Abstract
Ovarian cancer is mainly confined in peritoneal cavity and its metastasis is often associated with the formation of malignant ascites. As lysophosphatidic acid (LPA) is present at high levels in ovarian cancer patients’ ascites and potently stimulates cell migration, we reason that LPA-stimulated cell migration may play an important role in ovarian cancer metastasis. Here, we show that only those ovarian cancer cell lines with LPA migratory response undergo peritoneal metastatic colonization. LPA-stimulated cell migration is required for metastatic colonization because knockdown of LPA receptor 1 (LPAR1) abolishes this event. However, the difference in metastatic potentials is not caused by the absence of LPAR1 because both metastatic and non-metastatic lines express similar level of LPAR1. Instead, we find that LPA can only activate Rac in metastatic cells and that metastatic colonization of ovarian cancer cells necessitates Rac activity. These results thus suggest that LPA-induced Rac activation is a prerequisite for ovarian cancer metastasis. In metastatic cells, Rac activation is facilitated by SOS1/EPS8/ABI1 tri-complex and the integrity of this tri-complex is essential for LPA-stimulated cell migration and metastatic colonization. We show that at least one member of SOS1/EPS8/ABI1 tri-complex is absent in non-metastatic ovarian cancer cells and re-expressing the missing one conferred them with metastatic capability. Importantly, co-expression of SOS1, EPS8 and ABI1, but not the expression of any individual member of SOS1/EPS8/ABI1 tri-complex, correlates with advanced stages and shorter survival of ovarian cancer patients. Our study implicates that the integrity of SOS1/EPS8/ABI1 tri-complex is a determinant of ovarian cancer metastasis.
Keywords: Rac, cell migration, ovarian cancer metastasis
INTRODUCTION
Ovarian cancer has the highest mortality rate among gynecological cancers mainly due to complication of metastasis (1). Unlike other solid tumors that rely on the vasculature for metastasis, ovarian cancer is predominantly confined within the abdominal cavity and spread by direct extension to adjacent organs and/or disseminate throughout the peritoneal cavity (2, 3). A widely recognized behavior of ovarian cancer is its ability to seed the abdominal cavity with tumor implants, subsequent migration of tumor cells into peritoneum and underlying organs, and the formation of ascites (3, 4). Apparently, the ability of ovarian cancer cells to migrate is essential for ovarian cancer metastasis.
Cell migration is a complex process involving the reorganization of actin cytoskeleton that is facilitated by the members of the Rho GTPases including Rac, Cdc42 and Rho (5–7). In the process of cell migration, Rac facilitates the formation of actin-rich membrane ruffle, called lamellipodia, at the leading edge of migrating cells. Cdc42 regulates cell polarity and filopodia formation, thus controlling the direction of cell movement. Rho controls the formation of stress fibers and maintains focal adhesions at the rear of the cells (6). Ras has also been implicated to play an important role in cell migration because microinjection of Ras neutralizing antibody blocks cell migration (7) and forced expression of constitutively active Ras mutant enhances ovarian cancer cell migration (8). As Ras can activate Rac through either Tiam1 (9), βPIX (10) or SOS1/EPS8/ABI1 tri-complex (11, 12), it is likely that Ras may facilitate cell migration by regulating Rac activity.
Lysophosphatic acid (LPA) is a growth factor-like phospholipid and has been uniquely linked to ovarian malignancies. For example, LPA triggers protease production/activation (13, 14) and Cox-2 expression (15), thus facilitating ovarian cancer cell invasion. LPA also promotes angiogenesis by inducing the expression of various proangiogenic factors including VEGF (16), IL8 (17) and Groα (18). Moreover, LPA can be produced and secreted into peritoneal cavity by both ovarian cancer cells (19, 20) and mesothelial cells (21). Importantly, LPA is present at high levels in ovarian cancer patients’ ascites (22–25) and potently stimulates ovarian cancer cell migration (8, 26, 27). Therefore, ascite-borne LPA is likely to play a critical role in ovarian cancer metastasis by facilitating cell migration.
In this study, we demonstrated an excellent correlation between LPA migratory responses and metastatic potentials in a panel of ovarian cancer cell lines. Preventing LPA-stimulated cell migration by silencing LPAR1 diminished peritoneal metastatic colonization of ovarian cancer cells, implicating the importance of LPA-stimulated cell migration in ovarian cancer metastasis. LPA activates Rac only in metastatic cells and a signaling pathway consisting of Ras-SOS1/EPS8/ABI1 tri-complex mediates LPA-induced Rac activation. Interestingly, one or more members of SOS1/EPS8/ABI1 tri-complex is absent in non-metastatic ovarian cancer cells; however, re-expressing the missing member converts the non-metastatic lines to metastatic ones. Finally, we show that SOS1/EPS8/ABI1 co-expression, but not any one alone, correlates to advanced clinical stage and shorter survival of ovarian cancer patients.
MATERIALS AND METHODS
Cells, shRNAs and other reagents
All cells were maintained in DMEM containing 10% FCS at 37°C in a humidified incubator supplied with 5% CO2. The shRNA sequences for each target genes were designed using web-based Invitrogen Block-It program and subcloned into pLV-shRNA vector (Biosettia). Information for shRNA sequences, antibodies and other reagents are described in Supplementary Data.
Transwell cell migration assay
Transwell cell migration was assayed as previously described (28). Briefly, the lower phase of transwell was coated with 10µg/ml collagen I or laminin and serum-free medium with or without 10µM LPA was added to lower chambers. Serum-starved cells (105/well) were added to transwells and allowed to migrate for 4 hrs. Cells remained in transwells were removed with cotton swabs and cells attached on the lower phase of transwells were stained with crystal violet solution. The stained cells were solubilized with 10% acetic acid and quantitated on a microplate reader at 600 nm. To determine the involvement of Rac in cell migration, cells were infected with a lentiviral vector containing Rac1G12V or Rac1T17N for 2 days and then starved for 2 days followed by the analysis of cell migration. To determine the effect of silencing or forcing LPAR1, SOS1, EPS8 and ABI1 expression on cell migration, cells were infected with lentiviral vectors encoding either shRNAs or cDNAs of LPAR1, SOS1, EPS8 and ABI1 for 2 days and then starved for 2 days followed by the analysis of cell migration.
Peritoneal Metastatic Colonization Assay
Peritoneal metastatic colonization assays were performed as previously described (29, 30). Briefly, cells in the log-phase were trypsinized and resuspended in PBS. Six-week-old athymic female nude mice (Hsd:Athymic Nude-Foxn1nu, Harlan Spague Dawley) were intraperitoneally injected with 107 cells/mouse. To determine the role of LPAR1, SOS1, EPS8 and ABI1 in metastatic colonization, SKOV3 and HEY cells expressing shRNAs against those genes were intraperitoneally injected into mice. To determine the importance of Rac activity, cells expressing constitutively active Rac1 (Rac1G12V) or dominant negative Rac1 (Rac1T17N) were injected into mice. Five weeks after injection, the mice were sacrificed and autopsied. Visible metastatic implants were also collected and weighed. All procedures were approved by the Institution Animal Care Committee at Medical College of Georgia.
QRT-PCR
Total RNA was extracted from cells using Trizol (Invitrogen), treated by DNaseI and reverse transcribed with SuperScriptase II (Invitrogen). Generated cDNA was subjected to real-time PCR to measure LPAR1, LPAR2, LPAR3 and GAPDH levels with the respective TaqMan probes (Applied Biosystem). The expression levels were standardized by comparing the Ct values of target to that of GAPDH.
Rac, Cdc42 and Rho Activity Assays
Activation of Rac/Cdc42 and Rho was measured with the Rac/Cdc42 and the Rho activity assay kits (Cellbio Labs). To determine the effect of LPA on Rac, Cdc42 or RhoA activity, cells (2 × 106 cells/10-cm dish) were serum-starved for two days and then stimulated with 10µM LPA for various times. Cells were lysed and analyzed for Rac, Cdc42 or RhoA activity. To determine the effect of constitutively active Ras on Rac activity, HEY, IGROV1, OVCAR3 and SK-OV3 cells were infected with empty or H-RasG12V-containing retrovirus for 2 days followed by 2-day puromycin selection. Cells were lysed and analyzed of Rac activity. To determine how silencing Tiam1, SOS1, EPS8 and ABI1 affected LPA-induced Rac activation, HEY and SK-OV3 cells were incubated with lentiviral vectors containing the respective shRNAs for 2 days and starved for another 2 days. Cells were stimulated with 10µM LPA for 2 or 5 min followed by the analysis of Rac activity. To determine the effect of forced SOS1, EPS8 and ABI1 expression on LPA-induced Rac activation, TOV21G, IGROV1 and OVCAR3 cells were respectively infected with lentiviral vectors encoding SOS1, EPS8 and ABI1 for 2 days and starved for another 2 days. Cells were stimulated with 10µM LPA for 5 min followed by the analysis of Rac activity.
Immunofluorescence
Cells were cultured on 10µg/ml collagen I-coated coverslips overnight in serum-free condition and then treated with 10µM LPA for 30 min. Cells were fixed with 3% paraformadehyde and permeabilized with 1% Triton X-100 followed by 1-hr incubation with rhodomine-conjugated phalloidin. The polymerized actin were visualized by fluorescence microscope (Axiovert 200M, Zeiss).
Histochemistry
Specimens were collected from 64 epithelial ovarian cancer patients who underwent initial treatment at Zhongnan Hospital of Wuhan University from July 2002 to July 2006 (Their clinicopathological parameters were described in TABLE S1). Patients were followed up until November 2009. Overall survival was defined from initial treatment until cancer-related death or the last follow-up. Samples were processed in compliance with a protocol approved by the Ethics Committee of Wuhan University. All experiments conformed to the legal mandates implemented in China and written informed consent was received from each patient. The immunohistochemistry of SOS1, EPS8 and ABI1 was performed as previously described (31). Briefly, 4µm-thick sections of formalin-fixed/paraffin-embedded were deparaffinized and rehydrated. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide treatment. Sections were blocked with rabbit serum and incubated with antibodies against SOS1, EPS8 or ABI1. Antigens were visualized by streptavidin-biotin-peroxidase complex method. Immunostaining was evaluated by two pathologists without knowledge of patients’ clinical information. All three antigens were found to be localized in cytoplasm. Extent of immunostaining was graded based on the percentage of cells displaying staining. “−” is considered as negative staining (<10); “+”, “++” and “+++” were considered as positive staining (10–25%, 25–50% and >50% respectively).
Statistical Analysis
Statistical analyses of cell migration, metastatic implant weights and LPAR1 mRNA levels were performed by ANOVA and student t test. Chi-square test and Fisher's exact test were used to compare covariates between SOS1/EPS8/ABI1 staining and clinicopathological parameters. Survival curves were plotted according to the estimate of Kaplan and Meier. The log–rank test was used to determine the significance of differences in survival distribution. Statistical analyses were aided by SPSS (release 15.0; SPSS Inc). All statistical tests were two-sided and P < 0.05 was considered to be significant.
RESULTS
LPA migratory response is associated with ovarian cancer metastatic colonization
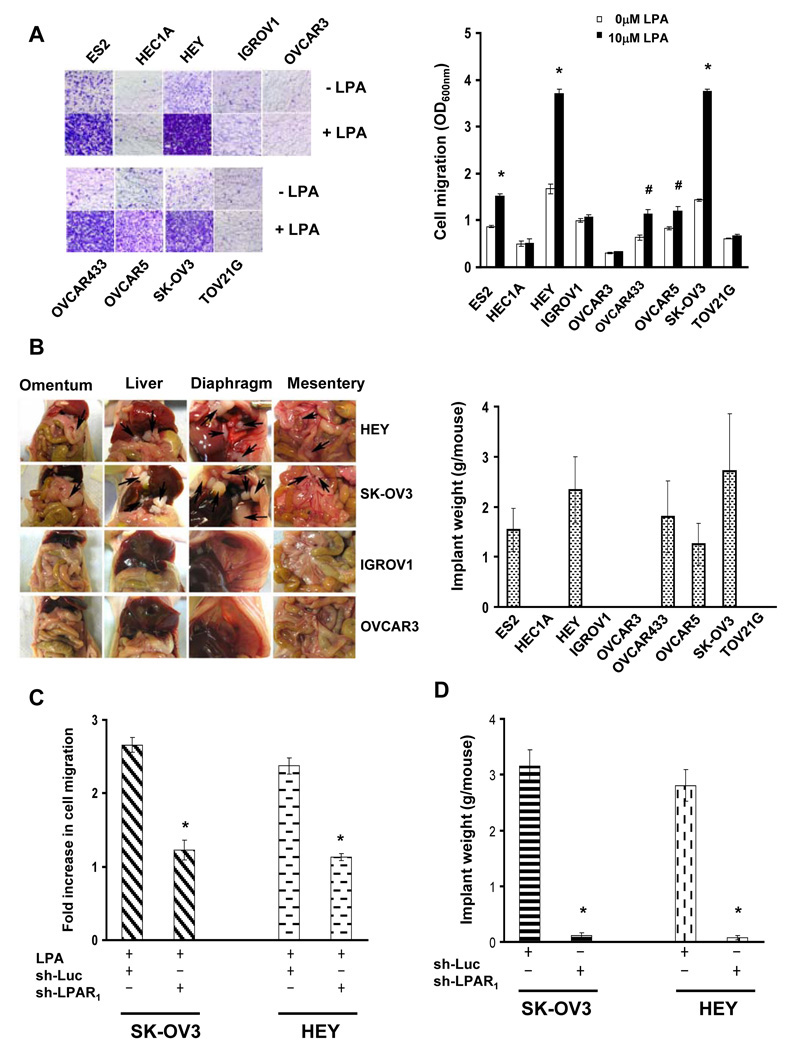
The fact that LPA levels are elevated in ovarian cancer patients’ ascites and LPA serves as a motility stimulator prompted us to hypothesize that LPA-stimulated cell migration is necessary for ovarian cancer metastasis. To test it, we first determined whether LPA migratory responses correlated to metastatic potentials in ovarian cancer cells. Transwell migration assay showed that LPA increased cell migration in ES2, HEY, OVCAR433, OVCAR5 and SK-OV3 lines on both collagen I- and laminin-coated surfaces but did poorly in HEC1A, IGROV1, OVCAR3 and TOV21G lines (Fig.1A and S1). With the aid of a well-established peritoneal seeding model (29, 30), we found that animals receiving LPA-responsive lines (ES2, HEY, OVCAR433, OVCAR5 and SK-OV3) displayed overt metastatic implants on omentum, liver, diaphragm and mesentery (Fig.1B and S2). In contrast, metastatic colonization was not detected in animals receiving LPA-unresponsive lines (HEC1A, IGROV1, OVCAR3 and TOV21G) (Fig.1B and S2). These results show that LPA migratory response correlates to metastatic potential in ovarian cancer cells.
Fig.1. LPA-stimulated cell migration and metastatic colonization of ovarian cancer cells.
A. Cell migration was analyzed using Transwells with or without 10µM LPA contained in the lower chambers. The lower phase of transwells was coated with 10µg/ml Collagen I. Images are stained cells on the lower phases. Cell migration is presented as the OD600nm. Data are means ± SE. n=3. *, P < 0.001 vs 0µM LPA; #, P < 0.05 vs 0µM LPA. B. Cells from various ovarian cancer lines were intraperitoneally injected to nude mice for 5 weeks to allow metastatic colonization. Images are the views of various areas in peritoneal cavity. Arrows point to metastatic implants. Metastatic implants were collected and weighed. Data are means ± SE. n=6. C. SK-OV3 and HEY cells were lentivirally transduced with luciferase or LPAR1 shRNA and subsequently analyzed for LPA-stimulated cell migration. Results are presented as Fold increase of cell migration [(LPA-stimulated cell migration)/(basal cell migration)]. Data are means ± SE. n=3. *, P < 0.001 vs sh-Luc. D. Control or LPAR1-knockdown cells were intraperitoneally injected to nude mice for 5 weeks. Metastatic implants were collected and weighed. Data are means ± SE. n=6. *, P < 0.001 vs sh-Luc.
LPA receptor subtype 1 (LPAR1) is known to mediate LPA-stimulated cell migration in various cell types including ovarian cancer cells (32–34). We lentivirally introduced LPAR1, LPAR2 or LPAR3 shRNA into metastatic SK-OV3 and HEY cells and confirmed that these shRNAs specifically reduced their respective target expression (Fig.S3). Knockdown of LPAR1 greatly inhibited LPA-stimulated cell migration in SK-OV3 and HEY cells (Fig.1C) while LPAR2 shRNA displayed slight inhibition and LPAR3 shRNA exhibited no effect on LPA-stimulated cell migration (Fig.S3). In the following experiment, we intraperitoneally injected control (luciferase shRNA) or LPAR1-knockdown cells into nude mice. Five weeks later, mice receiving control cells developed metastatic implants in their peritoneal cavities. However, mice receiving LPAR1-knockdown cells had much less metastatic colonization and the weight of implants was reduced over 90% in both SK-OV3 and HEY cells (Fig.1D and S4). These results suggest that LPA-stimulated cell migration is required for ovarian cancer metastasis.
Rac is activated by LPA only in metastatic ovarian cancer cells and required for metastatic colonization
To determine what caused the difference in LPA migratory responses between metastatic and non-metastatic cells, we initially measured the levels of LPAR1 mRNA in these lines. Except HEC1A that did not have detectable LPAR1 mRNA, metastatic and non-metastatic lines exhibited similar LPAR1 mRNA levels (Fig.S5). Subsequently, we lentivirally overexpressed LPAR1 in IGROV1, OVCAR3 and SK-OV3 cells. Although SK-OV3 cells with LPAR1 overexpression displayed greater LPA migratory response than the parental cells, enforced LPAR1 expression was unable to render non-metastatic IGROV1 and OVCAR3 cells responding to LPA for cell migration or undergoing metastatic colonization (Fig.S5). These results indicate that the defective LPA migratory response in non-metastatic lines occurs downstream of LPAR1.
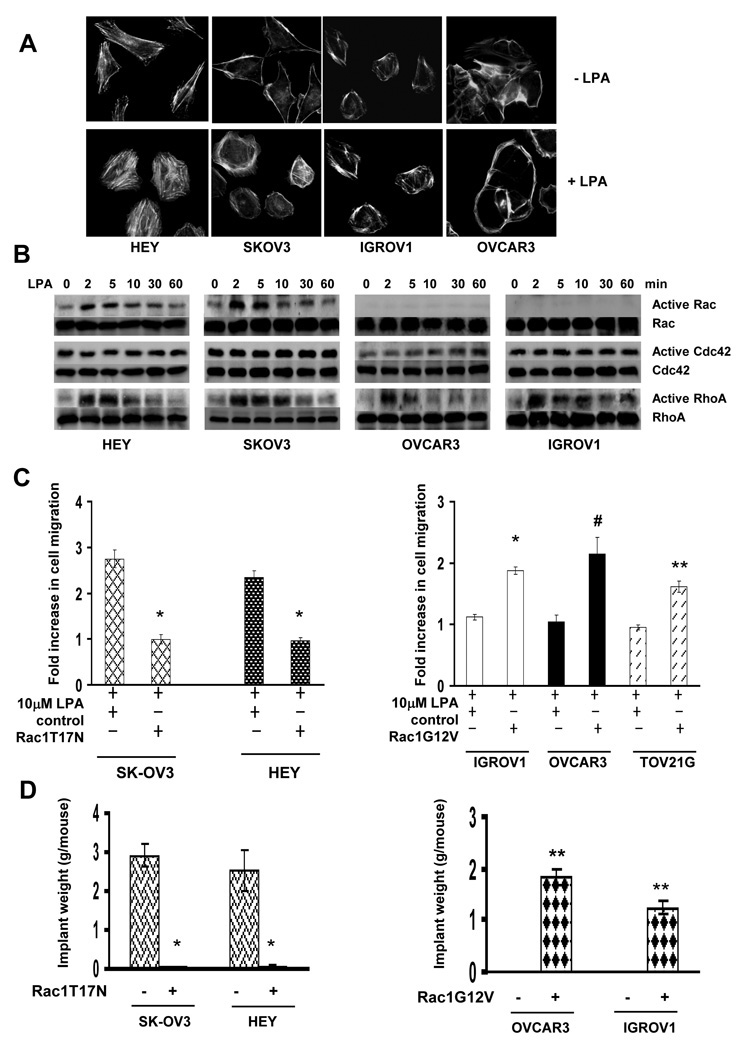
Because of the critical role of cytoskeleton reorganization in cell migration (26), we next examined the effect of LPA on cytoskeleton reorganization. Cells were treated with LPA followed by staining with rhodamine-conjugated phalloidin to detect F-actin. F-actin was observed on the plasma membrane of unstimulated cells (Fig.2A). Exposure to LPA led to dramatic actin reorganization in metastatic HEY and SK-OV3 cells but did little in non-metastatic IGROV1 and OVCAR3 cells (Fig.2A). As cytoskeleton reorganization is facilitated by Rac, Cdc42 and Rho (5), we examined the effect of LPA on their activities in these lines. Rho was similarly activated by LPA in all lines (Fig.2B). Cdc42 was slightly activated by LPA in OVCAR3 cells and unaffected in other lines (Fig.2B). In contrast, LPA significantly activated Rac in metastatic HEY and SK-OV3 cells but not in non-metastatic IGROV1 and OVCAR3 cells (Fig.2B). These results suggest that the inability of LPA to activate Rac may account for lacking LPA-induced cytoskeleton reorganization and cell migration in non-metastatic cells.
Fig.2. Rac regulates LPA-stimulated cell migration and metastatic colonization.
A. Cells were stimulated with 10µM LPA for 30 min followed by immunostaining with rhodamine-conjugated phalloidin. Cytoskeleton reorganization was visualized under a fluorescence microscope. B. Cells were stimulated with 10µM LPA for various times and then analyzed for Rac, Cdc42 and RhoA activities. C. SK-OV3 and HEY cells were lentivirally transduced with empty vector (control) or vector containing Rac1T17N while IGROV1, OVCAR3 and TOV21G cells were lentivirally transduced with empty vector (control) or vector containing Rac1G12V. Transduced cells were analyzed for LPA-stimulated cell migration. Results are presented as Fold increase of cell migration [(LPA-stimulated cell migration)/(basal cell migration)]. Data are means ± SE. n=3. *, P < 0.001 vs control; **, P < 0.005 vs control; #, P < 0.05 vs control. D. Rac1T17N-expressing SK-OV3, HEY or Rac1V12G-expressing OVCAR3 and IGROV1 cells were injected into nude mice. Five weeks after injection, metastatic implants were collected and weighed. Data are the mean ± SE. n=6. *, P < 0.001 vs control; **, P < 0.005 vs control.
To further investigate the association between Rac and LPA-stimulated cell migration, we inhibited Rac activity by introducing dominant negative Rac1 (Rac1T17N) in metastatic SK-OV3 and HEY cells. Forced Rac1T17N expression completely abolished LPA-stimulated cell migration in these cells (Fig.2C). In parallel, we expressed constitutively active Rac1 (Rac1G12V) in non-metastatic IGROV1, OVCAR3 and TOV21G lines and found that enforced Rac1G12V expression rendered them responsive to LPA for cell migration (Fig.2C).
We next injected dominant negative Rac1 (Rac1T17N)-expressing HEY or SK-OV3 cells into mice and found that Rac1T17N expression eliminated 100% and 98.35% metastatic colonization in mice respectively (Fig.S6A and 2D). Meanwhile, we injected constitutively active Rac1 (Rac1G12V)-expressing IGROV1 and OVCAR3 cells into mice. While there was no detectable metastatic implant in mice receiving control cells, implants were readily seen in mice receiving Rac1G12V-expressing cells (Fig.S6B and 2D). These results implicate that Rac is a key metastasis regulator.
SOS1/EPS8/ABI1 tri-complex is involved in LPA-induced Rac activation and metastatic colonization
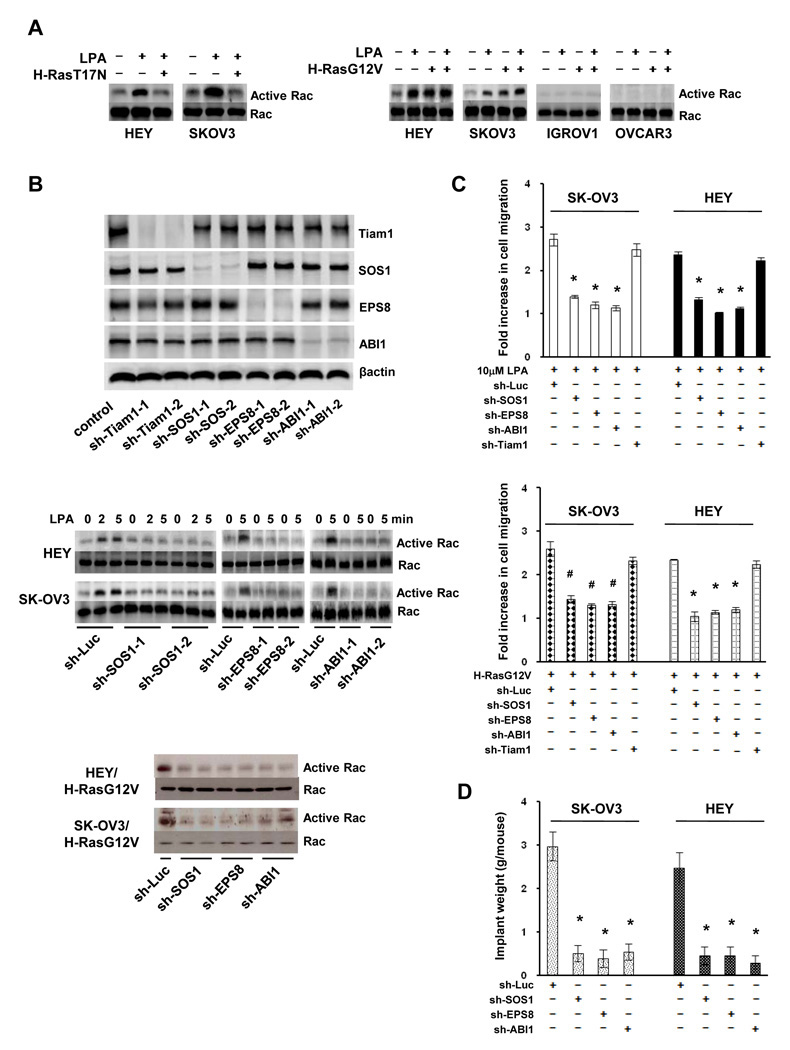
To characterize the signaling pathway mediating LPA-induced Rac activation, we turned our attention on Ras as LPA can activate Ras in metastatic ovarian cancer cells (8, 26) and Ras can activate Rac in various cell types (9, 11). We introduced dominant negative H-Ras (H-RasT17N) into HEY and SK-OV3 cells and found that inhibiting Ras activity abrogated LPA-induced Rac activation (Fig.3A), suggesting that Ras mediates LPA-induced Rac activation. We next treated non-metastatic IGROV1 and OVCAR3 cells with LPA and observed that LPA effectively activated Ras in both lines (Fig.S7), ruling out the possibility that inability of LPA to activate Ras led to the defect in Rac activation. In further experiment, we introduced constitutively active Ras (H-RasG12V) into ovarian cancer cells. H-RasG12V elevated Rac activity in metastatic HEY and SK-OV3 cells and LPA treatment did not further increase Rac activity (Fig.3A). In contrast, H-RasG12V was unable to activate Rac in non-metastatic IGROV1 or OVCAR3 cells (Fig.3A). These results suggest that Ras can only activate Rac in metastatic ovarian cancer cells.
Fig.3. SOS1/EPS8/ABI1 tri-complex participates in LPA-stimulated cell migration and metastatic colonization.
A. HEY, SK-OV3, IGROV and OVCAR3 cells were transduced with empty vector or retroviral vector containing H-RasT17N or H-RasG12V, then stimulated with 10µM LPA for 5 min and analyzed for Rac activity. B. HEY and SK-OV3 cells were transduced with lentiviral vector containing luciferase (control), Tiam1, SOS1, EPS8 or ABI1 shRNA for 4 days. An aliquot of transduced cells was subjected to immunoblotting to detect Tiam1, SOS1, EPS8 and ABI1 with the respective antibodies (only SK-OV3 cells were shown). Transduced cells were also stimulated with 10µM LPA for 2 or 5 min or infected with retrovirus containing H-RasG12V for 2 days followed by the analysis of Rac activity. C. Transduced SK-OV3 and HEY cells were assayed for LPA or H-RasG12V-induced cell migration. Results are presented as Fold increase of cell migration [(LPA-stimulated cell migration)/(basal cell migration)]. Data are means ± SE. n=3. *, P < 0.001 vs sh-Luc; #, P < 0.005 vs sh-Luc. D. Tranduced SK-OV3 and HEY cells were intraperitoneally injected to nude mice for 5 weeks. Metastatic implants were collected and weighed. Data are means ± SE. n=6. *, P < 0.001 vs sh-Luc.
Ras can activate Rac through Tiam1 (9), βPIX (10) or SOS1/EPS8/ABI1 tri-complex (11). We determined their potential involvement by individually silencing their expression in HEY and SK-OV3 cells (Fig.3B, S8A and S8D). Although Tiam1 shRNAs and βPIX siRNA pool effectively inhibited their respective target expression, they did not alter LPA- or H-RasG12V-induced Rac activation (Fig.S8A and S8D). In contrast, SOS1, EPS8 and ABI1 shRNAs all blocked LPA- or Ras-G12V-induced Rac activation (Fig.3B). Transwell migration assay also showed that knockdown of SOS1, EPS8 or ABI1, but not Tiam1, greatly decreased LPA-induced cell migration in SK-OV3 and HEY cells (left panel, Fig.3C). Similarly, disrupting SOS1/EPS8/ABI1 tri-complex blocked RasG12V-induced migration of SK-OV3 (right panel, Fig.3C). The inhibitory effect of SOS1/EPS8/ABI1 shRNAs on Rac activation and cell migration was specific since forcing the expression of respective murine counterparts largely restored these two events (data not shown). When SOS1-, EPS8- or ABI1-knockdown cells were analyzed for peritoneal metastatic colonization, we detected a significant reduction in the weight of metastatic implants in mice receiving knockdown cells compared to mice receiving the control cells (Fig.3D and S9).
SOS1/EPS8/ABI1 tri-complex is not intact in non-metastatic ovarian cancer cells
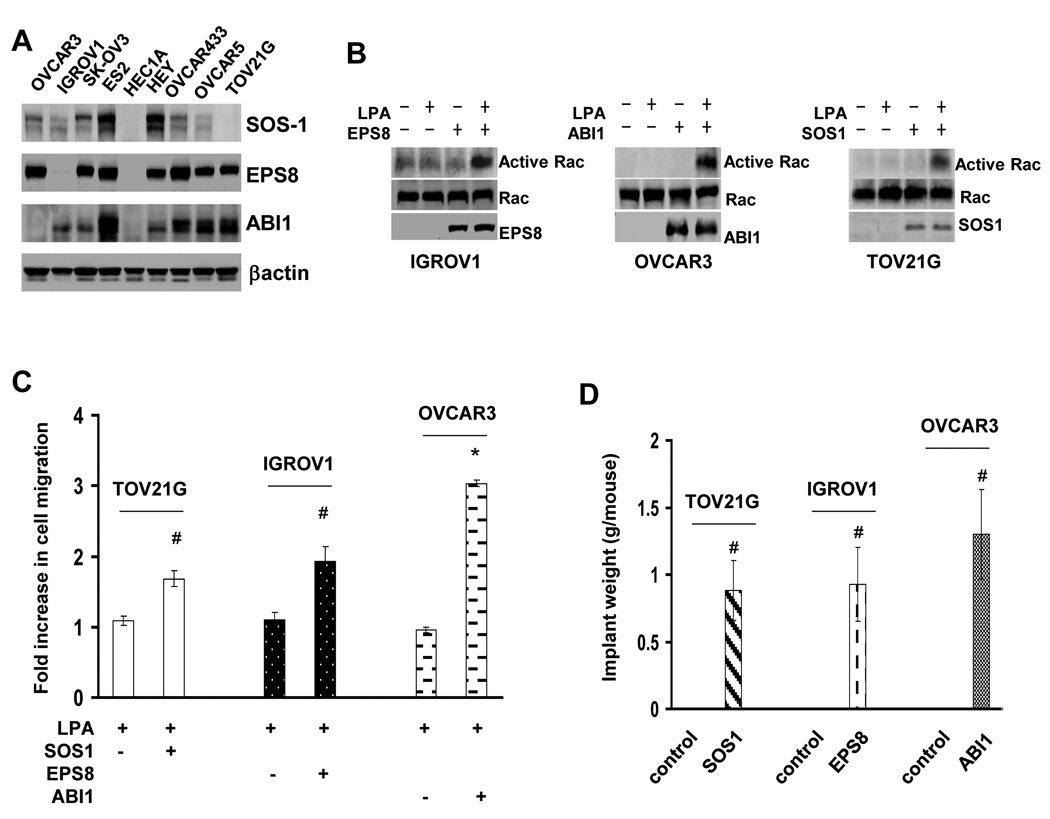
To further investigate the importance of SOS1/EPS8/ABI1 tri-complex in metastasis, we performed immunoblotting to examine SOS1, EPS8 and ABI1 expression in ovarian cancer lines. All members of this tri-complex were expressed in metastatic ES2, HEY, OVCAR433, OVCAR5 and SK-OV3 lines (Fig.4A). In contrast, at least one member of this tri-complex was absent in non-metastatic lines: EPS8 in IGROV1, ABI1 in OVCAR3, SOS1 in TOV21G line and all three in HEC1A line (Fig.4A). To determine whether the absence of SOS1/EPS8/ABI1 tri-complex member was responsible for the defect in LPA migratory response and metastatic colonization, SOS1, EPS8 and ABI1 were expressed in TOV21G, IGROV1 and OVCAR3 cells respectively. LPA both activated Rac (Fig.4B) and stimulated cell migration in TOV21G/SOS1, IGROV1/EPS8 and OVCAR3/ABI1 cells (Fig.4C). We next injected TOV21G/SOS1, IGROV1/EPS8, OVCAR3/ABI1 and the respective empty-vector control cells into nude mice followed by analyzing metastatic colonization. There was no detectable metastatic implant in mice receiving control cells (Fig.4D and S10). In contrast, implants were readily visible in the peritoneal cavities of mice that received TOV21G/SOS1, IGROV1/EPS8 or OVCAR3/ABI1 cells (Fig.4D and S10). These results further demonstrate the necessity of an intact SOS1/EPS8/ABI1 tri-complex in ovarian cancer metastasis.
Fig.4. Member of SOS1/EPS8/ABI1 tri-complex is absent in non-metastatic ovarian cancer cells.
A. Immunoblotting to detect SOS1, EPS8 and ABI1 in various ovarian cancer cell lines. B. IGROV1/EPS8, OVCAR3/ABI1 and TOV21G/SOS1 cells were stimulated with 10µM LPA for 5 min and then analyzed for Rac activation. C. IGROV1/EPS8, OVCAR3/ABI1 and TOV21G/SOS1 cells were analyzed for LPA-stimulated cell migration. Results are presented as Fold increase of cell migration [(LPA-stimulated cell migration)/(basal cell migration)]. Data are means ± SE. n=3. *, P < 0.001 vs control; #, P < 0.05 vs control. D. IGROV1/EPS8, OVCAR3/ABI1, TOV21G/SOS1 or the respective empty vector-transduced control cells were intraperitoneally injected to nude mice for 5 weeks. Metastatic implants were collected and weighed. Data are means ± SE. n=6. #, P < 0.05 vs control.
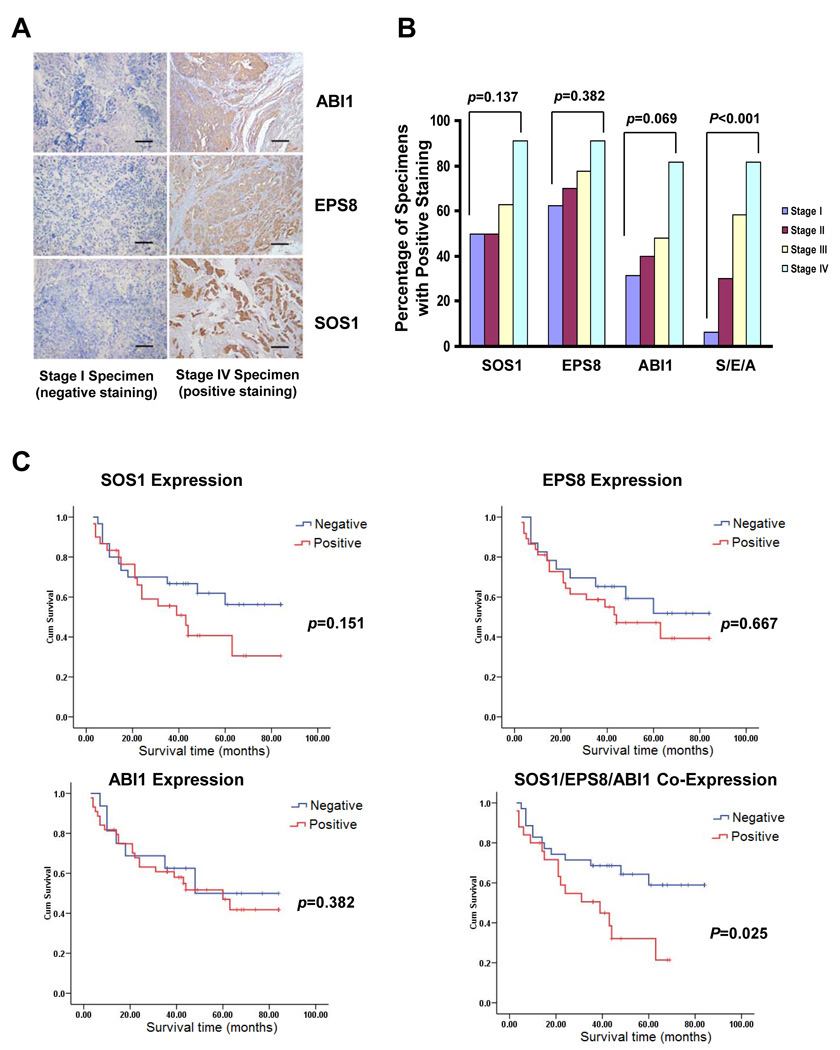
Co-expression of EPS8, ABI1 and SOS1 correlates with clinical malignancy of human ovarian cancer patients
As laboratory study may not recapitulate clinical ovarian malignancy, we extended our study by examining SOS1, EPS8 and ABI1 expression in 64 ovarian cancer specimens (Table S1). Immunohistochemistry showed that SOS1, EPS8 and ABI1 were positive in 48.4, 62.5 and 75% of the ovarian cancer specimens respectively (Fig.5A and 5B). When the expression status of individual member of SOS1/EPS8/ABI1 tri-complex was analyzed, we found that none of them was statistically correlated to age, histological types, pathological grades, clinical stages or patient survival (TABLE S2, Fig.5B and 5C).
Fig.5. SOS1/EPS8/ABI1 co-expression correlates to shorter survival of ovarian cancer patients.
A. Immunohistochemistry of SOS1, EPS8 and ABI1 on ovarian cancer specimens. Scale bars, 200µm. B. Percentage of various stage ovarian cancer specimens positive for SOS1, EPS8 and ABI1 expression. S/E/B: co-expression of SOS1, EPS8 and ABI1. C. Kaplan-Meier survival curves for 61 ovarian cancer patients, stratified based on individual SOS1, EPS8, ABI1 expression or SOS1/EPS8/ABI1 co-expression.
Our results with the established cell lines indicate that the integrity of SOS1/EPS8/ABI1 tri-complex is essential for ovarian cancer metastasis. We thus focused on the specimens that were immunoreactive for all three members of this tri-complex. Out of 64 samples, 26 specimens were stained positive for all three proteins and designated as “co-positive” (Table S1). The remaining specimens were negative for one or more of these proteins and designated as “co-negative”. The status of SOS1/EPS8/ABI1 co-expression was independent of age, histological types or pathological grade (Table 1). However, the “co-positive” percentage increased significantly along with the more advanced stages (stage I: 6.3%; II 30%; III: 48.1%; and IV: 81.8%; P < 0.001) (Fig.5B and Table 1). Moreover, “co-positive” also correlated with shorter patient survival (estimate mean survival of “co-negative”: 59.114 month vs “co-positive”: 36.537 month; P=0.025) (Fig.5C). These results indicate that the integrity of SOS1/EPS8/ABI1 tri-complex represents a reliable marker for ovarian cancer metastasis.
Table 1.
Correlation between the Status of SOS1/ABI1/EPS8 Co-Expression and Clinicopathological Parameters of Ovarian Cancer Patients
| Total No. of patients | Expression of Sos1/Eps8/Abi-1 | P value | ||
|---|---|---|---|---|
| “Co-Negative” (%) | “Co-Positive” (%) | |||
| Age * | 0.187 | |||
| ≤50 | 33 | 17 (51.5%) | 16 (48.5%) | |
| >50 | 31 | 21 (67.7%) | 10 (32.3%) | |
| Histological types | 0.844 | |||
| Serous | 38 | 21 (55.3%) | 17 (44.7%) | |
| Mucinous | 13 | 9 (69.2%) | 4 (30.8%) | |
| Endometroid | 8 | 5 (62.5%) | 3 (37.5%) | |
| Clear cell and Undifferentiated | 5 | 3 (60.0%) | 2 (40.0%) | |
| Pathologic Grade | 0.428 | |||
| Well differentiated | 21 | 14 (66.7%) | 7 (33.3%) | |
| Moderately differentiated | 24 | 15 (62.5%) | 9 (37.5%) | |
| Poorly differentiated | 19 | 9 (47.4%) | 10 (52.6%) | |
| Stage | 0.001 | |||
| I | 16 | 15 (93.8) | 1 (6.3%) | |
| II | 10 | 7 (70.0%) | 3 (30.0%) | |
| III | 27 | 14 (51.9%) | 13 (48.1%) | |
| IV | 11 | 2 (18.2%) | 9 (81.8%) | |
medium age 51(27 ~ 69)
DISCUSSION
LPA is present at high levels in ovarian cancer patients’ ascites (22–25) and can potently stimulate ovarian cancer cell migration (8, 27). In this study, we found that only those ovarian cancer cell lines with LPA migratory response undergo peritoneal metastatic colonization (Fig.1A and 1B), suggesting a potential link between LPA-stimulated cell migration and ovarian cancer metastasis. Although recent studies indicated that multiple LPA receptors may contribute to LPA-stimulated cell migration (21, 35), we showed that silencing LPAR1 largely abrogated both LPA-stimulated cell migration and metastatic colonization (Fig.1C and 1D). The discrepancy may be caused by the means to silence LPA receptors as synthetic siRNAs were used in these early studies while we used shRNAs for LPAR1 knockdown. A recent study reports that direct administration of LPA in vivo led to enhanced ovarian cancer metastasis and reduced animal survival (36). Enforced LPA receptor expression has also been shown to increase ovarian cancer aggressiveness in xenograft model (35). These findings all support the notion that LPA migratory response is a prerequisite for ovarian cancer metastasis.
Cell migration requires reorganization of actin cytoskeleton that is regulated by the members of Rho GTPases including Rac (37). We show that LPA only induces cytoskeleton reorganization in metastatic ovarian cancer cells (Fig.2A), indicating that members of Rho GTPase family may not be properly activated by LPA in non-metastatic cells. This possibility is substantiated since LPA only significantly activates Rac in metastatic cells (Fig.2B). Inhibiting Rac activity abolished LPA-stimulated cell migration and peritoneal metastatic colonization of metastatic cells while constitutively active Rac1 confers non-metastatic cells with LPA migratory response and the ability to metastasize (Fig.2C and 2D). Rac activation has been shown to correlate with hepatocellular carcinoma and breast cancer metastasis (38, 39). Our findings extend the importance of Rac activation into ovarian cancer metastasis.
Tiam1, βPIX and SOS1/EPS8/ABI1 tri-complex can mediate Ras-induced Rac activation (9–11). We show that silencing any member of SOS1/EPS8/ABI1 tri-complex, but not Tiam1 or βPIX, abrogates LPA or Ras-induced Rac activation, cell migration and peritoneal metastatic colonization (Fig.3B, 3C and 3D). SOS1/EPS8/ABI1 tri-complex has been shown to mediate EGF or PDGF-induced Rac activation and actin remodeling (40). This complex is also involved in PI3K-induced Rac activation (41). Our studies present another example of the usage of SOS1/EPS8/ABI1 tri-complex for Rac activation. Our observation with ovarian cancer cells is different from an early reports where LPA is shown to activate Rac through Tiam1 in murine fibroblasts (32). This difference may be attributed to species and cell lineage difference.
As silencing any member of SOS1/EPS8/ABI1 tri-complex is sufficient to diminish ovarian cancer cell migration and metastatic colonization, it indicates that the integrity of SOS1/EPS8/ABI1 tri-complex may determine ovarian cancer metastatic potentials. Indeed, metastatic lines express all three members of this tri-complex while at least one member is absent in non-metastatic lines (Fig.4A). Introducing the missing member in the respective line renders it capable of undergoing metastatic colonization (Fig.4D). EPS8 has been reported to promote fibrosarcoma and oral squamous carcinoma cell migration (42, 43). ABI1 can positively regulate breast cancer cell migration and invasion (44). Moreover, depletion of SOS1 blocks prostate cancer cell migration and invasion (45). Although only a single member was focused in these studies, they support an essential role of SOS1/EPS8/ABI1 tri-complex in cell migration and metastasis.
Recent studies indicate the clinical relevance of SOS1 and EPS8 expression in cancer progression. The level of SOS1 is elevated in prostate cancer specimens with high Gleason’s score (45). EPS8 overexpression is often detected in advanced stage of thyroid cancer (46), pancreatic cancer (47), oral squamous carcinoma (43) and pituitary tumors (48). To our knowledge, no study has been reported on the status of ABI1 in clinical cancer specimens. In this study, we detected SOS1, EPS8 and ABI1 expression in clinical ovarian cancer specimens (Fig.5A). However, the expression of any individual member of SOS1/EPS8/ABI1 tri-complex alone is not statistically associated with any clinicopathological parameters of patients (Table S2). Instead, we find that SOS1/EPS8/ABI1 co-expression is significantly correlated with advanced stage and shorter survival (Table 1, Fig.5B and 5C). This observation is in excellent agreement with the findings generated from the established cell lines that intact SOS1/EPS8/ABI1 tri-complex is required for ovarian cancer metastasis.
Our study demonstrates the importance of SOS1/EPS8/ABI1 tri-complex in ovarian cancer metastasis. Although we show it with the established ovarian cancer cell lines that may not fully simulate clinical setting, the consistency seen in multiple cell lines, the convergence of loss-and gain-of-function findings, and especially the excellent correlation observed between SOS1/EPS8/ABI1 co-expression and advanced disease stage/shorter patient survival strongly argue against any confounding influence derived from our experimental studies. Our studies suggest that the integrity of SOS1/EPS8/ABI1 tri-complex determines ovarian cancer metastasis and that therapeutic approach may be developed by targeting this tri-complex.
Supplementary Material
ACKNOWLEDGEMENTS
Grant support: NIH grants CA093926 and HL083335.
We thank Drs. Yan Xu and Tian-li Wang for providing ovarian cancer cell lines.
REFERENCES
- 1.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 2.Rose PG, Piver MS, Tsukada Y, Lau TS. Metastatic patterns in histologic variants of ovarian cancer. An autopsy study. Cancer. 1989;64:1508–1513. doi: 10.1002/1097-0142(19891001)64:7<1508::aid-cncr2820640725>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 4.Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 5.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 6.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 7.Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Phil Trans Royal Soc. 2000;355:965–970. doi: 10.1098/rstb.2000.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bian D, Su S, Mahanivong C, et al. Lysophosphatidic Acid Stimulates Ovarian Cancer Cell Migration via a Ras-MEK Kinase 1 Pathway. Cancer Res. 2004;64:4209–4217. doi: 10.1158/0008-5472.CAN-04-0060. [DOI] [PubMed] [Google Scholar]
- 9.Lambert JM, Lambert QT, Reuther GW, et al. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002;4:621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 10.Shin EY, Shin KS, Lee CS, et al. Phosphorylation of p85 beta PIX, a Rac/Cdc42-specific guanine nucleotide exchange factor, via the Ras/ERK/PAK2 pathway is required for basic fibroblast growth factor-induced neurite outgrowth. J Biol Chem. 2002;277:44417–44430. doi: 10.1074/jbc.M203754200. [DOI] [PubMed] [Google Scholar]
- 11.Scita G, Nordstrom J, Carbone R, et al. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- 12.Nimnual AS, Yatsula BA, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 13.Fishman DA, Liu Y, Ellerbroek SM, Stack MS. Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res. 2001;61:3194–3199. [PubMed] [Google Scholar]
- 14.Li H, Ye X, Mahanivong C, Bian D, Chun J, Huang S. Signaling mechanisms responsible for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. J Biol Chem. 2005;280:10564–10571. doi: 10.1074/jbc.M412152200. [DOI] [PubMed] [Google Scholar]
- 15.Symowicz J, Adley BP, Woo MM, Auersperg N, Hudson LG, Stack MS. Cyclooxygenase-2 functions as a downstream mediator of lysophosphatidic acid to promote aggressive behavior in ovarian carcinoma cells. Cancer Res. 2005;65:2234–2242. doi: 10.1158/0008.5472.CAN-04-2781. [DOI] [PubMed] [Google Scholar]
- 16.Hu YL, Tee MK, Goetzl EJ, et al. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst. 2001;93:762–768. doi: 10.1093/jnci/93.10.762. [DOI] [PubMed] [Google Scholar]
- 17.Fang X, Yu S, Bast RC, et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 18.Lee Z, Swaby RF, Liang Y, et al. Lysophosphatidic acid is a major regulator of growth-regulated oncogene alpha in ovarian cancer. Cancer Res. 2006;66:2740–2748. doi: 10.1158/0008-5472.CAN-05-2947. [DOI] [PubMed] [Google Scholar]
- 19.Eder AM, Sasagawa T, Mao M, Aoki J, Mills GB. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res. 2000;6:2482–2491. [PubMed] [Google Scholar]
- 20.Shen Z, Belinson J, Morton RE, Xu Y, Xu Y. Phorbol 12-myristate 13-acetate stimulates lysophosphatidic acid secretion from ovarian and cervical cancer cells but not from breast or leukemia cells. Gynecol Oncol. 1998;71:364–368. doi: 10.1006/gyno.1998.5193. [DOI] [PubMed] [Google Scholar]
- 21.Ren J, Xiao YJ, Singh LS, et al. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006;66:3006–3014. doi: 10.1158/0008-5472.CAN-05-1292. [DOI] [PubMed] [Google Scholar]
- 22.Westermann AM, Havik E, Postma FR, et al. Malignant effusions contain lysophosphatidic acid (LPA)-like activity. Ann Oncol. 1998;9:437–442. doi: 10.1023/a:1008217129273. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Shen Z, Wiper DW, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Gaudette DC, Boynton JD, et al. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin Cancer Res. 1995;1:1223–1232. [PubMed] [Google Scholar]
- 25.Xu Y, Fang XJ, Casey G, Mills GB. Lysophospholipids activate ovarian and breast cancer cells. Biochem J. 1995;309:933–940. doi: 10.1042/bj3090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bian D, Mahanivong C, Yu J, et al. The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene. 2006;25:2234–2244. doi: 10.1038/sj.onc.1209261. [DOI] [PubMed] [Google Scholar]
- 27.Sengupta S, Xiao YJ, Xu Y. A novel laminin-induced LPA autocrine loop in the migration of ovarian cancer cells. FASEB J. 2003;17:1570–1572. doi: 10.1096/fj.02-1145fje. [DOI] [PubMed] [Google Scholar]
- 28.Huang S, New L, Pan Z, Han J, Nemerow GR. Urokinase plasminogen activator/urokinase-specific surface receptor expression and matrix invasion by breast cancer cells requires constitutive p38alpha mitogen-activated protein kinase activity. J Biol Chem. 2000;275:12266–12272. doi: 10.1074/jbc.275.16.12266. [DOI] [PubMed] [Google Scholar]
- 29.Yamada SD, Hickson JA, Hrobowski Y, et al. Mitogen-activated protein kinase kinase 4 (MKK4) acts as a metastasis suppressor gene in human ovarian carcinoma. Cancer Res. 2002;62:6717–6723. [PubMed] [Google Scholar]
- 30.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–454. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 31.Su S, Li Y, Luo Y, et al. Proteinase-activated receptor 2 expression in breast cancer and its role in breast cancer cell migration. Oncogene. 2009;28:3047–3057. doi: 10.1038/onc.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Leeuwen FN, Olivo C, Grivell S, Giepmans BN, Collard JG, Moolenaar WH. Rac activation by lysophosphatidic acid LPA1 receptors through the guanine nucleotide exchange factor Tiam1. J Biol Chem. 2003;278:400–406. doi: 10.1074/jbc.M210151200. [DOI] [PubMed] [Google Scholar]
- 33.Yamada T, Sato K, Komachi M, et al. Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1. J Biol Chem. 2004;279:6595–6605. doi: 10.1074/jbc.M308133200. [DOI] [PubMed] [Google Scholar]
- 34.Hama K, Aoki J, Fukaya M, et al. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem. 2004;279:17634–17639. doi: 10.1074/jbc.M313927200. [DOI] [PubMed] [Google Scholar]
- 35.Yu S, Murph MM, Lu Y, et al. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. 2008;100:1630–1642. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Wang D, Zhang H, et al. Lysophosphatidic acid stimulates cell migration, invasion, and colony formation as well as tumorigenesis/metastasis of mouse ovarian cancer in immunocompetent mice. Mol Cancer Ther. 2009;8:1692–1701. doi: 10.1158/1535-7163.MCT-08-1106. [DOI] [PubMed] [Google Scholar]
- 37.Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 38.Lee TK, Poon RT, Yuen AP, et al. Rac activation is associated with hepatocellular carcinoma metastasis by up-regulation of vascular endothelial growth factor expression. Clin Cancer Res. 2006;12:5082–5089. doi: 10.1158/1078-0432.CCR-05-2794. [DOI] [PubMed] [Google Scholar]
- 39.Schnelzer A, Prechtel D, Knaus U, et al. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 40.Sini P, Cannas A, Koleske AJ, Di Fiore PP, Scita G. Abl-dependent tyrosine phosphorylation of Sos-1 mediates growth-factor-induced Rac activation. Nat Cell Biol. 2004;6:268–274. doi: 10.1038/ncb1096. [DOI] [PubMed] [Google Scholar]
- 41.Innocenti M, Frittoli E, Ponzanelli I, et al. Phosphoinositide 3-kinase activates Rac by entering in a complex with Eps8, Abi1, and Sos-1. J Cell Biol. 2003;160:17–23. doi: 10.1083/jcb.200206079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funato Y, Terabayashi T, Suenaga N, Seiki M, Takenawa T, Miki H. IRSp53/Eps8 complex is important for positive regulation of Rac and cancer cell motility/invasiveness. Cancer Res. 2004;64:5237–5244. doi: 10.1158/0008-5472.CAN-04-0327. [DOI] [PubMed] [Google Scholar]
- 43.Yap LF, Jenei V, Robinson CM, et al. Upregulation of Eps8 in oral squamous cell carcinoma promotes cell migration and invasion through integrin-dependent Rac1 activation. Oncogene. 2009;28:2524–2534. doi: 10.1038/onc.2009.105. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Navab R, Iakovlev V, et al. Abelson interactor protein-1 positively regulates breast cancer cell proliferation, migration, and invasion. Mol Cancer Res. 2007;5:1031–1039. doi: 10.1158/1541-7786.MCR-06-0391. [DOI] [PubMed] [Google Scholar]
- 45.Timofeeva OA, Zhang X, Ressom HW, et al. Enhanced expression of SOS1 is detected in prostate cancer epithelial cells from African-American men. Int J Oncol. 2009;35:751–760. [PMC free article] [PubMed] [Google Scholar]
- 46.Griffith OL, Melck A, Jones SJ, Wiseman SM. Meta-analysis and meta-review of thyroid cancer gene expression profiling studies identifies important diagnostic biomarkers. J Clin Oncol. 2006;24:5043–5051. doi: 10.1200/JCO.2006.06.7330. [DOI] [PubMed] [Google Scholar]
- 47.Welsch T, Endlich K, Giese T, Buchler MW, Schmidt J. Eps8 is increased in pancreatic cancer and required for dynamic actin-based cell protrusions and intercellular cytoskeletal organization. Cancer Lett. 2007;255:205–218. doi: 10.1016/j.canlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Xu M, Shorts-Cary L, Knox AJ, Kleinsmidt-DeMasters B, Lillehei K, Wierman ME. Epidermal growth factor receptor pathway substrate 8 is overexpressed in human pituitary tumors: role in proliferation and survival. Endocrinol. 2009;150:2064–2071. doi: 10.1210/en.2008-1265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.