Abstract
Purpose
In order to clarify the contemporary clinical epidemiology of renal cell carcinoma (RCC), we present trends in the clinical presentation and management of patients enrolled in a population-based case-control study.
Materials and Methods
The National Cancer Institute conducted a population-based case–control study in metropolitan Detroit and Chicago from 2002 through 2007. For 1,136 patients with RCC who consented to both an epidemiological interview and medical record review, we ascertained detailed information regarding social and medical history, methods of RCC detection and diagnosis, cancer severity, and treatment(s) received. From these data, we assessed the demographic and cancer-specific characteristics of study cases, as well as trends in their clinical presentation, diagnosis, and treatment.
Results
Most patients with RCC had localized or regional tumors, including 52% with tumor size ≤ 4 cm. The proportion of asymptomatic cases increased from 35% in 2002 to 50% in 2007 (p< 0.001). Hypertension (58%) and diabetes (17%) were common among cases, and 24% percent of patients had at least 2 significant comorbid conditions at the time of RCC diagnosis. While the use of laparoscopic surgery increased over time (p < 0.001), fewer than 1 in 5 patients received nephron-sparing surgery.
Conclusions
The proportion of patients presenting with small, asymptomatic renal cell carcinomas continues to increase. A majority of these cases are still treated with radical nephrectomy, although increasingly via a laparoscopic approach. Because most patients with small RCCs have one or more renal function-relevant comorbidities, there is an imperative to increase utilization of nephron-sparing surgery.
Keywords: Kidney cancer, epidemiology, comorbidity, nephron-sparing surgery
Introduction
National incidence rates for renal cell carcinoma (RCC) have risen steadily during the past two decades.1–4 This trend is mediated mainly by an increase in the frequency of patients with early-stage cancers, and disproportionately affects African-Americans.1,2 Seeking to better understand these epidemiological observations, the National Cancer Institute (NCI) initiated the United States Kidney Cancer Study (KCS).5 This population-based case-control study—which completed accrual in 2007—will elucidate specific genetic and environmental risk factors contributing to the incidence pattern of RCC, particularly among African-Americans. The current report presents demographic and cancer-specific characteristics of the cases enrolled in the KCS, and describes patterns in their clinical presentation and management. These data not only set the stage for forthcoming KCS analyses, but also provide unique and important insight regarding the clinical epidemiology of RCC in the twenty-first century.
Materials and Methods
Study overview
The KCS is a population-based case–control study conducted in the metropolitan areas of Detroit, Michigan (Wayne, Oakland, and Macomb Counties) and Chicago, Illinois (Cook County) from 2002 through 2007. Eligible cases for this study included resident Caucasian and African-American men and women aged 20 to 79 years, newly diagnosed with RCC from February 1, 2002 through July 31, 2007 in Detroit, or January 1, 2003 through December 31, 2003 in Chicago. In Detroit, potential cases were identified through the Metropolitan Detroit Cancer Surveillance System, an NCI Surveillance, Epidemiology, and End Results (SEER) program member. In Chicago, investigators identified potential cases from pathology reports issued at hospitals in Cook County and its adjacent communities.
Upon identification of an eligible case, KCS investigators contacted the physician of record to determine if the individual should not be contacted (e.g., deceased, too ill) regarding participation. In the absence of a “do not contact” recommendation, investigators contacted potential cases via postal mail. The recruitment letter explained that the study involved a 90-minute epidemiological interview, as well as the optional provision of saliva and blood samples. The letter also offered participants compensation for their time and effort. One to two weeks after the initial mailing, study coordinators placed follow-up phone calls to potential enrollees to answer questions, request participation, and schedule a time for the interview and specimen collections. For each participating case, KCS investigators obtained written informed consent at the time of the in-person interview. At this time, investigators also requested consent to access and review relevant medical records, pathology reports, and tissue samples. The study was approved by the Institutional Review Boards at all participating institutions.
During the study interval, KCS investigators identified 1,918 men and women diagnosed with RCC who resided in the two study regions. Among this sample, 1,217 (63.5%) and 1,136 (59.2%) consented to the interview or the interview and medical record review, respectively. The latter group (i.e., those patients consenting to both the interview and medical record review) represents the analytic cohort for the current report.
Study variables
For each case in the analytic cohort, KCS personnel ascertained demographic and clinical characteristics from the following sources: 1) medical record review; 2) participant interview; and 3) SEER tumor registry data (Detroit cases only). Patient demographics (e.g., age, sex), medical history (including presenting signs and symptoms and comorbidities), methods of RCC detection and diagnosis (e.g., imaging studies, biopsies), clinical and pathological characteristics of the kidney cancer, and treatment(s) received were abstracted from the medical record. The participant interview provided additional demographic information (e.g., marital status) and detailed medical history regarding multiple renal function-relevant comorbidities including diabetes, hypertension, urolithiasis, and chronic renal insufficiency. In contrast to the medical record abstraction, which assessed the presence or absence of specific conditions at the time of RCC diagnosis, the interview elicited lifetime history regarding the presence or absence of specific conditions two or more years prior to RCC diagnosis. For Detroit cases, we also used routinely-collected variables from the SEER program to ascertain additional cancer-specific information, including tumor size.
Using these data, we classified each case into one of three symptom categories at presentation: asymptomatic, local symptoms (i.e., flank or abdominal pain or mass, hematuria), or systemic symptoms (e.g., weight loss, night sweats). This categorization scheme has been used previously to demonstrate an association between extent of symptoms at diagnosis and long-term survival among patients with RCC.6,7 We also calculated a modified Charlson Comorbidity Index (includes the following chronic diseases: AIDS, cerebrovascular disease, invasive non-skin cancer other than RCC, chronic pulmonary disease, congestive heart failure, connective tissue disease, chronic renal failure, dementia, diabetes mellitus, liver cirrhosis, myocardial infarction, hemiplegia, peripheral vascular disease, and ulcer disease) for each case based on data from both the medical record review and patient interview.8 For analytic purposes, we also defined three categories of surgical treatments: 1) open radical nephrectomy (ORN); 2) laparoscopic radical nephrectomy (LRN); and 3) nephron-sparing surgery (NSS). The latter category includes both partial nephrectomy and energy-ablative therapies (performed by any approach).
Statistical analyses
As a first analytic step, we generated summary statistics describing both the demographic and cancer-specific characteristics of the KCS cases, as well as trends in their clinical presentation, diagnosis, and treatment. We then used Chi-squared testing to assess time trends in the frequency of asymptomatic presentation, and in the utilization of specific surgical therapies. Because there were relatively few Chicago cases, we also performed sensitivity analyses based only on the Detroit cases. All statistical testing was two-sided and performed at the 5% significance level (SAS v9.1, SAS Institute, Cary, NC).
Results
The analytic cohort comprised 1,136 RCC subjects who consented to and completed both the epidemiological interview and medical record review (Table 1). The average age at diagnosis was younger for the analytic cohort compared with the 762 cases who did not consent to both interview and medical record review (58.6 vs 60.9 years, p < 0.001); otherwise, the two groups were similar with respect to race (29% vs 32% African-American), gender (41% vs 38% female), study site (84% vs 83% Detroit) and year of diagnosis (all p-values > 0.10). For Detroit cases, moreover, the distributions of tumor stage, size, and histology were similar between the analytic cohort and the complete sample of identified cases (p-values > 0.10, data not shown).
Table 1.
Demographic and clinical characteristics of patients with RCC
| N (%) | |
|---|---|
| Total | 1,136 (100) |
| Demographic Characteristics | |
| Study site | |
| Detroit | 951 (84) |
| Chicago | 185 (16) |
| Year of diagnosis | |
| 2002 | 171 (15) |
| 2003 | 364 (32) |
| 2004 | 231 (20) |
| 2005 | 216 (19) |
| 2006/2007 | 154 (14) |
| Age at diagnosis (years) | |
| Mean (SD) | 58.6 (11.4) |
| Median (range) | 59 (26–79) |
| 20 – 44 | 139 (12) |
| 45 – 54 | 269 (24) |
| 55 – 64 | 355 (31) |
| 65 – 74 | 275 (24) |
| 75 – 79 | 98 (9) |
| Sex | |
| Male | 673 (59) |
| Female | 463 (41) |
| Race | |
| White | 810 (71) |
| Black | 326 (29) |
| Marital Status a | |
| Married | 703 (62) |
| Not married | 432 (38) |
| Primary insurance*,b | |
| Uninsured | 37 (3) |
| Public | 446 (40) |
| Private | 639 (57) |
| Educational attainment | |
| <= 11 years HS | 185 (16) |
| HS grad or equivalent | 395 (35) |
| 1–3 years college | 303 (27) |
| 4 years college or more | 253 (22) |
| Clinical Characteristics | |
| Body Mass Index c | |
| <25 | 224 (20) |
| 25 – <30 | 407 (36) |
| 30 – <35 | 276 (25) |
| 35+ | 218 (19) |
| Tumor size†,d | |
| < 4 cm | 483 (52) |
| 4 – 7 cm | 256 (27) |
| > 7 cm | 195 (21) |
| Tumor Stage e | |
| Local | 794 (81) |
| Regional | 143 (15) |
| Distant | 47 (5) |
| Tumor Histology | |
| Clear cell | 838 (74) |
| Papillary | 154 (14) |
| Chromophobe | 70 (6) |
| Cystic RCC | 54 (5) |
| Other histology | 20 (2) |
| Tumor Grade (Fuhrman Grade) f | |
| FG 1 | 125 (14) |
| FG 2 | 483 (53) |
| FG 3 | 247 (27) |
| FG 4 | 57 (6) |
Missing values:
1
14
11
4
17
152
g - 224
Public includes both Medicare and Medicaid
Tumor size data available only for Detroit patients
Among the analytic cohort, 951 (84%) and 185 (16%) cases accrued in the Detroit and Chicago study sites, respectively. A majority of KCS cases were white, married, insured, and younger than 65 years at the time of kidney cancer diagnosis (Table 1). In terms of clinical characteristics, most patients had localized or regional tumors, including more than 50% with tumor size ≤ 4 cm. Seventy four percent of patients had a classic clear cell RCC; patients with clear cell RCC (22%) were more likely than those with papillary (13%) or chromophobe (3%) tumors to have regional or distant disease at the time of diagnosis (p<0.01).
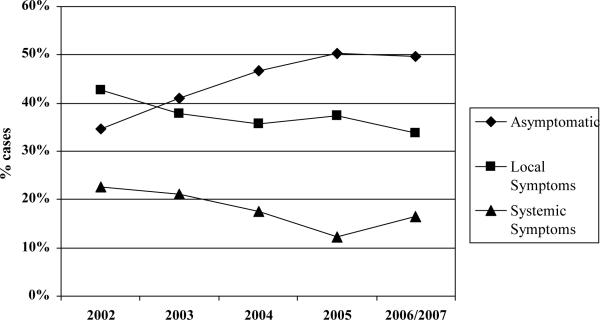
Figure 1 depicts the annual proportion of patients presenting with no, local, or systemic symptoms attributable to RCC. The proportion of cases with asymptomatic tumors increased progressively from 35% in 2002 to 50% in 2007 (Figure 1, p-value for trend < 0.001). In terms of diagnostic studies, computed tomography, magnetic resonance imaging and ultrasonography were employed as the sole pre-treatment imaging modality for 42%, 2%, and 2% of cases, respectively; 39% of cases underwent more than one imaging evaluation from the time of diagnosis through receipt of initial therapy. Only 14% of KCS cases underwent diagnostic biopsy of either the primary tumor or a suspected metastasis.
Figure 1.
Trends in the clinical presentation of patients with RCC by year of diagnosis *
* p-value for trend < 0.001; includes 6 cases from 2007
Among the analytic cohort, 24% had ≥ 2 of the comorbid conditions comprising the Charlson Index at the time of diagnosis (Table 2). A history of hypertension (58%) or diabetes (17%) were commonly reported by the KCS cases during the epidemiological interview, and 23% of cases reported having at least 2 prevalent conditions that may threaten long-term renal function (e.g., diabetes, urolithiasis, nephrotic syndrome).
Table 2.
Medical comorbidities among patients with RCC
| N (%) | |
|---|---|
| Overall Comorbidity‡ (Modified Charlson Index Score) | |
| 0 | 465 (49) |
| 1 | 255 (27) |
| 2 | 141 (15) |
| 3+ | 90 (9) |
| History of Renal-Relevant Comorbidities § | |
| Hypertension | 661 (58) |
| Diabetes mellitus | 190 (17) |
| Renal insufficiency a | 47 (4) |
| Urolithiasis b | 116 (10) |
| Analgesic nephropathy c | 3 (0.3) |
| Nephrotic syndrome | 31 (3) |
| Pyelonephritis d | 38 (3) |
| History of prior dialysis | 35 (3) |
| History of kidney transplant | 15 (1) |
| Count Of Renal-Relevant Comorbidities | |
| 0 | 393 (35) |
| 1 | 485 (43) |
| 2 | 180 (16) |
| 3+ | 78 (7) |
Charlson Index Score calculated based on medical record review, available only for Detroit cases
Based on participant self-report during epidemiological interview; specific comorbidities reported to be present at least 2 years prior to interview
Missing values:
1
5
1
5
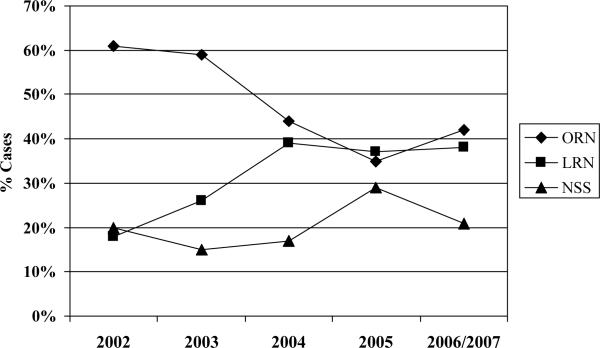
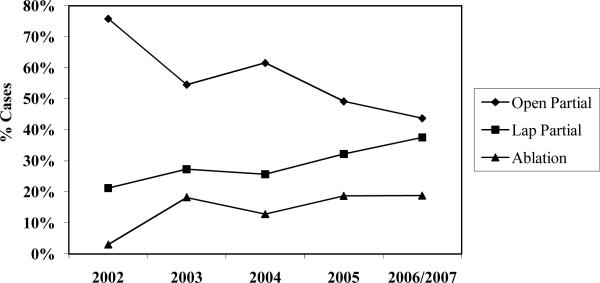
Ninety-six percent (n=1,093) of cases underwent surgical therapy for RCC. Nearly three in four patients underwent radical nephrectomy as primary surgical therapy; the proportion of radical nephrectomies performed laparoscopically increased significantly during the study interval (p<0.001), plateauing at approximately 40% of cases from 2004 through 2006 (Figure 2). The proportion of patients treated with nephron-sparing surgery was 20% or lower for each year in the study interval except 2005 (29%) (Figure 2). Among Detroit cases treated with a nephron-sparing surgical approach (n=185), the proportion of patients treated with laparoscopic partial nephrectomy increased from 21% in 2002 to 38% in 2006 (Figure 3, p=0.005). Our principal findings did not change substantively in sensitivity analyses based only on Detroit cases.
Figure 2.
Surgical treatments for patients with RCC by year of diagnosis *
* p-value for trend in laparoscopic radical nephrectomy < 0.001; includes 6 cases from 2007
Abbreviations: ORN: open radical nephrectomy; LRN: laparoscopic radical nephrectomy; NSS: nephron-sparing surgery
Figure 3.
Distribution of nephron-sparing surgical treatments for Detroit patients by year of diagnosis *
* p-value for trend < 0.005; includes 2 cases from 2007
Discussion
We describe the demographic and clinical characteristics of patients with RCC who enrolled in the population-based U.S. Kidney Cancer Study. Thirty percent of KCS cases were African-American, and a majority had small (i.e., ≤ 4 cm), localized renal cell carcinomas diagnosed before 65 years of age. Additional demographic and cancer-specific characteristics of KCS cases are similar to those reported for other large samples of patients with RCC,9–11 supporting the generalizability of this and future KCS analyses.
The principal findings from the current analysis provide important insight regarding the contemporary clinical epidemiology of RCC. For instance, these data provide population-based confirmation that the proportion of patients presenting with asymptomatic RCC increased steadily during the early 21st century. As described previously, this trend reflects—at least partially—the ability of advanced medical imaging to detect tumors well before the manifestation of clinical signs or symptoms.11–13 Although patients with asymptomatic RCC enjoy more favorable survival outcomes following surgical therapy,14 recent data suggest that many incidentally-detected tumors follow an indolent clinical course and that expedient surgical removal may therefore not be imperative in all cases.15–17 In this context, the increasing frequency of small, asymptomatic tumors among the population-based KCS sample highlights the urgent need for additional studies that clarify optimal treatment algorithms for patients with early-stage kidney cancer, including the comparative effectiveness of excisional, ablative, and expectant treatment options.18
Because we assessed comorbidity via patient self-report and detailed medical record review (rather than administrative or claims data), our findings also clarify more precisely the burden of concurrent medical disease among contemporary patients with RCC. We observed that 15% of patients have at least two significant comorbidities at the time of RCC diagnosis. Once long-term survival outcomes are available for this patient cohort, these detailed comorbidity data may prove invaluable to understanding and predicting competing causes of death among patients considering surgical treatment for kidney cancer.15,19 More immediately, this information informs ongoing debates surrounding the appropriate treatment intensity (versus expectant approaches) for populations of patients with early-stage kidney cancer.16,20,21
Furthermore, by characterizing accurately the non-trivial prevalence of diabetes (17%), hypertension (58%), and other renal function-relevant comorbidities (23%) among patients with kidney cancer, our findings provide essential context for efforts aimed at improving surgical practice patterns. Namely, there is growing consensus that nephron-sparing surgery represents optimal therapy for patients with RCC who are at heightened risk for chronic kidney disease, including those with hypertension and/or diabetes.18,22,23 Thus, while the increasing utilization of minimally-invasive kidney cancer surgery seems encouraging at first glance, enthusiasm for this trend is tempered by the concern that some patients who underwent laparoscopic radical nephrectomy (especially those with tumors ≤ 4 cm) may have been better served by a nephron-sparing intervention. In particular, our finding that—even in 2006—nearly 80% of patients with kidney cancer underwent total kidney removal underscores the presence of persistent barriers to surgeons' adoption of nephron-sparing interventions.24 Accordingly, future KCS analyses will seek to clarify further the potential influence of providers and their practice environments on initial treatment choices among patients with early-stage kidney cancer.
The primary limitation of this study relates to its potential generalizability to the larger population of patients with RCC in the United States. Our report includes data only for African-Americans and Caucasians; the clinical epidemiology of RCC may differ among other race/ethnic groups in the United States. Moreover, despite being population-based, the data reported herein are limited to cases diagnosed in metropolitan Chicago or Detroit, raising the possibility that our findings are not representative of patients with RCC in other geographic locations. An additional limitation is that our case cohort includes only patients with pathologically-confirmed RCC. As a result, our estimates of symptoms at presentation may be biased by the exclusion of patients with small renal masses (mainly small RCCs) who choose expectant management and who may be more likely to be asymptomatic. Finally, not all of the contacted cases consented to study participation, making our analyses susceptible to possible selection biases (although we noted no substantial differences in the measured characteristics of participants and non-participants). These limitations notwithstanding, this report advances our understanding of the contemporary clinical epidemiology of RCC. Moreover, forthcoming KCS analyses will provide additional insight regarding the diagnosis, treatment, and survival of this unique patient cohort.
Conclusions
The proportion of patients presenting with small, asymptomatic renal cell carcinomas continues to increase. A majority of these cases are still treated with radical nephrectomy, although increasingly via a laparoscopic approach. Because most patients with small RCCs have one or more renal function-relevant comorbidities, there is an imperative to increase utilization of nephron-sparing surgery.
Acknowledgments
This work was supported by the National Institutes of Health (NIH- N02-CP-11004); and the Edwin Beer Research Fellowship in Urology and Urology-Related Fields from the New York Academy of Medicine to D.C.M.
References
- 1.Chow WH, Devesa SS, Warren JL, et al. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 2.Vaishampayan UN, Do H, Hussain M, et al. Racial disparity in incidence patterns and outcome of kidney cancer. Urology. 2003;62:1012. doi: 10.1016/j.urology.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Hock LM, Lynch J, Balaji KC. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J Urol. 2002;167:57. [PubMed] [Google Scholar]
- 4.Wallen EM, Pruthi RS, Joyce GF, et al. Kidney cancer. J Urol. 2007;177:2006. doi: 10.1016/j.juro.2007.01.126. [DOI] [PubMed] [Google Scholar]
- 5.Colt JS, Wacholder S, Schwartz K, et al. Response rates in a case-control study: effect of disclosure of biologic sample collection in the initial contact letter. Ann Epidemiol. 2005;15:700. doi: 10.1016/j.annepidem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Patard JJ, Leray E, Cindolo L, et al. Multi-institutional validation of a symptom based classification for renal cell carcinoma. J Urol. 2004;172:858. doi: 10.1097/01.ju.0000135837.64840.55. [DOI] [PubMed] [Google Scholar]
- 7.Patard JJ, Leray E, Rodriguez A, et al. Correlation between symptom graduation, tumor characteristics and survival in renal cell carcinoma. Eur Urol. 2003;44:226. doi: 10.1016/s0302-2838(03)00216-1. [DOI] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Miller DC, Hollingsworth JM, Hafez KS, et al. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006;175:853. doi: 10.1016/S0022-5347(05)00422-2. [DOI] [PubMed] [Google Scholar]
- 10.Scoll BJ, Wong YN, Egleston BL, et al. Age, tumor size and relative survival of patients with localized renal cell carcinoma: a surveillance, epidemiology and end results analysis. J Urol. 2009;181:506. doi: 10.1016/j.juro.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 12.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51:203. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 13.Parsons JK, Schoenberg MS, Carter HB. Incidental renal tumors: casting doubt on the efficacy of early intervention. Urology. 2001;57:1013. doi: 10.1016/s0090-4295(01)00991-8. [DOI] [PubMed] [Google Scholar]
- 14.Patard JJ, Dorey FJ, Cindolo L, et al. Symptoms as well as tumor size provide prognostic information on patients with localized renal tumors. J Urol. 2004;172:2167. doi: 10.1097/01.ju.0000141137.61330.4d. [DOI] [PubMed] [Google Scholar]
- 15.Russo P, Jang TL, Pettus JA, et al. Survival rates after resection for localized kidney cancer: 1989 to 2004. Cancer. 2008;113:84. doi: 10.1002/cncr.23520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 17.Jewett MA, Zuniga A. Renal tumor natural history: the rationale and role for active surveillance. Urol Clin North Am. 2008;35:627. doi: 10.1016/j.ucl.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 18.American Urological Association: Guideline for management of the clinical stage I renal mass. 2009 [Google Scholar]
- 19.Hollingsworth JM, Miller DC, Daignault S, et al. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007;109:1763. doi: 10.1002/cncr.22600. [DOI] [PubMed] [Google Scholar]
- 20.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma--a meta-analysis and review. J Urol. 2008;179:1227. doi: 10.1016/j.juro.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Mattar K, Jewett MA. Watchful waiting for small renal masses. Current Urology Reports. 2008;9:22. doi: 10.1007/s11934-008-0006-3. [DOI] [PubMed] [Google Scholar]
- 22.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo P, Huang W. The medical and oncological rationale for partial nephrectomy for the treatment of T1 renal cortical tumors. Urol Clin North Am. 2008;35:635. doi: 10.1016/j.ucl.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Miller DC, Saigal CS, Banerjee M, et al. Diffusion of surgical innovation among patients with kidney cancer. Cancer. 2008;112:1708. doi: 10.1002/cncr.23372. [DOI] [PMC free article] [PubMed] [Google Scholar]