Abstract
This study examined the within-person relationship between sleep and cognitive functioning. Fifty community-dwelling African Americans (age range = 50 - 80 years) were asked to report their sleep duration and quality the previous evening and to complete cognitive measures over 8 occasions within a 2-3 week period. A within-person daily change in sleep duration was significantly associated with worse global cognitive performance. The greater an individual deviated away from his/her average sleep duration on a particular day, the more likely his/her performance would decline. These results demonstrate that the sleep-cognition relationship can be observed at a within-person level of analysis.
Sleep complaints are common among older adults (Blay, Andreoli, & Gastal, 2007; Reid, Martinovich, Finkel, Statsinger, Golden, Harter et al., 2006), particularly within African Americans (Durrence and Lichstein 2006; Kripke, Brunner, Freeman, Hendrix, Jackson, Masaki, & Carter, 2001). Cross-sectional studies have suggested that sleep difficulties in older adults are associated with impaired performance on tasks of memory (Bastien, Fortier-Brochu, Rioux, LeBlanc, Daley, & Morin, 2003; Gamaldo, Allaire, & Whitfield, 2008; Schmutte, Harris, Levin, Zweig, Katz, & Lipton, 2007), executive functioning (Hart, Morin, & Best, 1995; Nebes, Buysse, Halligan, Houck, & Monk, 2009), language (Fenn, Nusbaum, and Margoliash, 2003) and global cognitive functioning (Nebes et al., 2009), Cross-sectional studies, however, focus on interindividual differences and do not necessarily reflect within-person behavioral changes and the underlying processes for these behaviors (Molenaar & Campbell, 2009; Sliwinski & Hofer, 1999). Micro-longitudinal research, however, that focuses on intraindividual variability, within-person short-term changes in behavior, can offer a reliable and useful illustration of an individual’s typical level of functioning as well as short-term changes in functioning. More importantly, it allows for the direct examination of within-person relationships among constructs. Several studies exploring intraindividual variability have suggested a dynamic within-person coupling relationship between cognition and health factors. For example, variability in cognition has shown to correlate with fluctuations in blood pressure (Gamaldo, Weatherbee, Allaire, 2008), vision (Weatherbee, Gamaldo, & Allaire, 2009) and stress (Neupert, Almeida, Mroczek, & Spiro, 2006).
Previous research has found that sleep quantity and quality are not consistent and can vary across nights (Frey, Badia, & Wright, 2004; Knutson, Rathouz, Yan, Liu, & Lauderdale, 2007), which may differentially impact cognition the following day (Frey et al., 2004). Less consistent sleep/wake periods and shifts in the cycle have been observed in older adults (Ancoli-Israel, Ayalon, & Salzman, 2008). Older adults may attempt to delay their sleep onset to a bedtime that is more consistent with the societal norms, but still wake up early due to the shift in the circadian rhythm (Ancoli-Israel et al., 2008). Likewise, the shift in the circadian rhythm may cause older adults to take more naps, which, in turn, may increase their difficultly to fall asleep at night as well as their ability to remain asleep (Ancoli-Israel et al., 2008). Consequently, shifts in the circadian rhythm may lead to elders experiencing insufficient sleep duration/quality and potential cognitive deficits the following day. Thus, a coupling relationship between daily fluctuations in sleep and cognition may occur because both are reflected in waking behaviors associated with circadian rhythmicity (Durmer & Dinges, 2005). According to the state instability hypothesis (Doran, Van Dongen, Dinges, 2001; Durmer & Dinges, 2005), sleep deprivation disrupts an individual’s sleep cycle, particularly within the homeostatic sleep-initiating mechanisms, and, in turn, increases variability in cognitive performance. Frey and colleagues (2004) supported this hypothesis by observing greater trail-to-trail variability and worse mean performance on reaction time, processing speed, and task switching measures as result of sleep deprivation. The current study, however, wants to expand upon these findings and explore whether day-to-day cognitive variability is associated with day-to-day changes in cognition. Given both low sleep duration (≤ 6 hours of sleep) and high sleep duration (≥ 8 hours of sleep) have been associated with worse health (Kripke, Ancoli-Israel, Fell, Mason, Klauber, & Kaplan, 1991) and earlier mortality (Kripke, Garfinkel, Wingard, Klauber, & Marler, 2002), the current study plans to explore a non-linear coupling within-person sleep-cognition relationship.
A common cause underlying the within-person sleep-cognition relationship may be concurrent physical and/or psychological ailments (Dew et al., 2003). Concurrent ailments are likely to worsen sleep as well as impact changes in overall health and cognition. The current study attempts to account for this issue by controlling for preexisting health status. Another common cause underlying the within-person sleep-cognition relationship may be an individual’s sleeping habits. The Pittsburgh Sleep Quality Index (PSQI), a commonly used measure of sleep habits within the past month, is a reliable and consistent measure of sleep habits over several days (Backhaus, Junghanns, Broocks, Riemann, & Hohagen, 2002) and appears to be associated with poor cognitive functioning (Nebes et al., 2009). Thus, the within-person relationship between sleep duration and cognition may be moderated by sleep habits. Individuals who have typically have good sleeping habits may have more difficulty compensating cognitively on those occasions when they have gotten a poor night’s rest, while individuals who typically have poor sleeping habits may have less difficulty compensating cognitively after a poor night’s rest. Increased levels of stress might be a proxy of sleep difficulties (Hall et al., 2008; Pallesen et al., 2002). Within-person fluctuations in stress have been observed and appear to be associated with within-person fluctuations in cognitive performance (Neupert et al., 2006; Neupert, Mroczek, & Spiro, 2008; Sliwinski, Symth, Hofer, & Stawski, 2006). Life event stressors (i.e. death of spouse or diagnosis of major illnesses), which are likely to occur in old age, may also be associated with daily changes in stress levels, sleep patterns, and cognitive performance. The current study examines whether life event stressors might moderate the coupling relationship between sleep and cognition.
The current study extends previous work on sleep and cognition in African Americans in four ways. First, the study determined the amount of within- and between-person variability found in sleep duration and quality. It was expected that daily assessments over the course of 2-3 weeks would reveal substantial intraindividual variability in older adults’ sleep duration as well as sleep quality. Second, it examined the within-person relationship between sleep duration and cognition as well as the within-person relationship between sleep quality and cognition. Third, it examined whether the within-person relationship between sleep duration/quality and cognition is moderated by sleep habits within the last month. Finally, it examined whether or not the within-person relationship between sleep duration/quality and cognitive functioning would be moderated by life event stressors.
Method
Participants
The current study recruited 50 (39 women and 11 men) independently living, community dwelling African American older adults ranging in age from 50 to 80 years (M = 65.40, SD = 8.53) from the senior housing facilities in Baltimore, Maryland. Participants’ average monthly income was $950 (SD = $500; range = < $100 - >$2300) and the average years of education was 11.62 years (range = 6–18, SD = 2.38 years).
Demographics and Health Measures
The current analyses included the demographic variables age, education, and gender. Chronic health conditions were measured by a subjective assessment asking participants to indicate whether they had been diagnosed with any of a series of specified illnesses or diseases. A cardiovascular risk factors (CVRFS) composite score was created by summing the report of diabetes, cardiovascular disease, high blood pressure, stroke, heart attack, angina, and circulation problems. The CVRFS variable had scores that ranged from 0 (no risk factors) to 7 (more risk factors). In addition, a comorbid health illnesses (Non-CVRFS) composite score was created by summing the report of the remaining reported health illnesses that included arthritis, broken hip, asthma, gout, gallbladder trouble, stomach ulcers, thyroid trouble, tuberculosis, kidney trouble, and cancer. The Non-CVRFS variable had scores that ranged from 0 (no comorbid health illnesses) to 10 (high comorbid health illnesses). Both the CVRFS and Non-CVRFS variables were included in the analyses as covariates.
The Elderly Life Stress Inventory (ELSI; Aldwin, 1990) was used to assess self-reported stress at baseline testing. This questionnaire asked participants to indicate on a scale of 0 (did not occur) to 5 (extremely stressful) the extent to which they have experienced a number of different stress events (i.e. death of spouse, major personal injury, and retirement) during the past year.
The Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1988) was used to assess participants’ typical sleep habits within the last month only at baseline testing session. Questions were designed to assess seven components, including sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, sleep medications, and daytime dysfunction due to sleep habits. The index has been shown to be strongly reliable (Cronbach’s α = .83; Buysse et al., 1988) and to have relatively high sensitivity (89.6%) and specificity (86.5%) in differentiating good/bad sleep habits. Global sleep quality scores range from 0 (good sleeper) to 21 (poor sleeper).
At baseline and each follow-up testing session, participants were asked the number of hours slept the previous night at each testing session (i.e. sleep duration). Given that PSQI is designed to assess sleep habits or quality within the last month and not necessarily within the last 24 hours, participants were asked at baseline and each follow-up testing session to describe their sleep quality from 0 (extremely poor) to 5 (extremely good).
Cognitive Measures
A cognitive battery was administered to assess a broad range of abilities. Executive functioning was assessed using The Stroop Task (Trenerry, Crosson, DeBoe, & Leber, 1989) and the Clock Drawing Test (CDT; Freedman, Leach, Kaplan, Winocur, Shulman, & Delis, 1994), which measures a participant’s ability to draw a clock to a specified time (i.e. 10 after 11, 3:25, 10 after 9, 6:55, 10 after 6, 1:45, 5 after 4, and 9:40). Clocks were scored based on a 10-point scoring system (Manos & Wu, 1994; Shulman, Shedletsky, & Silver, 1986). Declarative memory was assessed using the Rey Auditory Verbal Learning Task (AVLT; Rey, 1941). Perceptual speed was assessed using the Number Comparison test (Ekstrom, French, Harman, & Derman, 1976). Inductive reasoning was assessed using the Letter Series test (Thurstone, 1962), Language was assessed using the Boston Naming Task (BNT; Goodglass & Kaplan, 1983). Verbal Fluency (Goodglass & Kaplan, 1983; Benton & Hamsher, 1976) was also used to assess language as well as semantic memory and executive function.
Procedure
Participants were assessed on eight occasions at their apartment or an empty room (i.e. office and library) located in a senior high-rise facility over 2-3 week period. At initial testing, participants were consented, and administered the baseline assessments. To reduce practice effects, eight alternate versions of the AVLT, Number Comparison, Letter Series, and CDT as well as four alternate versions of Constructions, BNT, Letter Fluency, and Semantic Fluency were administered. The tasks with four alternate versions were administered to the participants in a random order. Alternate versions of the Stroop task were not included in the testing booklets. With the exception of the baseline assessment, alternate versions of the measures were counterbalanced across the follow-up occasions.
The initial testing session lasted approximately 2 – 2 ½ hours and the daily sessions lasted 1 ½ – 2 hours. Participants were compensated a total of $120 for the completion of all 8 sessions within the two-week time period.
Cognitive Composites
All of the cognitive tests were standardized to a mean of 50 and standard deviation of 10. A global composite score was created by taking the mean of all the tests because there was an overall significant positive correlation between the measures across all occasions with (median r = .25. range = .13 - .50). The influence of age and education was residualized from the global composite, and this composite was standardized (M = 50, SD = 10) again.
Results
Although 50 participants were tested at the baseline assessment, 3 participants withdrew (1 male; 2 females) before completing all 8 daily assessments. On average, participants reported a relatively good sleep quality for the daily sleep quality question (M = 3.51, SD = 1.32). Participants’ average PSQI score, however, was approximately 7 (SD = 4), which would be considered poor sleep habits. Furthermore, participants reported approximately 6 hours of sleep (SD = 1.99). The stress scale distribution was positively skewed, therefore a median split was performed to create two levels of stress: low stress (8 or less) and stressed (9 or more).
Brief Description of Multilevel Modeling
Two multi-level models (MLM) were run to examine the association between sleep duration and global cognition. In the first model, the “coupling parameters” (level 1 predictor), between-person parameters (or level 2), and covariates (i.e. linear time, quadratic time, gender, cardiovascular risk factors, and other comorbid health illnesses) were included in the model to assess potential main effects. Two within-person level 1 predictors of interest (i.e. daily sleep duration and daily sleep quality) were used to estimate the coupling parameters. The level 1 predictors represented a participants’ sleep duration/quality on a particular occasion deviated from their average sleep duration/quality across all testing occasions (see Figure 1; Raudenbush & Bryk, 2002). For example, a positive beta coefficient, β1, would suggest that the more an individual’s hours of sleep deviates below his/her average on a given occasion, the worse his/her cognitive performance would be than his/her average. The quadratic effects of daily sleep duration and quality were also included in the model by squaring the within-person sleep variables.
Figure 1.
Multi-level Linear Modeling Equation.
Four level 2 predictors of interest were included in the model which included mean sleep duration across the 8 occasions, mean sleep quality across the 8 occasions as well as scores on the PSQI and the dichotomous life event stressor variable. Several interactions (i.e. between-person interactions and cross-level interactions) were added to the initial model in a second model to assess potential moderating relationships (see Figure 1).
Intraclass Correlations
The within- and between-person variability in sleep quantity was assessed using the intraclass correlation (ICC), an intercept only model. Results indicated that 48% of the total variance in sleep duration was within-person (σ2 = 1.98, z = 12.96, p < .01)_while 52% was between-persons (τ00 = 2.12, z = 4.35, p < .01). In contrast, 54% of the total variance in sleep quality was within-person variance (σ2 = 0.93, z = 0.07, p < .01) while 46% was between-persons (τ00 = 0.81, z = 0.19, p < .01). To assure that there was a sufficient amount of within- and between-person variance in the cognitive dependent variables to conduct our subsequent MLM analyses (Raudenbush & Bryk, 2002), ICCs were also calculated for the cognitive construct. The results suggested that there was significant within-person (24%; σ2 = 23.72, z = 13.02, p < .01) and between-person (76%; τ00 = 76.68, z = 4.76, p < .01) variance in global cognition.
Coupling of Sleep and Cognitive Functioning
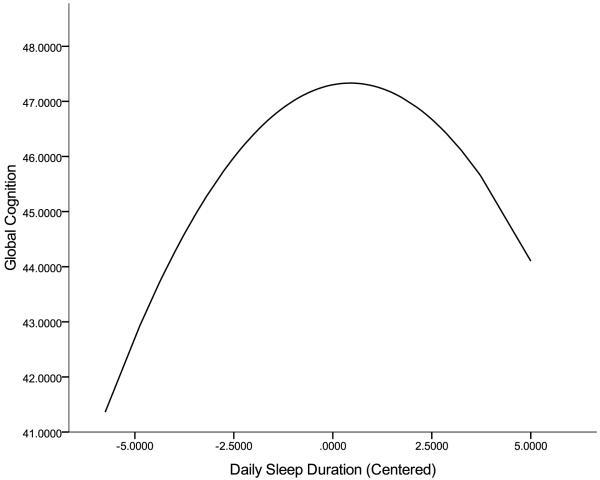
In the first model testing only main effects, the quadratic effect of daily sleep duration was a significant and negative predictor of performance on global cognition (see Table 1), suggesting that on those days when participants reported either fewer or several more hours of sleep than on average, they tended to perform worse. As illustrated in Figure 2, cognitive performance appeared to become increasingly worse the more an individual deviated from his/her average hours of sleep across the occasions. The linear and quadratic effects of mean sleep duration were not significant predictors of performance across the cognitive domains. Likewise, the linear and quadratic effects of daily or mean sleep quality were not significant predictors of performance across the cognitive domains. This model accounted for 18% of the within-person (σ2 = 19.41, z = 11.72, p < .01) and 34% of the between-person (τ00 = 50.62, z = 3.78, p < .01) variance in global cognition. In the second model, none of the interactions were significant (σ2 = 19.56, z = 1.68, p < .01; τ00 = 50.48, z = 13.44, p < .01).
Table 1.
Sleep Unstandardized Coefficients (and Standard Errors) of Daily Within-Person Effects and Between-Person Effects for Global Cognition
| Step 1 | Step 2 | |
|---|---|---|
| Within-person | ||
| Daily Sleep Duration (Linear) | 0.14 (0.19) | 0.12 (0.20) |
| Daily Sleep Duration (Quadratic) | −0.16* (0.07) | −0.14 (0.08) |
| Daily Sleep Quality (Linear) | −0.31 (0.33) | −0.19 (0.35) |
| Daily Sleep Quality (Quadratic) | 0.02 (0.20) | 0.03 (0.20) |
| Time (Linear) | 0.66 (0.37) | 0.73 (0.38) |
| Time (Quadratic) | 0.01 (0.05) | 0.00 (0.05) |
| Between-person | ||
| Mean Sleep Duration (Linear) | −0.17 (0.83) | −0.18 (0.83) |
| Mean Sleep Duration (Quadratic) | 0.40 (0.32) | 0.40 (0.32) |
| Mean Sleep Quality (Linear) | 1.27 (2.01) | 1.28 (2.01) |
| Mean Sleep Quality (Quadratic) | −0.37 (1.00) | −0.36 (1.00) |
| PSQI | 0.06 (0.39) | 0.06 (0.39) |
| Stress | −2.89 (2.67) | −2.91 (2.67) |
| Gender | −6.78* (3.05) | −6.72* (3.05) |
| CVRFS | −4.36** (0.93) | −4.35** (0.93) |
| Non-CVRFS | 2.26 (1.17) | 2.28 (1.17) |
| Interactions | ||
| Daily Sleep Duration x PSQI | 0.04 (0.05) | |
| Daily Sleep Duration x Stress | −0.59 (0.47) | |
| Daily Sleep Quality x PSQI | −0.12 (0.08) | |
| Daily Sleep Quality x Stress | 0.37 (0.61) | |
| Pseudo R2 between | 34% | 34% |
| Pseudo R2 within | 18% | 18% |
Note:
p < .05
p<.01
CVRFS = Cardiovascular Risk Factors. Non-CVRFS = Non Cardiovascular Risk Factors/Other Health Conditions.
Figure 2.
Quadratic Effect of Daily Sleep Duration for Global Cognition Function
Discussion
On average, our sample of African American older adults reported sleeping 6 hours and poor sleeping habits. Significant within- and between-person variability was observed in both sleep duration and sleep quality, which further supports using models to estimate both within- and between-person effects of sleep and cognition. Consistent with the previous literature (Allaire & Marsiske, 2005; Hultsch, MacDonald, & Dixon, 2002), the current study observed significant within- and between-person variability in global cognition. A dynamic within-person relationship was observed between sleep duration and global cognitive performance, in that an individual’s performance tended to decline on those days his/her sleep duration was lower or extremely greater than his/her average sleep duration across our 8 assessments.
These findings support the previous cross-sectional research suggesting that sleep is associated with cognitive performance in older adults (Gamaldo et al., 2008; Nebes et al., 2009). Both high and low sleep duration is associated with cognitive performance, which supports a non-linear relationship between sleep duration and cognition. Short sleep duration (i.e. 6 hours or less a night) as well as sleep deprivation has been shown to be associated with worse cognitive performance (Chee & Choo, 2004; Thomas et al., 2006), particularly on tasks of attention, working memory, processing speed, and/or executive function. Kripke and colleagues (2002) observed that individuals who reported sleeping 8 hours or more had an increase mortality risk. The explanation for this relationship is still speculative, but sleeping more than 8 hours has shown to be associated with older age and low physical activity (Stranges et al., 2008), both of which are associated with cognitive functioning. The reports of sleeping more than 8 hours may also be reflective of poor health status (Alvarez & Ayas, 2007). The postsleep inertia or “worn out sleep” (Horne, 1991) may also be a potential explanation for the results. Individuals who report sleeping extra hours of sleep tend to report feeling sleepier than before sleeping the extra hours (Horne, 1991). Similar to sleep deprivation, sleeping too many hours may be associated with increased sleepiness and reduced task engagement as reflected in worse cognitive performance.
A within-person relationship between sleep quality and cognitive performance was not observed. Furthermore, sleep habits, as measured by the PSQI, or life stressors did not appear to moderate the within-person coupling relationship between duration/quality and cognitive performance. These lack of findings may have been a result of the limited sample size. Even though life stressors did not appear to moderate the within-person sleep-cognition relationship, it is still possible that daily changes in stress due acute and/or chronic life events may better explain the within-person sleep-cognition relationship than our single assessment of the experience of life event stressors. Given that daily stressors have shown to be associated with worse cognitive performance on the same day (Sliwinski et al., 2006), it is likely fluctuations in daily stress levels may have better explained the relationship between daily sleep and cognition.
There are several limitations of the current study that should be noted. Aside from the relatively small sample, the participants represented a rather homogenous group of African American elders. Factors associated with our sample’s SES and residency, such as poor diet, poor health, and/or unsafe environmental conditions, may be associated with daily changes in sleep and cognition. Consequently, the generalizability of our findings beyond this sample to the broader population of African Americans older adults as well as European Americans should be made with caution. A third limitation is our measurement of previous stressful experiences as a single assessment, which more than likely did not accurately capture participants’ current level of stress. A fourth limitation is that the current study relied on subjective rather than objective assessments of sleep. Subjective measures of sleep are more likely to capture cognitive (i.e. attention and motivation) and emotional (i.e. anxiety) functioning than objective or physiological measures of sleep, which may better capture the biological necessity to sleep (Danker-Hopfe, Kraemer, Dorn, Schmidt, Ehlert, & Herrmann, 2001). Future research should explore whether the current results are replicated with the inclusion of objective sleep measures, which may potentially address whether the sleep-cognition relationship is due to a biological or behavioral influence. A fifth limitation is that daytime sleepiness was not assessed, even though daytime sleepiness is associated with long (Horne, 1991) and short sleep durations (Bixler, Vgontzas, Lin, Calhoun, Vela-Bueno, & Kales, 2005), old age (Bixler et al., 2005) as well as cognitive performance (Ohayon & Vecchierini, 2002). A final limitation is that assessment of health illnesses does not account for acute physical ailments that may have occurred during our daily assessments. These limitations should be weighed against the relative difficulty in obtaining repeated cognitive assessments on so many occasions separated by such short intervals. Despite these limitations, the current study did observe a significant relationship between sleep duration and cognitive performance, which has also been observed in the previous literature.
The current study’s results suggest the importance of identifying and treating health issues that may be common in African Americans to help preserve daily cognitive functioning. Given our sample of older African Americans typically reported poor sleep habits, sleep difficulties may explain cognitive functioning within this population. Overall, the current study reveals a promising direction for future research to implement models that estimate both between-person and within-person effects of sleep not only within minority populations, but also across racial groups. Furthermore, future research should explore whether the within-person coupling relationship between sleep duration/quality and cognitive functioning may signify early cognitive impairment.
Acknowledgments
This study was funded by a NIH Supplement to Promote Diversity awarded to Alyssa Gamaldo and by a NIA grant awarded to Keith Whitfield (RO1 AG024108-01). This writing of this was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/PAG
Contributor Information
Alyssa A. Gamaldo, National Institute of Health
Jason C. Allaire, North Carolina State University
Keith E. Whitfield, Duke University
References
- Aldwin CM. The Elders Life Stress Inventory: Egocentric and nonegocentric stress. In: Stephens MAP, Crowther JH, Hobfall SE, Tennenbaum DL, editors. Stress and coping in later life families. Hemisphere Publishing Corporation; New York, NY: 1990. pp. 49–69. [Google Scholar]
- Allaire JC, Marsiske M. Intraindividual variability may not always indicate vulnerability in elders’ cognitive performance. Psychology and Aging. 2005;20:390–401. doi: 10.1037/0882-7974.20.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez GG, Ayas NT. The impact of daily sleep duration on health: A review of the literature. Progress in cardiovascular nursing. 2007;19:56–59. doi: 10.1111/j.0889-7204.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Ayalon L, Salzman C. Sleep and the elderly: normal variations and common sleep disorders. Harvard Review of Psychiatry. 2008;16:279–286. doi: 10.1080/10673220802432210. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Fortier-Brochu É, Rioux I, LeBlanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia. Relationship between objective and subjective measures. Journal of Psychosomatic Research. 2003;54:39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. de S. Multilingual Aphasia Examination. University of Iowa; Iowa City: 1976. [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: The role of sleep apnea, age, obesity, diabetes, and depression. The Journal of Clinical Endocrinology & Metabolism. 2005;90:4510–4515. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- Blay SL, Andreoli SB, Gastal FL. Chronic painful physical conditions, disturbed sleep and psychiatric morbidity: Results from an elderly survey. Annals of Clinical Psychiatry. 2007;19:169–174. doi: 10.1080/10401230701468099. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1988;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. The Journal of Neuroscience. 2004;24:4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker-Hopfe H, Kraemer S, Dorn H, Schmidt A, Ehlert I, Herrmann WM. Time-of-day variations in different measures of sleepiness (MSLT, pupillography, and SSS) and their interrelations. Psycholophysiology. 2001;38:828–835. [PubMed] [Google Scholar]
- Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, Houck PR, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosomatic Medicine. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–267. [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Durrence H. Heith, Lichstein Kenneth L. The sleep of African Americans: A comparative review. Behavioral Sleep Medicine. 2006;4:29–44. doi: 10.1207/s15402010bsm0401_3. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Derman D. Kit of factor-referenced cognitive tests. Educational Testing Service; Princeton, NJ: 1976. [Google Scholar]
- Fenn KM, Nusbaum HC, Margoliash D. Consolidation during sleep of perceptual learning of spoken language. Nature. 2003;425:614–616. doi: 10.1038/nature01951. [DOI] [PubMed] [Google Scholar]
- Freedman M, Leach L, Kaplan E, Winocur G, Shulman KI, Delis DC. Clock drawing: A neuropsychological analysis. Oxford University Press; New York, NY: 1994. [Google Scholar]
- Frey DJ, Badia P, Wright KP. Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. Journal of Sleep Research. 2004;13:305–315. doi: 10.1111/j.1365-2869.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- Gamaldo AA, Allaire JC, Whitfield KE. The relationship between reported problems falling asleep and cognition among African American elderly. Research on Aging. 2008;30:752–767. [Google Scholar]
- Gamaldo AA, Weatherbee SR, Allaire JC. Exploring the within-person coupling of blood pressure and cognition in the elderly. Journal of Gerontology: Psychological Sciences. 2008;63:P386–P389. doi: 10.1093/geronb/63.6.p386. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination (BDAE) Lea and Febiger; Philadelphia, Pennsylvania: 1983. [Google Scholar]
- Hall M, Buysse DJ, Nofzinger EA, Reynolds CF, Thompson W, Mazumdar S, et al. Financial strain is a significant correlate of sleep continuity disturbance in late life. Biological Psychology. 2008;77:217–222. doi: 10.1016/j.biopsycho.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart RP, Morin CM, Best AM. Neuropsychological performance in elderly insomnia patients. Aging and Cognition. 1995;2:268–278. [Google Scholar]
- Horne JA. Dimensions to sleepiness. In: Monk TH, editor. Sleep, Sleepiness and Performance. John Wiley & Sons; Chichester, New York, Brisbane, Toronto, Singapore: 1991. pp. 169–196. [Google Scholar]
- Hultsch DF, MacDonald SWS, Dixon RA. Variability in reaction time performance of younger and older adults. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2002;57:101–115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Ancoli-Israel S, Fell RL, Mason WJ, Klauber MR, Kaplan O. Health risk of insomnia. In: Peter JH, Penzel T, Podszus T, Von Wichert P, editors. Sleep and health risk. Springer-Verlag; Berlin: 1991. pp. 547–554. [Google Scholar]
- Kripke DF, Brunner R, Freeman R, Hendrix SL, Jackson RD, Masaki K, Carter RA. Sleep complaints of postmenopausal women. Clinical Journal of Women’s Health. 2001;1:244–252. doi: 10.1053/cjwh.2001.30491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Archives of General Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA Study. Sleep. 2007;30:793–796. doi: 10.1093/sleep/30.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manos PJ, Wu R. The ten point clock test: A quick screen and grading method for cognitive impairment in medical and surgical patients. International Journal of Psychiatry Medicine. 1994;24:229–244. doi: 10.2190/5A0F-936P-VG8N-0F5R. [DOI] [PubMed] [Google Scholar]
- Molenaar PCM, Campbell CG. The new person-specific paradigm in psychology. Current Directions in Psychological Science. 2009;18:112–117. [Google Scholar]
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TM. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. Journal of Gerontology: Psychological Sciences. 2009;10:1–8. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert SD, Almeida DM, Mroczek DK, Spiro A. Daily stressors and memory failures in a naturalistic setting: Findings from the VA Normative Aging Study. Psychology and Aging. 2006;21:424–429. doi: 10.1037/0882-7974.21.2.424. [DOI] [PubMed] [Google Scholar]
- Neupert SD, Mroczek DK, Spiro A. Neuroticism moderates the daily relation between stressors and memory failures. Psychology and Aging. 2008;23:287–296. doi: 10.1037/0882-7974.23.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Vecchierini M-F. Daytime sleepiness and cognitive impairment in the elderly population. Archives of Internal Medicine. 2002;162:201–208. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- Pallesen S, Nordhus IH, Kvale G, Havik OE, Nielsen GH, Johnsen BH, et al. Psychological characteristics of elderly insomniacs. Scandinavian Journal of Psychology. 2002;43:425–432. doi: 10.1111/1467-9450.00311. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Sage Publications; Thousand Oaks, CA: 2002. [Google Scholar]
- Reid KJ, Martinovich Z, Finkel S, Statsinger J, Golden R, Harter K, et al. Sleep: A marker of physical and mental health in the elderly. American Journal of Geriatric Psychiatry. 2006;14:860–866. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- Rey A. Psychological examination of traumatic encephalopathy. Archives de Psychologie. 1941;28:286–340. [Google Scholar]
- Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: Results from the Bronx Aging Study. Behavioral Sleep Medicine. 2007;5:39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- Shulman KI, Shedletsky R, Silver IL. The challenge of time: clock-drawing and cognitive function in the elderly. International Journal of Geriatric Psychiatry. 1986;1:135–140. [Google Scholar]
- Sliwinski M, Hofer S. How strong is the evidence for mediators of age-related memory loss? Gerontology. 1999;45:351–354. doi: 10.1159/000022120. [DOI] [PubMed] [Google Scholar]
- Sliwinski MJ, Symth JM, Hofer SM, Stawski RS. Intraindividual coupling of daily stress and cognition. Psychology and Aging. 2006;21:545–557. doi: 10.1037/0882-7974.21.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges S, Dorn JM, Shipley MJ, Kandala N-B, Trevisan M, Miller MA, et al. Correlates of short and long sleep duration: A cross-cultural comparison between the United Kingdom and the United States. American Journal of Epidemiology. 2008;168:1353–1364. doi: 10.1093/aje/kwn337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. Effects of 24 h of sleep deprivation on waking human regional brain activity. Journal of Sleep Research. 2008;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Thurstone TG. Primary mental ability for Grades 9-12. Science Research Associates; Chicago, IL: 1962. Rev. ed. [Google Scholar]
- Trenerry MR, Crosson D, DeBoe J, Leber WR. Stroop neurological screening test. Psychological Assessment Resources; Odessa, FL: 1989. [Google Scholar]