Rituximab, a chimeric monoclonal antibody against the CD20 antigen, increased survival when it was added to chemotherapy for patients with systemic, CD20+ diffuse large B-cell (DLBCL) non-Hodgkin lymphoma (NHL). Consequently, it is now a standard component of the treatment for these patients. Approximately 90% of primary CNS lymphomas (PCNSL), a rare extranodal variant of NHL, are CD20+ DLBCL. Rituximab has been incorporated into some treatment regimens for newly diagnosed and relapsed PCNSL, although it is not known whether this agent will improve outcomes to the extent that it has for patients with systemic DLBCL.1,2 Rituximab may not traverse the normal blood–brain barrier (BBB) and this could limit the effectiveness of this agent in PCNSL. Rituximab concentrations in CSF are 0.1% of plasma levels when it is administered at a standard IV dose of 375 mg/m2, suggesting poor BBB penetration.3 Moreover, in a report of 4 patients with PCNSL administered I123-labeled rituximab, there was weak tumor uptake in only one out of 4 patients.4 However, in another study of the 90Y-labeled anti-CD20 antibody ibritumomab tiuxetan target accumulation of the antibody was observed in 4 out of 6 patients with PCNSL assessed by SPECT imaging with 111In-labeled ibritumomab tiuxetan.5 The latter report is consistent with the hypothesis that rituximab may achieve therapeutic concentrations in regions of a brain tumor manifesting contrast enhancement secondary to BBB disruption.
Level of evidence.
This is a Class III case series of 12 patients with PCNS lymphoma treated with rituximab, with MRI responses achieved in 36% of patients and extension of median progression-free survival to 57 days (95% CI 29–175 days), overall survival to 20.9 months (95% CI 2.9–47 months).
Methods.
This pilot study was conducted by the National Cancer Institute–sponsored New Approaches to Brain Tumor Therapy (NABTT) CNS Consortium to determine the response rate to rituximab monotherapy in patients with recurrent or refractory PCNSL (NCT00072449). Rituximab was administered at a dose of 375 mg/m2 as a single IV infusion every week for up to 8 weeks. MRI scans were performed every 2 months and radiographic responses were determined using standard criteria.6 Responses were confirmed with a follow-up MRI 1 month after first declaration of complete response (CR) or partial response (PR).
Results.
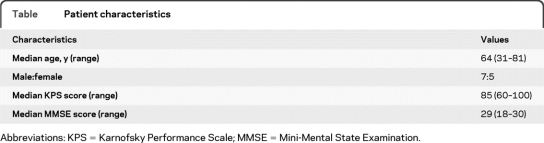
Twelve patients were enrolled at 4 NABTT institutions. Patient characteristics are enumerated in the table. The median time from initial diagnosis of PCNSL to relapse was 19 months (3.1–64.3 months). All patients had failed prior methotrexate-based treatment. The median number of rituximab infusions for patients treated on this study was 6 (range 3–8). Confirmed responses were achieved in 4/11 (36%) patients (3 CR, 1 PR). One additional patient achieved a CR but died of infection 63 days after starting rituximab and before the response could be confirmed on follow-up MRI. Including the latter patient resulted in an unconfirmed response proportion of 5/12 (42%). One patient was on dexamethasone at the time of radiographic PR but steroids were subsequently discontinued. No other responding patients were on corticosteroids at the time of rituximab treatment. The median progression-free survival was 57 days (95% confidence interval [CI] 29–175 days) and the median overall survival was 20.9 months (95% CI 2.9–47 months). Ten patients have developed progressive disease and 8 patients have died. The median progression-free survival and overall survival for the 4 patients achieving a confirmed radiographic response were 7.6 (5.7 to 36.2) months and 47 (9.1 to 47) months. One of these patients remains in remission. Toxicity was modest with 4 episodes of grade 3–4 toxicities possibly related to rituximab (allergic reaction, fatigue, anxiety, back pain).
Table.
Patient characteristics
Abbreviations: KPS = Karnofsky Performance Scale; MMSE = Mini-Mental State Examination.
Discussion.
This report is the largest series of patients with PCNSL treated with IV rituximab monotherapy. Radiographic responses were observed in approximately one-third of enrolled patients and these responses were durable in some. These data provide evidence of activity of IV rituximab monotherapy in patients with PCNSL and support the incorporation of this agent into chemotherapy regimens for this rare form of NHL. Further studies are required to determine the optimal dose and schedule of rituximab in this clinical setting.
Footnotes
Disclosure: Dr. Batchelor has served on scientific advisory boards for Acceleron Pharma, Exelixis Inc., ImClone Systems, and Genentech, Inc.; has received funding for travel and speaker honoraria from Merck Serono; serves on the editorial boards of the Journal of Clinical Oncology and Oncologist; has received publishing royalties from Up To Date in Oncology (Up To Date, Inc., 2007–2009); has received research support from the NIH and the Angiogenesis Foundation; and has served as a consultant in a medico-legal case. Dr. Grossman serves on scientific advisory boards for Roche, Merck Serono, and Diffusion Pharmaceuticals; serves on the editorial boards of the Journal of Clinical Oncology and Neuro-oncology; and is Principal of and holds stock in Axxia Pharmaceuticals, LLC, which is developing a patent re: Subcutaneous hydromorphone implant for the treatment of cancer pain. Dr. Mikkelsen serves on scientific advisory boards for Antisense Therapeutics Limited and Eisai Inc.; serves on speakers' bureaus for and has received speaker honoraria from Merck Serono, Schering-Plough Corp., Roche, and Genentech, Inc.; serves on the editorial board of Neuro-oncology; and is listed as an inventor on patents re: An anti-angiogenic kringle and its mutants, Specific therapy using integrin ligands for treating cancer, and New stabilized TRAIL conjugate for imaging, targeting and therapy of gliomas. Dr. Ye reports no disclosures. Dr. Desideri receives research support from the NIH/NCI. Dr. Lesser serves on scientific advisory boards for AstraZeneca and Genentech, Inc.; serves on the editorial boards of the Journal of Clinical Oncology, Current Treatment Options in Oncology, and as Editor of CNS Section for Current Treatment Options in Oncology; serves on the speakers' bureau for Prostraken; and receives research support from EMD Serono, Inc., the NIH, and a CCCWFU and Section on Hematology and Oncology Partner Grant.
References
- 1. Shah G, Yahalom J, Correa D, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol 2007;25:4730–4735 [DOI] [PubMed] [Google Scholar]
- 2. Enting R, Demopoulos A, DeAngelis L, Abrey L. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology 2004;63:901–903 [DOI] [PubMed] [Google Scholar]
- 3. Rubenstein JL, Rosenberg J, Damon L. High-dose methotrexate plus rituximab (Anti-CD20) monoclonal antibody in the treatment of primary CNS lymphoma. Society for Neuro-Oncology fourth annual meeting, Scottsdale, AZ, November 17–21, 1999 Abstract [Google Scholar]
- 4. Dietlein M, Pels H, Schulz H, et al. Imaging of central nervous system lymphomas with I123-labeled rituximab. Eur J Haematol 2005;74:348–352 [DOI] [PubMed] [Google Scholar]
- 5. Maza S, Kiewe P, Munz DL, et al. First report on a prospective trial with yttrium-90-labeled ibritumomab tiuxetan (Zevalin) in primary CNS lymphoma. Neurooncol 2009;11:423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macdonald DR, Cascino TL, Schold SC, Cairncross J. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277–1280 [DOI] [PubMed] [Google Scholar]