Abstract
Objective:
Neuronal nitric oxide synthase (nNOS), normally expressed at the sarcolemmal membrane, is known to be mislocalized to the sarcoplasm in several forms of muscular dystrophy. Our objectives were to characterize further the range of patients manifesting aberrant nNOS sarcolemmal immunolocalization and to study nNOS localization in animal models of nondystrophic myopathy.
Methods:
We carried out a retrospective cross-sectional study. We performed immunofluorescent staining for nNOS on biopsy specimens from 161 patients with acquired and nondystrophin inherited neuromuscular conditions. The localization of sarcolemmal nNOS correlated with mobility and functional status. Muscle specimens from mouse models of steroid-induced and starvation-related atrophy were studied for qualitative and quantitative nNOS expression.
Results:
Sarcolemmal nNOS staining was abnormal in 42% of patients with inherited myopathic conditions, 25% with acquired myopathic conditions, 57% with neurogenic conditions, and 93% with hypotonia. Interestingly, we found significant associations between mobility status or muscle function and sarcolemmal nNOS expression. Furthermore, mouse models of catabolic stress also demonstrated mislocalization of sarcolemmal nNOS.
Conclusion:
Our analyses indicate that nNOS mislocalization is observed in a broad range of nondystrophic neuromuscular conditions associated with impaired mobility status and catabolic stress. Our findings suggest that the assessment of sarcolemmal localization of nNOS represents an important tool for the evaluation of muscle biopsies of patients with a variety of inherited and acquired forms of neuromuscular disorders.
Neuronal nitric oxide synthase (nNOS) is one of a family of nitric oxide synthases, including endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS), that synthesize nitric oxide (NO).1 A muscle-specific alternatively spliced isoform of nNOS, nNOSμ, is the major source of NO in adult muscle.2 nNOS catalytic activity and NO signaling, regulated by calcium signaling and Ca2+ calmodulin (CaM) binding, has multiple roles in muscle as it modulates contractile force, oxidative stress, blood flow, mitochondrial biogenesis, glucose metabolism, muscle mass, and fatigue response.1,3–11 In skeletal muscle, nNOS localizes to the sarcolemma via direct binding to α-1-syntrophin, a member of the dystrophin-glycoprotein complex (DGC), and interaction with dystrophin.12–14 Unsurprisingly, several forms of muscular dystrophy with disruption of the DGC, including Duchenne muscular dystrophy and several limb girdle muscular dystrophies (LGMD 2C, 2D, and 2E), display loss of nNOS at the sarcolemma.12,15 Less intuitively but potentially revealing a fundamental role in the pathophysiology of weakness, previous research showed that patients with non-dystrophin-related muscular dystrophies (e.g., LGMD2B, MDC1A) and other forms of inherited and inflammatory myopathies exhibited mislocalization of sarcolemmal nNOS.11
To characterize further muscle disorders with aberrant nNOS localization, we retrospectively analyzed 161 patients with a range of neuromuscular disorders to determine the status of nNOS localization. We evaluated nNOS localization and the phenotype of patients to establish associations between nNOS status and clinical features. The insights from this clinical survey led us to evaluate nNOS localization in animal models of catabolic stress.
METHODS
Standard protocol approvals, registrations, and patient consents.
Johns Hopkins Institutional Review Board (IRB) approved the study, and at the time of biopsy all patients or their guardians signed a consent form permitting the use of excess muscle tissue in IRB-approved research. A collaborator in Germany provided 3 muscle specimens from patients diagnosed with critical illness myopathy with similar approval by their primary physician and local IRB. The Animal Care and Use Committee of Johns Hopkins University School of Medicine approved all experiments on the animal models.
Patients.
We performed a retrospective cross-sectional study on muscle biopsy specimens collected by the Johns Hopkins Neuromuscular Pathology Laboratory from 1985 to 2009 from 158 patients aged 1 month to 85 years. We categorized patients based on diagnosis, mobility, and functional status for further analysis (see e-Methods on the Neurology® Web site at www.neurology.org).
Mice.
We purchased male, 2-month-old C57 B/6 mice from Jackson Laboratories (Bar Harbor, ME) and treated them with dexamethasone or starved them for 48 hours (see e-Methods).
Squirrels.
The captive breeding colony at the University of Wisconsin Oshkosh provided 13-lined ground squirrels (Ictidomys tridecemlineatus). We used hibernation-naïve summer squirrels and squirrels under torpor for 2–5 months (see e-Methods).
Histology and immunofluorescence.
Patient, mouse, and squirrel skeletal muscle samples were processed according to normal procedures. For a detailed description of the processing, histology, and immunostaining, see e-Methods. Assessment of nNOS immunolocalization was performed blinded to the patient's clinical diagnosis and mobility and functional status.
Immunoblot analysis.
Western blot analysis was performed on protein from flash frozen mouse skeletal muscle (see e-Methods).
Real-time PCR.
Real-time PCR of nNOS, eNOS, and iNOS was completed using standard methods (see e-Methods).
Statistics.
We investigated the correlation between mobility and sarcolemmal nNOS localization using a χ2 statistic. To evaluate the relationship between functional status and nNOS mislocalization, we used Kruskal-Wallis equality-of-populations rank test. Individual pairwise comparisons were then performed between all subgroups. We corrected for multiple tests with a Bonferroni adjustment defining significance as p ≤ 0.008. In all other cases, significance was determined by unpaired 2-tailed t tests with significance defined as p ≤ 0.05. All values are expressed as mean ± SEM.
RESULTS
Mislocalization of sarcolemmal nNOS in a variety of neuromuscular disorders.
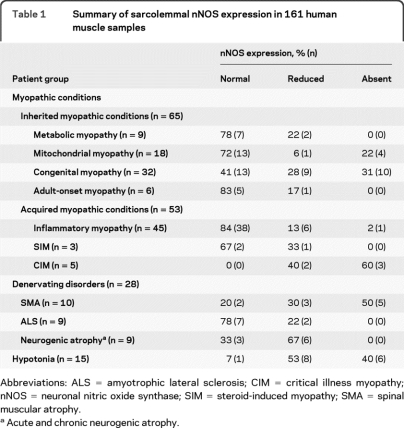
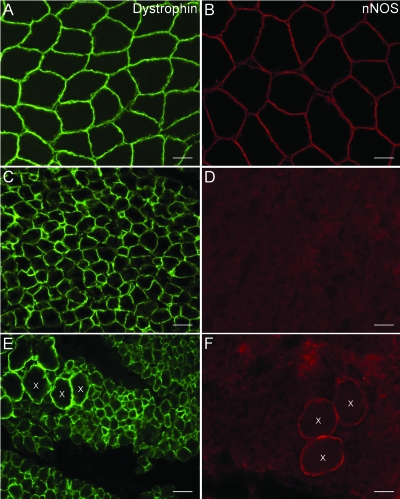
We investigated 161 muscle biopsy specimens broadly classified as from patients with either a myopathic (118/161, 73%) or a denervating (28/161, 17%) neuromuscular disorder. A third heterogeneous group of patients, classified as having hypotonia (15/161, 9%), were those who lacked a defined muscular or CNS etiology. Myopathic disorders were categorized further according to the groups outlined in table 1. All patient samples had normal expression of dystrophin and various members of the DGC (figure 1 and figure e-1, A–F). Collectively, less than half of 161 patient biopsies (43%, 70/161) demonstrated abnormal nNOS localization. Among patients with inherited myopathic conditions, 42% (27/65) had abnormal sarcolemmal nNOS localization as compared to nNOS localization in a healthy individual (figure 1B). A majority of the congenital myopathy subset (59%, 19/32) revealed mislocalization of nNOS at the sarcolemma (figure 1D). The group of patients with acquired myopathic conditions had abnormal sarcolemmal nNOS expression in 25% (13/53) of samples. This group consisted primarily of patients with inflammatory myopathy, in which a large percentage expressed normal nNOS immunolocalization (84%, 38/45). In contrast, although the number was limited, all samples from patients with critical illness myopathy showed reduced or absent sarcolemmal nNOS staining (figure 2 A).
Table 1.
Summary of sarcolemmal nNOS expression in 161 human muscle samples
Abbreviations: ALS = amyotrophic lateral sclerosis; CIM = critical illness myopathy; nNOS = neuronal nitric oxide synthase; SIM = steroid-induced myopathy; SMA = spinal muscular atrophy.
Acute and chronic neurogenic atrophy.
Figure 1. Expression pattern of neuronal nitric oxide synthase (nNOS) and dystrophin in neuromuscular disorders.
Muscle biopsies of a normal individual (A), a patient with congenital myopathy (C), and a patient with spinal muscular atrophy (E) exhibit normal dystrophin localization at the sarcolemma. In contrast, both patient samples (D and F) demonstrate loss of nNOS at the sarcolemma as compared to a biopsy from a healthy individual (B). Hypertrophic fibers are marked by X. The scale bar is 100 μm.
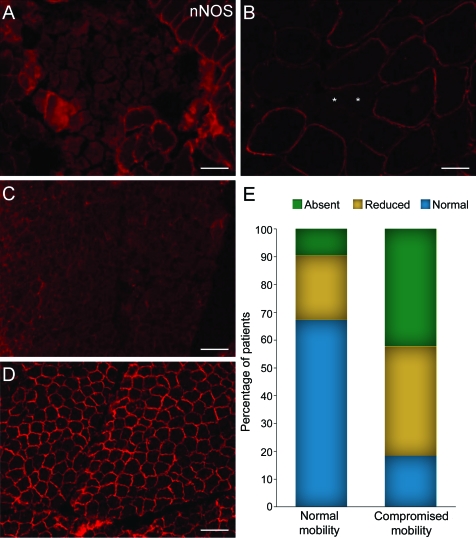
Figure 2. Neuronal nitric oxide synthase (nNOS) mislocalization in patients with atrophic fibers or impaired mobility.
Patients diagnosed with critical illness myopathy (CIM) (A) or amyotrophic lateral sclerosis (ALS) (B) demonstrate absent or reduced sarcolemmal nNOS. Patients with ALS show mislocalization of nNOS in smaller, atrophic fibers. Asterisks indicate atrophic fibers. Scale bar is 70 μm. A muscle biopsy from an immobile hypotonic patient (C) shows absent nNOS staining at the sarcolemma as compared to the maintenance of sarcolemmal nNOS in a mobile hypotonic patient (D). Scale bar is 100 μm. (E) Patients with compromised mobility have a higher percentage of biopsies showing reduced or absent nNOS staining at the sarcolemma as compared to patients with impaired mobility.
Overall, 57% (16/28) of samples from patients with denervating conditions had abnormal nNOS localization. A total of 80% (8/10) of biopsies from patients with spinal muscular atrophy (SMA) had a reduction or loss of sarcolemmal nNOS with half (5/10) showing complete loss of nNOS at the sarcolemma. Some patients with acquired neuropathies or motor neuron disease showed reduction but not absence of sarcolemmal nNOS (44%, 8/18). Interestingly, in several samples from patients with denervating disorders, the hypertrophic fibers (indicating innervated muscle fibers) maintained sarcolemmal nNOS expression, while the grouped or scattered atrophic fibers, likely representing denervated fibers, demonstrated mislocalization of nNOS (figure 1F and figure 2B).
Patients with hypotonia were those with severe neuromuscular impairment and without a primary diagnosis. The underlying causes for the neuromuscular disorder in this heterogeneous group of patients are, most likely, unidentified single gene disorders affecting the CNS, skeletal muscle, or both. Remarkably, almost all patients with hypotonia demonstrated abnormal (reduced or absent) staining (93%, 14/15) with 40% (6/15) having completely absent staining. Only one patient with hypotonia exhibited normal staining for sarcolemmal nNOS (figure 2, C and D), and upon review of the clinical data this is the only patient with hypotonia who had decreased muscle tone but normal mobility.
Our analyses suggest that nNOS mislocalization is observed in a broad range of neuromuscular conditions associated with abnormal skeletal muscle pathology, confirming previous observations.11 Moreover, the variability of nNOS localization among a wide range of conditions as well as with a given disease category indicates that nNOS mislocalization is a common phenomenon occurring independent of the primary cause of the disease.
Mislocalization of nNOS at the sarcolemma correlates with mobility and functional status.
Given the findings in our patients with hypotonia and the wide range of disorders with nNOS mislocalization, we hypothesized that overlapping clinical features, specifically immobility, may correlate to abnormal nNOS localization. Blinded to nNOS immunolocalization, we divided 154 patients with clinical data sufficient for categorization into 2 groups based on mobility status. Then, mobility was correlated to nNOS expression at the sarcolemma (figure 2E). Grouping the patient biopsies by mobility status identified a seemingly key factor in nNOS immunolocalization: 67% of the patients with normal mobility had normal sarcolemmal nNOS expression, while 82% of patients with compromised mobility demonstrated reduced or absent sarcolemmal nNOS staining (χ2 = 33.2, p < 0.001).
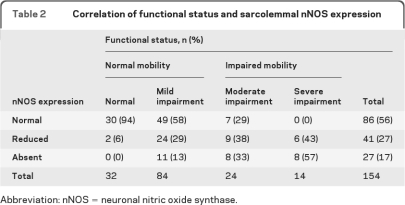
Notably, a large minority (32%) of mobile patients demonstrated mislocalized nNOS. To analyze this group further, the clinical information of the same 154 patients was of sufficient detail and quality to permit blinded ordinal assignment of patients into 4 groups based upon level of neuromuscular function at the time of biopsy (table 2). Using the Kruskal-Wallis rank test, the global correlation between functional status and sarcolemmal nNOS expression was highly significant (p = 0.0001). Individual pairwise comparisons were then performed comparing each category. Compared to normal subjects, patients with mild, moderate, and severe impairment were more likely to exhibit nNOS mislocalization (p values 0.003, 0.0001, and 0.0001). Of the 32% of patients with abnormal sarcolemmal nNOS and normal mobility, most exhibited moderately impaired muscle function. Furthermore, none of the patients with impaired mobility and preserved sarcolemmal nNOS demonstrated severely compromised muscle function. Our data indicate that impaired mobility and muscle function significantly correlates to abnormal localization of nNOS. The correlation between nNOS localization and mobility or function is not perfect, however: the residual variation may be due to the subjectivity of class assignment, the possibility of other factors influencing nNOS localization, the presence of other mechanisms associated with impaired mobility or muscle function, or a combination of causes.
Table 2.
Correlation of functional status and sarcolemmal nNOS expression
nNOS = neuronal nitric oxide synthase.
nNOS mislocalization in mouse models of acquired muscle atrophy.
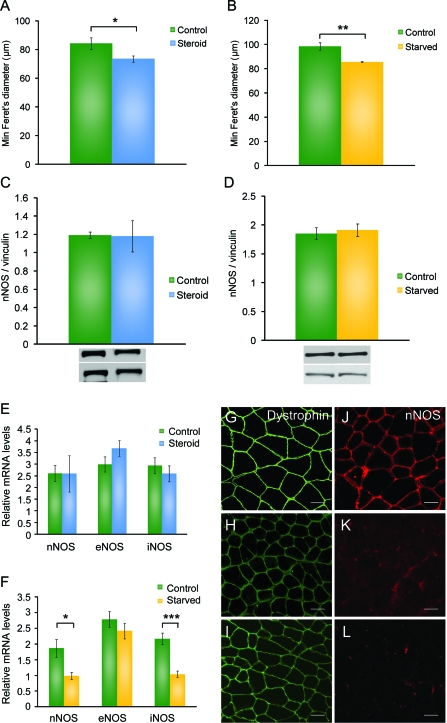
Although mobility seems to be a major factor related to nNOS mislocalization, our patient data reveal that other factors may be associated with the loss of sarcolemmal nNOS in patients with normal mobility. Two mouse models—high-dose corticosteroids therapy and short-term starvation—demonstrate muscle atrophy without compromised mobility providing a method to assess nNOS localization in mobile mice affected by catabolic stress. Mice treated with steroids or starved for 48 hours showed significant decreases in overall body mass and in normalized wet skeletal muscle mass (data not shown). Morphometric analysis of skeletal muscle specimens of both models demonstrate muscle atrophy, as defined by a significant decrease in mean minimal Feret fiber diameter as compared to age-matched controls (n = 5 for each group) (figure 3, A and B). Immunofluorescence staining for dystrophin, α-sarcoglycan, and α-1-syntrophin showed normal dystrophin localization suggestive of an intact DGC complex (figure 3, G–I, and figure e-1, G–N). However, both steroid-treated and starved mice showed absent or severely reduced sarcolemmal nNOS staining (figure 3, J–L). Real-time PCR for NOS family proteins (nNOS, eNOS, iNOS) revealed no significant differences in expression levels of any of the 3 transcripts in steroid-treated mice (n = 8 for each group) (figure 3E). Moreover, Western blot analysis for nNOS, iNOS, and eNOS showed no differences in protein levels (n = 5 for each group) (figure 3C and figure e-2, A and B). Starved mice exhibited a 1-fold decrease of nNOS and iNOS transcript expression as compared to wild-type mice (n = 9 for controls, n = 7 for starved) (figure 3F). However, the protein level of nNOS, iNOS, and eNOS revealed no differences between control and starved mice (n = 4 for each group) (figure 3D and figure e-2, C and D). These data demonstrate that abnormal localization of nNOS occurs in mice with severe muscle atrophy even if overall mobility is preserved, supporting the notion that, in addition to impaired mobility, other triggers such as catabolic stress may be associated with sarcolemmal loss of nNOS.
Figure 3. Loss of sarcolemmal neuronal nitric oxide synthase (nNOS) in steroid-induced myopathy and starvation-related atrophy mouse models.
(A) As compared to normal, steroid-treated mice have a decrease in fiber size using mean minimal Feret diameter (μm). (B) Starved mice also demonstrate smaller fibers. Western blot analysis revealed no changes in nNOS protein expression with representative bands shown and mean optical density using vinculin as a loading control for steroid-treated (C) and starved mice (D). (E) Relative expression of NOS transcripts in skeletal muscle of steroid-treated shows no significant differences. (F) Relative expression of NOS transcripts in skeletal muscle of starved mice shows significant decreases in nNOS and inducible nitric oxide synthase (iNOS). All graphs are the mean with error bars representing the SEM. Significant values from 2-tailed Student t tests include *p < 0.05, **p < 0.01, ***p < 0.001. Dystrophin staining in wild-type (G), steroid-treated (H), and starved mice (I) showed no abnormalities. However, as compared to controls (J), steroid-treated (K) and starved mice (L) reveal absent or reduced sarcolemmal nNOS staining. Scale bar is 200 μm. eNOS = endothelial nitric oxide synthase.
Skeletal muscle nNOS localization is maintained during hibernation.
We utilized skeletal muscle specimens from hibernating 13-lined ground squirrels to evaluate the impact of immobility and catabolic stress on nNOS localization in the context of maintained muscle homeostasis and integrity. These animals are obligate hibernating mammals that are protected against skeletal muscle atrophy during hibernation (Andres-Mateos et al., manuscript under review). Despite hibernating for 5 months with almost complete immobility and no caloric intake, sarcolemmal expression of nNOS was preserved (figure e-3). These data together with our patient and mouse data indicate that biochemical control of nNOS localization is complex and, importantly, that preserved sarcolemmal nNOS may be significant in maintaining muscle homeostasis.
DISCUSSION
Expression of sarcolemmal nNOS is lost in a variety of patients and animal models with inherited and acquired forms of neuromuscular disorders despite preserved dystrophin expression. Our analyses provide evidence that localization of nNOS is influenced by abnormal muscle states that include immobility and catabolic stress as well as structural defects and denervation.9,12,15,16 Previous research implicated mislocalization of nNOS in a variety of downstream, pathologic consequences affecting muscle homeostasis and performance.1,5,9–11 Thus, assessment of sarcolemmal localization of nNOS may represent an informative tool in the evaluation of muscle biopsies of patients with a variety of inherited and acquired forms of neuromuscular disorders.
The mechanisms regulating nNOS localization at the sarcolemma localization require further elucidation. Earlier research implicated nNOS mislocalization as a mechanical sensor of reduced mobility in mice.9 Analyses of mobility and nNOS localization in our patients confirm that loss of nNOS at the sarcolemma significantly correlates with compromised mobility in a variety of neuromuscular disorders. In addition, our observation that mislocalization of nNOS also occurs in some patients with preserved mobility and in our animal models of catabolic stress which maintain mobility suggests that other factors are associated with loss of sarcolemmal nNOS. Our gene expression and protein analyses demonstrate that loss of sarcolemmal nNOS is not due to deceased expression of nNOS, confirming previous observations.9,10,16 In our samples, unlike dystrophin-deficient muscular dystrophies, dystrophin and other proteins are present at the sarcolemma providing the structural backbone for nNOS localization.1 Downregulation of the α subunit in l-type calcium channels recently has been shown to result in redistribution of sarcolemmal nNOS suggesting that calcium signaling may be involved in nNOS localization.10 Loss of nNOS expression at the sarcolemma may be related to disruption of the DGC, denervation, immobility, or catabolic stress, alone or in combination, or may reflect other yet unidentified pathways.9,12,15,16
Based on our clinical survey, the range of disorders affected and the variability within disease categories suggests that nNOS mislocalization plays a role in secondary pathophysiologic disease processes. Multiple lines of evidence demonstrated that NO signaling plays a critical role in skeletal muscle homeostasis by impacting vasomodulation, muscle atrophy or autophagy, and mitochondrial function (see following discussion).1,5,9–11 Furthermore, recent results from the mutant superoxide dismutase 1 transgenic mouse model of amyotrophic lateral sclerosis (ALS) indicate that nNOS dislocation occurs early in the progression of the disease and before extensive muscle atrophy.16 These findings together with our own observations that loss of sarcolemmal nNOS correlates with impaired muscle function and mobility indicate that mislocalization of nNOS contributes to the development of muscle atrophy, weakness, and fatigue rather than simply reflecting an observation secondary to end-stage muscle disease.
The localization of nNOS at the sarcolemma controls its catalytic activity and consequently NO signaling in skeletal muscle, and alterations in the localization of nNOS affect NO signaling.1 For example, in mdx mice, a model of dystrophin-deficient muscular dystrophy, where sarcolemmal nNOS is lost, NO signaling fails to increase in response to exercise.17 Loss of sarcolemmal nNOS in mdx mice restricts cyclic guanosine monophosphate (cGMP)–mediated vasodilation leading to impairment of postexercise vasomodulation and significant muscle fatigue. Treatment with the phosphodiesterase 5 (PDE5) inhibitor, sildenafil, targets cGMP, a secondary messenger of NO signaling, and rescues this phenomenon.11 However, recent evidence suggests that muscle fatigue may be related to the loss of a novel nNOS splice variant localized to the Golgi apparatus affecting skeletal muscle integrity.18 nNOS may also affect fatigability through its positive allosteric interaction with phosphofructokinase (PFK). Although localization of nNOS was not addressed specifically, expression of a muscle-specific nNOS transgene in mdx mice recovers running endurance and enhances glycogen metabolism with upregulation of PFK activity.8
NO signaling also affects skeletal muscle weakness and fatigue by regulating mitochondria biogenesis and function.19,20 Although other NOS isoforms, particularly eNOS and mitochondrial NOS, likely are involved in NO regulation of mitochondria, research in nNOS-null mice shows that NO derived from nNOS is a factor in mitochondrial biogenesis and function.5,21 In addition, mice lacking nNOS have abnormally swollen mitochondria and decreased matrix density.18 In patients with sporadic ALS, decreases in mitochondrial activity positively correlate with decreases in nNOS expression and activity, suggesting a potential beneficial role of altered NO signaling on mitochondrial function.22
In addition to impacting muscle fatigability, mislocalization of nNOS activates atrophy and autophagy processes in skeletal muscle. Models of disuse atrophy such as hind-limb suspension and denervation demonstrate nNOS-mediated atrophy via activation of forkhead box, subgroup O (FoxO) transcription factors.9 Disuse atrophy was less severe in mice treated with a nNOS inhibitor or nNOS-null mice but not in eNOS-null mice.9 Activation of the autophagy pathway through FoxO transcription factors also arises from alterations in calcium signaling and the loss of sarcolemmal nNOS in dihydropyridine receptor α-1S subunit mutant mice.10 Interestingly, mobility of dihydropyridine receptor α-1S subunit mutant mice was not significantly diminished, supporting our own observations that other changes to muscle homeostasis can be associated with loss of sarcolemmal nNOS expression. Abnormalities of nNOS-mediated signaling pathways alter vasomodulation and mitochondrial biogenesis and function and activate atrophy and autophagy pathways contributing to muscle weakness and fatigue.
As loss of sarcolemmal nNOS results in muscle fatigue and myofiber atrophy and as they are common features of a variety of neuromuscular conditions, it is reasonable to posit that targeting of maintenance of normal nNOS localization or NO signaling may be of therapeutic benefit. Markedly, hypertrophic or normal-sized (innervated) fibers from biopsies from patients with neuropathic conditions reveal normal nNOS localization as compared to atrophic, noninnervated muscle fibers. Furthermore, hibernating squirrels, despite extreme conditions of immobility and catabolic stress, maintain sarcolemmal nNOS and do not experience muscle atrophy, and thus represent an intriguing model to evaluate nNOS regulation. In light of these observations and the era of personalized medicine, it is important to emphasize that 7 patients with compromised mobility and maintained normal sarcolemmal nNOS expression showed moderately decreased, but not severely impaired, muscle function. While our retrospective chart analyses did not contain sufficient data to characterize further the clinical status of these patients, it is tempting to hypothesize that these patients harbor important information about potential benefits of preserved nNOS expression on disease progression and phenotype. In addition to regulating nNOS localization, manipulating NO signaling may ameliorate the negative consequences of nNOS mislocalization. In mouse models, administration of the PDE5 inhibitor, sildenafil, alleviated postexercise muscle fatigue, while chemical inhibition of nNOS catalytic activity prevented FoxO-regulated atrophy in a hind-limb immobilization model.9,11 Future therapeutic strategies directed toward preservation of sarcolemmal nNOS or aberrant NO signaling may prove beneficial for a broad group of patients with neuromuscular disorders.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Christopher Dorsey, the senior histology technician at Johns Hopkins Hospital's Neuromuscular Pathology Laboratory, for technical assistance.
- ALS
- amyotrophic lateral sclerosis
- CaM
- Ca2+ calmodulin
- cGMP
- cyclic guanosine monophosphate
- DGC
- dystrophin-glycoprotein complex
- eNOS
- endothelial nitric oxide synthase
- FoxO
- forkhead box, subgroup O
- iNOS
- inducible nitric oxide synthase
- IRB
- Institutional Review Board
- LGMD
- limb girdle muscular dystrophy
- nNOS
- neuronal nitric oxide synthase
- NO
- nitric oxide
- PDE5
- phosphodiesterase 5
- PFK
- phosphofructokinase
- SMA
- spinal muscular atrophy
Editorial, page 940
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. E.L. Finanger Hedderick and J.L. Simmers.
DISCLOSURE
Dr. Finanger Hedderick receives research support from the NIH/NCRR. J.L. Simmers, A. Soleimani, Dr. Andres-Mateos, and Dr. Marx report no disclosures. Dr. Files receives research support from the NIH. Dr. King serves on the editorial boards of Clinical and Translational Science and the American Journal of Respiratory and Critical Care Medicine; has a patent pending re: Distinct lymphocyte subsets required for resolution of acute lung injury; and receives research support from the NIH/NHLBI. Dr. Crawford receives research support from the Muscular Dystrophy Association, Ataxia Telangiectasia Children's Project, and the Spinal Muscular Atrophy Foundation. Dr. Corse reports no disclosures. Dr. Cohn receives research support from the NIH and the Muscular Dystrophy Association.
REFERENCES
- 1. Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 2001;81:209–237 [DOI] [PubMed] [Google Scholar]
- 2. Silvagno F, Xia H, Bredt DS. Neuronal nitric-oxide synthase-mu, an alternatively spliced isoform expressed in differentiated skeletal muscle. J Biol Chem 1996;271:11204–11208 [DOI] [PubMed] [Google Scholar]
- 3. McAllister RM, Delp MD, Thayer KA, Laughlin MH. Muscle blood flow during exercise in sedentary and trained hypothyroid rats. Am J Physiol 1995;269:H1949–H1954 [DOI] [PubMed] [Google Scholar]
- 4. Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires alpha-syntrophin-mediated sarcolemmal localization of neuronal nitric oxide synthase. Circ Res 2003;92:554–560 [DOI] [PubMed] [Google Scholar]
- 5. Wadley GD, Choate J, McConell GK. NOS isoform-specific regulation of basal but not exercise-induced mitochondrial biogenesis in mouse skeletal muscle. J Physiol 2007;585:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roberts CK, Barnard RJ, Scheck SH, Balon TW. Exercise-stimulated glucose transport in skeletal muscle is nitric oxide dependent. Am J Physiol 1997;273:E220–225 [DOI] [PubMed] [Google Scholar]
- 7. Ross RM, Wadley GD, Clark MG, Rattigan S, McConell GK. Local nitric oxide synthase inhibition reduces skeletal muscle glucose uptake but not capillary blood flow during in situ muscle contraction in rats. Diabetes 2007;56:2885–2892 [DOI] [PubMed] [Google Scholar]
- 8. Wehling-Henricks M, Oltmann M, Rinaldi C, Myung KH, Tidball JG. Loss of positive allosteric interactions between neuronal nitric oxide synthase and phosphofructokinase contributes to defects in glycolysis and increased fatigability in muscular dystrophy. Hum Mol Genet 2009;18:3439–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki N, Motohashi N, Uezumi A, et al. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J Clin Invest 2007;117:2468–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pietri-Rouxel F, Gentil C, Vassilopoulos S, et al. DHPR alpha1S subunit controls skeletal muscle mass and morphogenesis. EMBO J 2010;29:643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kobayashi YM, Rader EP, Crawford RW, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 2008;456:511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 1995;82:743–752 [DOI] [PubMed] [Google Scholar]
- 13. Brenman JE, Chao DS, Gee SH, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 1996;84:757–767 [DOI] [PubMed] [Google Scholar]
- 14. Lai Y, Thomas GD, Yue Y, et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest 2009;119:624–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crosbie RH, Barresi R, Campbell KP. Loss of sarcolemma nNOS in sarcoglycan-deficient muscle. FASEB J 2002;16:1786–1791 [DOI] [PubMed] [Google Scholar]
- 16. Suzuki N, Mizuno H, Warita H, Takeda S, Itoyama Y, Aoki M. Neuronal NOS is dislocated during muscle atrophy in amyotrophic lateral sclerosis. J Neurol Sci 2010;294:95–101 [DOI] [PubMed] [Google Scholar]
- 17. Asai A, Sahani N, Kaneki M, Ouchi Y, Martyn JA, Yasuhara SE. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS One 2007;2:e806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Percival JM, Anderson KN, Huang P, Adams ME, Froehner SC. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest 2010;120:816–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clementi E, Nisoli E. Nitric oxide and mitochondrial biogenesis: a key to long-term regulation of cellular metabolism. Comp Biochem Physiol A Mol Integr Physiol 2005;142:102–110 [DOI] [PubMed] [Google Scholar]
- 20. Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand 1998;162:401–409 [DOI] [PubMed] [Google Scholar]
- 21. Schild L, Jaroscakova I, Lendeckel U, Wolf G, Keilhoff G. Neuronal nitric oxide synthase controls enzyme activity pattern of mitochondria and lipid metabolism. FASEB J 2006;20:145–147 [DOI] [PubMed] [Google Scholar]
- 22. Soraru G, Vergani L, Fedrizzi L, et al. Activities of mitochondrial complexes correlate with nNOS amount in muscle from ALS patients. Neuropathol Appl Neurobiol 2007;33:204–211 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.