Abstract
Background:
Guillain-Barré syndrome (GBS) has a highly diverse clinical course and outcome, yet patients are treated with a standard therapy. Patients with poor prognosis may benefit from additional treatment, provided they can be identified early, when nerve degeneration is potentially reversible and treatment is most effective. We developed a clinical prognostic model for early prediction of outcome in GBS, applicable for clinical practice and future therapeutic trials.
Methods:
Data collected prospectively from a derivation cohort of 397 patients with GBS were used to identify risk factors of being unable to walk at 4 weeks, 3 months, and 6 months. Potential predictors of poor outcome (unable to walk unaided) were considered in univariable and multivariable logistic regression models. The clinical model was based on the multivariable logistic regression coefficients of selected predictors and externally validated in an independent cohort of 158 patients with GBS.
Results:
High age, preceding diarrhea, and low Medical Research Council sumscore at hospital admission and at 1 week were independently associated with being unable to walk at 4 weeks, 3 months, and 6 months (all p 0.05–0.001). The model can be used at hospital admission and at day 7 of admission, the latter having a better predictive ability for the 3 endpoints; the area under the receiver operating characteristic curve (AUC) is 0.84–0.87 and at admission the AUC is 0.73–0.77. The model proved to be valid in the validation cohort.
Conclusions:
A clinical prediction model applicable early in the course of disease accurately predicts the first 6 months outcome in GBS.
Guillain-Barré syndrome (GBS) is a monophasic polyradiculoneuropathy with a highly variable clinical severity and outcome. IV immunoglobulin (IVIg) and plasma exchange (PE) are beneficial in patients who are severely affected, although one-third recover incompletely.1 These patients require more effective treatment, but the clinical diversity and the rarity of the disease hamper good and well-powered randomized controlled trials in this patient group. To identify early patients with a poor outcome, who are eligible for additional treatment, prognostic models are needed. Prognostic models can also increase the power of therapeutic studies by adjusting for prognostic factors.2 Ultimately, such prediction models can be used to individualize therapy in accordance with the expected outcome.
Previous studies have identified patient characteristics associated with poor outcome in GBS.3–10 The Erasmus GBS Outcome Score (EGOS) is a prognostic model based on age, diarrhea, and GBS disability score at 2 weeks after hospital admission that accurately predicts the chance of being able to walk independently at 6 months.8 However, prognostic models to optimize treatment in GBS should be applicable in the earliest phase of the disease, when treatment is considered to be most effective. Such models should also be designed to predict the primary endpoints used in most treatment trials in GBS; i.e., the clinical recovery on the GBS disability score at 4 weeks.11–15 The aim of the current study was to develop a readily applicable prognostic model for accurate selection of patients with a poor prognosis, based on clinical information available in the first week of hospital admission.
METHODS
Patients.
Data collected prospectively from a cohort of 397 patients with GBS were used to identify predictors for outcome. This derivation cohort consisted of patients who had been included in 2 treatment trials and one pilot study. The first study was a multicenter double-blind randomized controlled trial; this included 147 patients between 1985 and 1991 and compared PE with IVIg.12 The second study was a pilot study in 25 Dutch patients to determine the additional therapeutic effect of methylprednisolone (MP) to IVIg.16 This combination was tested in the third study: a multicenter double-blind randomized controlled trial in 225 patients included between 1994 and 2000.15 Most patients were included in Dutch hospitals, the others in 2 German and 2 Belgian hospitals. All 3 studies used the same inclusion and exclusion criteria. Inclusion criteria were fulfillment of the National Institute of Neurological Disorders and Stroke diagnostic criteria for GBS,17 inability to walk unaided 10 meters across an open space (GBS disability score 3 or more), and onset of weakness within 2 weeks before randomization. Exclusion criteria were age below 6 years, pregnancy, previous GBS, known severe allergic reaction to properly matched blood products, known selective IgA deficiency, previous steroid therapy, severe concurrent disease, inability to attend follow-up, or contraindications for corticosteroid treatment (not in first trial).
To validate the model, we used data collected prospectively from a cohort of 191 patients enrolled in a pilot study18 and an observational study19 in patients with GBS, both performed in the Netherlands. The pilot study evaluated the additional therapeutic effect of mycophenolate mofetil to IVIg and MP in 27 patients included between 2002 and 2005. The same inclusion and exclusion criteria were used as in the derivation cohort. Between 2005 and 2008, 164 patients with GBS were included in the observational study, which assessed pain and autonomic dysfunction (GRAPH study).19 Patients with a mild form of GBS (able to walk throughout the course of the disease) (n = 33) were also included in this study, but not used for validation.
Patient characteristics were described in more detail in the trial and survey reports.12,15,16,18,19
Standard protocol approvals, registrations, and patient consents.
Approval was received by an ethical standards committee on human experimentation for each of the studies mentioned above. Written informed consent was received from all patients.
Data collection.
All data were collected prospectively. At hospital admission, information was obtained regarding age, gender, diarrhea, or symptoms of an upper respiratory tract infection in the 4 weeks preceding onset of weakness, day of onset of weakness, cranial nerve dysfunction, Medical Research Counsel (MRC) sumscore,20 GBS disability score,21 and sensory deficits. In addition, data on the MRC sumscore and GBS disability score were collected at day 7 of hospital admission. The MRC sumscore is defined as the sum of MRC scores of 6 different muscles measured bilaterally, which results in a sumscore ranging from 0 (tetraplegic) to 60 (normal; appendix e-1 on the Neurology® Web site at www.neurology.org).20 The GBS disability score is a widely accepted scale for assessing the functional status of patients with GBS, ranging from 0 (normal) to 6 (death; appendix e-1).21 Pretreatment serum samples obtained within 4 weeks of onset of weakness were used for serologic screening to identify recent infections with Campylobacter jejuni and cytomegalovirus (CMV).
Age and MRC sumscore were categorized to facilitate the applicability in clinical practice. Categories were based on even group sizes and predictive ability.
Outcome measures.
This study used walking ability as outcome measure. Poor outcome was defined as the inability to walk unaided 10 meters across an open space (GBS disability score of 3 or higher). Outcome was assessed at 4 weeks, 3 months, and 6 months after inclusion in one of the studies. An additional outcome measure in this study was the improvement of one or more points on the GBS disability score in the first 4 weeks after inclusion. No improvement was considered as poor outcome. Both outcome measures have been used as primary endpoint in previous treatment trials in GBS.11–15
Model development.
Potential prognostic factors of outcome at 4 weeks, 3 months, and 6 months after inclusion were first analyzed in the derivation cohort by univariable logistic regression analysis. Statistically significant predictors for poor outcome at all timepoints were further analyzed for their independent predictive value using multivariable logistic modeling.
Missing values were imputed using a multiple imputation method.22 Odds ratios (OR) were used to express the strength of prognostic effects and were compared between the imputed and the complete case analyses. Predictive value was also measured using the likelihood ratio χ2 test (LR chi2), to account for the prevalence of the predictor. Variables that added significant predictive information were selected for use in a multivariable model. A p value <0.05 was considered to be significant.
The model was fitted using the ability to walk unaided at 4 weeks after hospital admission as outcome measure. The model was constructed based on the multivariable logistic regression coefficients in the derivation dataset.
Predictive performance of the model was quantified with respect to discrimination (area under the receiver operating characteristic curve [AUC]). The AUC ranges from 0.5 to 1.0 for sensible models. The internal validity of the model was assessed by bootstrapping techniques, including both the selection of predictors and estimation of the coefficients.22 The model was externally validated in an independent validation cohort of patients with GBS. Model performance in the validation set was quantified with respect to discrimination (AUC) and calibration. Calibration was assessed graphically by plotting observed frequencies against predicted probabilities.
Statistical analyses used SPSS version 15.0 for Windows, Stata version 11, and R statistical software (version 2.7, using the Design library).
RESULTS
Three (<1%) of the 397 patients in the derivation cohort died in the first week after hospital admission and were excluded from the current study. In this cohort, the primary endpoint was missing at 3 months for 3 (<1%) patients and at 6 months for 12 (3%) patients. Fifty-five percent had a poor outcome at 4 weeks, 30% at 3 months, and 19% at 6 months after hospital admission. In the validation cohort, none of the patients died in the first week of follow-up. Due to the slightly different follow-up structure of the observational study, outcome was unavailable for 38 (24%) patients at 4 weeks, 14 (9%) patients at 3 months, and 7 (4%) patients at 6 months after hospital admission. These patients were excluded from the study. Of the remaining patients in the validation cohort, 54% had poor outcome at 4 weeks, 29% at 3 months, and 15% at 6 months after hospital admission.
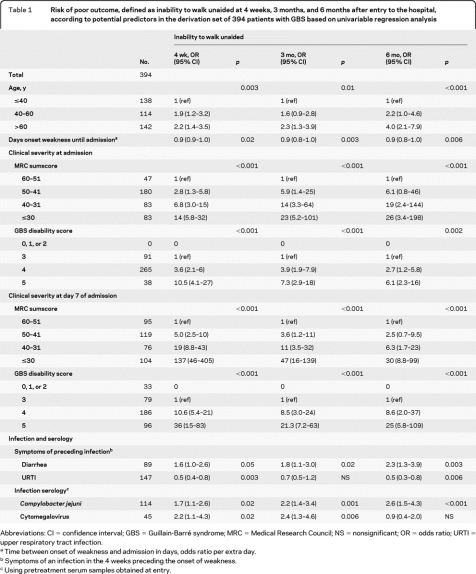
Gender, bulbar and facial weakness, sensory deficit, and pain were not significantly correlated with outcome (table e-1). In univariate analysis, 6 predictors of outcome—at 4 weeks, 3 months, and 6 months—were identified: age, disease progression (expressed as number of days between onset of weakness and hospital entry), MRC sumscore and GBS disability score, diarrhea in the 4 weeks preceding GBS, and C jejuni serology (all p = 0.05–0.001) (table 1 and table e-1). C jejuni serology was excluded for multivariable analysis because in clinical practice serology results will be difficult to obtain shortly after hospital admission. For further modeling, the MRC sumscore was selected over the GBS disability score, because the model using the MRC sumscore had a substantially better performance (LR statistic 69.75 vs 46.49 at admission and 195.27 vs 154.35 at 1 week). Disease progression lost its predictive ability when analyzed in a multivariable model with age, diarrhea, and MRC sumscore. The results of the multivariable analyses of the remaining prognostic factors are shown in table 2.
Table 1.
Risk of poor outcome, defined as inability to walk unaided at 4 weeks, 3 months, and 6 months after entry to the hospital, according to potential predictors in the derivation set of 394 patients with GBS based on univariable regression analysis
Abbreviations: CI = confidence interval; GBS = Guillain-Barré syndrome; MRC = Medical Research Council; NS = nonsignificant; OR = odds ratio; URTI = upper respiratory tract infection.
Time between onset of weakness and admission in days, odds ratio per extra day.
Symptoms of an infection in the 4 weeks preceding the onset of weakness.
Using pretreatment serum samples obtained at entry.
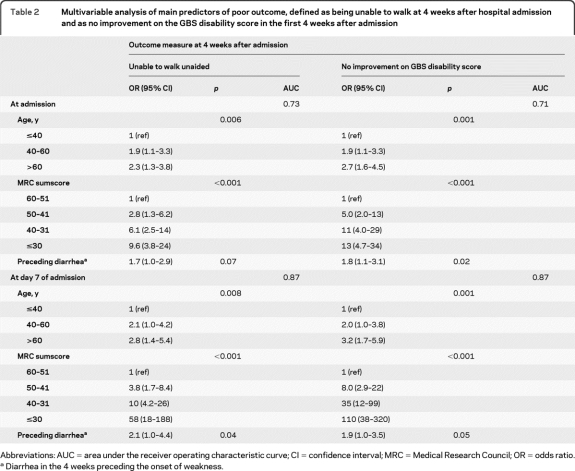
Table 2.
Multivariable analysis of main predictors of poor outcome, defined as being unable to walk at 4 weeks after hospital admission and as no improvement on the GBS disability score in the first 4 weeks after admission
Abbreviations: AUC = area under the receiver operating characteristic curve; CI = confidence interval; MRC = Medical Research Council; OR = odds ratio.
Diarrhea in the 4 weeks preceding the onset of weakness.
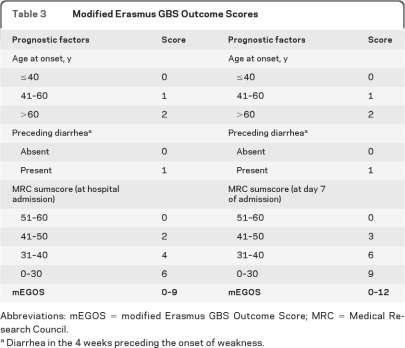
Age, diarrhea, and MRC sumscore were used to develop the model for clinical practice. This model was a modification of the previously developed EGOS.8 In contrast to the EGOS, this modified EGOS (mEGOS) can be applied already at hospital admission and at day 7 of hospital admission. When used at admission, the mEGOS scores ranged from 0 to 9 with 4 categories for the MRC sumscore, 3 categories for age, and 2 categories for preceding diarrhea (table 3 and figure 1A). The predictive ability of the model was better when used at day 7 of admission, because the MRC sumscore at this timepoint predicts outcome more accurately. Therefore, the MRC sumscore was weighted stronger in the mEGOS when used at 1 week and the scores range from 0 to 12 (table 3 and figure 1B).
Table 3.
Modified Erasmus GBS Outcome Scores
Abbreviations: mEGOS = modified Erasmus GBS Outcome Score; MRC = Medical Research Council.
Diarrhea in the 4 weeks preceding the onset of weakness.
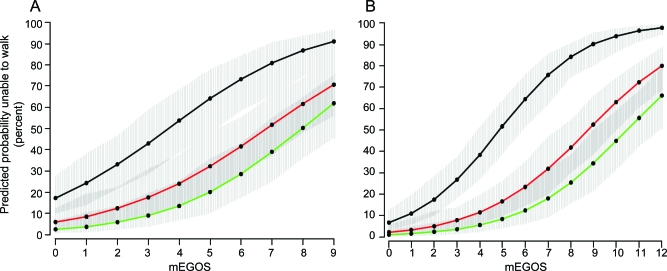
Figure 1. Predicted fraction of patients unable to walk independently according to modified Erasmus GBS Outcome Score (mEGOS).
Predicted fraction of patients unable to walk independently at 4 weeks (black lines), 3 months (red lines), and 6 months (green lines) on the basis of the mEGOS at hospital admission (A) and at day 7 of admission (B). The gray areas around the colored lines represent 90% confidence intervals.
The performance of mEGOS when used at admission was good for prediction of outcome at 4 weeks (AUC 0.73), at 3 months (AUC 0.73), and at 6 months (AUC 0.77) and was excellent when used at day 7 of admission, with AUCs for predicting outcome at these 3 timepoints of 0.87, 0.84, and 0.84, respectively. The mEGOS was validated in an independent cohort and showed a good calibration (figure e-1) and a good discriminative ability for predicting outcome at all 3 timepoints (admission: AUC = 0.75, 0.73, and 0.75; 1 week: AUC = 0.81, 0.70, and 0.77).
Age, preceding diarrhea, and MRC sumscore in multivariable analysis were also independently associated with another endpoint that is frequently used in therapeutic trials in GBS: the improvement of one or more points on the GBS disability score at 4 weeks after hospital admission (table 2). In addition, the mEGOS model predicted the failure to improve on the GBS disability score at 4 weeks with high accuracy (AUC of 0.71 and 0.87).
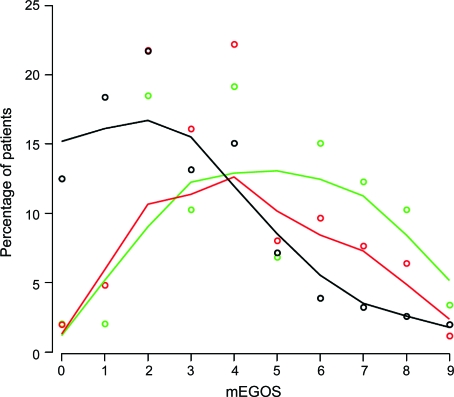
The current model can also be used to compare populations of patients included in various therapeutic trials and for covariate adjustment. To illustrate this, we compared 3 study populations12,15,19 with respect to the distribution of the patients over the mEGOS categories (figure 2). The figure illustrates the ability of mEGOS to make a distinction between different GBS populations with respect to prognosis. The patients included in the observational study had an overall better prognosis, as was expected because of the different inclusion criteria, which allowed the inclusion of mildly affected patients.
Figure 2. Comparing 3 therapeutic study populations with respect to prognostic factors at hospital admission using modified Erasmus GBS Outcome Score (mEGOS).
Points represent the percentages of patients with a specific mEGOS in a therapeutic trial comparing plasma exchange vs IV immunoglobulin (IVIg) (green), a therapeutic trial comparing IVIg/placebo vs IVIg/methylprednisolone (red), and an observational study (black). Smoothed lines represent the distribution of the study population over the total mEGOS.
DISCUSSION
The variation in clinical severity and outcome between patients with GBS hampers optimizing of treatment, because heterogeneous study populations will reduce the statistical power of treatment trials. New therapies and treatment modalities for GBS may not further improve outcome in patients who already recover sufficiently after standard treatment. Therefore, selective treatment trials should focus on a more homogeneous subgroup of patients with poor recovery despite current standard treatment. In this study a prognostic model is presented which early identifies patients with poor outcome and can be used for future therapeutic trials. The main predictors of being unable to walk independently at 4 weeks, 3 months, and 6 months were MRC sumscore, age, and preceding diarrhea in our study. Based on these predictors, a model was constructed which proved to be valid in an independent cohort of patients with GBS. The model is applicable at hospital admission as well as at day 7 of hospital admission and is therefore suitable to study treatments which should be started immediately as well as after standard treatment in patients with poor prognosis. The model may provide a first step toward individualized treatment in GBS.
This mEGOS originates from the EGOS, which can be applied in clinical practice at 2 weeks after hospital admission to predict outcome at 6 months and is based on the predictors age, preceding diarrhea, and GBS disability score.8 The EGOS is a simple, accurate, and validated prognostic model, but less suited for treatment development because of the delay of 2 weeks and the predicted outcome measure. The mEGOS was primarily designed for future treatment studies in GBS and for this application has important advantages. First, the mEGOS model can be applied already in the first week of admission, when treatment is considered to be most effective. Second, the mEGOS predicts reaching independent walking or improving on the GBS disability score at 4 weeks, which are the 2 primary endpoints most frequently used in therapeutic trials in GBS. Third, the mEGOS also accurately predicts long-term GBS disability scores, which were important secondary endpoints in previous trials. Because of these features, the mEGOS model can be used for early identification of patients with poor prognosis for future selective therapeutic studies. In addition, this model can be used for covariate adjustment, which is a powerful tool in heterogeneous patient populations to estimate the effect of treatment in individuals and to increase the statistical power of therapeutic trials.2,23,24 For example, adjustment for the effect of age on outcome results in an estimated treatment effect for a patient of a given age instead of an average age. When the results of these selective trials in patients with poor prognosis are positive, the mEGOS may also be used to individualize treatment of patients with GBS in routine clinical practice.
Our study confirms that poor outcome is associated with older age,4,5,7,8,10 rapid disease progression,7,10 severe disease indicated by GBS disability score or MRC sumscore,3,4,7,8 preceding diarrhea, positive C jejuni serology,3,5,8 positive CMV serology,9 and no symptoms of a preceding respiratory tract infection.3–4 Two of these studies used partly the same data as in this study.8–9 For the purpose of this study, we selected age, preceding diarrhea, and MRC sumscore, which are readily available at hospital admission of the patient. Prognostic biomarkers may further improve those models in the future. Promising candidates are infection serology, antiganglioside antibodies, and serum IgG level increase after IVIg treatment, which were all related to outcome.3,5,6,8,9 The need for accurate prediction models for outcome has also been acknowledged for traumatic brain injury25 and for stroke.26,27 These neurologic conditions resemble GBS in the sense that they are acute and monophasic and have a highly variable clinical course.
Our study had several limitations. First, the prognostic model was derived from cohorts of Dutch Caucasians, which may restrict the application to those patients. Second, information on outcome at 4 weeks was not available in 24% of patients from the validation cohort. For this cohort data were used from an observational study, in which 4 weeks was not a standardized evaluation timepoint. However, percentages of patients with a poor outcome at 4 weeks in the derivation and validation cohort were comparable (55% and 54%), so it is unlikely that this caused bias. A third limitation is that the model only predicts the ability to walk independently, and not the full ordinal GBS disability scores, as this would have provided maximum statistical power.28 However, this specific outcome measure we used is highly relevant for patients and was previously used by most therapeutic trials in GBS. Finally, EMG may have prognostic relevance in GBS, as indicated by several studies3–5,7,10; unfortunately, EMG was not performed systematically in the current study. Future studies are needed to define if EMG has additional value for predicting outcome already at the day of hospital admission.
The mEGOS is an accurate and validated model for prediction of outcome at several timepoints in the first 6 months after onset of GBS. An important advantage above existing models is that the mEGOS can be used in the early phase of disease when the process of nerve damage is ongoing and possibly reversible. This model predicts commonly used trial endpoints in GBS and can be used to conduct new trials selectively in patients with poor outcome. In addition, the model can be used to compare patient populations with respect to prognostic factors and expected outcome. This model may assist clinicians in optimizing treatment for individual patients with GBS.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Dutch Guillain-Barré Study Group and the Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group for providing the data for this analysis.
- AUC
- area under the receiver operating characteristic curve
- CMV
- cytomegalovirus
- EGOS
- Erasmus GBS Outcome Score
- GBS
- Guillain-Barré syndrome
- IVIg
- IV immunoglobulin
- LR
- likelihood ratio
- mEGOS
- modified Erasmus GBS Outcome Score
- MP
- methylprednisolone
- MRC
- Medical Research Counsel
- OR
- odds ratio
- PE
- plasma exchange
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. C. Walgaard, H.F. Lingsma, and Dr. E.W. Steyerberg.
DISCLOSURE
Dr. Walgaard receives research support from the Dutch Prinses Beatrix Fonds. H.F. Lingsma receives research support from the NIH. Dr. Ruts reports no disclosures. Dr. van Doorn has served on scientific advisory boards for Octapharma AG and Talecris Biotherapeutics; has received funding for travel and speaker honoraria from Baxter International Inc.; serves on the editorial board of the Journal of the Peripheral Nervous System; received a departmental research grant from Baxter International Inc.; and has received research support from Baxter International Inc., the Dutch Prinses Beatrix Fonds, and the GBS-CIDP Foundation International. Dr. Steyerberg serves as an Associate Editor for Medical Decision Making, PLos One, and the European Journal of Clinical Investigation; receives publishing royalties for Clinical Prediction Models (Springer, 2009); and receives research support from the NIH and the Netherlands Organization for Scientific Research. Dr. Jacobs has received funding for travel from Baxter International Inc.; and has received research support from the Netherlands Organization for Health Research and Development, the Prinses Beatrix Fonds, and GBS-CIDP Foundation International.
REFERENCES
- 1. van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol 2008;7:939–950 [DOI] [PubMed] [Google Scholar]
- 2. Roozenbeek B, Maas AI, Lingsma HF, et al. Baseline characteristics and statistical power in randomized controlled trials: selection, prognostic targeting, or covariate adjustment? Crit Care Med 2009;37:2683–2690 [DOI] [PubMed] [Google Scholar]
- 3. The Italian Guillain-Barré Study Group The prognosis and main prognostic indicators of Guillain-Barré syndrome: a multicentre prospective study of 297 patients. Brain 1996;119:2053–2061 [PubMed] [Google Scholar]
- 4. Chio A, Cocito D, Leone M, Giordana MT, Mora G, Mutani R. Guillain-Barré syndrome: a prospective, population-based incidence and outcome survey. Neurology 2003;60:1146–1150 [DOI] [PubMed] [Google Scholar]
- 5. Hadden RD, Karch H, Hartung HP, et al. Preceding infections, immune factors, and outcome in Guillain-Barré syndrome. Neurology 2001;56:758–765 [DOI] [PubMed] [Google Scholar]
- 6. Kuitwaard K, de Gelder J, Tio-Gillen AP, et al. Pharmacokinetics of intravenous immunoglobulin and outcome in Guillain-Barré syndrome. Ann Neurol 2009;66:597–603 [DOI] [PubMed] [Google Scholar]
- 7. McKhann GM, Griffin JW, Cornblath DR, Mellits ED, Fisher RS, Quaskey SA. Plasmapheresis and Guillain-Barré syndrome: analysis of prognostic factors and the effect of plasmapheresis. Ann Neurol 1988;23:347–353 [DOI] [PubMed] [Google Scholar]
- 8. van Koningsveld R, Steyerberg EW, Hughes RA, Swan AV, van Doorn PA, Jacobs BC. A clinical prognostic scoring system for Guillain-Barré syndrome. Lancet Neurol 2007;6:589–594 [DOI] [PubMed] [Google Scholar]
- 9. Visser LH, van der Meché FG, Meulstee J, et al. Cytomegalovirus infection and Guillain-Barré syndrome: the clinical, electrophysiologic, and prognostic features: Dutch Guillain-Barré Study Group. Neurology 1996;47:668–673 [DOI] [PubMed] [Google Scholar]
- 10. Winer JB, Hughes RA, Osmond C. A prospective study of acute idiopathic neuropathy: I: clinical features and their prognostic value. J Neurol Neurosurg Psychiatry 1988;51:605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. French Cooperative Group on Plasma Exchange in Guillain-Barré syndrome Efficiency of plasma exchange in Guillain-Barré syndrome: role of replacement fluids. Ann Neurol 1987;22:753–761 [DOI] [PubMed] [Google Scholar]
- 12. van der Meché FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome: Dutch Guillain-Barré Study Group. N Engl J Med 1992;326:1123–1129 [DOI] [PubMed] [Google Scholar]
- 13. Guillain-Barré Syndrome Steroid Trial Group Double-blind trial of intravenous methylprednisolone in Guillain-Barré syndrome. Lancet 1993;341:586–590 [PubMed] [Google Scholar]
- 14. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. Lancet 1997;349:225–230 [PubMed] [Google Scholar]
- 15. van Koningsveld R, Schmitz PI, van der Meché FG, Visser LH, Meulstee J, van Doorn PA. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain-Barré syndrome: randomised trial. Lancet 2004;363:192–196 [DOI] [PubMed] [Google Scholar]
- 16. The Dutch Guillain-Barré Study Group Treatment of Guillain-Barré syndrome with high-dose immune globulins combined with methylprednisolone: a pilot study. Ann Neurol 1994;35:749–752 [DOI] [PubMed] [Google Scholar]
- 17. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol 1990;27(suppl):S21–S24 [DOI] [PubMed] [Google Scholar]
- 18. Garssen MP, van Koningsveld R, van Doorn PA, et al. Treatment of Guillain-Barré syndrome with mycophenolate mofetil: a pilot study. J Neurol Neurosurg Psychiatry 2007;78:1012–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruts L, Drenthen J, Jacobs BC, van Doorn PA, Dutch GBS Study Group Distinguishing acute-onset CIDP from fluctuating Guillain-Barré syndrome: a prospective study. Neurology 2010;74:1680–1686 [DOI] [PubMed] [Google Scholar]
- 20. Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 1991;14:1103–1109 [DOI] [PubMed] [Google Scholar]
- 21. Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial prednisolone in acute polyneuropathy. Lancet 1978;2:750–753 [DOI] [PubMed] [Google Scholar]
- 22. Steyerberg EW. Clinical Prediction Models, 1st ed. New York: Springer-Verlag; 2008 [Google Scholar]
- 23. Hernandez AV, Steyerberg EW, Habbema JD. Covariate adjustment in randomized controlled trials with dichotomous outcomes increases statistical power and reduces sample size requirements. J Clin Epidemiol 2004;57:454–460 [DOI] [PubMed] [Google Scholar]
- 24. Steyerberg EW, Bossuyt PM, Lee KL. Clinical trials in acute myocardial infarction: should we adjust for baseline characteristics? Am Heart J 2000;139:745–751 [DOI] [PubMed] [Google Scholar]
- 25. Lingsma HF, Roozenbeek B, Steyerberg EW, Murray GD, Maas AI. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol 2010;9:543–554 [DOI] [PubMed] [Google Scholar]
- 26. Reid JM, Gubitz GJ, Dai D, et al. Predicting functional outcome after stroke by modelling baseline clinical and CT variables. Age Ageing 2010;39:360–366 [DOI] [PubMed] [Google Scholar]
- 27. Uchino K, Billheimer D, Cramer SC. Entry criteria and baseline characteristics predict outcome in acute stroke trials. Stroke 2001;32:909–916 [DOI] [PubMed] [Google Scholar]
- 28. Maas AI, Steyerberg EW, Marmarou A, et al. IMPACT recommendations for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics 2010;7:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.