INTRODUCTION
Asthma and atopic diseases have been increasing in prevalence in westernized countries.1 The reasons for this increase are unknown. In addition, the mechanisms that drive the development of atopic disease are unknown, although genetic and other hypotheses have been suggested 2, 3. One association that has been well documented is that severe respiratory paramyxoviral infections early in life lead to greatly increased risk of asthma and atopic disease.4, 5 We undertook these studies to see if exposure to a non-viral antigen during a respiratory viral infection was sufficient to drive the development of atopic disease.
Sendai virus (SeV), the murine parainfluenza virus type 1, is a paramyxovirus and natural rodent pathogen. In a strain dependent fashion, mice that survive a severe infection with SeV develop long-lasting IL-13 dependent mucous cell metaplasia and airway hyper-reactivity.6 We have shown that the acute development of post-viral atopic disease (occurring within the first 3 weeks post viral inoculation) depended upon expression of the high-affinity receptor for IgE (FcεRI) on lung conventional dendritic cells (cDC).7 Cross-linking of FcεRI by anti-viral IgE led to production of CCL28, a chemokine that attracted Th2 cells in an antigen non-specific fashion.7, 8 While these initial observations were made in mice, we have shown that the cDC FcεRI-CCL28 axis appears operative in humans.9 Further, it has been shown that expression of FcεRI on human peripheral blood cDC is increased by respiratory viral infection.10 Therefore, while not completely validated in the human, critical portions of the cDC FceRI-CCL28 pathway appear intact in both mouse and man.
We also have shown that during SeV infection, the resident lung cDC became mature and activated, which suggests that they could present antigen to passing T cells.11 Therefore, we hypothesized that the mature and activated cDC would be able to present non-viral bystander antigen to Th2 cells that had been recruited in an antigen non-specific fashion during the infection. Further, this would lead to an atopic response against the non-viral antigen, characterized by specific IgE production, increased mucous cell metaplasia, and airway hyper-reactivity. Therefore, we undertook these studies to test this hypothesis and see if mice exposed to ovalbumin once during SeV infection would make IgE against OVA without the addition of an adjuvant; and, if they were able to make IgE, would a subsequent exposure to the antigen lead to enhanced airway hyper-reactivity and mucous cell metaplasia (i.e. clinical disease in the mouse model).
We now report that a single exposure to a non-viral antigen during an active anti-viral immune response is sufficient to drive significant and marked IgE production against the non-viral antigen. We also show that generation of IgE is dependent, at least partly, on expression of FcεRI. Moreover, post-viral airway hyper-reactivity and mucous cell metaplasia were augmented in wild-type mice exposed to and subsequently challenged by the non-viral antigen. Interestingly, while the standard mouse ovalbumin asthma model does not appear to be fully dependent upon IgE, the augmented disease seen in our model is dependent upon both IgE and FcεRI.12, 13 These results have important implications for how atopic disease might develop and provide direction for future investigations into the development of post-viral atopic disease.
METHODS
Mouse generation and handling
C57BL6 mice were obtained from The Jackson Laboratory. Mice deficient in FcεRIα (FcεRIα–/–) on a C57BL6 background were a kind gift of J.P. Kinet (Harvard Medical school, Boston, MA). IgE-deficient mice (IgE–/–) on a C57BL6 background were generated by backcrossing IgE–/– Balb/c mice (kind gift from H. Oettgen; Harvard Medical School, Boston, MA) 10+ generations into C57BL6 mice. Mice 6-20 wks old were used for all experiments. Mice were housed and handled and experiments performed according to protocols approved by the Institutional Animal Care and Use Committee.
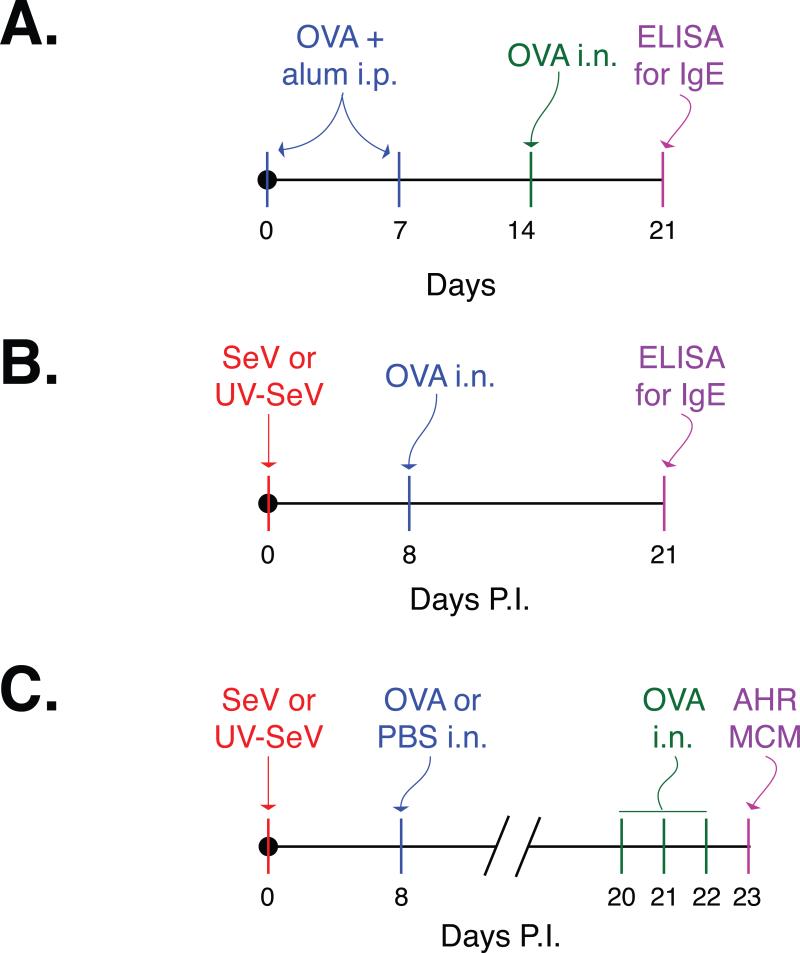
Some mice received two intraperitoneal (i.p.) injections of 20 μL of 1 mg/mL ovalbumin (OVA) plus alum (1:1) one week apart, followed by an intranasal (i.n.) challenge with OVA on day 14 as described (figure 1A).14 Other mice were inoculated i.n. with 2×105 pfu SeV (Strain 52; American Type Culture Collection) or UV-inactivated SeV, followed eight days later by a single i.n. OVA administration (15 μl of a 2 mg/ml solution; figure 1B). Mice in all groups were monitored daily for weight and activity. Twenty-one days after SeV inoculation or initial i.p. OVA/Alum administration, OVA specific IgE was determined in the serum by standard ELISA. In some mice, i.n. OVA (15 μl of 2 mg/ml solution) was administered daily on day 20, 21, 22. On day 23, airway resistance was measured and mice were sacrificed for evaluation of mucous cell metaplasia (figure 1C). We limited our experiments to day 23 so that our findings would not be confused by the chronic alternatively activated macrophage disease that develops by 7 weeks post inoculation in C57BL6 mice.15
Figure 1. Study design.
The generation of OVA specific IgE was assessed after two different sensitization/challenge models. (A) The traditional mouse model that involves two intraperitoneal (i.p.) injections of ovalbumin (OVA) plus alum one week apart, followed by an intranasal (i.n.) challenge with OVA on day 14.14 In this model, OVA specific IgE in the serum is determined one week later by ELISA. (B) To determine if exposure to a non-viral antigen (OVA) during a viral infection leads to IgE production against the non-viral antigen, mice were inoculated with Sendai virus (SeV) or UV-inactivated SeV (UV-SeV). Eight days later, OVA was administered i.n. On day 21 post inoculation (p.i.) OVA specific IgE was determined in the serum using ELISA. (C) To see if this anti-OVA IgE was sufficient to induce allergic airway disease, mice that were treated as in (B) were challenged with OVA i.n. on p.i. days 20, 21, and 22, and airway hyper-reactivity and mucous cell metaplasia assessed the following day.
Measurement of IgE
Standard ELISA was performed as previously reported.7 Briefly, 96-well MaxiSorp (Nalge Nunc) plates were coated overnight with OVA. After being washed, mouse sera was added to the wells, and OVA specific IgE was determined using a detection anti-IgE antibody (BDPharMingen). Purified mouse OVA specific IgE (clone 2C6, Abd Serotec) was used to construct a standard curve in order to calculate concentration of the anti-OVA IgE.
Histochemistry
Mouse lung (at 25 cm H2O pressure) was fixed in 10% zinc formalin and processed by the Medical College of Wisconsin histology core for standard Hematoxylin and Eosin (H & E) and Periodic acid-Schiff (PAS) staining. Quantification of mucous cells was performed by a blinded observer counting the number of PAS+ cells per mm of basement membrane using Image-J software (NIH) as previously reported.7
Real-time PCR assay
mRNA was isolated with Trizol (Sigma-Aldrich) from which cDNA was generated with the QuantiTect reverse transcription kit (Qiagen) as per manufacturer's instructions. Quantitiative real-time PCR (qPCR) assays were performed using TaqMan fast master mix and the StepOnePlus PCR system (Applied Biosystems). All qPCR data are presented as Muc5ac copy number normalized to GAPDH copy number. GAPDH and Muc5ac primer probe sets were obtained as previously reported.7
Airway reactivity measurements
Invasive measurement of airway hyper-reactivity (resistance) after exposure to nebulized vehicle (PBS) or doubling concentrations of methacholine (0.625–10 mg/ml) was done using the flexiVent system per the manufacturer's instructions (Scireq, Montreal Canada).
Statistical analyses
Unless otherwise stated, all data are presented as the mean ± SEM. Student's t test was used to assess statistical significance of differences between two means for data that are normally distributed. Mann-Whitney U test was used for comparison of two medians for data that are clearly not normally distributed. For comparison of airway hyper-reactivity data repeated measures ANOVA with Tukey's multiple comparison test was used. In all cases, significance was set at p < 0.05.
RESULTS
A single exposure to a non-viral antigen during a severe paramyxoviral respiratory infection is sufficient to drive specific IgE production against the non-viral antigen
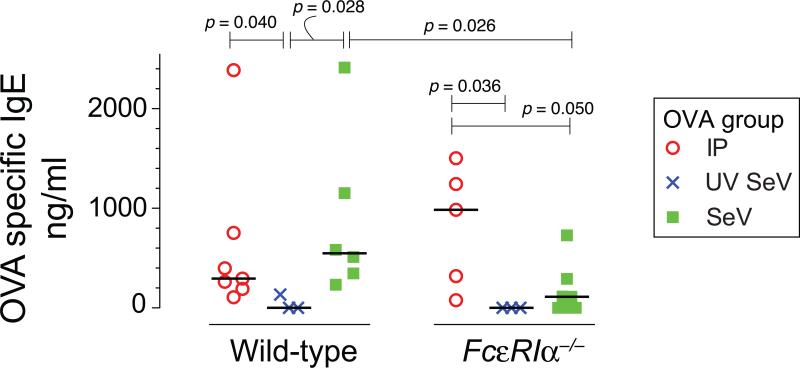
We first examined whether a single i.n. exposure to a non-viral antigen during a SeV infection was sufficient to drive IgE production against this non-viral antigen (see Figure 1A, B for design). Wild-type mice were inoculated with SeV and on day 8 post inoculation (p.i.), when viral-induced upregulation of FcεRI on lung cDC is well developed, a single dose of OVA was administered i.n. This single exposure to OVA without an adjuvant was sufficient to produce significant amounts of specific IgE against OVA when compared to mice inoculated with UV-SeV before receiving OVA i.n. on day 8 p.i. (548 (317-1470) vs. 0 (0-133) ng/ml, median (IQR), p=0.028, Fig. 2). The level of anti-OVA IgE produced was actually greater than that seen with the traditional two i.p. injections of OVA and alum, followed by i.n. OVA exposure (Fig. 1A for design; Fig. 2 for results).
Figure 2. Non-viral antigen exposure during a severe paramyxoviral infection drives specific IgE production and is partially dependent upon FcεRI.
Wild-type mice or mice deficient in the high affinity receptor for IgE, FcεRIα–/–, were treated as indicated in figure 1A, B. The concentrations of serum anti-OVA IgE were analyzed and individual values are shown. IP = the design in figure 1A; UV SeV and SeV refer to the design in figure 1B. The number of mice per group are as follows: Wild-type, IP = 7, UV SeV = 3, SeV = 6; FcεRIα–/–, IP = 5, UV SeV = 3, SeV = 7. Bar = median, significant and trending p values are shown (Mann Whitney U test.).
Development of IgE against a non-viral antigen during a paramyxoviral infection is dependent on FcεRI expression
When we performed the same experiment using mice genetically deficient in the high-affinity receptor for IgE, FcεRIα–/– mice, we saw a significant reduction in the level of OVA specific IgE produced (Fig. 2). In fact, only 2 of 5 FcεRIα–/– mice made any appreciable anti-OVA IgE, while all 6 of the wild type mice made anti-OVA IgE.
Exposure to a non-viral antigen during SeV infection is sufficient to drive IgE mediated augmented airway hyper-reactivity
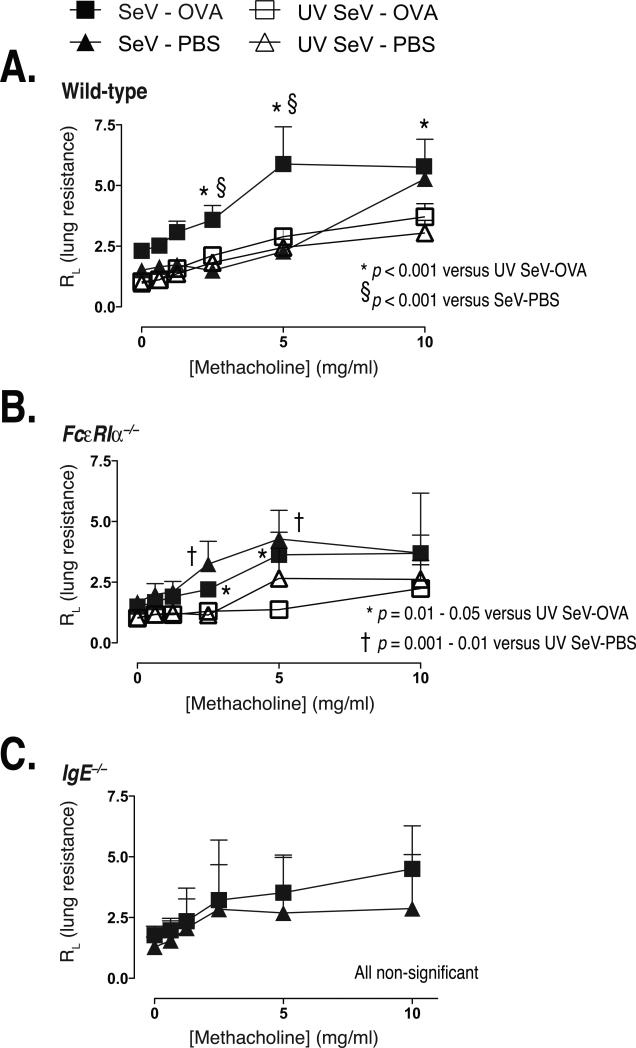
We next wanted to determine if our model could lead to clinically relevant disease in mice exposed to OVA during a SeV infection. The design of these experiments is presented in Figure 1C. Mice were inoculated with SeV or UV-SeV i.n. and then treated with either OVA or PBS i.n. on day 8 p.i. SeV. Subsequently, all mice were challenged with OVA i.n. on days 20, 21, and 22 p.i., with airway resistance to a methacholine challenge performed on day 23. In wild-type mice, airway resistance was significantly increased in those mice infected with SeV and exposed to OVA at day 8 p.i. SeV when compared to mice given PBS at day 8 p.i. (Fig. 3A). As expected, mice inoculated with UV-SeV did not develop airway hyper-reactivity regardless of whether they received OVA or PBS at day 8 p.i. FcεRIα–/– mice developed a modest increase in airway hyper-reactivity when infected with SeV, but the administration of OVA or PBS at day 8 p.i. had little further effect (Fig. 3B). These data suggested that the OVA mediated augmentation of airway hyper-reactivity required expression of FcεRI.
Figure 3. Non-viral antigen exposure during a severe paramyxoviral infection induces airway hyperreactivity through an IgE-dependent mechanism.
Mice were treated as outlined in figure 1C and airway resistance to a graded methacholine challenge was measured after 3 OVA i.n. challenges. Note that all mice received 3 OVA challenges regardless of the sensitization group (PBS or OVA). (A) Wild-type mice developed significantly increased airway resistance only when they had been exposed to ovalbumin during an active viral infection (SeV-OVA). The number of mice per group were: SeV-OVA = 7, SeV-PBS = 3, UV SeV-OVA = 8, UV SeV-PBS = 8. (B) This response appeared to depend upon the high affinity receptor for IgE, since FcεRIα–/– mice did not exhibit this increased airway hyper-reactivity. The number of mice per group were: SeV-OVA = 6, SeV-PBS = 7, UV SeV-OVA = 6, UV SeV-PBS = 8. (C) This response was IgE dependent, as IgE–/– mice failed to demonstrate increased airway hyper-reactivity when given OVA during an active viral infection. The number of mice per group were: SeV-OVA = 4, SeV-PBS = 3. All data are presented as mean +/- SEM of airway resistance for each dose of methacholine. Statistical comparisons are as indicated using repeated measures ANOVA with Tukey's multiple comparison test.
To test whether IgE played a role in this response, we examined the response in IgE deficient mice (IgE–/–). Similar to what was seen with the FcεRIα–/– mice, no increase in airway hyper-reactivity was seen in the IgE–/– mice when given OVA (Fig 3C), supporting the idea that IgE and FcεRI are important in this model.
Exposure to a non-viral antigen during SeV infection is sufficient to drive IgE mediated augmentation of mucous cell metaplasia
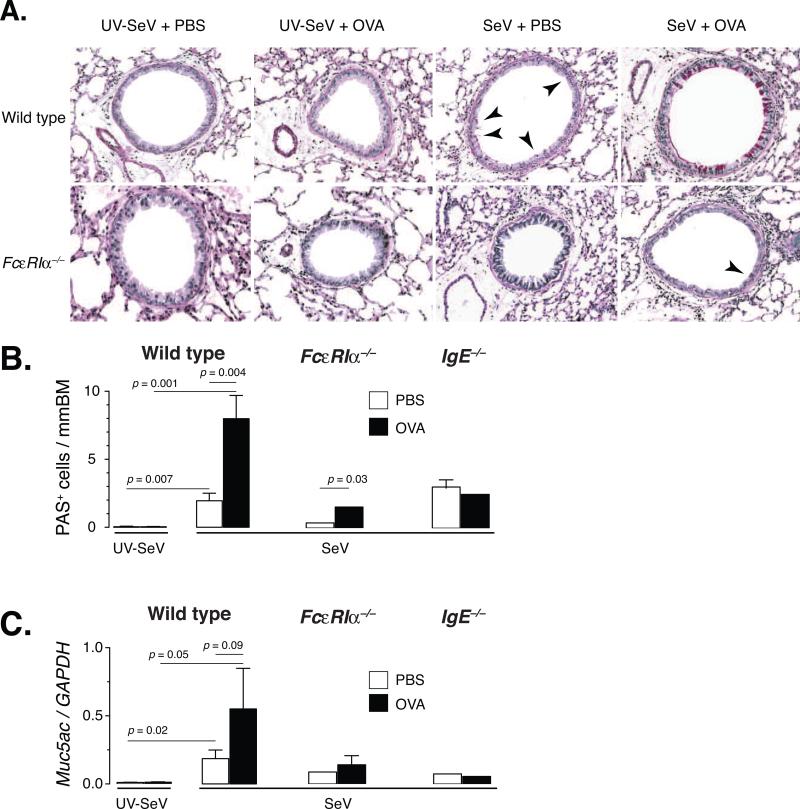
Since mucous cell metaplasia is one of the cardinal features of asthma and clearly has been shown to be dependent upon IL-13 in the SeV model, we examined the development of mucous cell metaplasia after exposure and challenge to a non-viral antigen. Using mice treated as outlined above, we quantified mucous cell metaplasia in lung sections from mice inoculated with SeV or UV-SeV followed by OVA or PBS i.n. on day 8 p.i. and OVA i.n. challenge daily on days 20, 21, and 22, and sacrificed for lung harvest the next day. As expected, SeV infection in wild-type mice induced PAS+ mucous cell metaplasia (Fig 4). However, a significant and marked increase in mucous cells and Muc5ac mRNA was seen in wild-type mice if they were exposed to OVA on day 8 p.i. (Fig. 4). In FcεRIα–/– mice, there was a significant but very small increase in PAS+ mucous cells in the OVA treated group compared to PBS treated (Fig. 4B). However, this increase was not seen in the level of Muc5ac mRNA (Fig. 4C). Further evidence for the IgE-FceRI axis being important for mucous cell metaplasia came from the fact that the IgE–/– mice demonstrated no increased level of mucous cells (either by PAS staining or mRNA) with OVA exposure (Fig. 4B, C).
Figure 4. Non-viral antigen exposure during a severe paramyxoviral infection induces mucous cell metaplasia primarily through an FcεRI-dependent process.
(A) Representative PAS staining of lung sections from C57BL6 or FcεRIα–/– mice inoculated with either 2×105 pfu SeV or UV-SeV i.n., exposed to OVA or PBS i.n. on day 8 p.i., and challenged with OVA i.n. daily as outlined in figure 1C. SeV infection induced a modest level of PAS positive cells (red staining cells in bronchial epithelium, indicated by arrowheads) in wild-type mice, but exposure to OVA during the viral infection greatly enhanced this response (the arrowheads were left off due to the much greater level of staining). Mice deficient in FcεRIα had very few PAS positive cells in their airways (see arrowhead), regardless of treatment. (B) Quantification of the number of PAS positive cells per mm BM in the indicated mouse strain inoculated with UV-SeV (wild-type only) or SeV and then given PBS (open bars) or OVA (filled bars) and treated as in (A). (C) Level of expression of Muc5ac gene product in whole lung (normalized to GAPDH levels; right graphs) for each of the groups as discussed in (B). Data are presented as mean +/- SEM, with the number of mice as outlined in the legend to figure 3. Statistical comparisons are as indicated using two-tailed, unpaired Student's t-test.
Taken together, these data demonstrate that a single exposure to a non-viral antigen during SeV infection is sufficient to drive IgE production against this antigen. Further, the development of airway hyper-reactivity and mucous cell metaplasia after subsequent challenge with OVA depends upon both IgE and FcεRI, even though this response has been reported to be IL-13 T cell dependent in the traditional OVA/alum model.12
DISCUSSION
The mechanisms underlying the development of atopy remain unclear, although a strong association between early life severe respiratory viral infection and atopy has been documented.4, 5, 16 Our prior studies have suggested that severe paramyxoviral infection is sufficient to drive an IL-13 dependent atopic response; however, these studies did not explore whether the atopic response could spread to non-viral bystander antigens.7 The results of our current study support the hypothesis that a single exposure to non-viral antigen during the anti-viral response is sufficient to drive atopic sensitization against the antigen. Although previous studies have examined the role of exposure to non-viral antigens during a viral infection, these studies have either sensitized the mice by intraperitoneal injection prior to viral exposure or exposed the mice to daily OVA for 10 days prior to measurement of outcomes, with less than ideal physiologic correlation.17-19 To our knowledge, our study is the first to examine the effect of a single antigenic exposure without adjuvant, and, therefore, we think this model is more reflective of what may occur in humans with severe respiratory viral infections.
The presence of IgE does not in itself indicate atopic disease. It is only the development of clinical disease upon subsequent exposure to the antigen, that atopic disease can truly be diagnosed. In this vein, we showed that the IgE generated against the non-viral antigen in our model was sufficient to induce a marked increase in mucus cell metaplasia and airway hyper-reactivity upon re-exposure to the allergen. Based on our prior studies, we believe that the IgE was able to bind and signal through FcεRI, as the presence of both IgE and FcεRI were critical for the exacerbation of the phenotype at the subsequent allergen challenge. This is a novel finding as previous reports on the development of airway hyper-reactivity and mucus cell metaplasia in the i.p. OVA/alum model have been shown to be primarily dependent upon T cells and IL13.12, 13 In fact, our prior studies have shown that mice infected with SeV make a robust anti-SeV IgE response. We believe that this anti-SeV IgE may be critical for the recruitment of Th2 cells in an antigen non-specific manner. Once recruited to the airways, these Th2 cells could respond to any non-viral antigen that is encountered (such as OVA in our model). This sensitization phase will then leave the mouse susceptible to a subsequent exposure to the non-viral antigen, and the development of atopic disease. Our current model of how exposure to a non-viral antigen during a viral infection drives the development of initial atopic disease is shown in the online supplement (eFig. 1). In fact, since cross-linking IgE on lung cDC drives further recruitment of Th2 cells, this model predicts that there could be development of an amplification loop (the so-called “atopic cycle”) that would drive further atopic sensitization.3
One caveat to our current studies is the fact that SeV infection itself drives airway hyper-reactivity and mucous cell metaplasia.6 In our current study we found that OVA exposure and challenge exacerbated this baseline increased airway disease. Therefore, we believe this response is due to the OVA exposure on top of the viral infection. The exacerbated atopic disease due to OVA exposure involved both increased airway hyper-reactivity and mucous cell metaplasia. This is interesting, because we have previously reported that FcεRIα–/– mice do not develop mucous cell metaplasia with SeV infection, although in that study we did not examine airway hyper-reactivity.7 Therefore, it appears that the mechanisms behind IL-13 dependent mucous cell metaplasia (which is FcεRIα dependent) and airway hyper-reactivity (not FcεRIα dependent based on our current results) are disparate, at least at 21 days p.i. SeV. This supports a prior study that examined the genetics of airway hyper-reactivity and mucous cell metaplasia and found these two traits segregate separately in the SeV model.20
The data from our mouse model provide the first direct evidence that development of atopy may be due to environmental exposures during a severe respiratory viral infection. Based on these studies we would predict that infants hospitalized with a severe paramyxoviral infection would likely develop initial sensitization to indoor allergens. In fact, it has been shown that young children tend to develop IgE against perennial allergens earlier than seasonal allergens.21 Whether specific allergen sensitivity in humans can be directly related to environmental allergen exposure awaits further study. Our model utilizes a severe paramyxoviral infection to initiate the allergic sensitization, but it is not known if other viruses or less severe infections are capable of driving a similar response. In fact, in addition to paramyxoviruses (RSV, parainfluenza 1 and 3), rhinoviruses have been associated with the development of asthma, and it is tempting to speculate that similar mechanisms are active during rhinovirus infections.3 Given our finding that exposure to antigen during a viral infection can lead to atopic sensitization against the antigen, we cannot help but wonder if the increase in atopic disease seen in the westernized world could be related to an increase in population density and possibly an increase in respiratory viral infections. Finally, our study helps elucidate the mechanisms involved in the translation of viral infection into atopic disease, and lays the groundwork for future studies exploring therapeutic strategies to reduce or prevent the development of atopic disease.
Supplementary Material
Online supplemental information
Development of atopy: severe paramyxoviral infection is sufficient to induce atopic disease against non-viral antigens in a mouse model
Dorothy S. Cheung, MD, Sarah J. Ehlenbach, BS, Tom Kitchens, BS, Desire A. Riley, BS, and Mitchell H. Grayson, MD
eFigure 1. Proposed model of development of atopy. (A) Viral respiratory infection drives expression of FcεRI on lung cDC, and IgE against virus leads to cross-linking of FcεRI on the cDC. This leads to release of chemoattractant (CCL28) for the recruitment of Th2 cells in an antigen non-specific fashion. (B) Exposure to a non-viral antigen could lead to expansion of Th2 cells, which would instruct B cells to make non-viral antigen specific IgE. (C) Subsequent exposure to the antigen would lead to clinical relevant atopic disease. If IgE against the non-viral antigen is cross-linked on the cDC in this second exposure, then further antigen non-specific recruitment of Th2 cells could occur, leading to an “atopic cycle”. DC refers to conventional dendritic cells.
ACKNOWLEDGEMENTS
The authors wish to thank Drs. Jack Gorski, Michael Holtzman, and Charles Parker for their support and discussion, as well as Drs. Kinet and Oettgen for the kind gift of mice.
Funding sources: NIH HL087778 and the Children's Research Institute of the Children's Hospital of Wisconsin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Hurme M, Pessi T, Karjalainen J. Genetics of inflammation and atopy. Ann Med. 2003;35:256–8. doi: 10.1080/07853890310000682. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Grayson MH. The role of viruses in the development and exacerbation of atopic disease. Ann Allergy Asthma Immunol. 2009;103:181–6. doi: 10.1016/S1081-1206(10)60178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigurs N. Epidemiologic and clinical evidence of a respiratory syncytial virus-reactive airway disease link. Am J Respir Crit Care Med. 2001;163:S2–6. doi: 10.1164/ajrccm.163.supplement_1.2011109. [DOI] [PubMed] [Google Scholar]
- 5.Sigurs N. A cohort of children hospitalised with acute RSV bronchiolitis: impact on later respiratory disease. Paediatr Respir Rev. 2002;3:177–83. doi: 10.1016/s1526-0542(02)00191-4. [DOI] [PubMed] [Google Scholar]
- 6.Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest. 2002;110:165–75. doi: 10.1172/JCI14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grayson MH, Cheung D, Rohlfing MM, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med. 2007;204:2759–69. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephens R, Randolph DA, Huang G, Holtzman MJ, Chaplin DD. Antigen-nonspecific recruitment of Th2 cells to the lung as a mechanism for viral infection-induced allergic asthma. J Immunol. 2002;169:5458–67. doi: 10.4049/jimmunol.169.10.5458. [DOI] [PubMed] [Google Scholar]
- 9.Khan SH, Grayson MH. Cross-linking IgE augments human conventional dendritic cell production of CC chemokine ligand 28. J Allergy Clin Immunol. 2010;125:265–7. doi: 10.1016/j.jaci.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subrata LS, Bizzintino J, Mamessier E, et al. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J Immunol. 2009;183:2793–800. doi: 10.4049/jimmunol.0900695. [DOI] [PubMed] [Google Scholar]
- 11.Grayson MH, Ramos MS, Rohlfing MM, et al. Controls for Lung Dendritic Cell Maturation and Migration during Respiratory Viral Infection. J Immunol. 2007;179:1438–48. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- 12.Hamelmann E, Cieslewicz G, Schwarze J, et al. Anti-interleukin 5 but not anti-IgE prevents airway inflammation and airway hyperresponsiveness. Am J Respir Crit Care Med. 1999;160:934–41. doi: 10.1164/ajrccm.160.3.9806029. [DOI] [PubMed] [Google Scholar]
- 13.Hamelmann E, Tadeda K, Oshiba A, Gelfand EW. Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model. Allergy. 1999;54:297–305. doi: 10.1034/j.1398-9995.1999.00085.x. [DOI] [PubMed] [Google Scholar]
- 14.Pauwels RA, Brusselle GJ, Kips JC. Cytokine manipulation in animal models of asthma. Am J Respir Crit Care Med. 1997;156:S78–S81. doi: 10.1164/ajrccm.156.4.12-tac-1. [DOI] [PubMed] [Google Scholar]
- 15.Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–40. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt PG, Rowe J, Kusel M, et al. Toward improved prediction of risk for atopy and asthma among preschoolers: a prospective cohort study. J Allergy Clin Immunol. 2010;125:653–9. 9 e1–9 e7. doi: 10.1016/j.jaci.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Peebles RS, Jr., Hashimoto K, Collins RD, et al. Immune interaction between respiratory syncytial virus infection and allergen sensitization critically depends on timing of challenges. J Infect Dis. 2001;184:1374–9. doi: 10.1086/324429. [DOI] [PubMed] [Google Scholar]
- 18.Peebles RS, Jr., Hashimoto K, Morrow JD, et al. Selective cyclooxygenase-1 and -2 inhibitors each increase allergic inflammation and airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 2002;165:1154–60. doi: 10.1164/ajrccm.165.8.2106025. [DOI] [PubMed] [Google Scholar]
- 19.Peebles RS, Jr., Sheller JR, Johnson JE, Mitchell DB, Graham BS. Respiratory syncytial virus infection prolongs methacholine-induced airway hyperresponsiveness in ovalbuminsensitized mice. J Med Virol. 1999;57:186–92. doi: 10.1002/(sici)1096-9071(199902)57:2<186::aid-jmv17>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Patel AC, Morton JD, Kim EY, et al. Genetic segregation of airway disease traits despite redundancy of calcium-activated chloride channel family members. Physiol Genomics. 2006;25:502–13. doi: 10.1152/physiolgenomics.00321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calabria CW, Dice J. Aeroallergen sensitization rates in military children with rhinitis symptoms. Ann Allergy Asthma Immunol. 2007;99:161–9. doi: 10.1016/S1081-1206(10)60640-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online supplemental information
Development of atopy: severe paramyxoviral infection is sufficient to induce atopic disease against non-viral antigens in a mouse model
Dorothy S. Cheung, MD, Sarah J. Ehlenbach, BS, Tom Kitchens, BS, Desire A. Riley, BS, and Mitchell H. Grayson, MD
eFigure 1. Proposed model of development of atopy. (A) Viral respiratory infection drives expression of FcεRI on lung cDC, and IgE against virus leads to cross-linking of FcεRI on the cDC. This leads to release of chemoattractant (CCL28) for the recruitment of Th2 cells in an antigen non-specific fashion. (B) Exposure to a non-viral antigen could lead to expansion of Th2 cells, which would instruct B cells to make non-viral antigen specific IgE. (C) Subsequent exposure to the antigen would lead to clinical relevant atopic disease. If IgE against the non-viral antigen is cross-linked on the cDC in this second exposure, then further antigen non-specific recruitment of Th2 cells could occur, leading to an “atopic cycle”. DC refers to conventional dendritic cells.