Abstract
The translocation of bacteria and bacterial products into the circulation contributes to alcoholic liver disease. Intestinal bacterial overgrowth is common in patients with alcoholic liver disease. The aims of our study were to investigate bacterial translocation, changes in the enteric microbiome, and its regulation by mucosal antimicrobial proteins in alcoholic liver disease. We used a mouse model of continuous intragastric feeding of alcohol or an isocaloric diet. Bacterial translocation occurred prior to changes observed in the microbiome. Quantitative changes in the intestinal microflora of these animals were assessed first by conventional culture techniques in the small and large intestine. Although we found no difference after 1 day or 1 week, intestinal bacterial overgrowth was observed in the gastrointestinal tract of mice fed alcohol for 3 weeks as compared to control liquid diet fed mice. Because less than 20% of all gastrointestinal bacteria are able to be cultured by conventional methodologies, we performed massively parallel pyrosequencing to further assess the qualitative changes in the intestinal microbiome following alcohol exposure. Sequencing of 16S rRNA genes revealed a relative abundance of Bacteroidetes and Verrucomicrobia bacteria in mice fed alcohol compared with a relative predominance of Firmicutes bacteria in control mice. With respect to the host’s transcriptome, alcohol feeding was associated with downregulation in gene and protein expression of bactericidal c-type lectins Reg3b and Reg3g in the small intestines. Treatment with prebiotics partially restored Reg3g protein levels, reduced bacterial overgrowth and lessened alcoholic steatohepatitis. In conclusion, alcohol feeding is associated with intestinal bacterial overgrowth and enteric dysbiosis. Intestinal antimicrobial molecules are dysregulated following chronic alcohol feeding contributing to changes in the enteric microbiome and to alcoholic steatohepatitis.
Keywords: alcoholic liver disease, microbiome, dysbiosis, bacterial translocation
Introduction
Alcohol abuse is the most important cause of liver cirrhosis in industrialized countries. Alcoholic liver disease is characterized by fatty liver (steatosis), which may progress to alcoholic hepatitis, fibrosis, and cirrhosis (1, 2). Unfortunately, there are no effective antifibrotic treatments. Patients who progressed to cirrhosis have a poor prognosis and liver transplantation is often indicated. Increased mortality in patients with liver cirrhosis is most often attributed to direct complications resulting from the loss of liver function, variceal hemorrhage as a sequela of portal hypertension, and the development of hepatocellular carcinoma. However, a significant percentage of patients also succumb to bacterial infections with an infection-attributed mortality of 30% to 50% (3–5). Bacterial translocation, defined as the passage of viable endogenous bacteria or their products from the intestinal tract through the epithelial mucosa to the mesenteric lymph nodes (MLN), systemic circulation, or extraintestinal organs, is considered an important mechanism for the incidence of infections including spontaneous bacterial peritonitis and sepsis in cirrhotic patients (6).
Bacterial translocation not only causes severe infections in cirrhotic patients, but it may also cause progression of early alcoholic liver injury and fibrosis. Plasma levels of lipopolysaccharide (LPS) or endotoxin, a major component of the Gram-negative bacterial outer-membrane, increases with the severity of liver dysfunction in cirrhotic patients, and are also significantly higher in patients with chronic hepatitis than in healthy subjects (7). Endotoxemia is significantly higher in patients with alcoholic cirrhosis than in patients with non-alcoholic cirrhosis (8, 9). In addition, endotoxemia is also frequent in patients with mild forms of alcoholic hepatitis without evidence of fibrosis or cirrhosis (1, 10). Toll-like receptor 4 (TLR4) as the cellular LPS receptor is one of seven genes associated with increased risk of developing cirrhosis in patients with chronic hepatitis C (11). Several studies addressed a role for LPS signaling in experimental alcohol-induced liver disease. Selective intestinal decontamination with antibiotics (polymyxin B and neomycin) decreases plasma endotoxin levels and prevents alcoholic liver injury (12–14). Mice deficient in CD14 as the cellular co-receptor for LPS are resistant to alcohol-induced liver injury (15). The most convincing evidence supporting a role for LPS in alcoholic liver injury are studies using TLR4 mutant C3H/Hej mice. Hepatic steatosis, inflammation, and necrosis are strongly reduced in the TLR4 mutant C3H/Hej strain following ethanol administration as compared to wildtype mice (16). It was postulated that LPS binds to hepatic Kupffer cells via TLR4 with resulting induction of TNF to induce hepatocyte damage.
Intestinal bacterial overgrowth is more commonly found in patients with alcoholic liver disease relative to healthy individuals. Both aerobic and anaerobic microorganisms were higher in jejunal aspirates in patients with chronic alcohol abuse (17, 18). However, the microbiome associated with alcoholic liver disease has never been assessed in detail. The aims of our study were to investigate bacterial translocation, changes in the enteric microbiome, and its regulation by mucosal antimicrobial proteins in alcoholic liver disease.
Material and Methods are described in the Supplementary Materials and Methods section.
Results
Intragastric ethanol feeding results in hepatic injury and steatosis
To characterize the dynamics of bacterial translocation and changes in the enteric microbiome, the intragastric feeding model of continuous ethanol infusion in C57/B6 mice was used for 1 day, 1 week, and 3 weeks. The ethanol dose is continuously increased until the end of the experiment (final alcohol delivered, 30.9 g/kg per day; 40% of total calories). Mice fed an isocaloric diet served as controls. Micro- and macrovesicular steatosis occurred after 1 week following alcohol administration as compared to control mice. Hepatic fat accumulation was markedly higher following 3 weeks of continuous ethanol feeding (Fig. 1A). Plasma Alanine Aminotransferase (ALT) levels as measures for liver injury were similar in alcohol and control-fed mice after one day of alcohol feeding (Fig. 1B). One and three weeks of enteral ethanol treatment significantly increased ALT levels, indicating acute liver damage (Fig. 1B). To investigate whether chronic ethanol feeding results in fibrogenesis, hepatic collagen α1(I) mRNA was measured. Collagen α1(I) mRNA was significantly induced as compared to control fed mice (Fig. 1C), suggesting stellate cell activation as the major source of extracellular matrix-producing cell type in chronic liver disease. Deposition of extracellular matrix proteins indicative of chronic liver disease was not observed after 3 weeks of alcohol feeding (not shown).
Figure 1. Intragastric alcohol feeding results in steatohepatitis in mice.
Mice were fed alcohol or an isocaloric diet via an intragastric feeding tube. (A) Representative photomicrographs of livers are shown. (B) Plasma ALT levels were measured. (C) Hepatic gene expression of collagen α1(I) was determined by RT-QPCR and normalized to 18S gene expression. Values are presented relative to control fed animals. *p<0.05, when mice fed alcohol are compared to mice fed an isocaloric control diet. Data represent the mean ± SEM of six control or alcohol fed mice at each time point.
Bacterial translocation following chronic alcohol administration
As a measure of bacterial translocation across the mucosal barrier, bacteria were quantitatively cultured in mesenteric lymph nodes, the first organ encountered in the translocation route from the gastrointestinal tract, and in the systemic circulation following continuous enteral ethanol treatment. There was no significant difference of culture positive mesenteric lymph nodes after 24hrs, 1 week, or 3 weeks of ethanol exposure as compared to isocaloric control fed mice (Fig. 2A). Depending on the immune competence in the mesenteric lymph nodes, bacteria can further spread to the blood. One week of ethanol feeding produced a significant increase in positive blood cultures of ethanol-fed mice as compared to control-fed mice, but blood cultures were negative again 3 weeks after ethanol feeding (Fig. 2B). Positive blood and mesenteric lymph node cultures following control and ethanol feeding for one week were sequenced. We found that the types of translocated bacteria were very similar between control and alcohol animals. These consisted mostly of non-pathogenic bacteria such as Lactococcus, Pediococcus, and Bacillus licheniformis in the control animals, and Lactobacillus, Enterobacter, Lactococcus, Pediococcus, Brevibacillus, and Enterococcus in alcohol fed animals. As an additional marker of bacterial translocation, plasma LPS levels were measured. LPS was similar in control and alcohol fed animals after 24 hrs; after 1 week and 3 weeks of ethanol feeding, there was a trend towards higher endotoxin levels as compared to control animals (Fig. 2C). Absolute LPS levels were higher in all mice with an intragastric tube compared to mice without this tube (not shown).
Figure 2. Bacterial translocation to mesenteric lymph nodes or to blood following enteric administration of alcohol.
Aerobic bacteria were quantified in (A) the mesenteric lymph nodes and (B) the blood in mice fed with alcohol or an isocaloric diet (n=4–17 animals per time point). *p<0.05 as compared to control fed mice. CFU = colony forming unit. (C) Endotoxin levels were measured and are presented relative to control fed animals for each time point (n=5–10 animals per time point).
Bacterial overgrowth is pronounced in the proximal small intestine following ethanol administration
The effect of ethanol or isocaloric control diet feeding on bacterial contents of the small intestine (proximal, mid, and distal third) and large intestine (cecum and colon) was assessed by conventional culture techniques. Aerobic bacteria were cultured on 5% blood agar plates, whereas anaerobic bacteria were grown on Brucella blood agar plates under anaerobic conditions. The number of aerobic (Fig. 3A) and anaerobic bacteria (Fig. 3B) increased 3 weeks following ethanol feeding as compared to control animals, but not after one day or one week of alcohol feeding (data not shown). The increase was pronounced in the small intestine. To confirm these results by a culture independent method, the total bacterial load was measured in the cecum by quantitative PCR using universal bacterial primer sets. The cecal number of total bacteria was significantly higher in animals fed ethanol for 3 weeks as compared to control animals (Fig. 3C). Taken together, bacterial translocation occurred prior to changes observed in the microbiome.
Figure 3. Enteric alcohol administration produces intestinal bacterial overgrowth.
Total aerobic bacteria (A) and anaerobic bacteria (B) were quantified by culture in the gastrointestinal tract of mice fed alcohol or an isocaloric control diet for three weeks. Data represent the mean ± SEM of twelve control or alcohol fed mice at each time point. *p<0.05, when compared to control treated mice. CFU = colony forming unit. (C) Total bacterial load in the cecum was determined by QPCR using universal bacteria primers. Values are presented relative to control fed animals. *p<0.05, when mice fed alcohol are compared to mice fed an isocaloric control diet. Data represent the mean ± SEM of five control or alcohol fed mice at each time point.
Dysbiosis following the onset of alcoholic liver disease analyzed by massively parallel pyrosequencing
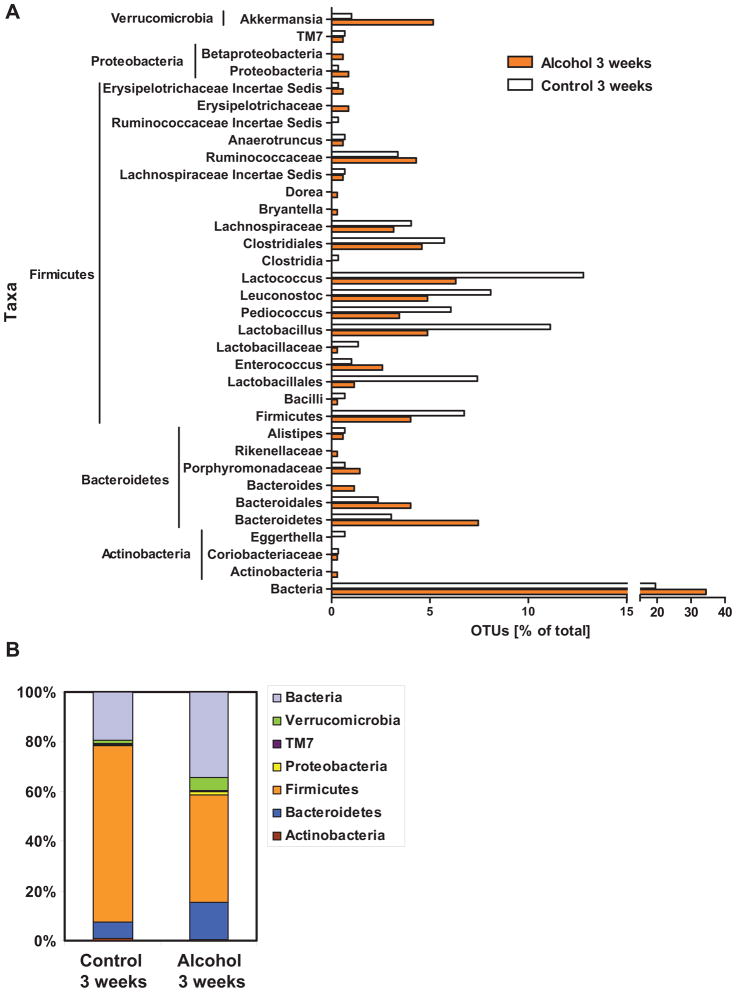
Only a minority of the enteric bacteria can be cultured by conventional culture techniques (37). To assess qualitative changes in the intestinal microbiome, massively parallel pyrosequencing was applied to cecal contents of mice subjected to continuous alcohol or isocaloric diet feeding for 3 weeks. To rule out genetic, dietary, and environmental factors that influence the microbiome (38), we used littermates fed the same defined control or alcohol diet with the same daily calorie intake and housed in the same vivarium. The cecum was chosen, since fecal material can be harvested in sufficient amounts. We found that the mice in the alcohol treated group had reduced operational taxonic units (OTUs) of several Firmicutes, namely Lactococcus, Pediococcus, Lactobacillus and the Leuconostoc genera. There was an increase in the number of OTUs of unknown bacteria, Verrucomicrobia, and Bacteroidetes, such as Bacteroidales, Bacteroides, and Porphyromonadaceae in the alcohol treated group (Fig. 4A and B). To evaluate similarity among the samples, principle component analysis was performed (Fig. 4C). Two methods of performing PCA were used and both gave a similar result, showing that the treated and control groups cluster separately and that the control samples cluster more tightly than do the alcohol treated samples. For the R analysis, the first principal component accounted for 97% of the total variance and consisted mainly of the orders Lactobacillales, Verrucomicrobiales, and Clostridiales. Thus, in addition to the observed quantitative changes, chronic alcohol administration produces distinct changes in the enteric microbiome leading to dysbiosis.
Figure 4. Effects of alcohol on microbial diversity of the mouse cecum.

(A) 16S rRNA from the mouse cecum was sequenced using 454 Titanium technology. Experiment-specific OTU representative sequences (97% identity) were classified using the Ribosomal Database Project (RDP) classifier and plotted. Orange bars indicate OTUs containing the alcohol treated group (3 mice, distributed among 349 OTUs) and white bars indicate OTUs containing the isocaloric control group (3 mice, distributed among 297 OTUs). (B) The graph demonstrates the percentages of each community contributed by the indicated phyla. (C) Scatter plots of Unifrac (left) and R (right) PCA. The alcohol treated samples are in blue while the isocaloric control samples are in red.
Antimicrobial molecule expression of Reg3b and Reg3g are suppressed following ethanol feeding
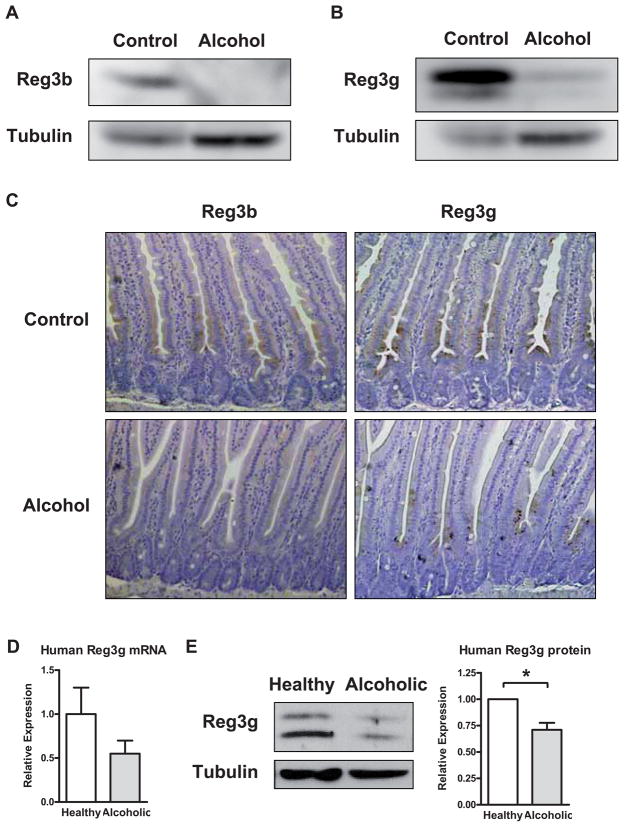
Multiple factors control the bacterial load of the small and large intestine including host antimicrobial peptides and proteins that are secreted by epithelial cells and Paneth cells. To gain insight into the expression and regulation of enteric antimicrobial molecules, their gene expression pattern was evaluated along the mouse intestine by RT-QPCR. Gene expression of regenerating islet-derived 3 beta (Reg3b) and gamma (Reg3g) were reduced in every segment of the small intestine of mice fed alcohol for 1 or 3 weeks as compared to control mice (Fig. 5A–D). The inhibition was pronounced in the proximal small intestine, the site with the largest relative increase in luminal and adherent bacteria. Suppression of Reg3b and Reg3g gene expression was not due to a nonspecific toxic effect of alcohol on epithelial cells, as other antimicrobial molecules such as defensin alpha 5 (Defcr5) did not differ significantly in mice after 3 weeks of enteral feeding with a control or an ethanol containing diet (Fig. 5E). In addition, H&E stained sections of the small intestine did not show major pathological abnormalities of the epithelial cell lining (not shown). Protein expression of Reg3b and Reg3g was also inhibited in the proximal small intestine (jejunum) of mice receiving alcohol for 3 weeks as assessed by Western blotting (Fig. 6A and B) or immunohistochemistry (Fig. 6C). Reg3b and Reg3g proteins were mostly expressed in enterocytes (Fig. 6C). Thus, a decrease in the antimicrobial proteins Reg3b and Reg3g following chronic alcohol exposure might contribute to the enteric dysbiosis observed following alcohol administration.
Figure 5. Reg3b and Reg3g gene expression are suppressed by alcohol.
Total RNA was prepared from intestinal segments of mice fed with alcohol or isocaloric diet for 1 week (A and C) or 3 weeks (B,D,E). Expression of the Reg3b (A and B), Reg3g (C and D), and Defensin alpha 5 (E) gene were measured by RT-QPCR by using 18S as an internal control. Data represent the mean ± SEM of five control or alcohol fed mice at each time point. *p<0.05, when compared to control treated mice.
Figure 6. Protein expression of Reg3b and Reg3g is downregulated in the jejunum by alcohol.
(A and B) Protein extracts from the proximal small intestine (jejunum) of mice on either an alcohol diet or isocaloric control diet for 3 weeks were analyzed by Western blotting with Reg3b or Reg3g antibodies. Tubulin was used as a loading control. Images are representative of one Western blot, which was reproduced in three independent experiments using different animals each time. (C) Immunohistochemical detection of Reg3b and Reg3g in paraffin-embedded proximal small intestinal sections in mice following alcohol or control feeding for 3 weeks. A representative section is shown. (D) Gene expression of human Reg3g was determined by RT-QPCR and normalized to 18S gene expression in duodenal biopsies from patients with chronic alcohol abuse (n=10) and healthy controls (n=10). Values are presented relative to healthy controls and represent the mean ± SEM. (E) Reg3g and tubulin protein expression in duodenal biopsies from patients with chronic alcohol consumption (n=6) and healthy controls (n=6) were analyzed by Western blot analysis. A representative Western blot image is shown. Densitometry of Western blot images with paired control and alcoholic samples was performed. Values are presented relative to healthy controls and represent the mean ± SEM; *p<0.05.
To investigate whether alcohol ingestion downregulates Reg3g levels in patients with alcohol abuse, duodenal biopsies from chronic alcoholics who still actively drank prior to admission were compared to biopsies from healthy subjects. Reg3g gene (Fig. 6D) and protein expression (Fig. 6E) were inhibited in duodenal biopsies obtained form alcoholic patients as compared to the healthy controls.
Prebiotics restore Reg3g levels, reduce intestinal bacterial overgrowth and ameliorate alcoholic steatohepatitis
Intestinal Reg3g levels increase after colonization of germfree mice with B. thetaiotaomicron, a prominent symbiotic member of the human microbiota (39). Several studies have demonstrated a beneficial effect of probiotic Lactobacillus strains on experimental alcoholic liver disease (40, 41). In an attempt to restore Reg3g levels in our model of alcohol feeding, we have chosen to use prebiotic fructooligosaccharides (FOS). Prebiotics are complex short-chain saccharides that cannot be digested by pancreatic and brush-border enzymes, but can be selectively used and fermented by the commensal microbiota. They stimulate probiotic bacteria such as Lactobacilli and Bifdobacteria (42). When mice were administered ethanol and FOS continuously through the intragastric feeding tube, intestinal levels of the antimicrobial protein Reg3g were maintained at almost the original level (Fig. 7A). However, alcohol-induced inhibition of Reg3b protein was not affected by FOS (not shown). This partial restoration of Reg3g decreased intestinal bacterial overgrowth (Fig. 7B) and reduced alcoholic steatohepatitis in mice fed ethanol and FOS (Fig. 7C). This was confirmed by lower plasma ALT (Fig. 7D) and hepatic triglyceride levels (Fig. 7E) in mice administered prebiotics in addition to ethanol as compared to ethanol alone.
Figure 7. Prebiotics improve alcoholic steatohepatitis by inducing Reg3g expression and reducing intestinal bacterial overgrowth.
Mice were fed an isocaloric diet, alcohol, or alcohol and fructooligosaccharides (FOS) via an intragastric feeding tube for 3 weeks. (A) Reg3g protein expression was analyzed in the proximal small intestine (jejunum) by Western blotting. Tubulin was used as a loading control. Images are representative of one Western blot, which was reproduced in four independent experiments using different animals each time. (B) Total aerobic bacteria (upper graph) and anaerobic bacteria (lower graph) were quantified by culture in the mid small intestine. Data represent the mean ± SEM of 5–6 mice per group; *p<0.05. CFU = colony forming unit. (C) Representative photomicrographs of H&E stained livers are shown. (D) Plasma ALT levels were measured. Data represent the mean ± SEM of 4–14 mice per group; *p<0.05. (E) Hepatic triglyceride content was determined. Data represent the mean ± SEM of 5–10 mice per group; *p<0.05.
Discussion
Our study uses an intragastric model of continuous alcohol or isocaloric diet feeding to describe the dynamics of liver disease, bacterial translocation, and intestinal dysbiosis. Steatosis and steatohepatitis occur one week following alcohol administration at a similar time when translocation of live bacteria to the systemic circulation is observed. Intestinal bacterial overgrowth of both aerobic and anaerobic bacteria is evident after 3 weeks of alcohol feeding, which is pronounced in the small intestine. As only 20% of all bacteria can be cultured by conventional techniques (43), we used 454 pyrosequencing to demonstrate qualitative changes of the microbiome following alcohol administration, characterized by a decrease in Firmicutes and an increase in Bacteroidetes. Alcohol induces a downregulation of the host antimicrobial proteins Reg3b and Reg3g. Finally, we demonstrate that partial restoration of Reg3g protein levels with prebiotics reduces bacterial overgrowth and ameliorates alcoholic steatohepatitis.
There is strong evidence in support of the concept that gut-derived endotoxin as a marker for bacterial translocation plays a central role in the initiation and progression of alcohol induced liver injury. First, plasma endotoxin levels are increased in patients with alcoholic liver disease (1, 10), and a correlation between alcohol ingestion and increased systemic levels of endotoxin has also been demonstrated in animal models of alcohol-induced liver injury (14, 44, 45). Second, selective intestinal decontamination with antibiotics prevents experimental alcoholic liver injury (12–14). Third, mice with genetic deletions in the LPS signaling pathway are resistant to alcohol-induced liver damage (15, 16).
The question arises whether bacterial translocation is dependent on qualitative and/or quantitative changes in the intestinal microbiome. It was noted that increased intestinal permeability with subsequent endotoxemia occurs 4 weeks following daily alcohol administration and prior to the development of alcoholic steatohepatitis in rats (46). We have shown that translocation of bacteria to the systemic circulation occurs prior to the onset of intestinal bacterial overgrowth. Endotoxin plasma levels and the number of translocated viable bacteria to mesenteric lymph node showed a higher trend in alcohol fed animals after 1 week and 3 weeks. One possibility why we did not observe a statistically significant difference is related to the nature of the mouse model of intragastric tube feeding. The catheter extends through a skin wound into the stomach that makes the animals prone to have transloaction at baseline. However, when bacterial overgrowth in the small intestine is induced experimentally, it results in hepatic injury mediated by translocated bacterial products (47). Thus, based on these studies, increased intestinal permeability with subsequent translocation of bacterial products or bacteria likely occurs very early in alcohol-induced liver disease. One possible mediator to increase intestinal permeability is ethanol itself, as acute ingestion of alcohol alters the epithelial barrier in the colon through ethanol oxidation into acetaldehyde by the colonic microflora and results in activation of mast cells (48). As described, bacterial overgrowth occurs three weeks following alcohol administration. As the overall amount of enteric endotoxin load increases, one could speculate that plasma endotoxin levels subsequently increase given a preexisting disrupted mucosal barrier. This might contribute to liver disease progression. Thus, alcohol-induced dysbiosis does not cause intestinal permeability with resulting endotoxemia, but it might increase systemic levels of endotoxin to perpetuate later disease stages.
Recently, gut bacterial microbiota fingerprinting using length heterogeneity PCR was applied to an animal model of alcoholic steatohepatitis. Consistent with our results, there is evidence for qualitative changes in the composition of the intestinal microflora in the colon following daily alcohol consumption in rats (41). However, the identity of bacteria has not been addressed in this study. We have advanced these findings by performing 454 massively parallel pyrosequencing of the intestinal contents of mice following an isocaloric diet or alcohol feeding for 3 weeks. Technical advances have helped to characterize the gastrointestinal microflora in its deep biodiversity and its functional contribution to the host biology by examination of the 16S ribosomal RNA genes used in taxonomical classification of bacteria. We found striking qualitative changes in the overall composition of the enteric microbiome associated with alcohol consumption. Following alcohol feeding there was an overall decrease in Firmicutes, while the relative abundance of Bacteroidetes and Verrucomibrobia increased in mice fed alcohol. Interestingly, Lactobacillus was strongly suppressed and almost absent in mice fed alcohol for 3 weeks as compared to control fed animals. This now provides a rationale for the beneficial effect of various probiotic Lactobacillus strains in experimental models of alcoholic liver disease. Feeding a Gram-positive probiotic Lactobacillus strain with subsequent displacement of Gram-negative bacteria protected mice from ethanol-induced liver injury with a concurrent decrease in systemic endotoxin levels (40, 41). This is a very promising example of how directed manipulation of the microbiome, and specifically of one microorganism within the gastrointestinal tract, may yield health benefits. This also demonstrates how remodeling of microbial communities associated with disease can be used for prevention or therapy.
The exact reason for intestinal bacterial overgrowth and enteric dysbiosis is unknown. Several factors contribute to the homeostasis of the intestinal microbial community, such as gastric acid secretion and small and large bowel motility. Intestinal epithelial cells are the main interface between the host and the intestinal microflora. Epithelial cells have many important functions in maintaining a symbiotic relationship between the host and the microflora. One of these functions is the secretion of antimicrobial effector molecules as part of the mucosal innate immune system (49). As we have reported, Reg3g is a secreted c-type lectin with potent bactericidal activity that is expressed in intestinal epithelial and Paneth cells, with highest levels found in the ileum (22, 23). Similarly, Reg3b has antimicrobial activity and has been implicated in intestinal homeostasis (50). Here we demonstrate that chronic alcohol exposure suppresses the gene and protein expression of Reg3b and Reg3g, which might contribute to quantitative and qualitative changes in the enteric flora following chronic alcohol feeding. Interestingly, the lowest levels of Reg3b and Reg3g were observed in the proximal small intestine, where the bacterial overgrowth was most pronounced and luminal alcohol concentrations are highest. Restoration of Reg3g levels using prebiotics decreases intestinal bacterial overgrowth and ameliorates alcoholic steatohepatitis. This dysregulation of the mucosal innate immune system demonstrates a novel link between alcohol and enteric dysbiosis.
Ongoing research initiatives including those by our group are expected to provide new insights into the metagenomics, transcriptomics, and metabolomics in alcoholic liver disease. The gut transcriptome and metabolome will help identify key substrates and signaling mediators to explain microbe-microbe and microbe-host interactions. This knowledge will facilitate rationale attempts to manipulate commensal microflora and to target specific microorganisms and metabolites by dietary supplements such as probiotics, antibiotics, prebiotics, and synbiotics.
Acknowledgments
We thank Akiko Ueno and Hasmik Mkrtchyan from the Animal Core facility of the Southern California Research Center for Alcoholic Liver and Pancreatic Diseases (ALPD) and Cirrhosis, at the University of Southern California for performing animal studies described in this study. We also thank Shibu Yooseph, Monika Bihan, and Doug Rusch at JCVI, and Bryan White at the University of Illinois at Urbana-Champaign for help with PCA. This study was supported in part by NIH grants K08 DK081830 (to BS) and RO1 DK072237 (to DAB). This study was also supported by the Pilot Project Program of the Southern California Research Center for ALPD and Cirrhosis (P50AA11999) funded by the National Institute on Alcohol Abuse and Alcoholism (to BS).
Abbreviations
- CFU
colony-forming unit
- Defcr5
defensin alpha 5
- FOS
fructooligosaccarides
- MLN
mesenteric lymph nodes
- LPS
lipopolysaccharide
- OTU
Operational Taxonomic Unit
- Reg3
regenerating islet-derived 3
- TLR
Toll-like receptor
References
- 1.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S–171S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brann OS. Infectious complications of cirrhosis. Curr Gastroenterol Rep. 2001;3:285–292. doi: 10.1007/s11894-001-0051-2. [DOI] [PubMed] [Google Scholar]
- 4.Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102:1510–1517. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 5.Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718–725. doi: 10.1136/gut.2004.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 7.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 8.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 9.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 10.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 11.Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, Abar OT, Rowland CM, Catanese JJ, Leong DU, Sninsky JJ, Layden TJ, Wright TL, White T, Cheung RC. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46:297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto N, Yamashina S, Kono H, Schemmer P, Rivera CA, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Thurman RG. Development of a new, simple rat model of early alcohol-induced liver injury based on sensitization of Kupffer cells. Hepatology. 1999;29:1680–1689. doi: 10.1002/hep.510290633. [DOI] [PubMed] [Google Scholar]
- 13.Enomoto N, Ikejima K, Yamashina S, Hirose M, Shimizu H, Kitamura T, Takei Y, Sato, Thurman RG. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res. 2001;25:51S–54S. doi: 10.1097/00000374-200106001-00012. [DOI] [PubMed] [Google Scholar]
- 14.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 15.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 16.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 17.Bode JC, Bode C, Heidelbach R, Durr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30–34. [PubMed] [Google Scholar]
- 18.Casafont Morencos F, de las Heras Castano G, Martin Ramos L, Lopez Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1996;41:552–556. doi: 10.1007/BF02282340. [DOI] [PubMed] [Google Scholar]
- 19.Tsukamoto H, Mkrtchyan H, Dynnyk A. Intragastric ethanol infusion model in rodents. In: Nagy L, editor. Alcohol Methods in Molecular Medicine. Humana Press; 2008. pp. 33–48. [DOI] [PubMed] [Google Scholar]
- 20.French SW. Intragastric ethanol infusion model for cellular and molecular studies of alcoholic liver disease. J Biomed Sci. 2001;8:20–27. doi: 10.1007/BF02255967. [DOI] [PubMed] [Google Scholar]
- 21.Benyacoub J, Rochat F, Saudan KY, Rochat I, Antille N, Cherbut C, von der Weid T, Schiffrin EJ, Blum S. Feeding a diet containing a fructooligosaccharide mix can enhance Salmonella vaccine efficacy in mice. J Nutr. 2008;138:123–129. doi: 10.1093/jn/138.1.123. [DOI] [PubMed] [Google Scholar]
- 22.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, Dematteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou HH, Holmes MH. DNA sequence quality trimming and vector removal. Bioinformatics. 2001;17:1093–1104. doi: 10.1093/bioinformatics/17.12.1093. [DOI] [PubMed] [Google Scholar]
- 25.Kunin V, Engelbrektson A, Ochman H, Hugenholtz P. Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ Microbiol. 2010;12:118–123. doi: 10.1111/j.1462-2920.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- 26.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and environmental microbiology. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics. 1987;43:783–791. [PubMed] [Google Scholar]
- 28.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middelbos IS, Vester Boler BM, Qu A, White BA, Swanson KS, Fahey GC., Jr Phylogenetic characterization of fecal microbial communities of dogs fed diets with or without supplemental dietary fiber using 454 pyrosequencing. PLoS One. 2010;5:e9768. doi: 10.1371/journal.pone.0009768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tacke F, Gabele E, Bataille F, Schwabe RF, Hellerbrand C, Klebl F, Straub RH, Luedde T, Manns MP, Trautwein C, Brenner DA, Scholmerich J, Schnabl B. Bone morphogenetic protein 7 is elevated in patients with chronic liver disease and exerts fibrogenic effects on human hepatic stellate cells. Dig Dis Sci. 2007;52:3404–3415. doi: 10.1007/s10620-007-9758-8. [DOI] [PubMed] [Google Scholar]
- 32.Horz HP, Vianna ME, Gomes BP, Conrads G. Evaluation of universal probes and primer sets for assessing total bacterial load in clinical samples: general implications and practical use in endodontic antimicrobial therapy. J Clin Microbiol. 2005;43:5332–5337. doi: 10.1128/JCM.43.10.5332-5337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnabl B, Kweon YO, Frederick JP, Wang XF, Rippe RA, Brenner DA. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology. 2001;34:89–100. doi: 10.1053/jhep.2001.25349. [DOI] [PubMed] [Google Scholar]
- 34.Kodama Y, Taura K, Miura K, Schnabl B, Osawa Y, Brenner DA. Antiapoptotic effect of c-Jun N-terminal Kinase-1 through Mcl-1 stabilization in TNF-induced hepatocyte apoptosis. Gastroenterology. 2009;136:1423–1434. doi: 10.1053/j.gastro.2008.12.064. [DOI] [PubMed] [Google Scholar]
- 35.Kodama Y, Kisseleva T, Iwaisako K, Miura K, Taura K, De Minicis S, Osterreicher CH, Schnabl B, Seki E, Brenner DA. JNK1 from Hematopoietic Cells Mediates Progression from Hepatic Steatosis to Steatohepatitis and Fibrosis in Mice. Gastroenterology. 2009;137(4):1467–1477. doi: 10.1053/j.gastro.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badaoui A, De Saeger C, Duchemin J, Gihousse D, de Timary P, Starkel P. Alcohol dependence is associated with reduced plasma and fundic ghrelin levels. Eur J Clin Invest. 2008;38:397–403. doi: 10.1111/j.1365-2362.2008.01947.x. [DOI] [PubMed] [Google Scholar]
- 37.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006;4:e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 41.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolida S, Gibson GR. Prebiotic capacity of inulin-type fructans. J Nutr. 2007;137:2503S–2506S. doi: 10.1093/jn/137.11.2503S. [DOI] [PubMed] [Google Scholar]
- 43.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol. 1993;142:367–373. [PMC free article] [PubMed] [Google Scholar]
- 45.Tamai H, Kato S, Horie Y, Ohki E, Yokoyama H, Ishii H. Effect of acute ethanol administration on the intestinal absorption of endotoxin in rats. Alcohol Clin Exp Res. 2000;24:390–394. [PubMed] [Google Scholar]
- 46.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414–423. doi: 10.1016/0016-5085(90)90833-m. [DOI] [PubMed] [Google Scholar]
- 48.Ferrier L, Berard F, Debrauwer L, Chabo C, Langella P, Bueno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168:1148–1154. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dessein R, Gironella M, Vignal C, Peyrin-Biroulet L, Sokol H, Secher T, Lacas-Gervais S, Gratadoux JJ, Lafont F, Dagorn JC, Ryffel B, Akira S, Langella P, Nunez G, Sirard JC, Iovanna J, Simonet M, Chamaillard M. Toll-like receptor 2 is critical for induction of Reg3 beta expression and intestinal clearance of Yersinia pseudotuberculosis. Gut. 2009;58:771–776. doi: 10.1136/gut.2008.168443. [DOI] [PubMed] [Google Scholar]