Abstract
Multifunctional dendrimers bearing two or more surface functionalities have the promise to provide smart drug delivery devices that can for example combine tissue targeting and imaging or be directed more precisely to a specific tissue or cell type. We have developed a concise synthetic methodology for efficient dendrimer assembly and heterobifunctionalization based on three sequential azide-alkyne cycloadditions. The methodology is compatible with biologically important compounds rich in chemical functionalities such as peptides, carbohydrates and fluorescent tags. In the approach, a strain promoted azide-alkyne cycloaddition (SPAAC) between polyester dendrons modified at the focal point with an azido and 4-dibenzocyclooctynol (DIBO) moiety provided dendrimers bearing terminal and TMS-protected alkynes at the periphery. The terminal alkynes were outfitted with azido-modified polyethylene glycol (PEG) chains or galactosyl residues using CuI catalyzed azide-alkyne cycloadditions (CuAAC). Next, a one-pot TMS-deprotection and second click reaction of the resulting terminal alkyne with azido-containing compounds gave multifunctional dendrimers bearing complex biologically active moieties at the periphery.
Keywords: dendrimers, carbohydrates, peptides, synthetic methods, drug delivery, click chemistry
Introduction
Dendrimers are emerging as promising materials for the development of imaging devices and drug and gene delivery vehicles.[1] Attractive properties of dendrimers include chemical homogeneity, tunability of biodistribution and pharmacokinetics by regulating size and controlled degradation by judicious choice of dendrimer chemistry.[2] Furthermore, the typical architecture of dendrimers results in the formation of cavities, which can entrap pharmaceutically active substances.[3] Moreover, the surface of dendrimers can be modified by prodrugs, imaging modules such as fluorescent tags, CT and MRI contrast agents,[4] polyethylene glycol to increase water solubility and improve biocompatibility,[5] and by cell tissue targeting ligands such as folic acid or RGD peptides to increase therapeutic efficiency.[6] Surface modification of dendrimers with a targeting device benefits from high multivalent densities, which will strengthen ligand-receptor binding as a result of a cluster effect.[7] A particularly attractive approach for surface modification of dendrimers is a CuI catalyzed 1,3-dipolar cycloaddition of azides with terminal alkynes (CuAAC) to give stable 1,2,3-triazoles.[8] CuAAC combines exceptional chemoselectivity with a lack of byproducts and high yields. It has been used to efficiently derivatize dendrimers with unprotected peptides,[9] carbohydrates,[10] and other complex compounds.[11]
It is to be expected that dendrimers modified by several different peripheral entities can combine functions such as tissue targeting and imaging or be directed more precisely to a specific tissue or cell type.[12] Usually, multifunctional dendrimers are prepared by a random chemical coupling reaction, which unfortunately leads to unwanted dispersity.[13] A more attractive approach uses dendritic molecules or polymers having two or more orthogonal functionalities or protecting groups.[14] In particular, azides or alkynes for CuAAC combined with hydroxyls for etherification[15] or aldehydes for hydrazone formation[16] have been successfully employed as sets of the orthogonal functionalities. Dendrimers have also been multifunctionalized using CuAAC in a sequential manner. In this approach, polyester dendrimers modified by mannoside-targeting moieties and coumarin fluorescent tags were prepared by starting with a dendrimer having peripheral alcohols and isopropylidene acetals.[17] The alcohols of the dendrimer could be modified by terminal alkynes, which could then be coupled with azide-modified coumarin. Removal of isopropylidene acetals gave alcohols and a repetition of alkyne formation and CuAAC led to the controlled introduction of peripheral mannosides. More recently, bifunctional[18] and trifunctional[19] dendrimers were constructed by click reaction followed by coupling of azide bearing dendron to the dendrimer core, thus enabling a surface modification by a second CuAAC.
Despite many attractive features of these methods, the limited chemoselectivity of conventional functional groups such as alcohol, amine, carboxylic acids and carbonyls and in some cases the relatively large number of chemical steps for orthogonal group installation, places restrictions on the type of functionality that can be attached to a dendritic surface.
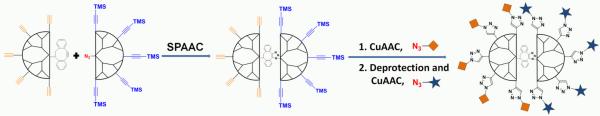
We report here a versatile approach for selective surface modification of dendrimers by a strain-promoted alkyne-azide cycloaddition (SPAAC)[20] between two dendrons modified by a focal dibenzocyclooctyne or azide and having peripheral alkynes or TMS-protected alkynes, respectively. This ligation exploits a selective reaction of a strained alkyne with an azide in the presence of terminal alkynes.[21] The terminal alkynes can, however, be selectively modified with an azide-containing moiety using a CuI catalyst. In a third step, a second type of surface functionality can be installed in a controlled manner by removal of the TMS-protecting groups followed by another CuAAC (Figure 1). The excellent chemoselectivity of SPAAC and CuAAC ensures that a wide variety of functionalities, such as biological relevant carbohydrates and peptides, can be attached in a controlled manner to the periphery of dendrimers.
Figure 1.
General concept of multifunctional dendrimer synthesis by three consecutive click reactions.
Results and Discussion
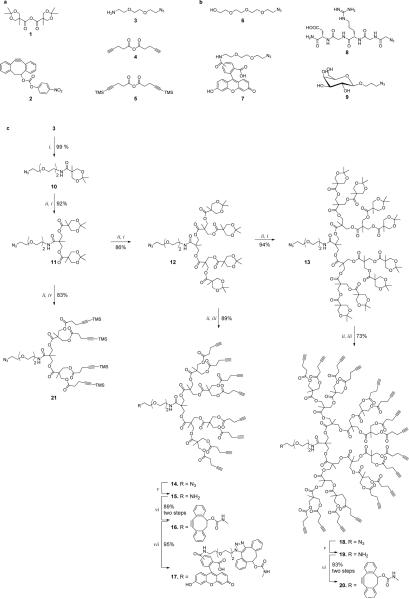
First, we examined whether a strain-promoted alkyne-azide cycloaddition can be utilized for the ligation of two dendrons. Such a reaction is challenging due to steric hindrance at dendron focal points, which may render couplings inefficient leading to low yields and loss of expensive dendrons. Thus, we synthesized generation three- and four-dendrons 14, 16, 18 and 20 having a polyester dendritic framework based on 2,2-bis(hydroxymethyl) propionic acid (bis-MPA)[22] and bearing an azide or a 4-dibenzocyclooctynol (DIBO) moiety[20c] at the focal point (Scheme 1). A polyester framework was selected because of its intrinsic biodegradability[23] and good solubility in organic solvents.[24] DIBO was used because it reacts fast with azido-containing compounds in the absence of a metal catalyst, can be prepared by a simple synthetic approach, is nontoxic and can easily be attached to a variety of probes.
Scheme 1.
a) Dendron building blocks. b) Azides for dendrimer derivatization. c) Synthesis of dendrons, introduction of dibenzocyclooctyne and copper free click reaction. Reaction conditions: i. isopropylidene-2,2-bis(methoxy)propionic anhydride (1), DMAP, Py, DCM, 0°C then RT, 12–18 h. ii. DOWEX® H+ resin, MeOH, 50°C, 2–24 h. iii. pent-4-ynoic anhydride (4), DMAP, Py, DCM, 0°C then RT, 18 h. iv. 5-(trimethylsilyl)pent-4-ynoic anhydride (5), DMAP, Py, DCM, 0°C then RT, 18 h. v. PMe3 10 eq., THF:H2O, 9:1 v/v, 3 h; vi. 2, DMF, DIPEA, 48 h; vii. 7, THF, 2 h.
Dendron synthesis started with a coupling of 2-(2-(2-azidoethoxy)ethoxy)ethanamine[25] (3) with isopropylidene protected bis-MPA anhydride 1[22] in the presence of pyridine and dimethyaminopyridine (DMAP) in DCM to give amide 10, which was treated with Dowex H+ resin in MeOH to remove the isopropylidene acetals and reveal alcohols. Each subsequent generation was introduced by reaction of hydroxyls with anhydride 1 followed by removal of the isopropylidene protecting groups. In this way, polyester dendrons 11, 12 and 13 were synthesized having masked alcohols at the periphery and an azide at the focal point. After deprotection of the isopropylidene acetals of 12 and 13, peripheral alkynes were introduced by treatment with pent-4-ynoic anhydride (4) to give 14 and 18, respectively. Alternatively, treatment of 11 with Dowex-H+ followed by reaction of the resulting alcohols with 5-(trimethylsilyl)pent-4-ynoic anhydride (5) gave 21, which has alkynes protected by trimethylsilyl (TMS) groups. All transformation proceeded in high yield leaving the important azide moiety at the focal point of the dendrons intact.
The azido-containing dendrons 14 and 18 were the starting material for the preparation of the DIBO containing derivatives 16 and 20, respectively. Thus, reduction of the azides of 14 and 18 with trimethylphosphine in a mixture of THF and water gave the corresponding amines 15 and 19, which were immediately treated with the activated carbonate of DIBO (2)[20c] to provide the requisite compounds 16 and 20 in yields of 89 and 93%, respectively.
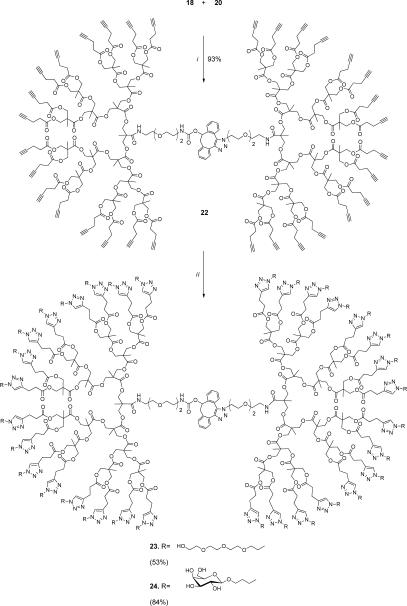
Having azide- and DIBO-modified dendrons at hand, attention was focused on SPAAC-mediated ligation[26] of these derivatives. Gratifyingly, reaction of the G4 dendrons 18 and 20 in THF at room temperature proceeded smoothly and gave, after a reaction time of 24 h, symmetrical dendrimer 22 in a yield of 93% (Scheme 2). Also, the focal DIBO moiety of 16 could be employed for installing a fluorescent probe and reaction with azido-modified fluorescein 7 gave derivative 17. Importantly, the copper free coupling required only a stoichiometric quantity of dendrons.
Scheme 2.
Dendrimer assembly via SPAAC, followed by CuAAC mediated derivatization. Reaction conditions: i. THF, 24 h; ii. 6 or 9, CuSO4, Na ascorbate, TBTA, THF:H2O, 18 h.
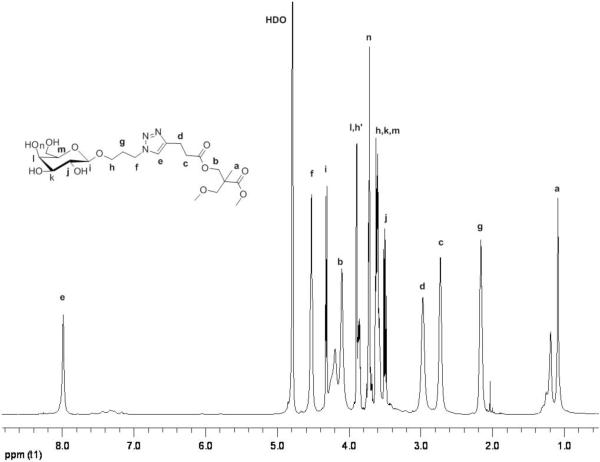
In the presence of a catalytic amount of CuSO4, tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA) and sodium ascorbate in a mixture of THF and water, the alkynes of 17 and 22 could be reacted with azido-containing compounds (see supporting information for modification of 17 and G3-G3 symmetrical dendrimer 32). For example, peripheral modified dendrimers 23 and 24 were obtained in good yields by reaction of 22 with 2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethanol[27] (6) and azido-containing galactoside[28] 9, respectively (Scheme 2). The 1H-NMR spectra of the CuAAC products showed characteristic triazole signals (8 ppm), integration of which along with the unique CH2–triazole signals (4.5 ppm) of the galactosyl or tetraethyleneglycol residues, yielded in each case thirty-two triazole residues per dendrimer molecule (Figure 2). Complete surface derivatization of compound 20 was additionally confirmed by quantitative sugar analysis (31.8±0.2 galactosyl residues).
Figure 2.
1H-NMR spectrum of glycodendrimer 24 (D2O, 500 MHz).
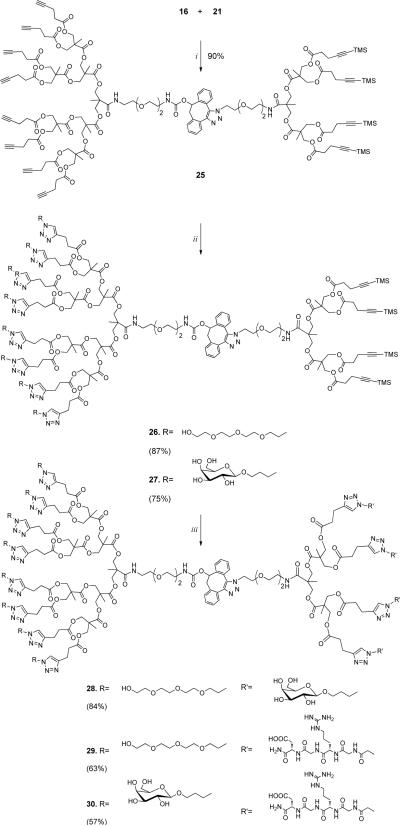
Next, we explored whether SPAAC can facilitate coupling of two dendrons having either terminal or TMS-protected alkynes to give dendrimers that can be modified in a controlled manner by two different surface entities. In this respect, a number of studies have shown that CuAAC can be performed without affecting a TMS-protected alkyne.[29] However, the TMS protecting group can easily be removed by reagents such as tetrabutylammonium fluoride (TBAF) or silver salts and the resulting terminal alkyne be used in a subsequent click reaction. The challenge for using this methodology for dendrimer modification is that multiple TMS-protected alkynes have to stay intact during dendrimer assembly and the first CuAAC. Thus, SPAAC mediated coupling of 16 with 21 in THF for 5 h gave clean formation of the asymmetrical dendrimer 25, which has terminal and TMS-protected alkynes at its periphery (Scheme 3). Next, 25 was subjected to azido-containing tetraethyleneglycol 6 in the presence of a catalytic amount of CuI and N,N-diisopropylethylamine (DIPEA). Under these conditions the terminal alkynes underwent a clean cycloaddition and after a reaction time of 4 h and purification by LH-20 size exclusion column chromatography, dendrimer 26 was obtained in a yield of 87%. Similarly, a reaction between asymmetrical dendrimer 25 and unprotected galactoside 9 afforded glycodendrimer 27 in a yield of 75%. Careful analysis of the structures of 25, 26 and 27 by 1H-NMR and MALDI ToF revealed that the TMS-protected alkynes had remained intact during the SPAAC and CuAAC click reactions. Partial desilylation was, however, observed when a combination of CuSO4 and sodium ascorbate was used for the CuAAC.
Scheme 3.
Three consecutive AAC reactions leading to bifunctionalized dendrimers. Reaction conditions: i. THF, 5 h; ii. 6, CuI, DIPEA, THF, 4 h; iii. 8 or 9, CuF2, MeOH or MeOH:H2O, 40°C, 8–40 h.
Previously, we found that CuF2 can efficiently unmask TMS-modified alkynes and promote cycloadditions with azides.[30] Fortunately, this protocol could be employed for the modification of 26 and reactions with RGD peptide 8 and galactoside 9 proceeded smoothly when methanol was used as a solvent at a temperature of 40°C, to give bifunctional dendrimers 28 and 29, respectively. Bifunctional dendrimer 30 bearing unprotected galactoside residues and RGD peptides was obtained in a similar manner by treatment of glycodendrimer 27 with peptide 8 with CuF2 in methanol-water mixture at 40°C. The absence of characteristic TMS proton signals in the 1H-NMR and correct integral areas of sugar and peptide protons indicated complete derivatization of dendrimers 26 and 27.
Conclusion
We have developed a convenient approach for dendrimer assembly and peripheral functionalization using three consecutive azide-alkyne cycloadditions. Strain promoted azide-alkyne cycloaddition was established as an effective and chemoselective method for coupling of dendrons to give symmetrical and asymmetrical dendrimers bearing alkynes on the periphery. Differentiated terminal and TMS-protected peripheral alkynes were efficiently modified with different combinations of model PEG, galactosyl and peptide-azides, bearing no protecting groups. The methodology is compatible with compounds that are rich in chemical functionalities such as peptides, carbohydrates and fluorescent tags. Furthermore only three consecutive steps are required for dendron coupling and installment of two-different surface entities. Recently, photo-,[31] thiol-ene,[32] and strain-promoted alkyne-nitrone[33] click reactions have been introduced, which also display excellent chemoselectivity and it is to be expected that integration of these reactions in the approach reported here will give easy access to even more complex dendritic structures.
Experimental Section
General methods
All chemicals and dry solvents were purchased from Sigma-Aldrich unless stated otherwise. All esterification, amidation, Staudinger reduction, CuSO4 and CuI mediated reactions were carried out under an atmosphere of argon. Reactions were performed at room temperature (20–22 °C), unless stated otherwise. Reactions were monitored by Thin Layer Chromatography (TLC) using aluminum backed silica gel 60 (F254) plates, visualized using UV (254 nm) and potassium permanganate and cerium molybdate dips as appropriate. Flash chromatography was carried out using silica gel G60 (SiliCycle, 60–200μm 60 Å) as the stationary phase. Solid-Phase Peptide Synthesis (SPPS) was performed on a Applied Biosystems, ABI 433A peptide synthesizer equipped with UV-detector using Ná-Fmoc-protected amino acids and 2-(1H-benzotriazole-1-yl)-oxy-1,1,3,3-tertamethyl hexafluorophosphate (HBTU) / 1-hydroxybenzotriazole (HOBt) as the activating reagents. Reverse Phase HPLC was performed on an Agilent 1200 series system equipped with an automated injector, UV-detector, fraction-collector and Agilent Zorbax Eclipse XD8-C18 column (5 μm, 9.4 × 250 mm). NMR spectra were recorded on Varian Mercury (300, 500 MHz) spectrometers at 25°C. Chemical shifts are reported in ä units, parts per million (ppm) downfield from TMS, spectra are referenced by solvent signals. Coupling constants (J) are measured in Hertz (Hz) and are unadjusted. Splitting patterns are designed as follows: s – singlet, d – doublet, t – triplet, dd – doublet of doublets, dt – doublet of triplets, td – triplet of doublets, m – multiplet, br – broad. Mass spectra were obtained using MALDI-ToF instruments (Applied Biosystems 4700 Proteomics Analyzer, Bruker Microflex LT Mass Spectrometer) with 2,5-dihydroxybenzoic acid or á-cyano-4-hydroxycinnamic acid as a matrix. Sugar analysis was performed on DIONEX ICS-3000 HPAEC chromatograph using deionizer water and 200 mM NaOH as an eluent.
General procedure for the synthesis of dendrons 10, 11, 12, 13, 14, 18 and 21 by sequential isopropylidene acetal removal and ester formation
Dowex® 50WX8-200 H+ ion-exchange resin (2–4 g) was added to the solution of isopropylidene protected dendron (G1 to G4) in MeOH (10 mL) and the resulting suspension was stirred for 2–24 h at 50°C. The reaction mixture was filtered and the resin was washed with MeOH (3×10mL). The combined filtrates were concentrated under reduced pressure to give hydroxyl terminated dendron which was used in the next step without further purification. Hydroxyl terminated dendron or amine 3, DMAP and pyridine were dissolved in DCM (5–10 mL). The mixture was cooled to 0°C and a solution of a suitable anhydride 1, 4 or 5 in DCM (10–15 mL) was added to the mixture in small portions over 10 min. The reaction mixture was then allowed to warm to room temperature and stirred for 12–18 h. The solution was diluted with DCM (50–100 mL) washed with water (50–100 mL), sat. aq. NaHCO3 (50–100 mL), sat aq. CuSO4 (for anhydrides 1 and 5, 50–100 mL) or 0.1 M HCl (for anhydride 4, 2×50 mL) and brine (50–100 mL). The organic layer was dried (MgSO4), filtered and the solvent was evaporated under reduced pressure. The residue was purified by column chromatography on silica gel. Analytical data for dendrons 10, 11, 12, 13, 14, 18 and 21 are reported in the supporting information.
General procedure for the installation of a cyclooctynol moiety for the preparation of 16 and 20
Azido-containing dendron (0.06 mmol) was dissolved in a mixture of THF and H2O (9:1, v/v, 5 mL). A 1 M solution of trimethylphosphine in THF (0.61 mL, 0.61 mmol) was added to the solution and the resulting mixture was stirred for 3 h. The solution was concentrated in vacuo and coevaporated with toluene (3×10 mL). The resulting yellow residue and 11,12-didehydro-5,6-dihydrodibenzo[a,e]cycloocten-5-yl carbonic acid 4-nitrophenyl ester[20c] (2) (35 mg, 0.09 mmol) were dissolved in DMF (5 mL). DIPEA (0.04 mL, 0.24 mmol) was added to the solution and the resulting mixture was stirred for 48 h. Evaporation of the solvent under reduced pressure gave a residue, which was purified by silica gel column chromatography. Analytical data for dendrons 16 and 20 are reported in the supporting information.
General procedure for Cu-free ligation of dendrons to give 17, 22 and 25
Cyclooctynol-modified dendron and azido-containing dendron (or azide 7) were dissolved in THF (10 μmol/mL) and the resulting mixture was stirred for 2–24 h. The solvent was evaporated under reduced pressure and the residue was purified by silica gel column chromatography. Analytical data for dendrimers 17, 22 and 25 are reported in the supporting information.
General procedure for CuAAC reactions using CuSO4 and Na ascorbate for the preparation of 23 and 24
Alkynylated dendrimer, azide 6 or 9 and tris[(1-benzyl-1H-1,2,3 -triazol-4-yl)methyl]amine (TBTA) were dissolved in THF (5 μmol/mL of dendrimer). 0.1 M Solution of (+)-sodium L-ascorbate and 0.1 M solution of CuSO4 in water were added to the mixture. The reaction mixture was stirred for 18 h. Solvent was evaporated and the residue was purified via HPLC. Fractions of interest were combined and lyophilized. Analytical data for dendrimers 23 and 24 are reported in the supporting information.
General procedure for CuAAC reaction using CuI for the preparation of 26 and 27
Dendrimer 25, azide 6 or 9, CuI and DIPEA were dissolved in THF (0.01 mmol/mL). The reaction mixture was stirred for 4–20 h and the solvent was evaporated in vacuo. The residue was purified by SEC on Sephadex® LH-20 gel (MeOH:DCM, 1:1, v/v). Analytical data for dendrimer 26 are reported in the supporting information.
General procedure for CuF2 mediated click reaction for the preparation of 28, 29 and 30
Dendrimer 26, azide 9 or azido-peptide 8 and CuF2 were dissolved in MeOH (4 μmol/mL). The reaction mixture was stirred at 40°C until completion of the reaction (monitored by MALDI-TOF MS). The solvent was evaporated and the residue was purified by HPLC to give after lyophilization of appropriate fractions the product. Analytical data for dendrimers 28 and 29 are reported in the supporting information.
Dendrimer 25 (isomers)
Prepared from G3 dendron 16 (36.9 mg, 20 μmol) and G2 dendron 21 (25.0 mg, 22 μmol) according to the general procedure for Cu-free ligation of dendrons. The reaction mixture was stirred for 5 h. Silica gel column chromatography (40% acetone in hexanes) gave dendrimer 25 (53 mg, 90%) as viscous oil: 1H NMR (500 MHz, CD6CO, 25°C, TMS): ä= 7.68–7.15 (m, 10 H, CH, NH), 6.55–6.46 (m, 1 H, NH), 6.22–5.97 (m, 1 H, CH), 4.65–4.56 (m, 2 H, CH2), 4.34–4.28 (m, 40 H, CH2), 4.01–3.79 (m, 2 H, CH2), 3.79–2.79 (m, 22 H, CH2), 2.60–2.46 (m, 48 H, CH2), 2.36 (s, 8 H, CH), 1.37–1.28 (m, 30 H, CH3), 0.11 ppm (s, 36 H, CH3); MS (MALDI-TOF): m/z: calcd for C151H200N6O48NaSi4: 3000.2 [M+Na]+; found: 3001.3.
Dendrimer 27 (isomers)
Prepared from dendrimer 25 (15.0 mg, 5.0 μmol) using: 3-azidopropyl β-D-galactopyranoside (9) (21.0 mg, 80.0 μmol), CuI (0.8 mg, 4.5 μmol) and DIPEA (4 μL, 20.0 μmol) according to the general procedure for CuAAC reaction using CuI. The reaction mixture was stirred for 20 h. SEC purification gave 27 as transparent oil (19 mg, 75%): 1H NMR (500 MHz, CD3OD, 25°C, TMS): δ= 7.83 (s, 8 H, CH), 7.66–7.19 (m, 8 H, CH), 6.15–5.92 (m, 1 H, CH), 4.60–4.50 (m, 18 H, CH2), 4.25–4.10 (m, 48 H, CH2, CH), 4.01–3.24 (m, 88 H, CH2, CH), 3.01–2.91 (m, 16 H, CH2), 2.81–2.67 (m, 16 H, CH2), 2.57–2.44 (m, 16 H, CH2), 2.21–2.08 (m, 16 H, CH2), 1.31–1.12 (m, 30 H, CH3), 0.10 (s, 36 H, CH3); MS (MALDI-TOF, most abundant mass): m/z: calcd for C223H336N30O96Si4Na: 5108.1 [M+Na]+; found: 5108.3.
Dendrimer 30 (isomers)
Dendrimer 27 (13.0 mg, 2.6 μmol), azido-RGD peptide 8 (7.5 mg, 15.4 μmol) and CuF2 (2.1 mg, 20.8 μmol) were dissolved in MeOH:H2O mixture 1:1 v/v (0.5 mL). The reaction mixture was stirred for 40 h at 40°C and the solvent was evaporated. The residue was purified by HPLC (t = 23.7 min) to give after lyophilization 30 (10.0 mg, 57%) as a white foam. 1H NMR (500 MHz, D2O, 25°C, TMS): δ= 7.81 (s, 12 H, CH), 7.59–7.11 (m, 8 H, CH), 6.12–5.80 (m, 1 H, CH), 5.27 (s, 8 H, CH2), 4.73 (dd, 3J(H,H) = 7.2, 5.6 Hz, 4 H, CH), 4.52–4.43 (m, 18 H, CH2), 4.37–3.84 (m, 84 H, CH2, CH), 3.79–3.25 (m, 72 H, CH2, CH), 3.17 (t, 3J(H,H) = 6.8 Hz, 8 H, CH2), 2.99–2.83 (m, 32 H, CH2), 2.79–2.62 (m, 24 H, CH2), 2.21–2.06 (m, 16 H, CH2), 1.92–1.72 (m, 8 H, CH2), 1.70–1.56 (m, 8 H, CH2), 1.32–0.99 (m, 30 H, CH3); MS (MALDI-TOF, MW, linear mode): m/z: calcd for C275H413N74O124 [M+H]+ = 6739.6; found: 6720.7.
Supplementary Material
Acknowledgments
This research was supported by the National Cancer Institute of the US National Institutes of Health (Grant No. R01CA088986, G.-J.B.).
Footnotes
Supporting information is available.
References
- [1].a) Lee CC, MacKay JA, Fréchet JMJ, Szoka FC. Nat. Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]; b) Cho K, Wang X, Nie SM, Chen Z, Shin DM. Clin. Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- [2].Fox ME, Szoka FC, Fréchet JMJ. Accounts Chem. Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].D'Emanuele A, Attwood D. Adv. Drug Deliv. Rev. 2005;57:2147–2162. doi: 10.1016/j.addr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- [4].Menjoge AR, Kannan RM, Tomalia DA. Drug Discov. Today. 2010;15:171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- [5].Gajbhiye V, Kumar PV, Tekade RK, Jain NK. Curr. Pharm. Design. 2007;13:415–429. [Google Scholar]
- [6].Astruc D, Boisselier E, Ornelas C. Chem. Rev. 2010;110:1857–1959. doi: 10.1021/cr900327d. [DOI] [PubMed] [Google Scholar]
- [7].Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Jr., Holl MMB. Chem. Biol. 2007;14:107–115. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- [8].a) Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; Meldal M, Tornøe CW. Chem. Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]; c) Franc G, Kakkar AK. Chem. Soc. Rev. 2010;39:1536–1544. doi: 10.1039/b913281n. [DOI] [PubMed] [Google Scholar]; d Hein JE, Fokin VV. Chem. Soc. Rev. 2010;39:1302–1315. doi: 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Pieters RJ, Rijkers DTS, Liskamp RMJ. QSAR Comb. Sci. 2007;26:1181–1190. [Google Scholar]; b) Yim CB, Boerman OC, de Visser M, de Jong M, Dechesne AC, Rijkers DT, Liskamp RM. Bioconjug. Chem. 2009;20:1323–1331. doi: 10.1021/bc900052n. [DOI] [PubMed] [Google Scholar]
- [10].a) Chabre YM, Roy R. Curr. Top. Med. Chem. 2008;8:1237–1285. doi: 10.2174/156802608785848987. [DOI] [PubMed] [Google Scholar]; b) Wang SK, Liang PH, Astronomo RD, Hsu TL, Hsieh SL, Burton DR, Wong CH. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3690–3695. doi: 10.1073/pnas.0712326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Carlmark A, Hawker C, Hult A, Malkoch M. Chem. Soc. Rev. 2009;38:352–362. doi: 10.1039/b711745k. [DOI] [PubMed] [Google Scholar]
- [12].a) Caplan MR, Rosca EV. Ann. Biomed. Eng. 2005;33:1113–1124. doi: 10.1007/s10439-005-5779-1. [DOI] [PubMed] [Google Scholar]; b) Paleos CM, Tsiourvas D, Sideratou Z, Tziveleka L. Curr. Top. Med. Chem. 2008;8:1204–1224. doi: 10.2174/156802608785848996. [DOI] [PubMed] [Google Scholar]
- [13].Shi X, Majoros IJ, Patri AK, Bi X, Islam MT, Desai A, Ganser TR, Baker JR., Jr. Analyst. 2006;131:374–381. doi: 10.1039/b515624f. [DOI] [PubMed] [Google Scholar]
- [14].a) Steffensen MB, Simanek EE. Angew. Chem. Int. Ed. 2004;43:5178–5180. doi: 10.1002/anie.200460031. [DOI] [PubMed] [Google Scholar]; b) Goodwin AP, Lam SS, Fréchet JMJ. J. Am. Chem. Soc. 2007;129:6994–6995. doi: 10.1021/ja071530z. [DOI] [PubMed] [Google Scholar]; c) Antoni P, Hed Y, Nordberg A, Nyström D, von Holst H, Hult A, Malkoch M. Angew. Chem. Int. Ed. 2009;48:2126–2130. doi: 10.1002/anie.200804987. [DOI] [PubMed] [Google Scholar]
- [15].Feng X, Taton D, Ibarboure E, Chaikof EL, Gnanou Y. J. Am. Chem. Soc. 2008;130:11662–11676. doi: 10.1021/ja7103119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Goyal P, Yoon K, Weck M. Chem. Eur. J. 2007;13:8801–8810. doi: 10.1002/chem.200700129. [DOI] [PubMed] [Google Scholar]
- [17].Wu P, Malkoch M, Hunt JN, Vestberg R, Kaltgrad E, Finn MG, Fokin VV, Sharpless KB, Hawker CJ. Chem. Commun. 2005:5775–5777. doi: 10.1039/b512021g. [DOI] [PubMed] [Google Scholar]
- [18].Deguise I, Lagnoux D, Roy R. New J. Chem. 2007;31:1321–1331. [Google Scholar]
- [19].Ornelas C, Weck M. Chem. Commun. 2009:5710–5712. doi: 10.1039/b913139f. [DOI] [PubMed] [Google Scholar]
- [20].a) Agard NJ, Prescher JA, Bertozzi CR. J. Am. Chem. Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]; b) Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. J. Am. Chem. Soc. 2008;130:11486–11493. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ning XH, Guo J, Wolfert MA, Boons GJ. Angew. Chem. Int. Ed. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kele P, Mezö G, Achatz D, Wolfbeis OS. Angew. Chem. Int. Ed. 2009;48:344–347. doi: 10.1002/anie.200804514. [DOI] [PubMed] [Google Scholar]
- [22].a) Ihre H, Hult A, Frechet JMJ, Gitsov I. Macromolecules. 1998;31:4061–4068. [Google Scholar]; b) Malkoch M, Malmström E, Hult A. Macromolecules. 2002;35:8307–8314. [Google Scholar]
- [23].Padilla De Jesús OL, Ihre HR, Gagne L, Fréchet JM, Szoka FC., Jr. Bioconjug. Chem. 2002;13:453–461. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- [24].Our initial studies using lysine dendrons indicated low solubility of alkyne terminated dendrons which complicated purification
- [25].Iyer SS, Anderson AS, Reed S, Swanson B, Schmidt JG. Tetrahedron Lett. 2004;45:4285–4288. [Google Scholar]
- [26].Jewett JC, Bertozzi CR. Chem. Soc. Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Park KD, Liu R, Kohn H. Chem. Biol. 2009;16:763–772. doi: 10.1016/j.chembiol.2009.06.005. [DOI] [PubMed] [Google Scholar]
- [28].Ladmiral V, Mantovani G, Clarkson GJ, Cauet S, Irwin JL, Haddleton DM. J. Am. Chem. Soc. 2006;128:4823–4830. doi: 10.1021/ja058364k. [DOI] [PubMed] [Google Scholar]
- [29].a) Aucagne V, Leigh DA. Org. Lett. 2006;8:4505–4507. doi: 10.1021/ol061657d. [DOI] [PubMed] [Google Scholar]; b) Gramlich PME, Warncke S, Gierlich J, Carell T. Angew. Chem. Int. Ed. 2008;47:3442–3444. doi: 10.1002/anie.200705664. [DOI] [PubMed] [Google Scholar]
- [30].Friscourt F, Ledin PA, Boons GJ. 239th ACS National Meeting; San Francisco, CA, USA. March 21–25.2010. [Google Scholar]
- [31].Poloukhtine AA, Mbua NE, Wolfert MA, Boons GJ, Popik VV. J. Am. Chem. Soc. 2009;131:15769–15776. doi: 10.1021/ja9054096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dondoni A. Angew. Chem. Int. Ed. 2008;47:8995–8997. doi: 10.1002/anie.200802516. [DOI] [PubMed] [Google Scholar]
- [33].a) McKay CS, Moran J, Pezacki JP. Chem. Commun. 2010;46:931–933. doi: 10.1039/b921630h. [DOI] [PubMed] [Google Scholar]; b) Ning X, Temming RP, Dommerholt J, Guo J, Ania DB, Debets MF, Wolfert MA, Boons GJ, van Delft FL. Angew. Chem. Int. Ed. 2010;49:3065–3068. doi: 10.1002/anie.201000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.