Abstract
Glial cell line-derived neurotrophic factor (GDNF) activity in ventral tegmental area (VTA) mediates the time-dependent increases in cue-induced cocaine-seeking after withdrawal (incubation of cocaine craving). Here, we studied the generality of these findings to incubation of heroin craving. Rats were trained to self-administer heroin for 10 days (6-h/day; 0.075 mg/kg/infusion; infusions were paired with a tone-light cue) and tested for cue-induced heroin-seeking in extinction tests after 1, 11 or 30 withdrawal days. Cue-induced heroin seeking was higher after 11 or 30 days than after 1 day (incubation of heroin craving), and the time-dependent increases in extinction responding were associated with time-dependent changes in GDNF mRNA expression in VTA and nucleus accumbens. Additionally, acute accumbens (but not VTA) GDNF injections (12.5-μg/side) administered 1–3 h after the last heroin self-administration training session enhanced the time-dependent increases in extinction responding after withdrawal. However, the time-dependent increases in extinction responding after withdrawal were not associated with changes in GDNF protein expression in VTA and accumbens. Additionally, interfering with endogenous GDNF function by chronic delivery of anti-GDNF monoclonal neutralizing antibodies (600-ng/side/day) into VTA or accumbens had no effect on the time-dependent increases in extinction responding. In summary, heroin self-administration and withdrawal regulate VTA and accumbens GDNF mRNA expression in a time-dependent manner, and exogenous GDNF administration into accumbens but not VTA potentiates cue-induced heroin seeking. However, based on the GDNF protein expression and the anti-GDNF monoclonal neutralizing antibodies manipulation data, we conclude that neither accumbens nor VTA endogenous GDNF mediates the incubation of heroin craving.
Keywords: Extinction, reinstatement, relapse, drug self-administration, ventral tegmental area, nucleus accumbens, neurotrophic factors, GDNF, CDNF, MANF
Introduction
Relapse to heroin use in humans can occur after prolonged periods of abstinence and is often precipitated by exposure to cues previously associated with heroin use (O'Brien et al., 1986; Wikler, 1973). In studies using extinction and reinstatement procedures to model drug craving and relapse (Self and Nestler, 1998; Shaham et al., 2003), we and others reported that the rat’s response to drug cues progressively increases after withdrawal from cocaine (Grimm et al., 2001; Neisewander et al., 2000) and heroin (Shalev et al., 2001; Zhou et al., 2009). This phenomenon is termed incubation of drug craving (Lu et al., 2004c). Studies on mechanisms of incubation of drug craving were primarily performed using cocaine-trained rats (Conrad et al., 2008; Lu et al., 2005). In contrast, the mechanisms of incubation of heroin craving are unknown.
GDNF is a secreted protein (Lin et al., 1993) that supports the survival and function of VTA and substantia nigra midbrain dopamine neurons (Boger et al., 2006; Hoffer et al., 1994 ), which project to nucleus accumbens and dorsal striatum, respectively (Fallon and Moore, 1978). These midbrain dopaminergic projections have been implicated in drug reward and relapse, as assessed in animal models (Pierce and Kumaresan, 2006; Wise, 2009). Surprisingly, results from several studies indicate that manipulations that increase GDNF function in VTA and accumbens decrease drug reward, as assessed in drug self-administration and conditioned place preference (CPP) procedures (Carnicella and Ron, 2009; Ghitza et al., 2010). GDNF VTA injections decrease, while local anti-GDNF antibodies injections increase, cocaine CPP (Messer et al., 2000). Striatal transplantation of a simian virus-40 glial (SVG) cell line that produces GDNF decreases cocaine self-administration (Green-Sadan et al., 2003). Drug-priming- and cue-induced reinstatement of methamphetamine seeking is potentiated in GDNF heterozygote knockout mice (Yan et al., 2007), and these transgenic mice are also more susceptible to the alcohol deprivation effect (increased alcohol consumption after an abstinence period) (Carnicella et al., 2009a). In rats, VTA GDNF injections decrease operant alcohol self-administration and escalation of home-cage alcohol intake (Carnicella et al., 2009a; Carnicella et al., 2009b; He et al., 2005).
In contrast, we found that GDNF actions in VTA are critical for the development of incubation of cocaine craving (Lu et al., 2009). VTA injections of an adeno-associated virus (AAV) vector expressing rat GDNF on withdrawal day 1 increase cue-induced cocaine seeking on withdrawal days 11 and 31. Additionally, VTA GDNF injections immediately after the last cocaine self-administration session increase cue-induced cocaine seeking on withdrawal days 3 and 10. Finally, interfering with VTA GDNF function by chronic delivery of anti-GDNF monoclonal neutralizing antibodies via minipumps during withdrawal days 1-14 prevents the development of the time-dependent increases in cue-induced cocaine seeking (Lu et al., 2009).
Based on these results, we studied the role of VTA and accumbens GDNF in incubation of heroin craving using experimental procedures previously employed in our studies on the role of GDNF and brain-derived neurotrophic factor (BDNF) in incubation of cocaine craving (Grimm et al., 2003; Lu et al., 2004a; Lu et al., 2009). We first assessed whether the time-dependent increases in cue-induced heroin seeking after withdrawal are associated with time-dependent increases in GDNF expression in VTA and accumbens. We then assessed whether acute injections of exogenous GDNF into VTA or accumbens given immediately after the last self-administration session would potentiate cue-induced heroin seeking during early withdrawal (day 1). We hypothesized that GDNF injections would enhance heroin seeking during early withdrawal when drug seeking is lower than during late withdrawal periods (Shalev et al., 2001). Finally, we assessed whether endogenous GDNF plays a causal role in the development of incubation of heroin craving by interfering with endogenous GDNF function by chronic delivery of anti-GDNF monoclonal neutralizing antibodies into VTA or accumbens during the first two week weeks of withdrawal from heroin.
Material and Methods
Subjects
Male Long-Evans rats (final n=195, 300–400 g, Charles River, Raleigh, NC) were maintained under a reverse 12-h light–dark cycle (lights off at 9 am). Food and water were freely available in the home cage. Experimental procedures followed the guidelines of the “Principles of Laboratory Care” (National Institutes of Health publication No. 86-23, 1996) and were approved by the local Animal Care and Use Committee.
Surgical procedures
See supplemental online material section.
Intracranial injections and implantation of minipumps
See supplemental online material section.
Sample processing, quantitative real-time PCR, and Western blots
See supplemental online material section.
Behavioral procedures
The experiments consisted of three phases: self-administration training (6 h per day, fixed ratio 1 schedule, heroin unit dose of 0.075 mg/kg), withdrawal period, and extinction testing on withdrawal days 1, 11, and 30. The behavioral procedures were similar to those used in our more recent incubation studies (Conrad et al., 2008; Koya et al., 2009; Lu et al., 2004b) and studies of other investigators (Freeman et al., 2008; Hollander and Carelli, 2007; Lee et al., 2006; Sorge and Stewart, 2005). In those studies, we and other investigators assessed the incubation of drug craving in extinction tests in which rats are exposed to contextual cues previously associated with drug availability (e.g., houselight, lever extension), and responding on the previously active lever (or hole) results in contingent presentations of the discrete tone-light cue; this discrete cue serves as a conditioned reinforcer during testing (Robbins, 1975).
Training phase
The training procedures were similar to those used in our previous studies on incubation of cocaine craving (Koya et al., 2009; Lu et al., 2004b; Lu et al., 2005) and are described in detail in the Supplementary Online Material section.
Withdrawal phase
At the end of the self-administration training phase, the rats were returned to the animal colony room and handled 3 times per week during the withdrawal phase. The rats were then brought to the self-administration chambers on the mornings of the extinction tests, which were conducted 1 day, 11 days or 29–31 days (hereafter termed day 30) after the last heroin self-administration session.
Extinction tests
The extinction tests in the presence of the heroin-associated cues consisted of a single 1-h or 2-h extinction session on withdrawal days 1, 11, and 30. Experimental conditions were the same as those in the training phase, except that presses on the previously active lever were not reinforced with heroin. Tests started at the onset of the dark cycle and began with the insertion of the active lever and the illumination of the red house light, which remained on for the duration of the session. Active lever responses during testing resulted in contingent presentations of the tone-light cue that was previously paired with heroin infusions, but not heroin itself.
Exp. 1: Time-dependent increases in extinction responding after withdrawal from heroin
The purpose of this initial experiment was to establish that time-dependent increases in cue-induced heroin seeking in extinction tests (incubation of heroin craving) are reliably observed under the training procedures described above, which are different from those used in our original heroin study (Shalev et al., 2001). Two groups of rats (n=10–12 per group) were trained to self-administer heroin for 10 days. After training, one group of rats was given an extinction test on withdrawal day 1 while the other group was tested on withdrawal day 30 (between-groups comparison). Additionally, the rats tested on day 1 were retested on day 30 to determine whether time-dependent increases in cue-induced heroin seeking are reliably observed in a within-subjects design, which was used in subsequent experiments.
Exp. 2: Effect of heroin self-administration on GDNF mRNA and protein expression
The purpose of this experiment was to determine whether time-dependent increases in cue-induced heroin seeking (incubation of drug craving) after withdrawal are associated with time-dependent changes in GDNF mRNA and protein expression in VTA and accumbens. The mRNA levels of the two recently discovered neurotrophic factors CDNF and MANF (Lindholm et al., 2007; Petrova et al., 2003) were also analyzed to assess the specificity of the effect of heroin self-administration and subsequent withdrawal on GDNF mRNA expression. Initially, 4 groups of rats (n=7–8 per group) were used in a 2 (Training Drug: heroin or saline) × 2 (Withdrawal Day: 1 or 30) between-subjects factorial design. The rats were trained to self-administer heroin or saline for 10 days and returned to the animal colony room for their withdrawal period. On withdrawal day 1 or 30, the brains were rapidly extracted, frozen in −50°C isopentane, and stored at −80°C. Subsequently, GDNF mRNA and protein levels and mRNA levels of CDNF and MANF in VTA and accumbens were assessed as described above. Following this, two additional groups of saline- and heroin-trained rats (n=10 per group) were run under the same experimental conditions described above to assess GDNF mRNA and protein levels on withdrawal day 11.
Exp. 3. Effect of VTA and accumbens GDNF injections on extinction responding on withdrawal days 1, 11, and 30
The purpose of Exp. 3 was to determine whether a single GDNF injection into VTA or accumbens at the end of training would potentiate cue-induced heroin seeking after withdrawal from the drug (see introduction for the rationale for this experiment). We used a mixed experimental design with the between-subjects factor of GDNF Dose (0, 12.5 μg/site) and the within-subjects factor of Withdrawal Day (1, 11, 30). Four groups of rats (n=10–15 per group, two per brain region) were trained to self-administer heroin for 10 days. Rats were injected with either vehicle or GDNF (12.5 μg/side) into VTA or accumbens 1–3 h after the last heroin self-administration training session. After the injections, the rats were brought to the animal colony room. They were subsequently tested in 1-h extinction tests on withdrawal days 1, 11, and 30.
To determine the anatomical specificity (Wise and Hoffman, 1992) of the effect of accumbens GDNF injections (which significantly increased responding on withdrawal day 11), we trained two additional groups of rats (n=11 per group) and injected them with either vehicle or GDNF (12.5 μg/site) into dorsal striatum (2 mm above the effective accumbens site) 1–3 h after the last heroin self-administration training session. After the injections, the rats were brought to the animal colony room. The rats were then tested in 1-h extinction tests on withdrawal days 1 and 11. We did not assess extinction responding on day 30, because extinction responding after GDNF accumbens injections was not significantly different from the vehicle condition on day 30.
Exp. 4: Effect of chronic delivery of anti-GDNF antibodies into VTA and accumbens on extinction responding on withdrawal day 11
The purpose of Exp. 4 was to determine whether endogenous GDNF activity in VTA or accumbens mediates the development of time-dependent increases in cue-induced heroin seeking (incubation of heroin craving). For this purpose, we interfered with endogenous GDNF function in VTA or accumbens by chronic local delivery of anti-GDNF antibody via Alzet osmotic minipumps during withdrawal days 1–14. Four groups of rats (n=9–13 per group) were trained to self-administer heroin for 10 days. On withdrawal day 1, the rats were given a 1-h extinction test and were assigned to one of the two groups based on their active lever responding during this test. After the extinction test, the rats were implanted with osmotic minipumps that delivered either monoclonal mouse antibodies against GDNF (600-ng/side/d, R&D Systems, MAB212) or mouse control immunoglobulin G (IgG, R&D Systems, MAB002). These minipumps provided a constant infusion rate of 0.5 μl/h for up to 14 days. The rats underwent a 1-h extinction test on withdrawal day 11. The minipumps were removed after fourteen days, and their weights were measured to confirm proper function. The experimental design included the between-subjects factor of Minipump Condition (anti-GDNF, mouse IgG).
Statistical analyses
Data were analyzed using the statistical program SPSS (GLM procedure), and significant effects (p< 0.05) were followed by Fisher PLSD post-hoc tests. The dependent variables in the extinction tests were non-reinforced previously active lever presses and inactive lever presses. The appropriate within-subjects and between-subjects factors of the different statistical analyses are described in the Results section. In Exp. 2, the GDNF mRNA and protein data on withdrawal day 11 were analyzed separately from those on withdrawal days 1 and 30, because the two groups of rats in this condition were run 3 years after the groups assessed on days 1 and 30.
Results
Fig. 1A shows the mean±SEM number of heroin infusions during the training phase of Exp. 1-4 (total n=167) and the mean±SEM number of saline infusions during the training phase of Exp. 2 (total n=28). The different experiments were conducted over 3 years in different buildings (Exp 1-2, old building; Exp. 3-4, new building). Thus, we analyzed the heroin training data using the between-subjects factor of Experiment (Exp. 1, 2, 3, 4) and the within-subjects factor of Training Session (sessions 1–10); for 7 rats that were trained for only 9 days, the mean intake was used to estimate the missing day intake. This analysis revealed a main effect of Training Session (F (1,159) = 33.1, p < 0.01), reflecting increased heroin intake over days. The interaction of Training Session X Experiment (F (3,162) = 1.8, p > 0.05) was not significant, reflecting similar rates of acquisition in the different experiments. However, there was a significant main effect of Experiment (F (3,162) = 3.7, p < 0.01), reflecting different overall heroin intake in the different experiments. In Exp. 2, the saline-trained rats decreased their lever responding over the first 3–4 training days, and their total number of infusions was significantly lower than that of the heroin-trained rats in this experiment (heroin: 34±2 infusions/6 h/d, n=26; saline: 10±1 infusions/6 h/d, n=28; significant interaction of Training Drug Condition X Training Session (F(1,52) = 11.2, p < 0.01)). The groups in the different experiments were matched for their heroin (or saline) intake during training. Thus, non-significant group differences in heroin self-administration during training are not reported.
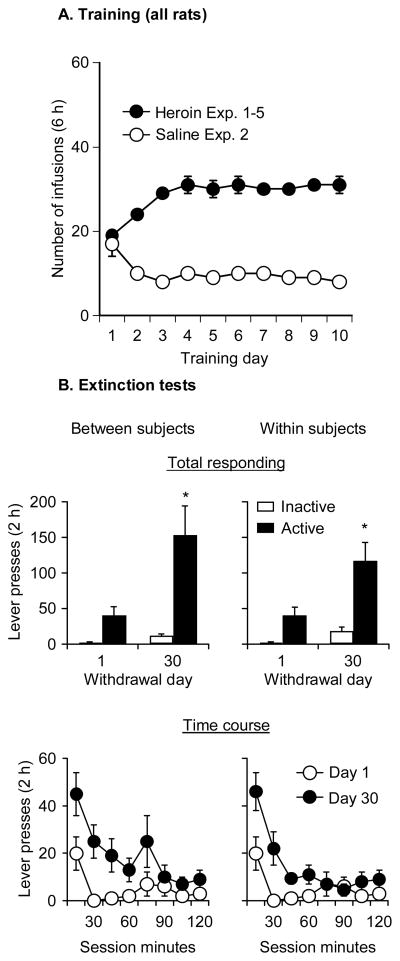
Figure 1. Heroin self-administration training and time-dependent increases in cue-induced heroin seeking in extinction tests (incubation of heroin craving).
(A) Training days 1–10. Data are mean±SEM number of heroin (0.075 mg/kg/injection) and saline infusions over the ten 6-h daily self-administration sessions. During training, lever presses were reinforced under a fixed-ratio-1 40-sec timeout reinforcement schedule; heroin injections were paired with a 5-sec tone-light cue. Data are from 167 heroin-trained rats from Exp. 1-4 and 28 saline-trained rats from Exp. 2. (B) Within- and between-subjects comparison of cue-induced heroin seeking. Upper panels: Mean±SEM responses per 2 h (sum of two 1-h sessions that were separated by 5 min) during the extinction tests in two groups of rats; one group was tested repeatedly on withdrawal days 1 and 30 (n=11) and the other group was tested on day 30 only (n=10). Lower panels: active lever responding during the extinction tests at 15 min intervals. During the extinction tests, heroin was not available and lever presses resulted in the presentation of a tone-light cue previously paired with heroin injections. * Different from withdrawal day 1, p< 0.05.
Exp. 1: Time-dependent increases in extinction responding after withdrawal from heroin
As mentioned in the Methods, the purpose of Exp. 1 was to establish that the time-dependent increases in cue-induced heroin seeking in extinction tests (incubation of heroin craving) are reliably observed under our current training procedures, which differ from those used in our initial study documenting this incubation phenomenon (Shalev et al., 2001). We found that, in rats given 10 days of extended daily access (6 h/day) to self-administered heroin, lever responding in the extinction tests was higher after 30 d than after 1 d of withdrawal from heroin; this effect was observed in both the between- and the within-subjects comparisons (Fig. 1B). The factors in the statistical analyses were Lever, Withdrawal Day, and Session Time (15 min intervals). In both the between-groups and the within-group comparisons, the statistical analysis revealed significant effects of Lever X Withdrawal Day (F(1,19) = 6.1 and F(1,10) = 8.1, p < 0.05, respectively) and Withdrawal Day X Session time (F(7,140) = 2.6 and F(7,70) = 5.1, p < 0.05, respectively). We also found modest time-dependent increases in inactive lever responding in both the between- and within-subjects comparison (p values<0.05, Fig. 1B). This increase likely reflects response generalization (Lu et al., 2004b); see Shalev et al. (2002) for a discussion on the interpretation of inactive lever data in extinction-reinstatement studies.
Exp. 2: Effect of heroin self-administration on GDNF mRNA and protein expression
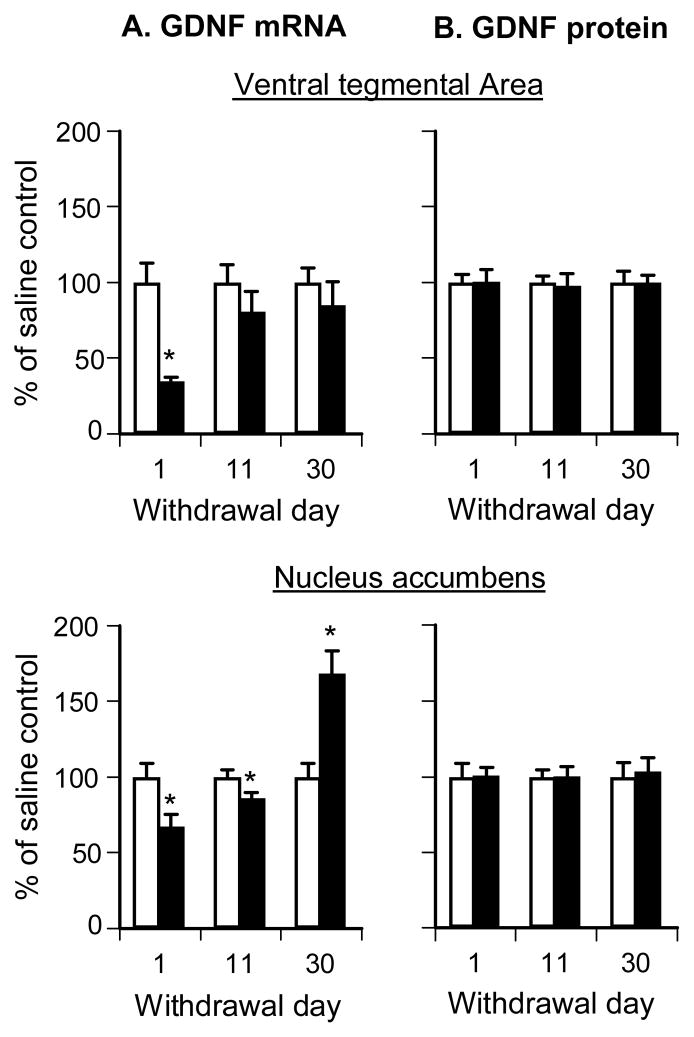
The initial aim of Exp. 2 was to determine whether the time-dependent increases in cue-induced heroin seeking after withdrawal observed in Exp. 1 (Fig. 1B) were associated with time-dependent changes in GDNF mRNA levels in accumbens and VTA. For this purpose, we trained 4 groups of rats to self-administer heroin or saline (a control condition), and their brains were taken for qPCR assessment after 1 or 30 withdrawal days. As can be seen in Fig. 2A, accumbens GDNF mRNA levels were decreased on withdrawal day 1 and increased on day 30; VTA GDNF mRNA levels were decreased on withdrawal day 1 and returned to normal drug-naïve levels on day 30. The statistical analyses revealed significant interactions of Training Drug X Withdrawal Day for both VTA and accumbens (F(1.27) = 6.0 and F(1,24) = 26.0, p < 0.05, respectively). In contrast, no group differences were observed for the mRNA levels of MANF and CDNF in VTA or accumbens (data not shown).
Figure 2. Time-dependent changes in GDNF mRNA but protein expression after withdrawal from heroin.
Rats self-administered heroin (0.075 mg/kg/injection, n=7-9 per group) or saline (n=8-10 per group) over ten 6-h daily sessions and brains were dissected on withdrawal days 1, 11 or 30. Real-time quantitative PCR and western blots were conducted from samples derived from VTA or nucleus accumbens. Data are presented as percent of the saline control (mean±SEM) within each withdrawal day. GDNF protein levels were normalized to tubulin. * Different from the corresponding saline group within each withdrawal day, p< 0.05.
To further characterize the time-dependent changes in GDNF mRNA in VTA and accumbens, and to gain insight to the biological significance of these changes at the protein level, we trained two additional groups of rats to self-administer heroin or saline and dissected their brains after 11 withdrawal days. We also developed a novel Western blot assay to accurately measure GDNF protein levels in small amounts of tissue from discrete brain areas of individual rats (Fig. 3). Because of the time difference (3 years) and location (new building) of the run with the withdrawal day 11 rats, their GDNF mRNA and protein data were analyzed separately from the original withdrawal day 1 and 30 rats. We found that in both VTA and accumbens, GDNF mRNA levels after 11 withdrawal days were modestly reduced (Fig. 2A); this effect was statistically significant in the accumbens samples (t14=2.5, p < 0.05) but not the VTA samples (p > 0.05). However, as can be seen in Fig. 2B, the profound time-dependent changes in GDNF mRNA after withdrawal from heroin did not translate to time-dependent changes in GDNF protein (p values >0.1 for the comparison between the saline and the heroin conditions within each withdrawal time point).
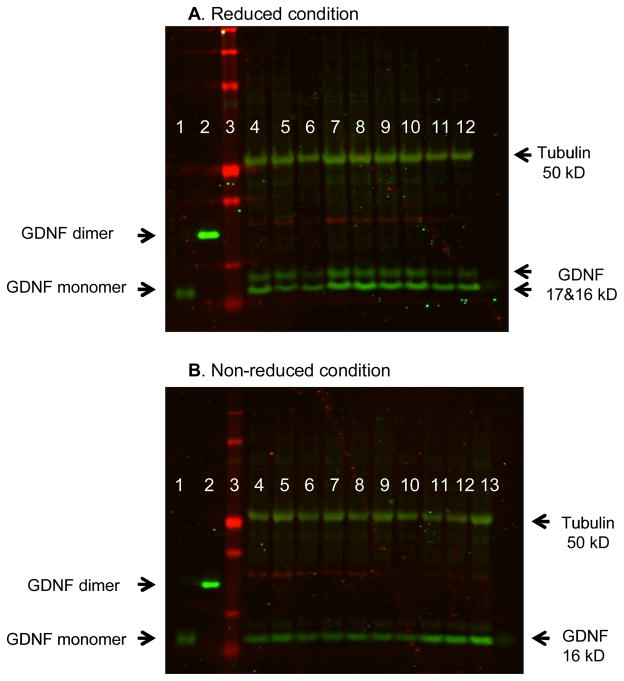
Figure 3. GDNF Western blot under reduced (A) and non-reduced conditions (B).
The Western blots are of nucleus accumbens of 10 saline-trained and 9 heroin-trained rats from the withdrawal day 11 groups; assays were performed under two different conditions: reduced and non-reduced. In the reduced condition (A), DTT was added to the final concentration of 20 mM in the lysis buffer before incubation at 45°C for 90 min. In the non-reduced condition (B), the lyses were without the DTT. A doublet band at the GDNF monomer region was observed in the reduced condition, possibly due to reduction of intramolecular disulfide bonds, and a single band was observed in the non-reduced condition. In the Western blot assays of the experimental rats (Fig. 3) we used the reduced condition to dissociate all potential GDNF dimers for accurate quantification of the GDNF monomer. Column numbers 1 to 12 in A refers to: 1: rhGDNF dimer under the non-reduced condition; 2: rhGDNF monomer under the reduced condition; 3: BioRad precision molecular weight marker; 4, 6, 8, 10: heroin-trained rats; 5, 7, 9, 11, 12: saline-trained rats. Column numbers 1 to 13 in B refer to: 1: rhGDNF dimer under the non-reduced condition; 2: rhGDNF monomer under reduced condition; 3: BioRad precision molecular weight marker; 4, 6, 8, 10, 12: heroin-trained rats; 5, 7, 9, 11, 13: saline-trained rats. Abbreviations: rhGDNF: recombinant human GDNF protein.
Exp. 3. Effect of VTA and accumbens GDNF injections on extinction responding on withdrawal days 1, 11, and 30
In Exp. 3 we assessed whether acute injections of exogenous GDNF into VTA or accumbens immediately after the last self-administration session would potentiate cue-induced heroin seeking during early withdrawal. We hypothesized that these GDNF injections would enhance heroin seeking during early withdrawal when drug seeking is lower than during late withdrawal periods (Shalev et al., 2001). The timeline for Exp. 3 is provided in Fig. 4. We found that accumbens, but not VTA, GDNF injections potentiated the time-dependent increases in extinction responding after withdrawal from self-administered heroin (Fig. 4). The statistical analyses included the between-subjects factor of GDNF Dose (0, 12.5 μg/side) and the within-subjects factors of Lever and Withdrawal Day. The critical statistic to support our conclusion is the significant triple interaction of GDNF Dose X Withdrawal Day X Lever (F(2, 54) =3.5, p < 0.05) for accumbens, but not VTA, injections (p > 0.1). For both VTA and accumbens injections, analyses revealed significant interactions of Lever X Withdrawal Day (F(2,40) = 13.3 and F(2, 54) = 24.6, p < 0.01, respectively), reflecting the more pronounced time-dependent increases in active versus inactive lever responding after withdrawal from heroin (Fig. 4), independent of the injection condition (vehicle versus GDNF).
Figure 4. Accumbens but not ventral tegmental area (VTA) injections of GDNF potentiate cue-induced heroin-seeking.
Data are mean±SEM responses per 1 h on the previously active lever (upper panels) and on the inactive lever (lower panels) during the extinction tests for cue-induced heroin seeking performed on withdrawal days 1, 11, and 30. During the extinction tests, heroin was not available and lever-presses resulted in the delivery of the tone-light cue previously paired with heroin injections. GDNF (12.5 μg/0.5μl/side) or vehicle (PBS) was injected bilaterally into VTA or accumbens 1-3 h after the last training session. (A) Timeline of the experiment, (B) VTA injections (n=10–12), (C) accumbens injections (n=14–16) * Different from vehicle, p< 0.05. SA, self-administration.
To determine the anatomical specificity of the effect of accumbens GDNF injections (which significantly increased responding on withdrawal day 11), we trained two groups of rats and injected them with either vehicle or GDNF into dorsal striatum (2 mm above the effective accumbens site) 1–3 h after the last heroin self-administration training session. We then assessed extinction responding in these rats on withdrawal days 1 and 11. We found that dorsal striatum GDNF injections had no effect on the time-dependent increases in extinction responding after withdrawal. For the vehicle injection condition, the mean±SEM number of lever presses during the 1-h extinction test on withdrawal days 1 and 11 were 25±8 and 65±10 (n=11). For the GDNF injection condition, the mean±SEM number of lever presses during the 1-h extinction tests on withdrawal days 1 and 11 were 37±10 and 82±17 (n=11). The statistical analysis revealed a significant effect of Lever X Withdrawal Day (F(1,20) = 17.8, p < 0.01), but not of GDNF Dose or interactions between GDNF Dose and Lever or Withdrawal Day. The significant Lever X Withdrawal Day interaction reflects the more pronounced effect of Withdrawal Day on active lever versus inactive lever responding.
Exp. 4: Effect of chronic delivery of anti-GDNF antibodies into VTA and accumbens on extinction responding on withdrawal day 11
In Exp. 4 we assessed whether endogenous GDNF in VTA or accumbens mediates the development of incubation of heroin craving by interfering with endogenous GDNF function using chronic delivery of anti-GDNF monoclonal neutralizing antibodies during the first two weeks of withdrawal from heroin. We interfered with GDNF function during early rather than late withdrawal because the magnitude of the time-dependent change in cue-induced heroin seeking is most pronounced during early withdrawal (see Fig. 3 and Shalev et al., 2001). We found that active lever responding was significantly higher after 11 withdrawal days than after 1 day (p<0.01). This effect was not reversed by chronic delivery of anti-GDNF antibodies into VTA or accumbens during this time period. VTA: on day 1 prior to minipump and injector implantation, the mean±SEM numbers of lever presses during the 1-h extinction test were 35±12 and 32±7 for the anti-GDNF antibodies group and the mouse IgG group, respectively (p > 0.1). On day 11, the mean±SEM numbers of lever presses for the two groups during the 1-h extinction test were 83±22 and 70±16, respectively (p > 0.1). Accumbens: on day 1 prior to minipump and injector implantation, the mean±SEM numbers of lever presses during the 1 h extinction test were 29±5 and 30±7 for the anti-GDNF antibodies group and the mouse IgG group, respectively (p > 0.1). On day 11, the mean±SEM numbers of lever presses for the two groups during the 1 h extinction test were 69±14 and 78±22, respectively (p>0.1).
Discussion
We found that time-dependent increases in cue-induced heroin seeking after withdrawal (incubation of heroin craving) were associated with time-dependent changes in GDNF mRNA expression in the VTA and accumbens. In the VTA, time-dependent expression changes were in the form of profound decreases on withdrawal day 1 and a return to drug-naive levels on day 11. In the accumbens, time-dependent expression changes were in the form of smaller decreases on day 1 and 11, and a significant increase on day 30. Additionally, accumbens but not VTA GDNF injections given immediately after the last self-administration session increased cue-induced heroin seeking after withdrawal; this delayed effect was significant on withdrawal day 11 but not on day 1 or day 30. However, the time-dependent increases in cue-induced heroin seeking were not associated with time-dependent increases in GDNF protein levels in VTA or accumbens. Additionally, interfering with endogenous GDNF function by chronic delivery of anti-GDNF monoclonal neutralizing antibodies into VTA or accumbens during the first 2 weeks of withdrawal had no effect on the development of incubation of craving. Thus, unlike incubation of cocaine craving, in which endogenous GDNF signaling plays a critical role (Lu et al., 2009), this signaling does not seem to mediate the incubation of heroin craving.
Methodological considerations
Several methodological issues should be considered in interpreting our data. One issue is the anatomical specificity of the GDNF injections. GDNF may diffuse away from the accumbens and act in dorsal sites due to a pressure gradient of the injection procedure (Wise and Hoffman, 1992). However, this possibility is unlikely given that we injected GDNF 2 mm above the effective accumbens site and found that these dorsal striatum injections were ineffective.
A second issue is the behavioral specificity of the effect of accumbens GDNF injections on cue-induced heroin seeking. Acute striatal or midbrain GDNF injections increase spontaneous locomotor activity for several weeks (Gash et al., 1998; Hudson et al., 1995; Martin et al., 1996). Thus, increased active lever responding after accumbens GDNF injections may be due to a nonspecific increase in activity. However, this possibility is unlikely because GDNF injections had no effect on inactive lever responding, which was modestly increased over time in both the vehicle- and the GDNF-injected rats. There are several interpretations of inactive lever data in extinction-reinstatement studies (Shalev et al., 2002). In our view, the modest time-dependent increases in inactive lever-presses in incubation studies (Lu et al., 2004c) are likely due to stimulus generalization of the attributes of the active lever cue. Stimulus generalization commonly occurs in learning tasks after prolonged time intervals between UCS+CS exposure and exposure to cues with stimulus attributes similar to the original CS (Riccio et al., 1992).
Another issue is the interpretation of the negative findings from Exp. 4 (chronic delivery of anti-GDNF antibodies). We interpret these data to suggest that endogenous GDNF does not contribute to incubation of heroin craving, because the chronic anti-GDNF antibodies regimen has effectively reversed GDNF-mediated behaviors in previous studies (He et al., 2005; Lu et al., 2009; Messer et al., 2000). However, these negative findings should be interpreted with caution, because it is possible that the results are due to incomplete blockade of GDNF actions under our experimental conditions.
A further issue concerns the brain site or sites that mediate exogenous GDNF’s effect on cue-induced heroin seeking after accumbens injections. Tomac et al. (1995) and Wang et al. (2010) demonstrated that when GDNF is injected into dorsal striatum or accumbens, it undergoes retrograde transport to the substantia nigra or VTA, respectively. Based on our results, it is unlikely that the VTA is the critical site; however, other accumbens projection areas such as basolateral amygdala and hippocampus (Groenewegen et al., 1999; McDonald, 1991) might be involved.
A final issue to consider is the dissociation between the effects of heroin self-administration and withdrawal on GDNF mRNA versus protein levels. This discrepancy might be due to the fact that protein expression is determined by multiple factors in addition to mRNA levels, including transcription and translation, alternative splicing, microRNA regulation, and protease processing. For example, during development the mRNA and protein expression of neurotrophins in different brain areas follow different time courses and are uncorrelated (Das et al., 2001). Additionally, there is evidence that drug exposure and subsequent withdrawal causes differential regulation of mRNAs and their target proteins (e.g., glutamate receptors) (Self, 2004; Wolf, 2003).
Nucleus accumbens, GDNF, and cue-induced heroin seeking
Our results on the effect of accumbens GDNF injections on cue-induced heroin seeking in extinction tests extend findings on the role of accumbens in cue-induced heroin seeking, as assessed in the extinction-reinstatement procedure (Crombag et al., 2008; Feltenstein and See, 2008). Reversible inactivation of accumbens core or shell or accumbens core injections of SCH 23390 (a D1-like receptor antagonist) decreased discrete cue-induced reinstatement of heroin seeking (Bossert et al., 2007; Rogers et al., 2008). Additionally, accumbens shell injections of either SCH 23390 or LY379268 (an mGluR2/3 agonist that decreases evoked glutamate release) decreased context-induced reinstatement of heroin seeking (Bossert et al., 2006; Bossert et al., 2007). Finally, accumbens core injections of SR 141716A (a CB1 receptor antagonist) decreased discriminative-cue-induced reinstatement of heroin seeking (Alvarez-Jaimes et al., 2008).
These results suggest a role of both accumbens core and shell in cue-induced reinstatement of heroin seeking. Here, we did not discriminate between the core and shell, but rather the injections were aimed at the border region between core and shell (Conrad et al., 2008). Thus, a future question is which accumbens sub-region is critical for GDNF’s effect on cue-induced heroin seeking in extinction tests. We speculate that both sub-regions are involved. In extinction tests after different withdrawal days, rats are exposed to an environmental context previously paired with drug injections (the self-administration chamber) and lever presses result in contingent presentations of the discrete tone-light cue (Lu et al., 2004b). As discussed above, both accumbens core and shell are critical for discrete cue-induced reinstatement of heroin seeking, and accumbens shell is critical for context-induced reinstatement.
The downstream mechanisms underlying the long-lasting effect of a single accumbens GDNF injection on cue-induced heroin seeking are unknown. We speculate that enhanced accumbens dopaminergic transmission is involved. Acute striatal GDNF injections cause long-lasting (several weeks) increases in DOPAC and HVA concentrations (dopamine metabolites), amphetamine- and potassium-evoked dopamine release, and tyrosine hydroxylase phosphorylation (Martin et al., 1996; Salvatore et al., 2004). Striatal GDNF injections also cause long-lasting increases in extracellular regulated kinase (ERK) phosphorylation (Salvatore et al., 2004), a measure of ERK activity. This effect is potentially relevant to the effects of accumbens GDNF injections on cue-induced heroin seeking. We previously identified a role of ERK activity in incubation of cocaine craving and GDNF-induced potentiation of this incubation. In the central amygdala, blockade of ERK activity by U0126 reversed enhanced cue-induced cocaine seeking 30 days after withdrawal from self-administered cocaine (Lu et al., 2005). Additionally, Li et al. (2008) reported that central amygdala U0126 injections reversed enhanced morphine conditioned place preference (CPP) 14 days after last drug exposure. In the VTA, U0126 injections reversed GDNF-induced potentiation of cue-induced cocaine seeking (Lu et al., 2009). Finally, in the accumbens, ERK is activated by drug and drug-associated cues and plays a role in conditioned drug effects (Beninger and Gerdjikov, 2004; Girault et al., 2007; Lu et al., 2006).
Concluding remarks
A decade ago, Messer et al. (2000) reported that heterozygote GDNF knockout mice demonstrated increased sensitivity to cocaine CPP, and that chronic minipump delivery of GDNF into VTA decreased cocaine CPP while local chronic delivery of anti-GDNF neutralizing antibodies increased cocaine-induced CPP. Subsequently, results from other studies provided further evidence that mesolimbic GDNF negatively regulates psychostimulant-, opiate- and alcohol-taking behaviors (Carnicella and Ron, 2009; Ghitza et al., 2010; Russo et al., 2009), but see (Airavaara et al., 2007). In contrast, our current and previous results (Lu et al., 2009) suggest that after extended access to heroin or cocaine self-administration, mesolimbic GDNF does not negatively regulate drug-seeking behaviors. Finally, VTA GDNF injections and chronic local delivery of anti-GDNF antibodies, which potentiate or inhibit, respectively, the development of incubation of cocaine craving (Lu et al., 2009), had no effect on incubation of heroin craving. These different results suggest that the neurobiological mechanisms of incubation of heroin and cocaine craving are not identical. This is a possible scenario based on evidence for differing mechanisms of opiate and psychostimulant self-administration (Ettenberg et al., 1982; Stinus et al., 1992) and the differential modulation of opiate versus psychostimulant self-administration and reward by environmental context (Caprioli et al., 2009; Caprioli et al., 2008).
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIH, DHHS). The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript. We thank S. Golden, Dr. F. Theberge and Dr. B. Hope for their help in conducting the experiments, and Drs. AC. Granholm and D. Ron for their help with the GDNF western blot assay.
References
- Airavaara M, Tuomainen H, Piepponen TP, Saarma M, Ahtee L. Effects of repeated morphine on locomotion, place preference and dopamine in heterozygous glial cell line-derived neurotrophic factor knockout mice. Genes Brain Behav. 2007;6:287–298. doi: 10.1111/j.1601-183X.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33:2483–2493. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Gerdjikov T. The role of signaling molecules in reward-related incentive learning. Neurotox Res. 2004;6:91–104. doi: 10.1007/BF03033301. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, Tomac AC, Granholm AC. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp Neurol. 2006;202:336–347. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Dubla A, Lucantonio F, Nencini P, Badiani A. Ambience and drug choice: cocaine- and heroin-taking as a function of environmental context in humans and rats. Biol Psychiatry. 2009;65:893–899. doi: 10.1016/j.biopsych.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Paolone G, Lucantonio F, Bari A, Nencini P, Badiani A. Opposite environmental regulation of heroin and amphetamine self-administration in the rat. Psychopharmacology (Berl) 2008;198:395–404. doi: 10.1007/s00213-008-1154-3. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, Janak PH, Ron D. GDNF is an endogenous negative regulator of ethanol-mediated reward and of ethanol consumption after a period of abstinence. Alcohol Clin Exp Res. 2009a;33:1012–1024. doi: 10.1111/j.1530-0277.2009.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009b;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D. GDNF--a potential target to treat addiction. Pharmacol Ther. 2009;122:9–18. doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag H, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Trans R Soc Lond B: Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KP, Chao SL, White LD, Haines WT, Harry GJ, Tilson HA, Barone S., Jr Differential patterns of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNA and protein levels in developing regions of rat brain. Neuroscience. 2001;103:739–761. doi: 10.1016/s0306-4522(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, Roberts DC, Vrana KE. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33:1807–1817. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gash DM, Gerhardt GA, Hoffer BJ. Effects of glial cell line-derived neurotrophic factor on the nigrostriatal dopamine system in rodents and nonhuman primates. Adv Pharmacol. 1998;42:911–915. doi: 10.1016/s1054-3589(08)60895-9. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010 doi: 10.1016/j.neubiorev.2009.11.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Green-Sadan T, Kinor N, Roth-Deri I, Geffen-Aricha R, Schindler CJ, Yadid G. Transplantation of glial cell line-derived neurotrophic factor-expressing cells into the striatum and nucleus accumbens attenuates acquisition of cocaine self-administration in rats. Eur J Neurosci. 2003;18:2093–2098. doi: 10.1046/j.1460-9568.2003.02943.x. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Mulder AB, Beijer AVJ, Wright CI, Lopez DA, Silva FH, Pennartz CM. Hippocampal and amygdaloid interactions in the nucleus accumbens. Psychobiology. 1999;27:149–164. [Google Scholar]
- He DY, McGough NN, Ravindranathan A, Jeanblanc J, Logrip ML, Phamluong K, Janak PH, Ron D. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci. 2005;25:619–628. doi: 10.1523/JNEUROSCI.3959-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer BJ, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, Lin LF, Gerhardt GA. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci Lett. 1994;182:107–111. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J, Granholm AC, Gerhardt GA, Henry MA, Hoffman A, Biddle P, Leela NS, Mackerlova L, Lile JD, Collins F, et al. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–432. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JM, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(S1):117–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, Shaham Y, Lu L. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci. 2008;28:13248–13257. doi: 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lindholm P, Voutilainen MH, Lauren J, Peranen J, Leppanen VM, Andressoo JO, Lindahl M, Janhunen S, Kalkkinen N, Timmusk T, Tuominen RK, Saarma M. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature. 2007;448:73–77. doi: 10.1038/nature05957. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004a;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004b;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004c;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, Harvey BK, Hoffer BJ, Shaham Y. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Miller G, Cullen T, Fischer N, Dix D, Russell D. Intranigral or intrastriatal injections of GDNF: effects on monoamine levels and behavior in rats. Eur J Pharmacol. 1996;317:247–256. doi: 10.1016/s0014-2999(96)00756-x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- Messer CJ, Eisch AJ, Carlezon WA, Jr, Whisler K, Shen L, Wolf DH, Westphal H, Collins F, Russell DS, Nestler EJ. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–257. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Ehrman RN, Ternes JW. Classical conditioning in human opioid dependence. In: Goldberg S, Stolerman I, editors. Behavioral analysis of drug dependence. Academic Press; Orlando: 1986. pp. 329–356. [Google Scholar]
- Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, Durocher Y, Commissiong JW. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci. 2003;20:173–188. doi: 10.1385/jmn:20:2:173. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Riccio DC, Ackil J, Burch-Vernon A. Forgetting of stimulus attributes: methodological implications for assessing associative phenomena. Psychol Bull. 1992;112:433–445. doi: 10.1037/0033-2909.112.3.433. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The potentiation of conditioned reinforcement by psychomotor stimulant drugs: a test of Hill's hypothesis. Psychopharmacologia. 1975;45:103–114. [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology 56 Suppl. 2009;1:73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Zhang JL, Large DM, Wilson PE, Gash CR, Thomas TC, Haycock JW, Bing G, Stanford JA, Gash DM, Gerhardt GA. Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. Journal of Neurochemistry. 2004;90:245–254. doi: 10.1111/j.1471-4159.2004.02496.x. [DOI] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(Suppl 1):242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–69. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology. 2005;183:210–217. doi: 10.1007/s00213-005-0160-y. [DOI] [PubMed] [Google Scholar]
- Stinus L, Cador M, Le Moal M. Interaction between endogenous opiods and dopamine within the nucleus accumbens. Ann N Y Acad Sci. 1992;654:254–273. doi: 10.1111/j.1749-6632.1992.tb25972.x. [DOI] [PubMed] [Google Scholar]
- Tomac A, Widenfalk J, Lin LF, Kohno T, Ebendal T, Hoffer BJ, Olson L. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci U S A. 1995;92:8274–8278. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Ahmadiantehrani S, He DY, Barak S, Kharazia V, Hamida SBAZ, Shippenberg TS, Ron D. Nucleus accumbens-derived GDNF Is a retrograde enhancer of dopaminergic tone in the mesocorticolimbic system. 2010. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence, implication of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Hoffman DC. Localization of drug reward mechanisms by intracranial injections. Synapse. 1992;10:247–263. doi: 10.1002/syn.890100307. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Effects of psychomotor stimulants on glutamate receptor expression. Methods Mol Med. 2003;79:13–31. doi: 10.1385/1-59259-358-5:13. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial-cell-line-derived neurotrophic factor mutant mice. Faseb J. 2007;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhang F, Liu H, Tang S, Lai M, Zhu H, Kalivas PW. Effects of training and withdrawal periods on heroin seeking induced by conditioned cue in an animal of model of relapse. Psychopharmacology. 2009;203:677–684. doi: 10.1007/s00213-008-1414-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.