Abstract
Purkinje fibers play an essential role in transmitting electrical impulses through the heart, but they may also serve as triggers for arrhythmias linked to defective intracellular calcium (Ca2+) regulation. Although prior studies have extensively characterized spontaneous Ca2+ release in nondriven Purkinje cells, little attention has been paid to rate-dependent changes in Ca2+ transients. Therefore we explored the behaviors of Ca2+ transients at pacing rates ranging from 0.125 to 3 Hz in single canine Purkinje cells loaded with fluo3 and imaged with a confocal microscope. The experiments uncovered the following novel aspects of Ca2+ regulation in Purkinje cells: 1) the cells exhibit a negative Ca2+-frequency relationship (at 2.5 Hz, Ca2+ transient amplitude was 66 ± 6% smaller than that at 0.125 Hz); 2) sarcoplasmic reticulum (SR) Ca2+ release occurs as a propagating wave at very low rates but is localized near the cell membrane at higher rates; 3) SR Ca2+ load declines modestly (10 ± 5%) with an increase in pacing rate from 0.125 Hz to 2.5 Hz; 4) Ca2+ transients show considerable beat-to-beat variability, with greater variability occurring at higher pacing rates. Analysis of beat-to-beat variability suggests that it can be accounted for by stochastic triggering of local Ca2+ release events. Consistent with this hypothesis, an increase in triggering probability caused a decrease in the relative variability. These results offer new insight into how Ca2+ release is normally regulated in Purkinje cells and provide clues regarding how disruptions in this regulation may lead to deleterious consequences such as arrhythmias.
Keywords: Ca2+ transients, pacing rate, conduction system, Ca2+ sparks, Ca2+ waves
Introduction
Purkinje fibers in heart are specialized networks of cells that play a critical role in electrical activity by rapidly conducting action potentials from the atrioventricular node to the ventricular endocardium [1]. Accordingly, research efforts have focused on determining the electrophysiological and conduction characteristics that allow them to fulfill this particular role.
Under pathological conditions, however, evidence suggests that spontaneous action potentials originating in the Purkinje network may serve as triggers for ventricular arrhythmias (eg. [2, 3]). Moreover, this triggered activity has been linked to intracellular Ca2+ regulation, whereby spontaneous release of Ca2+ from the sarcoplasmic reticulum (SR) during diastole leads to inappropriate membrane depolarization when Ca2+ is extruded from the cell via the Na+-Ca2+ exchanger [4, 5]. Understanding, and potentially preventing, these triggered arrhythmias therefore requires greater knowledge of the mechanisms of Ca2+ regulation in cells from the Purkinje network.
Prior work has established that the coupling of electrical excitation to SR Ca2+ release in Purkinje cells exhibits important qualitative differences compared with ventricular myocytes [6, 7]. In both cell types, Ca2+ entry through L-type Ca2+ channels triggers release of Ca2+ from SR stores, and intracellular Ca2+ transients reflect the contributions of both entry and release. Importantly, however, the extensive network of transverse (T) tubules in ventricular myocytes ensures that the pattern of SR Ca2+ release is relatively uniform throughout the cell [8, 9]. Since Purkinje cells are largely devoid of T-tubules [10, 11], SR Ca2+ release originates at the cell periphery where L-type Ca2+ channels in the cell membrane come in close contact with clusters of ryanodine receptors (RyRs) [4, 7]. Spontaneous SR Ca2+ release in quiescent canine Purkinje cells has been extensively characterized and shown to consist of local Ca2+ sparks, slightly larger macrosparks or Ca2+ wavelets, and occasional propagating cell-wide Ca2+ waves [4, 5, 12]. Electrical stimuli applied to resting cells have been shown to induce Ca2+ waves which propagate transversely from the cell periphery to the core [5]. Much less is known, however, about how patterns of SR Ca2+ release change with pacing rate in periodically-stimulated Purkinje cells. The goal of this study, therefore, was to investigate rate-dependent changes in Ca2+ transients, with particular attention to potentially de-stabilizing patterns of Ca2+ release. We found that canine Purkinje cells exhibit a negative Ca2+-frequency relationship, whereby large Ca2+ transients in the form of propagating Ca2+ waves occur at very low rates but only smaller, local events occur at higher rates. Moreover, we found, somewhat surprisingly, that Purkinje cells exhibit considerable inherent beat-to-beat variability in SR Ca2+ release, which can be accounted for by stochastic triggering of local Ca2+ release events.
Materials and Methods
Cell Isolation
Purkinje cells were enzymatically dispersed from the Purkinje fibers of the canine heart as previously described [10]. Briefly, Purkinje fibers were carefully dissected from RV and LV of canine hearts and subjected to enzyme incubation and dispersion. Purkinje cells studied here were isolated and did not show blebs or contraction bands.
Fluorescence Ca2+ imaging and solutions
Freshly isolated cells were loaded with the Ca2+-sensitive indicator fluo-3 AM for 30 minutes, then placed in an experimental chamber and imaged with a 40 x oil immersion objective. Recordings of intracellular [Ca2+] were obtained with a laser scanning confocal microscope (Zeiss LSM 510 Exciter) by exciting fluo-3 at 488 nm and recording fluorescence above 505 nm. Cellular fluorescence was measured in line-scan mode at a rate of 4 ms per line, and only cells that did not show spontaneous contractions were selected for investigation. To reduce artifacts from contractile motion, the following steps were taken: 1) the scan line was oriented transversely; 2) the scan line was drawn as close to the center of the cell as possible without scanning a nucleus; 3) the glass bottom of the chamber was coated with laminin to improve cell attachment and reduce motion. For all Ca2+ imaging experiments cells were continually superfused with Tyrode’s solution containing (in mM): NaCl 140, KCl 5.4, CaCl2 2, MgCl2 1, HEPES 10, glucose 10, pH 7.4. All experiments were performed at room temperature (22-24°C).
Electrical stimulation and Ca2+ transient recording
Purkinje cells were periodically stimulated by external field pulses applied through platinum electrodes located on opposite sides of the experimental chamber. Pacing cycle lengths ranged from 8 s to 0.25 s. To reduce the exposure of cells to laser light and allow for recordings over minutes, we configured the microscope to only scan during a brief period surrounding each electrical stimulus. For all recordings in this paper except those shown in Fig. 1, total recording time was reduced to about 170 ms (36 scan lines) for each Ca2+ transient. Among these 36 lines, 6 lines were obtained prior to electrical stimulation, and the remaining 30 lines defined the rising phase, peak, and initial declining phase of the Ca2+ transient. The spatial resolution was set to approximately 0.1 μm per pixel. In the experiment shown in Fig. 1, the total recording time was set about 300 ms to observe propagation of Ca2+ release into the cell core.
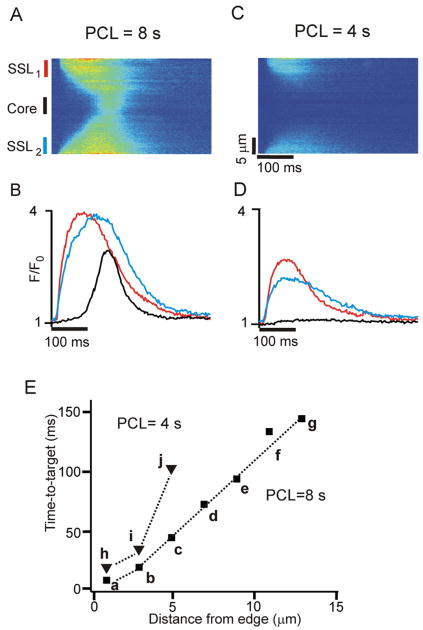
Figure 1.
Ca2+ waves occur at very low pacing rates; local subsarcolemmal (SSL) elevations in Ca2+ occur at higher pacing rates. (A) Space-time line scan image obtained at PCL = 8 s shows an early increase in [Ca2+] in SSL regions and a delayed increase in the core. (B) Local Ca2+ transients averaged over 5 μm at two SSL regions (red and blue) and in cell core (black), as indicated to the left of the image in (A). (C) and (D) Space-time image and local Ca2+ transients, respectively, obtained in the same cell at PCL = 4 s. Electrical stimulation induces elevations in Ca2+ only in the SSL regions. (E) Time-to-target plot of activation time versus distance from cell edge, with Ca2+ transients averaged over each 2 μm region.
Image analysis of Ca2+ transients
To compute beat-to-beat changes in the response to electrical stimulation, Ca2+ transients were measured at three spatial regions: two sub-sarcolemmal (SSL) regions, defined as the 5 μm closest to the cell edges [12], and in the cell core. We observed that the resting fluorescence (F0) prior to the initial stimulus was not uniform along the transversely-oriented scan line. Thus F0 was calculated as a function of spatial location x, and Ca2+ transients were normalized on a pixel-by-pixel basis. F0 was calculated before electrical stimulation commenced, and all subsequent Ca2+ transients were normalized to this value. This allowed us to observe changes in diastolic [Ca2+] caused by changes in pacing rate.
Ca2+ concentration calibration
To convert from normalized fluorescence to [Ca2+], we used the “pseudo-ratio” equation developed for single wavelength indicators [13]: [Ca2+] = KR/(K/[Ca2+]rest − R +1 ), where K is the affinity of the indicator for calcium, and R is the normalized fluorescence (F/F0). We set K = 0.7 μM, [Ca2+]rest = 0.1 μM.
Caffeine application
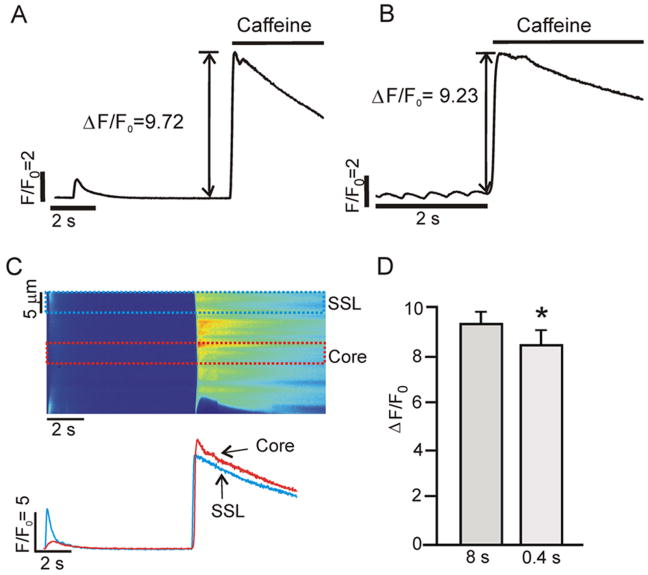
To assess the SR Ca2+ load at a fixed pacing cycle length (PCL), 20 mM Caffeine was rapidly applied to a single cell after at least 10 beats at PCL= 8 s and 20 beats at PCL= 0.4 s. The solution change was timed so that caffeine reached the cell approximately when the next stimulus would have occurred. SR Ca2+ load was approximated as the peak normalized change in fluorescence (ΔF/F0) upon caffeine application [14]. Changes in fluorescence induced by caffeine were normalized to resting fluorescence (F0) measured prior to electrical stimulation.
Statistics
Data are presented as mean ± s.e.m. Differences between groups were analyzed using a paired t test, with p < 0.05 considered statistically significant.
Results
Patterns of SR Ca2+ release are strongly rate-dependent
To understand rate-dependent changes in SR Ca2+ release in cardiac Purkinje cells, we recorded from periodically-stimulated cells using the line scan mode of the confocal microscope, with the scan line oriented in the transverse direction. Representative pseudo-images at two different rates are shown in Figure 1, with location on the y-axis and time on the x-axis. At an extremely low pacing rate (PCL = 8s; Fig. 1A), Ca2+ transients originated at the cell periphery (SSL) and then propagated into the cell core, exhibiting the V-shaped pattern typical of Ca2+ waves seen previously in myocytes lacking T-tubules [7, 15-18]. Strikingly different behavior was observed when this cell was paced at a slightly higher rate (PCL = 4 s; Fig. 1B). In this case SSL Ca2+ transients were smaller than at PCL = 8 s, and little increase in Ca2+ was observed in the cell core, consistent with failed Ca2+ wave propagation. SSL and core Ca2+ transients averaged over 5 μm regions illustrate this difference (Figs. 1C and 1D), and a “time-to-target” analysis of Ca2+ wave velocity (Fig. 1E) indicated that successful propagation to the cell core at PCL = 8 s was faster than failed, local diffusion seen at PCL = 4 s (84.5 μm/s versus 44.1 μm/s). Moreover, the linear relationship between location and activation time at PCL = 8 s is consistent with regenerative Ca2+ wave propagation whereas the progressively longer delays seen at PCL = 4 s are consistent with passive Ca2+ diffusion. Similar results were seen in n=9 cells, with the transition from successful waves to failed waves occurring at PCL = 4.9 ± 0.35 s. Ca2+ waves propagating into the cell core were never observed in cells pre-treated with ryanodine and thapsigargin to block SR Ca2+ uptake (Supplementary Figure S2). Together these results indicate that at extremely low pacing rates, Ca2+ release occurs via the regenerative propagation of local release events, whereas at high pacing rates only local elevations in [Ca2+] and simple diffusion away from the cell periphery is observed.
Purkinje cells exhibit a negative [Ca2+]-frequency relationship
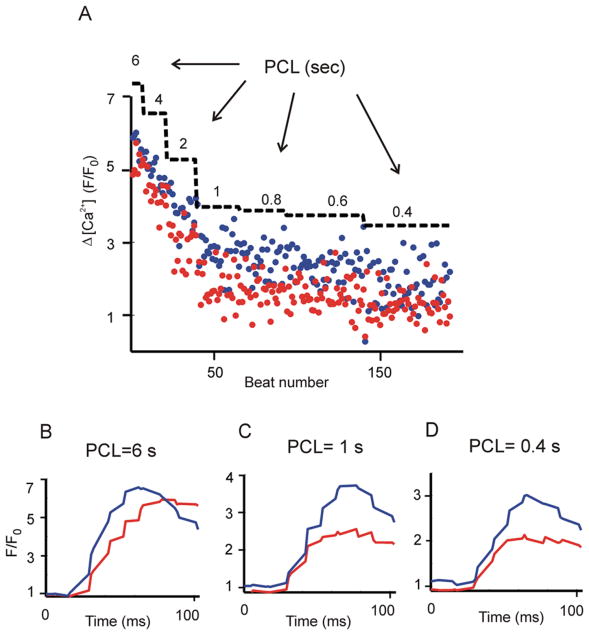
To investigate the rate dependence of Purkinje cell Ca2+ transients more systematically, we progressively increased the pacing rate while recording the rising phase of the SSL Ca2+ transients at each rate. Figure 2 displays the amplitudes of 192 consecutive Ca2+ transients recorded in an individual cell at PCLs ranging from 6 s to 0.4 s. The blue and red dots represent SSL Ca2+ transient amplitudes recorded just under the cell membrane at the two edges of the cell. An increase in rate caused a progressive decrease in Ca2+ transient amplitude at both locations. Averaged Ca2+ transients from the two edges at three values of PCL are shown in Fig. 2, B-D. This result shows that the [Ca2+]-frequency relationship in canine Purkinje cells is negative. Similar results were observed in n=7 cells. To our knowledge, this is the first such quantification of this relationship in this cell type.
Figure 2.
Purkinje cells exhibit a negative Δ[Ca2+] versus frequency relationship. A single Purkinje cell was paced with a protocol in which the PCL was progressively decreased and Ca2+ transients resulting from each stimulation were recorded. (A) Local SSL Ca2+ transients recorded from two edges of a cell. Amplitudes (Δ[Ca2+]= Peak−diastolic [Ca2+]) calculated at the two SSL regions are denoted by red and blue circles. PCLs are superimposed as a staircase to mark each cycle length regime. (B-D) Averaged SSL [Ca2+] transients at PCL= 6 s (B), 1 s (C), 0.4 s (D). All data were obtained at the same scan line in an individual cell.
Beat-to-beat variability persists at constant cycle length
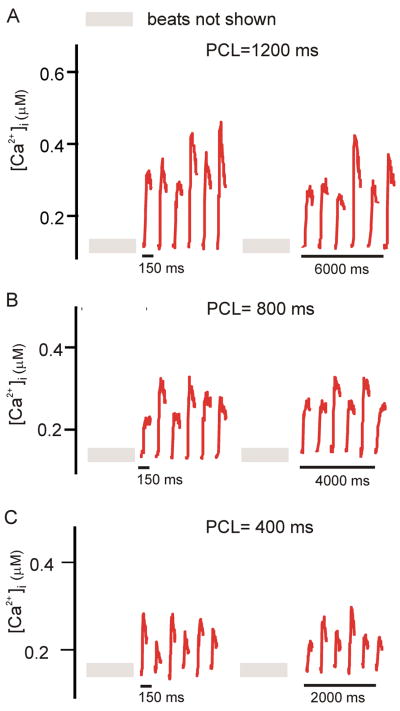
An additional observation from Fig. 2 is the fact that, at each sub-sarcolemmal region, the Ca2+ transient amplitude at a given PCL fluctuates considerably from beat to beat. In this experiment, some of the variability may have been caused by transient effects due to a recent abrupt change in the pacing rate. To clarify this issue, we performed additional experiments in which we recorded many (> 65) consecutive Ca2+ transients at a few PCLs. Figure 3 shows, for three values of PCL, two sets of six consecutive SSL Ca2+ transients: the first obtained after 30 beats at that PCL, and the second after an additional 23 beats. These results demonstrate that beat-to-beat variability in local SSL Ca2+ transient amplitude persists in Purkinje cells, even during sustained pacing at a constant cycle length.
Figure 3.
Beat-to-beat variability in the local SSL persists with steady pacing. (A), (B), and (C), respectively, show SSL Ca2+ transients at PCL=1200, 800, and 400 ms. Each panel shows two sets of six consecutive Ca2+ transients, the first obtained after 29 stimuli, and the second obtained after 23 additional stimuli. At each PCL, variability in SSL Ca2+ transient amplitude persists.
Relative variability of SSL Ca2+ transients is greater at higher pacing rates
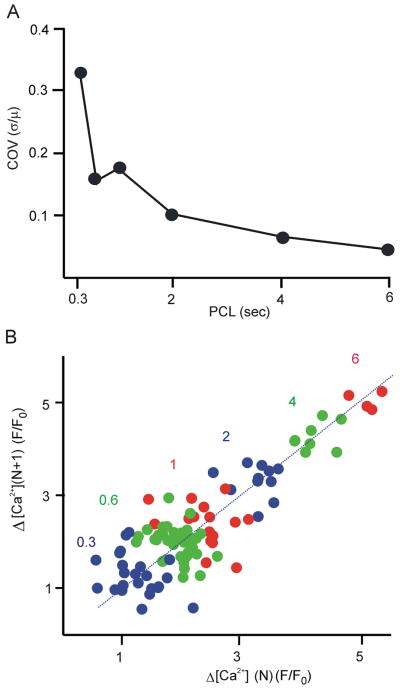
Results in Figs. 2 and 3 suggest that beat-to-beat variability in local Ca2+ transient amplitude is substantial in Purkinje cells. Two issues that are not clear, however, are: 1) how does this variability depend on PCL? and 2) do beat to beat changes in amplitude show a regular pattern such as alternans? To address these questions, we performed two additional analyses. To quantify variability, Fig. 4A displays, as a function of PCL, the coefficient of variation (COV), i.e. the standard deviation of local Ca2+ transient amplitude divided by the mean amplitude at that PCL. COV increases as PCL decreases; i.e. fast pacing leads to a greater relative variability in local Ca2+ transient amplitude. Figure 4B shows a map of Ca2+ transient amplitude at the current beat (ΔF(N)) versus Ca2+ transient amplitude on the subsequent beat (ΔF(N+1)). At low rates, data points are located in the upper right of the map, indicating larger amplitudes at these rates, and are aggregated close to the line of identity, indicating relatively constant beat-to-beat amplitude. At high rates, data points fill the space around the line of identity, with no obvious pattern. To explore in more detail whether Ca2+ transient amplitude on one beat influences the next beat, we calculated temporal persistence, a measure that allows for robust detection of small alternans, even in the presence of noise [19]. Temporal persistence in our data, however, was less than 20% (Supplementary Figure S3), close to the values that can occur in completely random sequences. Together these results suggest that the amplitude of the Nth beat has little influence on the amplitude of the (N+1)th beat. Thus, faster pacing leads to greater variability in SSL Ca2+ transients, but unlike regular alternating patterns seen in ventricular myocytes [20], beat-to-beat patterns are highly irregular.
Figure 4.
Faster pacing leads to an increase in the normalized Ca2+ transient variability, but an irregular pattern from one beat to the next. (A) Coefficient of variation (COV) in SSL Ca2+ transient amplitude, computed in a single cell as the standard deviation divided by the mean, and plotted as a function of PCL. (B) Map of SSL Ca2+ transient amplitude on the current beat versus amplitude on the next beat at several different PCLs as indicated.
Pacing rate influences SR Ca2+ load
The smaller local Ca2+ transients observed with fast pacing in Purkinje cells may result, at least in part, from a smaller SR Ca2+ load. To assess this hypothesis, we rapidly applied 20 mM caffeine after pacing cells at a constant rate. Figure 5A and B demonstrate results from a single cell indicating that SR Ca2+ load, quantified as ΔF/F0 averaged across the cell width in response to caffeine, is slightly smaller at PCL = 0.4 s compared with PCL = 8 s. Fig. 5C presents a line-scan image from a different cell illustrating that caffeine causes increases in Ca2+ in both the SSL regions and the cell core. Summary results (n=10 cells) in Fig. 5D show the modest (10%) decrease in caffeine-induced ΔF/F0 at PCL = 0.4 s compared with PCL = 8 s. Although the change in SR Ca2+ load reached statistical significance (p = 0.03), the decrease in load from slow to fast pacing was considerably smaller than the decrease in SSL Ca2+ transient amplitude (66%, n = 10 cells). When expressed as changes in Δ[Ca2+] rather than ΔF/F0, the mean data suggest that rapid pacing causes a 75% decrease in Ca2+ transient amplitude but only a 27% decrease in SR Ca2+ load. The results suggest, therefore, that mechanisms besides changes in SR Ca2+ load contribute to the negative Ca2+-frequency relationship in these cells.
Figure 5.
SR Ca2+ load during slow and fast pacing. (A) Example showing the increase in cytosolic [Ca2+] produced by rapid application of 20 mM caffeine after pacing at PCL = 8s. (B) Increase in cytosolic [Ca2+] in the same cell after pacing at 0.4 s. (C) Space time image of the response to caffeine at PCL = 8 s (top), and SSL and core Ca2+ transients (5 μm average; bottom). (D) Pooled data (n=10 cells) illustrating that SR Ca2+ load, approximated as ΔF/F0 induced by 20 mM caffeine, is slightly lower at PCL = 0.4 s than at PCL = 8 s (8.41 ± 0.63 versus 9.30 ± 0.52; p = 0.03).
A simple mathematical model suggests a mechanism underlying beat-to-beat variability
How does beat-to-beat variability occur in Purkinje cells? Based on our results, we hypothesize that the number of RyR clusters, or Ca2+ release units (CRUs), contributing to each recorded local Ca2+ transient is relatively small, and stochastic effects may therefore dominate the observed variability. In accordance with this hypothesis, we propose a simple model of Ca2+ transient amplitude variability based on binomial statistics. We assume that, in the sub-sarcolemmal region near the cell membrane: 1) the confocal microscope records from a volume containing N CRUs (Fig. 6A); 2) the CRUs are triggered independently; and 3) the amplitudes of the Ca2+ sparks from the triggered CRUs are identical. If, with each electrical stimulus, the probability of triggering a spark from a CRU is p, then the probability that exactly k Ca2+ sparks are triggered on a given beat is described by the kth term of the binomial distribution:
| (1) |
If A is the amplitude of a Ca2+ spark, then the mean and coefficient of variation (COV) of Ca2+ transients recorded from the imaging volume are
| (2) |
| (3) |
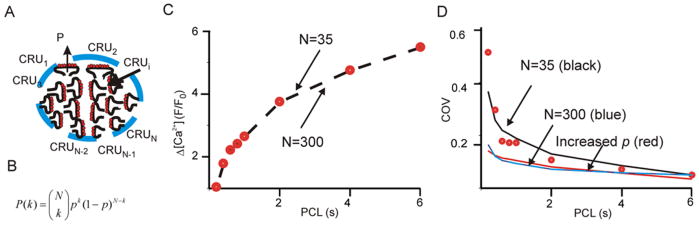
We sought to determine whether these predictions of mean Ca2+ transient amplitude (μ) and COV were consistent with our data. Since μ depends on the product A·N (Eq. 2), it follows that Ca2+ transient amplitude can be equally well-explained by a model with large A, small N, or one with small A, large N. In other words, based on mean amplitude alone, one cannot infer whether many CRUs are each producing small sparks, or few CRUs are each generating larger sparks. Beginning with μ obtained at PCL = 6 s, and assuming for simplicity that A does not depend on PCL, we determined the values of p at each PCL required to explain the Ca2+ transient mean amplitude data. Consistent with the above argument, Fig. 6C shows that results from a given cell could be equally well-fit by a model with N=35 (small) or one with N=300 (large). However, when we computed COV (Eq. 3) versus PCL for these parameters, we found that the model with N=35 fit the data well whereas the model with N=300 did not. Thus, the variability observed in our data can be explained by a simple model in which stochastic triggering of a limited number of release units contributes to the recorded signal.
Figure 6.
Results of simple mathematical model. (A) Diagram of N Ca2+ release units (CRUs). During an action potential each unit has a probability of p to be triggered and generate an event with amplitude A. (B) Assuming that triggering from each CRU is an independent event, the kth term of the binomial distribution describes the probability that k events are triggered. (C) Dots show the relationship between Ca2+ transient amplitude (averaged at one SSL of a cell) and PCL. The model can fit the data perfectly (dashed line) assuming either many release units, each with small amplitude (N=300), or fewer release units, each with larger amplitude (N=35). (D) COV data (black dots) from the same cell were compared with model results of N=35 (black solid line), N=300 (black dashed line) and N=35 with a 50% increased probability p (blue dashed line).
Variability decreases with increasing triggering probability
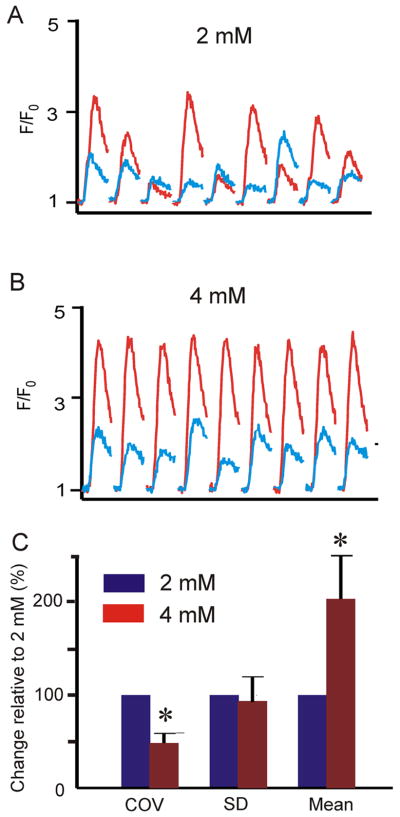
Although the model based on binomial statistics is extremely simple, it allows us to generate predictions that can be tested experimentally. For instance, how does an increase in p affect COV? Fig 6D shows that with N=35, an increase in p causes a decrease in COV at all values of PCL (which can also be seen by inspecting Eq. 3). We tested this prediction by increasing extracellular [Ca2+] ([Ca2+]o) from 2 mM to 4 mM. An important aspect of this prediction is the fact that Ca2+ spark amplitude A, which would be expected to increase at greater [Ca2+]o, does not influence COV (Eq. 3). In these experiments, PCL was fixed at 800 ms, a middle value in our range of cycle lengths. Many consecutive Ca2+ transients were recorded with [Ca2+]o = 2 mM (Fig. 7A), cells were perfused with [Ca2+]o = 4 mM for 5 minutes while pacing continued, then recording was resumed at the same scan line. Fig. 7B shows that at [Ca2+]o = 4 mM, Ca2+ transient amplitude increased, but the relative variability decreased at both edges of the cell, consistent with the model prediction. Summary data in Fig 7C show that COV decreased substantially (by 51 ± 11 %) and significantly (p < 0.001) with an increase in [Ca2+]o from 2 mM to 4 mM.
Figure 7.
An increase in triggering of Ca2+ release causes a decrease in relative Ca2+ transient variability. (A) [Ca2+] transients at two SSL regions with [Ca2+]o = 2 mM. (B) [Ca2+] transients measured from the same locations in the same cell with [Ca2+]o = 4 mM. (C) Pooled data (n=6 cells) illustrating that increasing [Ca2+]o causes a significant (p = 0.005) increase in mean SSL Ca2+ transient amplitude and a significant (p < 0.001) decrease in COV. Average COV at 4 mM is 49 ± 11% of the value at 2 mM. For these statistical comparisons, we treated the two SSL regions in each cell as independent events such that n=6 cells generated a total of n=12 data pairs for comparison.
Discussion
In this study, we have presented new results indicating how pacing rate influences the patterns of SR Ca2+ release observed in isolated, periodically-stimulated Purkinje cells. Electrical stimulation induced SSL Ca2+ release events which then propagated as Ca2+ waves to the core. However, when cells were paced at rates above roughly 0.25 Hz, only smaller, local SSL transients occurred, and wave propagation failed. A further increase in pacing rate caused a further decline in Ca2+ transient amplitude, accompanied by a modest decrease in SR Ca2+ load. With repetitive stimulation, considerable Ca2+ transient amplitude variability was observed, without any obvious beat-to-beat pattern. A simple mathematical model and a straightforward test of model predictions suggest that this variability may result from stochastic effects due to a small number of Ca2+ release sites contributing to the measured signal.
Negative Ca2+-frequency relationship
We observed, to our knowledge for the first time, that Ca2+ transient amplitude declines with increasing pacing rate in canine Purkinje cells, i.e. these cells exhibit a negative Ca2+-frequency relationship. In 1981 Wasserstrom & Ferrier measured a negative relationship between contractile force and pacing frequency in canine Purkinje fibers [21] – our study complements and extends this prior result by documenting changes in the patterns of SR Ca2+ release at different frequencies. For instance, we found that Ca2+ waves propagating from the periphery to the core occurred at low pacing rates, consistent with prior studies performed in cells lacking T-tubules [4, 7, 15, 17, 22], whereas release only occurred in the subsarcolemmal region at higher rates. Although most ventricular myocytes from larger mammals show a positive Ca2+-frequency relationship [14], some studies have suggested that this relationship is flat or negative in heart failure [23]. Since heart failure has been shown to lead to profound disruptions in the T-tubular system [24-26], an interesting question for future work is whether the loss of T-tubules per se causes changes in the Ca2+-frequency relationship of mammalian heart cells.
Although we have observed that SR Ca2+ load is smaller at PCL = 0.4 s compared with PCL = 8 s, this decrease is relatively modest (10%) compared with the reduction in SSL Ca2+ transient amplitude (66%). Even after taking into account the roughly cubic relationship between load and release measured in ventricular myocytes [27], it seems likely that other mechanisms are required to explain the decrease in Ca2+ transient amplitude. While determining the mechanisms underlying the Ca2+-frequency relationship was beyond the scope of this study, factors that may potentially contribute include: 1) decreased peak Ca2+ current at higher rates due to accumulating inactivation of L-type Ca2+ channels; 2) rate-dependent changes in transient outward current; and 3) greater refractoriness of the ryanodine receptor at higher rates. The last possibility is especially intriguing given: (1) the modest reduction in cellular SR Ca2+ load at high rates, and (2) the fact that dynamic local changes in SR [Ca2+] appear to control RyR refractoriness and recovery in ventricular muscle [28-30]. A potential explanation for the apparent discrepancy is that during fast pacing in Purkinje cells, Ca2+ has been taken into the SR by SERCA pumps but has not yet diffused to the junctions where it can be released. Measurements of local recovery of SR Ca2+ release [31] in Purkinje cells would be required to address this hypothesis. A second possibility is that control of RyR gating is fundamentally different in Purkinje cells, and a mechanism such as Ca2+-dependent inactivation, which appears to play no role in the ventricle [32], contributes to refractoriness during rapid pacing. Future studies can explore these interesting possibilities.
Beat-to-beat variability
We originally expected that rapid pacing of Purkinje cells would result in regular beat-to-beat changes in SR Ca2+ release, i.e. Ca2+ transient alternans as have been observed in ventricular myocytes [20, 33, 34]. Sustained beat-to-beat variability in the absence of Ca2+ transient alternans, which we found instead, was therefore somewhat unanticipated. This result becomes less surprising, however, when one considers that the confocal microscope in line-scan mode records from a small volume in the region near the cell membrane where Ca2+ entry and SR Ca2+ release occur. It therefore seems quite reasonable that the recorded Ca2+ transients reflect the triggering of a relatively small number of Ca2+ sparks.
Experiments performed in atrial [22] and ventricular myocytes [13] have similarly observed some beat-to-beat variability in SR Ca2+ release. Our analysis extends these previous observations by: 1) quantifying COV at different pacing rates, and 2) proposing a simple mathematical model to account for the experimental observations. Our model suggests that the increased relative variability at higher pacing rates may be a simple consequence of the negative force frequency relationship: i.e. a decrease in spark triggering probability p at higher rates causes an increase in variability. The model further allows us to conclude that if the decrease in mean Ca2+ transient amplitude μ were to occur by a different mechanism, for instance a decrease in spark amplitude A, then faster pacing could hypothetically be accompanied by either no change or an increase in COV (Supplementary Figure S4).
If variability indeed results from our proposed mechanism, it follows that an increase in p should decrease the variability. When we tested this prediction by increasing extracellular [Ca2+], a decrease in variability was observed (Fig. 7). These results therefore suggest that stochastic Ca2+ spark triggering may be sufficient to explain the beat-to-beat variability that we found. Moreover, the lack of a clear pattern in consecutive Ca2+ transient amplitudes (Fig. 4B and Supplementary Figure S3) additionally suggests that stochastic effects are the most important factor.
Implications for arrhythmogenesis
The negative Ca2+-frequency relationship and the dependence of Ca2+ wave propagation on pacing rate have important implications for Ca2+-triggered arrhythmias originating in the Purkinje system. Our results suggest that spontaneous propagating Ca2+ waves, which cause inappropriate membrane depolarization and can initiate arrhythmias [4, 35] are more likely to occur during slow rather than fast pacing. However, our results with rapid caffeine application indicate that SR stores in the cell core remain loaded with Ca2+ during rapid pacing. Moreover, since fast pacing causes only a small decline in total cellular SR Ca2+ load, this suggests that the rapidly-paced Purkinje cells may retain the ability to generate propagating Ca2+ waves. These considerations may help explain why arrhythmias can be frequently initiated after a pause in pacing [36]. During rapid pacing, the SR remains filled with Ca2+ even though triggered Ca2+ transients are small. A brief pause may allow RyRs to recover from refractoriness and thereby increase the probability that a spontaneous local Ca2+ release event will trigger a regenerative Ca2+ wave. Future studies can use more sophisticated pacing protocols to explore this hypothesis. Although a thorough understanding of the arrhythmic potential of Purkinje cells will also require consideration of factors such as β-adrenergic stimulation and disease states, the results presented here provide an important baseline for understanding future work.
An unresolved question, however, is how highly variable behavior at the sub-cellular level, as observed here, may be either pro-arrhythmic or anti-arrhythmic within the cardiac syncytium. Because beat-to-beat changes in local Ca2+ transients appeared to be essentially random, we speculate that these effects will still be present in the multicellular environment, but, due to averaging, variability per se will not be pro-arrhythmic. Future studies will be required to address these important issues.
Study Limitations
Several limitations should be considered. One is that all Ca2+ transient recordings were performed at room temperature. Although changes in temperature will certainly have quantitative effects on Ca2+ transient amplitude, this limitation is unlikely to influence the general trends that we observed. For instance, Boyden et al previously found that physiological temperature led to an increase in the frequency of Ca2+ release events, but comparable differences between healthy Purkinje cells and those that remained in the ischemic border zone [4, 5]. A second limitation is our use of the non-ratiometric dye fluo-3. Because we converted from fluorescence to [Ca2+] using the pseudo-ratio equation which required us to assume diastolic [Ca2+], our absolute Ca2+ transient amplitudes should be interpreted cautiously. We should note, however, that this limitation will not affect our observations regarding the relative changes in Ca2+ transient amplitude, beat-to-beat variability, and SR Ca2+ load made using consecutive measurements in individual cells. Finally, our calculations of SR Ca2+ load, computed as peak ΔF/F0 upon application of 20 mM caffeine, should be considered semi-quantitative, especially given that large increases in intracellular [Ca2+] may cause partial saturation of the indicator. More precise estimates of load could be obtained using fluorescent indicators trapped in the SR or by integrating Na+-Ca2+ exchange current in voltage-clamped cells.
Conclusions
In summary, our study provides new information regarding how pacing rate influences the patterns of SR Ca2+ release observed in canine Purkinje cells. We found that Ca2+ transients are larger at low versus high pacing rates. The decrease in local Ca2+ release during faster pacing was accompanied by an increase in beat-to-beat variability, which is potentially explained by a model in which stochastic triggering of a relatively small number of Ca2+ release units determines beat-to-beat changes. The results have implications both for normal Purkinje cell function and for potentially arrhythmogenic Ca2+ release that can occur in disease.
Supplementary Material
Acknowledgments
We would like to thank Frank Fabris for technical assistance. This work was supported by National Institutes of Health grants HL076230 (EAS) and HL67449 (PAB).
List of abbreviations
- PCL
pacing cycle length
- SSL
sub-sarcolemmal
- RyR
ryanodine receptors
- SR
sarcoplasmic reticulum
- T-tubule
Transverse tubule
- F0
resting fluorescence
- ΔF/F0
normalized change in fluorescence
- COV
coefficient of variation
- CRU
Ca2+ release unit
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
Footnotes
Disclosures None to declare.
Supplementary Materials Supplementary Figures associated with this manuscript illustrate: 1) the calculation of Ca2+ wave velocity (Figure S1); 2) the lack of Ca2+ wave propagation in a cell with SR Ca2+ release disabled (Figure S2); 3) analyses to determine whether Ca2+ transient amplitude on one beat influences the subsequent beat (Figure S3); and 4) additional calculations about how Ca2+ spark triggering may influence the rate dependence of COV (Figure S4).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyden PA, Hirose M, Dun W. Cardiac Purkinje cells. Heart Rhythm. 2010;7(1):127–35. doi: 10.1016/j.hrthm.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Bogun F, Good E, Reich S, Elmouchi D, Igic P, Tschopp D, et al. Role of Purkinje fibers in post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;48(12):2500–7. doi: 10.1016/j.jacc.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Cerrone M, Noujaim SF, Tolkacheva EG, Talkachou A, O’Connell R, Berenfeld O, et al. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2007;101(10):1039–48. doi: 10.1161/CIRCRESAHA.107.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyden PA, Pu J, Pinto J, Keurs HE. Ca2+ transients and Ca2+ waves in purkinje cells : role in action potential initiation. Circ Res. 2000;86(4):448–55. doi: 10.1161/01.res.86.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyden PA, Barbhaiya C, Lee T, ter Keurs HE. Nonuniform Ca2+ transients in arrhythmogenic Purkinje cells that survive in the infarcted canine heart. Cardiovasc Res. 2003;57(3):681–93. doi: 10.1016/s0008-6363(02)00725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dun W, Boyden PA. The Purkinje cell; 2008 style. J Mol Cell Cardiol. 2008;45(5):617–24. doi: 10.1016/j.yjmcc.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordeiro JM, Spitzer KW, Giles WR, Ershler PE, Cannell MB, Bridge JH. Location of the initiation site of calcium transients and sparks in rabbit heart Purkinje cells. The J Physiol. 2001;531(Pt 2):301–14. doi: 10.1111/j.1469-7793.2001.0301i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song LS, Guatimosim S, Gomez-Viquez L, Sobie EA, Ziman A, Hartmann H, et al. Calcium biology of the transverse tubules in heart. Ann N Y Acad Sci. 2005;1047:99–111. doi: 10.1196/annals.1341.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res. 2003;92(11):1182–92. doi: 10.1161/01.RES.0000074908.17214.FD. [DOI] [PubMed] [Google Scholar]
- 10.Boyden PA, Albala A, Dresdner KP., Jr Electrophysiology and ultrastructure of canine subendocardial Purkinje cells isolated from control and 24-hour infarcted hearts. Circ Res. 1989;65(4):955–70. doi: 10.1161/01.res.65.4.955. [DOI] [PubMed] [Google Scholar]
- 11.Di Maio A, Ter Keurs HE, Franzini-Armstrong C. T-tubule profiles in Purkinje fibres of mammalian myocardium. J Muscle Res Cell Motil. 2007;28(2-3):115–21. doi: 10.1007/s10974-007-9109-6. [DOI] [PubMed] [Google Scholar]
- 12.Stuyvers BD, Dun W, Matkovich S, Sorrentino V, Boyden PA, ter Keurs HE. Ca2+ sparks and waves in canine purkinje cells: a triple layered system of Ca2+ activation. Circ Res. 2005;97(1):35–43. doi: 10.1161/01.RES.0000173375.26489.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannell MB, Cheng H, Lederer WJ. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophysical journal. 1994;67(5):1942–56. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bers DM. Excitation–Contraction Coupling and Cardiac Contractile Force. 2. Dordrecht/Boston/London: Kluwer Academic Publishers; 2001. [Google Scholar]
- 15.Haddock PS, Coetzee WA, Cho E, Porter L, Katoh H, Bers DM, et al. Subcellular [Ca2+]i gradients during excitation-contraction coupling in newborn rabbit ventricular myocytes. Circ Res. 1999;85(5):415–27. doi: 10.1161/01.res.85.5.415. [DOI] [PubMed] [Google Scholar]
- 16.Kirk MM, Izu LT, Chen-Izu Y, McCulle SL, Wier WG, Balke CW, et al. Role of the transverse-axial tubule system in generating calcium sparks and calcium transients in rat atrial myocytes. The J Physiol. 2003;547(Pt 2):441–51. doi: 10.1113/jphysiol.2002.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brette F, Despa S, Bers DM, Orchard CH. Spatiotemporal characteristics of SR Ca2+ uptake and release in detubulated rat ventricular myocytes. J Mol Cell Cardiol. 2005;39(5):804–12. doi: 10.1016/j.yjmcc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Huser J, Lipsius SL, Blatter LA. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. The J Physiol. 1996;494(Pt 3):641–51. doi: 10.1113/jphysiol.1996.sp021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia Z, Bien H, Entcheva E. Detecting space-time alternating biological signals close to the bifurcation point. IEEE Trans Biomed Eng. 2010;57(2):316–24. doi: 10.1109/TBME.2009.2028652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B. Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophysical journal. 1999;77(6):2930–41. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasserstrom JA, Ferrier GR. Voltage dependence of digitalis afterpotentials, aftercontractions, and inotropy. Am J Physiol. 1981;241(4):H646–53. doi: 10.1152/ajpheart.1981.241.4.H646. [DOI] [PubMed] [Google Scholar]
- 22.Kockskamper J, Sheehan KA, Bare DJ, Lipsius SL, Mignery GA, Blatter LA. Activation and propagation of Ca2+ release during excitation-contraction coupling in atrial myocytes. Biophysical journal. 2001;81(5):2590–605. doi: 10.1016/S0006-3495(01)75903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sipido KR, Stankovicova T, Flameng W, Vanhaecke J, Verdonck F. Frequency dependence of Ca2+ release from the sarcoplasmic reticulum in human ventricular myocytes from end-stage heart failure. Cardiovasc Res. 1998;37(2):478–88. doi: 10.1016/s0008-6363(97)00280-0. [DOI] [PubMed] [Google Scholar]
- 24.Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, et al. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. The J Physiol. 2006;574(Pt 2):519–33. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, et al. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res. 2003;59(1):67–77. doi: 10.1016/s0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 26.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci U S A. 2006;103(11):4305–10. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trafford AW, Diaz ME, Eisner DA. Coordinated control of cell Ca2+ loading and triggered release from the sarcoplasmic reticulum underlies the rapid inotropic response to increased L-type Ca2+ current. Circ Res. 2001;88(2):195–201. doi: 10.1161/01.res.88.2.195. [DOI] [PubMed] [Google Scholar]
- 28.Sobie EA, Dilly KW, dos Santos Cruz J, Lederer WJ, Jafri MS. Termination of cardiac Ca(2+) sparks: an investigative mathematical model of calcium-induced calcium release. Biophys J. 2002;83(1):59–78. doi: 10.1016/s0006-3495(02)75149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terentyev D, Viatchenko-Karpinski S, Gyorke I, Volpe P, Williams SC, Gyorke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc Natl Acad Sci U S A. 2003;100(20):11759–64. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szentesi P, Pignier C, Egger M, Kranias EG, Niggli E. Sarcoplasmic reticulum Ca2+ refilling controls recovery from Ca2+-induced Ca2+ release refractoriness in heart muscle. Circ Res. 2004;95(8):807–13. doi: 10.1161/01.RES.0000146029.80463.7d. [DOI] [PubMed] [Google Scholar]
- 31.Sobie EA, Song LS, Lederer WJ. Local recovery of Ca2+ release in rat ventricular myocytes. The J Physiol. 2005;565(Pt 2):441–7. doi: 10.1113/jphysiol.2005.086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens SC, Terentyev D, Kalyanasundaram A, Periasamy M, Gyorke S. Intra-sarcoplasmic reticulum Ca2+ oscillations are driven by dynamic regulation of ryanodine receptor function by luminal Ca2+ in cardiomyocytes. The J Physiol. 2009;587(Pt 20):4863–72. doi: 10.1113/jphysiol.2009.175547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diaz ME, O’Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94(5):650–6. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 34.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94(8):1083–90. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 35.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88(11):1159–67. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 36.Leclercq JF, Maisonblanche P, Cauchemez B, Coumel P. Respective role of sympathetic tone and of cardiac pauses in the genesis of 62 cases of ventricular fibrillation recorded during Holter monitoring. Eur Heart J. 1988;9(12):1276–83. doi: 10.1093/oxfordjournals.eurheartj.a062444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.