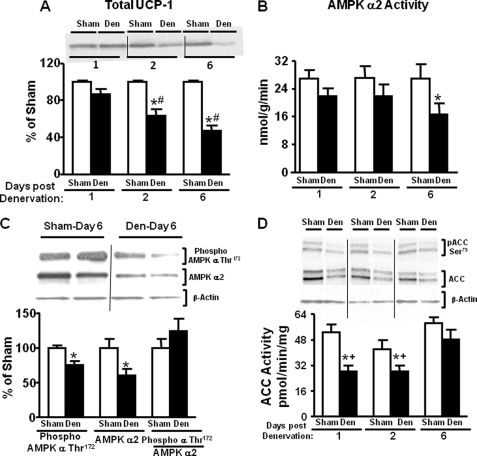
Unilateral denervation terminates neural inputs into the brown fat lobe. To determine whether denervation was successful in male FVB mice, UCP-1 protein levels were measured in the denervated and contralateral sham lobe of brown fat. UCP-1 protein decreased by 25% at 2 days after denervation and 45% by 6 days after denervation (Fig. 1A). There was no evidence of compensatory changes in the sham lobe (denervation hypersensitivity) as UCP-1 protein levels remained unaltered on the sham side at 1, 2, and 6 days after denervation (data not shown). We hypothesized that lack of sympathetic innervation would diminish AMPK activity. In FVB mice, AMPK α2 activity was decreased by 45% at 6 days after denervation but not at 1 or 2 days (Fig. 1B). This decline in activity was accompanied by a decrease in AMPK α catalytic subunit Thr172 phosphorylation (Fig. 1C), which appeared to be secondary to a 40% decline in total AMPK α2 protein level (Fig. 1C). The slightly greater decline in total α2 protein compared with phosphorylated α2 can be explained by the fact that the phosphorylated AMPK α Thr172 antibody detects phosphorylation of both α1 and α2 subunits. AMPK α1 activity remained unchanged in either BAT lobe at 1, 2, and 6 days after denervation (data not shown). The seemingly greater AMPK α1 activity compared with α2 activity in BAT is probably due to technical issues related to the specific AMPK α1 and α2 antibodies used in the assay.
FIGURE 1.
Effects of unilateral denervation (Den) on UCP1 protein and AMPK and ACC activity, protein levels, and phosphorylation in brown fat of 7–9-week-old male FVB mice. Immunoblotting of UCP1 (panel A), AMPK α2 activity (panel B), AMPK α subunit phosphorylation on Thr172 and AMPK α2 protein (panel C), and ACC activity and ACC phosphorylation on Ser79 and total ACC protein (panel D) are shown. ACC and AMPK protein levels are normalized to β-actin (panel C and panel D), which was not altered by the treatments. Data in panel C are 6 days post-denervation. Data are expressed as the mean ± S.E.; n = 10 per group. *. p < 0.05 versus sham same day; #, p < 0.05 versus denervated day 1; +, p < 0.05 versus denervated day 6. Food was withdrawn for 2 h before the sacrifice.
Once activated, AMPK phosphorylates and inactivates ACC. Activity of ACC was markedly diminished on days 1 and 2 after denervation before the decline in AMPK activity, suggesting early sympathetic regulation of ACC through an AMPK-independent mechanism (Fig. 1D). ACC protein was decreased by 30–35% on 1, 2, and 6 days after denervation. This most likely explains the decrease in ACC activity and phosphorylation at days 1 and 2 post-denervation (Fig. 1D). The changes in total and phosphorylated levels of ACC were observed in both isoforms (ACC2, upper band; 280 kDa; ACC1, lower band; 266 kDa, Fig. 1D). Both bands were quantified as alterations in phosphorylation of either isoform can have important metabolic effects. Phosphorylation levels of both isoforms were reduced secondary to changes in ACC1 and ACC2 protein levels. Six days after denervation, ACC activity was restored to contralateral sham levels despite decreased ACC protein levels. This indicates increased activity per molecule of ACC, which could result from the diminished AMPK α2 activity at this time point (Fig. 1D).
Are β-Adrenergic Receptors Involved in the Regulation of AMPK Activity?
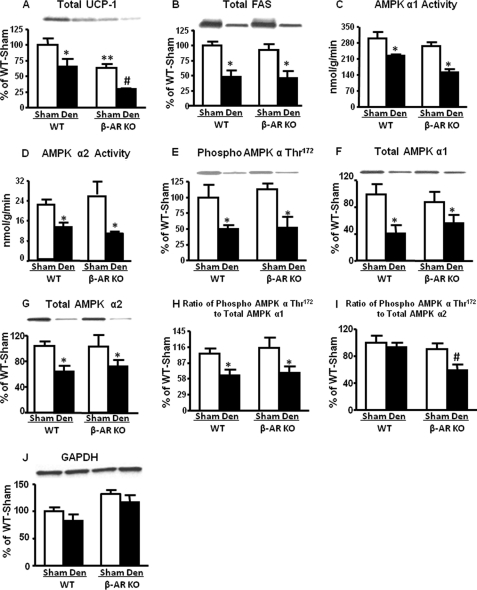
Next, we sought to determine which adrenergic receptor subtype(s) regulates BAT AMPK activity. We first studied mice lacking of β-adrenergic receptors (β-AR KO). No changes in body weight were observed 6 days after unilateral BAT denervation (data not shown). However, UCP-1 protein levels on the sham side of BAT in β-AR KO mice were 40% lower than on the sham side of WT (Fig. 2A), consistent with the fact that UCP-1 is under β-adrenergic control (20). Fatty acid synthase levels did not decrease in the sham lobe of β-AR KO mice when compared with WT (Fig. 2B). This indicates that the decrease in UCP-1 expression is not due to a global effect on protein levels. On the denervated side, UCP1 levels were decreased by 40–50% compared with the contralateral sham side in both genotypes (Fig. 2A), indicating some contribution of α-adrenergic or non-adrenergic pathways. Fatty acid synthase levels also decreased by 45% in the denervated lobes in both genotypes, indicating regulation by the autonomic nervous system (Fig. 2B).
FIGURE 2.
Effect of denervation on UCP1 and fatty acid synthase protein levels and AMPK activity and α subunit Thr172 phosphorylation in the brown fat of 7–9-week-old male WT and β-AR KO mice. All data are 6 days after denervation (Den). UCP-protein (panel A), fatty acid synthase protein (panel B), AMPK activity (panel C and D), Thr172 phosphorylation of AMPK α subunit (panel E), total AMPK α1 and α2 (panel F and G), and the ratio of phosphorylated to total AMPK α (panel H and I) are shown. Protein levels are normalized to GAPDH (panel J). Data are expressed as the mean ± S.E.; n = 12 per group. *, p < 0.05 versus respective sham; #, p < 0.05 versus all groups; **. p < 0.05 versus WT sham. Food was withdrawn for 2 h before the sacrifice.
In both genotypes denervation induced 25–45% decreases in AMPK α1 and α2 activities (Fig. 2, C and D) and corresponding decreases in phosphorylation of AMPK α at Thr172 (Fig. 2E) that most likely result from the down-regulation of AMPK α1 and α2 proteins (Fig. 2, F and G). The ratio of phosphorylated to total AMPK α1 was decreased by 30–40% after denervation in WT and KO mice (Fig. 2H), suggesting a decrease in the stoichiometry of phosphorylation of the AMPK α1 subunit in addition to decreased AMPK α1 protein in the denervated fat pad. However, the ratio of phosphorylated to total AMPK α2 subunit remained unchanged with denervation in WT, indicating that although AMPK α2 protein levels are reduced by denervation (Fig. 2I), the stoichiometry of phosphorylation is intact. Nevertheless, the biological effect is a reduction in the net AMPK α2 phosphorylation in BAT. Surprisingly, in β-AR KO mice the ratio of phosphorylated to total AMPK α2 subunit was decreased by 35% after denervation (Fig. 2I). This indicates that both AMPK α1 and α2 protein levels in BAT are neurally regulated (Fig. 2, F and G) but there are isoform-specific effects of neural innervation on the stoichiometry of AMPK catalytic subunit phosphorylation (Fig. 2, H and I).
Does the Denervation-induced Reduction in AMPK Involve Cidea?
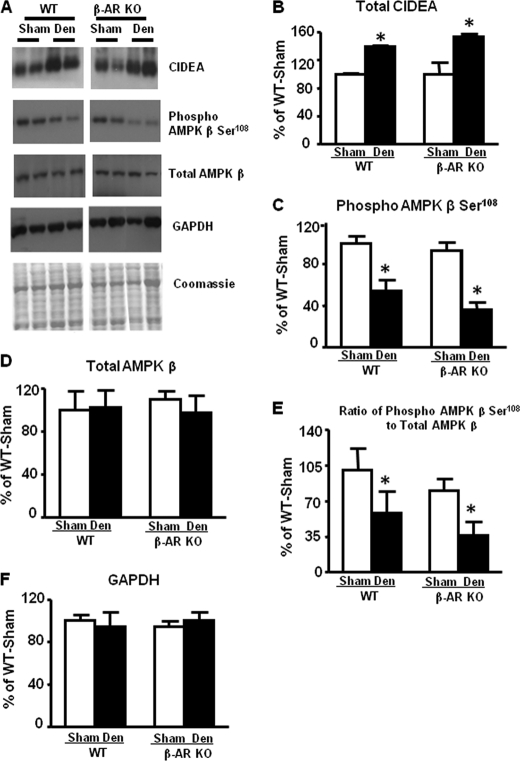
Cidea has been reported to regulate stability of the AMPK α subunit by interacting with the AMPK β subunit (28). Reduction in β subunits has been shown to result in decreased α subunit levels (28–30). To investigate the molecular mechanism by which denervation down-regulates AMPK protein and activity, we measured Cidea levels in BAT of WT and β-AR KO mice. Denervation increased Cidea protein levels 30–40% in both genotype (Fig. 3, A and B). Total AMPK β protein levels were not altered in either genotype (Fig. 3, A and D) after denervation, but AMPK β Ser108 phosphorylation was decreased (Fig. 3, A and C). This resulted in a decreased stoichiometric ratio of phosphorylated to total AMPK β in the denervated fat pads (Fig. 3E). This phosphorylation site on the AMPK β subunit is critical for the full activity of the AMPK heterotrimer (29). Conceivably Ser108 phosphorylation of the AMPK β subunit could affect AMPK α subunit binding and, therefore, its stability.
FIGURE 3.
Effect of denervation on CIDEA and AMPK β Ser108 phosphorylation and β subunit total protein levels in brown fat of 7–9-week-old male WT and β-AR KO mice. All data are 6 days after denervation (Den). CIDEA protein (panel A and B), Ser108 phosphorylation of AMPK β subunit (panel A and C), total AMPK β (panel A and D), and the ratio of phosphorylated to total AMPK β (panel E) are shown. Protein levels are normalized to GAPDH (panel F). Data are expressed as the mean ± S.E.; n = 3–4 per group. *, p < 0.05 versus respective sham. Food was withdrawn for 2 h before the sacrifice.
Does Acute Blockade of α- or β-Adrenoreceptor Signaling in Vivo Alter AMPK Activity in BAT in the Absence of Changes in Catalytic Subunit Levels?
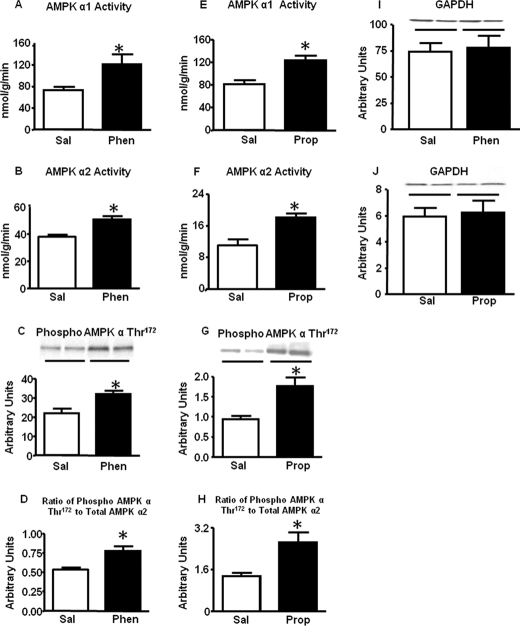
To determine whether adrenergic signaling could modulate AMPK activity without changes in subunit protein levels, we investigated the acute effects of adrenergic antagonists in vivo. Treatment of WT mice with phentolamine (α- adrenergic receptor antagonist) for 30 min resulted in a ∼25–30% increase in AMPK α1 and α2 activities in BAT (Fig. 4, A and B) compared with saline-treated mice. These changes were associated with augmented phosphorylation of AMPK α subunit at Thr172 (Fig. 4C) without changes in total AMPK α1 and α2 protein levels (data not shown). Thus, acute blockade of α-adrenergic signaling resulted in a 25% increase in the ratio of phosphorylated to total AMPK α2 (Fig. 4D) and AMPK α1 (data not shown). Surprisingly, pharmacological blockade of β-adrenergic receptors using propranolol also enhanced AMPK α1 (Fig. 4E) and α2 (Fig. 4F) activity and α subunit Thr172 phosphorylation (Fig. 4, G and H). To determine the mechanism for this activation of AMPK, we measured nucleotide levels in BAT. Tissue AMP levels and AMP-ATP ratio were increased in the BAT of mice treated with phentolamine or propranolol, and the effect of propranolol was substantially greater than that of phentolamine (Table 1). This may explain the increased AMPK activity and phosphorylation with these agents.
FIGURE 4.
Effect of pharmacological blockade of adrenergic receptors in vivo on AMPK activity and Thr172 phosphorylation and the ratio of Thr172 phosphorylated to total AMPK α in the brown fat of 7–9-week-old male WT mice. Mice were injected with either saline (Sal) or phentolamine (Phen) or propranolol (Prop) at a dose of 10 mg/kg intraperitoneally for 30 min after which animals were sacrificed by cervical dislocation, and BAT was frozen for activity and protein measurements. AMPK activity (panel A, B, E, and F), AMPK α Thr172 phosphorylation (panel C and G), and the ratio of phosphorylated to total AMPK α2 (panel D and H) are shown. Protein levels are normalized to GAPDH (panel I and J). Data are expressed as the mean ± S.E.; n = 8 per group; *, p < 0.05 versus saline. Food was withdrawn for 2 h before the sacrifice.
TABLE 1.
Adenine nucleotides (nmol/mg of protein), AMP/ATP, and ATP/ADP ratios in BAT of WT and β-AR KO mice treated with either phentolamine or propranolol
Male mice on chow diets were anaesthetized in the fed state, and BAT was isolated and immediately freeze-clamped and frozen in liquid nitrogen. β-AR KO, mice lacking β1,2,3-adrenergic receptors. Data are expressed as the mean ± S.E. n = 5–6 for each group.
| AMP | ADP | ATP | AMP/ATP | ATP/ADP | |
|---|---|---|---|---|---|
| WT | |||||
| Saline | 2.00 ± 0.08 | 2.43 ± 0.61 | 12.7 ± 0.6 | 0.16 ± 0.03 | 6.59 ± 1.3 |
| Phentolamine | 2.87 ± 0.07a | 3.34 ± 0.45 | 10.7 ± 0.7 | 0.26 ± 0.07a | 3.55 ± 0.6 |
| Propranolol | 3.88 ± 0.7a | 3.03 ± 0.22 | 11.9 ± 1.5 | 0.389 ± 0.11a | 4.04 ± 0.64 |
| β-AR KO | |||||
| Saline | 4.4 ± 1.01 | 3.87 ± 0.35 | 10.6 ± 2.5 | 0.61 ± 0.21 | 3.11 ± 0.9 |
| Phentolamine | 3.49 ± 0.41b | 3.78 ± 0.64 | 12.1 ± 1.7 | 0.31 ± 0.04b | 3.86 ± 0.9 |
a p < 0.05 vs. all other WT conditions.
b p < 0.05 vs. β-AR KO-saline.
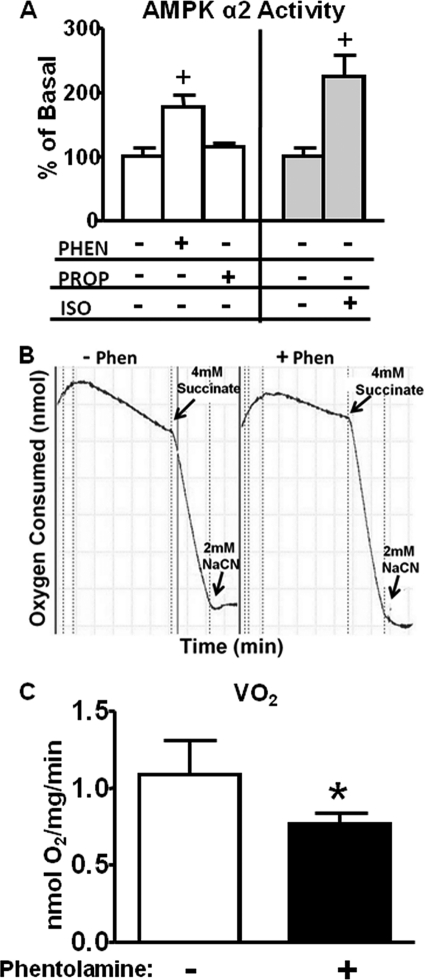
To determine whether the effect of pharmacological adrenergic blockade on AMPK phosphorylation and activity was directly at the level of BAT or indirect involving systemic changes such as altered blood pressure, heart rate, or other confounding effects, we performed studies in BAT explants. Incubation of BAT explants for 30 min with phentolamine enhanced AMPK α2 activity, whereas incubation with propranolol had no effect (Fig. 5A). Isoproterenol, a β-adrenergic agonist, had an even greater effect on AMPK α2 activity (Fig. 5A). To determine the biologic importance of the activation of AMPK by phentolamine, we measured O2 consumption in BAT explants. Phentolamine inhibited O2 consumption by 25–30% as reflected in the decreased downward slope of O2 consumption over time (Fig. 5B). This is quantitated and expressed per mg of tissue in Fig. 5C. Thus, α-adrenergic blockade activates AMPK α2 directly in BAT concurrent with inhibition of O2 consumption.
FIGURE 5.
Adrenergic signaling-mediated alterations in AMPK activity (panel A) and oxygen consumption (panel B and panel C) in brown fat explants from WT mice. Brown fat explants were isolated as described under “Experimental Procedures” and incubated with either vehicle (−), phentolamine (Phen, 100 μm), propranolol (Prop, 100 μm), or isoproterenol (Iso, 60 μm) for 30 min and then frozen for activity and protein measurements. For oxygen consumption studies, explants were incubated with vehicle (−) or 100 μm phentolamine for 30 min and then transferred to an oxygen electrode chamber, and oxygen consumption was measured in the absence and presence of succinate. Finally, NaCN was added to inhibit oxygen consumption. Data are expressed as the mean ± S.E.; n = 6–8 per group. +, p < 0.05 versus all other groups. In panel B a representative tracing of the oxygen consumption in the explants is shown. Panel C shows the mean data from n = 5–6 explant preparations in the absence (−) or presence (+) of phentolamine. *, p < 0.05 versus WT-vehicle.
Succinate was used to verify the viability of the samples. Succinate is a direct substrate for the tricarboxylic acid cycle. It enters the phospholipid bilayer of the mitochondrial membrane easily and stimulates state III respiration. In our studies the effect of succinate is comparable between vehicle and phentolamine-treated explants, suggesting that total tricarboxylic acid cycle activity is unaffected by phentolamine (Fig. 5B). Succinate-induced increases in O2 consumption persist in phentolamine-treated BAT explants, suggesting that the phentolamine effect to inhibit BAT O2 consumption is most likely upstream of the tricarboxylic acid cycle. Sodium cyanide inhibits respiration by acting on mitochondrial cytochrome oxidase (complex IV) and, thus, blocking electron transport. Here we used sodium cyanide to determine the specificity of the recorded O2 consumption.
Is the Effect of α-Adrenergic Receptor Blockade on AMPK Activity (Fig. 4, A–D) Due Directly to the Lack of α-Adrenergic Signaling or to Unopposed β-Adrenergic Signaling?
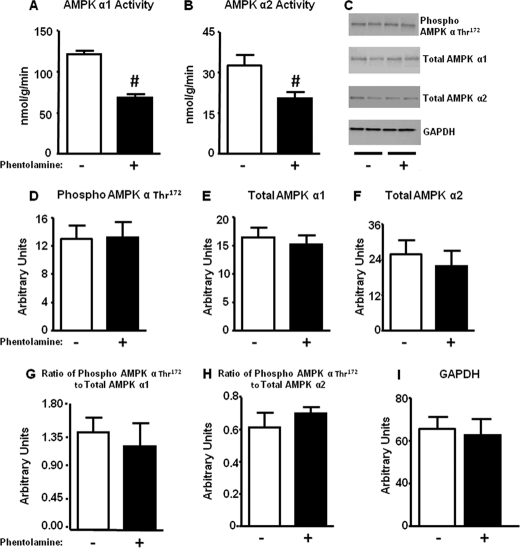
To investigate this, we treated β-adrenergic receptor KO mice with phentolamine. This decreased AMPK α1 and α2 activities by 40–50% compared with saline-treated KO mice (Fig. 6, A and B). Surprisingly, AMPK α Thr172 phosphorylation (Fig. 6, C and D) was unchanged. Total AMPK α1 (Fig. 6, C and E) and α2 (Fig. 6, C and F) catalytic subunit levels and the ratio of phospho to total AMPK α1 (Fig. 6G) or α2 (Fig. 6H) were also not altered. Therefore, reduced α-adrenergic signaling rather than unopposed β-adrenergic signaling appears to result in decreased AMPK activity.
FIGURE 6.
Acute α-adrenergic blockade in β-AR KO mice decreases AMPK activity in brown fat. Mice were injected with saline (−) or phentolamine at a dose of 8 mg/kg intraperitoneally and sacrificed after 30 min by cervical dislocation. BAT was isolated for activity and protein measurements. AMPK activity (panel A and B), Thr172 phosphorylation of AMPK α (panel C and D), total AMPK α1 and α2 (panel C, E, and F), and ratio of phosphorylated to total AMPK α1 and α2 subunit (panel G and H) are shown. Protein levels are normalized to GAPDH (panel C and I). Data are expressed as the mean ± S.E.; n = 7–8 for activity assays per group, and n = 5–6 for Western blot analysis per group. #, p < 0.05 versus saline-treated animals. Food was withdrawn for 2 h before the sacrifice.
What Is the Mechanism for Inhibition of AMPK Activity?
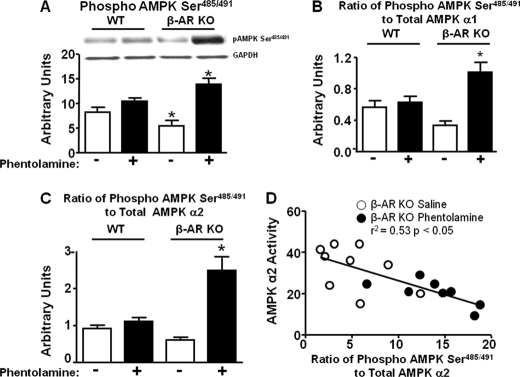
Because Ser485/491 phosphorylation of the AMPK α subunit can result in decreased AMPK activity (4, 5), we hypothesized that in BAT of β-AR KO mice treated with phentolamine, Ser485/491 phosphorylation of AMPK α may be increased. We assessed the control condition first. In WT BAT from phentolamine-treated mice, there was a tendency for a slight increase in Ser485/491 phosphorylation compared with saline-treated WT (Fig. 7A). Saline-treated β-AR KO mice showed a significant decrease in AMPK phosphorylation at Ser485/491 (Fig. 7A). Strikingly, in β-AR KO BAT, phentolamine increased serine phosphorylation by 2.5-fold compared with β-AR KO treated with saline and by 1.3-fold compared with WT phentolamine (Fig. 7A). The stoichiometry of phosphorylation of Ser485/491 to total AMPK α1 and α2 subunits was markedly increased in β-AR KO mice treated with phentolamine compared with WT treated with phentolamine and β-AR KO mice treated with saline (Fig. 7, B and C). This suggests that α-adrenergic receptor blockade in a setting of β-adrenergic receptor deficiency leads to Ser485/491 phosphorylation of AMPK. In β-AR KO mice, there was a robust inverse correlation between the ratio of Ser485/491 phosphorylation/total AMPK α2 and AMPK α2 activity in BAT (Fig. 7D).
FIGURE 7.
Phentolamine increases AMPK α Ser485/491 phosphorylation in BAT of β-AR KO but not WT mice. Mice were injected with saline (−) or phentolamine at a dose of 10 mg/kg intraperitoneally for WT and 8 mg/kg intraperitoneally for β-AR KO mice and sacrificed after 30 min by cervical dislocation. BAT was isolated for protein measurements. Phosphorylated AMPK α Ser485/491 (panel A), the ratio of Ser485/491 phosphorylated to total AMPK α1 (panel B), the ratio of Ser485/491 phosphorylated to total AMPK α2 (panel C), and the correlation of AMPK α2 activity with the ratio of Ser485/491 phosphorylation to total AMPK α2 (panel D) are shown. Protein levels are normalized to GAPDH (panel A). Data are expressed as the mean ± S.E.; n = 6–7 per group. *, p < 0.05 versus all groups. Food was withdrawn for 2 h before the sacrifice.
Does PKA or AKT Mediate Ser485/491 Phosphorylation of AMPK and Inhibition of AMPK Activity in Response to Adrenergic Signaling in BAT?
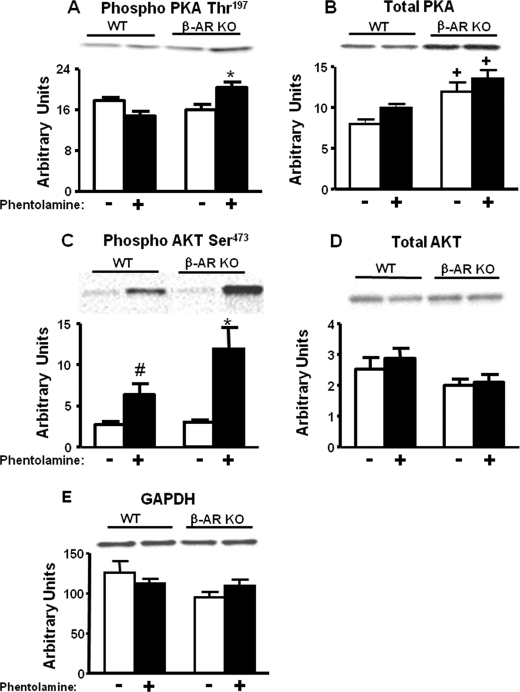
Because PKA and AKT have been shown to phosphorylate the AMPK α catalytic subunit at the Ser485/491 position in white adipocytes (6), perfused heart (5), and cultured cells (3, 4, 7), we sought to determine which upstream kinase phosphorylates AMPK at Ser485/491 in vivo in BAT of β-AR KO mice in response to phentolamine. Treatment of WT animals with phentolamine did not alter PKA Thr197 phosphorylation, a critical determinant for PKA activity (Fig. 8A). In BAT from saline-treated mice with genetic deficiency of β-adrenergic receptors, there were no changes in PKA Thr197 phosphorylation when compared with the saline-treated WT mice (Fig. 8A). However, phentolamine treatment of β-AR KO mice resulted in 35–40% increase in PKA Thr197 phosphorylation compared with all other groups (Fig. 8A). β-AR KO mice exhibited a 1.5-fold increase in total PKA protein (Fig. 8B). Therefore, the stoichiometry of PKA phosphorylation was not altered, although there was a net increase in phosphorylated PKA that may contribute to increased serine phosphorylation of AMPK (Fig. 8A).
FIGURE 8.
Changes in PKA and AKT phosphorylation in BAT of WT and β-AR KO mice treated with saline (−) or phentolamine (10 mg/kg, intraperitoneally for WT and 8 mg/kg, intraperitoneally for β-AR KO mice). Mice were sacrificed after 30 min by cervical dislocation, and BAT was isolated for protein measurements. Phosphorylated PKA Thr197 (panel A), total PKA (panel B), phosphorylated AKT Ser473 (panel C), and total AKT (panel D) are shown. Protein levels are normalized to GAPDH (panel E). Data are expressed as the mean ± S.E.; n = 6–7 per group. *, p < 0.05 versus all groups; +, p < 0.05 versus WT; #, p < 0.05 versus WT-saline. Food was withdrawn for 2 h before the sacrifice.
We determined whether AKT is also an upstream kinase mediating AMPK serine phosphorylation in β-AR KO in response to phentolamine. Ser473 is a key phosphorylation site that regulates AKT activity. Phentolamine treatment in WT animals increased AKT Ser473 phosphorylation by 2.3-fold compared with the saline-treated WT animals (Fig. 8C). Saline-treated β-AR KO mice did not demonstrate any changes in AKT Ser473 phosphorylation compared with saline-treated WT mice (Fig. 8C). However, in response to phentolamine, β-AR KO mice showed a 4-fold increase in AKT Ser473 phosphorylation compared with β-AR KO saline-treated mice (Fig. 8C). This phosphorylation was enhanced by 1.9-fold compared with WT mice treated with phentolamine (Fig. 8C). Changes in phosphorylation were independent of changes in total AKT protein (Fig. 8D). This suggests that in vivo α-adrenergic receptor blockade enhances AKT Ser473 phosphorylation in BAT. In a setting of β-adrenergic receptor deficiency, α-adrenergic receptor blockade has an even greater effect.
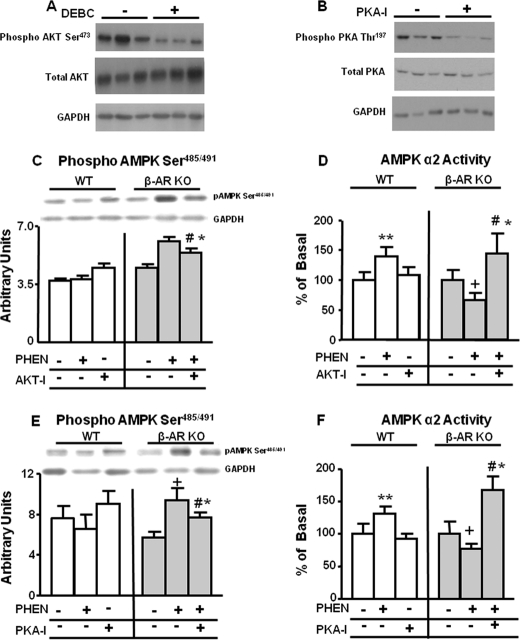
Our data indicate that both PKA and AKT may contribute to the increased Ser485/491 phosphorylation of AMPK in response to phentolamine in β-AR KO mice. To conclusively determine the importance of these upstream kinases, we carried out studies in BAT explants incubated with phentolamine in the presence or absence of an AKT inhibitor (DEBC) or a PKA inhibitor (PKA-I). We first assessed the effectiveness of these inhibitors on AKT and PKA phosphorylation in WT explants. DEBC inhibited AKT Ser473 phosphorylation by ∼40% (Fig. 9A) without any effect on total AKT protein. Similarly, PKA-I reduced PKA Thr197 phosphorylation by ∼50% (Fig. 9B) without any effect on total PKA protein. In WT explants, the AKT inhibitor alone had no effect on Ser485/491 phosphorylation of AMPK and did not influence the phentolamine effect (Fig. 9C). However, in β-AR KO explants, phentolamine increased AMPK Ser485/491 phosphorylation, and this effect was blunted but not abolished by AKT inhibition (Fig. 9C). In WT explants, phentolamine increased AMPK activity, and AKT inhibition alone had no effect (Fig. 9D). AKT inhibition also did not reduce phentolamine-stimulated AMPK activity (data not shown). In β-AR KO BAT explants, phentolamine decreased AMPK activity similar to the effect in vivo (Fig. 9D). AKT inhibition not only reversed this effect of phentolamine in explants but also increased AMPK activity above baseline (Fig. 9D). Total AMPK α2 protein levels remained unchanged in WT or β-AR KO BAT explants under these conditions (data not shown).
FIGURE 9.
Effect of inhibition of AKT and PKA on AMPK Ser485/491 phosphorylation and activity in BAT explants from WT and β-AR KO mice. BAT explants were isolated as described under “Experimental Procedures” and incubated with vehicle (−) or phentolamine (100 μm) alone or co-incubated with either DEBC-10 (3 μm) or PKA-I-14-22 (100 nm) for 30 min and then frozen for activity and protein measurements. Phosphorylated AKT Ser473 and total AKT (panel A), phosphorylated PKA Thr197 and total PKA (panel B), phosphorylated AMPK Ser485/491 (panel C and E), and AMPK α2 activity (panel D and F) are shown. Protein levels are normalized to GAPDH (panel A, B, C, and D). Data are expressed as the mean ± S.E.; n = 4–5 per group. **, p < 0.05 versus all other WT groups; +, p < 0.05 versus all other β-AR KO groups; #, p < 0.05 versus β-AR KO phentolamine group; *, p < 0.05 versus β-AR KO vehicle. Food was withdrawn for 2 h before the sacrifice.
Because the effect of AKT inhibition on AMPK Ser485/491 phosphorylation was partial, we sought to determine whether PKA was also necessary for Ser485/491 phosphorylation of AMPK. In WT BAT explants, neither phentolamine nor the PKA inhibitor had an effect on Ser485/491 phosphorylation of AMPK (Fig. 9E). However, in β-AR KO, phentolamine increased serine phosphorylation of AMPK, and PKA inhibition partially reversed this (Fig. 9E). In WT explants, phentolamine increased AMPK activity similar to the effect in BAT in vivo, and PKA inhibition alone had no effect on AMPK activity (Fig. 9F). However, in BAT explants from β-AR KO mice, phentolamine inhibited AMPK α2 activity (also similar to in vivo effects), and PKA inhibition not only reversed this effect but increased AMPK activity above baseline (Fig. 9F). Total AMPK α2 protein levels remained unchanged in WT or β-AR KO BAT explants with phentolamine or PKA inhibition (data not shown).
DISCUSSION
AMPK signaling is regulated by hormones and nutrients and is critical for many aspects of cellular metabolism (1, 2). For example, stimulation of AMPK in oxidative muscle is necessary for the leptin effect on fatty acid oxidation (8). These effects of leptin are blunted by denervation or blockade of α-adrenergic receptors (8). The mechanism by which the adrenergic nervous system regulates AMPK protein and activity in vivo remains unknown. In the current study we demonstrate that innervation of BAT is critical to maintain AMPK α protein at normal levels. In WT mice, chronic changes in sympathetic input alter AMPK α subunit protein levels either directly or indirectly. However, acute changes through short term pharmacological blockade of α-adrenergic receptors increase AMPK Thr172 phosphorylation and activity without altering AMPK α subunit levels. These acute changes in AMPK phosphorylation in BAT appear to result from inhibition of O2 consumption (Fig. 4, Table 1) and increased AMP levels.
Unlike in WT animals, in mice with a genetic lack of β-adrenergic receptors, α-adrenergic blockade decreases AMPK activity by increasing Ser485/491 phosphorylation, which has been suggested to inhibit AMPK activity (3–7). Previous studies on AMPK Ser485/491 phosphorylation used AMPK Thr172 phosphorylation as a surrogate measure of AMPK activity (4, 6–7). In those studies, increased AMPK Ser485/491 phosphorylation was associated with decreased AMPK Thr172 phosphorylation (4, 6–7). However, we find that changes in Ser485/491 phosphorylation are independent of alterations in AMPK Thr172 phosphorylation, whereas AMPK activity is affected. Thus, our data show that Ser485/491 phosphorylation of AMPK can regulate AMPK activity independent of Thr172 phosphorylation. AKT and PKA have been proposed to phosphorylate AMPK on these serine sites and inhibit AMPK activity (3–7). Our studies show that reducing both α- and β- adrenergic signaling in vivo activates AKT and PKA in BAT (Fig. 7, A and C), and both upstream kinases contribute to AMPK Ser485/491 phosphorylation and decreased AMPK activity. This is consistent with the findings in cultured cells (3–4, 6–7) and perfused heart (5). Thus, these findings expand our understanding of adrenergic regulation of AMPK activity and show for the first time that the adrenergic nervous system can alter AMPK subunit protein levels and phosphorylation of the α subunit at Ser485/491.
Neuronal Regulation of AMPK Catalytic Subunit Protein Levels
In our study we observe that in the BAT of FVB mice, AMPK α2 but not α1 protein levels decrease after denervation. In a very different model we also demonstrated neuronal regulation and isoform-specific regulation of the AMPK catalytic subunits. In mice with brain-specific knock-out of protein-tyrosine phosphatase 1B (PTP1B), which regulates leptin and insulin signaling, AMPK α2 protein and activity are increased in BAT with no change in AMPK α1 (31). This supports the finding that AMPK α2, and not α1, levels in BAT are neuronally regulated in some genetic backgrounds. However, in WT mice from a mixed genetic background, both isoforms were decreased in BAT after denervation. Similarly in mice on the same genetic background that were genetically engineered not to express β adrenergic receptors, both AMPK isoforms were also decreased by denervation. Thus, the isoform specificity of the denervation effects may depend on genetic background.
Only a few studies have shown alterations in AMPK catalytic subunit levels. Prolonged leptin treatment increases AMPK α2 subunit levels in soleus muscle (32), and exercise training increases α1 and α2 isoforms in liver and visceral adipose tissue (33, 34). Chronic (15 days) but not acute (24 h) cold exposure increases AMPK α1 protein in both WAT and BAT in mice, and at least in WAT these effects were reproduced by prolonged noradrenaline treatment (35). This suggests a role for adrenergic stimulation in long term regulation of AMPK α protein levels that agrees with our current studies in BAT. The mechanisms by which AMPK protein levels are regulated in these studies is unknown. Cidea, a fat-specific cytosolic protein, binds AMPK β and promotes its ubiquitination, resulting in decreased AMPK α subunit protein and AMPK activity (28). Our study demonstrates for the first time that Cidea levels are regulated by the adrenergic nervous system as denervation increases Cidea levels. This is associated with diminished phosphorylated, but not total, AMPK β. It is possible that altered AMPK β phosphorylation could result in changes in AMPK α subunit levels.
Acute Effects of Alterations in Adrenergic Signaling on AMPK Activity in BAT
Having observed that chronic alterations in adrenergic signaling lead to changes in protein levels of catalytic subunits of AMPK, we next assessed whether acute alterations in adrenergic signaling in vivo would impact AMPK phosphorylation and activity in the absence of any changes in protein levels. We demonstrate that acute blockade of either α- or β-adrenergic signaling in vivo rapidly enhances AMPK activity without changing total protein levels. The effect of α but not β pharmacological blockade of adrenergic receptors on AMPK activity in vivo is recapitulated in BAT explants and is, therefore, a direct effect and not secondary to systemic or neural alterations. In contrast to our findings that α-adrenergic blockade in BAT stimulates AMPK activity, in soleus (8) and heart (36, 37) α-adrenergic agonists activate AMPK α Thr172 phosphorylation. This provides further evidence that the effects of adrenergic signaling on AMPK activation are tissue-specific.
Mechanisms for Increased AMPK Activity in BAT after Acute α Adrenergic Blockade
The mechanism for rapid enhancement in AMPK activity with acute alterations in adrenergic signaling is likely to be increased the AMP levels and AMP to ATP ratio (Table 1). The increase in AMP levels with α-adrenergic blockade may be secondary to diminished oxygen consumption in BAT. A similar mechanism accounts for the effects of metformin, berberine, and rosiglitazone to increase AMPK activity (38). In agreement with a role for α-adrenergic receptors in oxygen consumption in BAT (39), incubation of BAT adipocytes and explants with phenylephrine, an α-adrenergic agonist, enhances mitochondrial oxygen consumption.
Our data suggest that in vivo in normal animals increased AMPK activity in BAT after acute blockade of α-adrenergic receptors involves two components, 1) a direct effect of α-adrenergic blockade to diminish oxygen consumption in BAT and increase AMP levels and 2) unopposed β-adrenergic signaling in a setting of α-adrenergic inhibition. Consistent with the latter, a β-adrenergic agonist activates AMPK in brown (17) and white adipocytes (16) in vitro and ex vivo (18).
Role of Adrenergic Receptors in Regulating AMPK Serine Phosphorylation and Activity in BAT
In BAT of mice lacking β-adrenergic receptors, baseline AMPK activity is unchanged in vivo compared with WT. However, α-adrenergic inhibition in β-AR KO mice diminished AMPK activity without decreasing AMPK α Thr172 phosphorylation or total protein. Thus, decreased AMPK activity may result from inhibitory phosphorylation of AMPK. Our study is the first to demonstrate adrenergic regulation of serine phosphorylation of AMPK. We show that changes in serine phosphorylation are inversely associated with AMPK activity both in vivo and in BAT explants, supporting the notion that phosphorylation on Ser485/491 is inhibitory.
Mechanisms for Alterations in Serine Phosphorylation of AMPK in BAT
Because AKT (4–6) or PKA (3, 7) can increase serine phosphorylation of AMPK α, we sought to determine which upstream kinase mediates AMPK serine phosphorylation in response to changes in adrenergic signaling. We show that in BAT of WT mice subjected to α-adrenergic inhibition, phosphorylation of AKT but not PKA is increased on sites necessary for kinase activation. This is consistent with the effect in white adipocytes whereby α-adrenergic inhibition allows β-adrenergic signaling to dominate and regulate AKT (40). In contrast to BAT, in the heart α-adrenergic stimulation (phenylephrine) activates AKT (41, 42). This again supports the notion of different effects in different tissues. Moreover, in WT animals subjected to α-adrenergic inhibition, AKT phosphorylation is not sufficient to increase serine phosphorylation of AMPK in BAT. Therefore, it seems plausible that inhibition of α-adrenergic pathway results in unopposed β-adrenergic stimulation, which promotes AMPK Thr172 phosphorylation and AMPK activation in WT animals. This theory is supported by our finding that α-adrenergic blockade in mice lacking β-adrenergic receptors decreases AMPK activity. The mechanism appears to be activation of AKT and PKA, which increases serine phosphorylation of AMPK, thereby inhibiting AMPK activity. Moreover, these effects of AKT and PKA were recapitulated in BAT explants and were blunted by AKT or PKA inhibitors. Thus, both AKT and PKA may play a role in adrenergic receptor-mediated alterations in serine phosphorylation of AMPK, thereby regulating AMPK activity.
In summary, our data demonstrate that the adrenergic nervous system regulates AMPK in vivo in an adrenergic receptor-dependent manner. Prolonged lack of sympathetic input leads to decreased AMPK activity secondary to changes in AMPK α but not AMPK β subunit levels. However, acute inhibition of α-adrenergic function enhances AMPK phosphorylation and activity through altered tissue oxygen consumption. We further demonstrate that changes in adrenergic signaling transduce signals through AKT and/or PKA that increase serine phosphorylation of AMPK α and inhibit its activity. Because AMPK modulators are being developed for many medical disorders, this study offers novel approaches to alter AMPK activity in a tissue-specific and isoform-specific manner to treat diseases such as obesity, type 2 diabetes, and cancer.
Acknowledgments
We are grateful to K. Catalano and D. Olson for comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants P01 DK56116 and the Physiology Core of P30 DK57521 (both to B. B. K.).
- AMPK
- AMP-activated protein kinase
- β-AR
- β-adrenergic receptor
- ACC
- acetyl-CoA carboxylase
- BAT
- brown adipose tissue.
REFERENCES
- 1. Xue B., Kahn B. B. (2006) J. Physiol. 574, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hardie D. G. (2007) Nat. Rev. Mol. Cell Biol. 8, 774–785 [DOI] [PubMed] [Google Scholar]
- 3. Hurley R. L., Barré L. K., Wood S. D., Anderson K. A., Kemp B. E., Means A. R., Witters L. A. (2006) J. Biol. Chem. 281, 36662–36672 [DOI] [PubMed] [Google Scholar]
- 4. Mankouri J., Tedbury P. R., Gretton S., Hughes M. E., Griffin S. D., Dallas M. L., Green K. A., Hardie D. G., Peers C., Harris M. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 11549–11554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horman S., Vertommen D., Heath R., Neumann D., Mouton V., Woods A., Schlattner U., Wallimann T., Carling D., Hue L., Rider M. H. (2006) J. Biol. Chem. 281, 5335–5340 [DOI] [PubMed] [Google Scholar]
- 6. Berggreen C., Gormand A., Omar B., Degerman E., Göransson O. (2009) Am. J. Physiol. Endocrinol. Metab. 296, E635–E646 [DOI] [PubMed] [Google Scholar]
- 7. Funahashi K., Cao X., Yamauchi M., Kozaki Y., Ishiguro N., Kambe F. (2009) Prostaglandins Other Lipid Mediat. 88, 31–35 [DOI] [PubMed] [Google Scholar]
- 8. Minokoshi Y., Kim Y. B., Peroni O. D., Fryer L. G., Müller C., Carling D., Kahn B. B. (2002) Nature 415, 339–343 [DOI] [PubMed] [Google Scholar]
- 9. Silva J. E. (2006) Physiol. Rev. 86, 435–464 [DOI] [PubMed] [Google Scholar]
- 10. Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., Kolodny G. M., Kahn C. R. (2009) N. Engl. J. Med. 360, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., Teule G. J. (2009) N. Engl. J. Med. 360, 1500–1508 [DOI] [PubMed] [Google Scholar]
- 12. Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerbäck S., Nuutila P. (2009) N. Engl. J. Med. 360, 1518–1525 [DOI] [PubMed] [Google Scholar]
- 13. Lefkowitz R. J., Cotecchia S., Kjelsberg M. A., Pitcher J., Koch W. J., Inglese J., Caron M. G. (1993) Adv. Second Messenger Phosphoprotein Res. 28, 1–9 [PubMed] [Google Scholar]
- 14. Hutchinson D. S., Bengtsson T. (2006) Diabetes 55, 682–690 [DOI] [PubMed] [Google Scholar]
- 15. Kishi K., Yuasa T., Minami A., Yamada M., Hagi A., Hayashi H., Kemp B. E., Witters L. A., Ebina Y. (2000) Biochem. Biophys. Res. Commun. 276, 16–22 [DOI] [PubMed] [Google Scholar]
- 16. Moule S. K., Denton R. M. (1998) FEBS Lett. 439, 287–290 [DOI] [PubMed] [Google Scholar]
- 17. Hutchinson D. S., Chernogubova E., Dallner O. S., Cannon B., Bengtsson T. (2005) Diabetologia 48, 2386–2395 [DOI] [PubMed] [Google Scholar]
- 18. Koh H. J., Hirshman M. F., He H., Li Y., Manabe Y., Balschi J. A., Goodyear L. J. (2007) Biochem. J. 403, 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clark H., Carling D., Saggerson D. (2004) Eur. J. Biochem. 271, 2215–2224 [DOI] [PubMed] [Google Scholar]
- 20. Bachman E. S., Dhillon H., Zhang C. Y., Cinti S., Bianco A. C., Kobilka B. K., Lowell B. B. (2002) Science 297, 843–845 [DOI] [PubMed] [Google Scholar]
- 21. Scarpace P. J., Matheny M. (1998) Am. J. Physiol. Endocrinol. Metab. 275, E259–E264 [DOI] [PubMed] [Google Scholar]
- 22. Minokoshi Y., Alquier T., Furukawa N., Kim Y. B., Lee A., Xue B., Mu J., Foufelle F., Ferré P., Birnbaum M. J., Stuck B. J., Kahn B. B. (2004) Nature 428, 569–574 [DOI] [PubMed] [Google Scholar]
- 23. Goodwin G. W., Taegtmeyer H. (1999) Am. J. Physiol. Endocrinol. Metab. 277, E772–E777 [DOI] [PubMed] [Google Scholar]
- 24. Ma S. W., Foster D. O. (1984) Can. J. Physiol. Pharmacol. 62, 949–956 [DOI] [PubMed] [Google Scholar]
- 25. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 26. Bak M. I., Ingwall J. S. (1994) J. Clin. Invest. 93, 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ström K., Hansson O., Lucas S., Nevsten P., Fernandez C., Klint C., Movérare-Skrtic S., Sundler F., Ohlsson C., Holm C. (2008) PLoS ONE 3, e1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qi J., Gong J., Zhao T., Zhao J., Lam P., Ye J., Li J. Z., Wu J., Zhou H. M., Li P. (2008) EMBO J. 27, 1537–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Warden S. M., Richardson C., O'Donnell J., Jr., Stapleton D., Kemp B. E., Witters L. A. (2001) Biochem. J. 354, 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steinberg G. R., O'Neill H. M., Dzamko N. L., Galic S., Naim T., Koopman R., Jørgensen S. B., Honeyman J., Hewitt K., Chen Z. P., Schertzer J. D., Scott J. W., Koentgen F., Lynch G. S., Watt M. J., van Denderen B. J., Campbell D. J., Kemp B. E. (2010) J. Biol. Chem. 285, 37198–37209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xue B., Pulinilkunnil T., Murano I., Bence K. K., He H., Minokoshi Y., Asakura K., Lee A., Haj F., Furukawa N., Catalano K. J., Delibegovic M., Balschi J. A., Cinti S., Neel B. G., Kahn B. B. (2009) Mol. Cell. Biol. 29, 4563–4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steinberg G. R., Rush J. W., Dyck D. J. (2003) Am. J. Physiol. Endocrinol. Metab. 284, E648–E654 [DOI] [PubMed] [Google Scholar]
- 33. Langfort J., Viese M., Ploug T., Dela F. (2003) Scand. J. Med. Sci. Sports 13, 169–174 [DOI] [PubMed] [Google Scholar]
- 34. Takekoshi K., Fukuhara M., Quin Z., Nissato S., Isobe K., Kawakami Y., Ohmori H. (2006) Metab. Clin. Exp. 55, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 35. Mulligan J. D., Gonzalez A. A., Stewart A. M., Carey H. V., Saupe K. W. (2007) J. Physiol. 580, 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jaswal J. S., Gandhi M., Finegan B. A., Dyck J. R., Clanachan A. S. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H1883–H1892 [DOI] [PubMed] [Google Scholar]
- 37. Xu M., Zhao Y. T., Song Y., Hao T. P., Lu Z. Z., Han Q. D., Wang S. Q., Zhang Y. Y. (2007) Sheng Li Xue Bao 59, 175–182 [PubMed] [Google Scholar]
- 38. Turner N., Li J. Y., Gosby A., To S. W., Cheng Z., Miyoshi H., Taketo M. M., Cooney G. J., Kraegen E. W., James D. E., Hu L. H., Li J., Ye J. M. (2008) Diabetes 57, 1414–1418 [DOI] [PubMed] [Google Scholar]
- 39. Borst S. E., Oliver R. J., Sego R. L., Scarpace P. J. (1994) Gen. Pharmacol. 25, 1703–1710 [DOI] [PubMed] [Google Scholar]
- 40. Moule S. K., Welsh G. I., Edgell N. J., Foulstone E. J., Proud C. G., Denton R. M. (1997) J. Biol. Chem. 272, 7713–7719 [DOI] [PubMed] [Google Scholar]
- 41. Clerk A., Sugden P. H. (1999) Am. J. Cardiol. 83, 64H–69H [DOI] [PubMed] [Google Scholar]
- 42. Morissette M. R., Cook S. A., Foo S., McKoy G., Ashida N., Novikov M., Scherrer-Crosbie M., Li L., Matsui T., Brooks G., Rosenzweig A. (2006) Circ. Res. 99, 15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]