Abstract
Bone marrow-derived mesenchymal stem cells (BM-MSCs) play a role in wound healing and tissue repair and may also be useful for organ regeneration. As we have demonstrated previously that A2A adenosine receptors (A2AR) promote tissue repair and wound healing by stimulating local repair mechanisms and enhancing accumulation of endothelial progenitor cells, we investigated whether A2AR activation modulates BM-MSC proliferation and differentiation. BM-MSCs were isolated and cultured from A2A-deficient and ecto-5′nucleotidase (CD73)-deficient female mice; the MSCs were identified and quantified by a CFU-fibroblast (CFU-F) assay. Procollagen α2 type I expression was determined by Western blotting and immunocytochemistry. MSC-specific markers were examined in primary cells and third-passage cells by cytofluorography. PCR and real time-PCR were used to quantitate adenosine receptor and CD73 expression. There were significantly fewer CFU-Fs in cultures of BM-MSCs from A2AR knockout (KO) mice or BM-MSCs treated with the A2AR antagonist ZM241385, 1 μM. Similarly, there were significantly fewer procollagen α2 type I-positive MSCs in cultures from A2AR KO and antagonist-treated cultures as well. In late passage cells, there were significantly fewer MSCs from A2A KO mice expressing CD90, CD105, and procollagen type I (P<0.05 for all; n=3). These findings indicate that adenosine and adenosine A2AR play a critical role in promoting the proliferation and differentiation of mouse BM-MSCs.
Keywords: CD73, CD105, CD34
INTRODUCTION
Bone marrow-derived mesenchymal stem cells (BM-MSCs) play a role in tissue repair and regeneration and represent an increasingly attractive population for cellular therapy protocols such as repairing infarcted myocardium, improving angiogenesis following stroke, and repairing bone and cartilage damage. Adult BM contains a minority population of MSCs that contributes to tissue regeneration. In addition, BM-MSCs can differentiate into adipocytes, osteoblasts, and chondrocytes and may also express characteristic genes of hepatocytes, cardiomyocytes, astrocytes, and neurons [1, 2].
Adenosine and adenosine A2A receptors (A2AR) play a critical role in wound healing; adenosine A2AR deletion impairs granulation tissue formation in healing wounds in healthy mice, and application of an adenosine A2AR agonist stimulates wound healing in normal mice and diabetic rats [3,4,5]. Although adenosine receptor-mediated stimulation of angiogenesis and matrix production by endothelial cells and fibroblasts present at the injured site contribute to the effect of adenosine A2AR on wound healing, increased application of an adenosine A2AR agonist stimulates recruitment of BM-derived endothelial progenitor cells to injured sites as well [6,7,8]. These endothelial progenitor cells contribute to enhanced angiogenesis in the healing wounds, and it is not unlikely that other BM-MSCs are recruited to healing wounds in greater numbers in adenosine A2AR agonist-treated animals.
Adenosine is generated by the dephosphorylation of adenine nucleotides, and in many tissues, adenine nucleotides are exported to the extracellular space, where they are dephosphorylated to AMP by the ecto-enzyme nucleoside triphosphate phosphohydrolase (CD39), and the AMP is dephosphorylated further to adenosine by ecto-5′ nucleotidase (CD73). In some settings, adenosine generated by CD73 plays an important tissue-protective role [9,10,11,12,13,14,15,16,17,18,19]. It is interesting to note that BM-MSCs express significant levels of CD73 and thus, are capable of generating significant quantities of adenosine in the extracellular space.
As CD73 and adenosine A2AR regulate each other reciprocally and as we had demonstrated previously that adenosine A2AR agonists promote recruitment of BM-MSCs to wounds and other sites, we determined whether adenosine A2AR regulated BM-MSC development. We report here that adenosine A2AR regulate the number of cells that differentiates as CFU-fibroblasts (CFU-Fs). Moreover, adenosine A2AR regulate the expression of differentiation antigens on the BM-MSCs that do develop. Moreover, our studies are consistent with prior studies demonstrating reciprocal regulation of CD73 by A2AR and vice versa.
MATERIALS AND METHODS
Isolation and expansion of mouse BM-MSCs
Mice
Female, 8- to 10-week-old B129 mice or adenosine A2AR knockout (KO) mice [20] on the same genetic background were used as the source of MSCs for these studies. At least three wild-type (WT) or KO mice were killed by CO2 for isolation of MSCs. The Institutional Animal Care and Use Committee of New York University School of Medicine (New York, NY, USA) approved these studies.
Isolation of MSCs
Soft tissue was gently dissected away from tibiae and femora, and the bones were crushed gently using a mortar and pestle to collect BM cells [21].
CFU-F assay
After removal of debris, BM cells were cultivated in six-well plates (Falcon, Becton Dickinson, Franklin Lakes, NJ, USA) at a concentration of 5 × 105 cells/ml in DMEM with high glucose concentration, 10% FBS (Atlanta Biological, Atlanta, GA, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco/Invitrogen, Carlsbad, CA, USA), and 0.8% methylcellulose (Gibco/Invitrogen). In cell cultures, half of the medium was refreshed every 3 days, and cultures were terminated on Day 10. At termination of the cultures, the medium was removed, and the plates were washed twice with PBS and fixed with methanol (10 min, room temperature). This was followed by staining of the CFU-Fs with a 1:10 dilution of Giemsa (Sigma-Aldrich, St. Louis, MO, USA) in Sörensen buffer. After a final rinse in tap water, colonies and cell numbers were counted using a standard inverted microscope. For the control A2AWT and A2AKO samples, clusters of cells were considered to be a colony if they contained 50 or more fibroblastoid cells [22, 23].
Flow cytometry
Mononuclear cells were isolated by centrifugation through Histopaque 1083 (Sigma-Aldrich) at 400 g for 30 min at 18°C. The cells were washed twice with PBS and seeded at 1 × 106 cells/ml in medium as above. The addition of methylcellulose to the cultures, as described originally by Brockbank et al. [22, 23], has the benefit of strongly reducing the outgrowth of contaminating macrophages. Cultures were incubated at 37°C in a 5% CO2 atmosphere. After 48 h, nonadherent cells, comprised mainly of hematopoietic cells, were removed by washing with PBS, and the remaining adherent population was cultured with medium alone or medium containing the A2AR agonist CGS21680 (1 μM) or the A2AR antagonist ZM241385 (1 μM). Confluent primary cultures were trypsinized, split 1:2, and passaged to a new culture. Subsequent passages were carried out when the culture approached confluence and split as needed to permit two subcultures per week.
Immunocytochemistry
Indirect immunocytochemical staining was performed using goat anti-mouse type I procollagen (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Cell cultures were washed and fixed in cold methanol and then permeabilized with 0.05% Triton X-100 in PBS. Nonspecific binding was suppressed by incubation with 5% normal serum (Vector Laboratories, Inc., Burlington, CA, USA) for 20 min. Following blocking, the cells were washed with 0.05% Tween 20, diluted in PBS, and incubated with the primary antibody, goat anti-mouse procollagen type I (1:200), for 2 h. Cells were then washed gently (×3) in PBS with 0.05% Tween 20. For detection, cells were incubated with the secondary antibodies: alkaline phosphatase-conjugated donkey anti-goat IgG (1:200, Santa Cruz Biotechnology) on a slowly plane-rotated plate for 45 min at room temperature. Cells were washed gently (×3) in PBS with 0.05% Tween 20 and developed by addition of Fast Red substrate system (Dako Cytomation, Carpenteria, CA, USA), according to the manufacturer’s protocol, and counterstained with hematoxylin. Controls included omission of the primary and/or secondary antibody and substitution of the primary antibody with an irrelevant antibody of the same species. Semiquantification of procollagen-positive cells was based on the average cell number of 10 high-power fields per well using a ×10 eyepiece and ×20 objective lens.
Phenotypic characterization
For flow cytometry staining, the adherent cells were detached from the plastic plates by trypsinization for 5 min at Day 7 of primary culture and at Day 5 after the third passage. Cells were then pelleted at 1000 g for 8 min, washed, and resuspended in PBS + 1% FCS + 0.1% sodium azide at a density of 10–20,000 cells/100 μl. Aliquots of cell suspension (50 μl) were then transferred to individual tubes, and nonspecific binding of receptors for IgG (FcR) was blocked by preincubation of the cells with FcR-blocking reagent (Miltenyi Biotec, Auburn, CA, USA). The cells were then labeled for 30 min on ice with the appropriate dilution of one of the following antibodies: goat anti-mouse procollagen type I-unconjugated, CD11b (FITC, eBioscience, San Diego, CA, USA), CD34 (FITC, eBioscience), CD44 (FITC, Abcam, Cambridge, MA, USA), CD45 (FITC, Abcam), CD73 (PE, Abcam), CD90 (allophycocyanin, Abcam), CD105 (FITC, R&D Systems, Minneapolis, MN, USA), and CD133 (FITC-labeled, eBioscience). For procollagen type I labeling, the cells were washed twice in staining buffer (PBS containing 2% FCS and 0.02% 1 M sodium azide) and resuspended in 100 μl Cytofix/Cytoperm (Becton Dickinson) for 20 min on ice. The fixed cells were washed twice in permeabilization buffer and stained with FITC-conjugated donkey anti-goat IgG for 60 min at 4°C in the dark. Finally, the cells were washed twice and resuspended in staining buffer. Nonimmune IgG of the corresponding class served as the negative control.
After washing in flow cytometry buffer, the cells were examined immediately or fixed in 0.5% formaldehyde (Polysciences, Warrenton, PA, USA) in PBS. Fluorescence was measured on a FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA, USA), and data were processed using FlowJo software (Tree Star, Inc., Ashland, OR, USA). At least 10,000 events were acquired for each sample. Viable cells were identified by gating on forward- and side-scatters, and the data are shown as logarithmic histograms of a representative single experiment or the mean ± sd of three experiments. Cell samples were considered positive for cluster of differentiation markers and procollagen type I, if at least 20% of the BM-MSCs/sample revealed higher fluorescence intensity than cells stained with the isotype-matched control antibody.
Western blot analysis
Cells were rinsed with ice-cold PBS, and total cell protein extracts were prepared using a cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA) containing protease inhibitors (2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin). Protein concentrations were measured by a bicinchoninic acid assay (Pierce, Rockford, IL, USA). Protein extracts (10 μg) were boiled in Laemmli sample buffer and subjected to SDS-PAGE (10% gels). Proteins were transferred to a nitrocellulose membrane (0.2 μm, Bio-Rad, Hercules, CA, USA) using a Bio-Rad gel-blotting apparatus. Membranes were stained with Poinceau Red to confirm transfer, followed by blocking of the membranes in 5% nonfat milk in a buffer consisting of 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 0.1% Tween 20 (TBST) for 1 h. Blots were probed with rabbit anti-mouse procollagen type I (1:1000 dilution; Santa Cruz Biotechnology). Following incubation with primary antibody, blots were exposed to goat anti-rabbit IgG antiserum conjugated to HRP (Gibco/Invitrogen) and developed with an ECL reagent (Amersham Biosciences, Piscataway, NJ, USA). Blots were then stripped and probed for β-actin (Abcam), as above.
RT-PCR and real-time quantitative PCR (qPCR) analyses for adenosine receptors and CD73
Total RNA from BM-MSCs was isolated using Trizol (Gibco/Invitrogen), following the manufacturer’s protocol. The PCR reaction mixture (50 μl) consisted of 2 μl cDNA in 1× PCR buffer [20 mM Tris-HCl (pH 8.4) and 50 mM KCl] containing 0.2 mM dNTP mix, 4.5 mM MgCl2, 100 ng each forward and reverse primer, and 1 U Platinum TaqDNA polymerase (Gibco/Invitrogen). sscDNA (cDNA) was synthesized and amplified using specific primers (forward and reverse): A1-ACAAAAACCAGTGGTGGAGTGA and TCTGTCCCCTCCCCTTGTC, A2A-TGAAGGCGAAGGGCATCA and GGGTCAGGCCGATGGC, A2B-ACGTGGCCGTGGGACTC and GCAGAAGCCCAAGCTGATG, A3-GAGACCTGCATCCTCCAGGTT and GGCCTGTTACAGGACCATCAA, CD73-CAAATCCCACACAACCACTG and TGCTCACTTGGTCACAGGAC, GAPDH-TCAACGGGAAGCCCATCA and CGGCCTCACCCCATTTG
PCR product was separated by electrophoresis on 2% agarose gels containing ethidium bromide (0.15 mg/ml), visualized with a UV transilluminator, and digitally photographed. The amplicon was quantitated densitometrically using Kodak Digital Science software.
Real-time qPCR analyses for adenosine receptors and CD73
Real-time PCRs were performed using the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), according to the instructions of the manufacturer, and carried out on the Mx3005Pt qPCR system (Stratagene, La Jolla, CA, USA). The reaction volume (25 uL) included 12.5 μL SYBR Green PCR Master Mix, 5 μL diluted cDNA (30 ng), and 0.2 μM each primer. Primers are described above. To standardize mRNA levels, we amplified GAPDH, a housekeeping gene, as an internal control. The amounts of target cDNA were determined using the comparative threshold (Ct) method. For each assay, standards, a no-template, and no-RT controls were included to verify the quality and cDNA specificity of the primers. Levels of RNA expression were determined according to the 2–ΔΔCt method. Briefly, expression results of a gene were normalized to internal control GAPDH and relative to a calibrator, consisting of the mean expression level of the corresponding gene in control samples as follows: 2–ΔΔCt = 2–[(Ct receptor gene–Ct GAPDH) KO MSCs–(Ct receptor gene–Ct GAPDH) calibrator]. The results from three independent repeat assays, performed in different plates, each using different cDNAs from the sample analyzed, were averaged to produce a single mean quantity value for each mRNA for each animal.
Statistical analysis
All experiments were repeated a minimum of three times, and qualitatively identical results were obtained. Differences between groups were analyzed by Student’s t-test or appropriate level of ANOVA. Statistical analyses were performed using Statistical Package for the Social Science (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered statistically significant. Where indicated, experimental data are reported as means ± sd of triplicate independent samples.
RESULTS
Adenosine A2AR promote CFU-F formation
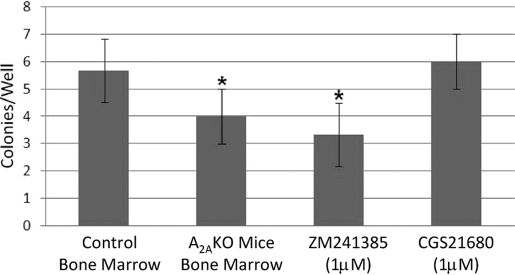
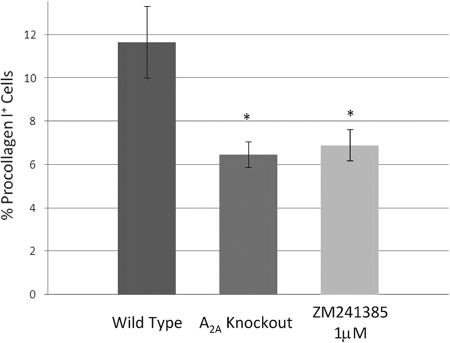
MSCs are characterized by their ability to form colonies comprising spindle-shaped cells deriving from a single cell (CFU-F assay). Interestingly, BM-MSCs cultured from adenosine A2AR KO mice formed significantly fewer colonies of fibroblasts (Fig. 1). Similarly, culture with an adenosine A2AR antagonist (ZM241385, 1 μM) diminished fibroblast colony formation as well, although culture with the A2A agonist did not enhance CFU-F formation. These findings suggest that endogenous adenosine production and ligation of A2AR play an important role in promoting maximal CFU-F formation, and no further increase could be stimulated by addition of an A2AR agonist.
Fig. 1.
The effect of adenosine A2AR deletion or blockade on CFU-F formation. CFU-Fs were cultured and assessed as described in Materials and Methods. The number of fibroblast colonies was counted in each well, and all determinations were carried out in triplicate using the cells from three different mice. Shown are means ± sd of these determinations. *, P < 0.05; Student’s t-test.
Adenosine receptor and CD73 mRNA expression
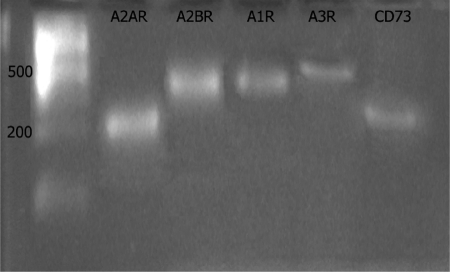
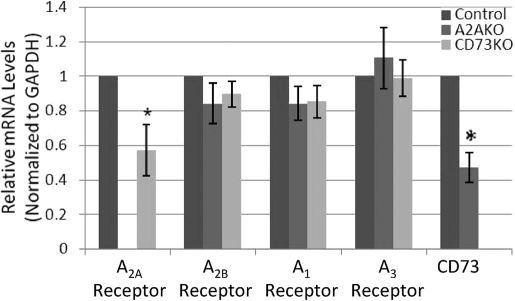
Messages for all four subtypes of adenosine receptors (A1, A2A, A2B, and A3) and CD73 were detectable in mRNA of MSCs derived from mouse BM (Fig. 2). Prior studies have demonstrated that CD73 and adenosine A2AR expression are mutually regulatory, and A2AR expression increases CD73 expression and vice versa [12, 24]. To determine whether expression of mRNA for these proteins was coordinated in BM-MSCs, we studied mRNA for CD73 and A2AR using real-time RT-PCR. Similar to what has been reported before, there was a significant decrease in mRNA for CD73 in cells from A2A KO mice and a marked reduction in mRNA for the A2AR in CD73 KO mice (Fig. 3).
Fig. 2.
Expression of mRNA for adenosine receptors and CD73 in third-passage murine MSCs. RNA was isolated from third-passage MSCs and reverse-transcribed to cDNA, as described. The cDNA was then subjected to PCR, as described, and electrophoresed through agarose.
Fig. 3.
Real-time RT-PCR analysis of adenosine receptors and CD73 mRNA level in third-passage MSCs. RNA was isolated from third-passage MSCs and reverse-transcribed to cDNA, as described. The cDNA was then subjected to real-time PCR using specific primers and expression levels calculated and normalized to an internal control (GAPDH). Results shown are the means (±sd) of relative mRNA expression. *, P < 0.05; n = 3; Student’s t-test.
Procollagen type I expression
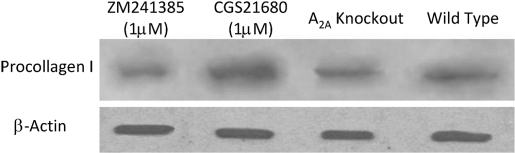
We have demonstrated previously that adenosine A2AR regulate collagen I expression by human dermal fibroblasts, hepatic stellate cells, and hepatic stellate cell lines [8, 25, 26]; thus, it seemed reasonable that adenosine A2AR would regulate collagen production by MSCs as well. We found that the adenosine A2AR agonist CGS21680 increased procollagen I protein levels significantly in cell lysates, and this increase was blocked completely by the adenosine A2AR antagonist ZM241385. Similarly, procollagen I levels were also diminished in the lysates of A2AR KO MSCs (Fig. 4). Indirect immunocytochemical staining for procollagen I in A2A KO MSCs demonstrated a significant decrease in the number of cells expressing procollagen I as compared with the WT MSCs, although the level of procollagen I on the surface of these cells was unchanged (Fig. 5), an observation that explains the diminished procollagen I in lysates detected by Western blot.
Fig. 4.
Procollagen type I protein expression in third-passage murine MSC lysates. Murine MSCs, isolated from WT or A2A KO mice were isolated and cultured, as described. In some cultures, the adenosine A2AR agonist CGS21680 or the A2AR antagonist ZM241385 was added to the cultures at a final concentration of 1 μM. Cell lysates were isolated and subjected to Western blot analysis, as described. Shown is a representative experiment of two.
Fig. 5.
Procollagen type I protein expression in third-passage murine MSCs, which were isolated from WT or A2A KO mice and were isolated and cultured as described. In some wells, the A2AR antagonist ZM241385 was added to the cultures of WT cells at a final concentration of 1 μM. Cells were then subjected to staining of intracellular procollagen I, as described, and the percentage of cells staining positive was quantitated by flow cytometry, as described. Shown are means ± sd of these determinations. *, P < 0.05, versus WT cells; n = 3; Student’s t-test.
Altered expression of MSC markers by cells from adenosine A2AR and CD73 KO mice
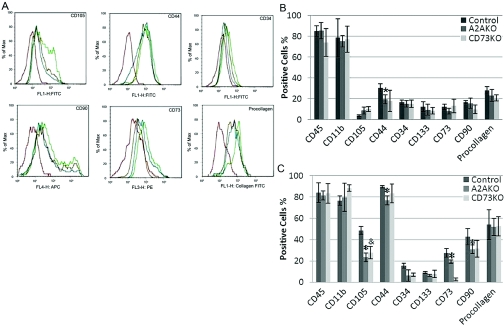
After 5 days of primary culture, WT cells express CD11b, CD44, CD45, and procollagen I but not CD34, CD133, CD73, CD90, or CD105. At this time-point, A2A KO cells do not express CD44 (Fig. 6A). Third-passage MSCs expressed all of the markers expressed in the primary cultures and increased expression of a number of these markers over time (Fig. 6, B and C). Of particular note, fewer than half as many CD73 KO and A2A KO cells expressed CD105 as WT cells (Fig. 6, B and C).
Fig. 6.
Expression of MSC markers on BM-derived MSCs. Murine MSCs, isolated from WT, CD73 KO, or A2A KO mice, were isolated and cultured, as described. Cells were then stained with appropriate antibodies and subjected to flow cytometric analysis. (A) Representative flow cytometric analysis of MSCs from mouse BM. Adenosine A2AR-deficient cells (blue), CD73-deficient cells (brown), WT cells (green), and cells stained with an isotype-matched control antibody (red). (B) Analysis of stem cell marker expression following 7 days of culture. All studies were carried out on the cells from three different mice. (C) Analysis of stem cell marker expression following 12 days of culture. All studies were carried out on the cells from three different mice. *, P < 0.01, versus WT cells, n = 3; &, P < 0.05, versus WT cells, n = 3.
DISCUSSION
This is the first study to directly evaluate the effect of adenosine signaling on regulation of BM-MSC proliferation and development. Our results demonstrate that endogenously produced adenosine, acting through A2AR, plays a significant role in promoting MSC development and protein expression. Moreover, our results confirm that adenosine A2AR and CD73 are coordinately regulated. These results are significant, as fibrocytes and endothelial progenitor cells, both derived from BM-MSCs, play an important role in wound healing and fibrosis, and adenosine A2AR are critical for normal wound healing and for pharmacologic stimulation of wound healing [3,4,5,6].
In our initial studies, we observed that ZM241385 (1 μM) diminished CFU-F development. Although previously reported to be selective for antagonism at A2AR, ZM241385 loses its specificity at higher concentrations and may also antagonize A2BR. In prior studies, we have observed that the concentration of ZM241385 used was selective for A2AR antagonism in the inhibition of thrombospondin-1 production by human vascular endothelial cells [7]. Although we did not confirm this finding specifically in the present study, the similarity of the effect of A2AR deletion on CFU-F development is strong evidence that the effect of ZM241385 was mediated by antagonism of A2AR. Nonetheless, it is possible that A2BR also play a role in the development of CFU-F and that the effects of ZM241385 result from antagonism of A2BR. Indeed, A2BR also play a role in collagen production and fibrosis [27,28,29,30].
The effect of endogenous adenosine and adenosine A2AR on CFU-F formation was studied here under relatively hyperoxic conditions (21% O2 in room air). As hypoxia increases adenosine release from cells (reviewed recently in ref. [31]), it is likely that the effect of adenosine and adenosine A2AR would be even more marked if it were studied under conditions of hypoxia (1–3% O2), and A2AR deletion or blockade would reduce CFU-F formation further to levels lower than those observed here. Nonetheless, even under the relatively hyperoxic conditions studied, there was a marked and significant reduction in CFU-F formation.
Perhaps the most significant finding of this study is the demonstration that adenosine A2AR deletion or blockade diminishes the number of CFU-Fs that develops from BM-MSCs. One explanation for this phenomenon is that adenosine, acting at the A2AR, may increase the proliferation of MSCs, as has been shown for other cell types [32,33,34,35,36,37,38]. Alternatively, adenosine A2AR stimulation has been shown to diminish apoptosis in other cell types and may thus permit increased CFU-F proliferation [39,40,41,42].
Previous studies by Evans and colleagues [15] demonstrated the presence of all four adenosine receptor subtypes in primary human BM stromal cells, similar to our findings with murine cells, and that these cells produce adenosine, which is released as the result of ATP catabolism. ATP is one of the most abundant molecules in cells, and adenine nucleotides may be released into the extracellular milieu following cellular stimulation, stress, or trauma. Extracellular adenine nucleotides are dephosphorylated to AMP by NTP dephosphorylase (CD39) and further dephosphorylated to adenosine by the plasma membrane-associated enzyme 5′-ectonucleotidase (CD73), also a marker for MSCs, which is reduced significantly in A2A KO MSCs.
The coordinate regulation of CD73 and adenosine A2AR suggests that the compensations mediated via these cell-surface proteins require increased capacity to generate extracellular adenosine as well [24]. Previous studies have demonstrated that adenosine A2AR expression and function are up-regulated by TNF-α, IL-1, and LPS [43,44,45,46,47] on endothelial cells and monocytes/monocytoid cell lines, whereas the effects of these cytokines on CD73 expression have not been established. In contrast, CD73 expression is increased by a variety of hormones, cytokines, and other factors, including hypoxia-inducible factor-1α [48,49,50,51,52,53,54,55,56,57]. It is likely that the regulation of adenosine A2AR and CD73 by inflammatory cytokines and other factors is coordinated as well and represents a mechanism for feedback inhibition of inflammation, hypoxia, and other potentially deleterious conditions.
Although most MSC markers were unaffected by A2AR deletion, the percentage of MSCs expressing CD105, endoglin, was reduced markedly in the A2A KO cells. Endoglin is an auxiliary receptor for TGF-β, expressed primarily on endothelium but also on MSCs (reviewed in ref. [58]), and this protein is highly expressed on tumor vasculature and other neo-vasculatures. We have not determined whether adenosine A2AR activation inhibits release of endoglin from cells or whether it stimulates endoglin expression, but it is likely that the reduction in endoglin contributes to the reduced MSC development observed in these studies.
It is interesting to note that agents that increase extracellular adenosine (AMP and dipyridamole) have been reported to protect BM from radiation and chemotherapy injury and to enhance responses to G-CSF following chemotherapy or radiation [59,60,61,62,63,64,65]. More recently, it was recognized that adenosine A1R and A3R [59,60,61] were involved in this phenomenon, and it is interesting to speculate that different adenosine receptors may modulate the proliferation and differentiation of different populations of BM stem cells. Although the effects of these different adenosine receptors on BM cells are clear, it is possible that the effects are indirect and mediated by downstream effects of adenosine and adenosine receptors on the synthesis and secretion of cytokines or other agents that regulate proliferation or apoptosis directly [66, 67].
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR56672, AR56672S1, and AR54897) and the NYU-HH Clinical and Translational Science Institute (UL1RR029893).
References
- Ting A E, Mays R W, Frey M R, Hof W V, Medicetty S, Deans R. Therapeutic pathways of adult stem cell repair. Crit Rev Oncol Hematol. 2008;65:81–93. doi: 10.1016/j.critrevonc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Togel F, Westenfelder C. Adult bone marrow-derived stem cells for organ regeneration and repair. Dev Dyn. 2007;236:3321–3331. doi: 10.1002/dvdy.21258. [DOI] [PubMed] [Google Scholar]
- Montesinos M C, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang C K, Hirschhorn R, Recht P A, Ostad E, Levin R I, Cronstein B N. Wound healing is accelerated by agonists of adenosine A2 (G α s- linked) receptors. J Exp Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor-Vega C, Desai A, Montesinos M, Cronstein B. Adenosine A2A agonists promote more rapid wound healing than recombinant human platelet derived growth factor (PDGF) Inflammation. 2002;26:19–24. doi: 10.1023/a:1014417728325. [DOI] [PubMed] [Google Scholar]
- Montesinos M C, Desai A C, Chen J F, Yee H, Schwarzschild M A, Fink J S, Cronstein B N. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol. 2002;160:2009–2018. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos M C, Shaw J P, Yee H, Shamamian P, Cronstein B N. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004;164:1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Victor-Vega C, Gadangi S, Montesinos M C, Chu C C, Cronstein B N. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005;67:1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- Chan E S, Fernandez P, Merchant A A, Montesinos M C, Trzaska S, Desai A, Tung C F, Khoa D N, Pillinger M H, Reiss A B, Tomic-Canic M, Chen J F, Schwarzschild M A, Cronstein B N. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 2006;54:2632–2642. doi: 10.1002/art.21974. [DOI] [PubMed] [Google Scholar]
- Lennon P F, Taylor C T, Stahl G L, Colgan S P. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L F, Eltzschig H K, Ibla J C, Van De Wiele C J, Resta R, Morote-Garcia J C, Colgan S P. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig H K, Thompson L F, Karhausen J, Cotta R J, Ibla J C, Robson S C, Colgan S P. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- Nemoto E, Kunii R, Tada H, Tsubahara T, Ishihata H, Shimauchi H. Expression of CD73/ecto-5′-nucleotidase on human gingival fibroblasts and contribution to the inhibition of interleukin-1α-induced granulocyte-macrophage colony stimulating factor production. J Periodontal Res. 2004;39:10–19. doi: 10.1111/j.1600-0765.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Bidzhekov K, Ozuyaman B, Fraemohs L, Liehn E A, Luscher-Firzlaff J M, Luscher B, Schrader J, Weber C. CD73/ecto-5′-nucleotidase protects against vascular inflammation and neointima formation. Circulation. 2006;113:2120–2127. doi: 10.1161/CIRCULATIONAHA.105.595249. [DOI] [PubMed] [Google Scholar]
- Montesinos M C, Takedachi M, Thompson L F, Wilder T F, Fernandez P, Cronstein B N. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: findings in a study of ecto-5′-nucleotidase gene-deficient mice. Arthritis Rheum. 2007;56:1440–1445. doi: 10.1002/art.22643. [DOI] [PubMed] [Google Scholar]
- Evans B A J, Elford C, Pexa A, Francis K, Hughes A C, Deussen A, Ham J. Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. J Bone Miner Res. 2006;21:228–236. doi: 10.1359/JBMR.051021. [DOI] [PubMed] [Google Scholar]
- Volmer J B, Thompson L F, Blackburn M R. Ecto-5′-nucleotidase (CD73)-mediated adenosine production is tissue protective in a model of bleomycin-induced lung injury. J Immunol. 2006;176:4449–4458. doi: 10.4049/jimmunol.176.7.4449. [DOI] [PubMed] [Google Scholar]
- Peng Z, Fernandez-Ferri P, Wilder T, Yee H, Chiriboga L, Chan E, Cronstein B. Ecto-5′-nucleotidase (CD73)-mediated extracellular adenosine production plays a critical role in hepatic fibrosis. FASEB J. 2008;22:2263–2272. doi: 10.1096/fj.07-100685. [DOI] [PubMed] [Google Scholar]
- Deaglio S, Dwyer K M, Gao W, Friedman D, Usheva A, Erat A, Chen J F, Enjyoji K, Linden J, Oukka M, Kuchroo V K, Strom T B, Robson S C. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson M A, Osswald H, Thompson L F, Unertl K, Eltzschig H K. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- Chen J F, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz M A, Fink J S, Schwarzschild M A. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin G A, Lambert J F, Moore B E, Carlson J E, Dooner M S, Abedi M, Cerny J, Quesenberry P J. Intrinsic hematopoietic stem cell/progenitor plasticity: inversions. J Cell Physiol. 2004;199:20–31. doi: 10.1002/jcp.10436. [DOI] [PubMed] [Google Scholar]
- Brockbank K G, Ploemacher R E, van Peer C M. An in vitro analysis of murine hemopoietic fibroblastoid progenitors and fibroblastoid cell function during aging. Mech Ageing Dev. 1983;22:11–21. doi: 10.1016/0047-6374(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Brockbank K G, Ploemacher R E. Quantitation of stromal and hemopoietic progenitors in spleen and femoral marrow derived from Steel (Slj/+ and Sl/Sld) mice and their normal littermates. Exp Hematol. 1983;11:467–474. [PubMed] [Google Scholar]
- Napieralski R, Kempkes B, Gutensohn W. Evidence for coordinated induction and repression of ecto-5′-nucleotidase (CD73) and the A2a adenosine receptor in a human B cell line. Biol Chem. 2003;384:483–487. doi: 10.1515/BC.2003.054. [DOI] [PubMed] [Google Scholar]
- Chan E S, Montesinos M C, Fernandez P, Desai A, Delano D L, Yee H, Reiss A B, Pillinger M H, Chen J F, Schwarzschild M A, Friedman S L, Cronstein B N. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che J, Chan E S, Cronstein B N. Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol Pharmacol. 2007;72:1626–1636. doi: 10.1124/mol.107.038760. [DOI] [PubMed] [Google Scholar]
- Dubey R K, Gillespie D G, Jackson E K. Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: role of A2B receptors. Hypertension. 1998;31:943–948. doi: 10.1161/01.hyp.31.4.943. [DOI] [PubMed] [Google Scholar]
- Chen Y, Epperson S, Makhsudova L, Ito B, Suarez J, Dillmann W, Villarreal F. Functional effects of enhancing or silencing adenosine A2b receptors in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2004;287:H2478–H2486. doi: 10.1152/ajpheart.00217.2004. [DOI] [PubMed] [Google Scholar]
- Murakami S, Hashikawa T, Saho T, Takedachi M, Nozaki T, Shimabukuro Y, Okada H. Adenosine regulates the IL-1 β-induced cellular functions of human gingival fibroblasts. Int Immunol. 2001;13:1533–1540. doi: 10.1093/intimm/13.12.1533. [DOI] [PubMed] [Google Scholar]
- Dubey R K, Gillespie D G, Zacharia L C, Mi Z, Jackson E K. A(2b) receptors mediate the antimitogenic effects of adenosine in cardiac fibroblasts. Hypertension. 2001;37:716–721. doi: 10.1161/01.hyp.37.2.716. [DOI] [PubMed] [Google Scholar]
- Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 α and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- Meininger C J, Schelling M E, Granger H J. Adenosine and hypoxia stimulate proliferation and migration of endothelial cells. Am J Physiol. 1988;255:H554–H562. doi: 10.1152/ajpheart.1988.255.3.H554. [DOI] [PubMed] [Google Scholar]
- Ethier M F, Chander V, Dobson J G., Jr Adenosine stimulates proliferation of human endothelial cells in culture. Am J Physiol. 1993;265:H131–H138. doi: 10.1152/ajpheart.1993.265.1.H131. [DOI] [PubMed] [Google Scholar]
- Sexl V, Mancusi G, Baumgartner-Parzer S, Schutz W, Freissmuth M. Stimulation of human umbilical vein endothelial cell proliferation by A2-adenosine and β 2-adrenoceptors. Br J Pharmacol. 1995;114:1577–1586. doi: 10.1111/j.1476-5381.1995.tb14942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexl V, Mancusi G, Holler C, Gloria-Maercker E, Schutz W, Freissmuth M. Stimulation of the mitogen-activated protein kinase via the A2A-adenosine receptor in primary human endothelial cells. J Biol Chem. 1997;272:5792–5799. doi: 10.1074/jbc.272.9.5792. [DOI] [PubMed] [Google Scholar]
- Sun L L, Xu L L, Nielsen T B, Rhee P, Burris D. Cyclopentyladenosine improves cell proliferation, wound healing, and hair growth. J Surg Res. 1999;87:14–24. doi: 10.1006/jsre.1999.5716. [DOI] [PubMed] [Google Scholar]
- Shimegi S. ATP and adenosine act as a mitogen for osteoblast-like cells (MC3T3–E1) Calcif Tissue Int. 1996;58:109–113. doi: 10.1007/BF02529732. [DOI] [PubMed] [Google Scholar]
- Shimegi S. Mitogenic action of adenosine on osteoblast-like cells, MC3T3–E1. Calcif Tissue Int. 1998;62:418–425. doi: 10.1007/s002239900454. [DOI] [PubMed] [Google Scholar]
- Walker B A, Rocchini C, Boone R H, Ip S, Jacobson M A. Adenosine A2a receptor activation delays apoptosis in human neutrophils. J Immunol. 1997;158:2926–2931. [PubMed] [Google Scholar]
- Mayne M, Fotheringham J, Yan H J, Power C, Del Bigio M R, Peeling J, Geiger J D. Adenosine A2A receptor activation reduces proinflammatory events and decreases cell death following intracerebral hemorrhage. Ann Neurol. 2001;49:727–735. doi: 10.1002/ana.1010. [DOI] [PubMed] [Google Scholar]
- Zhao Z Q, Budde J M, Morris C, Wang N P, Velez D A, Muraki S, Guyton R A, Vinten-Johansen J. Adenosine attenuates reperfusion-induced apoptotic cell death by modulating expression of Bcl-2 and Bax proteins. J Mol Cell Cardiol. 2001;33:57–68. doi: 10.1006/jmcc.2000.1275. [DOI] [PubMed] [Google Scholar]
- Apasov S, Chen J F, Smith P, Sitkovsky M. A(2A) receptor dependent and A(2A) receptor independent effects of extracellular adenosine on murine thymocytes in conditions of adenosine deaminase deficiency. Blood. 2000;95:3859–3867. [PubMed] [Google Scholar]
- Morello S, Ito K, Yamamura S, Lee K Y, Jazrawi E, Desouza P, Barnes P, Cicala C, Adcock I M. IL-1β and TNF-{α} regulation of the adenosine receptor (A2A) expression: differential requirement for NF-{κ}B binding to the proximal promoter. J Immunol. 2006;177:7173–7183. doi: 10.4049/jimmunol.177.10.7173. [DOI] [PubMed] [Google Scholar]
- Murphree L J, Sullivan G W, Marshall M A, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-κB in A(2A) adenosine receptor induction. Biochem J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoa N D, Montesinos M C, Reiss A B, Delano D, Awadallah N, Cronstein B N. Inflammatory cytokines regulate function and expression of adenosine A2A receptors in human monocytic THP-1 cells. J Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- Nguyen D K, Montesinos M C, Williams A J, Kelly M, Cronstein B N. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. J Immunol. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- Khoa N D, Postow M, Danielsson J, Cronstein B N. Tumor necrosis factor-{α} prevents desensitization of G{α}s-coupled receptors by regulating GRK2 association with the plasma membrane. Mol Pharmacol. 2006;69:1311–1319. doi: 10.1124/mol.105.016857. [DOI] [PubMed] [Google Scholar]
- Kiss J, Yegutkin G G, Koskinen K, Savunen T, Jalkanen S, Salmi M. IFN-β protects from vascular leakage via up-regulation of CD73. Eur J Immunol. 2007;37:3334–3338. doi: 10.1002/eji.200737793. [DOI] [PubMed] [Google Scholar]
- Buffon A, Wink M R, Ribeiro B V, Casali E A, Libermann T A, Zerbini L F, Robson S C, Sarkis J J. NTPDase and 5′ ecto-nucleotidase expression profiles and the pattern of extracellular ATP metabolism in the Walker 256 tumor. Biochim Biophys Acta. 2007;1770:1259–1265. doi: 10.1016/j.bbagen.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Li X, Zhou T, Zhi X, Zhao F, Yin L, Zhou P. Effect of hypoxia/reoxygenation on CD73 (ecto-5′-nucleotidase) in mouse microvessel endothelial cell lines. Microvasc Res. 2006;72:48–53. doi: 10.1016/j.mvr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Tamajusuku A S, Carrillo-Sepulveda M A, Braganhol E, Wink M R, Sarkis J J, Barreto-Chaves M L, Battastini A M. Activity and expression of ecto-5′-nucleotidase/CD73 are increased by thyroid hormones in vascular smooth muscle cells. Mol Cell Biochem. 2006;289:65–72. doi: 10.1007/s11010-006-9148-0. [DOI] [PubMed] [Google Scholar]
- Huang D Y, Vallon V, Zimmermann H, Koszalka P, Schrader J, Osswald H. Ecto-5′-nucleotidase (cd73)-dependent and -independent generation of adenosine participates in the mediation of tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol. 2006;291:F282–F288. doi: 10.1152/ajprenal.00113.2005. [DOI] [PubMed] [Google Scholar]
- Synnestvedt K, Furuta G T, Comerford K M, Louis N, Karhausen J, Eltzschig H K, Hansen K R, Thompson L F, Colgan S P. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsi K, Lawson C, Dominguez M, Taylor P, Yacoub M H, Smolenski R T. Regulation of ecto-5′-nucleotidase by TNF-α in human endothelial cells. Mol Cell Biochem. 2002;232:113–119. doi: 10.1023/a:1014806916844. [DOI] [PubMed] [Google Scholar]
- Narravula S, Lennon P F, Mueller B U, Colgan S P. Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J Immunol. 2000;165:5262–5268. doi: 10.4049/jimmunol.165.9.5262. [DOI] [PubMed] [Google Scholar]
- Airas L, Niemela J, Salmi M, Puurunen T, Smith D J, Jalkanen S. Differential regulation and function of CD73, a glycosyl-phosphatidylinositol-linked 70-kD adhesion molecule, on lymphocytes and endothelial cells. J Cell Biol. 1997;136:421–431. doi: 10.1083/jcb.136.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemela J, Henttinen T, Yegutkin G G, Airas L, Kujari A M, Rajala P, Jalkanen S. IFN-α induced adenosine production on the endothelium: a mechanism mediated by CD73 (ecto-5′-nucleotidase) up-regulation. J Immunol. 2004;172:1646–1653. doi: 10.4049/jimmunol.172.3.1646. [DOI] [PubMed] [Google Scholar]
- Ten Dijke P, Goumans M J, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11:79–89. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- Hofer M, Pospisil M, Znojil V, Holá J, Streitova D, Vacek A. Homeostatic action of adenosine A3 and A1 receptor agonists on proliferation of hematopoietic precursor cells. Exp Biol Med (Maywood) 2008;233:897–900. doi: 10.3181/0802-RM-43. [DOI] [PubMed] [Google Scholar]
- Hofer M, Vacek A, Pospíšil M, Holá J, Streitová D, Znojil V. Activation of adenosine A(3) receptors potentiates stimulatory effects of IL-3, SCF, and GM-CSF on mouse granulocyte-macrophage hematopoietic progenitor cells. Physiol Res. 2008 doi: 10.33549/physiolres.931454. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hofer M, Pospisil M, Znojil V, Hola J, Vacek A, Streitova D. Adenosine A(3) receptor agonist acts as a homeostatic regulator of bone marrow hematopoiesis. Biomed Pharmacother. 2007;61:356–359. doi: 10.1016/j.biopha.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Pospisil M, Hofer M, Znojil V, Vacha J, Netikova J, Hola J. Synergistic effect of granulocyte colony-stimulating factor and drugs elevating extracellular adenosine on neutrophil production in mice. Blood. 1995;86:3692–3697. [PubMed] [Google Scholar]
- Pospisil M, Hofer M, Znojil V, Vacha J, Netikova J, Hola J. Radioprotection of mouse hemopoiesis by dipyridamole and adenosine monophosphate in fractionated treatment. Radiat Res. 1995;142:16–22. [PubMed] [Google Scholar]
- Hofer M, Pospisil M, Netikova J, Znojil V, Vacha J, Hola J. Radioprotective efficacy of dipyridamole and AMP combination in fractionated radiation regimen, and its dependence on the time of administration of the drugs prior to irradiation. Physiol Res. 1995;44:93–98. [PubMed] [Google Scholar]
- Bohacek J, Hosek B, Pospisil M. Postirradiation administration of adenosine monophosphate combined with dipyridamole reduces early cellular damage in mice. Life Sci. 1993;53:1317–1324. doi: 10.1016/0024-3205(93)90577-p. [DOI] [PubMed] [Google Scholar]
- Hasko G, Nemeth Z H, Vizi E S, Salzman A L, Szabo C. An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-γ production and prevents lethality in endotoxemic mice. Eur J Pharmacol. 1998;358:261–268. doi: 10.1016/s0014-2999(98)00619-0. [DOI] [PubMed] [Google Scholar]
- Bar-Yehuda S, Madi L, Barak D, Mittelman M, Ardon E, Ochaion A, Cohn S, Fishman P. Agonists to the A3 adenosine receptor induce G-CSF production via NF-κB activation: a new class of myeloprotective agents. Exp Hematol. 2002;30:1390–1398. doi: 10.1016/s0301-472x(02)00962-1. [DOI] [PubMed] [Google Scholar]