Abstract
Limited osseointegration of current orthopaedic biomaterials contributes to the failure of implants such as arthroplasties, bone screws and bone grafts, which present a large socioeconomic cost within the United States. These implant failures underscore the need for biomimetic approaches that modulate host cell-implant material responses to enhance implant osseointegration and bone formation. Bioinspired strategies have included functionalizing implants with ECM proteins or ECM-derived peptides or protein fragments which engage integrins and direct osteoblast adhesion and differentiation. This review discusses 1) bone ECM composition and key integrins implicated in osteogenic differentiation, 2) the use of implants functionalized with ECM-mimetic peptides/protein fragments, and 3) growth-factor derived peptides to promote the mechanical fixation of implants to bone and to enhance bone healing within large defects.
1. Introduction
The limited biological performance of current orthopaedic implants, such as joint replacement prostheses, bone screws and bone grafts, presents a large and growing socioeconomic burden in the United States. For example, in 2004, the failure of replacement joints prompted 86,000 revision surgeries for hip and knee arthroplasties at a cost of $3.2 billion, and those surgery numbers are projected to exceed 3.6 million by 2030 1. Similarly, the loosening of screws for spinal implants and fracture fixation in osteoporotic patients are major clinical concerns, with high failure rates estimated to be 18–27% 2–4 and 5–23% 5–7 respectively. Furthermore, over 600,000 bone grafting procedures are performed annually in the U.S. to treat non-healing skeletal defects caused by traumatic injury and cancer 8–9. However, autografts, the gold standard of treatment, are limited by donor site supply and morbidity 10, and allografts are limited by increased resorption, poor mechanical properties and the risk of infection 9–10. Therefore, there is a significant need for improved orthopaedic materials which promote implant integration into host bone and enhance bone formation.
Bone contains multiple cells types such as osteoblasts, osteoclasts and osteocytes; osteoblasts are the major cell type responsible for bone formation. Osteoblasts differentiate from mesenchymal stem cells and osteoprogenitor cells found primarily in the bone marrow in a multi-step process in which the Cbfa1/Runx-2 transcription factor plays a crucial role 11. Stem cells differentiate into osteoprogenitors with limited self-renewal capacity, then to pre-osteoblasts with limited proliferation, and finally to mature osteoblasts, which secrete osteoid, the unmineralized organic component of bone matrix. As the deposited osteoid is mineralized, osteoblasts become trapped within lacunae as osteocytes, become bone lining cells, or die by apoptosis 12. Biomaterials which can modulate the response of host osteoblast and osteoprogenitor cells to the implant may be crucial to improving the mechanical fixation of implants and osteogenic capacity of bone grafts. For example, implant osseointegration, defined by the enhancement of new bone formation in direct contact with the implant as well as implant fixation within the first 2 years, has been shown to be predictive of the long-term success of implants 13–14. Therefore, materials that engage osteoblast receptors and induce peri-implant bone formation may effectively address the problems of arthroplasties and screw loosening. Similarly, although bone has an innate capacity to regenerate through intramembranous and endochondral ossification 15, in non- or delayed- unions, biomaterial grafts that augment this healing capacity by upregulating osteoblast-mediated bone formation may present viable alternatives to autografts.
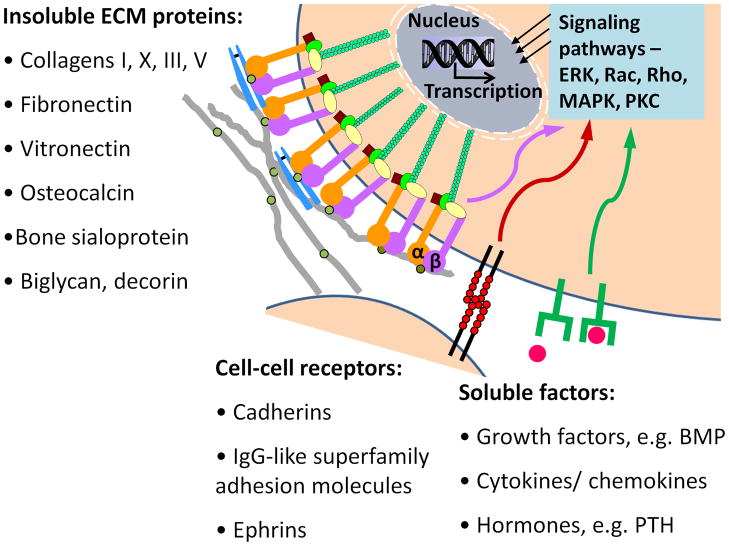
As successful orthopaedic biomaterials must support the adhesion, organization, differentiation and matrix mineralization of osteoblasts and osteoprogenitor cells, many strategies have focused on recapitulating natural biological cues which regulate these processes. Cell fates such as proliferation and differentiation are determined by a complex interplay of signals from the extracellular environment. These signals include (1) insoluble molecules within the extracellular matrix, (2) soluble and/or matrix-associated biochemicals such as systemic hormones or growth factors and cytokines that act locally, and (3) cell-cell receptors (Fig. 1). The ECM itself contains multiple types of insoluble molecules, forming a meshwork of structural proteins to which adhesive proteins, proteoglycans and glycosaminoglycans are associated 16. This complex biological supramolecular scaffold provides a compelling model for biomimetic strategies which mimic ECM protein, growth factor or hydroxyapatite mineral chemistry or architecture to create a synthetic matrix to control tissue-specific cell responses. Architectural ECM-mimetic approaches include nanofiber scaffolds that recapitulate the structure of proteins within ECM 17, substrates with features which mimic native ECM nanotopography 18, and composites which recreate the mineral content and mechanical properties of bone matrix 19–20. This review will focus on 1) bone ECM composition and key integrins implicated in osteogenic differentiation, 2) orthopedic biomaterials functionalized with ECM motifs, and 3) growth factor derived peptides.
Fig 1.
Bioactive signals found within the extracellular environment in bone.
2. Bone ECM composition and key integrins implicated in osteogenesis
The composition and spatial orientation of ECM varies for each tissue type. These differences in ECM composition/orientation may be useful in tailoring biomaterials to direct tissue-specific cellular responses as each type of ECM molecule may regulate cell differentiation differentially by interacting with specific cell receptors 21. In bone, the ECM consists of mainly of an organic phase known as osteoid, which constitutes approximately 20% of bone mass, and a mineral phase (Table 1). The organic fraction of bone consists of over 90% type I collagen 22, other minor collagens such as types III and V, and 5% non-collagenous proteins. The non-collagenous proteins in bone include osteocalcin, osteonectin, osteopontin, adhesion proteins such as fibronectin and vitronectin and proteoglycans such as versican, decorin and hyaluronan 23. The mineral phase of bone is composed of hydroxyapatite, a calcium phosphate compound. The bone matrix also sequesters growth factors, acting as a reservoir for soluble inductive signals such as bone morphogenic protein (BMP).
Table 1.
Composition of bone ECM.
| Molecular Weight | Function/regulates | Binds To | ||
|---|---|---|---|---|
| Organic (20% of bone mass) | Collagens | |||
| Type I | Range | Structural protein | Itg, TSP, OSN, OSP, BG, DC, BSP | |
| Type X | Range | Present in hypertropic cartilage | ||
| Type III | Range | Col fibril diameter | ||
| Type V | Range | Col fibril diameter | ||
| Adhesion proteins | ||||
| Fibronectin | ~ 400kD | Adhesion | Itg, Col, heparin, | |
| Thrombospondin | ~ 450 kD | Adhesion, bone formation | Ca, HAP, OSN | |
| Vitronectin | ~ 70kD | Adhesion | Itg, Col, heparin, | |
| Osteopontin | ~44-75kD | Adhesion, proliferation, resorption | Itg | |
| Osteonectin | ~35-45kD | HAP deposition, bone formation | Ca, HAP, Col, TSP | |
| Osteocalcin | ~5kD | Osteoclast activity | Ca | |
| Bone Sialoprotein | ~46-75kD | Adhesion, mineralization | Itg, Col | |
| Alkaline Phosphatase | ~80kD | Mineralization | - | |
| Proteoglycans | ||||
| Biglycan | ~270kD | Col fibril diameter | Col | |
| Decorin | ~150kD | Col fibril diameter | Col, TGF-β | |
| Inorganic (70% of bone mass) | Hydroxyapatite | - | Mechanical strength of bone | - |
Itg – Integrins, Col –Collagen, HAP –Hydroxyapatite, Ca –Calcium, TSP –Thrombospondin, OSN – Osteonectin, OSP – Osteopontin, BG – Biglycan, DC–Decorin, BSP – Bone Sialoprotein, TGF-β transforming growth factor- β.
Bone ECM serves both structural and biological functions, as the mineralized matrix accounts for the tissue’s mechanical properties while it also provides chemical cues that regulate bone cells and acts as a reservoir for ions 12. Collagen fibrils provide tensile strength to bone and are composed of collagen helices that assemble parallel to each other in a regular quarter-staggered pattern, creating 68 nm gaps between adjacent collagen molecules. Hydroxyapatite crystals, which make up 70% of bone, fill these gaps and are responsible for the compressive strength of bone 12. Bone ECM also regulates bone cells by providing ECM-integrin bonds that enable the formation of adhesive structures and activate signaling pathways which regulate cell spreading, survival and differentiation. However, as bone biology is not the focus of this article, the reader is referred to the following article 12. Recreating the biological function of ECM using bone ECM-specific adhesive signals such as collagen I, fibronectin and vitronectin may therefore be a powerful biomaterial strategy to enhance osteogenesis.
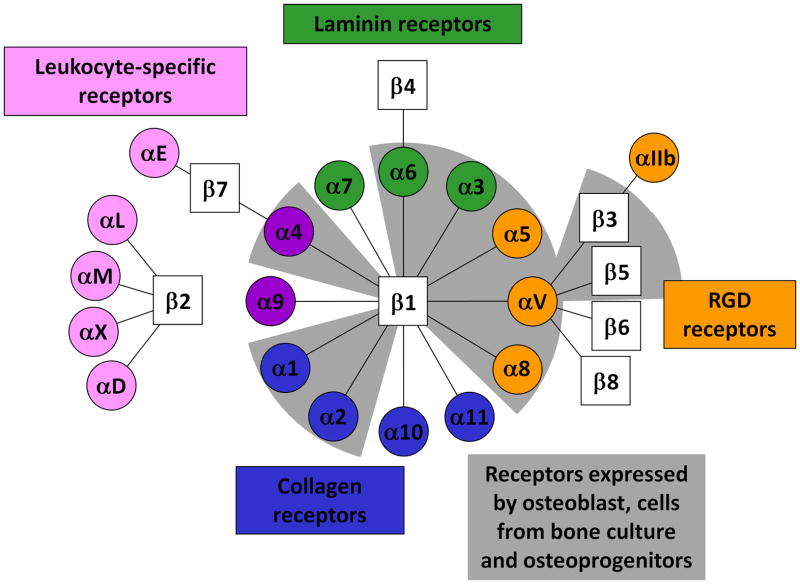
Integrins are a family of receptors that primarily mediate adhesion of cells to the extracellular matrix proteins such as collagen and fibronectin 24. Integrins are heterodimeric transmembrane proteins, each of which consists of α and β subunits. Currently, 8 β and 18 α integrin subunits are known, and these subunits associate to form 24 distinct αβ integrin combinations, each with unique binding characteristics (Fig. 2). X-ray crystallography analysis of integrin structure demonstrates a globular head connected to rod-like tails, and includes a flexible “knee” region that is involved in the activation state of the integrin. Integrins are capable of transducing signals in both directions across the cell membrane. For example, ‘outside-in’ signaling occurs when ECM ligation to integrins trigger intracellular signaling. Conversely, ‘inside-out’ signaling takes place when intracellular signals modulate integrin activation state and thus change its affinity for its extracellular ECM ligand 25. Upon ECM binding to their extracellular domains, integrins cluster and their cytoplasmic domains associate with both cytoskeletal and intracellular signal transduction molecules. The association of integrins with the cellular signaling network initiates downstream signaling cascades such as the FAK, protein kinase C, Rac, Rho and MAPK pathways. The coordinated clustering of ECM ligands, integrins and cytoskeletal components forms macromolecular aggregates known as focal adhesions on the inside and outside of the cell membrane 26. Because of the central roles of integrin-mediated adhesion to important cellular responses such as survival, growth, migration and differentiation 25,27–28, materials strategies that harness ECM-integrin interactions may play a key role in eliciting desired cellular responses in vivo.
Fig 2.
Integrin alpha and beta subunit combinations, binding specificity and expression in bone cells. Adapted from Hynes24.
The β1 sub-family integrins are the mostly highly expressed integrins in osteoprogenitors and osteoblasts and the predominant mediators of cell adhesion in these cells 29, although the β3 andβ5 subunits may be expressed as well 30–31. Alpha subunit expression data has been more inconsistent, with different combinations of α1, α2, α3, α4, α5 and αv subunits having been detected by immunohistochemistry in human and rat bone 30–34. The expression of the previously mentioned alpha subunits has also variously been determined by flow cytometry, immunoprecipitation, immunocytochemistry and Northern blot analysis on primary bone cultures 29–31,35–39. Although reports of alpha subunit and integrin heterodimer expression in osteoblasts have sometimes been contradictory, many studies have identified the α1β1, α2β1, α3β1, α5β1,αvβ3 integrins and their subunits in osteoblasts and bone cultures 29,33,37–38. A few isolated studies have also found osteoblast expression of α6β1 38,40, α8β1 41, αvβ135 and αvβ5 29. Integrin expression studies on osteoprogenitor cells have shown similar profiles as osteoblasts, as Gronthos et al. reported the detection of α1β1, α2β1, α5β1, α6β1, αvβ3 and αvβ5 on STRO-1 expressing human bone marrow stromal cells 42 (Table 2, Fig. 2).
Table 2.
Role of key integrins on function of osteoblasts, osteoblast-like cells or osteoprogenitors.
| Beta integrin subunits | Alpha integrin subunits | ECM ligand | Expression references | Functions in osteoblasts (OB), OB-like cells, or osteoprogenitors | Function references |
|---|---|---|---|---|---|
| Beta 1 | Alpha 1 | Col I, LN | [29–30], [32], [34–35], [42] | Differentiation | [48], [65] |
| Alpha 2 | Col I, LN | [29–30], [32], [34], [39], [42] | Adhesion, differentiation | [47–52], [54–55] | |
| Alpha 3 | FN, TN, Col | [29–30], [32], [34–35], [37], [39], [41] | Differentiation | [41] | |
| Alpha 4 | FN | [30–31], [33] | - | - | |
| Alpha 5 | FN | [29–30], [33–35], [37], [41– 42] | Adhesion, survival, differentiation, mechanical sensing, | [41], [57], [59–64] | |
| Alpha 6 | LN | [37], [40], [42] | - | - | |
| Alpha 8 | [41] | - | - | ||
| Alpha v | FN, VN | [33], [35], [39] | - | - | |
| Beta 3 | Alpha v | FN, VN, BSP, | [29–32], [35], [37], [41], [42] | Adhesion, inhibits differentiation, inhibits proliferation | [64], [66–67] |
| Beta 5 | Alpha v | OPN, TN VN | [29], [35], [42] | - | - |
2.1 β1 integrins
Blocking studies showed that β1-integrin-mediated adhesion contributes significantly to the adhesion strength of human bone marrow cells on fibronectin 43 as well as the adhesion of cells from human bone culture and human bone marrow stromal cells to collagen, laminin and fibronectin 42. Perturbation of β1-integrin function also inhibits matrix mineralization in human bone marrow cells 42. In addition, a glucocorticoid-induced reduction of β1 expression is correlated with inhibition of cell adhesion 44. Transgenic mice expressing a dominant-negative truncated β1 subunit in osteoblasts and osteocytes display reduced bone mass and increased bone porosity 45 as well as an alteration in tibial curvature and femoral torsional strength 46. The expression of β1 with altered function in the osteoblasts of these transgenic mice also results in impaired adhesion of osteoblasts in vitro 45.
2.1.1 α2β1
The α2β1 integrin is implicated in pro-osteogenic pathways as it is highly expressed by osteoblast-like cells and is a primary adhesion receptor used by osteoblast-like cells to adhere to collagen 29, the main organic component of bone. Several studies indicate that the interaction of α2β1 integrin with collagen I is a crucial signal for osteoblastic differentiation and matrix mineralization 47–52. For example, α2β1-mediated adhesion of mouse MC3T3-E1 pre-osteoblasts to collagen I activates Runx2/Cbfa1, a transcription factor that activates osteoblastic differentiation and matrix mineralization 51–52. α2β1 ligation to collagen I also induces the phosphorylation of focal adhesion kinase (FAK) and activation of extracellular signal-related kinase (ERK), which has been implicated in the regulation of osteoblast-specific gene expression and matrix mineralization 50–51,53–54. Furthermore, the collagen – α2β1 integrin interaction promotes an osteoblastic phenotype in rat multipotent bone marrow cells 47,49. Schneider et al also showed that perturbation of the α2β1 integrin resulted in a 95% reduction mineralization in an osteosarcoma cell line 55.
2.1.2 α5β1
The α5β1 integrin plays an important role in osteogenic differentiation as it is expressed by osteoblasts and osteoprogenitors, and promotes cell survival and matrix mineralization. α5β1 is stably expressed by osteoblasts during varying stages of osteogenesis 41 and is also expressed by bone marrow stromal cells 56. In addition, α5β1 also mediates cell attachment to fibronectin as well as fibronectin assembly 56. In mature cells, α5β1 binding is necessary for cell survival and a decrease in α5β1-fibronectin interaction leads to osteoblast apoptosis 57 through a caspase-dependent mechanism 58. α5β1 may also be involved in mechanical sensing by osteoblasts in vitro 59. Blockade of the α5β1 integrin inhibits bone-specific gene expression and mineralization in rat calvarial cultures 41,60, a rat osteosarcoma cell line 55, human osteoblast-like cells 61 and a mouse immature osteoblast-like cell-line 62. In human mesenchymal stromal cells (hMSC), priming the α5 subunit with an agonist or overexpression of the α5 subunit increases osteogenic capacity 63, while α5β1 blockade decreases the alkaline phosphatase activity of cells cultured on fibronectin 64.
2.1.3 α1β1 and α3β1
The α1β1 and α3β1 integrins also appear to play important roles in bone healing as α1 integrin knock-out mice display impaired fracture healing 65 and blockade of α3β1 inhibits mineralized nodule formation 41.
2.2 β3 integrins
2.2.1 αvβ3
While engagement of the αvβ3 integrin may support cell adhesion, it has a negative effect on the proliferation and differentiation of osteoprogenitors. Blocking of αvβ3 has been shown to enhance human MSC proliferation on fibronectin and fibronectin fragments 64. αvβ3 may also inhibit osteoblast differentiation and bone healing in vivo. A murine osteoblastic cell line made to overexpress human αvβ3 showed an increase in proliferation rate but a decrease in matrix mineralization 66. Furthermore, early fracture healing was accelerated in the tibiae of β3-null mice and twenty-three genes related to osteogenesis were upregulated at least two-fold in the β3-null mice 67. The αvβ3 integrin is also the major integrin receptor expressed by osteoclasts 68 and plays a major role in osteoclast adhesion 69, resorption 70 and sealing zone organization71.
Targeting materials to integrins such as α2β1 and α5β1 which are expressed by osteoblasts-like cells and regulate osteogenesis while preventing interactions with integrins which may inhibit osteoblastic differentiation such as αvβ3 may be a powerful molecular strategy for developing improved orthopaedic biomaterials.
3. Orthopaedic biomaterials functionalized with ECM motifs (Table 3)
Table 3.
ECM polymers, ECM-derived peptides and growth factor-derived peptides used in bone biomaterials.
| Types | Source | Itg. Spec. | MW (kD) | Sequence | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| In vitro | In vivo | ||||||||
| Adhesion/ diff. | Ectopic bone | Cranio-facial defect | Long bone defect | Implant integration | |||||
| Full-length natural polymers | |||||||||
| Collagen | Animal | ~300 | [78] | [76–77] | [72–75] | ||||
| Fibrin | Animal | ~340 | [79–82] | ||||||
| DCM | Animal | [87] | [88] | ||||||
| BSP | Animal | ~30–80 | [89] | ||||||
| ECM-derived peptides | |||||||||
| RGD (linear) | Many | αvβ3 , others | ~1 | [104–105], [109] | |||||
| RGD (cyclic) | Many | αvβ3, others | ~1 | [75], [103], [106–108] | |||||
| GFOGER | Col I | α2β1 | ~13.5 | GGYGGGPC(GPP)5GFOGER(GPP)5GPC/GPC(GPP)5GFOGER(GPP)5GPC | [136–138], [140] | [99] | [138] | ||
| P15 | Col I | α2β1 | ~1.5 | GTPGPQGIAGQRGVV | [140], [144], [146] | [100], [147–149] | [150] | ||
| DGEA | Col I | ~0.5 | DGEA | [47], [142] | |||||
| FNIII7-10 | FN | α5β1 | ~55 | [97], [110], [114] | [110], [114] | ||||
| FNIII9-10 | FN | α2β1 | [64] | ||||||
| RGD-PHSRN | FN | ~1.5 | RGDG13PHSRN/ G3PHSRNG6RGDG | [115–116] | |||||
| FHRRIKA | BSP | ~1 | CGGFHRRIKA | [117–121] | |||||
| KRSR | FN, VN, BSP,TN, OPN | ~0.5–0.9 | KRSR/ KRSRGYC | [122–126] | |||||
| BSP (278– 293) | BSP | ~2 | YESENGEPRGDNYRAYC | [127] | |||||
| (351–359) HVP | VN | ~1 | FRHRNRKGY | [128–129], [131] | [128], [130] | ||||
| ODP | OPN | ~1.5 | DVDVPDGRGDSLAYG | [132] | |||||
| HBP12 | Heparin | ~1.5 | VRRSKHGARKDR | [133] | |||||
| Growth-factor derived peptides | |||||||||
| p24 | BMP-2 (73–92) | ~2.5 | SKIPKASSVPTELSAISTLYLDDD/KIPKASSVPTELSAISTLYL | [157–158] | [155–157], [159] | [155,] [160–161] | |||
| OPD | BMP-2 (30–34) | ~0.5 | DWIVA | [162] | |||||
Itg. Spec.– Integrin Specificity, MW (kD) –Molecular Weight (kiloDaltons), Adhesion/Diff. –Adhesion/ Differentiation, HA –Hyaluronic Acid, DCM –Decellularized Matrix, BSP –Bone Sialoprotein, Col I –Collagen I, FN –Fibronectin, VN –Vitronectin, TN –Thrombospondin, OPN – Osteopontin, BMP –Bone Morphogenic Protein.
3.1 Full-length natural ECM polymers
Due to the important regulatory role that ECM molecules play on cellular responses in vivo, full-length ECM proteins have been studied as potential adhesive scaffolds for bone defect healing and implant integration. These ECM polymers include collagen 72–78, fibrin 79–82, hyaluronic acid 83–86, decellularized matrix 87–88 as well as bone sialoprotein 89 (Table 3). Methods used to functionalize titanium implants with ECM polymers include protein adsorption from solution 75,85, injection of protein solution into a porous implant 74, dip-coating and covalent tethering 73. For the treatment of bone defects, ECM implants have been used in the form of crosslinked membranes 77, sponges 78, gels 80, demineralized bone particles 87 or cut pieces of small intestinal submucosa 88. Although naturally derived ECM molecules have demonstrated some degree of success in selected studies 72–73,88, the widespread use of natural ECM macromolecules in orthopaedic applications has been hindered by several factors. First, full-length ECM polymers have low solubility, are costly to extract and purify in large quantities, suffer from batch-to-batch variation and potentially suffer from immunogenicity. Furthermore, it is challenging to modify, characterize and control the presentation of natural ECM biomaterials.
3.2 ECM-derived adhesive peptides/proteins
The above-mentioned limitations of full-length ECM molecules have spurred the use of ECM-derived peptides or recombinant fragments that incorporate the minimal functional sequence of their parent protein 90 in order to convey bioactivity to implant materials. In contrast to ECM polymers, these peptides and protein fragments may be synthesized in larger quantities (and via chemical synthesis or recombinant protein expression), immobilized on non-fouling surfaces at high densities, and may be tailored in composition for specific applications. While natural ECM proteins such as collagens and fibronectin are large macromolecules consisting of thousands of amino acids, only a few short peptide sequences within these polymers serve as integrin recognition and binding sequences that trigger downstream processes such as adhesion, signaling and spreading. For example, in collagens I, II and III, cells bind to the GFOGER 91–92 peptide sequence while in fibronectin, the RGD 93, PHSRN 94, REDV95, and LDV 96 sequences are responsible for cell binding. As a result, short peptide sequences such as these, as well as ECM-derived protein fragment such as FNIII7-10 are used to biofunctionalize titanium surfaces and bone tissue engineering scaffolds. In addition to the primary sequence of these peptide ligands, the structure or conformation of the ligand is a critical factor in their ability to binding to integrin receptors and trigger signaling pathways. Common peptide/protein fragment functionalization methods for titanium implants include simple adsorption or covalent immobilization onto titanium surfaces. Peptides may be presented on a non-fouling background by covalently tethering them to protein resistant polymer coatings such as poly(ethylene glycol) 97–98. Peptide modification strategies for bone regeneration within defects include adsorption to polymer scaffolds 99 or bone matrix 100. Both the tethering density and the stability of these peptide ligands on the implant surfaces are critical considerations in their performance.
3.2.1 RGD
RGD is an adhesive peptide sequence found in many ECM molecules including fibronectin, vitronectin, bone sialoprotein and osteopontin 101. RGD can bind to multiple integrins suchαvβ3, αvβ1, α8β1, αvβ8, αvβ6, αvβ5 and αIIbβ3. However, for certain integrins, binding to RGD is strongly modulated by another sequence, such as the PHSRN synergy site for α5β1 94,102. Because RGD serves as a potent, promiscuous binding sequence, many biomaterial strategies have incorporated RGD as an adhesive ligand.
The application of linear RGD oligopeptides onto implant surfaces has generally failed to enhance functional osseointegration as determined by bone-implant contact and mechanical fixation in several independent studies 97–98,103–104. In addition, Bellis and coworkers demonstrated a negative effect for RGD peptides in bone formation and osseointegration responses to hydroxyapatite implants 105. In contrast to these studies, Soballe and colleagues did report enhancements in osseointegration for implants presenting cyclic RGD peptides 106–107. However, other studies using cyclic RGD have also failed to show improvements in implant fixation in rat tibiae 75 and canine mandibles 108. Direct comparison among these contradictory studies is confounded by differences in the presence of a non-fouling polymer coating to prevent non-specific adsorption of plasma proteins, the animal model used, as well as implant surface finish (i.e., roughness). It is worth noting that two studies in which RGD was presented on titanium implants in a controlled fashion from non-fouling background coating demonstrated no improvements in osseointegration 109–110, suggesting that RGD-functionalization is not effective at enhancing implant integration. Fewer RGD modified materials have been tested as bone grafts within defects, but in those studies, RGD does not promote bone formation and repair in vivo 111.
3.2.2 Fibronectin-mimetic protein fragments/peptides
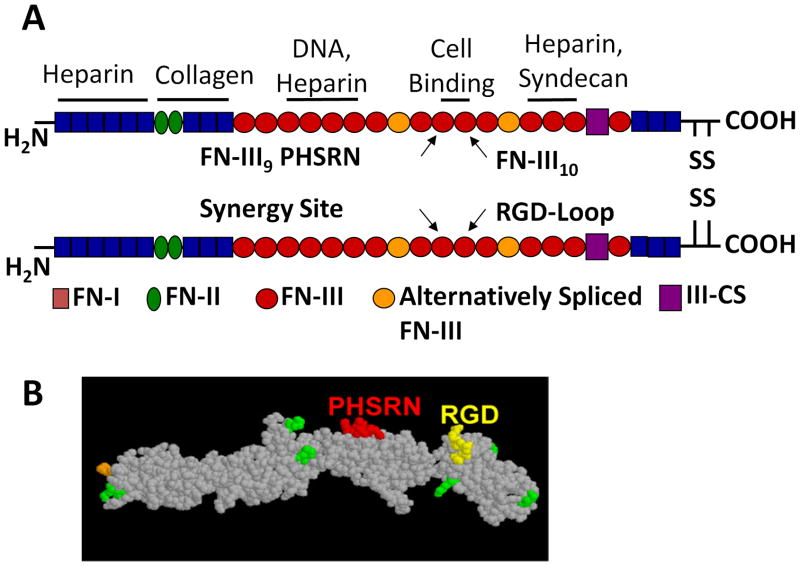
Fibronectin contains both the RGD adhesion site as well as a PHSRN synergy site. α5β1 binds to RGD in the presence of PHSRN in fibronectin with a forty-fold increase in affinity compared to RGD alone 94 (Fig. 3A). Each of these domains independently contributes little to binding, but, in combination, they synergistically bind to α5β1 to provide stable adhesion 102,112. In contrast, other integrins are unaffected by the synergy site and bind only to the RGD site within fibronectin with a lower affinity than α5β1113. Several fibronectin-derived peptides or fragments designed for biomaterial applications therefore recapitulate this interaction between α5β1 and the RGD and PHSRN sites.
Fig 3.
(A)Structure of plasma fibronectin and location of major binding sites. (B) Space-filling model of FNIII7-10 recombinant fragment of fibronectin.
3.2.2.1FNIII7-10
Our group has engineered a recombinant fragment of fibronectin, FNIII7-10, which encompasses the 7–10th repeats of native fibronectin and binds specifically to the α5β1 integrin (Fig. 3B). FNIII7-10 enhances both osteoblast adhesion strength and differentiation in vitro 110, as well as implant osseointegration in a rat cortical model when compared to titanium implants modified with RGD at an equivalent molar surface density 97. Furthermore, a simple adsorbed coating this fragment exhibits improved bone apposition and mechanical fixation to bone when compared to full-length fibronectin as fibronectin domains with antagonistic effects are excluded from the fragment 114.
3.2.2.2 FNIII9*-10
Martino et al. investigated the osteogenic potential of human MSCs on surfaces and hydrogels functionalized with full-length fibronectin (FN), fibronectin fragments (FNIII9–10 and FNIII10) and a more α5β1-specific mutated fibronectin fragment (FNIII9*-10) and demonstrated that FNIII9*-10 and FNIII9-10 supported higher MSC differentiation than FN 64. Interestingly, the level of osteoblastic differentiation for each fragment was correlated with its degree of binding specificity for the α5β1 integrin (FNIII9*-10 > FNIII9-10 > FNIII10), which supports other studies suggesting that α5β1 engagement may enhance osteogenesis97,110,114.
3.2.2.3 RGD-PHSRN oligopeptides
Synthetic peptides designed to co-present the RGD site and PHSRN synergy sites on the same molecule separated by polyglycine linkers result in increased adhesion and metabolic activity of primary rat calvarial osteoblasts 115 and human osteoblast-like cells 116 in vitro when compared to surfaces presenting RGD alone. However, whether these peptides enhance implant osseointegration and bone formation in vivo remains to be established.
3.2.3 Other ECM-derived peptides
Other ECM-derived peptides that have been found to enhance osteoblast adhesion and differentiation in vitro include FHRRIKA which is derived from the heparin binding site of bone sialoprotein 117–121, KRSR, which is a heparin binding sequence found on multiple ECM proteins 121–126, the bone sialoprotein derived BSP(278-293) 127, the human vitronectin peptide HVP (351-359) 128–131, an osteopontin derived peptide 132, and a heparin binding peptide, HBP12 133. While these ECM derived peptides have shown promise as bone biomaterials in vitro, more studies need to be done to demonstrate their osteogenic capacity in vivo as well.
3.2.3 Collagen-mimetic peptides
3.2.3.1 GFOGER
The hexapeptide sequence Gly-Phe-Hyp-Gly-Glu-Arg (GFOGER) is found on residues 502 507 of the α1(I) chain of type I collagen and serves as the major recognition site for α2β1 integrin binding 92,134–135. Our group engineered a Col I-mimetic GFOGER containing peptide, GGYGGGPC(GPP)5GFOGER(GPP)5GPC, which recapitulates the triple helical tertiary structure of native collagen as an adhesive ligand for biomaterials. Surfaces presenting adsorbed or covalently immobilized GFOGER peptide support equivalent levels of α2β1 integrin-mediated adhesion of HT1080 fibrosarcoma and MC3T3-E1 osteoblast-like cells as native collagen I 136 and also promote osteoblastic differentiation of MC3T3-E1 and primary bone marrow stromal cells in vitro 137–138. Furthermore, GFOGER enhances bone repair in vivo within rigorous critical-sized rat femur defect models without the delivery of cells or growth factors 139. GFOGER-functionalized titanium implants also enhance implant integration in a rat cortical model by improving peri-implant bone formation and implant fixation to bone 137–138. Surprisingly, an in vitro study by Hennessy et al. found that adsorption of a different triple-helical GFOGER sequence-containing peptide, GPC(GPP)5GFOGER(GPP)5GPC, did not improve human mesenchymal stem cell adhesion on hydroxyapatite disks 140, although cells cultured on GFOGER-treated tissue culture plastic showed levels of adhesion and spreading equivalent to full-length collagen I. This result contradicts other studies by our group and others which indicate that triple-helical peptides containing the GFOGER sequence support robust cell adhesion 92,135 and differentiation 137 and may possibly be due to low GFOGER adsorption to the hydroxyapatite disks or variations in the primary sequence of the GFOGER peptides used in these studies.
3.2.3.2 DGEA
The DGEA sequence has been suggested as the α2β1 recognition sequence in type I collagen 141, although a different study failed to demonstrate α2β1 mediated cell responses to DGEA 142. Soluble DGEA peptide inhibits the osteoblastic phenotype of rat bone marrow stromal cells cultured on type I collagen. DGEA coated hydroxyapatite disks have promoted cell adhesion and upregulated osteoblast marker expression in mesenchymal stem cells in vitro 140. However, surfaces modified with a CCGDGEAG peptide failed to support the adhesion of rat calvarial osteoblasts 143.
3.2.3.3 P15
P15 is a synthetic 15-amino acid peptide derived from the (766)GTPGPQGIAGQRGVV(780) sequence found in the α1(I) chain of type I collagen 144. Several studies have demonstrated that P15 enhances cell adhesion, osteoblastic gene expression and mineralization on anorganic bone matrix (ABM) in vitro 145–146 and accelerates early bone formation in porcine 100 and rat 147 cranial defects. In a head-to-head comparison of DGEA and P15 coated hydroxyapatite disks implanted into rat tibiae, both peptides improved new bone formation, but P15 failed to enhance bone implant contact 140. P15 peptide-coated ABM has also been used in human periodontal osseous defects 148–149 resulting in better clinical outcomes than open flap debridement alone, and has also been used in a pilot clinical study for long-bone defects 150. However, P15-coated ABM has not been compared with ABM alone in these human dental applications to determine the role of P-15 alone on the positive effects observed.
3.3 Growth factor-derived peptides
Many growth factors have a profound influence on bone formation including bone morphogenic proteins (BMPs), fibroblast growth factors (FGFs), insulin-like growth factors (IGFs), platelet-derived growth factors (PDGF), transforming growth factor- β (TFG-β) and epidermal growth factor (EGF) 151. BMPs in particular have been investigated as bone regenerative therapies as they regulate key steps in the process of bone morphogenesis, such as mitosis, chemotaxis, cartilage induction, osteoblastic differentiation and bone formation 152–154 .
3.3.1 BMP (73-92)
P24 is a 24-amino acid peptide, SKIPKASSVPTELSAISTLYLDDD, derived from amino acids 73-92 of BMP-2 which has been shown to enhance ectopic bone formation in vivo within poly-lactic-co-glycolic (PLGA) implants 155, hydroxyapatite/recombinant collagen/poly-lactic acid scaffolds 156 and PLGA/polyethylene oxide-aspartic acid scaffolds 157. In vitro studies with MSCs cultured with osteogenic media containing P24 peptide also showed higher alkaline phosphatase activity than cells in osteogenic media alone 158. Saito et al. have also shown that the similar 20-amino acid BMP-2 (73-92) peptide, KIPKASSVPTELSAISTLYL, sustains prolonged ectopic bone formation in rat calf muscle 159, and also accelerates bone healing in a rat tibial unicortical defect 160 and a rabbit radial unicortical defect 161.
3.3.2 Osteopromotive Domain (OPD)
Lee et al. identified a peptide sequence derived from BMP-2(30-34), DWIVA, termed the osteopromotive domain, which strongly supports human MSC attachment and enhances the alkaline phosphatase activity of human BMSCs in vitro 162 .
4. Conclusions and Outlook
Bone remodeling and host reactions to implants are complex processes in which osteoblasts and osteoprogenitors play important roles. Because host responses to implants are significantly influenced by the protein signals encountered by the osteoprogenitor/osteoblast receptors on the implant surface, biomaterial research efforts have focused on engineering biological recognition into materials using ECM-mimetic peptides and protein fragments. While many of the peptides reviewed here have shown promising results in vitro, their efficacy at enhancing bone healing within defects and promoting implant osseointegration must be further demonstrated within clinically relevant animal models (Table 3). Comparing results between studies using different ECM-mimetic peptides is also hindered by variations in peptide deposition method (adsorption/immobilization), the surface on which it is deposited (non-fouling, surface roughness), and the surface or matrix density of the peptide. The most rigorous head-to-head comparison of different peptides in promoting osteoblastic differentiation would study covalently immobilized ECM peptides presented at an equimolar density on a non-fouling background. The improved bone formation and osseointegration outcomes seen with α2β1-specific GFOGER andα5β1-specific FNIII7-10 and FNIII9*-10 suggest that engineering ECM ligands with specificity to integrins or other receptors implicated in promoting osteogenesis may be a valuable orthopaedic biomaterial strategy. We expect that ECM-mimetic bone biomaterial strategies should upregulate osteoblast bone formation in vivo by specifically engaging integrins and other receptors that trigger signaling cascades which enhance adhesion, proliferation and differentiation. Therefore, in the case of ligands which promote bone formation but were not designed with integrin specificity in mind, it may be valuable to charactarize the cell receptors which the ligands engage using antibody blocking studies in order to determine the cellular mechanisms of their effect. Other important future challenges in ECM-mimetic bioadhesion include using synergistic mixed ligand materials to harness integrin cross-talk, combining ECM motifs with surface topography or roughness as well multivalent ligand presentation to promote integrin clustering and signaling. The use of multivalent ECM-derived peptides with nanoscale control of ligand presentation may be a particularly powerful strategy, as we have recently demonstrated that materials functionalized with self-assembled dimeric, trimeric and pentameric constructs of FNIII7-10 on a protein-resistant background enhance in vitro cell signaling and differentiation, and improve the mechanical fixation of titanium implants by up to 250% in vivo compared to the monomer 163. Another important ECM-derived peptide strategy includes using matrix metalloproteinase cleavable peptide sequences in combination with ECM peptide motifs to allow cell-mediated degradation and cell invasion into polymer gels with small pore sizes 164–165.
Acknowledgments
The authors acknowledge support from the Agency for Science, Technology and Research (A*STAR, Singapore), the National Institutes of Health, the National Science Foundation and the Arthritis Foundation.
References
- 1.United States Bone and Joint Decade: The Burden of Musculoskeletal Diseases in the United States. Rosemont,IL: American Academy of Orthopaedic Surgeons; 2008. [Google Scholar]
- 2.Soini J, Laine T, Pohjolainen T, Hurri H, Alaranta H. Spondylodesis augmented by transpedicular fixation in the treatment of olisthetic and degenerative conditions of the lumbar spine. Clin Orthop Relat Res. 1993;297:111–6. [PubMed] [Google Scholar]
- 3.Ohlin A, Karlsson M, Duppe H, Hasserius R, Redlund-Johnell I. Complications after transpedicular stabilization of the spine. A survivorship analysis of 163 cases. Spine (Phila Pa 1976) 1994;19(24):2774–9. doi: 10.1097/00007632-199412150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Pihlajamaki H, Myllynen P, Bostman O. Complications of transpedicular lumbosacral fixation for non-traumatic disorders. J Bone Joint Surg Br. 1997;79(2):183–9. doi: 10.1302/0301-620x.79b2.7224. [DOI] [PubMed] [Google Scholar]
- 5.Stromsoe K. Fracture fixation problems in osteoporosis. Injury. 2004;35(2):107–13. doi: 10.1016/j.injury.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Moroni A, Hoang-Kim A, Lio V, Giannini S. Current augmentation fixation techniques for the osteoporotic patient. Scand J Surg. 2006;95(2):103–9. doi: 10.1177/145749690609500205. [DOI] [PubMed] [Google Scholar]
- 7.Lindner T, Kanakaris NK, Marx B, Cockbain A, Kontakis G, Giannoudis PV. Fractures of the hip and osteoporosis: the role of bone substitutes. J Bone Joint Surg Br. 2009;91(3):294–303. doi: 10.1302/0301-620X.91B3.21273. [DOI] [PubMed] [Google Scholar]
- 8.Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop. 2002;395:44–52. doi: 10.1097/00003086-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84–A(3):454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 10.De Long WG, Jr, Einhorn TA, Koval K, McKee M, Smith W, Sanders R, Watson T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649–58. doi: 10.2106/JBJS.F.00465. [DOI] [PubMed] [Google Scholar]
- 11.Franceschi RT, Ge C, Xiao G, Roca H, Jiang D. Transcriptional regulation of osteoblasts. Ann N Y Acad Sci. 2007;1116:196–207. doi: 10.1196/annals.1402.081. [DOI] [PubMed] [Google Scholar]
- 12.Principles of Bone Biology. 2002 [Google Scholar]
- 13.Branemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50(3):399–410. doi: 10.1016/s0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson KG, Karrholm J. RSA in the assessment of aseptic loosening. J Bone Joint Surg Br. 1996;78(1):1–3. [PubMed] [Google Scholar]
- 15.Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87(1–2):57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 16.Molecular biology of the cell. New York: Garland Science; 2002. [Google Scholar]
- 17.Kumbar SG, James R, Nukavarapu SP, Laurencin CT. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater. 2008;3(3):034002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- 18.Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro-and nanoscale to control cell function. Angew Chem Int Ed Engl. 2009;48(30):5406–15. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christenson EM, Anseth KS, van den Beucken JJ, Chan CK, Ercan B, Jansen JA, Laurencin CT, Li WJ, Murugan R, Nair LS, et al. Nanobiomaterial applications in orthopedics. J Orthop Res. 2007;25(1):11–22. doi: 10.1002/jor.20305. [DOI] [PubMed] [Google Scholar]
- 20.Dumbleton J, Manley MT. Hydroxyapatite-coated prostheses in total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(11):2526–40. doi: 10.2106/00004623-200411000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Aplin AE, Hogan BP, Tomeu J, Juliano RL. Cell adhesion differentially regulates the nucleocytoplasmic distribution of active MAP kinases. J Cell Sci. 2002;115(13):2781–90. doi: 10.1242/jcs.115.13.2781. [DOI] [PubMed] [Google Scholar]
- 22.Gelse K, Poschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531–46. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Robey PG, John PB, Lawrence GR, Gideon AR. Principles of Bone Biology. 2. San Diego: Academic Press; 2002. Bone Matrix Proteoglycans and Glycoproteins; pp. 225–237. [Google Scholar]
- 24.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 25.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 26.Petit V, Thiery JP. Focal adhesions: structure and dynamics. Biol Cell. 2000;92(7):477–94. doi: 10.1016/s0248-4900(00)01101-1. [DOI] [PubMed] [Google Scholar]
- 27.Bourdoulous S, Orend G, MacKenna DA, Pasqualini R, Ruoslahti E. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J Cell Biol. 1998;143(1):267–76. doi: 10.1083/jcb.143.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 29.Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. Journal of Bone and Mineral Research. 1997;12(8):1189–1197. doi: 10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- 30.Bennett JH, Carter DH, Alavi AL, Beresford JN, Walsh S. Patterns of integrin expression in a human mandibular explant model of osteoblast differentiation. Arch Oral Biol. 2001;46(3):229–38. doi: 10.1016/s0003-9969(00)00114-x. [DOI] [PubMed] [Google Scholar]
- 31.Grzesik WJ, Robey PG. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J Bone Miner Res. 1994;9(4):487–96. doi: 10.1002/jbmr.5650090408. [DOI] [PubMed] [Google Scholar]
- 32.Clover J, Dodds RA, Gowen M. Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci. 1992;103 ( Pt 1):267–71. doi: 10.1242/jcs.103.1.267. [DOI] [PubMed] [Google Scholar]
- 33.Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. J Bone Miner Res. 1993;8(5):527–33. doi: 10.1002/jbmr.5650080503. [DOI] [PubMed] [Google Scholar]
- 34.Ganta DR, McCarthy MB, Gronowicz GA. Ascorbic acid alters collagen integrins in bone culture. Endocrinology. 1997;138(9):3606–12. doi: 10.1210/endo.138.9.5367. [DOI] [PubMed] [Google Scholar]
- 35.Saito T, Albelda SM, Brighton CT. Identification of integrin receptors on cultured human bone cells. J Orthop Res. 1994;12(3):384–94. doi: 10.1002/jor.1100120311. [DOI] [PubMed] [Google Scholar]
- 36.Yu YM, Becvar R, Yamada Y, Reddi AH. Changes in the gene expression of collagens, fibronectin, integrin and proteoglycans during matrix-induced bone morphogenesis. Biochem Biophys Res Commun. 1991;177(1):427–32. doi: 10.1016/0006-291x(91)92001-z. [DOI] [PubMed] [Google Scholar]
- 37.Brighton CT, Albelda SM. Identification of integrin cell-substratum adhesion receptors on cultured rat bone cells. J Orthop Res. 1992;10(6):766–73. doi: 10.1002/jor.1100100604. [DOI] [PubMed] [Google Scholar]
- 38.Castoldi M, Pistone M, Caruso C, Puddu A, Filanti C, Piccini D, Tacchetti C, Manduca P. Osteoblastic cells from rat long bone. II: Adhesion to substrata and integrin expression in primary and propagated cultures. Cell Biol Int. 1997;21(1):7–16. doi: 10.1006/cbir.1996.0110. [DOI] [PubMed] [Google Scholar]
- 39.Pistone M, Sanguineti C, Federici A, Sanguineti F, Defilippi P, Santolini F, Querze G, Marchisio PC, Manduca P. Integrin synthesis and utilization in cultured human osteoblasts. Cell Biol Int. 1996;20(7):471–9. doi: 10.1006/cbir.1996.0062. [DOI] [PubMed] [Google Scholar]
- 40.Bruder SP, Jaiswal N, Ricalton NS, Mosca JD, Kraus KH, Kadiyala S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res. 1998;355(Suppl):S247–56. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- 41.Moursi AM, Globus RK, Damsky CH. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J Cell Sci. 1997;110 ( Pt 18):2187–96. doi: 10.1242/jcs.110.18.2187. [DOI] [PubMed] [Google Scholar]
- 42.Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001;28(2):174–81. doi: 10.1016/s8756-3282(00)00424-5. [DOI] [PubMed] [Google Scholar]
- 43.Athanassiou G, Deligianni D. Adhesion strength of individual human bone marrow cells to fibronectin. Integrin beta1-mediated adhesion. J Mater Sci Mater Med. 2001;12(10–12):965–70. doi: 10.1023/a:1012809115479. [DOI] [PubMed] [Google Scholar]
- 44.Gronowicz GA, McCarthy MB. Glucocorticoids inhibit the attachment of osteoblasts to bone extracellular matrix proteins and decrease beta 1-integrin levels. Endocrinology. 1995;136(2):598–608. doi: 10.1210/endo.136.2.7530648. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerman D, Jin F, Leboy P, Hardy S, Damsky C. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev Biol. 2000;220(1):2–15. doi: 10.1006/dbio.2000.9633. [DOI] [PubMed] [Google Scholar]
- 46.Globus RK, Amblard D, Nishimura Y, Iwaniec UT, Kim JB, Almeida EA, Damsky CD, Wronski TJ, van der Meulen MC. Skeletal phenotype of growing transgenic mice that express a function-perturbing form of beta1 integrin in osteoblasts. Calcif Tissue Int. 2005;76(1):39–49. doi: 10.1007/s00223-004-0309-4. [DOI] [PubMed] [Google Scholar]
- 47.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha 2 beta 1 integrin interaction. Journal of Cellular Physiology. 2000;184(2):207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 48.Jikko A, Harris SE, Chen D, Mendrick DL, Damsky CH. Collagen integrin receptors regulate early osteoblast differentiation induced by BMP-2. J Bone Miner Res. 1999;14(7):1075–83. doi: 10.1359/jbmr.1999.14.7.1075. [DOI] [PubMed] [Google Scholar]
- 49.Mizuno M, Kuboki Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J Biochem. 2001;129(1):133–8. doi: 10.1093/oxfordjournals.jbchem.a002824. [DOI] [PubMed] [Google Scholar]
- 50.Suzawa M, Tamura Y, Fukumoto S, Miyazono K, Fujita T, Kato S, Takeuchi Y. Stimulation of Smad1 transcriptional activity by Ras-extracellular signal-regulated kinase pathway: a possible mechanism for collagen-dependent osteoblastic differentiation. J Bone Miner Res. 2002;17(2):240–8. doi: 10.1359/jbmr.2002.17.2.240. [DOI] [PubMed] [Google Scholar]
- 51.Takeuchi Y, Suzawa M, Kikuchi T, Nishida E, Fujita T, Matsumoto T. Differentiation and transforming growth factor-beta receptor down-regulation by collagen-alpha2beta1 integrin interaction is mediated by focal adhesion kinase and its downstream signals in murine osteoblastic cells. J Biol Chem. 1997;272(46):29309–16. doi: 10.1074/jbc.272.46.29309. [DOI] [PubMed] [Google Scholar]
- 52.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273(49):32988–94. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 53.Tamura Y, Takeuchi Y, Suzawa M, Fukumoto S, Kato M, Miyazono K, Fujita T. Focal adhesion kinase activity is required for bone morphogenetic protein--Smad1 signaling and osteoblastic differentiation in murine MC3T3-E1 cells. J Bone Miner Res. 2001;16(10):1772–9. doi: 10.1359/jbmr.2001.16.10.1772. [DOI] [PubMed] [Google Scholar]
- 54.Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275(6):4453–9. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- 55.Schneider GB, Zaharias R, Stanford C. Osteoblast integrin adhesion and signaling regulate mineralization. J Dent Res. 2001;80(6):1540–4. doi: 10.1177/00220345010800061201. [DOI] [PubMed] [Google Scholar]
- 56.Van der Velde-Zimmermann D, Verdaasdonk MA, Rademakers LH, De Weger RA, Van den Tweel JG, Joling P. Fibronectin distribution in human bone marrow stroma: matrix assembly and tumor cell adhesion via alpha5 beta1 integrin. Exp Cell Res. 1997;230(1):111–20. doi: 10.1006/excr.1996.3405. [DOI] [PubMed] [Google Scholar]
- 57.Globus RK, Doty SB, Lull JC, Holmuhamedov E, Humphries MJ, Damsky CH. Fibronectin is a survival factor for differentiated osteoblasts. J Cell Sci. 1998;111 ( Pt 10):1385–93. doi: 10.1242/jcs.111.10.1385. [DOI] [PubMed] [Google Scholar]
- 58.Kaabeche K, Guenou H, Bouvard D, Didelot N, Listrat A, Marie PJ. Cbl-mediated ubiquitination of alpha5 integrin subunit mediates fibronectin-dependent osteoblast detachment and apoptosis induced by FGFR2 activation. J Cell Sci. 2005;118(Pt 6):1223–32. doi: 10.1242/jcs.01679. [DOI] [PubMed] [Google Scholar]
- 59.Salter DM, Robb JE, Wright MO. Electrophysiological responses of human bone cells to mechanical stimulation: evidence for specific integrin function in mechanotransduction. J Bone Miner Res. 1997;12(7):1133–41. doi: 10.1359/jbmr.1997.12.7.1133. [DOI] [PubMed] [Google Scholar]
- 60.Moursi AM, Damsky CH, Lull J, Zimmerman D, Doty SB, Aota S, Globus RK. Fibronectin regulates calvarial osteoblast differentiation. J Cell Sci. 1996;109 ( Pt 6):1369–80. doi: 10.1242/jcs.109.6.1369. [DOI] [PubMed] [Google Scholar]
- 61.Keselowsky BG, Wang L, Schwartz Z, García AJ, Boyan BD. Integrin alpha(5) controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness independent manner. J Biomed Mater Res A. 2007;80(3):700–10. doi: 10.1002/jbm.a.30898. [DOI] [PubMed] [Google Scholar]
- 62.Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A. 2005;102(17):5953–7. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamidouche Z, Fromigue O, Ringe J, Haupl T, Vaudin P, Pages JC, Srouji S, Livne E, Marie PJ. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc Natl Acad Sci U S A. 2009;106(44):18587–91. doi: 10.1073/pnas.0812334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA, Barker TH. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30(6):1089–97. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ekholm E, Hankenson KD, Uusitalo H, Hiltunen A, Gardner H, Heino J, Penttinen R. Diminished callus size and cartilage synthesis in alpha 1 beta 1 integrin-deficient mice during bone fracture healing. Am J Pathol. 2002;160(5):1779–85. doi: 10.1016/s0002-9440(10)61124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng SL, Lai CF, Blystone SD, Avioli LV. Bone mineralization and osteoblast differentiation are negatively modulated by integrin alpha(v)beta3. J Bone Miner Res. 2001;16(2):277–88. doi: 10.1359/jbmr.2001.16.2.277. [DOI] [PubMed] [Google Scholar]
- 67.Hu D, Lu C, Sapozhnikova A, Barnett M, Sparrey C, Miclau T, Marcucio RS. Absence of beta3 integrin accelerates early skeletal repair. J Orthop Res. 28(1):32–7. doi: 10.1002/jor.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nesbitt S, Nesbit A, Helfrich M, Horton M. Biochemical characterization of human osteoclast integrins. Osteoclasts express alpha v beta 3, alpha 2 beta 1, and alpha v beta 1 integrins. J Biol Chem. 1993;268(22):16737–45. [PubMed] [Google Scholar]
- 69.Horton MA, Dorey EL, Nesbitt SA, Samanen J, Ali FE, Stadel JM, Nichols A, Greig R, Helfrich MH. Modulation of vitronectin receptor-mediated osteoclast adhesion by Arg-Gly-Asp peptide analogs: a structure-function analysis. J Bone Miner Res. 1993;8(2):239–47. doi: 10.1002/jbmr.5650080215. [DOI] [PubMed] [Google Scholar]
- 70.McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105(4):433–40. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakamura I, Pilkington MF, Lakkakorpi PT, Lipfert L, Sims SM, Dixon SJ, Rodan GA, Duong LT. Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J Cell Sci. 1999;112 ( Pt 22):3985–93. doi: 10.1242/jcs.112.22.3985. [DOI] [PubMed] [Google Scholar]
- 72.Morra M, Cassinelli C, Meda L, Fini M, Giavaresi G, Giardino R. Surface analysis and effects on interfacial bone microhardness of collagen-coated titanium implants: a rabbit model. Int J Oral Maxillofac Implants. 2005;20(1):23–30. [PubMed] [Google Scholar]
- 73.Schliephake H, Aref A, Scharnweber D, Bierbaum S, Roessler S, Sewing A. Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation. Clin Oral Implants Res. 2005;16(5):563–9. doi: 10.1111/j.1600-0501.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 74.Svehla M, Morberg P, Bruce W, Walsh WR. No effect of a type I collagen gel coating in uncemented implant fixation. J Biomed Mater Res B Appl Biomater. 2005;74(1):423–8. doi: 10.1002/jbm.b.30256. [DOI] [PubMed] [Google Scholar]
- 75.Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006;27(32):5561–71. doi: 10.1016/j.biomaterials.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 76.Liu X, Li X, Fan Y, Zhang G, Li D, Dong W, Sha Z, Yu X, Feng Q, Cui F, et al. Repairing goat tibia segmental bone defect using scaffold cultured with mesenchymal stem cells. J Biomed Mater Res B Appl Biomater. doi: 10.1002/jbm.b.31622. [DOI] [PubMed] [Google Scholar]
- 77.Caiazza S, Colangelo P, Bedini R, Formisano G, De Angelis G, Barrucci S. Evaluation of guided bone regeneration in rabbit femur using collagen membranes. Implant Dent. 2000;9(3):219–25. doi: 10.1097/00008505-200009030-00007. [DOI] [PubMed] [Google Scholar]
- 78.d'Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 79.Ben-Ari A, Rivkin R, Frishman M, Gaberman E, Levdansky L, Gorodetsky R. Isolation and implantation of bone marrow-derived mesenchymal stem cells with fibrin micro beads to repair a critical-size bone defect in mice. Tissue Eng Part A. 2009;15(9):2537–46. doi: 10.1089/ten.tea.2008.0567. [DOI] [PubMed] [Google Scholar]
- 80.Kim SJ, Jang JD, Lee SK. Treatment of long tubular bone defect of rabbit using autologous cultured osteoblasts mixed with fibrin. Cytotechnology. 2007;54(2):115–20. doi: 10.1007/s10616-007-9084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karp JM, Sarraf F, Shoichet MS, Davies JE. Fibrin-filled scaffolds for bone-tissue engineering: An in vivo study. J Biomed Mater Res A. 2004;71(1):162–71. doi: 10.1002/jbm.a.30147. [DOI] [PubMed] [Google Scholar]
- 82.Perka C, Schultz O, Spitzer RS, Lindenhayn K, Burmester GR, Sittinger M. Segmental bone repair by tissue-engineered periosteal cell transplants with bioresorbable fleece and fibrin scaffolds in rabbits. Biomaterials. 2000;21(11):1145–53. doi: 10.1016/s0142-9612(99)00280-x. [DOI] [PubMed] [Google Scholar]
- 83.Solchaga LA, Dennis JE, Goldberg VM, Caplan AI. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J Orthop Res. 1999;17(2):205–13. doi: 10.1002/jor.1100170209. [DOI] [PubMed] [Google Scholar]
- 84.Paderni S, Terzi S, Amendola L. Major bone defect treatment with an osteoconductive bone substitute. Chir Organi Mov. 2009;93(2):89–96. doi: 10.1007/s12306-009-0028-0. [DOI] [PubMed] [Google Scholar]
- 85.Barros RR, Novaes AB, Jr, Papalexiou V, Souza SL, Taba M, Jr, Palioto DB, Grisi MF. Effect of biofunctionalized implant surface on osseointegration: a histomorphometric study in dogs. Braz Dent J. 2009;20(2):91–8. doi: 10.1590/s0103-64402009000200001. [DOI] [PubMed] [Google Scholar]
- 86.Lin H, Xu H, Zhang X, de Groot K. Tensile tests of interface between bone and plasma-sprayed HA coating-titanium implant. J Biomed Mater Res. 1998;43(2):113–22. doi: 10.1002/(sici)1097-4636(199822)43:2<113::aid-jbm5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 87.Kurkalli BG, Gurevitch O, Sosnik A, Cohn D, Slavin S. Repair of bone defect using bone marrow cells and demineralized bone matrix supplemented with polymeric materials. Curr Stem Cell Res Ther. 5(1):49–56. doi: 10.2174/157488810790442831. [DOI] [PubMed] [Google Scholar]
- 88.Suckow MA, Voytik-Harbin SL, Terril LA, Badylak SF. Enhanced bone regeneration using porcine small intestinal submucosa. J Invest Surg. 1999;12(5):277–87. doi: 10.1080/089419399272395. [DOI] [PubMed] [Google Scholar]
- 89.Graf HL, Stoeva S, Armbruster FP, Neuhaus J, Hilbig H. Effect of bone sialoprotein and collagen coating on cell attachment to TICER and pure titanium implant surfaces. Int J Oral Maxillofac Surg. 2008;37(7):634–40. doi: 10.1016/j.ijom.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 90.Shakesheff K, Cannizzaro S, Langer R. Creating biomimetic micro-environments with synthetic polymer-peptide hybrid molecules. J Biomater Sci Polym Ed. 1998;9(5):507–18. doi: 10.1163/156856298x00596. [DOI] [PubMed] [Google Scholar]
- 91.Emsley J, Knight CG, Farndale RW, Barnes MJ. Structure of the integrin alpha2beta1-binding collagen peptide. J Mol Biol. 2004;335(4):1019–28. doi: 10.1016/j.jmb.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 92.Knight CG, Morton LF, Onley DJ, Peachey AR, Messent AJ, Smethurst PA, Tuckwell DS, Farndale RW, Barnes MJ. Identification in collagen type I of an integrin alpha2 beta1-binding site containing an essential GER sequence. J Biol Chem. 1998;273(50):33287–94. doi: 10.1074/jbc.273.50.33287. [DOI] [PubMed] [Google Scholar]
- 93.Leahy DJ, Aukhil I, Erickson HP. 2.0 A crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84(1):155–64. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 94.Aota S, Nomizu M, Yamada KM. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994;269(40):24756–61. [PubMed] [Google Scholar]
- 95.Humphries MJ, Akiyama SK, Komoriya A, Olden K, Yamada KM. Identification of an alternatively spliced site in human plasma fibronectin that mediates cell type-specific adhesion. J Cell Biol. 1986;103(6 Pt 2):2637–47. doi: 10.1083/jcb.103.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Komoriya A, Green LJ, Mervic M, Yamada SS, Yamada KM, Humphries MJ. The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J Biol Chem. 1991;266(23):15075–9. [PubMed] [Google Scholar]
- 97.Petrie TA, Capadona JR, Reyes CD, Garcia AJ. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27(31):5459–70. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 98.Barber TA, Ho JE, De Ranieri A, Virdi AS, Sumner DR, Healy KE. Peri-implant bone formation and implant integration strength of peptide-modified p(AAM-co-EG/AAC) interpenetrating polymer network-coated titanium implants. J Biomed Mater Res A. 2007;80(2):306–20. doi: 10.1002/jbm.a.30927. [DOI] [PubMed] [Google Scholar]
- 99.Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, Guldberg RE, Garcia AJ. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials. 31(9):2574–2582. doi: 10.1016/j.biomaterials.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thorwarth M, Schultze-Mosgau S, Wehrhan F, Kessler P, Srour S, Wiltfang J, Andreas Schlegel K. Bioactivation of an anorganic bone matrix by P-15 peptide for the promotion of early bone formation. Biomaterials. 2005;26(28):5648–57. doi: 10.1016/j.biomaterials.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 101.Pytela R, Pierschbacher MD, Argraves S, Suzuki S, Ruoslahti E. Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymol. 1987;144:475–89. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- 102.Redick SD, Settles DL, Briscoe G, Erickson HP. Defining fibronectin's cell adhesion synergy site by site-directed mutagenesis. J Cell Biol. 2000;149(2):521–7. doi: 10.1083/jcb.149.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ho JE, Barber TA, Virdi AS, Sumner DR, Healy KE. The effect of enzymatically degradable IPN coatings on peri-implant bone formation and implant fixation. J Biomed Mater Res A. 2007;81(3):720–7. doi: 10.1002/jbm.a.31008. [DOI] [PubMed] [Google Scholar]
- 104.Ferris DM, Moodie GD, Dimond PM, Gioranni CW, Ehrlich MG, Valentini RF. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials. 1999;20(23–24):2323–31. doi: 10.1016/s0142-9612(99)00161-1. [DOI] [PubMed] [Google Scholar]
- 105.Hennessy KM, Clem WC, Phipps MC, Sawyer AA, Shaikh FM, Bellis SL. The effect of RGD peptides on osseointegration of hydroxyapatite biomaterials. Biomaterials. 2008;29(21):3075–83. doi: 10.1016/j.biomaterials.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elmengaard B, Bechtold JE, Soballe K. In vivo study of the effect of RGD treatment on bone ongrowth on press-fit titanium alloy implants. Biomaterials. 2005;26(17):3521–3526. doi: 10.1016/j.biomaterials.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 107.Elmengaard B, Bechtold JE, Soballe K. In vivo effects of RGD-coated titanium implants inserted in two bone-gap models. J Biomed Mater Res A. 2005;75(2):249–255. doi: 10.1002/jbm.a.30301. [DOI] [PubMed] [Google Scholar]
- 108.Schliephake H, Scharnweber D, Dard M, Rossler S, Sewing A, Meyer J, Hoogestraat D. Effect of RGD peptide coating of titanium implants on periimplant bone formation in the alveolar crest. An experimental pilot study in dogs. Clin Oral Implants Res. 2002;13(3):312–9. doi: 10.1034/j.1600-0501.2002.130312.x. [DOI] [PubMed] [Google Scholar]
- 109.Barber TA, Ho JE, De Ranieri A, Virdi AS, Sumner DR, Healy KE. Peri-implant bone formation and implant integration strength of peptide-modified p(AAM-co-EG/AAC) interpenetrating polymer network-coated titanium implants. J Biomed Mater Res A. 2007;80(2):306–320. doi: 10.1002/jbm.a.30927. [DOI] [PubMed] [Google Scholar]
- 110.Petrie TA, Raynor JE, Reyes CD, Burns KL, Collard DM, Garcia AJ. The effect of integrin-specific bioactive coatings on tissue healing and implant osseointegration. Biomaterials. 2008;29(19):2849–57. doi: 10.1016/j.biomaterials.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miljkovic ND, Cooper GM, Hott SL, Disalle BF, Gawalt ES, Smith DM, McGowan K, Marra KG. Calcium aluminate, RGD-modified calcium aluminate, and beta-tricalcium phosphate implants in a calvarial defect. J Craniofac Surg. 2009;20(5):1538–43. doi: 10.1097/SCS.0b013e3181b09c13. [DOI] [PubMed] [Google Scholar]
- 112.Garcia AJ, Schwarzbauer JE, Boettiger D. Distinct activation states of alpha5beta1 integrin show differential binding to RGD and synergy domains of fibronectin. Biochemistry. 2002;41(29):9063–9. doi: 10.1021/bi025752f. [DOI] [PubMed] [Google Scholar]
- 113.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309(5963):30–3. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 114.Petrie TA, Reyes CD, Burns KL, Garcia AJ. Simple application of fibronectin-mimetic coating enhances osseointegration of titanium implants. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Benoit DS, Anseth KS. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005;26(25):5209–20. doi: 10.1016/j.biomaterials.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 116.Kim TI, Jang JH, Lee YM, Ryu IC, Chung CP, Han SB, Choi SM, Ku Y. Design and biological activity of synthetic oligopeptides with Pro-His-Ser-Arg-Asn (PHSRN) and Arg-Gly-Asp (RGD) motifs for human osteoblast-like cell (MG-63) adhesion. Biotechnology Letters. 2002;24(24):2029–2033. [Google Scholar]
- 117.Healy KE, Rezania A, Stile RA. Designing biomaterials to direct biological responses. Ann N Y Acad Sci. 1999;875:24–35. doi: 10.1111/j.1749-6632.1999.tb08491.x. [DOI] [PubMed] [Google Scholar]
- 118.Rezania A, Healy KE. Integrin subunits responsible for adhesion of human osteoblast-like cells to biomimetic peptide surfaces. J Orthop Res. 1999;17(4):615–23. doi: 10.1002/jor.1100170423. [DOI] [PubMed] [Google Scholar]
- 119.Rezania A, Healy KE. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol Prog. 1999;15(1):19–32. doi: 10.1021/bp980083b. [DOI] [PubMed] [Google Scholar]
- 120.Stile RA, Healy KE. Thermo-responsive peptide-modified hydrogels for tissue regeneration. Biomacromolecules. 2001;2(1):185–94. doi: 10.1021/bm0000945. [DOI] [PubMed] [Google Scholar]
- 121.Schuler M, Hamilton DW, Kunzler TP, Sprecher CM, de Wild M, Brunette DM, Textor M, Tosatti SG. Comparison of the response of cultured osteoblasts and osteoblasts outgrown from rat calvarial bone chips to nonfouling KRSR and FHRRIKA-peptide modified rough titanium surfaces. J Biomed Mater Res B Appl Biomater. 2009;91(2):517–27. doi: 10.1002/jbm.b.31425. [DOI] [PubMed] [Google Scholar]
- 122.Dee KC, Andersen TT, Bizios R. Design and function of novel osteoblast-adhesive peptides for chemical modification of biomaterials. J Biomed Mater Res. 1998;40(3):371–7. doi: 10.1002/(sici)1097-4636(19980605)40:3<371::aid-jbm5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 123.Dettin M, Conconi MT, Gambaretto R, Pasquato A, Folin M, Di Bello C, Parnigotto PP. Novel osteoblast-adhesive peptides for dental/orthopedic biomaterials. J Biomed Mater Res. 2002;60(3):466–71. doi: 10.1002/jbm.10066. [DOI] [PubMed] [Google Scholar]
- 124.Hasenbein ME, Andersen TT, Bizios R. Micropatterned surfaces modified with select peptides promote exclusive interactions with osteoblasts. Biomaterials. 2002;23(19):3937–42. doi: 10.1016/s0142-9612(02)00129-1. [DOI] [PubMed] [Google Scholar]
- 125.Nelson M, Balasundaram G, Webster TJ. Increased osteoblast adhesion on nanoparticulate crystalline hydroxyapatite functionalized with KRSR. Int J Nanomedicine. 2006;1(3):339–49. [PMC free article] [PubMed] [Google Scholar]
- 126.Balasundaram G, Webster TJ. Increased osteoblast adhesion on nanograined Ti modified with KRSR. J Biomed Mater Res A. 2007;80(3):602–11. doi: 10.1002/jbm.a.30954. [DOI] [PubMed] [Google Scholar]
- 127.Rapuano BE, Wu C, MacDonald DE. Osteoblast-like cell adhesion to bone sialoprotein peptides. J Orthop Res. 2004;22(2):353–61. doi: 10.1016/S0736-0266(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 128.Dettin M, Bagno A, Morpurgo M, Cacchioli A, Conconi MT, Di Bello C, Gabbi C, Gambaretto R, Parnigotto PP, Pizzinato S, et al. Evaluation of silicon dioxide-based coating enriched with bioactive peptides mapped on human vitronectin and fibronectin: in vitro and in vivo assays. Tissue Eng. 2006;12(12):3509–23. doi: 10.1089/ten.2006.12.3509. [DOI] [PubMed] [Google Scholar]
- 129.Bagno A, Piovan A, Dettin M, Chiarion A, Brun P, Gambaretto R, Fontana G, Di Bello C, Palu G, Castagliuolo I. Human osteoblast-like cell adhesion on titanium substrates covalently functionalized with synthetic peptides. Bone. 2007;40(3):693–9. doi: 10.1016/j.bone.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 130.Cacchioli A, Ravanetti F, Bagno A, Dettin M, Gabbi C. Human Vitronectin-Derived Peptide Covalently Grafted onto Titanium Surface Improves Osteogenic Activity: A Pilot In Vivo Study on Rabbits. Tissue Eng Part A. 2009;15(10):2917–26. doi: 10.1089/ten.TEA.2008.0542. [DOI] [PubMed] [Google Scholar]
- 131.Dettin M, Bagno A, Gambaretto R, Iucci G, Conconi MT, Tuccitto N, Menti AM, Grandi C, Di Bello C, Licciardello A, et al. Covalent surface modification of titanium oxide with different adhesive peptides: surface characterization and osteoblast-like cell adhesion. J Biomed Mater Res A. 2009;90(1):35–45. doi: 10.1002/jbm.a.32064. [DOI] [PubMed] [Google Scholar]
- 132.Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials. 2004;25(5):895–906. doi: 10.1016/s0142-9612(03)00602-1. [DOI] [PubMed] [Google Scholar]
- 133.Kim HE, Kim HW, Jang JH. Identification and characterization of a novel heparin-binding peptide for promoting osteoblast adhesion and proliferation by screening an Escherichia coli cell surface display peptide library. J Pept Sci. 2009;15(1):43–7. doi: 10.1002/psc.1098. [DOI] [PubMed] [Google Scholar]
- 134.Morton LF, Peachey AR, Zijenah LS, Goodall AH, Humphries MJ, Barnes MJ. Conformation-dependent platelet adhesion to collagen involving integrin alpha 2 beta 1-mediated and other mechanisms: multiple alpha 2 beta 1-recognition sites in collagen type I. Biochem J. 1994;299 ( Pt 3):791–7. doi: 10.1042/bj2990791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Knight CG, Morton LF, Peachey AR, Tuckwell DS, Farndale RW, Barnes MJ. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J Biol Chem. 2000;275(1):35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 136.Reyes CD, Garcia AJ. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. J Biomed Mater Res A. 2003;65(4):511–23. doi: 10.1002/jbm.a.10550. [DOI] [PubMed] [Google Scholar]
- 137.Reyes CD, Garcia AJ. Alpha2beta1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. J Biomed Mater Res A. 2004;69(4):591–600. doi: 10.1002/jbm.a.30034. [DOI] [PubMed] [Google Scholar]
- 138.Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28(21):3228–35. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, Guldberg RE, Garcia AJ. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials. 2010;31(9):2574–82. doi: 10.1016/j.biomaterials.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hennessy KM, Pollot BE, Clem WC, Phipps MC, Sawyer AA, Culpepper BK, Bellis SL. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials. 2009;30(10):1898–909. doi: 10.1016/j.biomaterials.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Staatz WD, Fok KF, Zutter MM, Adams SP, Rodriguez BA, Santoro SA. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991;266(12):7363–7. [PubMed] [Google Scholar]
- 142.McCann TJ, Mason WT, Meikle MC, McDonald F. A collagen peptide motif activates tyrosine kinase-dependent calcium signalling pathways in human osteoblast-like cells. Matrix Biol. 1997;16(5):273–83. doi: 10.1016/s0945-053x(97)90015-9. [DOI] [PubMed] [Google Scholar]
- 143.Harbers GM, Healy KE. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. J Biomed Mater Res A. 2005;75(4):855–69. doi: 10.1002/jbm.a.30482. [DOI] [PubMed] [Google Scholar]
- 144.Bhatnagar RS, Qian JJ, Gough CA. The role in cell binding of a beta-bend within the triple helical region in collagen alpha 1 (I) chain: structural and biological evidence for conformational tautomerism on fiber surface. J Biomol Struct Dyn. 1997;14(5):547–60. doi: 10.1080/07391102.1997.10508155. [DOI] [PubMed] [Google Scholar]
- 145.Bhatnagar RS, Qian JJ, Wedrychowska A, Sadeghi M, Wu YM, Smith N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng. 1999;5(1):53–65. doi: 10.1089/ten.1999.5.53. [DOI] [PubMed] [Google Scholar]
- 146.Nguyen H, Qian JJ, Bhatnagar RS, Li S. Enhanced cell attachment and osteoblastic activity by P-15 peptide-coated matrix in hydrogels. Biochem Biophys Res Commun. 2003;311(1):179–86. doi: 10.1016/j.bbrc.2003.09.192. [DOI] [PubMed] [Google Scholar]
- 147.Artzi Z, Kozlovsky A, Nemcovsky CE, Moses O, Tal H, Rohrer MD, Prasad HS, Weinreb M. Histomorphometric evaluation of natural mineral combined with a synthetic cell-binding peptide (P-15) in critical-size defects in the rat calvaria. Int J Oral Maxillofac Implants. 2008;23(6):1063–70. [PubMed] [Google Scholar]
- 148.Radhakrishnan S, Anusuya CN. Comparative clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) and open flap debridement (DEBR) in human periodontal osseous defects: a 6 month pilot study. J Int Acad Periodontol. 2004;6(3):101–7. [PubMed] [Google Scholar]
- 149.Bhongade ML, Tiwari IR. A comparative evaluation of the effectiveness of an anorganic bone matrix/cell binding peptide with an open flap debridement in human infrabony defects: a clinical and radiographic study. J Contemp Dent Pract. 2007;8(6):25–34. [PubMed] [Google Scholar]
- 150.Gomar F, Orozco R, Villar JL, Arrizabalaga F. P-15 small peptide bone graft substitute in the treatment of non-unions and delayed union. A pilot clinical trial Int Orthop. 2007;31(1):93–9. doi: 10.1007/s00264-006-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sikavitsas VI, Temenoff JS, Mikos AG. Biomaterials and bone mechanotransduction. Biomaterials. 2001;22(19):2581–93. doi: 10.1016/s0142-9612(01)00002-3. [DOI] [PubMed] [Google Scholar]
- 152.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16(3):247–52. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 153.Cunningham NS, Paralkar V, Reddi AH. Osteogenin and recombinant bone morphogenetic protein 2B are chemotactic for human monocytes and stimulate transforming growth factor beta 1 mRNA expression. Proc Natl Acad Sci U S A. 1992;89(24):11740–4. doi: 10.1073/pnas.89.24.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77(8):626–31. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 155.Duan Z, Zheng Q, Guo X, Yuan Q, Chen S. Experimental research on ectopic osteogenesis of BMP2-derived peptide P24 combined with PLGA copolymers. J Huazhong Univ Sci Technolog Med Sci. 2007;27(2):179–82. doi: 10.1007/s11596-007-0219-6. [DOI] [PubMed] [Google Scholar]
- 156.Wu B, Zheng Q, Guo X, Wu Y, Wang Y, Cui F. Preparation and ectopic osteogenesis in vivo of scaffold based on mineralized recombinant human-like collagen loaded with synthetic BMP-2-derived peptide. Biomed Mater. 2008;3(4):044111. doi: 10.1088/1748-6041/3/4/044111. [DOI] [PubMed] [Google Scholar]
- 157.Lin ZY, Duan ZX, Guo XD, Li JF, Lu HW, Zheng QX, Quan DP, Yang SH. Bone induction by biomimetic PLGA-(PEG-ASP)n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. J Control Release. 2010 doi: 10.1016/j.jconrel.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 158.Niu X, Feng Q, Wang M, Guo X, Zheng Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J Control Release. 2009;134(2):111–7. doi: 10.1016/j.jconrel.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 159.Saito A, Suzuki Y, Ogata S, Ohtsuki C, Tanihara M. Prolonged ectopic calcification induced by BMP-2-derived synthetic peptide. J Biomed Mater Res A. 2004;70(1):115–21. doi: 10.1002/jbm.a.30071. [DOI] [PubMed] [Google Scholar]
- 160.Saito A, Suzuki Y, Ogata S, Ohtsuki C, Tanihara M. Accelerated bone repair with the use of a synthetic BMP-2-derived peptide and bone-marrow stromal cells. J Biomed Mater Res A. 2005;72(1):77–82. doi: 10.1002/jbm.a.30208. [DOI] [PubMed] [Google Scholar]
- 161.Saito A, Suzuki Y, Kitamura M, Ogata S, Yoshihara Y, Masuda S, Ohtsuki C, Tanihara M. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an alpha-tricalcium phosphate scaffold. J Biomed Mater Res A. 2006;77(4):700–6. doi: 10.1002/jbm.a.30662. [DOI] [PubMed] [Google Scholar]
- 162.Lee JY, Choo JE, Choi YS, Suh JS, Lee SJ, Chung CP, Park YJ. Osteoblastic differentiation of human bone marrow stromal cells in self-assembled BMP-2 receptor-binding peptide-amphiphiles. Biomaterials. 2009;30(21):3532–41. doi: 10.1016/j.biomaterials.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 163.Petrie TA, Raynor JE, Dumbauld DW, Lee TT, Jagtap S, Templeman KL, Collard DM, Garcia AJ. Multivalent integrin-specific ligands enhance tissue healing and biomaterial integration. Sci Transl Med. 2010;2:45ra60. doi: 10.1126/scitranslmed.3001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim S, Chung EH, Gilbert M, Healy KE. Synthetic MMP-13 degradable ECMs based on poly(N-isopropylacrylamide-co-acrylic acid) semi-interpenetrating polymer networks. I. Degradation and cell migration. J Biomed Mater Res A. 2005;75(1):73–88. doi: 10.1002/jbm.a.30375. [DOI] [PubMed] [Google Scholar]
- 165.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100(9):5413–8. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]