Abstract
Glioblastoma multiforme (GBM) is a deadly primary brain tumor in adults, with a median survival of ~12–18 months post-diagnosis. Despite recent advances in conventional therapeutic approaches, only modest improvements in median survival have been achieved; GBM usually recurs within 12 months post-resection, with poor prognosis. Thus, novel therapeutic strategies to target and kill GBM cells are desperately needed. Our group and others are pursuing virotherapy and gene therapy strategies for the treatment of GBM. In this review, we will discuss various virotherapy and gene therapy approaches for GBM currently under preclinical and clinical evaluation including direct or conditional cytotoxic, and/or immunostimulatory approaches. We also discuss cutting-edge technologies for drug/gene delivery and targeting brain tumors, including the use of stem cells as delivery platforms, the use of targeted immunotoxins, and the therapeutic potential of using GBM microvesicles to deliver therapeutic siRNAs or virotherapies. Finally, various animal models available to test novel GBM therapies are discussed.
Introduction
Glioblastoma multiforme (GBM) is the most common type of brain tumor in adults, with a median survival of ~12–18 months post-diagnosis (Grossman et al., 2010; Wen and Kesari, 2008). The current standard of care for GBM patients consists of surgical resection, followed by radiotherapy and chemotherapy (Stupp et al., 2006; Wen and Kesari, 2008). Despite recent advances in conventional therapeutic approaches including the gamma knife (radiation) and temozolomide (chemotherapy) (Stupp et al., 2006; Stupp et al., 2005), GBM usually recurs within 12 months post-resection (Grossman et al., 2010; Wen and Kesari, 2008). Recurrent GBM tumors are usually comprised of tumor cells that are radiation and/or chemotherapy resistant and do not respond or do so poorly to further treatments. Interestingly, the susceptibility of tumor cells and the therapeutic benefit of temozolomide were shown to correlate with epigenetic silencing of the DNA repair enzyme O(6)-methylguanine-DNA-methyltransferase (MGMT) (Hegi et al., 2005); patients with a methylated MGMT promoter exhibit a more favorable therapeutic outcome when treated with temozolomide (Stupp et al., 2009). Nevertheless, novel therapeutic strategies to target residual tumor cells and prevent tumor recurrences are urgently needed (Curtin et al., 2005; Heimberger and Sampson, 2009; Wheeler and Black, 2009). Our group and others are pursuing virotherapy and gene therapy strategies for the treatment of GBM. In this review, we will discuss various virotherapy and gene therapy approaches for GBM that are currently under pre-clinical and clinical evaluation including cytotoxic, and/or immunostimulatory approaches. Also highlighted are cutting-edge technologies and approaches for the delivery of therapeutic agents and/or targeting of GBM cancer cells including using stem cells to target the vector at GBM cells and GBM microvesicles to deliver therapeutic siRNA. Various animal models available to test novel GBM therapies are also discussed.
Gene Therapy for Brain Tumors: Conditional Cytotoxic Approaches
Cytotoxic therapeutic approaches mainly consist of either delivering conditional or direct cytotoxic trans-genes (gene therapy) (Aboody et al., 2008; Aghi and Chiocca, 2006; Candolfi et al., 2009; Lawler et al., 2006), or delivery of conditionally replicating viral vectors that exclusively replicate in tumor cells and kill them (virotherapy) (Ferguson et al., 2010; Jiang et al., 2009; Markert et al., 2006). The most widely studied conditional cytotoxic transgene is Herpes Simplex Type 1 thymidine kinase (TK), which converts the prodrug ganciclovir (or valacyclovir) into the highly toxic deoxyguanosine triphosphate causing early chain termination of nascent DNA strands (Beltinger et al., 1999) . The by-stander effect of the TK approach relies on the passage of phosphorylated ganciclovir to neighboring cells through gap junctions, amplifying the cytotoxic effect of TK gene therapy (Mesnil and Yamasaki, 2000). This approach has been widely studied using either adenoviral or retroviral vectors in numerous clinical trials in the U.S. and Europe and has demonstrated modest increases in median survival (Germano et al., 2003; Immonen et al., 2004; Sandmair et al., 2000; Smitt et al., 2003; Trask et al., 2000). In April 2009, the U.K. based Ark Therapeutics released an update on promising results from a multi-center Phase III clinical trial using a first-generation adenoviral vector encoding TK (Cerepro®) (Ark Therapeutics, 2009; Osborne, 2008). Unfortunately, the European Medicines Agency (EMEA) recently rejected Ark Therapeutics’s marketing application after deciding that the study was statistically underpowered and failed to show sufficient efficacy in terms of postponing death or re-intervention. The decision by the EMEA is currently under appeal by Ark Therapeutics (Mitchell, 2010).
Gene Therapy Strategies to Deliver Targeted Immunotoxins
An attractive cytotoxic gene therapy approach for GBM consists of using non-replicating, first generation adenoviral vectors to deliver transgenes encoding for highly toxic proteins such as Pseudomonas exotoxin (PE), which disrupts protein translation in the target cell leading to cell death. To restrict the cytotoxicity of this approach to brain tumor cells, a targeted toxin was formulated by linking the PE toxin to human IL-13. 50–80% of human GBM cells overexpress a variant of the IL-13 receptor, i.e., IL13Rα2 (Okada et al., 2008; Wykosky et al., 2008), that differs from its physiological counterpart IL4R/IL13R, expressed in normal tissues (Hershey, 2003). A protein formulation of this targeted toxin approach, Cintredekin Besudotox, was tested in Phase I–III human clinical trials but due to the short half life of the hIL-13-PE protein formulation (Kawakami et al., 2004; Vogelbaum et al., 2007) multiple injections or continued delivery was necessary to achieve therapeutic effects (Kawakami et al., 2004; Kunwar et al., 2007). As a result, the Phase III trial studying the efficacy of convection-enhanced delivery (CED) of Cintredekin Besudotox compared with Gliadel wafers (GW) in adult patients with glioblastoma multiforme did not demonstrate a significant survival difference between the two treatment groups. The average intraparenchymal distribution of Cintredekin Besudotox ranged from 10 to 15 mm radially from the tip of catheter. Therefore, poor drug distribution could have contributed to the lack of significant clinical responses (Kunwar et al., 2010). To overcome the short half life of the hIL-13-PE protein formulation, we have used regulatable first generation adenoviral vectors to deliver IL-13.E13K, a mutated variant of the hIL13 (Debinski et al., 1998) with a high binding affinity to the GBM-associated IL13Rα2 (Candolfi et al., in press, 2010). In encouraging pre-clinical experiments in human GBM xenografts, we demonstrated that adenoviral vector mediated delivery of mhIL-13-PE led to tumor regression and long term survival in ~70% of the animals without causing apparent neurotoxicity (Candolfi et al., in press, 2010).
Virotherapy for Brain Tumors: Conditionally Replicating HSV Vectors
Using virotherapy approaches, tumor cell death is achieved by oncolytic virus replication within the neo-plastic cells, ultimately leading to tumor cell lysis. To achieve safe and effective oncolytic activity, an oncolytic vector must conditionally replicate within the target tumor cells with minimal toxicity to the surrounding normal brain. Oncolytic vectors are classified as either mesogenic (moderately pathogenic, capable of producing viable progeny and infecting adjacent cells) or lentogenic (attenuated non-pathogenic, produces defective progeny and is incapable of spreading between tissues) (Dey et al., 2010). Replicating, oncolytic viruses have been developed from numerous species of viruses.
Conditionally replicating Herpes Simplex Virus (HSV) vectors have been tested for the treatment of malignant glioma (Grandi et al., 2009). The most widely studied oncolytic HSV vector is G207, a genetically engineered HSV-1. It has deletion of γ34.5 gene at both alleles (Granelli-Piperno et al., 2000) and an insertion of the lacZ gene that prevents the expression of the UL 39 gene, which encodes for the large subunit of viral ribonucleotide reductase. As a result of these mutations, G207 can only replicate in rapidly dividing cells but not in quiescent cells. Furthermore, the HSV derived thymidine kinase gene was left intact in G207, thus delivery of G207 can be combined with prodrugs like ganciclovir to further increase the oncolytic effects of this approach. A Phase Ib clinical trial of G207 was recently published in which six patients with resectable, recurrent GBM were treated with two administrations of G207, the first administration was given via a direct injection into the tumor mass followed by surgical resection several days later. After tumor debulking, a second administration of G207 was given by multiple injections into the tumor cavity post surgical resection (Markert et al., 2009). Results from this trial demonstrated the high safety profile of multiple administrations of G207 with no evidence of encephalitis. Although the Phase Ib study was not designed to demonstrate therapeutic efficacy, viral replication was observed and limited evidence of anti-tumor activity was reported. Additional clinical trials of G207 are in the planning stages and second generation oncolytic HSV vectors are under pre-clinical development, such as vectors in which a single copy of the γ34.5 gene was re-introduced into the vector to enhance oncolytic replication (Kambara et al., 2005) and oncolytic HSV vectors genetically engineered to encode cytotoxic transgenes such as TNFα (Han et al., 2007), angiostatic transgenes such as Platelet Factor-4, extracellular fragment of brain-specific angiogenesis inhibitor 1, and shRNA specific for VEGF (Hardcastle et al., 2010; Liu et al., 2006; Yoo et al., 2007), or immuno-stimulatory transgenes such as IL-4 (Terada et al., 2006).
Virotherapy for Brain Tumors: Conditionally Replicating Adenovirus Vectors
Conditionally replicating adenoviruses can also be engineered to selectively replicate and lyse malignant cells. ONYX-015 and Ad5-Delta24 are two widely studied oncolytic adenoviruses (Chiocca et al., 2003; Fueyo et al., 2003; Jiang et al., 2009). ONYX-015 has a deletion in the E1B 55K gene that allows its replication in p53 defective tumor cells (Geoerger et al., 2002). Ad5-Delta24, can replicate and lyse cancer cells due to a 24-bp deletion in the E1 region responsible for binding the retinoblastoma (Rb) protein, which is often disrupted in human GBM (Fueyo et al., 2003; Fueyo et al., 2000). In a Phase I clinical study, ONYX-015 was safely administered into the tumor bed cavity post resection (Chiocca et al., 2004). Several groups are actively pursuing second generation oncolytic adenoviruses. One such example is Ad5-Delta24RGD, which has a genetically modified capsid that incorporates an Arg-Gly-Asp (RGD) motif into the HI loop of the viral fiber knob. The RGD motif enhances the virus’s affinity for αv integrins, which are abundant in glioma cells (Lamfers et al., 2002; Suzuki et al., 2001). Ad5-Delta24RGD has shown promise in pre-clinical studies using human GBM bearing xenograft nude mice in combination with low dose radiation (Lamfers et al., 2002). Other strategies include the use of tissue specific or glioma specific promoters including GFAP, nestin, human telomerase reverse transcriptase (hTERT), and survivin. One promising example of an oncolytic adenovirus incorporating tissue specific promoter technology is CRAd-Survivin-pk7, which contains a pk7 mutation in the adenovirus fiber and incorporates the survivin promoter driving E1A replication. Using human GBM xenografts, CRAd-Survivin-pk7 resulted in 67% long-term survival with evidence of enhanced adenovirus infectivity, decreased mitotic activity, and enhanced tumor apoptosis (Ulasov et al., 2007).
Virotherapy for Brain Tumors: Replication-competent Retrovirus (RCR) Vectors
Replication-competent retrovirus (RCR) vectors based on murine leukemia virus (MLV) exhibit unique characteristics. Due to its inability to infect quiescent cells, MLV based RCR exhibit high selectivity for tumor cells and has been shown to achieve highly selective and stable gene transfer throughout entire solid tumors in vivo. Most RCR vector genomes consist of an intact retrovirus genome including the transgene expression cassette containing an internal ribosome entry site (IRES) inserted immediately after the stop codon of the env gene (Jespersen et al., 1999; Logg et al., 2001). In contrast to oncolytic HSV and adenovirus, RCR are naturally non-cytolytic and their tumor killing power is derived from the incorporation of suicide transgene genes into the vector genome, which kill tumor cells in the presence of a prodrug. Examples of suicide genes used in RCR include the yeast cytosine deaminase (CD) gene, which converts the nontoxic prodrug 5-fluorocytosine (5FC) into the chemotoxin 5-fluorouracil (Hiraoka et al., 2007), and most recently Escherichia coli purine nucleoside phosphorylase (PNP), which results in potent cytotoxicity after administration of the prodrug fludarabine phosphate (F-araAMP) (Tai et al., 2010).
Virotherapy for Brain Tumors: Oncolytic Reovirus and Measles Virus Vectors
Finally, oncolytic reovirus and measles viral vectors are under development for GBM virotherapy. Reoviruses selectively replicate in glioma cells, where stimulation of RAS pathway by PDGFR or EGFR inhibits RNA-activated protein kinase activation, and thus viral proteins can be synthesized leading to tumor regression in preclinical studies using nude mice bearing intracranial human glioma xenografts in mice (Coffey et al., 1998). Delivery of live, replication competent, and genetically unmodified reovirus directly into the tumors of patients with malignant gliomas in a Phase I clinical trial demonstrated that oncolytic reoviruses are safe and well tolerated with no evidence of clinical encephalitis (Forsyth et al., 2008). Strains of the attenuated measles virus derived from the Edmonston vaccine lineage (MV-Edm) have been shown to preferentially infect and kill malignant cells while sparing the surrounding non-neoplastic tissues (Msaouel et al., 2009). The MV-Edm vector backbone has been engineered to express soluble marker peptides, such as the human carcinoembryonic antigen (CEA; MV-CEA) gene and the human thyroidal sodium iodide symporter (NIS; MV-NIS virus) gene to monitor the in vivo spread and elimination of the virus over time (Peng et al., 2002a; Peng et al., 2002b). In pre-clinical experiments, MV showed promising therapeutic efficacy in the U87, U118, and U251 glioma cell lines, with significant cytopathic effect being observed in all tumor cell lines tested (Phuong et al., 2003). A Phase I clinical trial of intratumoral and administration into the resection cavity of MV-CEA in patients with recurrent glioblastoma multiforme is currently recruiting patients (http://www.clinicaltrials.gov/ct2/show/NCT00390299).
Immune Challenges of the Brain Tumor Microenvironment
Immunotherapy is an alternative approach to direct cytotoxicity of GBM cells using gene therapy. The principle of immunotherapy approaches is the expectation that specific activated anti-tumor T cells would be able to eliminate any residual tumor cells that remain post-surgery, and thus inhibit tumor recurrence. In spite of the evidence that anti-brain tumor immunity can be induced through various immune-therapeutic approaches, i.e., vaccination (Broder et al., 2003; Choi et al., 2009; Heimberger et al., 2002; Liau et al., 2005; Yu et al., 2004), challenges to induce effective immune responses to eliminate GBM still remain. These include, a paucity of antigen presenting dendritic cells (DCs) within the brain parenchyma which may thus limit the capacity to stimulate an anti-tumor immune response, a lack of classic lymphatic outflow channels to allow activated DCs to exit the brain, the presence of immune suppressive regulatory T cells (Tregs), and immune suppressive cytokines, i.e., TGFβ (Gomez and Kruse, 2006; Learn et al., 2006; Yang et al., 2009). All these factors contribute to creating an immune suppressive tumor microenvironment. Further evidence has shown that a population of cells with immune suppressive activities, i.e., myeloid derived suppressor cells (MDSCs), play a major role in promoting immune suppression in several cancer models in rodents and also in human cancers (Gabrilovich and Nagaraj, 2009), thus contributing to poor priming of systemic immune responses against tumor antigens (Lowenstein, 2002). These immunosuppressive cells have also been recently described in human GBMs (Rodrigues et al., 2010).
Gene Therapy for Brain Tumors: Combining Immunotherapy and Cytotoxic Approaches
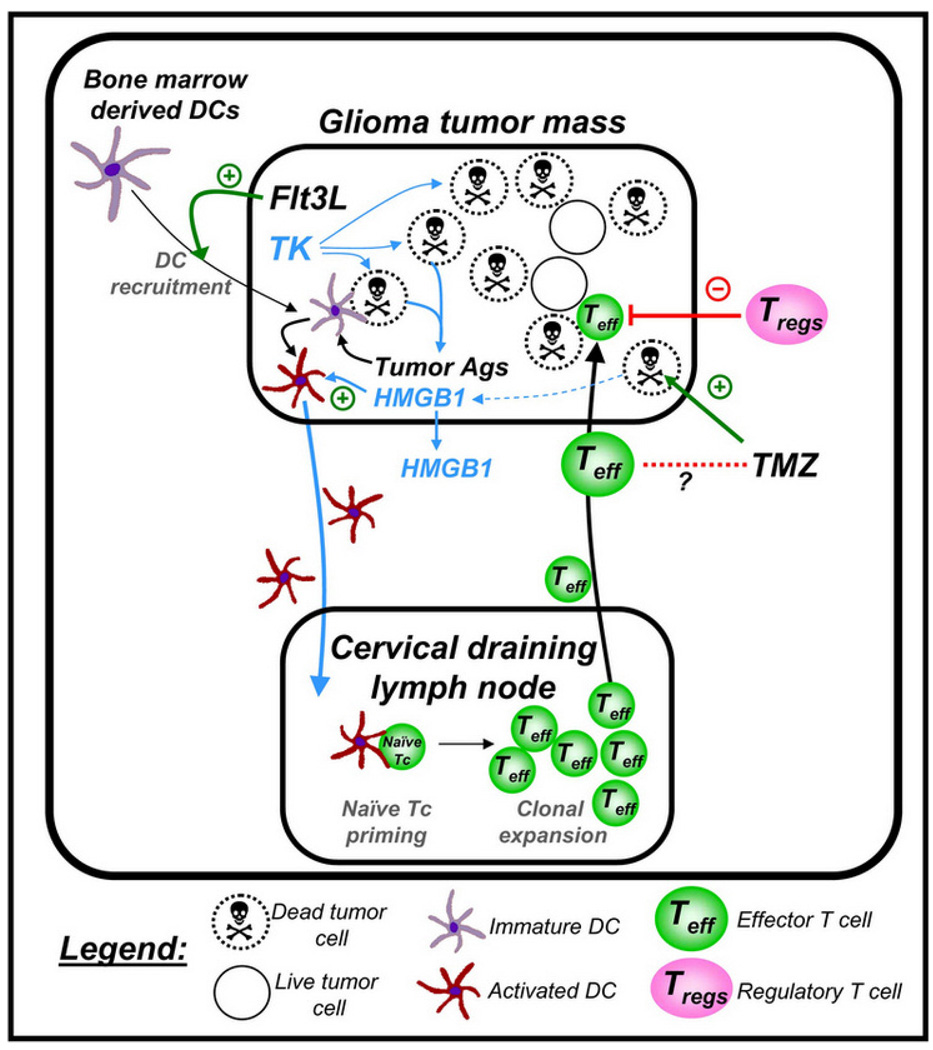
To prime an effective anti-tumor immune response from within the brain tumor microenvironment itself, our group has pioneered a novel gene therapy approach to recruit DCs to the brain parenchyma. The cytokine Flt3L increases the number of DCs in gastro-intestinal lymphoid tissue, lymph nodes, lung, peripheral blood, spleen, thymus, and bone marrow (Brawand et al., 2002; Diao et al., 2004), and these increases are higher than those achieved by GM-CSF, or by GM-CSF and IL-4 (Lynch et al., 1997; Pulendran et al., 1997). We have demonstrated that intracerebral or intratumoral delivery of Flt3L increased the levels of DCs within the normal brain tissue, or intracranial GBM tumors (Curtin et al., 2006; Larocque et al., 2010). However, to prime an immune response, DCs must be exposed to tumor antigens, take them up, process them, and migrate to local lymph nodes (LN) to present antigenic epitopes on MHC-I and MHC-II to naïve T-cells. Using first generation adenoviral vectors, we delivered Flt3L to recruit DCs into the brain tumor microenvironment and expose them in situ to tumor-derived antigens within the tumor mass generated by using HSV1 thymidine kinase + ganciclovir to induce tumor cell death, thus making brain tumor antigens available to the tumor infiltrating DCs (Figure 1). Using this approach, we have demonstrated therapeutic efficacy, induction of anti-tumor immune responses, and immunological memory in several transplantable, orthotopic, syngeneic models of GBM (Ali et al., 2005; Candolfi et al., 2009; Curtin et al., 2008; Curtin et al., 2009; Ghulam Muhammad et al., 2009; King et al., 2008; King et al., 2008). By enabling the in vivo intra-tumoral antigen loading of DCs, we aim to direct the immune response against a large repertoire of tumor antigens (Ali et al., 2005; Curtin et al., 2006). This therapeutic approach is scheduled to commence a Phase I dose-escalation study in early 2011 in 18 patients with primary GBM.
Figure 1.
Schematic depiction of the mechanism underlying the induction of a specific anti-GBM immune response using Flt3L/TK gene therapy. First generation adenoviral vectors encoding Flt3L and HSV1-TK are injected into an intracranial brain tumor; ganciclovir is administered systemically. Expression of HSV1-TK in the presence of ganciclovir mediates tumor cell death, releasing endogenous brain tumor antigens and danger signals, including HMGB1. Intratumoral expression of Flt3L recruits dendritic cells (DCs) into the brain tumor milieu where they take up brain tumor antigens released from the dying GBM cells and present them on their MHC complexes. This phenomenon is dependent on the TLR2/RAGE agonist HMGB1, which is released from dying tumor cells. The DCs loaded with brain tumor antigens migrate to the cervical draining lymph nodes where there present brain tumor antigens to naïve T cells, mediating a clonal expansion of brain tumor specific anti-GBM effector T cells. The GBM specific effector T cells then migrate back into the brain and kill residual GBM cells. Therapeutic efficacy of the approach is not diminished when mice are also treated with the chemotherapy temozolomide (TMZ).
Novel Therapeutic Delivery Platforms: Stem Cells and Exosomes
Exciting novel technologies are also emerging in the field of gene therapy and virotherapy for GBM. GBM tumor cells have been shown to release microvesicles (exosomes), which are endosomally derived 30–100 nm membranous vesicles (Al-Nedawi et al., 2009). Microvesicles containing mRNA, miRNA, and/or angiogenic proteins are taken up by surrounding normal cells, thus serving as a conduit of intercellular communication between cancer cells and their surrounding normal cells (Chen et al., 2010; Graner et al., 2009). The therapeutic potential of encoding anti-GBM specific siRNA and miRNA is being explored by several groups (Graner et al., 2009; Skog et al., 2008). Another exciting technology is the use of neural and mesenchymal stem cells to deliver oncolytic vectors to the tumor mass in the hope of improving therapeutic efficacy by overcoming the limited distribution of oncolytic vectors beyond the site of injection (Dembinski et al., 2010; Ferguson et al., 2010; Kranzler et al., 2009; Yong et al., 2009). Stem cells have been shown to selectively migrate to intracranial gliomas, invade tumor foci, and track single isolated tumor cells infiltrating into the surrounding normal brain parenchyma (Kendall et al., 2008; Zhao et al., 2008). Recently stem cells were shown to effectively deliver oncolytic adenovirus in mice bearing intracranial U87 brain tumors, even when administered at sites distant to the brain tumor mass (Sonabend et al., 2008).
Animal Models for Testing Novel GBM Therapeutics
To successfully test the therapeutic efficacy of novel GBM therapeutics, orthotopic animal models of GBM must be used that closely mimic the histopathological features and tumor microenvironment found in human GBMs. While transplantable syngeneic brain tumors share many histopathology characteristics with those of human GBMs, transplantable rodent tumors display low levels of normal brain tissue infiltration, a hallmark of human GBMs (Candolfi et al., 2007). Moreover, these tumors do not harbor the genetic lesions present in human tumors (i.e., PDGF and/or EGFR) and silencing of tumor suppressor genes (i.e., p53 and/or PTEN) (Ohgaki and Kleihues, 2007), but rather have a larger spectrum of heterogeneous alterations. Although orthotopic human GBM xenograft models in nude rodents display tissue infiltration, and contain genetic lesions of human GBM tumors, the human origin of these GBM cells requires their implantation into severely immune-compromised rodents (Giannini et al., 2005; Sarkaria et al., 2007; Xie et al., 2008). While these models are very useful for studying the molecular pathways involved in gliomagenesis (Dasgupta et al., 2009; Solomon et al., 2008a; Solomon et al., 2008b) and evaluating therapeutic efficacy of cytotoxic, anti-angiogenic, and other anti-GBM approaches (de Bouard et al., 2007; Dinca et al., 2008; Harding et al., 2006; Kitange et al., 2009; Kitange et al., 2008), it is impossible to assess the potential therapeutic efficacy of experimental immunotherapeutics because of the absence of an intact immune system. Within the past decade, several groups have used a variety of germline or virally encoded mutations to induce the development of endogenous brain models of gliomas (Gutmann, 2009; Huse and Holland, 2009; Reilly, 2009). Brain tumors of astrocytic and oligodendrocyte origins develop in transgenic mouse models with altered expression profiles of Ras, Ink4a/Arf, erbB, PTEN, and/or p53 (Charest et al., 2006; Kwon et al., 2008; Reilly et al., 2000; Uhrbom et al., 2002; Wei et al., 2006). RCAS vectors [Replication-Competent ASLV long terminal repeat (LTR) with a Splice acceptor] encoding Ras and AKT (Holland and Varmus, 1998) or PDGF-B (Dai et al., 2001) induced brain tumors of astrocytic origin in mice. Brain gliomas also develop when RCAS vectors are used to deliver Ras and or Akt into transgenic mice with PTEN or Ink4a/Arf knockouts (Hu et al., 2005; Uhrbom et al., 1998). MoMuLV retroviral vectors encoding PDGF-B were also used to induce tumors of oligodendrocyte origin in mice (Uhrbom et al., 2002). A retroviral vector encoding EGFR was used to generate tumors of oligodendrocyte tumors in neonatal mice (Ivkovic et al., 2008), and a retroviral vector expressing PDG F-B was used to generate gliomas in adult rats (Assanah et al., 2006). Most recently lentiviral vectors encoding activated Akt and Ras in wildtype or in Tp53−/+ knockout mice induced the development of brain tumors in adult mice (Marumoto et al., 2009). In all of these models, tumors appeared within 3–12 months post-tumor induction. Evaluation of therapeutic approaches using these endogenous brain tumor models has begun; in a recent study, the effects of radiotherapy and perifosine were assessed using three endogenous tumor models induced by RCAS delivery of proto-oncogenes (Hambardzumyan et al., 2008). Another recent approach to induce endogenous GBM uses the Sleeping Beauty (SB) transposable element to achieve integration of oncogenes in immune competent neonatal mice (Wiesner et al., 2009). Spontaneous brain tumors were induced by injecting an SB-tranposase encoding plasmid in combination with transposon DNA plasmids harboring several genetic lesions (AKT, N-RAS, EGRFvIII, and/or shRNA specific for p53) into the brain of neonatal mice (Wiesner et al., 2009). The histological characteristics of the tumors resembled many of the features encountered in human astrocytoma or GBM (Wiesner et al., 2009).
Conclusions
Glioblastoma multiforme constitutes a formidable therapeutic challenge, due in part to the infiltrative and aggressive nature of the tumor, the presence of the blood brain barrier, which restricts entry of therapeutic entities to the tumor area, the recurrent nature of the tumor, the paucity of antigen presenting cells and lymphatic drainage within the brain, and the immune suppressive nature of the tumor microenvironment. All these factors contribute to the short survival post-diagnosis and the lack of treatments that substantially prolong median survival of GBM patients. Gene therapy, which includes virotherapy and the use of stem cells and exosomes as novel platforms for therapeutic gene delivery, presents powerful, novel opportunities for developing adjuvant therapies for this devastating cancer. They include the delivery of direct and conditional cytotoxic genes, immunotoxins, oncolytic viruses, and immune-modulatory molecules to overcome immune suppression and mount an effective and specific antitumor immune response. These novel strategies, some of which are currently being tested in Phase I clinical trials, provide new hope for improved therapeutic outcomes for this devastating cancer.
Acknowledgments
We thank Drs. S. Melmed, L. Fine, and Mark Greene for their support and Dr. John Young and his staff at the Department of Comparative Medicine, Cedars-Sinai Medical Center.
Footnotes
Disclosure
The authors have no conflicts of interest to disclose.
Contributor Information
Kurt M. Kroeger, Gene Therapeutics Research Institute, Departments of Biomedical Sciences and Medicine, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA
A.K.M. Ghulam Muhammad, Gene Therapeutics Research Institute, Departments of Biomedical Sciences and Medicine, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA
Gregory J. Baker, Gene Therapeutics Research Institute, Departments of Biomedical Sciences and Medicine, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA
Hikmat Assi, Gene Therapeutics Research Institute, Departments of Biomedical Sciences and Medicine, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA
Mia K. Wibowo, Gene Therapeutics Research Institute, Departments of Biomedical Sciences and Medicine, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA
Weidong Xiong, Gene Therapeutics Research Institute, Departments of Biomedical Sciences and Medicine, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA
Kader Yagiz, Gene Therapeutics Research Institute, Departments of Biomedical Sciences and Medicine, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA
Marianela Candolfi, Gene Therapeutics Research Institute, Departments of Biomedical Sciences and Medicine, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA
Pedro R. Lowenstein, Gene Therapeutics Research Institute, Departments of Biomedical Sciences and Medicine, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA
Maria G. Castro, Gene Therapeutics Research Institute, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA and Departments of Medicine and Molecular & Medical Pharmacology, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, California 90095, USA
References
- Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15(10):739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- Aghi M, Chiocca EA. Gene therapy for glioblastoma. Neurosurg Focus. 2006;20(4):E18. [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8(13):2014–2018. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, Puntel M, Cheng Q, Prieto J, Ribas A, Kupiec-Weglinski J, van Rooijen N, Lassmann H, Lowenstein PR, Castro MG. Combined immunostim-ulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65(16):7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ark-Therapeutics. Cerepro® Phase III trial update: Analyses strengthen as more patients reach endpoint. 2009. [Accessed on Sept. 28, 2010]. Available online at: http://investors.arktherapeutics.com/servlet/HsPublic?context=ir.access&ir_option=RNS_NEWS&item=126418067393456&ir_client_id=4553&transform=newsitem_new. [Google Scholar]
- Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26(25):6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltinger C, Fulda S, Kammertoens T, Meyer E, Uckert W, Debatin KM. Herpes simplex virus thymidine kinase/ganciclovir-induced apoptosis involves ligand-independent death receptor aggregation and activation of caspases. Proc Natl Acad Sci U S A. 1999;96(15):8699–8704. doi: 10.1073/pnas.96.15.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawand P, Fitzpatrick DR, Greenfield BW, Brasel K, Maliszewski CR, De Smedt T. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J Immunol. 2002;169(12):6711–6719. doi: 10.4049/jimmunol.169.12.6711. [DOI] [PubMed] [Google Scholar]
- Broder H, Anderson A, Kremen TJ, Odesa SK, Liau LM. MART-1 adenovirus-transduced dendritic cell immunization in a murine model of metastatic central nervous system tumor. J Neurooncol. 2003;64(1–2):21–30. doi: 10.1007/BF02700017. [DOI] [PubMed] [Google Scholar]
- Candolfi M, Curtin JF, Nichols WS, Muhammad AG, King GD, Pluhar GE, McNiel EA, Ohlfest JR, Freese AB, Moore PF, Lerner J, Lowenstein PR, Castro MG. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85(2):133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolfi M, Kroeger KM, Muhammad AK, Yagiz K, Farrokhi C, Pechnick RN, Lowenstein PR, Castro MG. Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety. Curr Gene Ther. 2009;9(5):409–421. doi: 10.2174/156652309789753301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolfi M, Xiong W, Yagiz K, Liu C, Puntel M, Foulad D, Muhammad A, Zadmehr A, Ahlzadeh G, Kroeger K, Tesarfreund M, Lee S, Debinski W, Svendsen C, Rodriguez R, Lowenstein P, Castro M. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics: efficacy in the absence of neurotoxicity. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1008261107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest A, Wilker EW, McLaughlin ME, Lane K, Gowda R, Coven S, McMahon K, Kovach S, Feng Y, Yaffe MB, Jacks T, Housman D. ROS fusion tyrosine kinase activates a SH2 domain-containing phosphatase-2/phosphatidylinositol 3-kinase/mammalian target of rapamycin signaling axis to form glioblastoma in mice. Cancer Res. 2006;66(15):7473–7481. doi: 10.1158/0008-5472.CAN-06-1193. [DOI] [PubMed] [Google Scholar]
- Chen C, Skog J, Hsu CH, Lessard RT, Balaj L, Wurdinger T, Carter BS, Breakefield XO, Toner M, Irimia D. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip. 2010;10(4):505–511. doi: 10.1039/b916199f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, Kracher J, Grossman SA, Fisher JD, Carson K, Rosenblum M, Mikkelsen T, Olson J, Markert J, Rosenfeld S, Nabors LB, Brem S, Phuphanich S, Freeman S, Kaplan R, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Chiocca EA, Aghi M, Fulci G. Viral therapy for glioblastoma. Cancer J. 2003;9(3):167–179. doi: 10.1097/00130404-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Choi BD, Archer GE, Mitchell DA, Heimberger AB, McLendon RE, Bigner DD, Sampson JH. EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol. 2009;19(4):713–723. doi: 10.1111/j.1750-3639.2009.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282(5392):1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- Curtin JF, Candolfi M, Fakhouri TM, Liu C, Alden A, Edwards M, Lowenstein PR, Castro MG. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS ONE. 2008;3(4):e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J, Liu N, Candolfi M, Xiong W, Assi A, Yagiz K, Edwards M, Michelsen K, Kroeger K, Liu C, Muhammad A, Clark M, Arditi M, Comin-Anduix B, Ribas A, Lowenstein P, Castro M. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Medicine. 2009;6(1):e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, King GD, Barcia C, Liu C, Hubert FX, Guillonneau C, Josien R, Anegon I, Lowenstein PR, Castro MG. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176(6):3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, King GD, Candolfi M, Greeno RB, Kroeger KM, Lowenstein PR, Castro MG. Combining cytotoxic and immune-mediated gene therapy to treat brain tumors. Curr Top Med Chem. 2005;5(12):1151–1170. doi: 10.2174/156802605774370856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15(15):1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A, Raychaudhuri B, Haqqi T, Prayson R, Van Meir EG, Vogelbaum M, Haque SJ. Stat3 activation is required for the growth of U87 cell-derived tumours in mice. Eur J Cancer. 2009;45(4):677–684. doi: 10.1016/j.ejca.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bouard S, Herlin P, Christensen JG, Lemoisson E, Gauduchon P, Raymond E, Guillamo JS. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol. 2007;9(4):412–423. doi: 10.1215/15228517-2007-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debinski W, Gibo DM, Obiri NI, Kealiher A, Puri RK. Novel anti-brain tumor cytotoxins specific for cancer cells. Nat Biotechnol. 1998;16(5):449–453. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- Dembinski JL, Spaeth EL, Fueyo J, Gomez-Manzano C, Studeny M, Andreeff M, Marini FC. Reduction of nontarget infection and systemic toxicity by targeted delivery of conditionally replicating viruses transported in mesenchymal stem cells. Cancer Gene Ther. 2010;17(4):289–297. doi: 10.1038/cgt.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey M, Ulasov IV, Lesniak MS. Virotherapy against malignant glioma stem cells. Cancer Lett. 2010;289(1):1–10. doi: 10.1016/j.canlet.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J, Winter E, Chen W, Cantin C, Cattral MS. Characterization of distinct conventional and plasmacytoid dendritic cell-committed precursors in murine bone marrow. J Immunol. 2004;173(3):1826–1833. doi: 10.4049/jimmunol.173.3.1826. [DOI] [PubMed] [Google Scholar]
- Dinca EB, Lu KV, Sarkaria JN, Pieper RO, Prados MD, Haas-Kogan DA, Vandenberg SR, Berger MS, James CD. p53 Small-molecule inhibitor enhances temozolomide cytotoxic activity against intracranial glioblastoma xenografts. Cancer Res. 2008;68(24):10034–10039. doi: 10.1158/0008-5472.CAN-08-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SD, Ahmed AU, Thaci B, Mercer RW, Lesniak MS. Crossing the boundaries: stem cells and gene therapy. Discov Med. 2010;9(46):192–196. [PMC free article] [PubMed] [Google Scholar]
- Forsyth P, Roldan G, George D, Wallace C, Palmer CA, Morris D, Cairncross G, Matthews MV, Markert J, Gillespie Y, Coffey M, Thompson B, Hamilton M. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16(3):627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, Liu TJ, Jiang H, Lemoine MG, Suzuki K, Sawaya R, Curiel DT, Yung WK, Lang FF. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95(9):652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19(1):2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoerger B, Grill J, Opolon P, Morizet J, Aubert G, Terrier-Lacombe MJ, Bressac De-Paillerets B, Barrois M, Feunteun J, Kirn DH, Vassal G. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 2002;62(3):764–772. [PubMed] [Google Scholar]
- Germano IM, Fable J, Gultekin SH, Silvers A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol. 2003;65(3):279–289. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- Ghulam Muhammad AK, Candolfi M, King GD, Yagiz K, Foulad D, Mineharu Y, Kroeger KM, Treuer KA, Nichols WS, Sanderson NS, Yang J, Khayznikov M, Van Rooijen N, Lowenstein PR, Castro MG. Antiglioma immunological memory in response to conditional cyto-toxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15(19):6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL, Schroeder MA, James CD. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7(2):164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez GG, Kruse CA. Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther Mol Biol. 2006;10(A):133–146. [PMC free article] [PubMed] [Google Scholar]
- Grandi P, Peruzzi P, Reinhart B, Cohen JB, Chiocca EA, Glorioso JC. Design and application of oncolytic HSV vectors for glioblastoma therapy. Expert Rev Neurother. 2009;9(4):505–517. doi: 10.1586/ern.09.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A, Zhong L, Haslett P, Jacobson J, Steinman RM. Dendritic cells, infected with vesicular stomatitis virus-pseudotyped HIV-1, present viral antigens to CD4+ and CD8+ T cells from HIV-1-infected individuals. J Immunol. 2000;165(11):6620–6626. doi: 10.4049/jimmunol.165.11.6620. [DOI] [PubMed] [Google Scholar]
- Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23(5):1541–1557. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16(8):2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann DH. Modeling human brain tumors in mice. Brain Pathol. 2009;19(1):108–111. doi: 10.1111/j.1750-3639.2008.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22(4):436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZQ, Assenberg M, Liu BL, Wang YB, Simpson G, Thomas S, Coffin RS. Development of a second-generation oncolytic Herpes simplex virus expressing TNFalpha for cancer therapy. J Gene Med. 2007;9(2):99–106. doi: 10.1002/jgm.999. [DOI] [PubMed] [Google Scholar]
- Hardcastle J, Kurozumi K, Dmitrieva N, Sayers MP, Ahmad S, Waterman P, Weissleder R, Chiocca EA, Kaur B. Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Mol Ther. 2010;18(2):285–294. doi: 10.1038/mt.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding TC, Lalani AS, Roberts BN, Yendluri S, Luan B, Koprivnikar KE, Gonzalez-Edick M, Huan-Tu G, Musterer R, VanRoey MJ, Ozawa T, LeCouter RA, Deen D, Dickinson PJ, Jooss K. AAV serotype 8-mediated gene delivery of a soluble VEGF receptor to the CNS for the treatment of glioblastoma. Mol Ther. 2006;13(5):956–966. doi: 10.1016/j.ymthe.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- Heimberger AB, Archer GE, Crotty LE, McLendon RE, Friedman AH, Friedman HS, Bigner DD, Sampson JH. Dendritic cells pulsed with a tumor-specific peptide induce long-lasting immunity and are effective against murine intracerebral melanoma. Neurosurgery. 2002;50(1):158–164. doi: 10.1097/00006123-200201000-00024. discussion 164–156. [DOI] [PubMed] [Google Scholar]
- Heimberger AB, Sampson JH. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert Opin Biol Ther. 2009;9(8):1087–1098. doi: 10.1517/14712590903124346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111(4):677–690. doi: 10.1067/mai.2003.1333. quiz 691. [DOI] [PubMed] [Google Scholar]
- Hiraoka K, Kimura T, Logg CR, Tai CK, Haga K, Lawson GW, Kasahara N. Therapeutic efficacy of replication-competent retro-virus vector-mediated suicide gene therapy in a multifocal colorectal cancer metastasis model. Cancer Res. 2007;67(11):5345–5353. doi: 10.1158/0008-5472.CAN-06-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci U S A. 1998;95(3):1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Pandolfi PP, Li Y, Koutcher JA, Rosenblum M, Holland EC. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7(4):356–368. doi: 10.1593/neo.04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse JT, Holland EC. Genetically engineered mouse models of brain cancer and the promise of preclinical testing. Brain Pathol. 2009;19(1):132–143. doi: 10.1111/j.1750-3639.2008.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, Langford G, Murray N, Yla-Herttuala S. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10(5):967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Canoll P, Goldman JE. Constitutive EGFR signaling in oligodendrocyte progenitors leads to diffuse hyperplasia in postnatal white matter. J Neurosci. 2008;28(4):914–922. doi: 10.1523/JNEUROSCI.4327-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen T, Duch M, Carrasco ML, Warming S, Pedersen FS. Expression of heterologous genes from an IRES translational cassette in replication competent murine leukemia virus vectors. Gene. 1999;239(2):227–235. doi: 10.1016/s0378-1119(99)00402-3. [DOI] [PubMed] [Google Scholar]
- Jiang H, Gomez-Manzano C, Lang FF, Alemany R, Fueyo J. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9(5):422–427. doi: 10.2174/156652309789753356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65(7):2832–2839. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Kawakami M, Kioi M, Husain SR, Puri RK. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J Neurosurg. 2004;101(6):1004–1011. doi: 10.3171/jns.2004.101.6.1004. [DOI] [PubMed] [Google Scholar]
- Kendall SE, Najbauer J, Johnston HF, Metz MZ, Li S, Bowers M, Garcia E, Kim SU, Barish ME, Aboody KS, Glackin CA. Neural stem cell targeting of glioma is dependent on phosphoinositide 3-kinase signaling. Stem Cells. 2008;26(6):1575–1586. doi: 10.1634/stemcells.2007-0887. [DOI] [PubMed] [Google Scholar]
- King GD, Kroeger KM, Bresee CJ, Candolfi M, Liu C, Manalo CM, Muhammad AM, Pechnick RJ, Lowenstein PR, Castro MG. Flt3L in combination with HSV1-TK mediated gene therapy reverses brain tumor induced behavioral deficits. Mol Ther. 2008;16(4):682–690. doi: 10.1038/mt.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Muhammad AKM, Curtin JF, Barcia C, Puntel M, Liu C, Honig SB, Candolfi M, Mondkar S, Lowenstein PR, Castro MG. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol. 2008;10(1):19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitange GJ, Carlson BL, Mladek AC, Decker PA, Schroeder MA, Wu W, Grogan PT, Giannini C, Ballman KV, Buckner JC, James CD, Sarkaria JN. Evaluation of MGMT promoter methylation status and correlation with temozolomide response in orthotopic glioblastoma xenograft model. J Neurooncol. 2009;92(1):23–31. doi: 10.1007/s11060-008-9737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitange GJ, Carlson BL, Schroeder MA, Grogan PT, Lamont JD, Decker PA, Wu W, James CD, Sarkaria JN. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol. 2008 doi: 10.1215/15228517-2008-090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler J, Tyler MA, Sonabend AM, Ulasov IV, Lesniak MS. Stem cells as delivery vehicles for oncolytic adenoviral virotherapy. Curr Gene Ther. 2009;9(5):389–395. doi: 10.2174/156652309789753347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, Shaffrey M, Ram Z, Piepmeier J, Prados M, Croteau D, Pedain C, Leland P, Husain SR, Joshi BH, Puri RK. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, Sampson JH, Ram Z, Gutin PH, Gibbons RD, Aldape KD, Croteau DJ, Sherman JW, Puri RK. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25(7):837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhao D, Chen J, Alcantara S, Li Y, Burns DK, Mason RP, Lee EY, Wu H, Parada LF. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68(9):3286–3294. doi: 10.1158/0008-5472.CAN-07-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamfers ML, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J, Alemany R, Fueyo J, Curiel DT, Vassal G, Pinedo HM, Vandertop WP, Gerritsen WR. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62(20):5736–5742. [PubMed] [Google Scholar]
- Larocque D, Sanderson NS, Bergeron J, Curtin JF, Girton J, Wibowo M, Bondale N, Kroeger KM, Yang J, Lacayo LM, Reyes KC, Farrokhi C, Pechnick RN, Castro MG, Lowenstein PR. Exogenous fms-like tyrosine kinase 3 ligand overrides brain immune privilege and facilitates recognition of a neo-antigen without causing autoimmune neuropathology. Proc Natl Acad Sci U S A. 2010;107(32):14443–14448. doi: 10.1073/pnas.0913496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler SE, Peruzzi PP, Chiocca EA. Genetic strategies for brain tumor therapy. Cancer Gene Ther. 2006;13(3):225–233. doi: 10.1038/sj.cgt.7700886. [DOI] [PubMed] [Google Scholar]
- Learn CA, Fecci PE, Schmittling RJ, Xie W, Karikari I, Mitchell DA, Archer GE, Wei Z, Dressman H, Sampson JH. Profiling of CD4+, CD8+, and CD4+CD25+CD45RO+FoxP3+ T cells in patients with malignant glioma reveals differential expression of the immunologic transcriptome compared with T cells from healthy volunteers. Clin Cancer Res. 2006;12(24):7306–7315. doi: 10.1158/1078-0432.CCR-06-1727. [DOI] [PubMed] [Google Scholar]
- Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS, Cloughesy TF, Roth MD. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- Liu TC, Zhang T, Fukuhara H, Kuroda T, Todo T, Martuza RL, Rabkin SD, Kurtz A. Oncolytic HSV armed with platelet factor 4, an antiangiogenic agent, shows enhanced efficacy. Mol Ther. 2006;14(6):789–797. doi: 10.1016/j.ymthe.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Logg CR, Logg A, Tai CK, Cannon PM, Kasahara N. Genomic stability of murine leukemia viruses containing insertions at the Env-3′ untranslated region boundary. J Virol. 2001;75(15):6989–6998. doi: 10.1128/JVI.75.15.6989-6998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 2002;23(1):23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- Lynch DH, Andreasen A, Maraskovsky E, Whitmore J, Miller RE, Schuh JC. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nat Med. 1997;3(6):625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, Nabors LB, Markiewicz M, Lakeman AD, Palmer CA, Parker JN, Whitley RJ, Gillespie GY. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17(1):199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert JM, Parker JN, Buchsbaum DJ, Grizzle WE, Gillespie GY, Whitley RJ. Oncolytic HSV-1 for the treatment of brain tumours. Herpes. 2006;13(3):66–71. [PubMed] [Google Scholar]
- Marumoto T, Tashiro A, Friedmann-Morvinski D, Scadeng M, Soda Y, Gage FH, Verma IM. Development of a novel mouse glioma model using lentiviral vectors. Nat Med. 2009;15(1):110–116. doi: 10.1038/nm.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnil M, Yamasaki H. Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junc-tional intercellular communication. Cancer Res. 2000;60(15):3989–3999. [PubMed] [Google Scholar]
- Mitchell P. Ark’s gene therapy stumbles at the finish line. Nat Biotechnol. 2010;28(3):183–184. doi: 10.1038/nbt0310-183. [DOI] [PubMed] [Google Scholar]
- Msaouel P, Dispenzieri A, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr Opin Mol Ther. 2009;11(1):43–53. [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170(5):1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Low KL, Kohanbash G, McDonald HA, Hamilton RL, Pollack IF. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol. 2008;88(3):245–250. doi: 10.1007/s11060-008-9566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne R. Ark floats gene therapy’s boat, for now. Nat Biotechnol. 2008;26(10):1057–1059. doi: 10.1038/nbt1008-1057. [DOI] [PubMed] [Google Scholar]
- Peng KW, Facteau S, Wegman T, O’Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002a;8(5):527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002b;62(16):4656–4662. [PubMed] [Google Scholar]
- Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ, Mishra PK, Macura SI, Russell SJ, Galanis EC. Use of a vaccine strain of measles virus genetically engineered to produce carci-noembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63(10):2462–2469. [PubMed] [Google Scholar]
- Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraskovsky E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J Immunol. 1997;159(5):2222–2231. [PubMed] [Google Scholar]
- Reilly KM. Brain tumor susceptibility: the role of genetic factors and uses of mouse models to unravel risk. Brain Pathol. 2009;19(1):121–131. doi: 10.1111/j.1750-3639.2008.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet. 2000;26(1):109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, Parney IF. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12(4):351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M, Hurskainen H, Tyynela K, Turunen M, Vanninen R, Lehtolainen P, Paljarvi L, Johansson R, Vapalahti M, Yla-Herttuala S. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11(16):2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Yang L, Grogan PT, Kitange GJ, Carlson BL, Schroeder MA, Galanis E, Giannini C, Wu W, Dinca EB, James CD. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6(3):1167–1174. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitt PS, Driesse M, Wolbers J, Kros M, Avezaat C. Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol Ther. 2003;7(6):851–858. doi: 10.1016/s1525-0016(03)00100-x. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Kim JS, Cronin JC, Sibenaller Z, Ryken T, Rosenberg SA, Ressom H, Jean W, Bigner D, Yan H, Samuels Y, Waldman T. Mutational inactivation of PTPRD in glioblastoma multiforme and malignant melanoma. Cancer Res. 2008a;68(24):10300–10306. doi: 10.1158/0008-5472.CAN-08-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DA, Kim JS, Jenkins S, Ressom H, Huang M, Coppa N, Mabanta L, Bigner D, Yan H, Jean W, Waldman T. Identification of p18 INK4c as a tumor suppressor gene in glioblastoma multiforme. Cancer Res. 2008b;68(8):2564–2569. doi: 10.1158/0008-5472.CAN-07-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonabend AM, Ulasov IV, Tyler MA, Rivera AA, Mathis JM, Lesniak MS. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26(3):831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, Cairncross JG. Changing paradigms–an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11(2):165–180. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001;7(1):120–126. [PubMed] [Google Scholar]
- Tai CK, Wang W, Lai YH, Logg CR, Parker WB, Li YF, Hong JS, Sorscher EJ, Chen TC, Kasahara N. Enhanced efficiency of prodrug activation therapy by tumor-selective replicating retrovirus vectors armed with the Escherichia coli purine nucleoside phosphorylase gene. Cancer Gene Ther. 2010;17(9):614–623. doi: 10.1038/cgt.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada K, Wakimoto H, Tyminski E, Chiocca EA, Saeki Y. Development of a rapid method to generate multiple oncolytic HSV vectors and their in vivo evaluation using syngeneic mouse tumor models. Gene Ther. 2006;13(8):705–714. doi: 10.1038/sj.gt.3302717. [DOI] [PubMed] [Google Scholar]
- Trask TW, Trask RP, Aguilar-Cordova E, Shine HD, Wyde PR, Goodman JC, Hamilton WJ, Rojas-Martinez A, Chen SH, Woo SL, Grossman RG. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1(2):195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- Uhrbom L, Dai C, Celestino JC, Rosenblum MK, Fuller GN, Holland EC. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002;62(19):5551–5558. [PubMed] [Google Scholar]
- Uhrbom L, Hesselager G, Nister M, Westermark B. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 1998;58(23):5275–5279. [PubMed] [Google Scholar]
- Ulasov IV, Zhu ZB, Tyler MA, Han Y, Rivera AA, Khramtsov A, Curiel DT, Lesniak MS. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007;18(7):589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- Vogelbaum MA, Sampson JH, Kunwar S, Chang SM, Shaffrey M, Asher AL, Lang FF, Croteau D, Parker K, Grahn AY, Sherman JW, Husain SR, Puri RK. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery. 2007;61(5):1031–1037. doi: 10.1227/01.neu.0000303199.77370.9e. discussion 1037–1038. [DOI] [PubMed] [Google Scholar]
- Wei Q, Clarke L, Scheidenhelm DK, Qian B, Tong A, Sabha N, Karim Z, Bock NA, Reti R, Swoboda R, Purev E, Lavoie JF, Bajenaru ML, Shannon P, Herlyn D, Kaplan D, Henkelman RM, Gutmann DH, Guha A. High-grade glioma formation results from postnatal pten loss or mutant epidermal growth factor receptor expression in a transgenic mouse glioma model. Cancer Res. 2006;66(15):7429–7437. doi: 10.1158/0008-5472.CAN-06-0712. [DOI] [PubMed] [Google Scholar]
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- Wheeler CJ, Black KL. DCVax-Brain and DC vaccines in the treatment of GBM. Expert Opin Investig Drugs. 2009;18(4):509–519. doi: 10.1517/13543780902841951. [DOI] [PubMed] [Google Scholar]
- Wiesner SM, Decker SA, Larson JD, Ericson K, Forster C, Gallardo JL, Long C, Demorest ZL, Zamora EA, Low WC, SantaCruz K, Largaespada DA, Ohlfest JR. De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer Res. 2009;69(2):431–439. doi: 10.1158/0008-5472.CAN-08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykosky J, Gibo DM, Stanton C, Debinski W. Interleukin-13 receptor alpha 2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res. 2008;14(1):199–208. doi: 10.1158/1078-0432.CCR-07-1990. [DOI] [PubMed] [Google Scholar]
- Xie Q, Thompson R, Hardy K, DeCamp L, Berghuis B, Sigler R, Knudsen B, Cottingham S, Zhao P, Dykema K, Cao B, Resau J, Hay R, Vande Woude GF. A highly invasive human glioblastoma pre-clinical model for testing therapeutics. J Transl Med. 2008;6:77. doi: 10.1186/1479-5876-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2009 doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong RL, Shinojima N, Fueyo J, Gumin J, Vecil GG, Marini FC, Bogler O, Andreeff M, Lang FF. Human bone marrow-derived mes-enchymal stem cells for intravascular delivery of oncolytic aden-ovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69(23):8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JY, Kim JH, Kwon YG, Kim EC, Kim NK, Choi HJ, Yun CO. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther. 2007;15(2):295–302. doi: 10.1038/sj.mt.6300023. [DOI] [PubMed] [Google Scholar]
- Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64(14):4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- Zhao D, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, Kim SU, Aboody KS. Neural stem cell tropism to glioma: critical role of tumor hypoxia. Mol Cancer Res. 2008;6(12):1819–1829. doi: 10.1158/1541-7786.MCR-08-0146. [DOI] [PubMed] [Google Scholar]