Abstract
Background:
Individuals aged 80 years and older is the fastest growing segment of the population worldwide. To understand the biology behind increasing longevity, it is important to examine factors related to survival in this age group. The relationship between brain atrophy and survival after age 85 remains unclear.
Methods:
A population-based sample (n = 239) had head CT scans at age 85 and was then followed until death. Cortical atrophy and ventricular size were assessed. Statistical analyses included Cox proportional hazards models with time to death as the outcome and considering a large number of possible confounders, including baseline cognitive function, incident dementia, and somatic disorders.
Results:
Mean survival time (±SD) was 5.0 ± 3.6 years (range 0.10–19.8 years). Decreased survival was associated with temporal, and frontal atrophy, sylvian fissure width and a number of ventricular measures after adjustment for potential confounders. In participants without dementia at baseline (n = 135), decreased survival was associated with temporal lobe atrophy and bifrontal ratio. In those with dementia (n = 104), decreased survival was associated with third ventricle width, cella media ratio, and ventricle-to-brain and ventricle-to-cranial ratio.
Conclusions:
Several indices of brain atrophy were related to decreased survival after age 85, regardless of dementia status. Brain atrophy is rarely mentioned as a significant indicator of survival in the elderly, independent of traditional predictors such as cardiovascular disease or cancer. The biology behind the influence of brain atrophy on survival needs to be further scrutinized.
The number of elderly is increasing dramatically worldwide, particularly those aged ≥80 years.1 It is therefore important to study factors related to survival in this age group.
Leading causes of death in the elderly include cardiovascular diseases and cancer.2,3 Cognitive function is also a predictor of death.4 After age 85, dementia is the primary predictor of death, followed by male sex, cardiovascular diseases, cancer, and chronic obstructive lung disease.5 After age 95, dementia and cognitive performance are important contributors to 5-year mortality.6
Few studies have examined whether structural brain changes are important for survival. Two population-based studies7,8 examined brain atrophy in relation to survival in elderly subjects without dementia (mean ages around 74 years). In these studies the size of the lateral ventricles7 and CSF volume8 measured with MRI was associated with survival. A third study in elderly subjects without dementia reported that global and regional brain atrophy was associated with decreased survival between ages 78 and 85 years.9 None of these studies report on the association between brain atrophy and survival after age 85.
We studied a population of 85-year-olds who participated in CT scans and was followed until death. Our aim was to examine whether brain atrophy was related to survival independent of previously reported factors, such as dementia and cognitive function.
METHODS
The population sample and the examinations have been described in detail.10 We invited every second 85-year-old born July 1, 1901–June 30, 1902, and registered for census purposes in Gothenburg, to take part in a health survey, including psychiatric examinations, which took place in the participant's home or at the institution where the participant was resident. Of those, 494 (63.1%, 143 men, 351 women) accepted. There were no statistically significant differences between participants and nonparticipants regarding sex, marital status, 3-year mortality rate, and status as psychiatric outpatient or inpatient.10
All participants with dementia (n = 147) and a systematic subsample of 269 participants without dementia were invited to a CT scan. A total of 104 (70.7%) of those with dementia and 135 (50.2%) of those without participated. There were no significant differences between participants and nonparticipants in the CT scan according to dementia status with regards to any of the parameters studied (sex, registration as a psychiatric outpatient or inpatient, mental disorders, institutionalization, 3-year mortality, history of stroke, cardiovascular disorders, or mean systolic and diastolic blood pressure).11
Date of death was obtained from the Swedish population register. This is a national register including all individuals living in Sweden and Swedish citizens living abroad. The register is known to be complete regarding mortality data.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Ethics Committee for Medical Research at the University of Gothenburg. Informed consent was obtained from the participants, their relatives, or both.
The examinations have been described in detail10 and included medical history, physical examinations, neuropsychiatric examinations, CT scans, and interviews with close informants. In addition, medical records from psychiatric and geriatric institutions and outpatient departments in Gothenburg were examined by a psychiatrist.
The psychiatric examinations were semi-structured and included questions about background factors, ratings of psychiatric symptoms and signs, signs common in dementia, and the Mini-Mental State Examination (MMSE).12
After the examination, the participant was asked to give the interviewer permission to interview a close informant. If the participant was unable to give permission, close informants were identified in other ways. The informant interview was semi-structured and included questions about changes in the participant's behavior and intellectual function, background factors, and for participants with dementia, questions about age at onset and course.
Measurements of potential confounders at baseline.
Physical disorders (cardiac insufficiency, myocardial infarction, and chronic obstructive lung disease) were diagnosed using information from medical history and physical and laboratory examinations. History of stroke was based on information from self-reports, key informant interviews, and the Swedish Hospital Discharge Register.13 Information on cancer was obtained from the Swedish Cancer Registry. Diabetes mellitus was defined based on a physician's diagnosis, being on antidiabetes therapy, or having 2 fasting venous or capillary whole blood glucose values ≥7.0 mmol/L. Information on smoking (never vs ever smoker) and education (mandatory, i.e., 6 years, vs more than mandatory) was based on self-reports. Casual blood pressure was measured in the right arm in the seated position after 5 minutes rest using a mercury manometer. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were registered to the nearest 5 mm Hg. DBP was defined as Korotkoff phase 5. Hypertension was defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg or taking antihypertensive medication. Measurements of all potential confounders were made without knowledge of the results from the neuropsychiatric and CT examinations.
Diagnosis of dementia and depression.
Dementia was determined according to the criteria in the DSM-III-R14 using information from the psychiatric examination and the close informant interview.10 Incident dementia during follow-up was based on the same information at the examinations at ages 88, 90, 92, 95, 97, 99, and 100, and in those lost to follow-up from the Swedish Hospital Discharge Register according to the International Classification of Diseases (ICD) codes (ICD9 codes15 290A/B/E/W/X, 291B/C, 294A/B, 331A/B/C/F; ICD1016 codes F0.00–0.02/0.09–0.13/0.18–0.24/0.28/0.39/0.51/1.06–1.07, G3.0/3.00–3.01/3.08–3.09) and medical records if available. Major depressive syndrome during the last month was diagnosed according to DSM-III-R.17
CT scan.
CT was performed without contrast enhancement on a Philips Tomoscan 310 (n = 133) or on a General Electric 8800 (n = 106).11 The scans included 10-mm continuous slices, which were examined by experienced radiologists. The degree of cortical atrophy in the frontal, temporal, parietal, and occipital lobes was rated and categorized using a 3-point scale (normal, mild/moderate, or severe).18
The following linear distances were measured using a transparent metric ruler as described18: a) the bifrontal span of the lateral ventricle, b) the width of the lateral ventricles at the head of the caudate nucleus, c) the sum of the separate widths of the left and right sylvian fissures, d) the minimum width of the bodies of the lateral ventricles at the waist, and e) the width of the third ventricle. Ratios for a) to d) were determined by dividing the values obtained by the width of the brain at the level of the measurement, resulting in the following ratios: bifrontal ratio, bicaudate ratio, sylvian fissure ratio, and cella media ratio. The rating procedure was carried out separately for the subjective cortical ratings and the linear distances.
Ventricular and brain volumes were assessed using the following procedure. A hard copy of each scan was made and digitized by uniform background illumination and image captured using a Canon 600 power shot digital camera. Using the US NIH IMAGE program version 1.55,19 the inner table of the skull was traced following well-established procedures.20 Total intracranial volume was calculated by measuring the inner table of the skull, interpolating area between slices and calculating total cranial capacity. Volumes were calculated by interpolating slice thickness and separation to yield a single value for total intracranial volume. Similarly, total brain volume was based on thresholding brain parenchyma from CSF and bone and tracing the outer surface of the brain interpolating volume from slice thickness, gap between slices, and the number of slices from the cisterna magna to vertex. CSF was similarly thresholded separating it from brain parenchyma and the inner surface of the lateral, third, and fourth ventricles traced. Ventricular volume was the sum total of lateral, third, and fourth ventricular volume also interpolated as above. The ventricle-to-brain and ventricle-to-cranial ratios were calculated by dividing total ventricular volume with total brain volume or total intracranial volume, multiplying those ratios by 100 so that whole numbers could be used.
White matter lesions (WMLs) were defined as low-density areas in the periventricular and subcortical white matter.11 Decreased density was rated in relation to the attenuation of normal white matter and was defined as no, mild, moderate, or severe signal attenuation. WMLs were analyzed as no/mild vs moderate/severe signal attenuation.
The interrater reliabilities for different CT measures have been found to be good.21–23
The interobserver agreement for the presence vs absence of atrophy was studied in 140 CT scans rated by the radiologist who rated the scans in the present study and a neurologist trained in CT evaluations. The interobserver agreement was κ = 0.43 for temporal atrophy, κ = 0.34 for frontal atrophy, κ = 0.28 for occipital atrophy, and κ = 0.35 for parietal atrophy.22 Spearman correlations between the raters regarding linear measurements was 0.71 (p < 0.001) for bifrontal ratio, 0.63 (p < 0.001) for cella media ratio, 0.72 (p < 0.001) for bicaudate ratio, and 0.31 (p < 0.001) for sylvian fissure ratio. The interrater reliability for volumetric measures was 0.9 or greater.23 The correlations between the linear ventricular measurements and the volumetric ventricular measures were high (Spearman 0.61–0.68).
Statistical analyses.
Cox proportional hazards models were applied to analyze the association between brain atrophy and time to death, defined as the time from CT scan to death. Analyses were performed on the total sample and on groups stratified by dementia status at baseline. Weighted analyses taking into account the sampling fractions were done in analyses of the total sample. Ratings of cortical atrophy were analyzed as absence or presence of atrophy and as severe or not severe atrophy. The quantitative measures were analyzed as continuous data.
The measures of brain atrophy were first assessed in univariate Cox models. The variables that were significantly associated with mortality were further analyzed in multivariate models with potential confounders. Preselected confounders in multivariate models included sex, baseline MMSE, dementia at baseline or follow-up in the analyses of the total sample, and baseline MMSE score and dementia at follow-up in the analyses of those without dementia at baseline.
Other potential confounders were assessed in univariate analyses of the total sample, and in separate analyses of participants with and without dementia. Factors that were significantly associated with mortality (p < 0.05) in the univariate analyses were included as confounders in the multivariate analyses. The additional confounders which were included in the final analyses of the total sample included WMLs, SBP, stroke, gastrointestinal cancer, and chronic obstructive lung disease. In the analyses of those without dementia, the additional confounders were gastrointestinal cancer and chronic obstructive lung disease. In those with dementia, the confounders were sex, WMLs, and DBP.
Results from the Cox models are presented as hazard ratios (HR) and 95% confidence intervals (CI). HRs for quantitative measures are expressed per SD. The assumption of proportional hazards was tested by comparing the HR for the first 5 years (57% of all deaths) with the HR for the later period. No significant differences were found. Group differences in survival time are calculated using Student t test. Statistical analyses were performed using SPSS version 16.0. Results were considered statistically significant at a level of p < 0.05.
No data were imputed. Participants were thus included in all analyses for which they provided information.
RESULTS
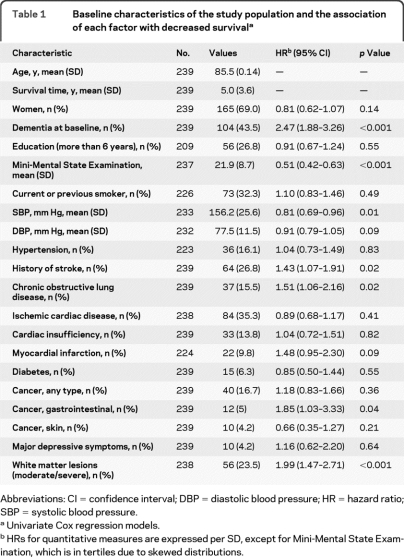
Mean survival time (±SD) was 5.0 ± 3.6 years (range 0.10–19.8 years). Survival time was 6.2 ± 3.9 years in participants without dementia (n = 135) and 3.4 ± 2.5 years (p < 0.001) in those with dementia (n = 104). New cases of dementia occurred during follow-up in 44 (32.6%) of 135 participants without dementia at baseline. Baseline characteristics and associations with survival are presented in table 1.
Table 1.
Baseline characteristics of the study population and the association of each factor with decreased survivala
Abbreviations: CI = confidence interval; DBP = diastolic blood pressure; HR = hazard ratio; SBP = systolic blood pressure.
Univariate Cox regression models.
HRs for quantitative measures are expressed per SD, except for Mini-Mental State Examination, which is in tertiles due to skewed distributions.
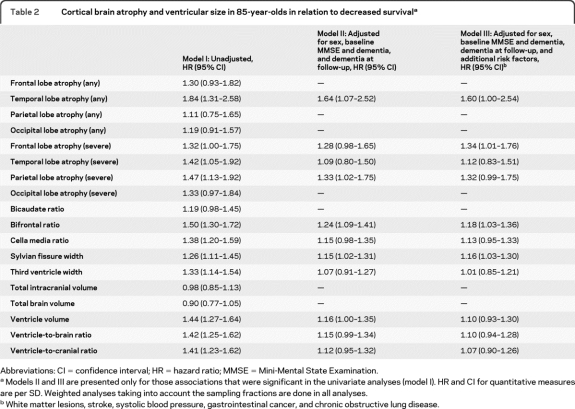
Survival in relation to brain atrophy in the total sample is presented in table 2 . Any temporal and severe frontal, as well as sylvian fissure width, and bifrontal ratio were associated with decreased survival adjusting for confounders (table 2, model III).
Table 2.
Cortical brain atrophy and ventricular size in 85-year-olds in relation to decreased survivala
Abbreviations: CI = confidence interval; HR = hazard ratio; MMSE = Mini-Mental State Examination.
Models II and III are presented only for those associations that were significant in the univariate analyses (model I). HR and CI for quantitative measures are per SD. Weighted analyses taking into account the sampling fractions are done in all analyses.
White matter lesions, stroke, systolic blood pressure, gastrointestinal cancer, and chronic obstructive lung disease.
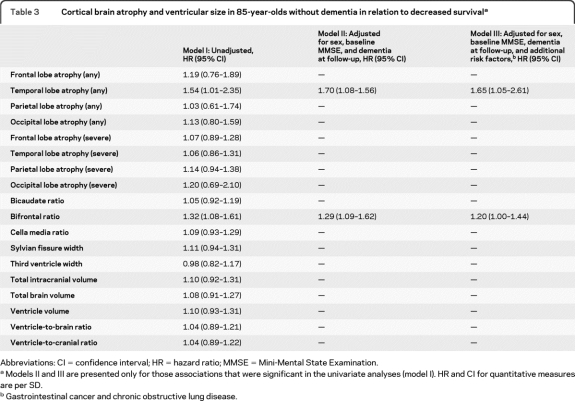
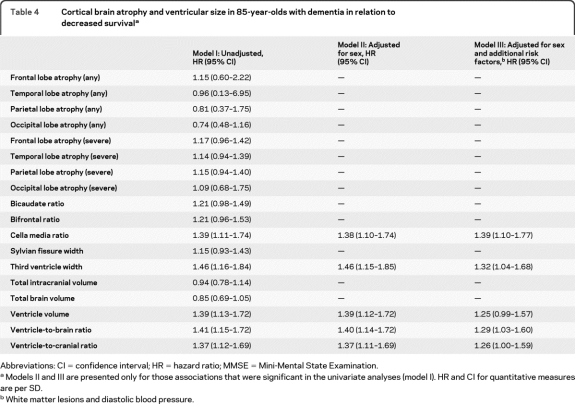
Table 3 shows survival in relation to brain atrophy in participants without dementia at baseline. In this group, temporal lobe atrophy and bifrontal ratio were associated with decreased survival adjusting for confounders (table 3, model III). Survival in relation to brain atrophy in participants with dementia at baseline is presented in table 4 . In those with dementia, cella media ratio, third ventricle width, and ventricle-to-brain and ventricle-to-cranial ratio were associated with decreased survival after adjusting for confounders (table 4, model III).
Table 3.
Cortical brain atrophy and ventricular size in 85-year-olds without dementia in relation to decreased survivala
Abbreviations: CI = confidence interval; HR = hazard ratio; MMSE = Mini-Mental State Examination.
Models II and III are presented only for those associations that were significant in the univariate analyses (model I). HR and CI for quantitative measures are per SD.
Gastrointestinal cancer and chronic obstructive lung disease.
Table 4.
Cortical brain atrophy and ventricular size in 85-year-olds with dementia in relation to decreased survivala
Abbreviations: CI = confidence interval; HR = hazard ratio; MMSE = Mini-Mental State Examination.
Models II and III are presented only for those associations that were significant in the univariate analyses (model I). HR and CI for quantitative measures are per SD.
White matter lesions and diastolic blood pressure.
There were no significant interaction effects of the different CT measures by dementia on mortality risk, except for third ventricle width (p = 0.016) and a tendency for total brain volume (p = 0.091).
Among the participants, 129 (54.0%) died of cardiovascular causes, 23 (9.6%) of psychiatric or nervous system diseases, 28 (11.7%) of respiratory system diseases, 27 (11.3%) of cancer, and 32 (13.4%) of other causes. The brain atrophy measures did not differ regarding causes of death.
DISCUSSION
We found that a number of measures of brain atrophy were associated with survival after age 85 in a population-based sample of 85-year-olds followed for 20 years. Temporal lobe atrophy and bifrontal ratio were associated with decreased survival in elderly without dementia, while cella media ratio, third ventricle width, and ventricle-to-brain and ventricle-to-cranial ratio were related to decreased survival in those with dementia. The associations were independent of a range of confounders. These results suggest that the influence of brain atrophy should be considered in studies of survival in the elderly.
Three previous population-based studies report that brain atrophy, assessed as ventricular enlargement,7 CSF volume,8 and global and regional brain volumes,9 were associated with decreased survival in elderly without dementia. Participants were approximately 7–10 years younger than in our study and follow-ups were limited to 6–12 years.7–9 In contrast, we followed all 85-year-olds until death. We chose to include also elderly with dementia as we aimed to examine the independent influence of brain atrophy on survival in the total population of 85-year-olds. In a memory clinic population, decreased survival was associated with global cortical atrophy in younger (<68 years) but not in older patients,24 which is in contrast to our findings. In support of our findings, cortical atrophy on CT was included in an index that predicted survival in patients with early Alzheimer disease.25
There are several possible reasons why brain atrophy may decrease survival. First, the brain regulates a number of functions important for survival, such as blood pressure, body temperature, appetite, energy balance, control of body fluids, electrolyte homeostasis, and cardiac function.26,27 We have previously shown that larger bifrontal ratio and frontal atrophy were associated with lower blood pressure in 85-year-olds without dementia,28 areas related to decreased survival in our study. Second, brain atrophy is related to midlife factors, such as obesity21 and hypertension.29 Brain atrophy may therefore be a marker of general poor health. However, we found that brain atrophy was related to decreased survival even after taking these and a number of other confounders into consideration. Third, brain atrophy may be a marker for incipient and manifest Alzheimer-type dementia,30 which is strongly related to decreased survival. We found that brain atrophy was associated with decreased survival even after controlling for incident dementia and baseline cognitive function. Fourth, brain atrophy is related to motor impairment31 and behavioral manifestations which may influence the ability to survive.
The cellular processes that may underlie brain atrophy on CT in the elderly include neurodegeneration32 and neuronal shrinkage.33 Brain atrophy is probably caused by a number of factors occurring during the lifespan, from prenatal and perinatal factors to factors occurring during adolescence and mid and late life. Several factors related to brain atrophy, e.g., midlife obesity21 and hypertension,29 are modifiable. Other factors potentially related to brain atrophy, e.g., perinatal care and nutrition, have improved during the last century. It needs to be elucidated to what regard this has contributed to increased survival during the last century.
The strengths of the study include the population-based sample, the comprehensive examinations performed by psychiatrists, the range of assessments of brain atrophy, the large number of possible confounders, and the possibility to follow all individuals until death. There are some limitations and methodologic issues. First, measures of brain atrophy were only done at one timepoint. Longitudinal studies are needed to examine whether changes in brain atrophy over time are related to survival. Second, only about half of those invited to have a CT scan accepted. However, this response rate is acceptable for such an examination in a population study of 85-year-olds. Furthermore, participants did not differ from nonparticipants regarding a number of factors, including mortality. Third, visual ratings and linear measurements are rather crude estimates of cortical atrophy. The interrater κ values for agreement between a neurologist and a radiologist were moderate for temporal lobe atrophy (κ = 0.43) and fair for the other cortical regions (κ = 0.28–0.35). If anything, the lack of perfect agreement likely attenuates the observed relationships. Nonetheless, visual rating of the temporal lobe has been found to be highly predictive for the discrimination of patients with Alzheimer disease vs control subjects.34 Fourth, we used CT rather than MRI. CT is more severely affected by beam hardening artifacts, which is particularly severe in the region of the medial temporal lobe.35 However, CT is comparable to MRI in detecting brain atrophy35,36 and may be more suitable than MRI for the elderly, as it is less sensitive to motion artifacts. CT is also the most used brain imaging tool worldwide. Atrophy on CT37 and MRI23,38 have been related to neuropathologic findings. Fifth, multiple comparisons were made, which may lead to false-positive findings. Conversely, the use of a correction for multiple comparisons may give rise to false-negative results. Our way to treat this problem is to make no adjustments for the number of comparisons but to give information on how many comparisons have been made and to emphasize that the findings should be considered only suggestive until further confirmed.39 Seventh, we examined a very old Swedish sample. Our findings might therefore not be generalized to younger age groups or other geographic areas.
Brain atrophy is rarely mentioned as a significant indicator of survival in the elderly, independent of traditional predictors such as cardiovascular disease or cancer. There are indications that brain function has improved over the last decades, as shown by better results on cognitive testing in successive birth cohorts of elderly.40 The biology behind the influence of brain atrophy on survival needs to be further scrutinized.
ACKNOWLEDGMENT
The authors thank Dr. Bo Palmertz (Department of Radiology, Sahlgrenska University Hospital, Gothenburg, Sweden), who was responsible for the CT examinations; Valter Sundh, Kristoffer Bäckman, and Tomas Marlow (Neuropsychiatric Epidemiology Unit, University of Gothenburg, Sweden), for statistical assistance; and Tracy Abildskov (Department of Psychology and Neuroscience Center, Brigham Young University, Provo, UT), for technical assistance with image analysis.
Footnotes
- CI
- confidence interval
- DBP
- diastolic blood pressure
- DSM-III-R
- Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised
- HR
- hazard ratio
- ICD
- International Classification of Diseases
- MMSE
- Mini-Mental State Examination
- SBP
- systolic blood pressure
- WML
- white matter lesion
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. P.J. Olesen and Dr. Ingmar Skoog.
DISCLOSURE
Dr. Olesen and Dr. Guo report no disclosures. Dr. Gustafson has received honoraria from the Albuquerque Area Indian Health Board and Shire plc and receives research support from the NIH and the Swedish Research Council. Dr. Börjesson-Hanson has served as a consultant for and received funding for travel and speaker honoraria from Pfizer Inc., Novartis, Janssen-Cilag, and Lundbeck; and serves on speakers' bureaus for Pfizer Inc. and Novartis. Dr. Sacuíu and Dr. Eckerström report no disclosures. Dr. Bigler serves as an Associate Editor of Brain Imaging and Behavior; receives research support from the NIH; and has given expert testimony in medico-legal cases. Dr. Skoog has served on scientific advisory boards for Pfizer Inc. and AstraZeneca; serves as Triage Editor for the European Journal of Psychiatry; has served on an editorial advisory board for International Psychogeriatrics; receives publishing royalties for Alzheimers sjukdom och andra kognitiva sjukdomar (English title: Alzheimer's Disease and Other Cognitive Disorders [Liber, 2003]); serves on speakers' bureaus for Shire plc, Janssen-Cilag, Pfizer Inc., Novartis, GE Healthcare, and Eisai Inc.; and has received research support from the Swedish Research Council, the Alzheimer's Association, and the Bank of Sweden Tercentenary Foundation.
REFERENCES
- 1. Kinsella K, He W. An Aging World: 2008 (Report No.: P95/09-1). Washington, DC: US Census Bureau; 2009 [Google Scholar]
- 2. Niederlaender E. Causes of death in the EU. Luxembourg: 2006 [Google Scholar]
- 3. World Health Organization The top ten causes of death. WHO Media Centre 2008;1–5 [Google Scholar]
- 4. Bosworth H, Siegler I. Terminal change in cognitive function: an updated review of longitudinal studies. Exp Aging Res 2002;28:299–315 [DOI] [PubMed] [Google Scholar]
- 5. Aevarsson O, Svanborg A, Skoog I. Seven-year survival rate after age 85 years: relation to Alzheimer disease and vascular dementia. Arch Neurol 1998;55:1226–1232 [DOI] [PubMed] [Google Scholar]
- 6. Börjesson-Hansson A, Gustafson D, Skoog I. Five-year mortality in relation to dementia and cognitive function in 95-year-olds. Neurology 2007;69:2069–2075 [DOI] [PubMed] [Google Scholar]
- 7. Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the Cardiovascular Health Study. Neurobiol Aging 2007;28:1307–1315 [DOI] [PubMed] [Google Scholar]
- 8. Ikram MA, Vernooij MW, Vrooman HA, Hofman A, Breteler MM. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging 2009;30:450–456 [DOI] [PubMed] [Google Scholar]
- 9. Staff RT, Murray AD, Ahearn T, et al. Brain volume and survival from age 78 to 85: the contribution of Alzheimer-type magnetic resonance imaging findings. J Am Geriatr Soc 2010;58:688–695 [DOI] [PubMed] [Google Scholar]
- 10. Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A. A population-based study of dementia in 85-year-olds. N Engl J Med 1993;328:153–158 [DOI] [PubMed] [Google Scholar]
- 11. Skoog I, Palmertz B, Andreasson LA. The prevalence of white-matter lesions on computed tomography of the brain in demented and nondemented 85-year-olds. J Geriatr Psychiatry Neurol 1994;7:169–175 [DOI] [PubMed] [Google Scholar]
- 12. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 13. Liebetrau M, Steen B, Skoog I. Depression as a risk factor for the incidence of first-ever stroke in 85-year-olds. Stroke 2008;39:1960–1965 [DOI] [PubMed] [Google Scholar]
- 14. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. Washington, DC: American Psychiatric Association; 1987 [Google Scholar]
- 15. Socialstyrelsen Klassifikation av sjukdomar 1987 Systematisk förteckning. Stockholm: Norstedts Tryckeri; 1987 [Google Scholar]
- 16. World Health Organization The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization; 1993 [Google Scholar]
- 17. Skoog I, Nilsson L, Landahl S, Steen B. Mental disorders and the use of psychotropic drugs in an 85-year-old urban population. Int Psychogeriatr 1993;5:33–48 [DOI] [PubMed] [Google Scholar]
- 18. De Leon MJ, Ferris SH, George AE, Reisberg B, Kricheff II, Gershon S. Computed tomography evaluations of brain-behavior relationships in senile dementia of the Alzheimer's type. Neurobiol Aging 1980;1:69–79 [DOI] [PubMed] [Google Scholar]
- 19. Rasband W. NIH-IMAGE, 1.55 ed. Washington, DC: US National Institutes of Health; 1994 [Google Scholar]
- 20. Bigler ED, Burr R, Gale S, et al. Day of injury CT scan as an index to pre-injury brain morphology. Brain Inj 1994;8:231–238 [DOI] [PubMed] [Google Scholar]
- 21. Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology 2004;63:1876–1881 [DOI] [PubMed] [Google Scholar]
- 22. Simoni M, Pantoni L, Pracucci G, et al. Prevalence of CT-detected cerebral abnormalities in an elderly Swedish population sample. Acta Neurol Scand 2008;118:260–267 [DOI] [PubMed] [Google Scholar]
- 23. Bigler ED, Tate DF. Brain volume, intracranial volume, and dementia. Invest Radiol 2001;36:539–546 [DOI] [PubMed] [Google Scholar]
- 24. Henneman WJ, Sluimer JD, Cordonnier C, et al. MRI biomarkers of vascular damage and atrophy predicting mortality in a memory clinic population. Stroke 2009;40:492–498 [DOI] [PubMed] [Google Scholar]
- 25. Claus JJ, van Gool WA, Teunisse S, et al. Predicting survival in patients with early Alzheimer's disease. Dement Geriatr Cogn Disord 1998;9:284–293 [DOI] [PubMed] [Google Scholar]
- 26. Tabarean I, Morrison B, Marcondes MC, Bartfai T, Conti B. Hypothalamic and dietary control of temperature-mediated longevity. Ageing Res Rev 2010;9:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKinley MJ, Gerstberger R, Mathai ML, Oldfield BJ, Schmid H. The lamina terminalis and its role in fluid and electrolyte homeostasis. J Clin Neurosci 1999;6:289–301 [DOI] [PubMed] [Google Scholar]
- 28. Skoog I, Andreasson LA, Landahl S, Lernfelt B. A population-based study on blood pressure and brain atrophy in 85-year-olds. Hypertension 1998;32:404–409 [DOI] [PubMed] [Google Scholar]
- 29. Swan GE, DeCarli C, Miller BL, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology 1998;51:986–993 [DOI] [PubMed] [Google Scholar]
- 30. Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology 2004;63:94–100 [DOI] [PubMed] [Google Scholar]
- 31. Guo X, Steen B, Matousek M, et al. A population-based study on brain atrophy and motor performance in elderly women. J Gerontol A Biol Sci Med Sci 2001;56:M633–M637 [DOI] [PubMed] [Google Scholar]
- 32. Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex 1998;8:295–300 [DOI] [PubMed] [Google Scholar]
- 33. Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol 1987;21:530–539 [DOI] [PubMed] [Google Scholar]
- 34. Wahlund LO, Julin P, Johansson SE, Scheltens P. Visual rating and volumetry of the medial temporal lobe on magnetic resonance imaging in dementia: a comparative study. J Neurol Neurosurg Psychiatry 2000;69:630–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frisoni GB. Structural imaging in the clinical diagnosis of Alzheimer's disease: problems and tools. J Neurol Neurosurg Psychiatry 2001;70:711–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wattjes M, Henneman W, van der Flier W, et al. Diagnostic imaging of patients in a memory clinic: comparison of MR imaging and 64-detector row CT. Radiology 2009;253:174–183 [DOI] [PubMed] [Google Scholar]
- 37. Rossi R, Joachim C, Smith A, Frisoni G. The CT-based radial width of the temporal horn: pathological validation in AD without cerebrovascular disease. Int J Geriatr Psychiatry 2004;19:570–574 [DOI] [PubMed] [Google Scholar]
- 38. Barkhof F, Polvikoski T, van Straaten E, et al. The significance of medial temporal lobe atrophy: a postmortem MRI study in the very old. Neurology 2007;69:1521–1527 [DOI] [PubMed] [Google Scholar]
- 39. Rothman KJ. Modern Epidemiology. Boston: Little, Brown; 1986 [Google Scholar]
- 40. Sacuíu SF, Gustafson D, Sjogren MJ, et al. Secular changes in cognitive predictors of dementia and mortality in 70-year-olds. Neurology 2010;75:779–785 [DOI] [PMC free article] [PubMed] [Google Scholar]