Abstract
Objective:
To improve understanding of TRPV4-associated axonal Charcot-Marie-Tooth (CMT) neuropathy phenotypes and their debated pathologic mechanism.
Methods:
A total of 17 CMT2C phenotypic families with vocal cord and diaphragmatic involvement and 36 clinically undifferentiated CMT2 subjects underwent sequencing analysis of the coding region of TRPV4. Functional studies of mutant proteins were performed using transiently transfected cells for TRPV4 subcellular localization, basal and stimulated Ca2+ channel analysis, and cell viability assay with or without channel blockade.
Results:
Two TRPV4 mutations R232C and R316H from 17 CMT2C families were identified in the ankyrin repeat domains. The R316H is a novel de novo mutation found in a patient with CMT2C phenotype. The family with R232C mutation had individuals with and without vocal cord and diaphragm involvement. Both mutant TRPV4 proteins had normal subcellular localization in HEK293 and HeLa cells. Cells transfected with R232C and R316H displayed increased intracellular Ca2+ levels and reversible cell death by the TRPV channel antagonist, ruthenium red.
Conclusion:
TRPV4 ankyrin domain alterations including a novel de novo mutation cause axonal CMT2. Individuals with the same mutation may have nondistinct CMT2 or have phenotypic CMT2C with vocal cord paresis. Reversible hypercalcemic gain-of-function of mutant TRPV4 instead of loss-of-function appears to be pathologically important. The reversibility of cell death by channel blockade provides an attractive area of investigation in consideration of treatable axonal degeneration.
Hereditary motor and sensory neuropathy 2C (HMSN2C) or Charcot-Marie-Tooth type 2C (CMT2C) is a dominantly inherited axonal neuropathy with diaphragmatic and vocal cord paresis.1 The occurrence of voice and diaphragmatic involvements provided for subclassification beyond the broad classification of HMSN2 (CMT2). Our initial chromosomal localization to 12q24.1-q24.32 and confirmatory localization3 further validated its differentiation from other CMT subtypes. This localization also led us to speculate that CMT2C was allelic to scapuloperoneal spinal muscular atrophy (SPSMA)4 and possibly other disorders which colocalized to this same chromosomal region including congenital distal spinal muscular atrophy (dSMA).2 In SPSMA, scapular wasting was clinically distinguishable from CMT2C but both had laryngeal palsy. In both disorders, childhood presentations had more severe disease with respiratory failure. Sensory nerve involvements were most apparent in CMT2C while only infrequent vibratory loss was observed in SPSMA. We recently identified 2 mutations in transient receptor potential vanilloid 4 (TRPV4) as causative in the originally described CMT2C (R269H) and SPSMA (R316C) kindreds.5 At the same time, 2 other TRPV4 mutations (R315W and R269C) were described by different groups.6,7 The R315W mutation was found in one family with diverse phenotypes, i.e., CMT2C, SPSMA, and dSMA. Collectively these mutations have also been found in other unrelated kindreds along with 2 additional mutations (R232C, V620I).8
TRPV4 is a transmembrane Ca2+-permeable, nonselective cation channel and responsive to mechanical force, osmotic concentration, and increased temperature.9 The potential for calcium neurotoxicity is emphasized by earlier reviews in neurodegeneration10 with suggested roles in AD,11 motor neuron disease,12 and PTEN-induced putative kinase (PINK1)– associated PD.13 Additionally, l-type calcium channel blockers have recently been shown as neuroprotective in development of idiopathic PD.14
Earlier functional analysis of TRPV4 has yielded conflicting results.5–7 Two studies5,6 showed mutant proteins have normal subcellular localization with neurotoxic gain of function from increased Ca2+ influx, whereas another study7 suggested TRPV4 mutants lost membrane localization and had decreased stimulus-dependent channel activity utilizing HeLa cells.7 Cell-line–specific differences may explain divergent results as we utilized HEK293 cells. All the studied mutations occurred in ankyrin repeat domains, raising the question of whether mutant proteins could lose the binding ability to PIP2, a cell membrane phospholipid, thus leading to the altered stimulus-dependent channel activity.15 Complex pathogenesis is further highlighted by the bone disorders associated with TRPV4 mutations including brachyolmia,16 spondylometaphyseal dysplasia Kozlowski type metatropic dysplasia,17 and now spondylo-epiphyseal dysplasia.18 The variable phenotypes such as short trunk, scoliosis, and small hands occur in these disorders but detailed neuromuscular testing has not been reported. Toxic gain of function of TRPV4 was suggested to be responsible for their pathogenesis, but a large number of mutations discovered in a wide spectrum of phenotypes including SPSMA, CMT2C, distal SMA, and HMN with vocal cord paralysis and clinically undifferentiated HMSN2 suggests that additional phenotype modifier or more complex pathogenic mechanism exists. Some individuals with CMT2C and SPSMA had bony abnormalities beyond what would be expected with a primary neurogenic process, possibly linking these primary bone disorders with neuronal degenerative pathogenesis.5,6
We report findings from exon sequencing of TRPV4 in 17 families with CMT2C phenotypes and 36 nondistinct CMT2 individuals without proximal vocal cord or diaphragm involvement. Protein localization experiments and functional analysis were performed using both HEK293 and HeLa cells to determine whether discovered mutants have loss or gain of TRPV4 function.
METHODS
Patients.
We identified 17 families with CMT2C phenotypes and 36 cases of nondistinct CMT2 individuals and families where genetic cause was unknown. The families were not known to be related. All persons had clinical examinations with sensory testing and nerve conductions verifying the axonal nature of their motor and sensory involvements with exclusion of acquired etiologies. Among patients with CMT2C, a shortness of breath, a breathy voice, or asthma–like wheeze on exertion was typical. Phrenic nerve conductions, diaphragm needle EMG, pulmonary function tests, direct visualization of vocal cords, and speech pathology were employed in ascertainment. Family history or clinical involvements were confirmed by direct examination in kindred evaluation.
CMT2C kindred 1 (R232C) with varied vocal cord involvement.
A 43-year-old man and his family, reported previously,19 presented with 4 years of diminished manual dexterity, difficulty walking, and a 2-year history of dysphonia. He had distal amyotrophy, pes cavus foot deformity, left lateral rectus palsy, and left vocal cord paralysis by visualization. Generalized areflexia and loss of sensory vibration sense were detected in the legs of all 3 affected. His mother and sister were asymptomatic, but distal muscle weakness was elicited and vocal cord and diaphragm involvement was absent. All 3 affected persons showed evidence of an axonal polyneuropathy with varied extent of sensory involvement on electrophysiology testing and no bony dysmorphic features (figure 1A).
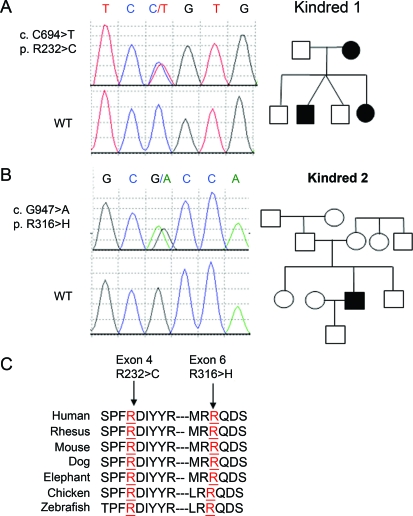
Figure 1. Mutations of TRPV4 in Charcot-Marie-Tooth (CMT) 2C and CMT2 pedigrees.
Wild-type (WT) sequences are shown in the lower panels of each case. All examined and affected carried the corresponding mutation not found in unaffected persons and our 800 controls. (A) Heterozygous mutation c.C694>T, resulting in amino acid change p.R232>C, was identified in exon 4 of the TRPV4 gene. The affected male had vocal cord involvement which was not found in his mother or sister. (B) Heterozygous mutation c.G947>A, resulting in amino acid change p.R316>H, was identified in exon 6 of the TRPV4 gene in this CMT2C de novo patient; the mutation was not found in his genetically confirmed parents or other family members. (C) Evolutionary conservation of amino acids in the mutated regions of TRPV4 among different species is shown. 232R and 316R are labeled in red and the surrounding TRPV4 amino acids sequences of human (H sapiens) and its orthologues in rhesus (Macaca mulatta), mouse (M musculus), dog (Canis lupus familiaris), elephant (Elephantidae), chicken (Gallus gallus), and zebrafish (Danio rerio) are aligned. The mutated amino acids are indicated by arrows on the top.
CMT2C kindred 2 (R316H).
A 30-year-old man presented with shortness of breath which limits him in mild exercise including walking. There was also a weakness of voice that precluded yelling dating back to his childhood. He suffered chronically with fatigue in long speeches with reduced volume. There was distal foot and hand atrophy. Sensory symptoms of paresthesias, dysesthesias, or sensory loss were denied; sensory loss was documented in distal extremities to light touch, vibration, temperature, and pain. He had moderate ankle dorsiflexion weakness and could not walk on his heels. Mild abductor weakness of the intrinsic hand muscles accompanied distal areflexia and proximal hyporeflexia. Quantitative sensory testing (heat, pain, cooling, and vibration) showed that large (256-Hz vibration) and small fiber sensory loss occurred in the feet and hands. He had a hoarseness of voice, and on direct visualization of vocal cords had complete paresis of the left vocal cord with diminished abduction on the right. Pulmonary function testing showed reduced functional vital capacity (FVC 55% of predicted) and forced expiratory volume (FEV1 52% of predicted) consistent with neuromuscular failure.
The electrophysiologic features were of a chronic motor predominant axonal denervating process with chronic reinnervation. The motor nerve conductions were preserved with peroneal compound motor action potential amplitude being 4.8 mV with conduction velocity of 49 m/s. Ulnar motor amplitude was 8.1 mV with conduction velocity of 67 m/s. The sural sensory amplitude was 7 μV with conduction velocity of 47 m/s. The radial and median sensory amplitudes were reduced at 15 and 8 μV with normal distal latencies. The phrenic responses were reduced in amplitude, 0.3 (left) and 0.8 mV (right), with associated prolonged latencies 9.0 and 9.3 msec. Blink responses R1, R2 ipsilateral to supraorbital trigeminal stimulation were normal. On needle examination motor units were long in duration with reduced recruitment more prominent in distal compared to proximal musculature, but also with diaphragm and paraspinal muscles involved.
His genetically confirmed parents were unaffected by neuropathy as determined by normal clinical examinations. His mother had relapsing-remitting multiple sclerosis with mild hemiparesis and no lower motor neuron findings. His 2 siblings and offspring were normal. No persons had bony abnormalities (figure 1B).
Expression vectors.
A human cDNA clone (IMAGE: 40125977) was used as a template. Two primers anchored with an XhoI (TRPV4-TP1:5′ctgtctcgagcaggcatggcggattccagcgaag-3′) and BamHI (TRPV4-TP2:5′-catcggatccctagagcggggcgtcatcagt-3′) were used to amplify the coding sequence. The amplified fragment was cloned into vector pBluescript-M13. The TRPV4 sequence was verified by sequencing. The R232C mutation was introduced into the vector by site-directed mutagenesis using a primer TRPV4-R232C:5′-cattaactcgcccttctgtgacatctactatcg-3′. The R316H was introduced by TRPV4-R316H: 5′-gaaggcggacatgcggcaccaggactcgcgagg-3′. The XhoI/BamHI fragment containing wild-type TRPV4, TRPV4R232C, and TRPV4R316H was cloned into the XhoI and BamHI sites of a dual expression vector pIRES2-ZsGreen1 (Clontech).
Expression of wild-type and mutant TRPV4.
HEK293T or HeLa cells were grown on glass coverslips in DMEM containing 10% serum at 37°C. The cells were transfected with wild-type TRPV4, TRPV4R232C, and TRPV4R316H using Lipofectamine 2000 (Invitrogen).
Intracellular Ca2+ measurements.
HEK293 were transfected, incubated with ruthenium red (10 μM) for 24 hours, and loaded with 3 μM Indo-1 AM for 45 minutes. Cultures were then rinsed and kept in the dark in HEPES buffer for 30 minutes to allow for dye de-esterification. Calcium flux was measured as a ratio of 405/510 fluorescence using a MoFlo cell sorter before and during treatment with 2 μM of TRPV4-specific agonist 4α-phorbol 12, 13-didecanoate (4αPDD), and analyzed using Summit (DakoCytomation). The argon-ion (488 nm) and krypton (UV) lasers were used for excitation. The GFP and propidium iodide (PI) signals were collected using 530/40 nm and 670/20 nm bandpass filters. Indo-1 signals were collected using 405/30 nm and 510/21 nm filters. Measurements were calibrated using the Grynkiewicz equation.20 Values for Rmin and Rmax were determined in Ca2+-free solution and high Ca2+-containing solution in the presence of 5 μM ionomycin. The dissociation constant (Kd) of 250 nM for Indo-1 AM was used for calculations. In all experiments, data were gated on cells that were PI-negative and showed high levels of GFP expression. Two-tailed unpaired Student t test (p < 0.0005) was used for statistical analysis.
Cell viability assay.
HEK293 or HeLa were transfected, incubated either with or without ruthenium red (20 μM) for 48 hours, and harvested in PBS containing 25 μg/mL PI (Calbiochem). All flow cytometric data were analyzed using a Dako Cytomation Cyan flow cytometer and Summit software (DakoCytomation) recording at least 50,000 events. The argon-ion 488 nm was used for excitation. The GFP and PI signals were collected using 530/40 nm and 680/30 nm filters. In all experiments, data were gated on cells showing high levels of GFP expression. Percent cell death was the percentage of GFP-positive cells that were also PI positive. Two-tailed unpaired Student t test (p < 0.05) was used for analysis.
RESULTS
DNA sequencing of 15 exons of TRPV4 in a total of 53 unrelated CMT2 probands with vocal cord paralysis including subjects with diaphragmatic weakness (n = 17) and undifferentiated CMT2 (n = 36) revealed 2 mutations. A c.694C>T mutation in exon 4, leading to p.R232C, was identified in kindred 1 (figure 1A). A c.947G>A mutation in exon 6, resulting in p.R316H, was found in a CMT2C individual (figure 1B). R316H has not been reported before and is a de novo mutation. The R232 and R316 are both highly conserved amino acids (figure 1C). These mutations were absent in our 800 control samples.
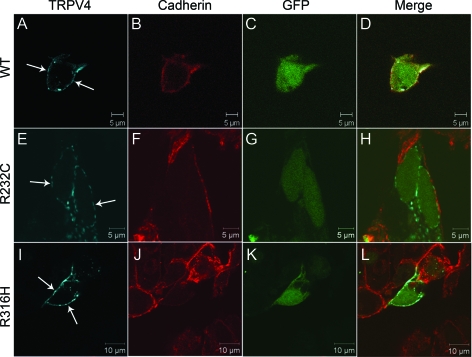
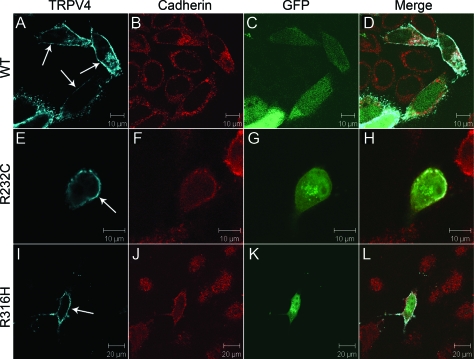
TRPV4 is a cation channel located on the cytoplasmic membrane. The R232C and R316H mutations are in the ankyrin-repeat domain (figure e-1 on the Neurology® Web site at www.neurology.org). To examine the impact of the mutations on targeting TRPV4 to the cytoplasmic membrane, we analyzed the subcellular distribution of TRPV4 with R232C (TRPV4232C) and R316H (TRPV4R316H) in transiently transfected HEK293 cells, using the human wild-type TRPV4 (wtTRPV4) as a control. We found these mutants had a similar pattern of subcellular localization comparing to the wtTRPV4 in the transfected HEK293 cells (figure 2), suggesting that these mutations may not interfere with channel assembly and intracellular trafficking. Previous studies have demonstrated that mutant TRPV4 channels have a physiologic distribution in HEK293 cells, but not in HeLa cells,7 suggesting a possibility of cell-type–specific distribution of the mutant TRPV4. To test this possibility, we further examined the localization of the mutant TRPV4 in HeLa cells and used wtTRPV4 as a control. We found that the mutants localized on the cytoplasmic membrane in HeLa cells as well (figure 3).
Figure 2. Localization of wild-type and mutant TRPV4 on the plasma membrane in HEK293 cells.
Confocal microscopy was performed using HEK293 cells transfected with plasmids pIRES2-ZsGreen1 containing wtTRPV4 (A–D), TRPV4R232C (E–H), and TRPV4R316H (I–L). Cells expressing exogenous TRPV4 were labeled by green fluorescent protein (GFP) (C, G, and K). TRPV4 is shown by blue (A, E, and I) and cadherin by red (B, F, and J). Merged images are shown on the right panels (D, H, and L). Arrows indicate TRPV4 signals on the plasma membrane.
Figure 3. Localization of wild-type and mutant TRPV4 on the plasma membrane in HeLa cells.
Confocal microscopy was performed using HeLa cells transfected with plasmids pIRES2-ZsGreen1 containing wtTRPV4 (A–D), TRPV4R232C (E–H), and TRPV4R316H (I–L). Cells expressing exogenous TRPV4 were labeled by green fluorescent protein (GFP) (C, G, and K). TRPV4 is shown by blue (A, E, and I) and cadherin by red (B, F, and J). Merged images are shown on the right panels (D, H, and L). Arrows indicate TRPV4 signals on the plasma membrane.
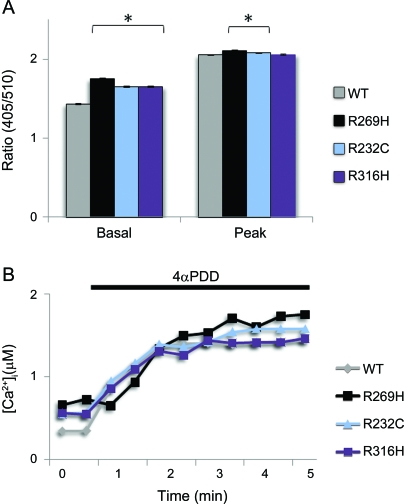
The TRPV4 channel responds to a large variety of stimuli. To test the effects of the mutations on calcium channel activity, we analyzed the channel activity in transiently transfected HEK293 cells. We examined the calcium channel activity of TRPV4 using Indo-1 AM Calcium Sensor Dye. Internal Indo-1 fluorescence ratio (405 nm/510 nm) was used as an indicator of the intracellular Ca2+ levels, which depend on Ca2+ influx. In addition to the 2 new mutations (TRPV4R232C, TRPV4R316H), wtTRPV4 and previously identified neuropathy-linked mutation (TRPV4R269H) were also included as controls. Similar to TRPV4R269H, TRPV4R232C and TRPV4R316H showed increased basal intracellular calcium concentration vs the wtTRPV4 (p < 0.0005) (figure 4). The calcium channel activity was further analyzed using TRPV4-specific agonist 4α-phorbol 12, 13-didecanoate (4αPDD). The wild-type and all the mutant TRPV4 channels could be activated by 4αPDD. Similar to TRPV4R269H, the TRPV4R232C mutation showed increased calcium channel activity compared to wtTRPV4 when activated by 4αPDD (p < 0.0005) (figure 4).
Figure 4. Effects of mutations on calcium channel activity of TRPV4.
(A) Internal fluorescence ratio in HEK293 cells transfected with WT, R269H, R232C, or R316H construct at rest or stimulated condition (2 μM 4αPDD) is given as basal (left) or peak (right) value (n >3,000 cells for each condition). Mean calcium responses before and during 4αPDD application are given. Significant difference was observed in TRPV4 mutants (R269H, R232C, or R316H) at basal level, and mutants (R269H or R232C) at activated condition when compared to WT-TRPV4 (2-tailed Student t test, *p < 0.005). (B) Application of 4αPDD induced an increase in intracellular calcium ([Ca2+]i). Error bars, mean ± SEM.
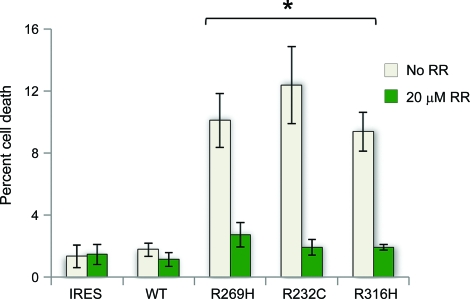
We then analyzed the effect of the mutant TRPV4 channels on cell viability. HEK293 and HeLa cells were transfected with above dual expression vectors. After 48 hours of incubation with or without ruthenium red (20 μM), cytotoxicity in GFP-positive cells was analyzed by flow cytometry. We observed a trend toward increased cell death in mutant TRPV4-expressing HEK293 without reaching statistical significance (figure e-2). We then further tested cell viability in HeLa cells. We observed that HeLa cells expressing TRPV4R232C and TRPV4R316H had markedly increased cell death when compared to the wtTRPV4-expressing cells (figure 5). Cells expressing TRPV4R269H, a previously identified mutation in our original CMT2C family, also led to increased cell death. All observed cell death could be suppressed by TRPV antagonist ruthenium red (figure 5).
Figure 5. Quantification of propidium iodide uptake in HeLa cells expressing wild-type and mutant TRPV4.
Transfected HeLa cells indicated an increase in cytotoxicity in mutant-expressing cells at 48 hours that is blocked by the TRPV channel blocker ruthenium red (RR). Data are averaged from at least 3 independent experiments. *p < 0.05, indicating significant differences when compared to WT-TRPV4 (2-tailed Student t test). Error bars, mean ± SEM.
DISCUSSION
Through screening 36 unrelated individuals with nondistinct CMT2 and 17 unrelated families with CMT2C phenotype having vocal cord and diaphragmatic involvement, we identified 2 TRPV4 mutations R232C and R316H. R316H mutation has not been reported before and is de novo. R232C is from a CMT2C family, but only the proband has vocal cord involvement whereas other affected do not. This family was previously localized to 12q23–243 and the variability of vocal cord involvement was discussed.19 The findings here emphasize the importance of considering TRPV4 mutation when vocal cord paralysis feature is not present in inherited axonal neuropathy, i.e., nondistinct clinical CMT2. The previous and the current study suggest the location of TRPV4 mutations does not account for this specific neurologic variability.5–8
This study highlights the genetic heterogeneity of dominantly inherited axonal neuropathies with vocal cord and diaphragmatic involvement. Specifically, only 2 out of 17 screened CMT2C families had identified mutations in TRPV4 and no TRPV4 mutation was found in 36 screened undifferentiated CMT2 families. Mutations in GDAP1 have been linked to vocal cord paresis with peripheral neuropathy but are uncommon in dominant or axonal cases. Most affected cases caused by GDAP1 mutation have recessive inheritance and demyelination.21 Also highlighted is that the lack of family history should not dissuade from consideration of CMT2C with the proper phenotype as seen in the individual with a de novo mutation R316H. Furthermore, incomplete penetrance or markedly variable expression may also obscure the genetic cause as seen in our original kindred with R269H mutation where repeated clinical examinations over time including specialized nerve conductions were required to identify very mildly affected persons and hence the accurately diagnosed affected status.2,5,8 Therefore penetrance should only be determined close to the end of life as symptoms among some may not occur until the eighth decade of life.2 Scoliosis and small hands were characteristic of our severely affected persons with R316C and R269H mutations5 but were not seen in the individuals described here with R316H and R232C mutations. The fact that TRPV4 can cause nondistinct CMT2 and CMT2C, SPSMA, and distal SMA as well as different bone dysplasias16 implies the existence of complex pathogenic mechanism related to TRPV4 mutation for varied phenotypes, and pathogenic effect of TRPV4 mutation may not exert through cytotoxic calcium influx alone.
To date, 7 mutations in TRPV4 have been identified in 15 families with axonal neuropathies. Six mutations located in the ankyrin-repeat domains, 5 showed segregation with the disease, and 2 de novo mutations. Our newly reported de novo mutation R316H combined with previous studies suggests codon 269R and 316R are both hot spots for axonal neuropathy-linked TRPV4 mutations. The substitutions of 269R or 316R by either cysteine or histidine cause CMT2C.
The R232C mutation was reported in 3 other families with axonal neuropathies8 in addition to the family described here. The TRPV4 mutations in the 3 initial reports were in the third and fourth ankyrin-repeat domains5–7 while R232C is in the second domain (figure 2). The only neuropathy mutation located outside of the ankyrin repeat domain is V620I,8 which was previously reported in brachyolmia.16 Neurologic examination and testing is not reported among the previously reported TRPV4-associated bone dysplasias. The V620I neuropathy case was a de novo mutation, raising the possibility of an additional hot spot in axonal neuropathy. This person had mild skeletal dysplasia features that suggested a possible overlap syndrome.
The previously reported discrepancies in the functional studies are addressed with our current experiments. Two studies showed TRPV4 mutations R269H, R316C, and R269C have normal cellular localization and increased calcium channel activity while another study showed R269H, R315W, and R316C had decreased calcium channel activity and abnormal cellular localization. Since different cell types were used, the cell-type–specific response could explain the conflicting data. To investigate whether gain of function or loss of function of TRPV4 mutations accounted for the abnormality, we performed functional analysis in both HEK 293 and HeLa cells. Using wtTRPV4 as control, we performed subcellular localization, channel activity, and cell viability assay for R232C, R316H, and R269H. All 3 mutations properly localize to the cell membrane and cause gain of function of TRPV4, resulting in increased intracellular calcium with decreased cell viability. The R269H mutant experiments reproduced the prior results. Our data indicate that an increased constitutive function, rather than increased response to agonist stimulation, is probably the key property gained. Significantly increased cell death was only observed in mutant TRPV4-expressing HeLa cells although cell viability was increased in both transfected HeLa and HEK293 cells after using TRPV antagonist ruthenium red to block the mutant channel. The data support that TRPV4 ankyrin repeat mutations exert their effects by dominant gain of function with normal cell membrane localization. Nevertheless we remain open to the possibility that other unidentified factors are involved in pathogenesis and ongoing work is required.
Intracellular hypercalcemia is a common pathway of diverse pathogenesis.10,22 Ruthenium red, the calcium channel blocker, has recently been shown to improve mitochondrial ATP levels and membrane potentials in neurons expressing mutant α-synuclein or PINK-1 associated with Parkinson.23 Optimism of a pharmacologic approach also comes from recent research where l-type calcium blockers have been suggested to reduce the chance of developing PD.14 Further work will be required to establish whether the observed hypercalcemia cytotoxicity manifests its effect in the cytoplasm or mitochondria. Calcium channel inhibitors provide a rational new approach for investigation of TRPV4-mediated axonal neuropathies.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Eric J. Ahlskog for helpful discussion. We appreciate help provided by the staff of the Robert H. Lurie Comprehensive Cancer Center Flow Cytometry Core Facility.
- CMT
- Charcot-Marie-Tooth
- dSMA
- distal spinal muscular atrophy
- HMSN2C
- hereditary motor and sensory neuropathy 2C
- PI
- propidium iodide
- SPSMA
- scapuloperoneal spinal muscular atrophy
- TRPV4
- transient receptor potential vanilloid 4.
Editorial, page 856.
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
C.J.K., P.J.D., T.S., and H.-X.D. conceived this project. C.J.K., Y.W., and L.H. performed sequencing analysis. Y.S., F.F., and H.X.D. performed functional analysis. C.J.K., M.D., G.N., M.E.M., A.C., and K.M.M. examined and collected family information and samples. C.J.K., H.-X.D., T.S., and P.J.D. analyzed the data and wrote the article.
DISCLOSURE
Dr. Klein, Dr. Shi, Dr. Fecto, Dr. Donaghy, Dr. Nicholson, Dr. McEntagart, Dr. Crosby, Dr. Wu, Dr. Lou, and Dr. McEvoy report no disclosures. Dr. Siddique serves on the scientific advisory board of NIH: Skeletal Muscle and Exercise Physiology (SMEP) Study Section; serves on the editorial boards of Neurogenetics and Amyotrophic Lateral Sclerosis; holds a patent re: Human α-tocopherol transport protein: compositions and methods; and receives research support from the NIH (NINDS and NIEHS), the Harold Post ALS Research Fund, the Les Turner ALS Foundation/Herbert C. Wenske Foundation, Vena Schaaf, Frank White ALS Research Fund, the Spastic Paraplegia Foundation, Inc., the Amyotrophic Lateral Sclerosis Association, the CVS/ALS Therapy Alliance, and the Blazeman Foundation for ALS. Dr. Deng reports no disclosures. Dr. Dyck receives research support from the NIH and the FDA.
REFERENCES
- 1. Dyck PJ, Litchy WJ, Minnerath S, et al. Hereditary motor and sensory neuropathy with diaphragm and vocal cord paresis. Ann Neurol 1994;35:608–615 [DOI] [PubMed] [Google Scholar]
- 2. Klein CJ, Cunningham JM, Atkinson EJ, et al. The gene for HMSN2C maps to 12q23–24: a region of neuromuscular disorders. Neurology 2003;60:1151–1156 [DOI] [PubMed] [Google Scholar]
- 3. McEntagart ME, Reid SL, Irrthum A, et al. Confirmation of a hereditary motor and sensory neuropathy IIC locus at chromosome 12q23-q24. Ann Neurol 2005;57:293–297 [DOI] [PubMed] [Google Scholar]
- 4. DeLong R, Siddique T. A large New England kindred with autosomal dominant neurogenic scapuloperoneal amyotrophy with unique features. Arch Neurol 1992;49:905–908 [DOI] [PubMed] [Google Scholar]
- 5. Deng HX, Klein CJ, Yan J, et al. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat Genet 2010;42:165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Landoure G, Zdebik AA, Martinez TL, et al. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat Genet 2010;42:170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Auer-Grumbach M, Olschewski A, Papic L, et al. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet 2010;42:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimon M, Baets J, Auer-Grumbach M, et al. Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain 2010;133:1798–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liedtke W. Molecular mechanisms of TRPV4-mediated neural signaling. Ann NY Acad Sci 2008;1144:42–52 [DOI] [PubMed] [Google Scholar]
- 10. Mattson MP. Calcium and neurodegeneration. Aging Cell 2007;6:337–350 [DOI] [PubMed] [Google Scholar]
- 11. Yang L, Wang Z, Wang B, Justice NJ, Zheng H. Amyloid precursor protein regulates Cav1.2 L-type calcium channel levels and function to influence GABAergic short-term plasticity. J Neurosci 2009;29:15660–15668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang M, Schuster JE, Fu R, Siddique T, Heckman CJ. Progressive changes in synaptic inputs to motoneurons in adult sacral spinal cord of a mouse model of amyotrophic lateral sclerosis. J Neurosci 2009;29:15031–15038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gandhi S, Wood-Kaczmar A, Yao Z, et al. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 2009;33:627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S. L-type calcium channel blockers and Parkinson disease in Denmark. Ann Neurol 2010;67:600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. Embo J 2008;27:2809–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rock MJ, Prenen J, Funari VA, et al. Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat Genet 2008;40:999–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krakow D, Vriens J, Camacho N, et al. Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia. Am J Hum Genet 2009;84:307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishimura G, Dai J, Lausch E, et al. Spondylo-epiphyseal dysplasia, Maroteaux type (pseudo-Morquio syndrome type 2), and parastremmatic dysplasia are caused by TRPV4 mutations Am J Med Genet A 2010;152A:1443–1449 [DOI] [PubMed] [Google Scholar]
- 19. Donaghy M, Kennett R. Varying occurrence of vocal cord paralysis in a family with autosomal dominant hereditary motor and sensory neuropathy. J Neurol 1999;246:552–555 [DOI] [PubMed] [Google Scholar]
- 20. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985;260:3440–3450 [PubMed] [Google Scholar]
- 21. Claramunt R, Pedrola L, Sevilla T, et al. Genetics of Charcot-Marie-Tooth disease type 4A: mutations, inheritance, phenotypic variability, and founder effect. J Med Genet 2005;42:358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farber JL. The role of calcium in cell death. Life Sci 1981;29:1289–1295 [DOI] [PubMed] [Google Scholar]
- 23. Marongiu R, Spencer B, Crews L, et al. Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson's disease by disturbing calcium flux. J Neurochem 2009;108:1561–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.