Abstract
Osteoblastic bone metastases are the most common metastases produced by human prostate cancers (PCa). Deregulated activity of WNT growth factors resulting from overexpression of the WNT inhibitor DKK-1 is known to contribute to formation of the osteoblastic component of PCa skeletal bone metastases. In this study, we report that DKK-1 knockdown in osteolytic human PCa cells unexpectedly delays the development of both soft tissue and osseous lesions. PCa cells deficient in DKK-1 expression did not increase canonical Wnt signaling in target osteoblast cell lines; however, DKK-1 knockdown PCa cells exhibited increased expression of the CDK inhibitor p21CIP1/WAF1 and a 32% increase in G1 arrest compared to control cells. Ablating p21CIP1/WAF1 in PCa cells deficient in DKK-1 was sufficient to rescue tumor growth. Collectively, our findings demonstrate that DKK-1 overexpression supports tumor growth in part by restricting expression of p21CIP1/WAF1 through a mechanism independent of canonical Wnt signaling.
Keywords: prostate cancer, DKK-1, skeletal metastasis, Wnt, p21
Introduction
Prostate cancer (PCa) remains a leading cause of cancer-related deaths in men within the United States (1). Men who die of PCa do so as a result of metastatic spread from the prostate to the dura, liver, lungs, and bone. Metastasis to the trabecular bone of the pelvis, femur and vertebral bodies are the most common and occur in over 80% of all men who die of PCa (2). Growth of PCa within the bone results in primarily osteoblastic (bone forming) lesions with underlying areas of osteolysis (bone resorption) (3). Numerous PCa-derived protein factors have been identified that can affect osteoblast development and differentiation in vitro (4), including Wnts (5), which may contribute to the formation of osteoblastic lesions in vivo.
Wnt proteins are a large family of soluble glycoproteins that control bone development and regulation of bone mass (6, 7). Wnts signal through three pathways; the canonical, the Wnt/Ca2+, and planar cell polarity pathways. In the canonical pathway, Wnts bind to a receptor complex composed of Lrp5 or 6 and Frizzled (FZD) proteins which leads to the stabilization and activation of the TCF transcriptional co-factor β-catenin. The activity of Wnt proteins is controlled by secreted antagonists including secreted FZD-related proteins (sFRP), Wnt inhibitory factor-1 (WIF-1), Cerberus, Sclerostin, and Dickkopfs (DKK) (8). sFRP, WIF-1, and Cerberus act as competitive inhibitors of FZD by sequestering Wnt factors and can therefore block both canonical and non-canonical Wnt pathways. Sclerostin and DKK-1, 2, and 4, in contrast, bind the Wnt co-receptors LRP 5 and 6 to block canonical Wnt signaling by removing LRP from the cell surface (9).
DKK-1 was first identified in Xenopus for its ability to induce complete head structures (10). It is encoded by a relatively small (3 kb) gene at Chr 10q11.2 whose expression is restricted to the bone in adult mice (11). The temporal and spatial regulation of Wnt activity by DKK-1 is essential for normal bone development. In the absence of DKK-1, murine embryos display a fusion and duplication of digits whereas DKK-1 over-expression in the chick results in distal truncation of the limb bud (12, 13). The osteoblast specific expression of DKK-1 in the mouse leads to severe osteopenia underscoring the importance of Wnt signaling in bone formation (11, 14).
We previously demonstrated that blocking Wnt activity through over-expression of DKK-1 led to increased osteolytic activity and PCa tumor growth within bone in vivo (5). Furthermore, we demonstrated that DKK-1 expression, while strongly present in primary tumors, declines in PCa bone metastases (15). This led us to hypothesize that declining DKK-1 levels in bone metastases unmasks PCa-mediated Wnt activity that would favor development of osteoblastic lesion. To test this hypothesis, we decreased DKK-1 activity in a murine model of PCa bone metastasis. We found that decreased DKK-1 activity in osteolytic PC-3 PCa cells did not induce bone formation but rather delayed the formation of osseous and subcutaneous lesions in vivo. Reduction of DKK-1 activity induced the expression of the cyclin-dependent kinase inhibitor p21CIP1/WAF1 (CDKN1A) suggesting that DKK-1 expression in PCa cells facilitates tumor establishment by promoting cell cycle progression.
Materials and Methods
Cells
Human PC-3 and Du145 PCa cells were obtained from the American Type Culture Collection (Rockville, MD) and were maintained as previously described (5). PC-3 luciferase cells were kindly provided by Alnawaz Rehemtulla (University of Michigan, Ann Arbor, MI). PC-3 DKK-1 shRNA 366 cells were generated from PC-3 cells as previously described (5). Additional control shRNA and DKK-1 shRNA transduced cells were generated by lentiviral transduction with pLKO.1 carrying a non-targeting shRNA or DKK-1 shRNA to position 796 (5′ GAAAGAAGGTCAAGTGTGT 3′). All cells were shown to be free of Mycoplasma by the PlasmTest mycoplasma detection method (Invivogen, San Diego, CA).
Generation of DKK-1/p21 double knock-down cells
pGIPZ plasmid DNA encoding a non-targeting shRNA control or p21-directed shRNAs that target p21 at positions 703 and 888 were obtained from Open Biosystems (Huntsville, AL). Plasmids were packaged into virus particles according to manufacture’s instructions and used to transduce PC-3 DKK-1shRNA 796-transduced cells. Puromycin resistant, GFP positive clones were then selected by limiting dilution.
In vivo animal model of bone metastasis
Tumor cells (5×105 cells/50 μl) were injected into the tibia of male nude mice at 5-6 weeks of age as described previously (5). Tumors were allowed to grow for 3 or 6 weeks. All animals were evaluated using Faxitron radiography (Faxitron x-ray Corp, Wheeling, IL). Radiographs were digitized and the percent osteolytic area was quantified as previously described (5). Injected tibiae and contralateral tibiae without tumors were removed, bone mineral density measured using a pDEXA Sabre scanner (Orthometrix, Inc, White Plains, NY), and processed for histology as previously described (5).
Subcutaneous tumor growth assay
Tumor cells (1×106 cells/100 μl) were injected into the subcutis of male nude mice at 5-6 weeks of age. Tumor diameter was measured biweekly in two axes using a caliper and tumor volumes calculated using the formula (min2 × max)/2. A repeated measures generalized linear model was used to test for a difference in the tumor growth rates between the two groups.
Intracardiac PCa experimental metastasis model
A purified, neutralizing monoclonal antibody to DKK-1 and isotype control were provided by Eli-Lilly (Indianapolis, IN). Male nude mice, 5-6 weeks of age, received biweekly intraperitoneal injections of 5 mg/kg antibody in 0.1 ml PBS for the length of the study. One week after the start of antibody injections, DKK-1+ PC-3-luc cells were injected into the left cardiac ventricle as previously described (16, 17). Six weeks post tumor cell injection, tumor burden was measured by Bioluminescent imaging using a Xenogen IVIS imaging system (Xenogen Corporation,Alameda, CA).
PCR analysis
The expression of p21, DKK-1, and β-actin was evaluated by quantitative PCR on a Roche Lightcycler 480 as previously described (18). The primers used were as follows: p21-1131F 5′ ATGAAATTCACCCCCTTTCC 3′; p21-1304R 5′ CCCTAGGCTGTGCTCACTTC 3′; axin2-3585F 5′ CCCAGGTTGATCCTGTGACT 3′; axin2-3823R 5′ AGGTGTGTGGAGGAAAGGTG 3′. PCR primers for DKK-1 and β–actin appear in (5).
Transient transfection
DKK-1 366 siRNA (5′ GGAATAAGTACCAGACCA 3′) was obtained from Thermo Scientific (Lafayette, CO). Fluorescein conjugated SignalSilence non-targeting control siRNA was used to control for transfection efficiency (Cell Signaling, Danvers, MA). On day 0, 2.5×105 PCa cells/2 ml complete medium were plated to 6 well plates and allowed to attach 24 hours. The following day (day 1), the cells were transfected with 125 pmoles of siRNA using Lipofectamine 2000 (Invitrogen) according to manufactures instructions. Twenty four hours later (day 2), the media was removed and replaced with complete medium or a 72 hour conditioned medium prepared from parental PC-3 cells. On day 3, whole cell lysates or total RNA were prepared and analyzed by western blot analysis or RT-Q-PCR, respectively.
Western blot for analysis
The expression of p21 and DKK-1 proteins were determined using western blotting of total cell lysates as described previously (5). Equal amounts of protein (30 μg/sample) were resolved using 12% SDS-PAGE. The filters were cut and separate pieces blotted with anti-p21 mAb (BD Biosciences, San Jose, CA), anti-DKK-1 pAb (R&D Systems, Minneapolis, MN), or anti-α-tubulin mAb (Sigma, St. Louis, MO).
Cell cycle analysis
5×105 cells were plated to 100 mm dishes and allowed to grow for 48 hours. Following this incubation, cells were trypsinized, washed 1x in PBS and fixed for 20 minutes in 0.5 ml/tube ice-cold absolute ethanol. Fixed cells were washed then stained with Propidium Iodide solution (50 μg/ml PI and 100 units RNAase A in PBS) for 20 minutes. The diploid DNA content was quantified using a Coulter FACS Scan flow cytometer (Beckman-Coulter, Fullerton, CA).
cDNA microarray analysis
The ONCOMINE database and gene microarray analysis tool, a repository for published cDNA microarray data (http://141.214.6.50/oncomine/main/index.jsp), was explored for mRNA expression of p21 and DKK-1 in clinical cases of prostate, breast, and ovarian cancer. Statistical analysis of differences was performed using ONCOMINE algorithms to account for the multiple comparisons among different studies as previously described (19).
Results
Blocking DKK-1 delays PCa establishment within the bone
We previously demonstrated that Wnts contribute to the osteoblastic component of PCa skeletal metastases (5). This conclusion was based on three principle lines of evidence: 1) PCa cells express the RNA for multiple Wnts, 2) expression of the Wnt inhibitor DKK-1 in osteoblastic C4-2B PCa cells stimulated severe osteolysis in vivo, and 3) shRNA mediated knock-down of DKK-1 in osteolytic PC-3 PCa cells stimulated osteoblast differentiation and mineralization of co-cultured bone marrow stromal cells. PC-3 DKK-1 shRNA 366 clone 3 cells are a stable clone isolated following calcium phosphate transfection with a shRNA molecule that targets position 366 of DKK-1 (5). These cells were shown to have >80% down-regulation of DKK-1 protein but equal cell proliferation rates in vitro compared to a DKK-1 directed but non-cleaving shRNA control-transfected clone (5). To characterize the effect of DKK-1 knock-down on the formation of osseous lesions, both PC-3 DKK-1 shRNA 366 and DKK-1 control shRNA-transfected cells were injected into the tibiae of male nude mice (intratibial injection).
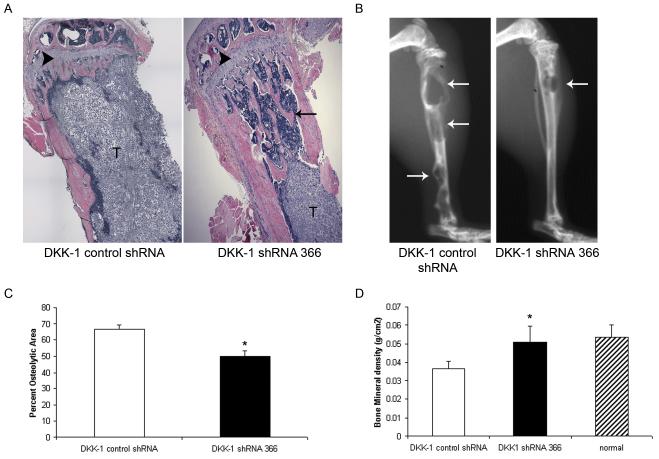
Based on the above data, it was expected that DKK-1 shRNA 366 cells would induce bone production following intratibial injection. However, DKK-1 knock-down cells were found to grow poorly in the bone of nude mice. Specifically, PC-3 DKK-1 shRNA 366 cells were found to form tumors with equal incidence (incidence DKK-1 control shRNA cells, 8/8; incidence of DKK-1 shRNA 366 clone 3 cells, 9/10) but developed smaller lesions compared to DKK-1+ control shRNA-transfected cells. Decreased tumor growth was evident by a decline in tumor volume based on histological evaluation (Figure 1a). Corresponding with the decreased tumor volume, there was a decline in bone remodeling as observed on radiographs (Figure 1b), a reduction in percent osteolytic area (Figure 1c), and an increase in bone mineral density of injected tibia (Figure 1d). Together, these data show that blocking DKK-1 expression reduced the intraosseous growth of PC-3 PCa cells, a cell line with abundant DKK-1 expression.
Figure 1. Blocking DKK-1 delays PCa establishment within the bone.
DKK-1 directed but non-cleaving control shRNA-transfected or DKK-1 shRNA 366-transfected cells (5×105 cells/50 μl) were directly injected into the tibiae of anesthetized male nude mice (10 mice/group). Tumors were allowed to grow for 6 weeks at which time the mice were sacrificed and bones processed for histological evaluation. A) Representative H&E histology of tibiae injected with DKK-1 control shRNA-transfected cells vs. DKK-1 shRNA 366 cells. Normal trabeculae (arrow), marrow (M), tumor (T), and growth plate (arrowhead). Original magnification, 20x. B) Corresponding Faxitron X-rays of tibiae injected with DKK-1 control shRNA cells or DKK-1 shRNA 366-transfected cells. Arrows indicate osteolytic activity. C) Percent osteolytic area of total tibial area. Radiographs from tumor-injected tibiae were digitized and the percent osteolytic area of the total tibial area was quantified. Data are presented as mean±standard error within each group; *p<0.002. vs. PC-3 DKK-1 control shRNA cells by t-test. D) Bones were subjected to DEXA to quantify bone mineral density. Normal tibiae (n=5), tibiae injected with PC-3 DKK-1 control shRNA cells (n=8) or PC-3 DKK-1 shRNA 366-transfected cells (n=10) were scanned. Data are presented as mean±standard error within each group; *p<0.005 compared to PC-3 DKK-1 control shRNA-transfected tibiae by t-test.
Blocking DKK-1 decreases PCa tumor burden in vivo
The molecular knock-down of DKK-1 using a shRNA molecule delayed PCa tumor establishment within the bone following direct injection. To determine whether the effect was specific to bone, PC-3 DKK-1 shRNA 366-transfected or DKK-1 control shRNA-transfected cells were injected into the subcutis of nude mice and the incidence and growth rate of the tumors measured. As before, the tumor incidence was equivalent in each group (4/4 DKK-1 control shRNA vs. 10/10 DKK-1 shRNA 366), however; the DKK-1 shRNA-transfected cells developed tumors at a much slower rate as reflected in both a significant decreased tumor growth rate and tumor weight at the experimental endpoint (Figure 2 a-b, respectively). These data not only suggest that DKK-1 expression promotes tumor growth and establishment but also indicate that the effect of DKK-1 knock-down was not specific to the bone.
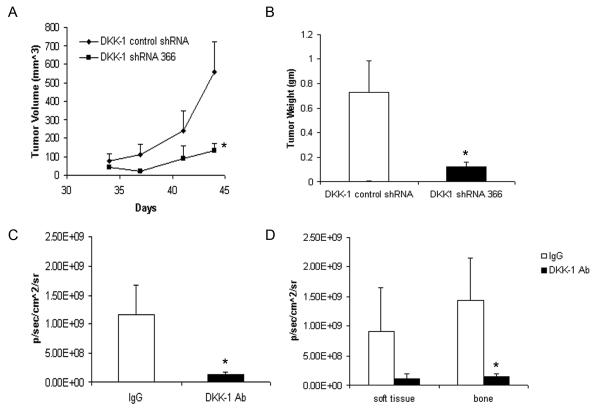
Figure 2. Blocking DKK-1 decreases PCa tumor burden in vivo.
DKK-1 control shRNA-transfected or DKK-1 shRNA 366-transfected cells (1×106 cells/100 μl) were directly injected into the hind flank of anesthetized male nude mice. A) Tumor diameter was measured in two axes using a caliper twice a week and tumor volume calculated. Shown is the tumor volume over time of DKK-1 control shRNA-transfected or DKK-1 shRNA 366-transfected cells; mean±standard error of subcutaneous tumors. *p<0.006 vs. DKK-1 control shRNA cells by a repeat measures generalized linear model. B) Subcutaneous tumor weight at experimental endpoint. After 6 weeks, the subcutaneous tumors were excised and weighed. Shown is the mean±standard error of subcutaneous tumors; *p<0.003 vs. DKK-1 control shRNA cells by t-test. C-D. DKK-1 neutralizing antibody: Beginning at Day 0, male Nu/Nu mice were injected with 5 mg/kg IgG isotype control or DKK-1 neutralizing antibody (I.P.) twice a week for seven total weeks. At Day 7, DKK-1+ PC-3 luciferase cells were injected into the left cardiac ventricle (2×105 cells/0.1 ml) to establish bone metastases. C) Total tumor burden was measured using bioluminescent imaging following the injection of luciferin. The data are presented as mean number of photons/sec/cm2 (± the standard error), 10 mice/group. *p<0.028 compared to IgG control mice by t-test. D) Tumor burden in soft tissue or bone lesions within each treatment group; *p<0.02 compared to IgG control mice by t-test.
To confirm that reduced DKK-1 expression suppresses tumor growth in soft tissue and bone, an alternative method to inhibit DKK-1 function was used. Specifically, a DKK-1 neutralizing antibody was employed to decrease systemic DKK-1 activity in an experimental metastasis model. PC-3 cells injected into the left cardiac ventricle (intracardiac injection) produce osteolytic bone lesion of the mandible, humerus, and tibia/femur as well as rare soft tissue lesions. As observed with the DKK-1 shRNA knock-down cells, DKK-1 neutralizing antibody did not alter tumor incidence compared to IgG control as the median number of lesions/mouse in each group was 3 (range 1-6). However, treatment with DKK-1 neutralizing antibody reduced the overall tumor burden 8.3-fold compared IgG control (Figure 2c). A similar reduction with DKK-1 antibody treatment was observed when separately comparing only bone lesions (Figure 2d). There was also a strong trend towards decreased formation of soft tissue lesions in DKK-1 antibody treated animals (Figure 2d) indicating that DKK-1 activity promotes tumor establishment at any site. Taken together, the data demonstrate that knock-down of DKK-1 activity with either a shRNA or neutralizing antibody reduced PCa tumor growth in vivo.
DKK-1 knock-down increases p21 expression and G1 arrest
The mechanism through which DKK-1 suppression leads to a reduction in tumor growth is unknown but may result from alterations in the rate of apoptosis and/or cell cycle. An increase in tumor cell apoptosis or a decrease in the rate of cell cycling would have a negative impact on tumor growth at any site. To investigate both of these possibilities, changes in the expression of apoptosis and cell cycle genes between parental and DKK-1 shRNA 366-transfected cells were compared using PCR arrays (Table 1). The top two cell cycle genes upregulated upon DKK-1 knock-down were the cyclin-dependent kinase inhibitors p21CIP1/WAF1 and p15INK4B, however, only p21 was found to change at both the RNA and protein levels and thus was explored further. To validate the effect of DKK-1 knock-out on CDKN expression independent of clonal cell selection, p21 expression was measured following the transient transfection of a DKK-1 siRNA oligonucleotide that targets position 366. Quantitative PCR analysis demonstrated that DKK-1 knock-down increased p21 RNA transcripts 2-fold in both PC-3 and Du145 DKK-1 siRNA-transfected PCa cells compared to control siRNA-transfected cells (Figure 3a). Further western blotting confirmed that the suppression of DKK-1 increased p21 protein levels without a corresponding increase in p27 in both cell lines suggesting that DKK-1 knock-down selectively induced p21 vs. other CDKNs (Figure 3b). To show the specificity of DKK-1 knock-down on p21 expression, an additional stable DKK-1 shRNA cell line was generated with a shRNA that targets DKK-1 at position 796. Again, to avoid clonal selection bias, a stable viral-transduced pool of cells was used for this analysis. As in siRNA transfected cells, transduction with the DKK-1 shRNA 796 decreased DKK-1 levels 10.8±0.7-fold and increased p21 expression 5.1±0.9-fold compared to control shRNA-transduced cells supporting that DKK-1 knock-down lead to increased p21 expression (Figure 3c). To confirm that the increase in p21 message was mediated by DKK-1, the ability of DKK-1 protein to restore p21 levels was measured. The addition of PC-3 conditioned medium to add back DKK-1 protein restored p21 expression to normal levels in DKK-1 siRNA-transfected cells thus confirming that DKK-1 directly effects p21 expression (Figure 3d). Together, these results demonstrate that the knock-down of DKK-1 increased p21 expression in PCa cells.
Table I.
Cell cycle genes elevated in DKK-1 knock-down cells
| Gene name | Parental vs. DKK-1 shRNA 366 (fold change) |
|---|---|
|
Cyclin-dependent kinase
inhibitor 1A (p21, Cip1) |
10.3 |
|
| |
|
Cyclin-dependent kinase
inhibitor 2B (p15, inhibits CDK4) |
4.2 |
|
| |
| Cyclin D2 | 4.1 |
|
| |
| Hect domain and RLD 5 | 3.1 |
|
| |
| Growth arrest and DNA- damage-inducible, alpha |
2.0 |
|
| |
| Retinoblastoma-like 2 (p130) | 1.9 |
|
| |
| DIRAS family, GTP-binding RAS-like 3 |
1.8 |
|
| |
| Tumor protein p53 (Li- Fraumeni syndrome) |
1.7 |
|
| |
| Cyclin-dependent kinase inhibitor 1B (p27, Kip1) |
1.6 |
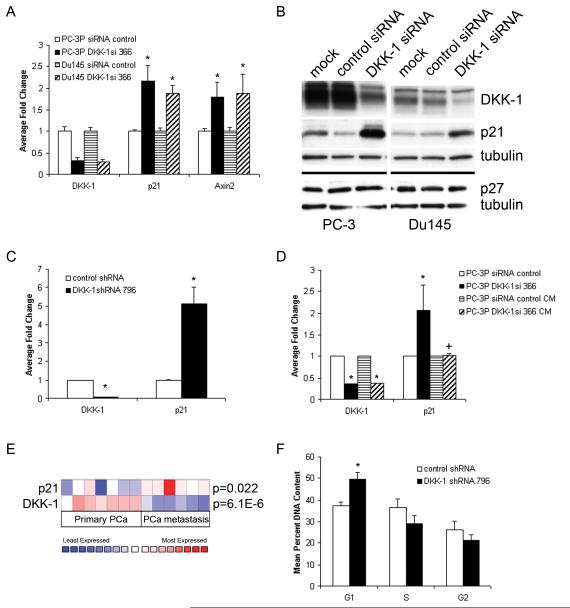
Figure 3. DKK-1 knock-down increases p21 expression and G1 arrest.
Parental PC-3 and Du145 PCa cells were transiently transfected with a control siRNA oligo or a DKK-1 366 targeting siRNA. After 48 hours, cells were lysed for both RNA and protein isolation. A) RT quantitative-PCR analysis of transfected cells for DKK-1, p21, or axin-2, mean±standard deviation of four experiments; *p<0.01 compared to control siRNA cells by t-test. B) Corresponding western blot of transfected cells. Membranes were blotted with antisera specific to DKK-1, p21, p27 or, as a control for loading, α-tubulin. C) RT-quantitative PCR analysis of DKK-1 and p21 expression in stable pools of DKK-1 shRNA 796-transduced cells. Shown is the fold change vs. control shRNA-transduced cells, mean±standard error of duplicate experiments; *p<0.004 vs. control shRNA cells by t-test. D) RT-quantitative PCR analysis of DKK-1 and p21 expression in PC-3 DKK-1 siRNA 366 or control siRNA-transfected cells treated with 72 hour PC-3 conditioned medium. Shown is the fold change vs. control shRNA-transduced cells, mean±standard deviation of duplicate experiments; *p<0.05 vs. control siRNA cells by t-test. E) The Oncomine cDNA microarray database was evaluated for p21 expression in human PCa tumor samples where DKK-1 was found to be decreased. Shown is a study comparing clinical cases of primary PCa vs. PCa metastases. F) Cell cycle analysis. Equal number of DKK-1 shRNA 796-transduced or control shRNA-transduced cells (5×105 cells) were plated at Day 0 and the percent DNA content was measured at Day 2 by flow cytometry following staining with Propidium Iodide. Shown is the mean percent DNA content, mean±standard error of three experiments; *p<0.01 vs. control shRNA cells by t-test.
To explore whether this observation extended to clinical PCa and other cancer cell types, the Oncomine cDNA microarray database was used to evaluate p21 expression in human tumor samples where DKK-1 was found to be decreased. In a study comparing clinical cases of primary PCa vs. PCa metastases, DKK-1 was dramatically decreased in PCa metastases vs. primary lesions, consistent with our analysis of PCa clinical samples using tissue microarrays (15) (Figure 3e). Compared to the primary lesions, p21 was significantly increased in PCa metastases consistent with our in vitro data and published reports by Navone et al (20). Further query of Oncomine revealed that reduced DKK-1 expression correlated with increased p21 levels in clinical cases of human breast cancer and ovarian cancer thus supporting that an inverse relationship between DKK-1 and p21 is a feature of many cancer cell types (data not shown).
To examine whether the increase in p21 contributed to suppression of cell cycle, DKK-1 shRNA 796 and control shRNA-transduced cells were compared for difference in cell cycle distribution using PI-flow cytometry. P21 is a known inhibitor of cyclin D-Cdk4/6 and cyclin E-Cdk2 which control cell cycle progression from G1 to S phase. An increase in p21, therefore, is expected to increase the number of cells in G1 and decrease the number of cells in either S or G2. Consistent with this fact, compared to control shRNA-transduced cells, the diploid DNA content at G1 in DKK-1 shRNA 796 knock-down cells was found to increase 32.2 ± 5.0% (Figure 3f) indicating that increased p21 expression following DKK-1 knock-down can lead to cell cycle arrest.
To test whether DKK-1-mediated changes in cell cycle directly affect PCa cell proliferation, 2-D attachment dependent growth rates were measured in vitro over 96 hours. Despite the modest yet reproducible increase in G1 fraction within DKK-1 shRNA 796-transduced cells, there was no decrease in attachment dependent growth compared to control shRNA-transduced cells (data not shown), consistent with our previous published reports (5). These data raise the possibility that p21 elevated cells are selected against during continuous culture. To remove possible selection bias, 2-D attachment dependent growth rates were compared in PC-3 and Du145 DKK-1 siRNA transiently-transfected cells. Consistent with the cell cycle data, DKK-1 siRNA-transfected cells showed a modest yet reproducible decrease in attachment dependent growth on plastic (Supplemental figure 1A and 1B). Moreover, DKK-1 knock-down did not appear to impact PCa cell survival under attachment independent conditions (supplemental figure 1C) suggesting that DKK-1 knock-down increases cell cycle arrest leading to suppression of cellular proliferation.
DKK-1 knock-down increased p21 expression through a mechanism independent of canonical Wnt-signaling
Given that DKK-1 is a known inhibitor of Wnt proteins that stabilize β-catenin, we hypothesized that β-catenin signaling mediates the observed increase in p21 and subsequent growth suppressive effects in PCa. Further, we previously demonstrated that PCa cells express the RNA for multiple canonical Wnts supporting that canonical Wnt signaling may be elevated in DKK-1 suppressed cells (5). Consistent with these findings, the β-catenin target gene axin-2 was elevated in DKK-1 knock-down cells compared to control siRNA-transfected cells (Figure 3a). However, the conditioned media from PC-3 DKK-1shRNA 796 cells failed to increase β-catenin activity in co-cultured ST-2 cells as measured by TOP-FLASH reporter assays; whereas, co-transfection of β-catenin cDNA or the addition of rWnt3a protein could activated TOP-FLASH (Supplemental figure 2A). PC-3 DKK-1shRNA 796 cells were further shown to have similar levels of total and phosphorylated Jun N-terminal kinase (JNK) compared to shRNA control-transduced cells suggesting that there were also no changes in non-canonical Wnt signaling within DKK-1 knock-down cells (Supplemental figure 2B). These findings suggest that DKK-1 knock-down increases p21 expression through a mechanism independent of canonical Wnt-signaling and are consistent with those of Sato et al who showed cytotoxic effects of DKK-1 neutralizing antibody in A549 lung cancer cells without a corresponding increase in either canonical or non-canonical Wnt signaling (21).
Because no changes in β-catenin or JNK transcriptional activity were observed, we investigated whether p21 was regulated at the post-transcriptional level. Analysis of p21 RNA transcripts demonstrated an increase in the steady state levels of p21 in DKK-1shRNA 796 cells without a corresponding increase in p21 RNA synthesis compared to control shRNA-transduced cells (Supplemental figure 2C). Viewed together, these data suggest that DKK-1 knock-down increased the stability of p21 RNA transcripts independent of Wnt signaling. The precise molecular mechanism through which decreased DKK-1 expression controls p21 levels via RNA stability are still under investigation. However, our data are consistent with a growth suppressive effect of DKK-1 knock-down through mRNA stabilization of p21.
Knock-down of p21 restores in vivo tumorigenicity of DKK-1 knock-down cells
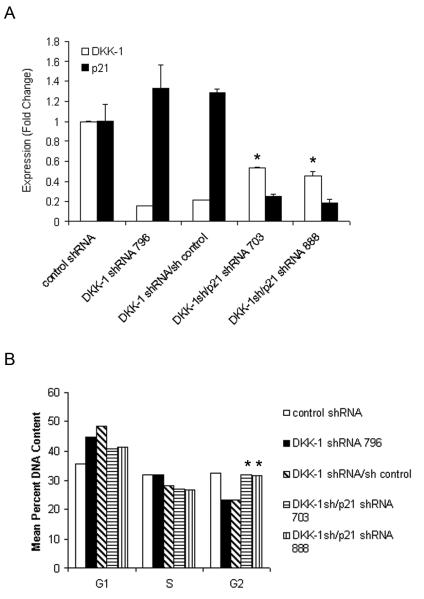
To directly test whether p21 participated in suppression of tumor growth following decreased DKK-1 activity, p21 expression was decreased in DKK-1 shRNA 796 cells through transduction with two different p21 targeting shRNAs. Quantitative PCR confirmed the successful knock-down of both DKK-1 and p21 mRNA compared to control shRNA-transduced cells (Figure 4a). As observed previously with DKK-1 single knock-out cells, DKK-1 shRNA/control shRNA-transduced cells had a 36.3% increase in G1 content compared to control shRNA cells. DKK-1 shRNA/p21 shRNA double knock-out cells showed a statistically significant 36.8±0.5% increase in G2 content, with modest reductions in G1 and S phase content (11.8±0.6% and 10.1±0.5%, respectively), compared to DKK-1 single knock-out cells suggesting that knockdown of p21 restored cell cycle progression in cells with decreased DKK-1 expression (Figure 4b).
Figure 4. Characterization of DKK-1/p21 double knock-down cells.
PC-3 DKK-1 shRNA 796 cells were transduced with a control shRNA or two shRNA specific to p21. A) RT quantitative-PCR analysis of transduced cells for DKK-1 or p21, mean±standard deviation of a representative experiment; *p<0.003 vs. control shRNA cells by t-test. B) Cell cycle analysis. Equal number of cells (5×105 cells) were plated at Day 0 and the percent DNA content was measured using flow cytometry at Day 2 following staining with Propidium Iodide; *p<0.0001 vs. DKK-1 shRNA cells by t-test.
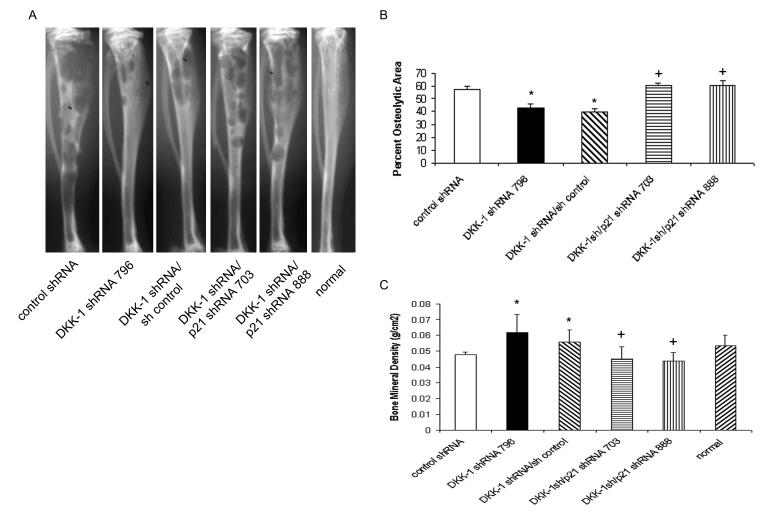
Upon direct injection into the tibiae of male nude mice (10 mice/group), DKK-1 single knock-out or DKK-1 shRNA/control shRNA-transduced cells grew poorly compared to control shRNA-transduced cells consistent with data obtained with DKK-1 shRNA 366 clones (Figure 5a-c). As in the previous experiments, the incidence of osseous tumors was 100% in all groups. Both the DKK-1 shRNA 796-transduced and DKK-1 shRNA/control shRNA-transduced cells had decreased osteolysis in the bone as evident on radiographs (Figs. 5a and b) and bone mineral density (Fig. 5c). Suppression of p21 in DKK-1 knock-down cells restored the tumorigenicity of DKK-1 knock-down cells by the same measures and were indistinguishable from control shRNA-transduced cells (Figure 5a-c). Specifically, knock-down of p21 increased osteolytic area 47.1% and decreased bone mineral density 24.4% compared to both DKK-1 shRNA 796-transduced and DKK-1 shRNA/control shRNA-transduced cells and was similar to that of control shRNA-transduced cells alone. Taken together, the data demonstrate that targeting DKK-1 delays PCa growth in vivo, in part, through p21.
Figure 5. Knock-down of p21 restores in vivo tumorigenicity of DKK-1 knock-down cells.
Control shRNA-transduced, DKK-1 shRNA 796-transduced, DKK-1 shRNA 796/control shRNA-transduced, or DKK-1 shRNA 796-transduced/p21shRNA-transduced cells (5×105 cells/50 μl) were directly injected into the tibiae of anesthetized male nude mice (10 mice/group). Tumors were allowed to grow for 3 weeks at which time the mice were sacrificed and bones radiographed and processed for histological evaluation. A) Representative Faxitron X-rays of injected tibia. B) Percent osteolytic area of total tibial area. Radiographs from tumor-injected tibiae were digitized and the percent osteolytic area of the total tibial area was quantified. Data are presented as mean±standard error within each group; *p<0.003 vs. PC-3 control shRNA cells; +p<0.002 vs. PC-3 DKK-1 shRNA 796 cells by t-test. C) Bones were subjected to DEXA to quantify bone mineral density. Data are presented as mean±standard error within each group; *p<0.01 compared to control shRNA-transduced tibiae; +p<0.001 vs. PC-3 DKK-1 shRNA 796-transduced tibiae by t-test.
As reduction of p21 expression in cells with reduced DKK-1 expression restored intraosseous growth, we wanted to determine if reduction of p21 alone could account for the restored growth in bone. Accordingly, Du145 control shRNA or p21 shRNA 888 single knock-down cells were evaluated in in vitro and in vivo growth assays. Quantitative PCR analysis confirmed that p21-directed shRNA specifically reduced p21 RNA transcript levels compared to shRNA control cells (Supplemental figure 3A). Despite the decrease in p21 expression, Du145 p21 shRNA 888-transduced cells did not display increased in vitro proliferation in cells expressing DKK-1 protein (Supplemental figure 3B). Consistent with this finding, p21 shRNA cells did not display increased intraosseous growth compared to control shRNA-transduced cells as indicated by no appreciable change in tumor volume, osteolytic area, or bone mineral density (Supplemental figure 4). Viewed together with the PC-3 cell in vivo data, these results suggest that knock-down of p21 alone is not sufficient to increase PCa cell growth when DKK-1 is present, however; the impact of p21 on tumor growth is realized only when DKK-1 expression is concomitantly down-regulated.
Discussion
The skeleton is the first site of reoccurrence in men who fail androgen deprivation therapy (22). Therefore, if improvements are to be realized in the treatment of men with PCa, a better understanding of the biology of bone metastases is needed. The Wnt/DKK-1 axis represents an important pathway that may contribute to tumor development within the bone and visceral sites. In the present study, we demonstrate that knock-down of DKK-1 delays the growth of PCa tumors in vivo. We further provide evidence that DKK-1 blockade increases the expression of the cyclin dependent kinase inhibitor p21CIP1/WAF1 through a mechanism independent of canonical Wnt-signaling resulting in increased cell cycle arrest. Finally, knock-down of p21 restores the tumorigenicity of DKK-1 knock-down cells supporting the hypothesis that DKK-1 expression in PCa cells facilitates tumor establishment by preventing suppression of cell cycle through induction of p21.
The hypothesis that the expression of a Wnt inhibitor, DKK-1, promotes tumor growth in vivo is consistent with a number of pieces of clinical data. The correlation between elevated serum expression of DKK-1 in patients with osteolytic lesions of multiple myeloma (MM) is well documented (23). Subsequently, increased DKK-1 expression is patient sera or tumor tissue has been correlated with lung cancer, hepatocellular carcinoma, esophageal squamous cell carcinoma, and osteosarcoma where high DKK-1 levels were shown to be an independent predictor of diminished overall survival and/or disease free survival within these patients (24-27). Concordantly, neutralizing antibodies to DKK-1 efficiently reduced MM-induced osteolysis and decreased MM tumor growth in bone within a rodent model of myelomatous bone disease (28-30). The mechanism for the in vivo inhibition of MM growth may result from inhibition of bone marrow stromal cell production of interleukin-6 or the Wnt-mediated expression of osteoprotegerin (28, 31, 32). Our data using a DKK-1 neutralizing antibody in PCa also demonstrated decreased tumor burden in bone but are extended to soft tissue metastases suggesting a general anti-tumor effect that we ascribe to deregulation of cell cycle.
General anti-tumor activity in PCa cells following DKK-1 knock-down may seem inconsistent with the well-described pro-oncogenic effect of Wnt/β-catenin signaling in colon cancer; however, in the case of PCa, it appears that Wnt signaling may have an anti-tumor effect in transformed cells. Consistent with this possibility is that expression of the Wnt-signaling mediator, β-catenin, is frequently lost during PCa progression. Specifically, analysis of over 500 clinical cases in 5 separate studies found that β-catenin expression decreased with increasing tumor grade and was lost in advanced PCa lesions and bone metastases (33-37). Decreased β-catenin was a poor prognostic factor for survival in each of these studies implying that canonical Wnt signaling impedes PCa tumor progression. Consistent with these findings, activating mutations in the canonical Wnt pathway are rare in PCa occurring in only 5-30% of PCa primary lesions (38). In our own study, β-catenin was found to redistribute from the nucleus to the cytoplasm in PCa metastases, concomitant with a decrease in DKK-1 expression, further supporting β-catenin loss in advanced PCa (15). In this light, it is interesting to note that DKK-1 expression in the serum of patients with gastric cancer, colorectal cancer, ovarian cancer, and cervical adenocarcinoma show decreased DKK-1 levels compared to unaffected individuals (26). These data suggest that canonical Wnt signaling is more important in these cancers and provide evidence that Wnt signaling is not universally oncogenic in all lesions at all stages. Although β-catenin is involved in colon cancer tumorigenesis, Wnt signaling appears to be tumor suppressive in advanced PCa cells. During review of this manuscript, Sato et al published that DKK-1 antibody reduced the in vivo growth of A549 lung cancer cells implanted in the subcutis (21). These studies suggested that DKK-1 suppression increased tumor cell apoptosis through and undefined, Wnt-independent mechanism. Our results are consistent with these findings and suggest that stabilization of p21 is a possible mechanism for the observed anti-tumor effect of DKK-1 knock-down in multiple cancer types.
Our data support a model in which DKK-1 knock-down delays PCa tumor establishment through increased cell cycle arrest. Although stable DKK-1 shRNA-transduced cells maintain a modest yet reproducible increase in both p21 and G1 content compared to control shRNA-transduced cells, we have been unable to demonstrate changes in attachment dependent growth in vitro in DKK-1 knock-down cells. It is unclear why these cells, which show markedly reduced growth in vivo, would have an increase in p21 protein without a decrease in in vitro proliferation. One possibility is that while DKK1 increases p21, it also my impact other factors that promote cell proliferation or decrease apoptosis which results in no overall change in cell growth as measured by in vitro assays. The divergent results may also indicate a role of DKK-1 in the host micro-environment that suppresses tumor cell growth independent of direct effects on the tumor cell. The fact that changes in in vitro growth were observed in transient DKK-1 knock-down cells raises the possibility that over time we may select against p21-elevated cells in continuous culture. Which of these possibilities or combination is relevant in PCa is unclear, however, our results are consistent with the model that DKK-1 knock-down increases cell cycle arrest and suppresses tumor growth through the induction of p21.
Our finding that p21 delays tumor growth in DKK-1 knock-down PCa cells is consistent with p21’s ability to induce cell cycle arrest and its ability to mediate tumor suppression induced by chemotherapeutic agents (39). Further, the over-expression of p21 in human melanoma cells suppressed (40); whereas the knock-down of p21 in human PCa cells enhanced the growth of transfected cells in vivo (41). Transient transfection of p21 in PCa cell line was also found to suppress in vitro proliferation independent of androgen sensitivity (42). However, p21 has also been shown to have anti-apoptotic activity and likely plays a key role in cell survival following DNA damage by promoting the growth arrest that permits DNA repair (43). Moreover in PCa, p21 expression in primary lesions was associated with progression to androgen independence (20) and shorter overall survival in patients treated by androgen ablation therapy (44). Expression of p21 was also found to correlate with higher Gleason score and PSA failure following radiotherapy (45). In our own survey of DKK-1 expression in metastatic lesions from men who died of PCa, low DKK-1 expression was associated with longer overall survival (15). Whether p21 expression is higher in DKK-1 low expressing lesions and associated with better clinical outcome compared to DKK-1 high expressing tumors is currently unknown. Our observation that DKK-1 knock-down led to p21 upregulation is consistent with the observation that over-expression of DKK-1 in DKK-1 transgenic mice down-regulated p21 expression in hematopoietic stem cells (46). Viewed together, induction of p21 in PCa cells remains a likely mechanism for tumor suppression in DKK-1 knock-down cells.
In conclusion, we demonstrated that suppression of DKK-1 in PCa cells leads to a decrease in tumor growth thorough induction of p21 in vivo. These data suggest that DKK-1 expression in PCa is an early event necessary to support osteolysis and early tumor establishment. However as the tumor progresses, DKK-1 expression is lost allowing Wnt mediated bone formation. These data provide a possible mechanism for the clinical appearance of PCa bone metastases.
Supplementary Material
Acknowledgments
Supported in part by National Cancer Institute Grants P01 CA093900 and R01 CA071672.
References
- 1.Keller ET, Zhang J, Cooper CR, et al. Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev. 2001;20:333–49. doi: 10.1023/a:1015599831232. [DOI] [PubMed] [Google Scholar]
- 2.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–83. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 3.Roudier MP, Corey E, True LD, et al. Histological, immunophenotypic and histomorphometric characterization of prostate cancer bone metastases. Cancer Treat Res. 2004;118:311–39. doi: 10.1007/978-1-4419-9129-4_13. [DOI] [PubMed] [Google Scholar]
- 4.Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005;5:21–8. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 5.Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65:7554–60. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 6.Hall CL, Kang S, Macdougald OA, Keller ET. Role of wnts in prostate cancer bone metastases. J Cell Biochem. 2006;97:661–72. doi: 10.1002/jcb.20735. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y. Wnts and wing: Wnt signaling in vertebrate limb development and musculoskeletal morphogenesis. Birth Defects Res C Embryo Today. 2003;69:305–17. doi: 10.1002/bdrc.10026. [DOI] [PubMed] [Google Scholar]
- 8.Rubin JS, Barshishat-Kupper M, Feroze-Merzoug F, Xi ZF. Secreted WNT antagonists as tumor suppressors: pro and con. Front Biosci. 2006;11:2093–105. doi: 10.2741/1952. [DOI] [PubMed] [Google Scholar]
- 9.Mao B, Wu W, Davidson G, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–7. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 10.Glinka A, Wu W, Delius H, et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–62. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Sarosi I, Cattley RC, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–66. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Grotewold L, Ruther U. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J. 2002;21:966–75. doi: 10.1093/emboj/21.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–34. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald BT, Joiner DM, Oyserman SM, et al. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41:331–9. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate. 2008;68:1396–404. doi: 10.1002/pros.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun YX, Schneider A, Jung Y, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Lu Y, Dai J, et al. In vivo real-time imaging of TGF-beta-induced transcriptional activation of the RANK ligand gene promoter in intraosseous prostate cancer. Prostate. 2004;59:360–9. doi: 10.1002/pros.20019. [DOI] [PubMed] [Google Scholar]
- 18.Dai J, Hall CL, Escara-Wilke J, et al. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 2008;68:5785–94. doi: 10.1158/0008-5472.CAN-07-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fizazi K, Martinez LA, Sikes CR, et al. The association of p21((WAF-1/CIP1)) with progression to androgen-independent prostate cancer. Clin Cancer Res. 2002;8:775–81. [PubMed] [Google Scholar]
- 21.Sato N, Yamabuki T, Takano A, et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res. 70:5326–36. doi: 10.1158/0008-5472.CAN-09-3879. [DOI] [PubMed] [Google Scholar]
- 22.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–25. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 23.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 24.Lee N, Smolarz AJ, Olson S, et al. A potential role for Dkk-1 in the pathogenesis of osteosarcoma predicts novel diagnostic and treatment strategies. Br J Cancer. 2007;97:1552–9. doi: 10.1038/sj.bjc.6604069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makino T, Yamasaki M, Takemasa I, et al. Dickkopf-1 expression as a marker for predicting clinical outcome in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:2058–64. doi: 10.1245/s10434-009-0476-7. [DOI] [PubMed] [Google Scholar]
- 26.Sheng SL, Huang G, Yu B, Qin WX. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin Chem. 2009;55:1656–64. doi: 10.1373/clinchem.2009.125641. [DOI] [PubMed] [Google Scholar]
- 27.Yu B, Yang X, Xu Y, et al. Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol. 2009;50:948–57. doi: 10.1016/j.jhep.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Fulciniti M, Tassone P, Hideshima T, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–9. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heath DJ, Chantry AD, Buckle CH, et al. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res. 2009;24:425–36. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- 30.Yaccoby S, Ling W, Zhan F, et al. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–11. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glass DA, 2nd, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Gunn WG, Conley A, Deininger L, et al. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–91. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 33.Aaltomaa S, Karja V, Lipponen P, et al. Reduced alpha- and beta-catenin expression predicts shortened survival in local prostate cancer. Anticancer Res. 2005;25:4707–12. [PubMed] [Google Scholar]
- 34.Bismar TA, Humphrey PA, Grignon DJ, Wang HL. Expression of beta-catenin in prostatic adenocarcinomas: a comparison with colorectal adenocarcinomas. Am J Clin Pathol. 2004;121:557–63. doi: 10.1309/4470-49GV-52H7-D258. [DOI] [PubMed] [Google Scholar]
- 35.Bryden AA, Hoyland JA, Freemont AJ, et al. E-cadherin and beta-catenin are down-regulated in prostatic bone metastases. BJU Int. 2002;89:400–3. doi: 10.1046/j.1464-4096.2001.01712.x. [DOI] [PubMed] [Google Scholar]
- 36.Horvath LG, Henshall SM, Lee CS, et al. Lower levels of nuclear beta-catenin predict for a poorer prognosis in localized prostate cancer. Int J Cancer. 2005;113:415–22. doi: 10.1002/ijc.20599. [DOI] [PubMed] [Google Scholar]
- 37.Jaggi M, Johansson SL, Baker JJ, et al. Aberrant expression of E-cadherin and beta-catenin in human prostate cancer. Urol Oncol. 2005;23:402–6. doi: 10.1016/j.urolonc.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 38.Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:119–26. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Bishop WR, Liu M. Differential effects of cell cycle regulatory protein p21(WAF1/Cip1) on apoptosis and sensitivity to cancer chemotherapy. Drug Resist Updat. 2003;6:183–95. doi: 10.1016/s1368-7646(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 40.Yang ZY, Perkins ND, Ohno T, Nabel EG, Nabel GJ. The p21 cyclin-dependent kinase inhibitor suppresses tumorigenicity in vivo. Nat Med. 1995;1:1052–6. doi: 10.1038/nm1095-1052. [DOI] [PubMed] [Google Scholar]
- 41.Roy S, Singh RP, Agarwal C, et al. Downregulation of both p21/Cip1 and p27/Kip1 produces a more aggressive prostate cancer phenotype. Cell Cycle. 2008;7:1828–35. doi: 10.4161/cc.7.12.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugibayashi R, Kiguchi Y, Shimizu T, et al. Up-regulation of p21(WAF1/CIP1) levels leads to growth suppression of prostate cancer cell lines. Anticancer Res. 2002;22:713–9. [PubMed] [Google Scholar]
- 43.Weiss RH. p21Waf1/Cip1 as a therapeutic target in breast and other cancers. Cancer Cell. 2003;4:425–9. doi: 10.1016/s1535-6108(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 44.Omar EA, Behlouli H, Chevalier S, Aprikian AG. Relationship of p21(WAF-I) protein expression with prognosis in advanced prostate cancer treated by androgen ablation. Prostate. 2001;49:191–9. doi: 10.1002/pros.1134. [DOI] [PubMed] [Google Scholar]
- 45.Rigaud J, Tiguert R, Decobert M, et al. Expression of p21 cell cycle protein is an independent predictor of response to salvage radiotherapy after radical prostatectomy. Prostate. 2004;58:269–76. doi: 10.1002/pros.10329. [DOI] [PubMed] [Google Scholar]
- 46.Fleming HE, Janzen V, Lo Celso C, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–83. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.