Abstract
Traumatic brain injury (TBI) is a major risk factor for the subsequent development of epilepsy. Currently, chronic seizures after brain injury are often poorly controlled by available anti-epileptic drugs. Hypothermia treatment, a modest reduction in brain temperature, reduces inflammation, activates pro-survival signaling pathways, and improves cognitive outcome after TBI. Given the well-known effect of therapeutic hypothermia to ameliorate pathological changes in the brain after TBI, we hypothesized that hypothermia therapy may attenuate the development of post-traumatic epilepsy and some of the pathomechanisms that underlie seizure formation. To test this hypothesis, adult male Sprague Dawley rats received moderate parasagittal fluid-percussion brain injury, and then were maintained at normothermic or moderate hypothermic temperatures for 4 hr. At 12 weeks after recovery, seizure susceptibility was assessed by challenging the animals with pentylenetetrazole (PTZ), a GABAA receptor antagonist. PTZ elicited a significant increase in seizure frequency in TBI normothermic animals as compared to sham surgery animals and this was significantly reduced in TBI hypothermic animals. Early hypothermia treatment did not rescue chronic dentate hilar neuronal loss, nor did it improve loss of doublecortin-labeled cells in the dentate gyrus post-seizure. However, mossy fiber sprouting was significantly attenuated by hypothermia therapy. These findings demonstrate that reductions in seizure susceptibility after TBI are improved with post-traumatic hypothermia and provide a new therapeutic avenue for the treatment of post-traumatic epilepsy.
Keywords: epilepsy, mossy fiber, rat, traumatic brain injury
Introduction
A significant, debilitating consequence of traumatic brain injury (TBI) is the development of seizures (Annegers et al., 1998; Vespa et al., 1999). Nearly 40-50% of severe TBI patients develop epilepsy and brain injuries account for 20% of epilepsy (Herman, 2002; Garga & Lowenstein, 2006). Unfortunately, post-traumatic epilepsy is frequently intractable to standard anti-epileptic medications (Temkin et al., 2001; Loscher & Schmidt, 2002). Thus, it is important to develop therapeutic interventions targeting the pathological mechanisms that underlie post-traumatic epilepsy.
There are several parallel pathomechanisms of some forms of epilepsy and TBI. Hippocampal epilepsy with mesial temporal sclerosis and TBI both result in a stereotypical neurodegeneration pattern in the hippocampus that includes dentate hilus neuronal loss (Babb et al., 1991; Lowenstein et al., 1992; Bramlett et al., 1997; Golarai et al., 2001; Santhakumar et al., 2001; Grady et al., 2003; D’Ambrosio et al., 2004; Swartz et al., 2006). Hilar interneurons exert control on excitation levels in the dentate gyrus and loss of hilar neurons results in hyperexcitability changes contributing to future seizures (Sutula et al., 1989; Lukoyanov et al., 2004).
After both TBI and status epilepticus, cell division in the dentate gyrus is markedly affected. Double-labeling immunocytochemical studies using 5-bromo-deoxyuridine (BrdU) and neuronal nuclear protein (NeuN) has revealed that cell proliferation increases in the dentate gyrus and peaks within 1-2 weeks after TBI (Dash et al., 2001; Braun et al., 2002; Sun et al., 2005; Urrea et al., 2007). Similarly, after status epilepticus, neural progenitor cells proliferate in the dentate gyrus (Parent et al., 1997; Huttmann et al., 2003; Jessberger et al., 2005; Indulekha et al.). Although a subject of debate, some of these cells could develop neuronal phenotypes, and potentially project aberrant axons to the CA3 pyramidal cell region as well as into the dentate hilus (Parent et al., 1999; Jessberger et al., 2007; Shapiro et al., 2007; Nitta et al., 2008).
Another shared feature of hippocampal epilepsy and TBI is abnormal sprouting of the mossy fiber pathway in the dentate gyrus. In both human hippocampal epilepsy and TBI, mossy fiber sprouting has been observed at chronic time points (Sutula et al., 1988; Houser et al., 1990; Babb et al., 1991; Santhakumar et al., 2000; Golarai et al., 2001; Kharatishvili et al., 2006). Although not a cause of epilepsy, sprouting of the mossy fiber pathway increases the number of recurrent connections on dentate granule cells, further increasing hippocampal excitability (Lowenstein et al., 1992; Dudek et al., 1994; Represa et al., 1994; Coulter et al., 1996; Nadler, 2003; Morimoto et al., 2004).
Hypothermia treatment is a highly promising therapy that improves structural and functional outcome measures after experimental and clinical TBI (Polderman, 2008; Dietrich et al., 2009). Lowering brain temperature after a traumatic brain insult dramatically reduces histopathology, and also improves behavioral recovery (Clifton et al., 1991; Lyeth et al., 1993; Dietrich et al., 1994; Bramlett et al., 1995; Suzuki et al., 2003; Gao et al., 2010). In this study, we tested the hypothesis that hypothermia attenuates seizure susceptibility changes after TBI.
Material and methods
Traumatic brain injury model
Three experimental groups (n=49) were used for seizure assessment and histopathology analysis: normothermic sham surgery animals (n=17), normothermic TBI animals (n=16), and hypothermic TBI animals (n=16). Male Sprague Dawley rats (270-320 gm) were anesthetized with 3% halothane, 70% N2O, and 30% O2 and received a 4.8 mm craniotomy (3.8 mm posterior to bregma, 2.5 mm lateral to the midline) to anchor a plastic injury hub (3.5 mm inside diameter) over the right parietal cortex. Twenty-four hr after the craniotomy, the animals were re-anesthetized with 1.5% halothane, 70% N2O, and 30% O2 and intubated. Pancuronium bromide (0.5 mg/kg, intravenously) was administered to facilitate mechanical ventilation. Arterial blood pressure, blood gases, and blood pH were monitored for 30 min prior to and up to 4 hr after TBI to maintain physiological ranges of blood pH between 7.35-7.45, pCO2 between 35-40 mm Hg and pO2 between 105-140 mm Hg. After stabilization, the animals received a moderate (1.8-2.2 atmospheres) fluid-percussion pulse (22 msec pulse duration) or sham injury. Brain temperature was indirectly monitored with a probe placed in the left temporalis muscle and core temperature was monitored with a rectally placed thermistor. The temporalis muscle temperature has been shown in a previous study to be an accurate reflection of brain temperature in the range of 30-40°C (Jiang et al., 1991). Self-adjusting feedback warming lamps were used to control brain temperature. The brains of normothermic animals were maintained at 36.5-37.0°C and hypothermic animals were maintained at 33.0-33.6°C by gently blowing cooled air over the head. Hypothermia was initiated 30 min post-injury and maintained for 4 hr. The animals were allowed to slowly re-warm to normothermia in ambient temperature over 2 hr. All experiments were conducted according to protocols approved by the University of Miami Animal Care and Use Committee and carried out according to the NIH Guide for the Care and Use of Laboratory Animals.
Seizure susceptibility determination
At 12 weeks post-surgery, animals were allowed to habituate to the animal testing room in a clear plastic cage for 10 min prior to behavioral testing. Animals received pentylenetetrazole (PTZ, 30 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) intraperitoneally. This dose is based on previous reports and our preliminary results (not shown) evaluating a dose response curve of PTZ in naive 3 month old Sprague Dawley rats to determine a dose that is at the threshold of eliciting seizures in sham animals (Andre et al., 1998; Erakovic et al., 2001). After receiving PTZ, each animal was observed for 1 hr by an investigator who was blinded to the treatment groups to measure seizure frequency and seizure class. Seizure class was scored using a modified scale as follows (Racine, 1972): Class 1, myoclonic (brief shock-like jerks of a muscle or a group of muscles); Class 2, unilateral clonic (rhythmic, rapidly alternating contraction and relaxation of a muscle or muscle group) lasting less than 1 min; Class 3, bilateral clonic lasting less than 1 min; Class 4, bilateral clonic sustained, lasting more than 1 min; Class 5, tonic-clonic (all muscles stiffen with loss of postural control alternating with sustained clonic); Class 6, terminal tonic-clonic (class 5 that results in death).
Electrocorticography recordings
At 12 weeks after sham or TBI surgery, a separate, additional group of animals (sham n=5, TBI-normothermia n=5, TBI-hypothermia n=5) were analyzed only for seizure susceptibility after PTZ using both behavioral and electrocorticography (ECoG) recordings. Animals were anesthetized (3% isoflurane, 70% N2O, and 30% O2, 5 min) and three cortical screw electrodes (Plastics One Inc., Roanoke, VA, USA) were placed into the skull over the parietal cortex, two caudal to the craniotomy made for the TBI surgery (-3.8 mm bregma, 2.5 mm lateral of the midline), and one indifferent electrode over the contralateral hemisphere caudal to the center of the craniotomy. ECoG activity was recorded differentially between electrodes (E1 and E2) implanted ipsilateral to the injury site. The contralateral indifferent electrode (Ei) was connected to the cable shield and the amplifier ground The screw electrodes and their lead wires were cemented to the skull with dental acrylic. Upon recovery from anesthesia, the animals were placed in a clear plastic cage and the wires from the electrodes were attached to a flexible swivel and a differential preamplifier (CWE Inc., Ardmore, PA, USA). The electrical signals were amplified, filtered (1-30 Hz) and stored in digital form using a DATAQ DI-720 digitizer (DATAQ Instruments, Akron, OH, USA). After 5 min of baseline ECoG recordings, the animals received PTZ (30 mg/kg, intraperitoneally), and were simultaneously recorded for 60 min and scored for seizure number and class. One day after ECoG recordings and behavioral analysis, animals were perfused. During the perfusion and brain removal, all skulls and brains were inspected to ensure that penetration of the dura mater did not occur. One animal was discarded in the analysis due to an injury to the dura.
Stereology
At 24 hr after seizure determination, animals were anesthetized (3% halothane, 70% N2O, and 30% O2) and perfused with 0.2% sodium sulphide (80 mL), and then with 4% paraformaldehyde in phosphate-buffered saline (PBS, 350 mL). The brains were cryoprotected (30% sucrose in PBS) and sectioned on a freezing microtome (50 μm thick). Serial sections spaced 300 μm apart were immunostained with mouse anti-NeuN (1:500, Millipore, Temecula, CA, USA) or goat anti-doublecortin (1:500, C-18 and N-19, Santa Cruz Biotechnology, Santa Cruz, CA, USA) (Atkins et al., 2007b; Shapiro et al., 2007). Immunostaining was developed with anti-mouse or anti-goat IgG (1:200), ABC Elite (Vector Laboratories, Burlingame, CA, USA), and NiDAB (2.5% Nickle Ammonium Sulfate Acetate-Imidasole Buffer, 0.05% DAB, 0.001% H2O2, Vector Laboratories). For both antibodies, anti-NeuN and anti-doublecortin, antibody penetration through the entire section for all animals was verified prior to analysis. The dentate hilus and dentate granule cell layers were contoured at 5x using StereoInvestigator software 7.50.1 (MicroBrightField, Williston, VT, USA) with an Olympus BX51TRF microscope (Olympus America, Center Valley, PA, USA) by a blind observer. Sections between bregma levels -3.6 to -4.8 mm were chosen for analysis; this focused the cell counting analysis near the epicenter of the injury (bregma level -3.8 mm), and also these bregma levels were unequivocally identifiable in all animals. A counting grid of 50×50 μm was placed over the dentate hilus region, and a counting grid of 75×75 μm was used for the dentate granule cell layer. For sections immunostained with anti-NeuN, section thickness was 35 μm and the optical disector height was 25 μm with 5 μm guard zones. For sections immunostained with anti-doublecortin, section thickness was 30 μm, the optical disector height was 22 μm and the guard zones were 4 μm. Using a 50×50 μm counting frame for the dentate hilus and a 60×60 μm counting frame for the dentate granule cell layer, NeuN- or doublecortin-positive cells were counted in 25-90 randomly-placed sampling sites with a 63x, 1.42 NA objective. For dentate hilus cell counts, Q values ranged from 92-477, and CE2/CV2 values were 0.11, 0.57, and 0.18 for the sham, TBI-normothermia and TBI-hypothermia groups, respectively. For the doublecortin-positive cell counts, the Q range was 47-314, and CE2/CV2 values were 0.12 for the sham group, 0.15 for the TBI-normothermic group and 0.12 for the TBI-hypothermia group.
Images were taken with 20x and 60x objectives on an Olympus BX51TRF microscope (Olympus America) and montaged using the virtual slice module in the Neurolucida 7.50.1 software program (MicroBrightField).
TIMM staining
Sections were developed with 14% gum arabic, 2.5% citric acid, 2.3% trisodium citrate, 1.7% hydroquinone, and 0.08% silver nitrate (Seress & Gallyas, 2000). Sections were developed in parallel for each animal treatment, and then stopped simultaneously with 5% sodium thiosulfate. TIMM staining was scored by 4 investigators blinded to the treatment groups at bregma levels -3.3, -4.3 and -5.8 mm: 0, no TIMM granules; 1, sparse TIMM granules in the supragranular cell layer; 2, continuously distributed TIMM granules in the supragranular cell layer; 3, continuously distributed TIMM granules in the supragranular cell layer with patches of confluency; 4, a confluent dense band of TIMM granules in the supragranular cell layer; 5, a band as in 4 that extended into the inner molecular layer (Golarai et al., 2001). Images of the dentate gyrus were taken at 20x and 60x magnification at bregma level -4.3 mm.
Statistical analysis
Data presented are mean ± SEM. Results from the seizure number, seizure class, and TIMM scoring were analyzed using the Kruskal-Wallis ANOVA on Ranks test with post hoc Mann Whitney U t-test. Dentate hilar neuronal counts and doublecortin cell counts were analyzed using a one-way ANOVA with post hoc Tukey HSD t-test. Significance was set at P<0.05.
Results
Hypothermia reduces seizures after TBI
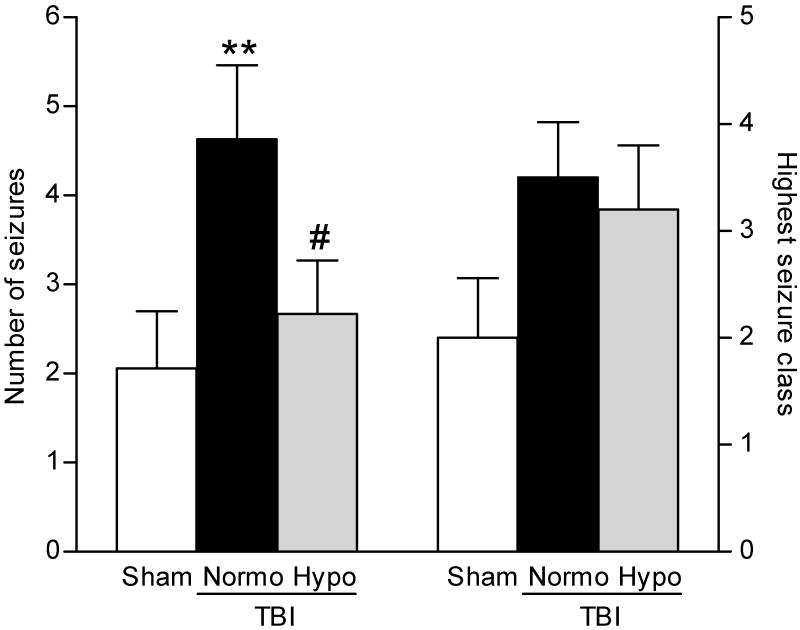
To determine if post-traumatic seizures are improved with hypothermia treatment, we assessed seizure susceptibility in rats after moderate parasagittal fluid-percussion brain injury (FPI) and normothermia or hypothermia treatment. Since moderate FPI does not typically elicit behaviorally visible, spontaneous seizures (Golarai et al., 2001; Santhakumar et al., 2001; Kharatishvili et al., 2006), we utilized the GABAA receptor antagonist PTZ to study seizure threshold by challenging the injured brain with a decrease in inhibition. Normothermic TBI animals exhibited a significant increase (H2 = 6.904, P<0.05) in total number of seizures as compared to sham surgery animals. There was a significant decrease (P<0.05) in the numbers of seizures observed in animals treated with post-traumatic hypothermia as compared to TBI normothermic animals (Fig. 1). There was also an increase, although not significant, in the highest seizure class reached in TBI normothermic animals as compared to sham surgery animals and this was not reduced with hypothermia treatment. Time for seizure onset was not statistically different for any animal group (sham 2.45±0.19 min, TBI normothermia 4.21±1.88 min, TBI hypothermia 3.12±0.24 min).
Fig. 1.
Seizure susceptibility was determined by challenging animals with a decrease in inhibition using PTZ (30 mg/kg, intraperitoneally), a GABAA receptor antagonist, at 12 weeks after recovery from brain surgery. There was a significant increase in the number of seizures exhibited by each TBI animal (**P<0.01) at 12 weeks after TBI (n=16) as compared to sham surgery animals (n=17). The increase in seizure numbers after TBI was not observed in TBI-hypothermic animals (n=16, #P<0.05 for TBI-normothermic versus TBI-hypothermic animals’ seizure number). Highest seizure class reached by each animal was increased, although not significantly, in both TBI-normothermic (n=16) and TBI-hypothermic animals (n=16) as compared to sham surgery animals (n=17). Data represent mean ± SEM.
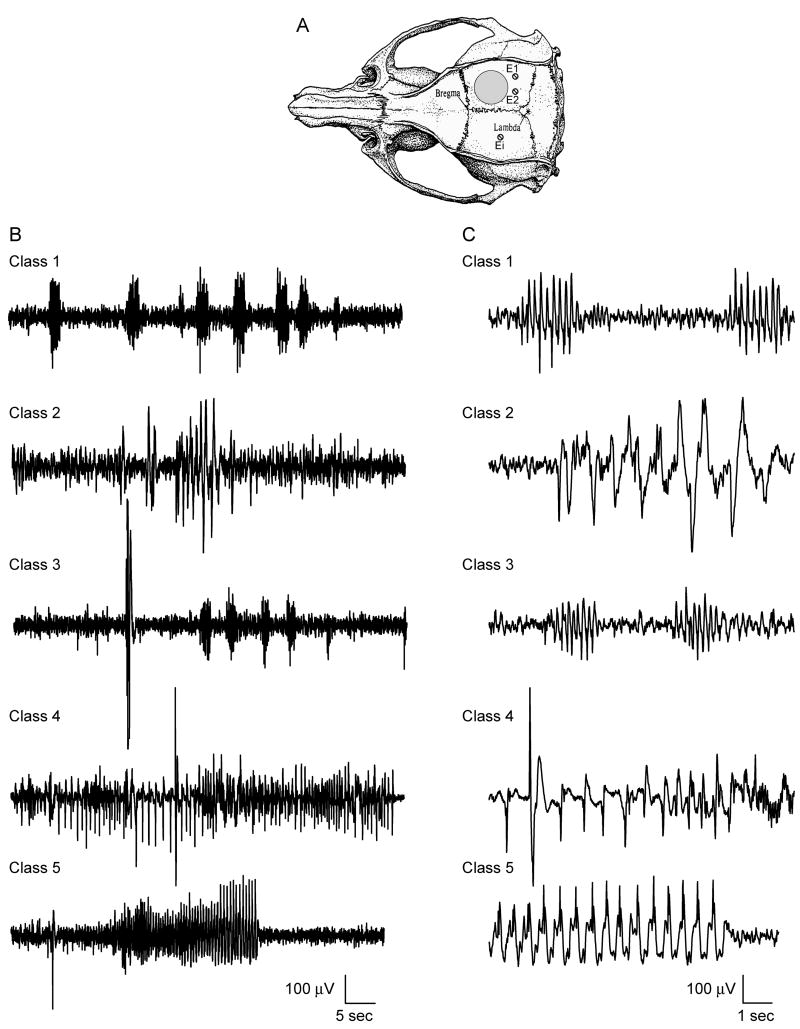
To ensure that the behavioral rating scale was accompanied by electrophysiological changes, ECoG recordings were performed in a separate, additional group of animals (Fig. 2). At 12 weeks after sham or TBI surgery, animals were implanted with electrodes in the skull over the parietal cortex. Baseline ECoG recordings were conducted for 5 min prior to PTZ administration (30 mg/kg, intraperitoneally), and for 60 min post-PTZ with simultaneous behavioral assessment. Seizure classes 1-5 identified by a behavioral scorer who was blinded to the ECoG recordings were associated with the ECoG recordings.
Fig. 2.
Representative ECoG recordings from animals at 12 weeks post-surgery. Electrodes were placed caudally from the craniotomy (E1 and E2) and the indifferent electrode (Ei) was placed in the contralateral hemisphere (A, adapted from Paxinos & Watson, 2005). A baseline recording was initiated 5 min prior to PTZ (30 mg/kg, intraperitoneally) and recordings were performed for 60 min (B). Simultaneous blinded behavioral scoring was conducted. For each seizure classification, periods of hyperexcitability consisting of high amplitude single spikes or combinations of single spikes and repetitive spike discharges, were observed on the ECoG records (C).
Dentate gyrus cell loss is not reduced with hypothermia therapy
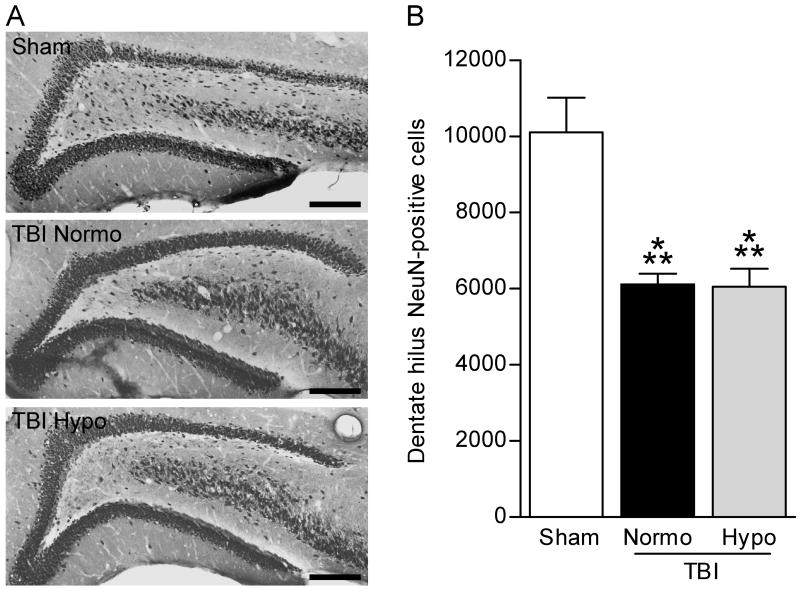
Because the FPI model results in stereotypical cell death in the dentate hilus, an area that exerts inhibitory control over the dentate gyrus, we determined if hypothermia reduced post-traumatic seizures by reducing dentate hilar neuronal death (Lowenstein et al., 1992; Bramlett et al., 1997; Golarai et al., 2001; Santhakumar et al., 2001; Grady et al., 2003; D’Ambrosio et al., 2004; Kharatishvili et al., 2006). After seizure assessment had been performed, the animals were perfused, and serial sections were analyzed by stereology to measure the numbers of NeuN-positive cells in the dentate hilus. As has been reported previously, we observed a significant (F2,40 = 13.39, P<0.001) decrease in surviving numbers of dentate hilar neurons in TBI normothermic animals as compared to sham surgery animals (Fig. 3) (Lowenstein et al., 1992; Golarai et al., 2001; Santhakumar et al., 2001; Grady et al., 2003; D’Ambrosio et al., 2004; Kharatishvili et al., 2006). However, in agreement with previous studies, we found that hypothermia treatment did not rescue the hilar cell loss observed after trauma (Bramlett et al., 1997).
Fig. 3.
Dentate hilus neuronal survival was not rescued with hypothermia treatment. The dentate gyrus was immunostained with NeuN to identify surviving neurons (A). Images were taken at 20X magnification and montaged using NeuroLucida. Both TBI-normothermic (TBI-N) and TBI-hypothermic (TBI-H) animals had fewer remaining NeuN-positive cells in the dentate hilus as compared to sham animals (Sham). Dentate hilar neurons were quantified by stereology (B). Both TBI-normothermic and TBI-hypothermic animals had fewer surviving dentate hilar neurons at 12 weeks post-injury as compared to sham surgery animals (Sham n=15, TBI-normo n=12, TBI-hypo n=16). ***P<0.001 for sham versus TBI-normothermic animals or TBI-hypothermic animals. Data represent mean ± SEM. Scale bars, 200 μm.
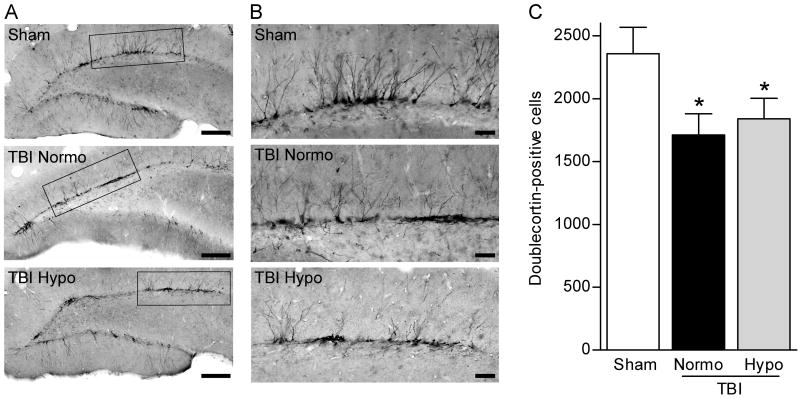
To determine if neurogenesis, another feature of epilepsy, is also affected by brain trauma at a chronic time point after injury, we examined the numbers of doublecortin-positive cells in the dentate gyrus at 12 weeks after FPI (Parent et al., 1997; Huttmann et al., 2003; Jessberger et al., 2005). There was a significant (F2,40 = 3.415, P<0.05) decrease in doublecortin-positive cells in both TBI normothermic and hypothermic animals post-seizure as compared to sham surgery animals (Fig. 4). A qualitative comparison suggests that the processes retained more lateral projections and this was observed in both normothermic and hypothermic TBI animals. Together these findings suggest that early hypothermia treatment does not prevent increases in seizure susceptibility by rescuing dentate gyrus cell loss after TBI.
Fig. 4.
Numbers of doublecortin-positive cells are decreased at chronic time points after brain injury and this decrease is not rescued by hypothermia therapy. Low magnification images (20X) of doublecortin immunostaining of the dentate gyrus at 12 weeks post-TBI (A). Higher magnification (60X) of doublecortin-positive cells revealed that the dendritic branches were more laterally oriented in the injured hippocampus as compared to the non-injured hippocampus (B). Quantification by stereology revealed that numbers of doublecortin-positive cells were significantly decreased in both TBI-normothermic (n=12) and TBI-hypothermic animals (n=16) as compared to sham animals (n=15) (C). *P<0.05 for sham versus TBI-normothermic animals or TBI-hypothermic animals. Data represent mean ± SEM. (A) Scale bars, 200 μm. (B) Scale bars, 50 μm.
Mossy fiber sprouting is attenuated by hypothermia treatment
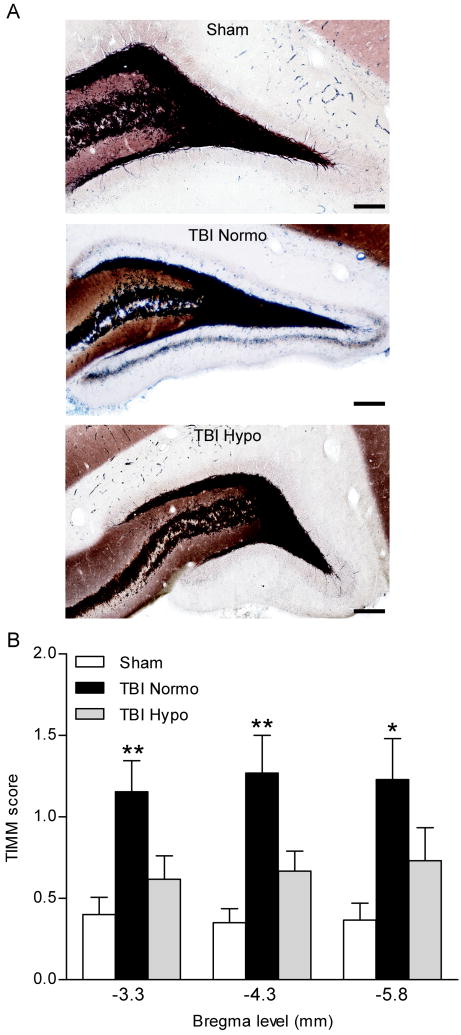
Mossy fiber sprouting occurs in patients with temporal lobe epilepsy, and is seen in experimental models of brain injury (Sutula et al., 1989; Houser et al., 1990; Babb et al., 1991; Santhakumar et al., 2000; Golarai et al., 2001; Kharatishvili et al., 2006). To determine if hypothermia therapy attenuates mossy fiber sprouting after FPI, we performed TIMM staining and scored for the amount of mossy fiber sprouting in TBI normothermic animals as compared to TBI hypothermic and sham surgery animals at 24 hr after seizure assessment with PTZ (Fig. 5). Previous work has shown that PTZ at 30 mg/kg does not induce mossy fiber sprouting within 24 hr of administration (Golarai et al., 2001). We found that mossy fiber sprouting was present at 12 weeks after brain injury, and this traumatic consequence was attenuated in TBI hypothermic animals (H8 = 29.93, P<0.001). These results indicate that hypothermia treatment may reduce the increases in seizure susceptibility by reducing aberrant axonal sprouting in the dentate gyrus.
Fig. 5.
TIMM staining of the dentate gyrus of the hippocampus. The mossy fiber pathway is clearly delineated in black. Mossy fiber sprouting onto the supragranular cell layer was observed in 12 week TBI-normothermic animals (TBI-N, arrows), and this was attenuated in 12 week TBI animals treated with hypothermia (TBI-H). Images (20 or 60X) of the dentate gyrus are shown at bregma level -3.8 mm (A). TIMM scores were significantly higher in TBI-normothermic animals (n=13) as compared to sham animals at all bregma levels assessed (C). No significant differences were found between sham animals (n=15) and TBI hypothermic animals (n=15), *P<0.05, **P<0.01 for TBI-normothermic animals versus sham animals. Data represent mean ± SEM. Scale bars, 200 μm.
Discussion
Developing a therapy to prevent seizure susceptibility after brain injury is of paramount importance given that current anti-epileptic medications are not completely sufficient to prevent post-traumatic epilepsy (Temkin, 2009). However, previous studies of experimental models of post-traumatic epilepsy have been hampered by the need to use a brain injury that is severe and even with severe brain injury, only a subset of animals eventually develop spontaneous seizures in the months to years after the TBI (Kharatishvili et al., 2006; Hunt et al., 2009; 2010; Kharatishvili & Pitkanen, 2010). These limitations have hindered the testing of therapeutic strategies to reduce the development of post-traumatic epilepsy. In this study, we report that seizure susceptibility can be reliably observed after moderate FPI by challenging the injured brain with a decrease in inhibitory control utilizing a GABAA receptor antagonist, PTZ.
Using this method to assess seizure susceptibility, we observed significant increases in seizure number in moderate TBI animals as compared to sham animals at 12 weeks post-injury. Furthermore, we were able to test the effectiveness of a highly promising therapy currently in TBI clinical trials to determine if post-traumatic seizure susceptibility can be prevented (Polderman, 2008). We found that hypothermia therapy, a modest reduction in brain temperature for only 4 hours after brain injury, significantly reduced the number of chronic seizures elicited by PTZ, as well as attenuated a pathological feature of epilepsy, mossy fiber sprouting. These results indicate that assessing seizure susceptibility may be an effective method to evaluate potential therapeutic strategies for post-traumatic epilepsy and thereby may be an important mechanism by which early cooling may improve outcome in TBI patients.
We found that the observed behavioral changes induced by PTZ were associated with abnormal electrical activity with ECoG recordings. However, since depth recordings within the hippocampus were not performed, we could not determine if isolated hippocampal seizures were affected by hypothermia therapy. In addition, frequencies above the β3F band were filtered, and gamma frequencies were not examined (Lehmkuhle et al., 2009).
Although hypothermia treatment reduced seizure frequency after TBI, seizure severity did not improve. Both seizure frequency and seizure severity correlate with poorer quality of life in epileptic patients, and reducing both aspects of epilepsy should be considered when developing a therapy for post-traumatic epilepsy (Bautista & Glen, 2009). The pathomechanisms of post-traumatic seizures are likely to be multifactorial, and given that hypothermia therapy reduced only one pathology feature, i.e. mossy fiber sprouting, our results suggest that a more prolonged duration of cooling and/or a combinatorial therapeutic strategy of hypothermia with a pharmacological agent may be required to target post-traumatic susceptibility to increases in both seizure frequency and severity (Margulies & Hicks, 2009).
As previously reported, hypothermia therapy did not prevent the loss of the vulnerable cell population of dentate hilar neurons that are rapidly and selectively lost after brain injury (Lowenstein et al., 1992; Bramlett et al., 1997; Golarai et al., 2001; Santhakumar et al., 2001; Grady et al., 2003; D’Ambrosio et al., 2004). One caveat of this interpretation is that we assessed dentate hilar neuronal loss 24 hr after seizure induction and assessment. Although none of our animals exhibited status epilepticus, it is possible that additional neuronal loss occurred as a result of the PTZ treatment, compounding the effects of the brain injury on hilar cell death (Ben-Ari, 1985; Buckmaster & Dudek, 1997; Borges et al., 2003). The loss of hilar cells could reflect both interneuronal cells as well as mossy cells since we assessed NeuN-positive cells (Amaral, 1978; Freund & Buzsaki, 1996). Both of these cell populations help the dentate gyrus act as a gatekeeper in preventing excessive excitatory stimulation (Cavazos et al., 1994; Sloviter, 1994; Buckmaster & Jongen-Relo, 1999). It is critical to develop a pharmacological therapy to prevent loss of both of these hilar cell populations, and possibly use in conjunction with hypothermia therapy.
Previous reports have demonstrated an increase in cell proliferation using BrdU-labeling, or other markers of immature neurons after brain injury (Dash et al., 2001; Braun et al., 2002; Sun et al., 2007; Urrea et al., 2007). However, in the hippocampus this increase does not last, as other studies have demonstrated that doublecortin-positive cells are decreased from 14 days to 6 weeks post-injury (Rola et al., 2006; Gao et al., 2008; Potts et al., 2009). To our knowledge, this is the first report of a loss of doublecortin-positive cells at 3 months after brain injury and induction of seizures. Although there was no difference in the loss of doublecortin-positive cell numbers between TBI-normothermic or TBI-hypothermic animals, given that we assessed doublecortin-positive cells 24 hr after a period of seizure induction, we cannot rule out that this loss is due, in part, to the induced period of seizures elicited by PTZ. Qualitatively, we observed that the remaining doublecortin-positive cells exhibited an immature morphology in TBI animals as compared to sham animals (Walter et al., 2007). After status epilepticus, one hypothesis is that newly generated granule cells could potentially project ectopically to the hilus, creating recurrent excitatory circuitry (Ribak et al., 2000; Austin & Buckmaster, 2004; Shapiro et al., 2007; Walter et al., 2007). Whether the surviving doublecortin-positive cells in the injured hippocampus develop hilar basal dendrites remains to be determined.
A common feature of hippocampal epileptogenesis is aberrant mossy fiber sprouting of dentate granule cells to the supragranular cell layer of the dentate gyrus (Sutula et al., 1989; Houser et al., 1990; Babb et al., 1991). We found that of all the pathomechanisms analyzed, only mossy fiber sprouting was suppressed by 4 hr of early post-traumatic hypothermia therapy. This result suggests that hypothermia selectively attenuated the molecular mechanisms that stimulate mossy fiber sprouting after brain trauma. Although the density of sprouting is modest, previous reports have found that TIMM scores ranging from 1-2 can correlate with significant hippocampal-dependent cognitive dysfunction (Cilio et al., 2003; Lukoyanov et al., 2004). The molecular determinants of mossy fiber sprouting are still unknown. One potential mechanism that hypothermia may have affected is semaphorin expression. Semaphorins are secreted proteins that are critically involved in neural development by sculpting axon growth by repulsive or attractive effects (Zhou et al., 2008). The mRNA levels of sema3A, F and C are decreased after status epilepticus and temporal lobe epilepsy and knockout mice of Sema3F are prone to seizure activity (Barnes et al., 2003; Holtmaat et al., 2003; Sahay et al., 2005). Hypothermia therapy has been reported to regulate transcription factors within the hippocampus and may have regulated gene transcription of semaphorins (Atkins et al., 2007a). Another possibility is that hypothermia altered brain-derived neurotrophic factor and trkB receptor signaling and we have previously observed significant effects of hypothermia on downstream targets of the trkB receptor (Dinocourt et al., 2006; Atkins et al., 2007a). However, mossy fiber sprouting is thought to be a consequence, not a causal factor, in the development of post-traumatic epilepsy (Cronin & Dudek, 1988; Sloviter, 1992; Zhang et al., 2002; Morimoto et al., 2004). Thus, hypothermia treatment likely had effects on other temperature-sensitive injury mechanisms that have been suggested to underlie seizure susceptibility (Dietrich et al., 2009).
Another pathological aspect of seizure susceptibility that hypothermia could have affected was electrophysiological alterations in the injured hippocampus. After brain injury, the dentate gyrus exhibits hyperexcitability, resulting from changes in voltage-gated ion channels and GABA receptors as well as impaired potassium buffering by astrocytes (Lowenstein et al., 1992; D’Ambrosio et al., 1999; Ross & Soltesz, 2000; Santhakumar et al., 2001; Griesemer & Mautes, 2007; Hunt et al., 2009). Current studies are assessing other electrophysiological alterations that may have also contributed to the reduction in seizure susceptibility by hypothermia therapy.
Given the low seizure threshold of the hippocampus and the many pathological changes that occur in the hippocampus after brain injury, it is likely that the PTZ-induced seizures involved the hippocampus. In human post-traumatic epileptic patients, between 35-62% have epilepsy originating in the temporal lobe (Diaz-Arrastia et al., 2000; Hudak et al., 2004). However, the overlying parietal cortex also exhibits neuronal loss, inflammation, astrogliosis, and circuit reorganization, suggesting that this damaged region may also be involved (D’Ambrosio et al., 2005; Kharatishvili et al., 2006; Kharatishvili & Pitkanen, 2010). In both injured regions, hypothermia rescues neuronal death as well as potentiates cell survival pathways, and it is unclear if hypothermia reduced seizure frequency by effects on the hippocampus, parietal cortex or other areas (Lotocki et al., 2006; Atkins et al., 2007a). The multiplicity of hypothermia’s effects to reduce pathology is perhaps its strongest therapeutic feature (Dietrich et al., 2009).
Therapeutic hypothermia is one of the few treatments that have been successfully translated to select patient populations (Marion & Bullock, 2009). For example, therapeutic hypothermia has benefited patients following cardiac arrest, postnatal infants with hypoxic insults, and in severe TBI patients in many single-institution clinical TBI studies (Marion et al., 1997; Hachimi-Idrissi et al., 2001; Bernard et al., 2002; Mayer, 2002; Gunn et al., 2005; Shankaran et al., 2005; Jiang et al., 2006; Polderman, 2008). However, hypothermia has not passed Phase III clinical trials for the treatment of TBI, and secondary complications arising from the use of systemic hypothermia require management (Hayashi, 2009; Polderman, 2009; Polderman & Herold, 2009). Translation of hypothermia animal studies to clinical trials remain challenging and further understanding of the therapeutic time window, optimal duration, and degree of cooling would greatly facilitate the development of hypothermia as a potential therapy for post-traumatic epilepsy.
The latency period for developing seizures is a time period of variable duration on the scale of months to years; however, the critical time window for therapeutics to attenuate the development of post-traumatic epilepsy has been proposed to be within 3 days of injury (Salazar et al., 1985; Graber & Prince, 2004). Although anti-epileptic drugs are often administered prophylactically in the hours to days after brain injury, they do not always suppress the development of chronic seizures (Temkin, 2009). We demonstrated that early post-traumatic hypothermia treatment for only 4 hr had a pronounced, long lasting effect on seizure susceptibility even when tested 12 weeks after brain injury. Our results demonstrate that hypothermia may be an efficacious and unique anti-seizure medication, perhaps both in the acute setting and in chronic stages after injury.
Acknowledgments
This work was supported by the National Institutes of Health (NS030291 and NS042133). We thank Stephanie DaSilva for technical assistance and Dr. Edward Green for helpful discussions.
Abbreviations
- BrdU
5-bromo-deoxyuridine
- ECoG
electrocorticography
- FPI
fluid-percussion brain injury
- NeuN
neuronal nuclear protein
- PBS
phosphate-buffered saline
- PTZ
pentylenetetrazole
- TBI
traumatic brain injury
References
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Andre V, Pineau N, Motte JE, Marescaux C, Nehlig A. Mapping of neuronal networks underlying generalized seizures induced by increasing doses of pentylenetetrazol in the immature and adult rat: a c-Fos immunohistochemical study. Eur J Neurosci. 1998;10:2094–2106. doi: 10.1046/j.1460-9568.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Oliva AA, Jr, Alonso OF, Chen S, Bramlett HM, Hu BR, Dietrich WD. Hypothermia treatment potentiates ERK1/2 activation after traumatic brain injury. Eur J Neurosci. 2007a;26:810–819. doi: 10.1111/j.1460-9568.2007.05720.x. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Oliva AA, Jr, Alonso OF, Pearse DD, Bramlett HM, Dietrich WD. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol. 2007b;208:145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin JE, Buckmaster PS. Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys. J Comp Neurol. 2004;476:205–218. doi: 10.1002/cne.20182. [DOI] [PubMed] [Google Scholar]
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- Barnes G, Puranam RS, Luo Y, McNamara JO. Temporal specific patterns of semaphorin gene expression in rat brain after kainic acid-induced status epilepticus. Hippocampus. 2003;13:1–20. doi: 10.1002/hipo.10041. [DOI] [PubMed] [Google Scholar]
- Bautista RE, Glen ET. Seizure severity is associated with quality of life independent of seizure frequency. Epilepsy Behav. 2009;16:325–329. doi: 10.1016/j.yebeh.2009.07.037. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD, Green EJ, Busto R. Chronic histopathological consequences of fluid-percussion brain injury in rats: Effects of post-traumatic hypothermia. Acta Neuropathol (Berl) 1997;93:190–199. doi: 10.1007/s004010050602. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Green EJ, Dietrich WD, Busto R, Globus MY, Ginsberg MD. Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. J Neurotrauma. 1995;12:289–298. doi: 10.1089/neu.1995.12.289. [DOI] [PubMed] [Google Scholar]
- Braun H, Schafer K, Hollt V. βIII tubulin-expressing neurons reveal enhanced neurogenesis in hippocampal and cortical structures after a contusion trauma in rats. J Neurotrauma. 2002;19:975–983. doi: 10.1089/089771502320317122. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Buckmaster PS, Jongen-Relo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci. 1999;19:9519–9529. doi: 10.1523/JNEUROSCI.19-21-09519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci. 1994;14:3106–3121. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilio MR, Sogawa Y, Cha BH, Liu X, Huang LT, Holmes GL. Long-term effects of status epilepticus in the immature brain are specific for age and model. Epilepsia. 2003;44:518–528. doi: 10.1046/j.1528-1157.2003.48802.x. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Jiang JY, Lyeth BG, Jenkins LW, Hamm RJ, Hayes RL. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cereb Blood Flow Metab. 1991;11:114–121. doi: 10.1038/jcbfm.1991.13. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Rafiq A, Shumate M, Gong QZ, DeLorenzo RJ, Lyeth BG. Brain injury-induced enhanced limbic epileptogenesis: Anatomical and physiological parallels to an animal model of temporal lobe epilepsy. Epilepsy Res. 1996;26:81–91. doi: 10.1016/s0920-1211(96)00044-7. [DOI] [PubMed] [Google Scholar]
- Cronin J, Dudek FE. Chronic seizures and collateral sprouting of dentate mossy fibers after kainic acid treatment in rats. Brain Res. 1988;474:181–184. doi: 10.1016/0006-8993(88)90681-6. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128:174–188. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci. 1999;19:8152–8162. doi: 10.1523/JNEUROSCI.19-18-08152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Diaz-Arrastia R, Agostini MA, Frol AB, Mickey B, Fleckenstein J, Bigio E, Van Ness PC. Neurophysiologic and neuroradiologic features of intractable epilepsy after traumatic brain injury in adults. Arch Neurol. 2000;57:1611–1616. doi: 10.1001/archneur.57.11.1611. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Globus MY, Ginsberg MD. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol (Berl) 1994;87:250–258. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Atkins CM, Bramlett HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma. 2009;26:301–312. doi: 10.1089/neu.2008.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinocourt C, Gallagher SE, Thompson SM. Injury-induced axonal sprouting in the hippocampus is initiated by activation of trkB receptors. Eur J Neurosci. 2006;24:1857–1866. doi: 10.1111/j.1460-9568.2006.05067.x. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Obenaus A, Schweitzer JS, Wuarin JP. Functional significance of hippocampal plasticity in epileptic brain: Electrophysiological changes of the dentate granule cells associated with mossy fiber sprouting. Hippocampus. 1994;4:259–265. doi: 10.1002/hipo.450040306. [DOI] [PubMed] [Google Scholar]
- Erakovic V, Zupan G, Varljen J, Laginja J, Simonic A. Altered activities of rat brain metabolic enzymes caused by pentylenetetrazol kindling and pentylenetetrazol--induced seizures. Epilepsy Res. 2001;43:165–173. doi: 10.1016/s0920-1211(00)00197-2. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gao G, Oda Y, Wei EP, Povlishock JT. The adverse pial arteriolar and axonal consequences of traumatic brain injury complicated by hypoxia and their therapeutic modulation with hypothermia in rat. J Cereb Blood Flow Metab. 2010;30:628–637. doi: 10.1038/jcbfm.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Deng-Bryant Y, Cho W, Carrico KM, Hall ED, Chen J. Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J Neurosci Res. 2008;86:2258–2270. doi: 10.1002/jnr.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garga N, Lowenstein DH. Posttraumatic epilepsy: A major problem in desperate need of major advances. Epilepsy Curr. 2006;6:1–5. doi: 10.1111/j.1535-7511.2005.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Greenwood AC, Feeney DM, Connor JA. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J Neurosci. 2001;21:8523–8537. doi: 10.1523/JNEUROSCI.21-21-08523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber KD, Prince DA. A critical period for prevention of posttraumatic neocortical hyperexcitability in rats. Ann Neurol. 2004;55:860–870. doi: 10.1002/ana.20124. [DOI] [PubMed] [Google Scholar]
- Grady MS, Charleston JS, Maris D, Witgen BM, Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: Analysis by stereological estimation. J Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Griesemer D, Mautes AM. Closed head injury causes hyperexcitability in rat hippocampal CA1 but not in CA3 pyramidal cells. J Neurotrauma. 2007;24:1823–1832. doi: 10.1089/neu.2006.0237. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Battin M, Gluckman PD, Gunn TR, Bennet L. Therapeutic hypothermia: From lab to NICU. J Perinat Med. 2005;33:340–346. doi: 10.1515/JPM.2005.061. [DOI] [PubMed] [Google Scholar]
- Hachimi-Idrissi S, Corne L, Huyghens L. The effect of mild hypothermia and induced hypertension on long term survival rate and neurological outcome after asphyxial cardiac arrest in rats. Resuscitation. 2001;49:73–82. doi: 10.1016/s0300-9572(00)00268-9. [DOI] [PubMed] [Google Scholar]
- Hayashi N. Management of pitfalls for the successful clinical use of hypothermia treatment. J Neurotrauma. 2009;26:445–453. doi: 10.1089/neu.2008.0648. [DOI] [PubMed] [Google Scholar]
- Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59:S21–26. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Gorter JA, De Wit J, Tolner EA, Spijker S, Giger RJ, Lopes da Silva FH, Verhaagen J. Transient downregulation of Sema3A mRNA in a rat model for temporal lobe epilepsy. A novel molecular event potentially contributing to mossy fiber sprouting. Exp Neurol. 2003;182:142–150. doi: 10.1016/s0014-4886(03)00035-9. [DOI] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak AM, Trivedi K, Harper CR, Booker K, Caesar RR, Agostini M, Van Ness PC, Diaz-Arrastia R. Evaluation of seizure-like episodes in survivors of moderate and severe traumatic brain injury. J Head Trauma Rehabil. 2004;19:290–295. doi: 10.1097/00001199-200407000-00003. [DOI] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN. Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp Neurol. 2009;215:243–252. doi: 10.1016/j.expneurol.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN. Regionally localized recurrent excitation in the dentate gyrus of a cortical contusion model of posttraumatic epilepsy. J Neurophysiol. 2010;103:1490–1500. doi: 10.1152/jn.00957.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, Steinhauser C, Gray WP. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci. 2003;18:2769–2778. doi: 10.1111/j.1460-9568.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- Indulekha CL, Sanalkumar R, Thekkuveettil A, James J. Seizure induces activation of multiple subtypes of neural progenitors and growth factors in hippocampus with neuronal maturation confined to dentate gyrus. Biochem Biophys Res Commun. 2010;393:864–871. doi: 10.1016/j.bbrc.2010.02.101. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Romer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol. 2005;196:342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD, Jr, Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27:9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JY, Lyeth BG, Clifton GL, Jenkins LW, Hamm RJ, Hayes RL. Relationship between body and brain temperature in traumatically brain-injured rodents. J Neurosurg. 1991;74:492–496. doi: 10.3171/jns.1991.74.3.0492. [DOI] [PubMed] [Google Scholar]
- Jiang JY, Xu W, Li WP, Gao GY, Bao YH, Liang YM, Luo QZ. Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:771–776. doi: 10.1038/sj.jcbfm.9600253. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Pitkanen A. Association of the severity of cortical damage with the occurrence of spontaneous seizures and hyperexcitability in an animal model of posttraumatic epilepsy. Epilepsy Res. 2010;90:47–59. doi: 10.1016/j.eplepsyres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle MJ, Thomson KE, Scheerlinck P, Pouliot W, Greger B, Dudek FE. A simple quantitative method for analyzing electrographic status epilepticus in rats. J Neurophysiol. 2009;101:1660–1670. doi: 10.1152/jn.91062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Schmidt D. New horizons in the development of antiepileptic drugs. Epilepsy Res. 2002;50:3–16. doi: 10.1016/s0920-1211(02)00063-3. [DOI] [PubMed] [Google Scholar]
- Lotocki G, de Rivero Vaccari JP, Perez ER, Alonso OF, Curbelo K, Keane RW, Dietrich WD. Therapeutic hypothermia modulates TNFR1 signaling in the traumatized brain via early transient activation of the JNK pathway and suppression of XIAP cleavage. Eur J Neurosci. 2006;24:2283–2290. doi: 10.1111/j.1460-9568.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: A potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoyanov NV, Sa MJ, Madeira MD, Paula-Barbosa MM. Selective loss of hilar neurons and impairment of initial learning in rats after repeated administration of electroconvulsive shock seizures. Exp Brain Res. 2004;154:192–200. doi: 10.1007/s00221-003-1658-3. [DOI] [PubMed] [Google Scholar]
- Lyeth BG, Jiang JY, Liu S. Behavioral protection by moderate hypothermia initiated after experimental traumatic brain injury. J Neurotrauma. 1993;10:57–64. doi: 10.1089/neu.1993.10.57. [DOI] [PubMed] [Google Scholar]
- Margulies S, Hicks R. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion D, Bullock MR. Current and future role of therapeutic hypothermia. J Neurotrauma. 2009;26:455–467. doi: 10.1089/neu.2008.0582. [DOI] [PubMed] [Google Scholar]
- Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, Wisniewski SR, DeKosky ST. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- Mayer SA. Hypothermia for neuroprotection after cardiac arrest. Curr Neurol Neurosci Rep. 2002;2:525–526. doi: 10.1007/s11910-002-0040-3. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28:1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- Nitta N, Heinrich C, Hirai H, Suzuki F. Granule cell dispersion develops without neurogenesis and does not fully depend on astroglial cell generation in a mouse model of temporal lobe epilepsy. Epilepsia. 2008;49:1711–1722. doi: 10.1111/j.1528-1167.2008.01595.x. [DOI] [PubMed] [Google Scholar]
- Parent JM, Tada E, Fike JR, Lowenstein DH. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J Neurosci. 1999;19:4508–4519. doi: 10.1523/JNEUROSCI.19-11-04508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; Burlington, MA, USA: 2005. [Google Scholar]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37:1101–1120. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- Potts MB, Rola R, Claus CP, Ferriero DM, Fike JR, Noble-Haeusslein LJ. Glutathione peroxidase overexpression does not rescue impaired neurogenesis in the injured immature brain. J Neurosci Res. 2009;87:1848–1857. doi: 10.1002/jnr.21996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Represa A, Niquet J, Pollard H, Khrestchatisky M, Ben-Ari Y. From seizures to neo-synaptogenesis: Intrinsic and extrinsic determinants of mossy fiber sprouting in the adult hippocampus. Hippocampus. 1994;4:270–274. doi: 10.1002/hipo.450040308. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol. 2000;428:240–253. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, Potts MB, Fike JR. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202:189–199. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Ross ST, Soltesz I. Selective depolarization of interneurons in the early posttraumatic dentate gyrus: involvement of the Na(+)/K(+)-ATPase. J Neurophysiol. 2000;83:2916–2930. doi: 10.1152/jn.2000.83.5.2916. [DOI] [PubMed] [Google Scholar]
- Sahay A, Kim CH, Sepkuty JP, Cho E, Huganir RL, Ginty DD, Kolodkin AL. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J Neurosci. 2005;25:3613–3620. doi: 10.1523/JNEUROSCI.5255-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35:1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Bender R, Frotscher M, Ross ST, Hollrigel GS, Toth Z, Soltesz I. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the ‘irritable mossy cell’ hypothesis. J Physiol. 2000;524(Pt 1):117–134. doi: 10.1111/j.1469-7793.2000.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Ratzliff AD, Jeng J, Toth Z, Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann Neurol. 2001;50:708–717. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- Seress L, Gallyas F. The use of a sodium tungstate developer markedly improves the electron microscopic localization of zinc by the Timm method. J Neurosci Methods. 2000;100:33–39. doi: 10.1016/s0165-0270(00)00227-2. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Figueroa-Aragon S, Ribak CE. Newly generated granule cells show rapid neuroplastic changes in the adult rat dentate gyrus during the first five days following pilocarpine-induced seizures. Eur J Neurosci. 2007;26:583–592. doi: 10.1111/j.1460-9568.2007.05662.x. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci Lett. 1992;137:91–96. doi: 10.1016/0304-3940(92)90306-r. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994;35:640–654. doi: 10.1002/ana.410350604. [DOI] [PubMed] [Google Scholar]
- Sun D, Colello RJ, Daugherty WP, Kwon TH, McGinn MJ, Harvey HB, Bullock MR. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J Neurotrauma. 2005;22:95–105. doi: 10.1089/neu.2005.22.95. [DOI] [PubMed] [Google Scholar]
- Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bramlett HM, Dietrich WD. The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184:1017–1026. doi: 10.1016/S0014-4886(03)00389-3. [DOI] [PubMed] [Google Scholar]
- Swartz BE, Houser CR, Tomiyasu U, Walsh GO, DeSalles A, Rich JR, Delgado-Escueta A. Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia. 2006;47:1373–1382. doi: 10.1111/j.1528-1167.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50(Suppl 2):10–13. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- Temkin NR, Jarell AD, Anderson GD. Antiepileptogenic agents: How close are we? Drugs. 2001;61:1045–1055. doi: 10.2165/00003495-200161080-00002. [DOI] [PubMed] [Google Scholar]
- Urrea C, Castellanos DA, Sagen J, Tsoulfas P, Bramlett HM, Dietrich WD. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor Neurol Neurosci. 2007;25:65–76. [PubMed] [Google Scholar]
- Vespa PM, Nuwer MR, Nenov V, Ronne-Engstrom E, Hovda DA, Bergsneider M, Kelly DF, Martin NA, Becker DP. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750–760. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter C, Murphy BL, Pun RY, Spieles-Engemann AL, Danzer SC. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J Neurosci. 2007;27:7541–7552. doi: 10.1523/JNEUROSCI.0431-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cui SS, Wallace AE, Hannesson DK, Schmued LC, Saucier DM, Honer WG, Corcoran ME. Relations between brain pathology and temporal lobe epilepsy. J Neurosci. 2002;22:6052–6061. doi: 10.1523/JNEUROSCI.22-14-06052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]