Abstract
Objective
To investigate prospectively whether autonomic nervous system (ANS) dysfunction and inflammation play a role in the increased cardiovascular disease (CVD)-related mortality risk associated with depression.
Methods
Participants in the Cardiovascular Health Study (n = 907; mean age, 71.3 ± 4.6 years; 59.1% women) were evaluated for ANS indices derived from heart rate variability (HRV) analysis (frequency and time domain HRV, and nonlinear indices, including detrended fluctuation analysis (DFA1) and heart rate turbulence). Inflammation markers included C-reactive protein, interleukin-6, fibrinogen, and white blood cell count). Depressive symptoms were assessed, using the 10-item Centers for Epidemiological Studies Depression scale. Cox proportional hazards models were used to investigate the mortality risk associated with depression, ANS, and inflammation markers, adjusting for demographic and clinical covariates.
Results
Depression was associated with ANS dysfunction (DFA1, p = .018), and increased inflammation markers (white blood cell count, p = .012, fibrinogen p = .043) adjusting for covariates. CVD-related mortality occurred in 121 participants during a median follow-up of 13.3 years. Depression was associated with an increased CVD mortality risk (hazard ratio, 1.88; 95% confidence interval, 1.23–2.86). Multivariable analyses showed that depression was an independent predictor of CVD mortality (hazard ratio, 1.72; 95% confidence interval, 1.05–2.83) when adjusting for independent HRV and inflammation predictors (DFA1, heart rate turbulence, interleukin-6), attenuating the depression-CVD mortality association by 12.7% (p < .001).
Conclusion
Autonomic dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression, but a large portion of the predictive value of depression remains unexplained by these neuroimmunological measures.
Keywords: depression, autonomic nervous system, inflammation, risk factors, cardiovascular disease, mortality
Introduction
Depression is associated with an increased risk of cardiovascular disease (CVD) (1–4). The pathophysiological mechanisms accounting for the relationship between depression and CVD risk remain unclear. These mechanisms may involve autonomic nervous system (ANS) dysfunction and inflammation. Prior cross-sectional studies have shown that depression is associated with autonomic dysfunction (5–7) and elevated inflammation markers (8–11), but the mediating role of these purported mechanisms has not been consistently established (12–16).
ANS activity in the direction of parasympathetic withdrawal and increased sympathetic activation is associated with increased cardiovascular mortality risk. Using heart rate variability (HRV) analyses, most studies have documented that reduced HRV is associated with poor cardiovascular outcomes, although negative studies have been reported as well, particularly when short-term HRV assessments are used (17–23). In addition, multiple studies (24–26) have shown adverse CVD outcomes in individuals with elevated levels of inflammation markers. The ANS plays an essential role in the bidirectional relationship between the central nervous and immune systems. Elevated parasympathetic nervous system activity is associated with attenuated immune system responsiveness to inflammatory stimuli (27). Noninvasive markers of sympathovagal imbalance, particularly reduced HRV power and high-frequency HRV, are associated with higher concentrations of systemic inflammation markers (e.g., interleukin [IL]-6, tumor necrosis factor-α, and fibrinogen) (28–36). Some evidence indicates that the association between ANS activity and inflammation is stronger in depressed versus nondepressed individuals (28), but this may, in part, be explained by CVD as a common underlying factor. Additional research is needed to examine whether these autonomic and inflammation-related processes are involved in the relationship between depression and CVD progression.
The present cohort study examined whether depressive symptoms are cross sectionally associated with HRV-based indices of ANS dysfunction and inflammation markers. We also investigated whether associations between autonomic dysfunction and inflammation are stronger in depressed versus nondepressed individuals. The main goal of this investigation was to examine whether these biobehavioral factors were independently predictive of CVD-related mortality, and to determine the extent to which the increased risk of CVD mortality associated with depression is influenced by ANS dysfunction and inflammation.
Methods
Participants
A detailed description of the design and recruitment procedures of the Cardiovascular Health Study (CHS) has been described previously (29,37,38). The main CHS cohort (n = 5201), aged ≥65 years, was enrolled from a sample of Medicare-eligible individuals from four communities in the United States, between April 1989 and May 1990. Exclusion criteria for the CHS were: hospice treatment, wheel-chair bound in the home, and radiation or chemotherapy for cancer. Data for the CHS minority cohort (n = 687, recruited in 1992/1993) were not included because simultaneous assessments of ambulatory electrocardiogram (ECG) and inflammation markers were not available for the added cohort.
The CHS was approved by the Institutional Review Boards of the participating study sites. All participants gave their informed consent. Data from the baseline CHS assessment year (1988–1989) were used for the present analyses.
Of the 5,201 CHS participants, 1,424 (27.3%) participants were randomly selected for an ancillary investigation (directed by P.K.S.) involving ambulatory ECG monitoring (29,38). The ambulatory ECG registration was used for measurement of HRV-based ANS indices. Demographic and clinical factors in the CHS subcohort with ambulatory ECG recordings did not differ from the total CHS cohort (38). Valid recordings were returned by 1384 (97.1%) of 1424 of which 1198 (86.6%) were usable for HRV analyses. Participants with a positive history of CVD (290 of 1198, 24.2%) were excluded from the present analyses to avoid potential confounding effects of CVD and CVD-related interventions on ANS indices and inflammation markers. Absence of CVD was based on history of coronary artery disease (i.e., myocardial infarction, coronary artery bypass surgery, coronary angioplasty, hospitalization for anginal symptoms), cerebrovascular accidents, or history of transient ischemic accidents. A flowchart of participant selection is presented in Figure 1. A total of 908 CVD-free participants with valid ambulatory ECG recordings were included in this investigation; participant characteristics are presented in Table 1.
Figure 1.
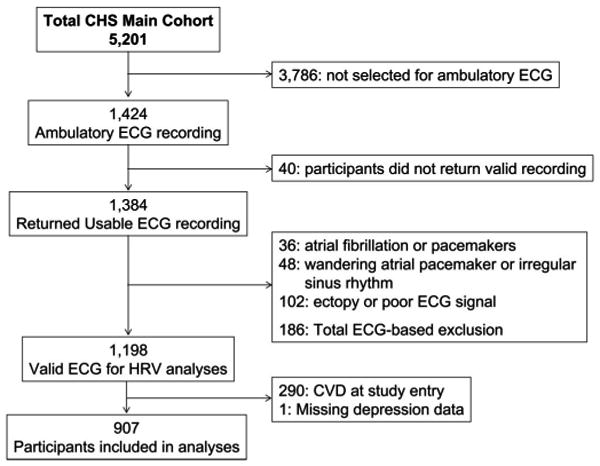
Flowchart of participant selection. ECG = electrocardiogram; HRV = heart rate variability; CVD = cardiovascular disease.
TABLE 1. Demographic and Clinical Characteristics Related to Depression Status.
| Not Depressed (CES-D <8) n = 776 |
Depressed (CES-D ≥8) n = 131 |
p | |
|---|---|---|---|
| Age | 71.3 ± 4.5 | 71.3 ± 5.2 | .95 |
| Sex, female (%) | 447 (57.6) | 90 (68.7) | .017 |
| Race, African American (%) | 29 (3.7) | 4 (3.1) | .70 |
| Systolic blood pressure (mm Hg) | 134.5 ± 20.8 | 132.6 ± 20.1 | .34 |
| Diastolic blood pressure (mm Hg) | 70.7 ±11.0 | 70.5 ± 10.0 | .78 |
| Diabetes status (%) | |||
| IFG | 93 (12.0) | 18 (13.8) | |
| Diabetes | 102 (13.2) | 14 (10.8) | .67 |
| Subclinical CVD (%) | 389 (50.1) | 64 (48.9) | .79 |
| Smoking status (%) | |||
| Former | 336 (43.3) | 47 (35.9) | |
| Current | 70 (9.0) | 19 (14.5) | .083 |
| BMI (kg/m2) | 26.6 ± 4.0 | 26.5 ± 4.6 | .82 |
| Physical activity (kcal/wk) | 2180 ± 2217 | 1691 ± 1749 | .017 |
| β-adrenergic blocking agents (%) | 80 (10.3) | 9 (6.9) | .22 |
| Antidepressive medication (%) | 18 (2.3) | 15 (11.5) | <.001 |
CES-D = Centers for Epidemiological Studies Depression scale; IFG = impaired fasting glucose; CVD = cardiovascular disease; BMI = body mass index.
Data are presented as mean ± standard deviation or N and (%).
Assessment of Depressive Symptoms
Depressive symptoms were assessed, using the 10-item Centers for Epidemiologic Studies Depression (CES-D) scale (39), previously validated in adults >65 years of age (40). The magnitude of depressive symptoms was analyzed as a continuous variable based on the CES-D scores (potential range, 0–30) and categorically using a previously validated cutoff score (≥8) for the presence of depression (1,40). Valid CES-D data were available for 907 (99.8%) of 908 participants, and the observed CES-D scores ranged from 0 to 29 (mean, 4.5 ± 4.4) with a reliability coefficient (Cronbach's α) of 0.75 (11).
Ambulatory ECG Monitoring and ANS Indices
ECGs were recorded on Del Mar Avionics tape recorders which have a calibrated timing signal; ECGs were processed, using an ambulatory ECG analyzer (MARS 8000, GE-Marquette, Milwaukee, Wisconsin). Only normal-to-normal (N-N) beats with uniformly detected onsets were included in the HRV analyses. N-N intervals outside of the range limits between the longest and shortest validated N-N interval were excluded from the analyses (e.g., ectopic beats and blocked atrial premature contractions). HRV was calculated from beat-to-beat files of the 24-hour recordings and exported to a Sun Enterprise 450 server (Sun Microsystems, Santa Clara, California), using validated software as described previously (29,38). For the present analyses, both 24-hour and daytime (i.e., between 8 AM and 8 PM) analyses of the ECG recordings were used. The daytime analyses were included in addition to standard 24-hour HRV indices to minimize bias related to poor sleep and other confounds of nocturnal physiological assessments in the elderly.
Of the 1,424 participants who were equipped with an ambulatory ECG monitor, 1,384 (97%) participants returned tapes with usable signals for HRV analyses. Exclusions from the HRV analyses were: atrial fibrillation or pacemakers (n = 36); wandering atrial pacemaker or irregular sinus rhythm precluding accurate identification of normal beats (n = 48), ectopy or poor ECG signals resulting in insufficient data for 24-hour frequency domain HRV analyses (n = 102). Of the remaining 1,198 participants with >18 hours of ≥80% valid N-N intervals, 908 participants were free of CVD at study entry.
Frequency and Time Domain HRV Analysis
Frequency domain analysis was performed, using previously described methods (19,29,38). Fast Fourier Transformation was used to determine HRV components: very low frequency (VLF, 0.0033–0.040 Hz), low frequency (LF, 0.04–0.15 Hz), and high frequency (HF, 0.15–0.40 Hz), expressed in ln (ms2). LF-HRV and HF-HRV were determined as the average over 5-minute periods to reduce artifacts related to nonstationarity. LF-HRV power reflects both sympathetic and parasympathetic modulation of heart rate, whereas the HF-HRV power primarily reflects vagal modulation of heart rate (19). Total power was based on the 0.0033-Hz to 0.40-Hz frequency range and calculated for the entire recording. Time-domain-based standard deviation of the NN intervals (SDNN) is equivalent to the total power HRV index and was included to enable comparison with other research reports in this area. Other time-domain HRV indices were: the root mean square of successive differences of NN intervals (RMSSD), in milliseconds (ms), the percent of successive NN interval differences >50 ms, in percent (19). RMSSD and PNN50 primarily reflect parasympathetic influence on the heart, and the SDNN reflects all sources of variability in heart rate (19).
Nonlinear HRV
Nonlinear HRV analysis quantifies the randomness or degree of self-similarity of heart rate patterns at different time scales (21). Detrended fluctuation analysis (DFA) determines the short-term self-similar properties of the NN interval time series. Details of this method have been described previously (29,38,41,42). The short-term fractal scaling exponent was determined for 4 to 11 beat sequences of N-N interval data (DFA1). DFA1 = 0.5 indicates a random signal, and DFA1 = 1.5 indicates totally correlated signals. DFA data were analyzed as continuous variables and as dichotomized measure based on a cutoff of <1.0 (29,38). Reduced DFA1 reflects a breakdown of fractal heart rate patterns that occur during activation of both sympathetic and parasympathetic nervous system outflow (43).
Heart rate turbulence (HRT) quantifies the response of the sinus node to premature ventricular complexes (PVCs) (29,38,44,45). Two indices are calculated: turbulence onset (TO) and turbulence slope (TS). In healthy hearts, PVCs are followed by a brief sinus tachycardia. The percent change in N-N interval of the two post-PVC beats was compared with the two beats preceding the PVC. In healthy hearts, this index is negative or zero, and a TO of >0% is therefore considered abnormal. TS quantifies the oscillation in heart rate (tachycardia, bradycardia, then return to baseline) that follows a PVC as the largest fitted slope of the N-N intervals between any 5 beats within 15 beats of the PVC. Turbulence was examined as a continuous and categorical variable (TO of >0% and TS of <3 ms/beat) (29,38). These indices require ≥5 PVCs for calculation and are determined as an average of all of the PVCs on the recording. Participants with <5 ventricular premature beats were categorized as having normal heart rate turbulence values (21,29,38).
Blood Chemistry
Processing
Phlebotomy methods, handling of the samples, and quality assurance have been described previously (46,47). Samples were obtained in the morning (between 7:30 AM and 10 AM) after an overnight fast and centrifuged at 4°C within 40 minutes of venipuncture. Citrated plasma was used for analyses of fibrinogen and serum for the C-reactive protein (CRP) and IL-6 assays. Aliquots were frozen at −70°C until analysis. Analyses on whole blood (white blood cell count [WBC]) were conducted at local laboratories.
Inflammation Markers
High-sensitivity CRP was assessed with an ultra-sensitive enzyme-linked immunosorbent assay, using purified protein and polyclonal anti-CRP antibodies (48). The coefficient of variation (CV) for CRP is 7.6%. IL-6 was assayed, using an enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, Minnesota; CV = 6.3%) (26,49,50). Plasma fibrinogen was measured as the rate of clot formation, using a semiautomated modified method described by Clauss using a BBL Fibrometer (Becton-Dickinson, Franklin Lakes, NJ). Calibration reference plasma was used as standard, and results were confirmed by participating in the College of American Pathologists' comprehensive coagulation quality assurance program, as previously described (51). The mean monthly CV was 3.09%. WBC count was assessed, using automated cell counters at the local laboratories (47). The interassay CV was 5.50%.
Covariates: Clinical Measures and Cardiovascular Risk Factors
Covariates were selected based on their reported associations with depression, HRV indices, or inflammation markers. Baseline clinical examinations included standardized assessments of blood pressure, diabetes, the presence of isolated subclinical cardiovascular disease, use of β blocking and antidepressive medications, weight, height, smoking status, and level of physical activity. Diabetes was defined as a fasting glucose of ≥126 mg/dL or use of hypoglycemic agents (pills and/or insulin), and impaired fasting glucose was defined as glucose between 100 to 125 mg/dL (37,51). Isolated subclinical cardiovascular disease (i.e., markers of CVD in the absence of any form of clinical CVD) was defined as composite index of the following: ankle-arm index ≤0.9, common carotid intima-media thickness in >1.20 mm, maximum carotid stenosis ≥50%, major ECG abnormality (ventricular conduction defect, major Q-wave abnormalities, left ventricular hypertrophy, isolated ST-T wave abnormalities, atrial fibrillation, first-degree atrioventricular block), or left ventricular ejection fraction characterized as borderline or abnormal (52). The use of β-blocking agents was included as a covariate in the statistical analyses because β-adrenergic blocking agents may result in depressive symptoms and can alter HRV-based ANS indices. Smoking status was categorized as current, former, and nonsmoker. Physical activity was based on self-reported activity levels, using the modified Minnesota Leisure-Time Activities questionnaire (53,54), which evaluated frequency and duration of 15 different activities during the prior 2 weeks; converted into kcal/week as described previously (55).
Ascertainment of Cause of Mortality During Follow-Up
The protocol for the assessment of adverse cardiovascular outcomes and mortality has been previously described (56). The Events Subcommittee of the CHS adjudicated cardiovascular cause of mortality by review of the medical record, death certificate, or through review of autopsy reports and the Medicare database during 15 years of follow-up. There was 100% ascertainment of vital status. Cause of death was defined as cardiovascular if the primary reason for mortality was determined to be cardiac or cerebrovascular if the underlying cause of mortality was determined to be cardiac or cerebrovascular as adjudicated by the CHS Events Subcommittee (56).
Statistical Analyses
Data are presented as mean ± standard deviation for all clinical variables and mean ± standard error of the mean for blood chemistry data. Comparisons between depressed (CES-D ≥8) versus nondepressed (CES-D <8) participants (1,40) were made, using multivariate analyses of variance and subsequent t tests or χ2 tests. If data were not normally distributed, logarithmic (HRV and blood chemistry values) or square root (CES-D) transformations were applied before parametric statistical analyses. Associations between depressive symptoms as continuous variable with HRV and inflammation markers were examined, using regression analysis with CES-D scores as continuous predictor variable. Multivariable analyses were used to determine whether the associations of depression with HRV indices and inflammation markers were independent of covariates using analyses of covariance for continuous variables and multivariable logistic regression for dichotomous variables. Covariate selection was based on theoretical plausible confounding factors (age, systolic blood pressure, diabetes, body mass index [BMI], and use of β-adrenergic medications) and sample-specific measures that were significantly associated with depression (sex and physical activity).
Predictors of CVD mortality were examined, using Cox proportional hazards models. Unadjusted HRs are presented first. HRV and inflammation markers were examined as continuous measures, using standardized Z scores to facilitate comparison between the predictor variables that were measured using different scales. Risks based on previously established cutoff values (CES-D, DFA1, and turbulence measures) or median splits (if no such cutoff values were available) are presented as well. We then examined the magnitude of change in the association between depression and CVD mortality when continuous HRV and inflammation markers were added to the Cox regression models. Specifically, to evaluate whether a covariate changed the strength of association between depressive symptoms and CVD mortality, we calculated the percentage change in the effect size (log HR) for depression after adjustment for the covariate from the unadjusted log HR (12,57). To rule out artifacts related to different sample sizes between the two nested models (i.e., depression and depression + covariate), participants with missing values for the covariate were excluded for each of the % log HR estimates. Positive values for the % change in log HR indicate potential confounding or mediation, and negative values indicate suppressor effects. Variables that resulted in >5% change in the log HR for depression were considered to influence the association between depression and CVD mortality (12).
To avoid multicollinearity resulting from the multiple interrelated HRV measures (VLF-HRV, LF-HRV, HF-HRV, total HRV power, SDNN, RMSSD, PNN50, DFA1, HRT onset, HRT slope, heart rate), a stepwise multivariable approach was used to select the HRV parameters that independently predicted CVD mortality (for 24-hour and daytime HRV separately). A similar approach was used to identify the independently predictive inflammation markers (WBC, IL-6, CRP, and fibrinogen). Multivariable analyses were then used for examining depression and independent HRV indices in the first covariate set. The second set included inflammation markers. Analyses were subsequently adjusted for demographic measures (age, sex), CV risk factors (systolic blood pressure, diabetes mellitus, subclinical CVD), medication use (β-blocking agents), and health behaviors (physical activity, smoking status, and BMI).
Mediational analyses were performed, examining HRV and inflammation markers that were a) related to depression at p < .10, and b) associated with CVD mortality at p < .10 (58,59). A two-sided p < .05 was used as a cutoff for statistical significance.
Results
Characteristics of the study participants are shown in Table 1. Depression was more common in women (p = .017) and was associated with reduced physical activity levels (p =.017). Depression was not associated with traditional CVD risk factors or subclinical disease (p > .2). Antidepressive medication use was more common in depressed participants (p < .001) and involved predominantly tricyclic antidepressants. The use of β blockade did not differ between participants with versus without depression (p = .22).
Association of Depression with ANS Indices and Inflammation Markers
Table 2 shows the relationship between depression status and HRV indices of ANS function. The overall multivariate analysis of variance (MANOVA) for all HRV-based measures combined was significant for daytime observations (F for Wilks' Lambda (11, 895) = 1.87, p = .040), but not for 24-hour HRV (F for Wilks' Lambda (11, 882) = 1.24, p = .26). When adjusting for covariates, MANOVA results remained similar (daytime p = .026 and 0.33, respectively). Depression was associated with significantly (p = .017) greater prevalence of reduced daytime DFA1 (short-term fractal scaling exponent <1), but not to other HRV indices. Analyses of depressive symptoms as continuous variable revealed the same results (odds ratio for DFA1 <1, 1.05; 95% confidence interval [CI], 1.01–1.08, p = .014 per CES-D unit0.5); the correlation with continuous DFA1 was marginally significant (r = −.056, p = .093). The association between depression status and reduced daytime DFA1 <1 remained significant (odds ratio, 1.64; 95% CI, 1.09–2.45; p = .017), when adjusting for age, sex, diabetes, systolic blood pressure, BMI, physical activity, and use of β-adrenergic blocking agents (Table 2). None of the 24-hour HRV indices were significantly associated with depression status in covariate-adjusted models. Participants with depression did not display more premature ventricular complexes than nondepressed participants (206 ± 1049 versus 167 ± 694; p = .89).
TABLE 2. Association Between Depression and Heart Rate Variability Indices During 24 Hour and Daytime Observations.
| 24 Hour HRV | Daytime HRV | |||||||
|---|---|---|---|---|---|---|---|---|
| Not Depressed (CES-D <8) n = 776 |
Depressed (CES-D ≥8) n = 131 |
p Unadjusted | p Adjusteda | Not Depressed (CES-D <8) n = 776 |
Depressed (CES-D ≥8) n = 131 |
p Value Unadjusted | p Adjusteda | |
| VLF-HRV | 6.92 ± 0.64 | 6.79 ± 0.62 | .036 | .13 | 6.72 ± 0.63 | 6.61 ± 0.63 | .059 | .14 |
| LF-HRV | 5.87 ± 0.83 | 5.78 ± 0.82 | .26 | .53 | 0.57 ± 0.78 | 5.47 ± 0.79 | .17 | .25 |
| HF-HRV | 4.81 ± 1.04 | 4.74 ± 1.04 | .49 | .61 | 4.42 ± 1.03 | 4.35 ± 0.96 | .49 | .64 |
| Total power | 9.55 ± 0.58 | 9.51 ± 0.52 | .46 | .70 | 9.09 ± 0.60 | 9.10 ± 0.57 | .94 | .57 |
| SDNN | 124.7 ± 35.5 | 121.9 ± 31.3 | .41 | .69 | 98.3 ± 30.3 | 98.3 ± 29.0 | .98 | .55 |
| RMSSD | 27.5 ± 20.8 | 26.8 ± 21.9 | .72 | .90 | 23.9 ± 18.4 | 22.4 ± 13.8 | .39 | .56 |
| PNN50 | 6.01 ± 8.84 | 5.27 ± 7.58 | .37 | .50 | 4.81 ± 8.71 | 3.98 ± 6.15 | .29 | .42 |
| DFA1 <1.0 | 247 (31.8%) | 43 (32.8%) | .82 | .98 | 233 (30.0%) | 53 (40.5%) | .017 | .017 |
| HRT onset >0 | 109 (14.0%) | 17 (13.0%) | .74 | .86 | 40 (5.2%) | 4 (3.1%) | .30 | .24 |
| HRT slope | 130 (17.0%) | 24 (18.5%) | .69 | .58 | 123 (15.9%) | 20 (15.3%) | .98 | .92 |
| Heart rate | 73.8 ± 9.0 | 73.9 ± 7.4 | .92 | .66 | 79.2 ± 10.2 | 79.4 ± 8.4 | .91 | .70 |
HRV = heart rate variability; CES-D = Centers for Epidemiological Studies Depression scale; VLF = very low frequency; LF = low frequency; SDNN = standard deviation of the NN intervals; RMMSD = square root of mean square successive difference of NN intervals; PNN50 = percent of successive NN interval differences >50 ms; DFA1 = detrended fluctuation analysis for the short-term fractal scaling exponent; HRT = heart rate turbulence.
Units: VLF, LF, HF and total power HRV in ln ms2; SDNN and RMSSD in ms; and PNN50 in %; HRT onset >0%.
HRT slope, <3 ms/beat; heart rate, beats/min.
Adjusted for age, sex, systolic blood pressure, diabetes, body mass index, physical activity (kcal/wk), and use of β-adrenergic blocking agents.
Data are presented as mean ± standard deviation or N and (%).
Results for inflammation markers are shown in Table 3. The multivariate (MANOVA) main effect for depression on inflammation markers was significant (F for Wilks' Lambda (4, 812) = 2.57, p = .037) when adjusting for covariates (age, sex, diabetes, systolic blood pressure, BMI, physical activity and use of β-blocking agents. Covariate-adjusted analyses revealed that depression was associated with elevated WBC, p = .015, and fibrinogen, p = .042 (Table 3).
TABLE 3. Relationship Between Depression and Inflammation Markers.
| Not Depressed (CES-D <8) n = 776 |
Depressed (CES-D ≥8) n = 131 |
p Unadjusted | p Adjusted | |
|---|---|---|---|---|
| WBC (103/mm3) | 6.04 ± 0.07 | 6.61 ± 0.32 | .033 | .015 |
| IL-6 (pg/mL) | 1.96 ± 0.06 | 2.11 ± 0.16 | .21 | .082 |
| CRP mg/L) | 3.23 ± 0.23 | 4.02 ± 0.88 | .072 | .063 |
| Fibrinogen (mg/dL) | 312.3 ± 2.2 | 323.0 ± 0.4 | .051 | .042 |
Adjusted for age, sex, systolic blood pressure, diabetes, body mass index, physical activity (kcal/wk), and use of β-adrenergic blocking agents.
IL-6 = interleukin 6; CRP = C-reactive protein; WBC = white blood cell count.
Data are presented as mean ± standard error of the mean.
Relationship Between ANS Indices and Inflammation Markers
The relationship between HRV indices and inflammation markers is displayed in Table 4. Data are presented for the 24-hour and daytime HRV indices. Inverse associations were found between VLF-HRV and LF-HRV with CRP, IL-6, and WBC. A 24-hour total power HRV and SDNN were related to all inflammation markers. Furthermore, 24-hour DFA1 was related to IL-6 and CRP, and HRT slope to WBC and IL-6. Data for daytime HRV displayed a similar pattern of inverse relationships between HRV and inflammation markers.
TABLE 4. Association Between HRV-Based Autonomic Nervous System Indices and Inflammation Markers.
| WBC | IL-6 | CRP | Fibrinogen | |
|---|---|---|---|---|
| 24-hr HRV | ||||
| VLF-HRV (ln ms2) | −0.135** | −0.120** | −0.182** | −0.056 |
| LF-HRV (ln ms2) | −0.104** | −0.105** | −0.148** | −0.043 |
| HF-HRV (ln ms2) | −0.082* | −0.040 | −0.069* | −0.026 |
| Total power HRV (ln ms2) | −0.196** | −0.150** | −0.211** | −0.100** |
| SDNN (ms) | −0.195** | −0.134** | −0.203** | −0.111** |
| RMSSD (ms) | −0.050 | −0.002 | −0.071* | −0.054 |
| PNN50 (%) | −0.031 | 0.024 | −0.058 | −0.063 |
| DFA1 | −0.040 | −0.112** | −0.093** | −0.023 |
| HRT onset (%) | 0.036 | −0.012 | −0.010 | 0.035 |
| HRT slope (ms/beat) | −0.077* | −0.120** | −0.058 | −0.056 |
| Heart rate (bpm) | 0.125** | 0.037 | 0.123** | 0.048 |
| Daytime HRV | ||||
| VLF-HRV (ln ms2) | −0.155** | −0.134** | −0.191** | −0.062 |
| LF-HRV (ln ms2) | −0.127** | −0.120** | −0.172** | −0.046 |
| HF-HRV (ln ms2) | −0.081 | −0.013 | −0.077 | −0.019 |
| Total power HRV (ln ms2) | −0.144** | −0.04 | −0.149** | −0.058 |
| SDNN (ms) | −0.128** | −0.037 | −0.139** | −0.059 |
| RMSSD (ms) | −0.043 | 0.023 | −0.069* | −0.045 |
| PNN50 (%) | −0.029 | 0.028 | −0.063 | −0.052 |
| DFA1 | −0.054 | −0.131** | −0.091** | −0.02 |
| HRT onset (%) | 0.032 | −0.006 | −0.023 | 0.018 |
| HRT slope (ms/beat) | −0.053 | −0.152** | −0.051 | −0.047 |
| Heart rate (bpm) | 0.081 | −0.001 | 0.083 | 0.023 |
HRV = heart rate variability; WBC = white blood cell count; IL = interleukin; CRP = C-reactive protein; VLF = very low frequency; LF = low-frequency; HF = high frequency; SDNN = standard deviation of the NN intervals; RMSSD = square root of mean square successive difference of RR intervals; PNN50 = percent of successive RR interval differences >50 ms; DFA1 = detrended fluctuation analysis for the short-term fractal scaling exponent.
p < .05;
p < .01.
Relationships were similar in depressed and nondepressed participants. A few differential patterns were observed. In nondepressed individuals, 24-hour DFA1 was inversely associated with WBC (r = −.076, p = .035), IL-6 (r = −.126, p < .001), and CRP (r = −.139, p < .001), which was not observed in depressed individuals (p >.10) (p interaction = .011, .042, and .012, respectively). Among depressed participants, HF-HRV was inversely related to WBC (r = −.292, p = .001), IL-6 (r = −.233, p = .010), and CRP (r = −.205, p = .020), which was not observed in nondepressed individuals (p interaction = <.001, .025, and .040, for WBC, IL-6, and CRP, respectively). Results for daytime HRV indices stratified by depression status were similar (data not shown).
Increased Cardiovascular Mortality Risk Associated With Depression; Role of ANS and Inflammation Markers
A total of 352 (38.8%) participants died during follow-up (median follow-up duration, 13.3 years (range, 78 days–14.1 years). Of these deaths, 121 (34.4%) were classified as resulting from cardiovascular causes (median time until CVD death, 7.9 years; range, 78 days–13.8 years).
As shown in Figure 2, presence of depression was associated with increased risk of CVD-related mortality (HR, 1.88; 95% CI, 1.23–2.86). This association remained significant after adjustment for age, sex, systolic blood pressure, diabetes, BMI, physical activity (kcal/week), and use of β-adrenergic blocking agents (HR, 2.11; 95% CI, 1.37–3.24) and additional adjustments for predictors of CVD death (smoking status and subclinical CVD: HR, 2.09; 95% CI, 1.36–3.24). The level of depression severity (i.e., CES-D as continuous variable), was also significantly predictive of CVD mortality after adjustment for covariates (HR, 1.04; CI, 1.01–1.09 per CES-D unit).
Figure 2.
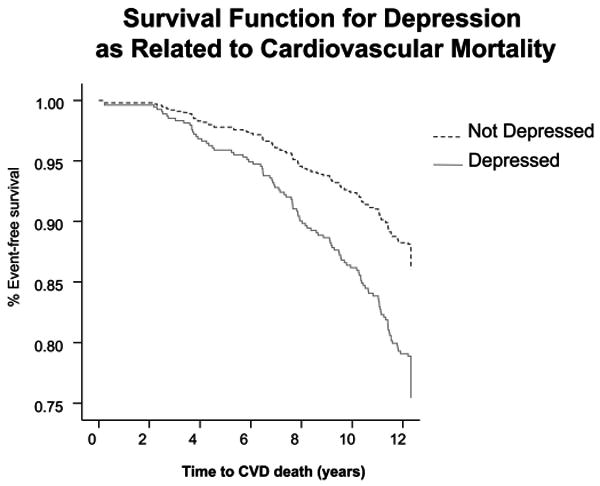
Relationship between depression and cardiovascular disease (CVD) mortality (Kaplan Meyer survival curve). Depression was associated with a significantly elevated risk of cardiovascular mortality: hazard ratio, 1.88, 95% confidence interval, 1.23–2.86.
Table 5 shows the unadjusted predictive value of depression, HRV indices and inflammation markers for CVD mortality. HRV indices significantly associated with CVD mortality were VLF-HRV, LF-HRV, total HRV power, SDNN, RMSSD (only as continuous measure), DFA1, HRT onset, and HRT slope. Because these HRV indices are interrelated, we used a stepwise model to determine which HRV measures independently predicted CVD mortality. The 24-hour DFA1 and HRT slope were independent HRV-based predictors of mortality and were used in subsequent multivariable analyses. Data for daytime HRV measures were similar, with VLF-HRV, DFA1, and HRT slope emerging as independent HRV-based predictors of mortality.
TABLE 5. Predictive Value of Depression, HRV Indices, and Inflammation Markers for Cardiovascular Mortality.
| Dichotomizeda | Continuousb | |
|---|---|---|
| Depression (CES-D ≥8) | 1.88 (1.23–2.86) | 1.18 (1.00–1.41) |
| Autonomic indices (24-hr) | ||
| VLF-HRV (<1000 ms) | 1.44 (1.01–2.07) | 0.71 (0.59–0.86) |
| LF-HRV (<340 ms) | 1.54 (1.07–2.21) | 0.80 (0.66–0.97) |
| HF-HRV (<110 ms) | 1.05 (0.73–1.50) | 1.01 (0.83–1.20) |
| Total power (< 14400 ms) | 1.53 (1.06–2.19) | 0.78 (0.65–0.93) |
| SDNN (<120 ms) | 1.71 (1.19–2.46) | 0.77 (0.63–0.94) |
| RMSSD (<22 ms) | 0.79 (0.56–1.14) | 1.17 (1.01–1.34) |
| PNN50 (<2.9%) | 0.91 (0.64–1.30) | 1.11 (0.95–1.29) |
| DFA1 (<1) | 2.01 (1.40–2.87) | 0.69 (0.59–0.82) |
| HRT onset (>0%) | 2.43 (1.61–3.65) | 1.26 (1.07–1.47) |
| HRT slope (<3 ms/beat) | 2.66 (1.82–3.90) | 0.62 (0.46–0.83) |
| Heart rate (>74 beats/min) | 1.14 (0.79–1.62) | 1.10 (0.92–1.32) |
| Autonomic indices (daytime) | ||
| VLF-HRV (<840 ms) | 2.01 (1.38–2.91) | 0.64 (0.54–0.77) |
| LF-HRV (<260 ms) | 1.48 (1.03–2.13) | 0.70 (0.58–0.85) |
| HF-HRV (<73 ms) | 1.06 (0.74–1.51) | 0.99 (0.82–1.19) |
| Total power (<8900 ms) | 1.44 (1.01–2.07) | 0.78 (0.65–0.94) |
| SDNN (<94 ms) | 1.54 (1.07–2.22) | 0.79 (0.64–0.96) |
| RMSSD (<19 ms) | 1.14 (0.80–1.63) | 1.15 (0.99–1.33) |
| PNN50 (<1.8%) | 1.05 (0.74–1.50) | 1.13 (0.98–1.31 |
| DFA1 (<1) | 2.30 (1.61–3.29) | 0.65 (0.56–0.76) |
| HRT onset (>0%) | 2.79 (1.56–4.97) | 1.15 (0.99–1.34) |
| HRT slope (<3 ms/beat) | 2.93 (2.00–4.29) | 0.58 (0.42–0.79) |
| Heart rate (>80 beats/min) | 1.05 (0.74–1.50) | 1.03 (0.87–1.24) |
| Inflammation markers | ||
| CRP (>1.71 mg/L) | 1.52 (1.06–2.19) | 1.24 (1.04–1.47) |
| IL-6 (>1.57 pg/mL) | 2.01 (1.35–2.98) | 1.49 (1.25–1.77) |
| Fibrinogen (>305 mg/dL) | 1.18 (0.82–1.69) | 1.25 (1.04–1.50) |
| WBC (>5.8 103/mm3) | 1.40 (0.98–2.02) | 1.16 (0.98–1.37) |
HRV = heart rate variability; CES-D = Centers for Epidemiological Studies Depression scale; VLF = very low frequency; LF = low-frequency; HF = high frequency; SDNN = standard deviation of the NN intervals; RMSSD = square root of mean square successive difference of RR intervals; PNN50 = percent of successive NN interval differences >50 ms; DFA1 = detrended fluctuation analysis for the short-term fractal scaling exponent; HRT = heart rate turbulence; CRP = C-reactive protein; IL = interleukin; WBC = white blood cell count.
Unadjusted = hazard ratios based for each predictor separately. HRV indices refer to 24-hr measures.
Dichotomized based on previously validated cutoff values (CES-D, DFA1, HRT) or median split.
Continuous values were transformed to Z scores in order to enable direct comparison between hazard ratios.
Data are presented as hazard ratio and 95% confidence interval in parenthesis.
Among the inflammation markers, CRP and IL-6 were associated with CVD mortality. Multivariable stepwise analyses of the inflammation markers indicated that IL-6 was the only inflammation marker independently associated with mortality.
Figure 3 displays the relative reduction in predictive value of depression for CV mortality when adjusting for each of the 24-hour ANS and inflammation markers. Reductions in the predictive value of depression (>5%) occurred with adjustments for 24-hour VLF (8.9%) and DFA1 (6.8%). Reductions for daytime HRV indices were stronger: VLF (11.0%), LF (6.0%), DFA1 (8.2%). Figure 3 also shows that inflammation markers reduced the predictive value of depression (IL-6, 10.2%; CRP, 6.5%; and fibrinogen, 6.7%).
Figure 3.
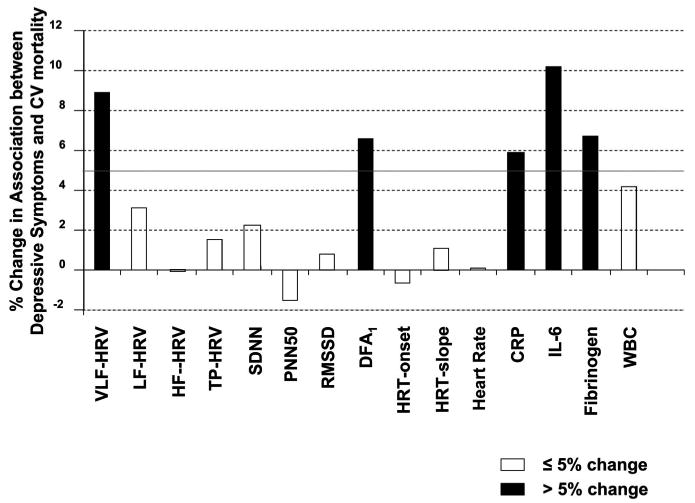
Changes in the strength of the association between depression and cardiovascular (CV) mortality (% log hazard ratio) after adjustment for autonomic nervous system measures (24-hour heart rate variability-based indices) and inflammation markers. A cutoff of 5% was used to indicate a significant mediation effect (solid bars). VLF = very low frequency; HRV = heart rate variability; LF = low frequency; HF = high frequency; SDNN = standard deviation of the normal-normal intervals; PNN50 = percent of successive NN interval differences >50 ms; RMSSD = root mean square successive difference of NN intervals; DFA1 = detrended fluctuation analysis; HRT = heart rate turbulence; CRP = C-reactive protein; IL = interleukin; WBC = white blood cell.
Multivariable analyses were used to examine the combined risk of depression, HRV indices and inflammation markers for CVD mortality (Table 6). Specifically, adding independent 24-hour HRV indices (DFA1 and HRT slope) significantly improved a model that included depression (Δ χ2 = 24.3, p < .001), and the association of depression with CVD mortality was reduced by 7.4%. Addition of IL-6 (i.e., the independent inflammation marker) further improved the model (Δ χ2 = 13.3, p < .001), reducing the predictive value of depression by another 7.9% of the remaining variance (depression remained significantly predictive of CVD mortality (HR, 1.72) (Table 6, model 1). The reduction in the predictive value of these 24-hr HRV and inflammation markers combined was 12.7%. When further adjusting for demographic, CVD risk factors, use of β-adrenergic blocking agents and health behaviors, results remained similar (Table 6, model 2). When examining the independent daytime-HRV indices and inflammation markers (i.e., VLF-HRV, DFA1 HRT slope, and IL-6), the reduction in the predictive value attributable to these factors was stronger (18.4% versus 12.7%). Results were similar when analyses were repeated while excluding participants on antidepressive medications (data not shown).
TABLE 6. Multivariable Adjusted Association Between Depression, HRV Indices and Inflammation Markers as Related to the Prediction of Cardiovascular Mortality.
| Unadjusted | Model 1 Mutually Adjusted | Model 2 Fully Adjusted | |
|---|---|---|---|
| Depression | 1.88 (1.23–2.86) | 1.72 (1.05–2.83) | 2.11 (1.26–3.55) |
| DFA1 | 0.69 (0.59–0.82) | 0.69 (0.57–0.84) | 0.79 (0.64–0.97) |
| HRT slope | 0.58 (0.42–0.79) | 0.70 (0.51–0.95) | 0.76 (0.56–1.04) |
| IL-6 | 1.49 (1.25–1.77) | 1.44 (1.19–1.74) | 1.39 (1.12–1.72) |
HRV = heart rate variability; Unadjusted = hazard ratios based for each predictor separately; Model 1 = Multivariable model: depression, autonomic nervous system indices, and inflammation markers adjusted for each other. Variables selected based on stepwise models (see text for details). Model 2 = Multivariate model adjusted for demographics (age, sex and race) CV risk factors: (systolic blood pressure, diabetes, body mass index, subclinical disease), use of β-adrenergic blocking agents, and health behaviors (smoking status and physical activity). DFA1 = detrended fluctuation analysis for the short-term fractal scaling exponent; HRT = heart rate turbulence; IL = interleukin.
Data are presented as hazard ratio and 95% confidence interval in parenthesis.
Mediational analyses were conducted examining the HRV and inflammation markers that were associated with depression (Tables 2 and 3), as well as CVD mortality (Table 5) at p < .10 in adjusted models; i.e., daytime DFA1, IL-6, CRP, and fibrinogen). The addition of these variables reduced the predictive value of depression by 13.9%, and depression remained an independent predictor of CVD mortality (HR, 1.68; 95% CI, 1.06–2.65). Adding the demographic and clinical measures to the multivariable model, a 7.7% reduced risk of depression was observed when adding DFA1, IL-6, CRP, and fibrinogen to a model that included depression and covariates.
Discussion
This study demonstrates that depression is associated with selected indices of ANS dysfunction (daytime DFA1) and inflammation markers (WBC count and fibrinogen) in individuals >65 years of age free of clinical CVD. Depression, ANS dysfunction, and inflammation markers were additive and independent risk factors for long-term CVD mortality. Only a small percentage of the predictive value of depression was attributable to autonomic dysfunction and inflammation markers, and further investigations are needed to explain the biobehavioral mechanisms by which depression predicts adverse CVD outcome.
Results from this study are consistent with prior observations that depression is associated with indicators of ANS dysfunction (5–7) and elevated inflammation markers (8–11). The associations between depression and these ANS and inflammation markers were relatively weak and only significant at p < .05 for daytime DFA1, fibrinogen, and WBC (Tables 2 and 3) in multivariable models. This finding was not a consequence statistical Type I error, as multivariate models indicated a significant effect for depression on the combined HRV indices as well as inflammation markers. Some evidence indicates that the depression-HRV relationships are not as strong in healthy individuals compared with CVD patients, and negative findings have been reported in CVD patients as well (28). Restriction of HRV analyses to daytime observations was associated with stronger depression-HRV associations, but the typical HRV indices associated with depression (VLF-HRV, HF-HRV, total power [equivalent to SDNN], and RMSSD) did not reveal significant results for either daytime or 24-hour analyses. The relatively small effect size may, in part, be related to the selection criteria, excluding individuals with a known history of CVD, and processes related to aging.
Prior studies have suggested that inflammation markers explain only a small portion of the risk associated with depressive symptoms for adverse cardiovascular outcomes (12,13), and that depression and inflammation markers are additive and independent risk factors of these outcomes (14–16). Arbelaez et al. (60) observed a significantly elevated risk of stroke associated with depression only when inflammation markers (CRP) were elevated. The present findings indicate that the cardiovascular mortality risk associated with depression is reduced by 12.7% when adjusting for 24-hour DFA1, 24-hour HRT slope, and IL-6, and by 18.4% when adjusting for daytime VLF-HRV, DFA1 HRT slope, and IL-6. These percentages indicate a significant (p < .01) but modest effect (<20%) of ANS dysfunction and inflammation (Fig. 3). We note here that these percentages are largely explained by the predictive value of these measures for CVD mortality, as the cross-sectional associations with depression were minimal.
HRV-based indices of autonomic dysfunction were associated with higher levels of inflammation markers (Table 4), which is consistent with other investigations (27–32,36). Patterns of association between ANS indices and inflammation markers were similar in depressed and nondepressed individuals. However, HF-HRV was related to lower inflammation markers in depressed but not in nondepressed participants, which is consistent with other observations (28). We also found that nonlinear markers (DFA1) were inversely associated with inflammation markers in nondepressed but not in depressed individuals. It may, therefore, be that depression status needs to be considered in the selection of optimal HRV-based markers for ANS dysregulation.
Limitations
The present longitudinal study used psychological and biological measures that are feasible in large-scale cohort studies. The observed effect sizes of the associations between depression with ANS and inflammation markers were relatively small. HRV data could have been influenced by nonstationarity and artifacts related to differences in breathing characteristics. More sensitive measures may have resulted in stronger associations with depression (e.g., interview-based evaluations for depression, peroneal sympathetic nerve registration for sympathetic nervous system activity, and flow-cytometry-based measures for inflammatory processes). However, such measures are impractical in large-scale epidemiological studies.
Mediation analyses were conducted based on predictors that were assessed at baseline only. Repeated measures of simultaneously assessed depression, autonomic and inflammation markers are needed to further document the mediational pathways (58). It is also possible that the long duration of follow-up may have obscured some of the transient mechanisms that play a role in the elevated CVD risk associated with depression. Prospective studies linking a risk factor to a disease outcome generally measure the risk factor at one time point (usually baseline), assuming relatively stable levels of the predictor and hence small within-individual variation over time. The predictive value of IL-6 for CVD mortality is stronger in the first 2 years post assessment compared with events that occur later during follow-up (61). No such time-dependent effects were seen for depression in this study (Fig. 2). We have also shown that the 5-year changes in HRV indices are relatively small (21,29). Depression may wax and wane over time, but the inflammation-related correlates of depression may persist after depression remits (9). Further research is needed on the time trajectories of behavioral and biological risk factors for adverse CVD outcomes.
The low prevalence of clinically severe depression and limited use of antidepressive medications in depressed individuals (11.5%) may, in part, reflect selection biases specific to community-based epidemiological studies. In addition, antidepressive medications, such as tricyclics, may have been used for conditions other than depression (e.g., sleep disorders or neuropathic pain) and may be associated with cardiac conduction delay, orthostatic hypotension, and other cardiovascular changes that can adversely affect CVD progression. Results from this study may not be generalizable to individuals <65 years of age and those with clinical CVD. Additional studies are needed to examine the neuroimmunological correlates of clinical depression and the effects of antidepressive interventions in the elderly as well as younger populations.
Clinical Implications
Results from this study suggest that the long-term adverse cardiovascular consequences of depression may be partially explained by ANS dysfunction and inflammation. These findings are important because clinical trials in patients with established CVD have shown that improvements in depression by either pharmacological (62–64) or psychological interventions (65) are modest, and generally not followed by significant reductions in cardiovascular outcomes (65,66). Subgroups of depressed individuals with excess cardiovascular risk may, therefore, exist based on neuroimmunological activation. Further research is needed to explore whether interventions that combine multiple pathways are beneficial in reducing the adverse cardiovascular risk related to depression. A multifactorial approach may improve optimal identification and treatment of patients who are at high-risk of cardiovascular morbidity and mortality.
Acknowledgments
This research was supported, in part, by contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, Grants U01 HL080295 and R0-1 HL62181 and HL66149 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke.
- ANS
autonomic nervous system
- LF
low frequency
- CI
confidence interval
- PNN50
percent of successive RR interval differences >50 ms
- CRP
C-reactive protein
- CES-D
Centers for Epidemiological Studies Depression scale
- PVC
premature ventricular complex
- RMSSD
root mean square successive difference of RR intervals
- CHS
Cardiovascular Health Study
- CVD
cardiovascular disease
- SD
standard deviation
- DFA1
detrended fluctuation analysis
- SDNN
standard deviation of the NN intervals
- HF
high frequency
- HR
hazard ratio
- HRT
heart rate turbulence
- TO
turbulence onset
- HRV
heart rate variability
- TS
turbulence slope
- VLF
very low frequency
- IL-6
interleukin-6
- WBC
white blood cell count
Footnotes
A full list of principal Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
References
- 1.Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–8. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–74. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 3.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45:637–51. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–53. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 5.Carney RM, Howells WB, Blumenthal JA, Freedland KE, Stein PK, Berkman LF, Watkins LL, Czajkowski SM, Steinmeyer B, Hayano J, Domitrovich PP, Burg MM, Jaffe AS. Heart rate turbulence, depression, and survival after acute myocardial infarction. Psychosom Med. 2007;69:4–9. doi: 10.1097/01.psy.0000249733.33811.00. [DOI] [PubMed] [Google Scholar]
- 6.Glassman AH, Bigger JT, Gaffney M, van Zyl LT. Heart rate variability in acute coronary syndrome patients with major depression: influence of sertraline and mood improvement. Arch Gen Psychiatry. 2007;64:1025–31. doi: 10.1001/archpsyc.64.9.1025. [DOI] [PubMed] [Google Scholar]
- 7.van Zyl LT, Hasegawa T, Nagata K. Effects of antidepressant treatment on heart rate variability in major depression: a quantitative review. Biopsychosoc Med. 2008;2:12. doi: 10.1186/1751-0759-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranjit N, ez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, Ni H. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167:174–81. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 9.Kop WJ, Gottdiener JS. The role of immune system parameters in the relationship between depression and coronary artery disease. Psychosom Med. 2005;67:537–41. doi: 10.1097/01.psy.0000162256.18710.4a. [DOI] [PubMed] [Google Scholar]
- 10.Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychol Bull. 1993;113:472–86. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- 11.Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, Newman A, Hirsch C, Tracy RP. Inflammation and coagulation factors in persons >65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89:419–24. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 12.Whooley MA, de Jonge J, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–88. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN, National Heart, Lung, and Blood Institute Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–50. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 14.Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, Ferrieres J, Amouyel P, Bingham A, Montaye M, Ruidavets JB, Haas B, Evans A, Jouven X, Ducimetiere P. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2005;111:2299–305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 15.Nabi H, Singh-Manoux A, Shipley M, Gimeno D, Marmot MG, Kivimaki M. Do psychological factors affect inflammation and incident coronary heart disease: the Whitehall II Study. Arterioscler Thromb Vasc Biol. 2008;28:1398–406. doi: 10.1161/ATVBAHA.108.167239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladwig KH, Marten-Mittag B, Lowel H, Doring A, Koenig W. C-reactive protein, depressed mood, and the prediction of coronary heart disease in initially healthy men: results from the MONICA-KORA Augsburg Cohort Study 1984–1998. Eur Heart J. 2005;26:2537–42. doi: 10.1093/eurheartj/ehi456. [DOI] [PubMed] [Google Scholar]
- 17.Buccelletti E, Gilardi E, Scaini E, Galiuto L, Persiani R, Biondi A, Basile F, Silveri NG. Heart rate variability and myocardial infarction: systematic literature review and metanalysis. Eur Rev Med Pharmacol Sci. 2009;13:299–307. [PubMed] [Google Scholar]
- 18.Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–71. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 19.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 20.Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufman PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, Van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 21.Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electrophysiol. 2005;16:13–20. doi: 10.1046/j.1540-8167.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- 22.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103:2072–7. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 23.Zareba W, Moss AJ. Noninvasive risk stratification in postinfarction patients with severe left ventricular dysfunction and methodology of the MADIT II noninvasive electrocardiology substudy. J Electrocardiol. 2003;36(Suppl):101–8. doi: 10.1016/j.jelectrocard.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 25.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, Tracy RP. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 26.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 27.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 28.Frasure-Smith N, Lesperance F, Irwin MR, Talajic M, Pollock BG. The relationships among heart rate variability, inflammatory markers and depression in coronary heart disease patients. Brain Behav Immun. 2009;23:1140–7. doi: 10.1016/j.bbi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Stein PK, Barzilay JI, Chaves PH, Traber J, Domitrovich PP, Heckbert SR, Gottdiener JS. Higher levels of inflammation factors and greater insulin resistance are independently associated with higher heart rate and lower heart rate variability in normoglycemic older individuals: the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:315–21. doi: 10.1111/j.1532-5415.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 30.Carney RM, Freedland KE, Stein PK, Miller GE, Steinmeyer B, Rich MW, Duntley SP. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosom Res. 2007;62:463–7. doi: 10.1016/j.jpsychores.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088:361–72. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- 32.Lanza GA, Sgueglia GA, Cianflone D, Rebuzzi AG, Angeloni G, Sestito A, Infusino F, Crea F, Maseri A. Relation of heart rate variability to serum levels of C-reactive protein in patients with unstable angina pectoris. Am J Cardiol. 2006;97:1702–6. doi: 10.1016/j.amjcard.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 33.Pizzi C, Manzoli L, Mancini S, Costa GM. Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function. Eur Heart J. 2008;29:1110–7. doi: 10.1093/eurheartj/ehn137. [DOI] [PubMed] [Google Scholar]
- 34.Hamer M, Steptoe A. Association between physical fitness, parasympathetic control, and proinflammatory responses to mental stress. Psychosom Med. 2007;69:660–6. doi: 10.1097/PSY.0b013e318148c4c0. [DOI] [PubMed] [Google Scholar]
- 35.Lanza GA, Pitocco D, Navarese EP, Sestito A, Sgueglia GA, Manto A, Infusino F, Musella T, Ghirlanda G, Crea F. Association between cardiac autonomic dysfunction and inflammation in type 1 diabetic patients: effect of beta-blockade. Eur Heart J. 2007;28:814–20. doi: 10.1093/eurheartj/ehm018. [DOI] [PubMed] [Google Scholar]
- 36.Lampert R, Bremner JD, Su S, Miller A, Lee F, Cheema F, Goldberg J, Vaccarino V. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am Heart J. 2008;156:759–7. doi: 10.1016/j.ahj.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy R. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 38.Stein PK, Barzilay JI, Domitrovich PP, Chaves PM, Gottdiener JS, Heckbert SR, Kronmal RA. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: the Cardiovascular Health Study. Diabet Med. 2007;24:855–63. doi: 10.1111/j.1464-5491.2007.02163.x. [DOI] [PubMed] [Google Scholar]
- 39.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 40.Andresen EM, Malmgren JA, Carter WB, Patrick DL, Radloff LS. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 41.Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 42.Iyengar N, Peng CK, Morin R, Goldberger AL, Lipsitz LA. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol. 1996;271:R1078–84. doi: 10.1152/ajpregu.1996.271.4.R1078. [DOI] [PubMed] [Google Scholar]
- 43.Tulppo MP, Kiviniemi AM, Hautala AJ, Kallio M, Seppänen T, Mäkikallio TH, Huikuri HV. Physiological background of the loss of fractal heart rate dynamics. Circulation. 2005;112:314–9. doi: 10.1161/CIRCULATIONAHA.104.523712. [DOI] [PubMed] [Google Scholar]
- 44.Ghuran A, Reid F, La Rovere MT, Schmidt G, Bigger JT, Jr, Camm AJ, Schwartz PJ, Malik M. Heart rate turbulence-based predictors of fatal and nonfatal cardiac arrest (The Autonomic Tone and Reflexes After Myocardial Infarction substudy) Am J Cardiol. 2002;89:184–90. doi: 10.1016/s0002-9149(01)02198-1. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt G, Malik M, Barthel P, Schneider R, Ulm K, Rolnitzky L, Camm AJ, Bigger JT, Jr, Schomig A. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390–6. doi: 10.1016/S0140-6736(98)08428-1. [DOI] [PubMed] [Google Scholar]
- 46.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–70. [PubMed] [Google Scholar]
- 47.Bovill EG, Bild DE, Heiss G, Kuller LH, Lee MH, Rock R, Wahl PW. White blood cell counts in persons aged 65 years or more from the cardiovascular health study. correlations with baseline clinical and demographic characteristics. Am J Epidemiol. 1996;143:1107–15. doi: 10.1093/oxfordjournals.aje.a008687. [DOI] [PubMed] [Google Scholar]
- 48.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–8. [PubMed] [Google Scholar]
- 49.Jenny NS, Tracy RP, Ogg MS, Luong lA, Kuller LH, Arnold AM, Sharrett AR, Humphries SE. In the elderly, interleukin-6 plasma levels and the -174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:2066–71. doi: 10.1161/01.atv.0000040224.49362.60. [DOI] [PubMed] [Google Scholar]
- 50.Zakai NA, Katz R, Jenny NS, Psaty BM, Reiner AP, Schwartz SM, Cushman M. Inflammation and hemostasis biomarkers and cardiovascular risk in the elderly: the Cardiovascular Health Study. J Thromb Haemost. 2007;5:1128–35. doi: 10.1111/j.1538-7836.2007.02528.x. [DOI] [PubMed] [Google Scholar]
- 51.Tracy RP, Bovill EG, Yanez D, Psaty BM, Fried LP, Heiss G, Lee M, Polak JF, Savage PJ. Fibrinogen and factor VIII, but not factor VII, are associated with measures of subclinical cardiovascular disease in the elderly. Results from the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1995;15:1269–79. doi: 10.1161/01.atv.15.9.1269. [DOI] [PubMed] [Google Scholar]
- 52.Kuller L, Borhani N, Furberg C, Gardin J, Manolio T, O'Leary D, Psaty B, Robbins J. Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. Am J Epidemiol. 1994;139:1164–79. doi: 10.1093/oxfordjournals.aje.a116963. [DOI] [PubMed] [Google Scholar]
- 53.Siscovick DS, Fried L, Mittelmark M, Rutan G, Bild D, O'Leary DH. Exercise intensity and subclinical cardiovascular disease in the elderly. The Cardiovascular Health Study. Am J Epidemiol. 1997;145:977–86. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 54.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–50. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 55.Hirsch CH, Fried LP, Harris T, Fitzpatrick A, Enright P, Schulz R. Correlates of performance-based measures of muscle function in the elderly: the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 1997;52:M192–200. doi: 10.1093/gerona/52a.4.m192. [DOI] [PubMed] [Google Scholar]
- 56.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 57.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 58.Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001;158:848–56. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 59.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 60.Arbelaez JJ, Ariyo AA, Crum RM, Fried LP, Ford DE. Depressive symptoms, inflammation, and ischemic stroke in older adults: a prospective analysis in the cardiovascular health study. J Am Geriatr Soc. 2007;55:1825–30. doi: 10.1111/j.1532-5415.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 61.Jenny NS, Yanez ND, Psaty BM, Kuller LH, Hirsch CH, Tracy RP. Inflammation biomarkers and near-term death in older men. Am J Epidemiol. 2007;165:684–95. doi: 10.1093/aje/kwk057. [DOI] [PubMed] [Google Scholar]
- 62.Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, Mclvor M. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–9. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 63.Lesperance F, Frasure-Smith N, Koszycki D, Laliberte MA, van Zyl LT, Baker B, Swenson JR, Ghatavi K, Abramson BL, Dorian P, Guertin MC. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297:367–79. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- 64.Honig A, Kuyper AM, Schene AH, van Melle JP, de Jonge P, Tulner DM, Schins A, Crijns HJ, Kuijpers PM, Vossen H, Lousberg R, Ormel J, MIND-IT Investigators Treatment of post-myocardial infarction depressive disorder: a randomized, placebo-controlled trial with mirtazapine. Psychosom Med. 2007;69:606–13. doi: 10.1097/PSY.0b013e31814b260d. [DOI] [PubMed] [Google Scholar]
- 65.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289:3106–16. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 66.van Melle JP, de Jonge P, Honig A, Schene AH, Kuyper AM, Crijns HJ, Schins A, Tulner D, van den Berg MP, Ormel J, MIND-IT Investigtors Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460–6. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]


