Abstract
Objective
Generalized Anxiety Disorder (GAD) is common in older adults and can be treated with SSRIs. Genetic variation in the serotonin transporter gene promoter region is posited to be associated with SSRI efficacy: two polymorphisms (5HTTLPR s/l and rs25531 g/a) form a haplotype with the La combination having higher transcription activity than other haplotypes. We hypothesized that GAD patients with no La haplotypes (La-) have lower SSRI treatment efficacy than those with 1-2 La haplotypes (La+).
Method
The study enrolled subjects aged 60 and older with a principal diagnosis of GAD, into a twelve-week, randomized trial of escitalopram vs. placebo. One hundred-fifty subjects were genotyped for the serotonin transporter promoter region haplotype and were divided into La- and La+ genotype groups; the primary analyses were done in European-Americans only (N=125; 59 escitalopram and 66 placebo).
Results
Escitalopram had no efficacy in the La- group versus moderate efficacy in the La+ group. This genetic moderation of SSRI efficacy was due to a higher placebo response in La- subjects, compared to La+ subjects. Drug concentration did not affect the genetic results. Exploratory analyses suggest that La- subjects had greater variability of anxiety symptoms unrelated to treatment.
Conclusions
The serotonin transporter promoter haplotype is associated with variability in SSRI efficacy for late-life GAD. The variability may result from a genetic effect on anxiety symptom variability unrelated to treatment, rather than a pharmacodynamic effect that has been previously assumed. Further research is needed to understand the pharmacogenetic mechanism of this haplotype.
INTRODUCTION
Generalized Anxiety Disorder (GAD) a common and impairing disorder in older adults (1). Selective serotonin reuptake inhibitors (SSRIs) are efficacious for late-life GAD (2-4) and are increasingly used for anxiety in this age group (5). Variation in the serotonin transporter gene (SLC6A4), the target of SSRIs, has been posited as a potentially significant source of SSRI treatment response variability (6). Within SLC6A4, two promoter polymorphisms form a haplotype associated with high vs. low transcription of the gene. The 5HTTLPR is an insertion/deletion polymorphism which results in a short (S) vs. long (L) polymorphism, and rs25531 is an a/g single nucleotide polymorphism located just upstream of the 5HTTLPR. Only the La haplotype is associated with high SLC6A4 transcription (7), with all others (i.e. Sg, Sa and Lg) are associated with low SLC6A4 transcription. It is postulated that this genetic association with SSRI efficacy is due to pharmacodynamic changes; i.e., those with two low activity haplotypes have lower production of serotonin transporters, hence fewer drug targets (8).
A meta-analysis of placebo controlled studies in depression found that genetic variation in the serotonin transporter promoter is associated with treatment response variability with SSRIs (8). These haplotypes were examined in the STAR*D study and were not associated with citalopram treatment outcome (7); however, a separate analysis of the same dataset (9) reported an association with remission for a haplotype consisting of 5HTTLPR, rs25531, and a variable nucleotide tandem repeat variation (VNTR) in intron 2 (haplotype S-a-12).
In a placebo-controlled study which demonstrated efficacy of the SSRI escitalopram for the acute treatment of GAD in older adults (4), we genotyped the serotonin transporter promoter haplotype. We hypothesized that individuals with two low activity alleles (La-) would have a lower SSRI efficacy than those with 1-2 high activity alleles (La+). Additionally, as research suggests that serotonin transporter promoter variability may affect the SSRI concentration-response curve (10), and as pharmacokinetic variability may be particularly relevant to older adults (11), we gathered escitalopram levels to examine whether lower efficacy of escitalopram in those with two low activity alleles was associated with escitalopram concentration.
MATERIALS AND METHODS
Study design and participants
Subjects were participants in a randomized placebo-controlled trial of escitalopram for the treatment of GAD in adults aged 60 and older (4). All subjects had a principal diagnosis of GAD, diagnosed by the Structured Clinical Interview for DSM-IV Axis I disorders (12), and a score of 17 or greater in the Hamilton Anxiety Rating Scale (Ham-A)(13) and be free of dementia or other neurodegenerative disease, unstable medical illness, current substance abuse, or lifetime history of a psychotic disorder or bipolar disorder; comorbid anxiety and depressive disorders were allowed if GAD was the principal diagnosis and anxiety was not due to medical illness. Participants were recruited in 2005-2007 from primary care practices, specialty mental health practices, and by self-referral. Antidepressant or anxiolytic coprescription was not allowed, with the exception of benzodiazepines (up to 2mg/day equivalent of lorazepam) if already in use. The study was approved by the University of Pittsburgh Institutional Review Board and all subjects provided written informed consent.
Subjects were randomized to escitalopram or placebo for 12 weeks using a permuted-block, 1:1 randomized list generated by a study statistician. The starting dose was 10mg daily; after four weeks, for subjects who did not achieve response, the dose was increased to 20mg daily, as tolerated.
Outcome Measures
Participants were seen weekly for the first four weeks and then every other week. Outcome assessments for this pharmacogenetic analysis were the Clinical Global Impressions-Improvement scale (CGI(14)) and the Penn State Worry Questionnaire (PSWQ)(15). The main outcome was the CGI, which incorporates symptom rating scale data, clinical observations, and the patient's report (4). Interrater reliability for the CGI and Ham-A was adequate (intraclass correlation coefficient: CGI, 0.89; Ham-A, 0.96).
Genotyping
DNA was extracted from blood using standard procedures. For genotyping the serotonin transporter polymorphisms we followed a protocol which allows for genotyping of the SLC6A4 promoter haplotype (16). In short, this is a triplex PCR protocol followed by double restriction endonuclease digestion, which determines the phase-certain 5-HTTLPR and rs25531 (combined as Sa, Sg, La, and Lg haplotypes).
Pharmacokinetics
Plasma samples for escitalopram levels were obtained at week 2, 8, and 12. We assessed escitalopram concentrations using high performance liquid chromatography with ultraviolet detection (17).
Statistical Analysis
Analyses were conducted using SAS 9.1 and Stata 9. The primary analytic strategy for this measure was an evaluation of cumulative response, defined as CGI 1-2, for escitalopram vs. placebo, separated by genotype. As a secondary analytic strategy we examined the efficacy of escitalopram for improvement in self-reported worry severity (using the PSWQ) via mixed effects repeated measures modeling within each genetic group. For these analyses, we divided subjects into two genetic groups, depending on whether they had no La alleles (La-) or 1-2 La alleles (La+), similar to previous work in depression (18-20).
Nonlinear mixed effects modeling was used for the population pharmacokinetic analysis using the NONMEM computer program (Version 5, level 1.1; University of California at San Francisco, CA, USA)(21) to calculate normalized average daily exposure for each subject at a given dose. From this model, the variable utilized in this study was average escitalopram concentration at the modal dose (i.e., the dose that the subject stayed on during the major part of their participation in the study) (22). We used only parent compound levels, as metabolites appear to have little to no pharmacological effect in vivo (23).
RESULTS
Patient Flow and Baseline Characteristics
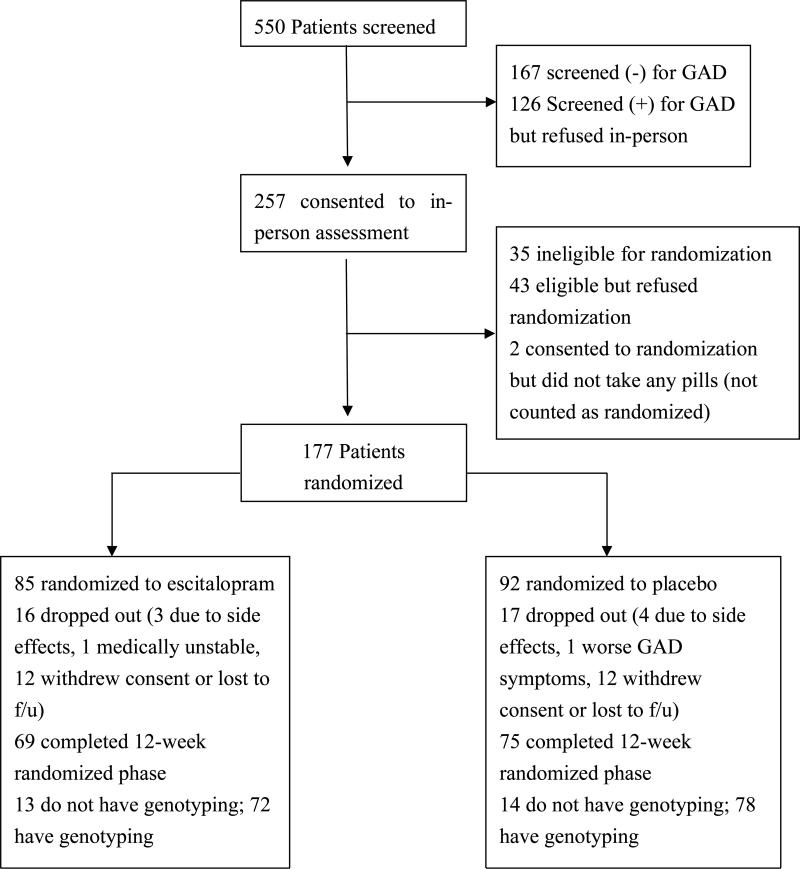
Figure 1 shows patient recruitment and flow through the study. Of 177 participants randomized to treatment, one refused genotyping. Another 19 subjects dropped out of the study before blood could be obtained for genotyping, 5 samples could not provide DNA, and 2 DNA samples could not be genotyped at the 5HTTLPR, leaving 150 subjects for whom treatment and genotyping data are available. Most dropouts were due to patient refusal to continue, and there were no differences between treatment groups in dropouts or reason for dropout. Analyses focused on the Caucasian-only subset of this sample (n=125) to reduce confounding by population stratification, and were repeated in the full sample (n=150) which included 25 minorities.
Figure 1.
Study participant flow
Haplotype frequencies for 5-HTTLPR/rs25531 combinations were as follows: S[a] 40.3%, S[g] 0%, L[a] 48.3%, L[g] 11.3%. Frequencies of these alleles or haplotypes were similar to previously-published research (16) and genotypes showed no departure from Hardy-Weinberg Equilibrium.
For all treatment effect analyses, we compared two groups: La- (i.e., all low-activity haplotypes- Sa and Lg - were combined), n=37 (18 taking escitalopram, 19 placebo); and La+ (i.e., 1-2 La alleles), n=88 (41 escitalopram, 47 placebo). The sample characteristics were as follows: age, mean (SD) 71.5 (8.0) years, age of GAD onset mean (SD) 38.9 (27.1) years, duration of GAD, mean (SD) 383 (331) months, gender 62% female; baseline Ham-A mean (SD) 22.9 (4.7), PSWQ mean (SD) 57.2 (12.7), Mini-Mental State Examination score mean (SD) 28.3 (1.7), Cumulative Illness Rating Scale-Geriatrics score 9.0 (4.1). Further description of the subjects, including comorbidity, is available (4). There were no significant differences in any baseline characteristic by number of La haplotypes.
Relationship of genotype to treatment outcome
Response
We found a significant association of serotonin transporter genotype with treatment efficacy, as defined by escitalopram vs. placebo response. Response rate in the La+ group was significantly higher for escitalopram (68%; 95% confidence interval 55-71%) than placebo (42%; 95% CI 30-57%; p=0.02). In contrast, response for escitalopram in the La- group (77%; 95% CI 58-92%) was not different than placebo (78%; 95% CI 59-91%, p=0.96).
Symptomatic improvements
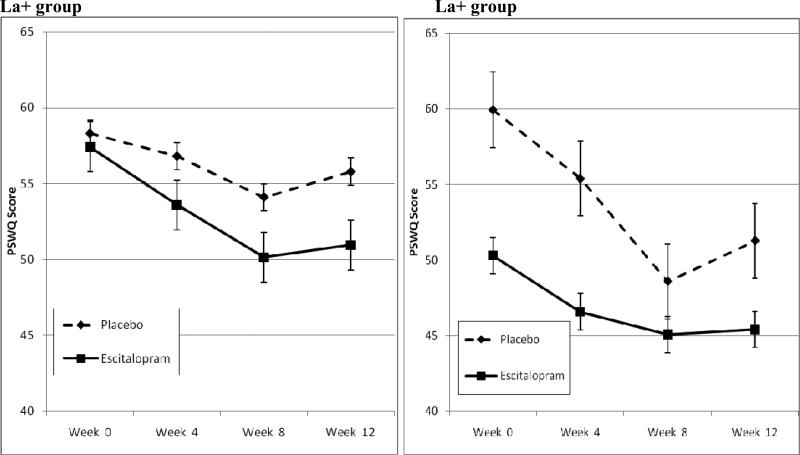
Figure 2 shows changes in PSWQ in the escitalopram and placebo groups, separated by genotype group. Using mixed-effects repeated measures methods, the group × time interaction was significant in the La+ group, but not significant in the La- group. Thus, we found the same genetic relationship with escitalopram's efficacy in these analyses of self-reported worry severity as was seen in time-to-response analyses.
Figure 2. Symptomatic improvements in the Penn State Worry Questionnaire (PSWQ): treatment effect size by genotype group.
In the La+ allele group, there was a significantly greater improvement in the Penn State Worry Questionnaire with escitalopram than placebo (N's 41 escitalopram, 47 placebo, treatment group × time interaction β=-0.41 [standard error=.14, p=0.004], while in the no La group, there were no differences (N's 18 escitalopram, 19 placebo, treatment group × time interaction β=.25 [standard error=.23, p=0.274]). In the La+ group, superiority of escitalopram to placebo in reduction in PSWQ score was 4.6 points (95% CI 1.6-7.5, effect size [d] 0.54). In contrast, in the La- group there was no difference between escitalopram and placebo (effect size -0.11). The difference in effect size between La+ and La- groups was significant (p<0.001).
We also examined effects based on the presence of 0, 1, or 2 La haplotypes; results showed similar results for the 1 La group (i.e., La + Lg or Sa) N= 26 escitalopram, 26 placebo; group × time interaction p=0.05) and 1 La group (i.e., La/La; N=15 escitalopram, 21 placebo; group × time interaction p=0.04); however, they did not show similar results for the response analysis (1 La group p=0.001; 1 La group p=0.92); thus, there was no evidence of a more favorable drug:placebo separation in the 2 La group compared to the 1 La group.
We also recoded genotype based on S vs L genotype alone, in line with previous research (6). The analyses showed similar results: those with the S/S genotype (n=25) had an escitalopram vs. placebo cumulative response rate of 76% vs. 70% (p=0.70), while those with the S/L or L/L genotype (n=100) had a escitalopram vs. placebo response rate of 66% vs. 47% (p=0.05). Similarly, PSWQ improvement in the S/S subjects was not different between escitalopram and placebo (group × time interaction in mixed effect model p=0.25), while PSWQ changes in the S/L or L/L subjects showed greater improvement with escitalopram than with placebo (group × time interaction p=0.01). The differences in escitalopram vs. placebo response by genotype were similar to, though slightly smaller than, the same comparisons using the La- vs. La+ differentiation.
Association of the La- versus La+ group with response and symptomatic improvements was re-examined with the entire sample (n=150 including 24 African-Americans and one Asian-American). The size and direction of the effects did not change: response for the entire sample favored escitalopram over placebo (p=0.009) for the La+ group but not in the La- group (p=0.97). Similarly, for symptomatic changes, in the La+ group, superiority of escitalopram to placebo in reduction in PSWQ score was 5.3 points (95% CI 2.3-8.4, effect size 0.63, while in the La- group there was no difference between escitalopram and placebo (effect size 0.16).
Pharmacokinetic-Pharmacogenetic relationships
We calculated average escitalopram concentration at the modal dose (i.e., the dose that subjects were taking for the greatest duration, typically 20mg during weeks 4 to 12). For the entire sample (n=69), mean concentration was 40.7ng/ml (standard deviation 35.3; range 8.6–295.7). We examined association of drug concentration with response in the overall group of escitalopram-treated subjects and by genotype. We found no relationship of drug concentration with response: subjects who achieved a response (n=47) had a mean concentration of 40.7ng/ml (SD 35.3), while those who did not achieve response during the study (n=22) had mean concentration of 40.0ng/ml (SD 25.1); t=0.09, p=0.93. We also found no relationship of drug concentration with response in either genetic group: La-, responders (n=19) 46.9ng/ml (SD 46.2), nonresponders (n=6) 36.2ng/ml (SD 15.0); La+, responders (n=28) 36.4ng/ml (SD 25.5), nonresponders (n=14) 44.4ng/ml (SD 29.1). PSWQ change in these subjects similarly failed to show an effect of drug concentration, either in the entire group or in each genotype group (data not shown).
Exploratory analysis of association of genotype with longitudinal course of symptoms
Because we found that treatment response variability associated with genotype appeared mainly due to effects on the placebo response (i.e., higher in the La- group than the La+ group), we hypothesized that the La- group were more likely to have fluctuations in anxiety level irrespective of treatment (consistent with prior literature (24)). We conducted an exploratory examination of whether the La- group was more likely to show symptomatic worsening of symptoms (irrespective of treatment assignment). We defined worsening as an increase in the CGI score after week 0. This hypothesis was supported: in the La- group, 59% of subjects (22/37) worsened, compared to 38% (33/88) of the La+ group (exact p=0.04). Thus, the finding suggested that the La- group had greater fluctuations in anxiety symptoms, possibly undermining a drug-placebo separation.
DISCUSSION
Summary
We found that genetic variation in the serotonin transporter promoter is associated with variability in SSRI treatment efficacy for late-life GAD: haplotypes associated with lower transcriptional activity of the promoter were associated with lower drug-placebo separation, supporting our hypothesis. However, the basis for our hypothesis was a pharmacodynamic mechanism, which is not supported by our finding. Specifically, pharmacogenetic research has postulated that association of the serotonin transporter polymorphisms with SSRI efficacy is due to pharmacodynamic changes; i.e., those with two low activity alleles have low transcription activity, resulting in lower production of serotonin transporters, hence fewer drug targets. However, in our study the response variability associated with genetic variation appeared to be mainly in placebo response, as response to escitalopram was not different between genotypes. Of note, the higher placebo response with the S or Lg allele has been suggested in previous research in geriatric depression (25). This genetic association with placebo response cannot be a pharmacodynamic effect.
We carried out an exploratory analysis to examine alternative explanations for our finding. One explanation is that the genetic variation in the serotonin transporter predicted a difference in illness course, not in drug effect. La- individuals have altered corticolimbic affective regulation resulting in a “stress reactive” phenotype (26). Such individuals have more fluctuations in anxiety (27), increased mood symptoms in the context of life stressors (27), and a more robust biological stress response (28,29). Such fluctuation may appear as placebo “response” or abrupt symptomatic exacerbation in a clinical trial, undermining treatment efficacy, and our exploratory analyses support this: La- individuals showed not only more response but more symptomatic worsening, compared to La+ individuals. These fluctuations irrespective of treatment suggest a greater stress reactivity that was not reduced by SSRIs, which is also suggested by a recent report in depression finding that the pharmacogenetic effect of the serotonin transporter is strongly influenced by life events (30). If so, it is possible that this subgroup would be better treated with psychotherapy targeting affective regulation (31). However, this was an exploratory analysis which requires further examination over longer periods of treatment.
Another important characteristic of this study was the inclusion of population pharmacokinetic modeling, as recommended for pharmacogenetic studies of antidepressants (32,33) and may be particularly relevant in elderly persons, in whom more variable drug concentrations may confound pharmacogenetic results (11). We did not find a relationship of escitalopram level with treatment response in any genetic group. This disagrees with a late-life depression study, in which paroxetine concentrations were positively associated with early changes in depressive symptoms in those with the S allele (10).
Limitations of this study should be noted. First, we had a relatively small sample size (e.g., 37 subjects in the Caucasian-only La- group); thus, the findings may be due to low power in the La- subgroup rather than a true genetic difference in drug-placebo separation. Second, we did not gather data on experiences (e.g., life events or social modulators) or psychological modulators that may have accounted for the treatment-unrelated fluctuations in anxiety that we observed. Finally, results may not be generalizable beyond older adults with GAD; such individuals are more likely to have medical illnesses or take medications which may interact with genetic factors to affect response, and this study was not designed to test such alternatives.
In summary, this study of older adults with GAD found that genetic variation in the serotonin transporter promoter predicts SSRI efficacy. However, the genetic variability appeared to occur in placebo response, not drug response, so our findings do not support a pharmacodynamic hypothesis of this genetic effect. A genetic effect on illness course may explain the results, and a study which employs multiple methods of measuring treatment outcome, and also measures other environmental factors (such as life events) is needed, as per recent recommendations for pharmacogenetic studies (34). More research into the mechanism of the serotonin transporter haplotype with respect to treatment outcome is necessary prior to the development and eventual implementation of genomic medicine in anxiety disorders.
Table 1.
Baseline variables by genotype group
| overall n=125 | No La alleles n=37 | 1 or 2 La alleles n=88 | Chi square/ t/ Wilcoxon value | p-value | |
|---|---|---|---|---|---|
| age | 71.5(SD 8.0) | 70.4 (SD 7.1) | 72.0 (SD 8.3) | 1.06 | 0.29 |
| gender | |||||
| male | n=47(38%) | n=19 (51%) | n=28 (32%) | 4.24 | 0.04 |
| female | n=78(62%) | n=18 (49%) | n=60 (68%) | ||
| Ham-A | 22.9(SD 4.7) | 23.0 (SD 5.6) | 22.9 (SD 4.2) | 2233 | 0.60 |
| MMSE | 28.3(SD 1.7) | 28.4 (SD 1.6) | 28.3 (SD 1.8) | 2385 | 0.77 |
| PSWQ | 57.2(SD 12.7) | 55.4 (SD 13.7) | 57.9 (SD 12.3) | 1.00 | 0.32 |
| CIRS score | 9.0(SD 4.1) | 8.5 (SD 4.6) | 9.2 (SD 3.9) | 0.81 | 0.42 |
| Age onset | 38.9(SD 27.1) | 39.1(SD 25.5) | 38.8 (SD 27.9) | 2071 | 0.95 |
| Duration, mo | 383 (SD 331) | 367 (SD 316) | 390 (SD 340) | 2078 | 0.98 |
Disclosures and acknowledgements
Results were presented in part at the American Association for Geriatric Psychiatry annual meeting, Honolulu, HI, March 2009.
Dr. Lenze is a consultant for Fox Learning Systems, was a consultant for the Veteran's Medical Research Foundation from 2007-2008, and receives research support from Forest Laboratories, Bristol Myer Squibb, and Wyeth. Dr. Pollock has served on the advisory board of Forest Laboratories and is a faculty member of the Lundbeck International Institute. He is currently a consultant for Lundbeck and Wyeth.
This research was supported by National Institutes of Health grant numbers R01 MH070547, the Center for Mental Health Services Research (P30 MH068579; principal investigator: Enola Proctor, Ph.D.), the Advanced Center for Interventions and Services Research in Late-life Mood Disorders (P30 MH71944; principal investigator: Charles F. Reynolds III, M.D.), the John A. Hartford Center of Excellence in Geriatric Psychiatry (principal investigator: Reynolds), and the UPMC endowment in geriatric psychiatry (Reynolds). Forest Laboratories Inc., which holds the U.S. patent for escitalopram, provided escitalopram and matching placebo for the study. Neither NIH nor Forest had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Funding sources: NIH, Forest Laboratories, Hartford Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bryant C, Jackson H, Ames D. The prevalence of anxiety in older adults: methodological issues and a review of the literature. J Affect Disord. 2008;109(3):233–250. doi: 10.1016/j.jad.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Lenze EJ, Mulsant BH, Shear MK, et al. Efficacy and tolerability of citalopram in the treatment of late-life anxiety disorders: results from an 8-week randomized, placebo-controlled trial. Am J Psychiatry. 2005;162(1):146–150. doi: 10.1176/appi.ajp.162.1.146. [DOI] [PubMed] [Google Scholar]
- 3.Schuurmans J, Comijs H, Emmelkamp PM, et al. A randomized, controlled trial of the effectiveness of cognitive-behavioral therapy and sertraline versus a waitlist control group for anxiety disorders in older adults. Am J Geriatr Psychiatry. 2006;14(3):255–263. doi: 10.1097/01.JGP.0000196629.19634.00. [DOI] [PubMed] [Google Scholar]
- 4.Lenze EJ, Rollman BL, Shear MK, et al. Escitalopram for older adults with Generalized Anxiety Disorder: a placebo-controlled trial. JAMA. 2009;301(3):296–303. doi: 10.1001/jama.2008.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5–13. doi: 10.1097/JGP.0b013e31815aff5c. [DOI] [PubMed] [Google Scholar]
- 6.Serretti A, Kato M, De Ronchi D, et al. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12(3):247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- 7.Hu X- Z, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotrich FE, Pollock BG, Ferrell RE. Polymorphism of the serotonin transporter: implications for the use of selective serotonin reuptake inhibitors. Am J Pharmacogenomics. 2001;1(3):153–164. doi: 10.2165/00129785-200101030-00001. [DOI] [PubMed] [Google Scholar]
- 9.Mrazek DA, Rush AJ, Biernacka JM, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):341–51. doi: 10.1002/ajmg.b.30816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotrich FE, Pollock BG, Kirshner M, et al. Serotonin transporter genotype interacts with paroxetine plasma levels to influence depression treatment response in geriatric patients. J Psychiatry Neurosci. 2008;33(2):123–130. [PMC free article] [PubMed] [Google Scholar]
- 11.Pollock BG. The pharmacokinetic imperative in late-life depression. J Clin Psychopharmacol. 2005;25(4 Suppl 1):S19–23. doi: 10.1097/01.jcp.0000162809.69323.66. [DOI] [PubMed] [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version. Administration Booklet; Washington, DC: 1996. [Google Scholar]
- 13.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 14.Guy W, US Dept. of Health EaWpA-, Rev. Ed. NIMH; Rockville, MD: 1976. ECDEU Assessment Manual for Psychopharmacology. [Google Scholar]
- 15.Meyer TJ, Miller ML, Metzger RL, et al. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 16.Wendland JR, Martin BJ, Kruse MR, et al. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11(3):224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 17.Foglia JP, Sorisio D, Kirshner M, et al. Quantitative determination of paroxetine in plasma by high-performance liquid chromatography and ultraviolet detection. J Chromatogr B Biomed Sci Appl. 1997;693(1):147–151. doi: 10.1016/s0378-4347(97)00010-8. [DOI] [PubMed] [Google Scholar]
- 18.Huezo-Diaz P, Uher R, Smith R, et al. Moderation of antidepressant response by the serotonin transporter gene. Br J Psychiatry. 2009;195(1):30–38. doi: 10.1192/bjp.bp.108.062521. [DOI] [PubMed] [Google Scholar]
- 19.Arias B, Catalan R, Gasto C, et al. 5-HTTLPR polymorphism of the serotonin transporter gene predicts non-remission in major depression patients treated with citalopram in a 12-weeks follow up study. J Clin Psychopharmacol. 2003;23(6):563–567. doi: 10.1097/01.jcp.0000095350.32154.73. [DOI] [PubMed] [Google Scholar]
- 20.Zanardi R, Benedetti F, Di Bella D, et al. Efficacy of paroxetine in depression is influenced by a functional polymorphism within the promoter of the serotonin transporter gene. J Clin Psychopharmacol. 2000;20(1):105–107. doi: 10.1097/00004714-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Beal SL, Sheiner LB, Group SNP . University of California; San Francisco, CA: 1992. NONMEM Users Guides. [Google Scholar]
- 22.Jin Y, Pollock BG, Frank E, et al. The effect of reporting methods for dosing times on the estimation of pharmacokinetic parameters of escitalopram. J Clin Pharmacol. 2009;49(2):176–184. doi: 10.1177/0091270008327538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sogaard B, Mengel H, Roa N, et al. The pharmacokinetics of escitalopram after oral and intravenous administration of single and multiple doses to healthy subjects. J Clin Pharmacol. 2005;45(12):1400–1406. doi: 10.1177/0091270005280860. [DOI] [PubMed] [Google Scholar]
- 24.Gunthert KC, Conner TS, Armeli S, et al. Serotonin transporter gene polymorphism (5-HTTLPR) and anxiety reactivity in daily life: a daily process approach to gene-environment interaction. Psychosom Med. 2007;69(8):762–768. doi: 10.1097/PSY.0b013e318157ad42. [DOI] [PubMed] [Google Scholar]
- 25.Durham LK, Webb SM, Milos PM, et al. The serotonin transporter polymorphism, 5HTTLPR, is associated with a faster response time to sertraline in an elderly population with major depressive disorder. Psychopharmacology (Berl) 2004;174(4):525–529. doi: 10.1007/s00213-003-1562-3. [DOI] [PubMed] [Google Scholar]
- 26.Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59(10):888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 28.Gotlib IH, Joormann J, Minor KL, et al. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Hara R, Schroder CM, Mahadevan R, et al. Serotonin transporter polymorphism, memory and hippocampal volume in the elderly: association and interaction with cortisol. Mol Psychiatry. 2007;12(6):544–555. doi: 10.1038/sj.mp.4001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandelli L, Marino E, Pirovano A, et al. Interaction between SERTPR and stressful life events on response to antidepressant treatment. Eur Neuropsychopharmacol. 2009;19(1):64–67. doi: 10.1016/j.euroneuro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Hawley LL, Ringo Ho MH, Zuroff DC, et al. Stress reactivity following brief treatment for depression: differential effects of psychotherapy and medication. J Consult Clin Psychol. 2007;75(2):244–256. doi: 10.1037/0022-006X.75.2.244. [DOI] [PubMed] [Google Scholar]
- 32.Lotrich FE, Bies RR, Smith GS, et al. Relevance of assessing drug concentration exposure in pharmacogenetic and imaging studies. J Psychopharmacol. 2006;20(4 Suppl):33–40. doi: 10.1177/1359786806066044. [DOI] [PubMed] [Google Scholar]
- 33.Serretti A, Kato M, Kennedy JL. Pharmacogenetic studies in depression: a proposal for methodologic guidelines. Pharmacogenomics J. 2008;8(2):90–100. doi: 10.1038/sj.tpj.6500477. [DOI] [PubMed] [Google Scholar]