Abstract
Objective:
To identify the prevalence and severity of fatigue and predicting factors for severe fatigue in autosomal dominant spinocerebellar ataxia (SCA).
Methods:
We studied a cross-section of 123 patients with SCA. Six functional scales were used in a self-assessment: the Fatigue Severity Scale (FSS); the Beck Depression Inventory (BDI); the Rotterdam Handicap Scale (RHS); the Short Form–36 health survey, distinguishing a norm-based physical and mental component score (Nb-PCS and Nb-MCS); the Pittsburgh Sleep Quality Index (PSQI); and the Epworth Sleepiness Scale (ESS). A subset of 58 patients was clinically evaluated, measuring severity of ataxia with the Scale for the Assessment and Rating of Ataxia and cognitive functioning with the Mini-Mental State Examination.
Results:
Severe fatigue (FSS ≥5) was present in 69% of patients and FSS value correlated with the scores on RHS, Nb-PCS, Nb-MCS, BDI, PSQI, and ESS. There was no relation with disease duration, gender, or medication use. Multivariate analysis revealed that Nb-PCS and BDI were the best independent predictors for severe fatigue. Interestingly, the presence of visual symptoms was related to FSS value in the clinically evaluated subgroup.
Conclusion:
Fatigue is a severe and disabling symptom in adult patients with SCA, even early in the course of disease. Physical functioning and depression are the strongest predictors of fatigue. In treatment strategies, all treatable factors for fatigue should be addressed, especially depression, visual symptoms, and sleeping disorders.
Cerebellar ataxias represent a heterogeneous group of neurodegenerative disorders, distinguishing hereditary and sporadic ataxias. The majority of adult-onset hereditary ataxias are constituted by autosomal dominant cerebellar ataxias (ADCAs), also referred to as spinocerebellar ataxias (SCAs), with SCA1, SCA2, SCA3, and SCA6 as the most common genotypes.1–3 In 30% of Dutch patients with ADCA, no SCA genotype can be identified.4
Cerebellar ataxia is the predominant symptom in all patients with SCA.5,6 In our experience, adults with cerebellar ataxia often complain of fatigue, limiting quality of life apart from physical impairment. To our knowledge, fatigue has been studied in 2 small series of patients with Friedreich ataxia and SCA3,7,8 but without data about predictors of fatigue. Severity of ataxia and noncerebellar symptoms and depression have been shown to predict the health status in patients with SCA 1, 2, 3, and 6 in a European multicenter study, but fatigue was not investigated.9
In neurologic disorders like multiple sclerosis (MS), Parkinson disease (PD), head trauma, stroke, or neuromuscular disorders, fatigue is increasingly recognized as an important cause of disability.10–16 Predicting factors of fatigue are depression (MS, PD, stroke), physical impairment (MS, neuromuscular disorders), but not disease duration.11,14,15,17,18
We aimed to investigate the prevalence of fatigue and its predicting factors in Dutch patients with SCA. Severity of fatigue was quantified with the Fatigue Severity Scale (FSS). We collected data on disease-specific factors (disease duration, severity of ataxia, physical impairment, SCA subtype) and factors known to be related to fatigue (medication use, sleep disturbances, depression, quality of life).
METHODS
Patient assessments.
Patients aged 18 years and older were recruited between March and October 2009 from our outpatient clinic (n = 16) and from the Dutch ADCA patient foundation, by a written invitation. We included patients with ADCA displaying one of the known SCA mutations and patients with positive family history but without identifiable SCA genotype, since these SCA-negative patients with ADCA represent a significant proportion of SCAs. The clinical diagnosis was established by one of the neurologists (E.B., J.v.S.) or it was verified by reviewing medical records. Patients were asked to complete a questionnaire including demographic data, diagnosis, onset age, comorbidity and use of medication, the Dutch version of the Fatigue Severity Scale (FSS), the Rotterdam Handicap Scale (RHS), the Medical Outcomes Study 36-item Short Form health survey (SF-36), the Beck Depression Inventory (BDI), the Pittsburgh Sleep Quality Index (PSQI), and the Epworth Sleepiness Scale (ESS).
We were especially interested in medication use and comorbidity known to be related to fatigue: drugs like β-adrenergic blockers, antidepressants, or sedatives, and disorders like thyroid disease, anemia, cardiac failure, and malignancies. We did not perform new blood tests on thyroid status, hemoglobin level, or other metabolic disturbances.
The FSS is a 9-item questionnaire with a score ranging from 1 (“strongly disagree”) to 7 (“strongly agree”) for each item. The mean FSS value of the 9 inquiries ranges from 1 (“no signs of fatigue”) to 7 (“most disabling fatigue”). A mean FSS value of 5 or higher indicates severe fatigue, the FSS value of 5 being the 95th percentile in healthy controls.14,19,20 The RHS comprises 9 items, resulting in a total score between 9 (“unable to fulfill any applicable task or activity”) to 36 (“able to fulfill all applicable tasks or activities”).21,22 Both FSS and RHS have been validated in patients with peripheral and central nervous disorders, and have shown good internal consistency, test-retest reliability, and discriminative validity.14,19,20
The SF-36 is a well evaluated, widely used health status survey, comprising 8 domains: physical functioning, role limitations due to physical health problems, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and general mental health. We used the standard version. All domain scores were transformed to norm-based scores, related to the 8 domain means and standard deviations derived from a Dutch community23; summation resulted in a norm-based Physical Component Summary (Nb-PCS) measure (domains 1–4) and a norm-based Mental Component Summary (Nb-MCS) measure (domains 5–8). Higher summary scores indicate higher levels of functioning or well-being.24
We included the long form of the BDI with a total score ranging from 0 to 63, defining scores from 0–9 as normal, 10–15 as minimal depression, 16–19 as mild to moderate depression, 20–29 as moderate to severe depression, and 30–63 as severe depression.25,26 In PD, BDI has proven to be a reliable measure of depression despite the inclusion of somatic items, not just reflecting disease severity.25,26 To study fatigue in relation to the presence of sleep disturbances, we included the PSQI and the ESS. The PSQI assesses sleep quality and disturbances over a 1-month time interval, using 7 components, resulting in a global score from 0 to 21. A global score >5 indicates poor sleep quality.27 The ESS measures sleep propensity in 8 different daily situations, resulting in a total score ranging from 0 (no excessive daytime sleepiness) to 24 (severe excessive daytime sleepiness).28
All patients were requested in the informed consent letter to participate in a consecutive clinical evaluation at our clinic. Sixty-two patients were willing to participate and were examined by the same neurologist (E.B.). History in these patients specifically addressed factors causing or relieving fatigue, presence of gait and limb ataxia, visual complaints, dysarthria, dysphagia, and level of impairment. The severity of ataxia was assessed with the 8-item Scale for the Assessment and Rating of Ataxia (SARA), with a sumscore ranging from 0 (absence of ataxia) to 40 (most severe ataxia).6 Global cognitive functioning was expressed in the Mini-Mental State Examination (MMSE), with a maximum score of 30.29
Standard protocol approvals, registrations, and patient consents.
The study was approved by the ethics committee of the Erasmus MC University Medical Center and informed consent was obtained from all participants.
Statistical analysis.
We estimated the need of including 97 patients to reach a power of 0.80 in analyzing whether duration of the disease, depression, and disease severity have the potential to predict the level of fatigue by multiple regression analysis, assuming an α level of 0.05 (2-tailed) and further assuming that the explained variance of these 3 predictive values with respect to fatigue is expected to be 10% above and beyond the estimated 10% of the gender and age as confounding factors.
Outcome for specific ataxia diagnosis was recoded in 4 categories (ADCA, SCA3, SCA6, and other SCA mutation) reflecting the most frequent SCA subtypes in our population. ADCA addresses the category of patients with SCA without a mutation in the known SCA genes. Other SCA mutation refers to patients with alternative SCA genotypes apart from SCA3 or SCA6. Associations between variables and FSS values were tested using Spearman correlation test for continuous variables and the Mann-Whitney U test or Kruskal-Wallis test, as appropriate, for categorical variables.
Since fatigue had a skewed distribution which could not be transformed, we dichotomized fatigue for the predictive model, with FSS <5 reflecting mild to moderate fatigue and FSS ≥5 reflecting severe fatigue. To identify variables from the self-assessment that were associated with FSS, χ2 tests or Fisher exact tests for categorical variables and Mann-Whitney U test for continuous variables were performed. Variables with a significance of p ≤ 0.15 were considered as candidate variables for multivariate logistic regression analysis and were all entered, except for Nb-MCS. The reason to leave out this variable lies in the fact that the items of the vitality domain of the Nb-MCS are similar to the FSS items, which will obviously lead to a prediction of FSS score. Subsequently, variables with the highest p value were eliminated step by step, until the fit of the model decreased significantly (based on the likelihood ratio test).
Evaluation was performed with SPSS 15.0 for Windows (SPSS Inc., 2007).
RESULTS
Clinical characteristics.
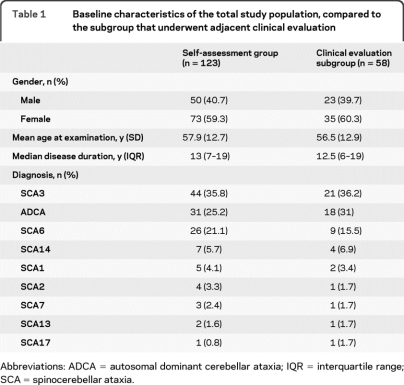
We distributed 365 self-assessment questionnaires, to which 157 patients responded (response rate 41%). We had to exclude 34 individuals (21% of responders): 28 patients had an alternative or uncertain diagnosis, in 4 individuals data were incomplete, 1 patient was deceased, and 1 was unable to fill in the assessment. Eventually, we enrolled 123 patients (78% of responders) and 58 patients participated in the subsequent clinical evaluation (47% of enrolled patients). Baseline characteristics of the overall study population and the clinically evaluated subgroup are similar (table 1). The proportion of women (59.3%) is higher than men (40.7%): p = 0.035. The mean age at examination is 57.9 years and the median disease duration 13 years. ADCA (SCA without identified gene mutation), SCA3, and SCA6 were the most frequent diagnoses.
Table 1.
Baseline characteristics of the total study population, compared to the subgroup that underwent adjacent clinical evaluation
Abbreviations: ADCA = autosomal dominant cerebellar ataxia; IQR = interquartile range; SCA = spinocerebellar ataxia.
Fatigue.
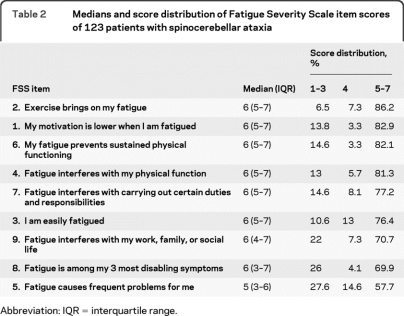
The median FSS value in our patients was 5.8 (interquartile range 4.6–6.6), with 69% of the patients having a median FSS score of 5 or higher, indicating severe fatigue. The median FSS item scores and their score distributions are presented in table 2. The largest proportion of scores ≥5 is seen in FSS item 2: “Exercise brings on my fatigue,” one of the items relating fatigue to physical functioning. Nearly 70% of the patients classified fatigue among their 3 most disabling symptoms (scoring 5 or higher on FSS item 8, agreeing with this quote).
Table 2.
Medians and score distribution of Fatigue Severity Scale item scores of 123 patients with spinocerebellar ataxia
Abbreviation: IQR = interquartile range.
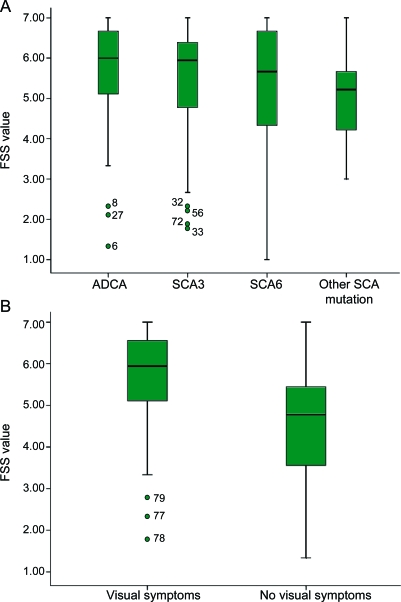
There was no significant relation between gender, age, disease duration, or SCA subtype and FSS value (table 3; figure, A). Medication use and comorbidity were not significantly related to FSS value (table 3). RHS and Nb-PCS, reflecting physical function, were significantly correlated with FSS value (table 3). However, SARA, indicating severity of ataxia, did not correlate with FSS in the clinically evaluated subgroup (p = 0.94). In this subgroup, patients with visual symptoms (symptomatic nystagmus or diplopia) were more fatigued than patients without visual complaints (p = 0.017; figure, B). BDI, reflecting depression, and both PSQI and ESS were significantly related to FSS value (table 3), but not cognitive functioning on MSSE (p = 0.94).
Table 3.
Correlations (Spearman rank correlation coefficient and corresponding p value) between the FSS and basic characteristics, physical and mental functioning, and sleep in patients with spinocerebellar ataxia, followed by p values between FSS and categorical variables of gender, medication use, and specific diagnosis
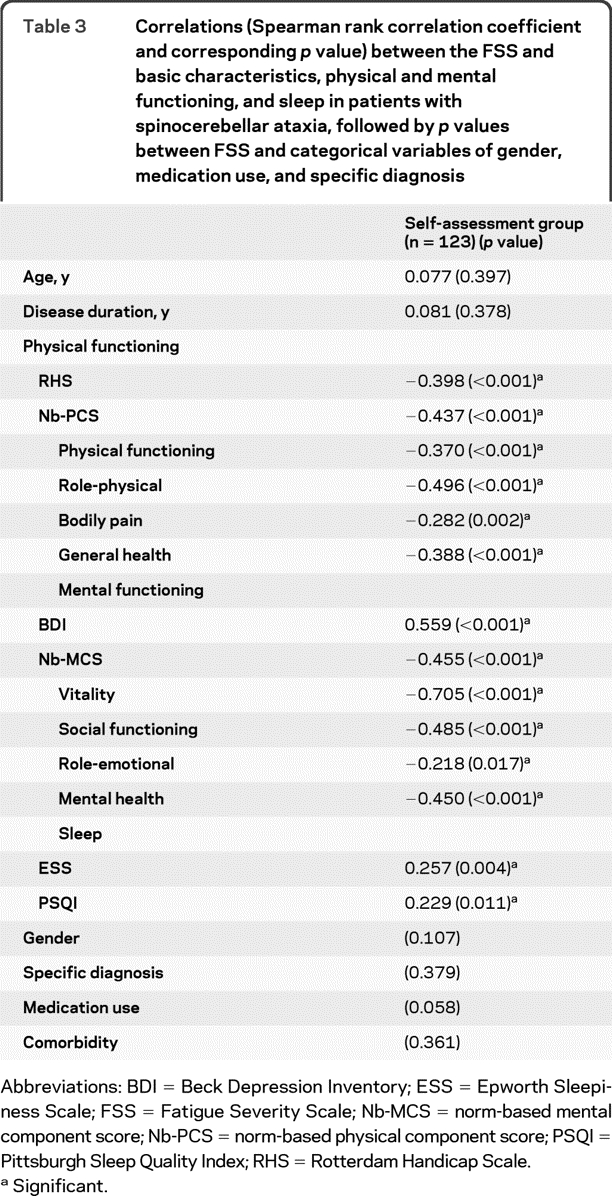
Abbreviations: BDI = Beck Depression Inventory; ESS = Epworth Sleepiness Scale; FSS = Fatigue Severity Scale; Nb-MCS = norm-based mental component score; Nb-PCS = norm-based physical component score; PSQI = Pittsburgh Sleep Quality Index; RHS = Rotterdam Handicap Scale.
Significant.
Figure. Box plot of median Fatigue Severity Scale (FSS) values (95% confidence interval) in (A) 4 diagnostic subgroups of cerebellar ataxia and (B) the clinically evaluated patients with cerebellar ataxia, with or without visual symptoms.
ADCA = autosomal dominant cerebellar ataxia; SCA = spinocerebellar ataxia.
Predictors of fatigue.
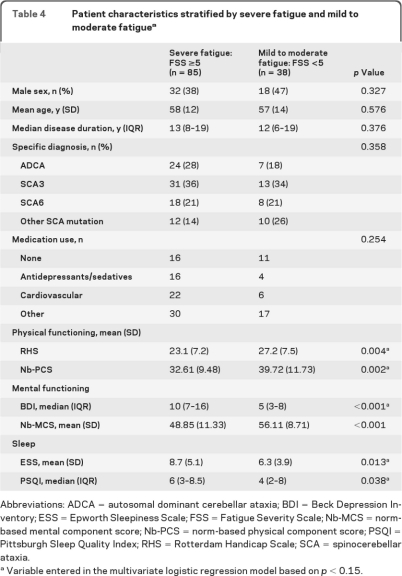
When our population was divided into patients with mild to moderate fatigue (FSS <5, n = 38) and severe fatigue (FSS ≥5, n = 85), again, physical functioning, mental functioning, and sleep were significantly related to fatigue (table 4).
Table 4.
Patient characteristics stratified by severe fatigue and mild to moderate fatiguea
Abbreviations: ADCA = autosomal dominant cerebellar ataxia; BDI = Beck Depression Inventory; ESS = Epworth Sleepiness Scale; FSS = Fatigue Severity Scale; Nb-MCS = norm-based mental component score; Nb-PCS = norm-based physical component score; PSQI = Pittsburgh Sleep Quality Index; RHS = Rotterdam Handicap Scale; SCA = spinocerebellar ataxia.
Variable entered in the multivariate logistic regression model based on p < 0.15..
These variables were then entered in the multivariate logistic regression model, except for Nb-MCS, revealing that the Nb-PCS and BDI were the best independent predictors for severe fatigue (FSS ≥5). Each point increase (improvement) on the Nb-PCS score was associated with a 5% decrease (OR 0.95; 95% CI 0.91–0.99, p = 0.030) in risk of severe fatigue, whereas each point increase (deterioration) on the BDI score resulted in a 16% (OR 1.16; 95% CI 1.06–1.27, p = 0.001) increase in risk of severe fatigue.
DISCUSSION
This study demonstrates that fatigue is a prominent and disabling symptom in adult patients with SCA, with 69% of our population displaying a FSS value of 5 and higher, indicating severe fatigue, and nearly 70% classifying fatigue among their 3 most disabling symptoms. The median FSS value of 5.8 in our population is evidently higher than the median FSS value of 2.9 in a previously described, healthy control group of 133 patients with similar age distribution (mean 54.2 years, SD 14.8) and percentage of women (47.8%).20
We are aware that the response rate of 41% may indicate a sampling bias, however, regarding the distribution of age, disease duration, and SCA subtypes, our study matches the population of a previous study on self-rated health status, representing 304 members of the Dutch ADCA patient federation.30
Physical functioning and depression appear to be the strongest predictive factors for the presence of severe fatigue. Strikingly, we did not find a significant correlation between FSS value and severity of ataxia (SARA), although the latter was significantly related to physical functioning scored by RHS and Nb-PCS (data not shown). This implies that physical functioning, related to fatigue, is not only determined by cerebellar ataxia. Indeed, subgroup analysis of clinically evaluated patients showed significantly more severe fatigue in patients with symptomatic nystagmus or diplopia, visual symptoms not addressed by the SARA.
We did not find a relation between fatigue and gender, age, or disease duration, which means fatigue was also present in young patients and was an early symptom in some of the patients with SCA. This early onset of fatigue may be related to visual symptoms as an early disease symptom. Furthermore, depression, predicting fatigue, is not related to duration or severity of disease. Notably, disease duration was not related to self-rated health status in a recent European multicenter study in 526 patients with SCA 1, 2, 3, and 6, also displaying depression to be a stronger predictor of compromised health status than the severity of ataxia and noncerebellar symptoms.9
Depression (BDI ≥16), although a predictor of fatigue in our study, is present in a minority (22%) of our fatigued patients. Depression may cause fatigue, whereas depression may also result from an impaired quality of life. A compromised quality of life was established in the European SCA population9; the health report on 304 Dutch cerebellar ataxia patients also described significantly lower scores on the physical and social functional status, mental health, and vitality items of the SF-36.30
Furthermore, we found significant correlation of fatigue with sleep disturbances, with 47% of our population having a PSQI >5, indicating a poor sleep quality. Correlation with ESS implicates that fatigue results in excessive daytime sleepiness.
The high prevalence of severe fatigue is in line with the observations in 2 previous studies on fatigue in a small group of 28 patients with SCA3 and in a group of 130 patients with Friedreich ataxia: in Friedreich ataxia, both severity of disease and disease duration were related to fatigue. However, these studies did not investigate predicting factors of fatigue.7,8
The present results show strong similarities with studies on fatigue in other neurologic disorders like MS, demonstrating severe fatigue in 64%–83% of patients,10,16,17 and like PD, with fatigue being an early symptom and a major complaint in about half of the patients16,31 and also various neuromuscular disorders.20,32 Furthermore, similar predictive factors are described in these disorders: depression in MS, PD, and stroke, and physical impairment or physical (in)activity in MS and neuromuscular disorders.15,17,18,20,31,33,34
Fatigue is defined by the difficulty in initiating or sustaining voluntary mental and physical activities. Peripheral fatigue is similar to muscle fatigability, resulting from disorders of the peripheral nerve system, whereas central fatigue is caused by lesions in the CNS, disturbing cognitive processing and limiting the ability to sustain concentration and mental tasks (mental fatigue). Psychiatric disorders like depression also cause central fatigue by loss of motivation.10,14
Since the cerebellum plays a fundamental role in action control and motor learning and since the nonmotor functions of the cerebellum, related to executive control, attention, memory, learning, visuospatial abilities, and language, are increasingly recognized,2 it may also play an important role in the model of central fatigue. Interestingly, several patients ascribed fatigue to mental slowness, especially in multitasking. This may well be due to mental fatigue. Further research is needed to analyze the role of the cerebellum in central fatigue. Specific neurophysiologic methods can be used to assess peripheral fatigue in combination with central fatigue.14,35 Mental fatigue can be evaluated by neuropsychological assessment. It would be of high interest to investigate both strategies in patients with cerebellar ataxia.
As nycturia, nocturnal muscle cramps, and periodic limb movements are described in SCA3,36 further studies are needed to analyze causes of sleep disturbances in cerebellar ataxia.
Treatment strategies for fatigue in cerebellar ataxia should focus on treatable causes of sleep disturbances or underlying vital depression, and on minimizing visual symptoms. In addition, it will be important to optimize physical functioning and endurance, for which rehabilitation programs are essential. A prospective study on exercise programs, reducing fatigue in MS, and immune-mediated polyneuropathy14,37,38 is necessary to evaluate efficacy in cerebellar ataxia. Finally, pharmacotherapeutics like amantadine and modafinil, showing variable effects on fatigue in other neurologic disorders,14 should be evaluated in cerebellar ataxia.
Footnotes
- ADCA
- autosomal dominant cerebellar ataxia
- BDI
- Beck Depression Inventory
- ESS
- Epworth Sleepiness Scale
- FSS
- Fatigue Severity Scale
- MMSE
- Mini-Mental State Examination
- MS
- multiple sclerosis
- Nb-MCS
- norm-based mental component score
- Nb-PCS
- norm-based physical component score
- PD
- Parkinson disease
- PSQI
- Pittsburgh Sleep Quality Index
- RHS
- Rotterdam Handicap Scale
- SARA
- Scale for the Assessment and Rating of Ataxia
- SCA
- spinocerebellar ataxia
- SF-36
- Short Form–36
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. E. Brusse, Dr. H.J. van Duivenvoorden, and Dr. M. Brusse-Keizer.
DISCLOSURE
Dr. Brusse serves on the Medical Advisory Board of the Dutch ADCA patient foundation. Dr. Brusse-Keizer and Dr. Duivenvoorden report no disclosures. Dr. van Swieten receives/has received research support from the AFTD (Association for Frontotemporal Dementias), the Dioraphte Foundation, the Hersenstichting, and the Nuts Ohra Foundation.
REFERENCES
- 1. Brusse E, Maat-Kievit JA, van Swieten JC. Diagnosis and management of early- and late-onset cerebellar ataxia. Clin Genet 2007;71:12–24 [DOI] [PubMed] [Google Scholar]
- 2. Manto M, Marmolino D. Cerebellar ataxias. Curr Opin Neurol 2009;22:419–429 [DOI] [PubMed] [Google Scholar]
- 3. Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol 2010;9:885–894 [DOI] [PubMed] [Google Scholar]
- 4. van de Warrenburg BP, Sinke RJ, Verschuuren-Bemelmans CC, et al. Spinocerebellar ataxias in the Netherlands: prevalence and age at onset variance analysis. Neurology 2002;58:702–708 [DOI] [PubMed] [Google Scholar]
- 5. Schmitz-Hubsch T, Coudert M, Bauer P, et al. Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms. Neurology 2008;71:982–989 [DOI] [PubMed] [Google Scholar]
- 6. Schmitz-Hubsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 2006;66:1717–1720 [DOI] [PubMed] [Google Scholar]
- 7. Friedman JH, Amick MM. Fatigue and daytime somnolence in Machado Joseph disease (spinocerebellar ataxia type 3). Mov Disord 2008;23:1323–1324 [DOI] [PubMed] [Google Scholar]
- 8. Epstein E, Farmer JM, Tsou A, et al. Health related quality of life measures in Friedreich ataxia. J Neurol Sci 2008;272:123–128 [DOI] [PubMed] [Google Scholar]
- 9. Schmitz-Hubsch T, Coudert M, Giunti P, et al. Self-rated health status in spinocerebellar ataxia–results from a European multicenter study. Mov Disord 2010;25:587–595 [DOI] [PubMed] [Google Scholar]
- 10. Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet 2004;363:978–988 [DOI] [PubMed] [Google Scholar]
- 11. Friedman JH, Brown RG, Comella C, et al. Fatigue in Parkinson's disease: a review. Mov Disord 2007;22:297–308 [DOI] [PubMed] [Google Scholar]
- 12. Kos D, Kerckhofs E, Nagels G, D'Hooghe MB, Ilsbroukx S. Origin of fatigue in multiple sclerosis: review of the literature. Neurorehabil Neural Repair 2008;22:91–100 [DOI] [PubMed] [Google Scholar]
- 13. Lerdal A, Bakken LN, Kouwenhoven SE, et al. Poststroke fatigue: a review. J Pain Symptom Manage 2009;38:928–949 [DOI] [PubMed] [Google Scholar]
- 14. de Vries JM, Hagemans ML, Bussmann JB, van der Ploeg AT, van Doorn PA. Fatigue in neuromuscular disorders: focus on Guillain-Barré syndrome and Pompe disease. Cell Mol Life Sci 2010;67:701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schepers VP, Visser-Meily AM, Ketelaar M, Lindeman E. Poststroke fatigue: course and its relation to personal and stroke-related factors. Arch Phys Med Rehabil 2006;87:184–188 [DOI] [PubMed] [Google Scholar]
- 16. van Hilten JJ, Hoogland G, van der Velde EA, Middelkoop HA, Kerkhof GA, Roos RA. Diurnal effects of motor activity and fatigue in Parkinson's disease. J Neurol Neurosurg Psychiatry 1993;56:874–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lerdal A, Celius EG, Krupp L, Dahl AA. A prospective study of patterns of fatigue in multiple sclerosis. Eur J Neurol 2007;14:1338–1343 [DOI] [PubMed] [Google Scholar]
- 18. Penner IK, Bechtel N, Raselli C, et al. Fatigue in multiple sclerosis: relation to depression, physical impairment, personality and action control. Mult Scler 2007;13:1161–1167 [DOI] [PubMed] [Google Scholar]
- 19. Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res 2004;56:157–170 [DOI] [PubMed] [Google Scholar]
- 20. Merkies IS, Schmitz PI, Samijn JP, van der Meche FG, van Doorn PA. Fatigue in immune-mediated polyneuropathies: European Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology 1999;53:1648–1654 [DOI] [PubMed] [Google Scholar]
- 21. Merkies IS, Schmitz PI, van der Meche FG, et al. Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. J Neurol Neurosurg Psychiatry 2002;72:596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hagemans ML, Laforet P, Hop WJ, et al. Impact of late-onset Pompe disease on participation in daily life activities: evaluation of the Rotterdam Handicap Scale. Neuromuscul Disord 2007;17:537–543 [DOI] [PubMed] [Google Scholar]
- 23. Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 1998;51:1055–1068 [DOI] [PubMed] [Google Scholar]
- 24. Ware JE J, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME, eds. User's Manual for the SF-36v2 Health Survey 2nd ed. Lincoln, RI: QualiMetric Incorporated; 2007 [Google Scholar]
- 25. Spreen O, Strauss E. Adaptive behaviour and personality: Beck Depression Inventory (BDI). In: A Compendium of Neuropsychological Tests: Administration, Norms and Commentary, 2nd ed. New York: Oxford University Press, Inc.; 1998:602–607 [Google Scholar]
- 26. Nolen WA, Dingemans PMAJ. [Meetinstrumenten bij stemmingsstoornissen.] Tijdschrift Psychiatrie 2004;46:681–686 [Google Scholar]
- 27. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213 [DOI] [PubMed] [Google Scholar]
- 28. Miletin MS, Hanly PJ. Measurement properties of the Epworth Sleepiness Scale. Sleep Med 2003;4:195–199 [DOI] [PubMed] [Google Scholar]
- 29. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922–935 [DOI] [PubMed] [Google Scholar]
- 30.Van den Berg MB, Schaap HB.[ADCA en de kwaliteit van leven.] [Accessed April 18, 2010]. Available at: http://www.ataxie.nl/ataxie/wetenschappelijk_onderzoek/adca_en_de_kwaliteit_van_leven.doc.
- 31. Herlofson K, Larsen JP. Measuring fatigue in patients with Parkinson's disease: the Fatigue Severity Scale. Eur J Neurol 2002;9:595–600 [DOI] [PubMed] [Google Scholar]
- 32. Kalkman JS, Zwarts MJ, Schillings ML, van Engelen BG, Bleijenberg G. Different types of fatigue in patients with facioscapulohumeral dystrophy, myotonic dystrophy and HMSN-I: experienced fatigue and physiological fatigue. Neurol Sci 2008;29(suppl 2):S238–S240 [DOI] [PubMed] [Google Scholar]
- 33. Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord 2001;16:507–510 [DOI] [PubMed] [Google Scholar]
- 34. Hagemans ML, van Schie SP, Janssens AC, van Doorn PA, Reuser AJ, van der Ploeg AT. Fatigue: an important feature of late-onset Pompe disease. J Neurol 2007;254:941–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zwarts MJ, Bleijenberg G, van Engelen BG. Clinical neurophysiology of fatigue. Clin Neurophysiol 2008;119:2–10 [DOI] [PubMed] [Google Scholar]
- 36. D'Abreu A, Franca M, Jr, Conz L, et al. Sleep symptoms and their clinical correlates in Machado-Joseph disease. Acta Neurol Scand 2009;119:277–280 [DOI] [PubMed] [Google Scholar]
- 37. Garssen MP, Bussmann JB, Schmitz PI, et al. Physical training and fatigue, fitness, and quality of life in Guillain-Barré syndrome and CIDP. Neurology 2004;63:2393–2395 [DOI] [PubMed] [Google Scholar]
- 38. Voet NB, van der Kooi EL, Riphagen II, Lindeman E, van Engelen BG, Geurts A. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev 2010:CD003907. [DOI] [PubMed] [Google Scholar]