Abstract
Background:
Neuroinflammation may contribute to the pathogenesis of Parkinson disease (PD). Use of nonsteroidal anti-inflammatory drugs (NSAID) in general, and possibly ibuprofen in particular, has been shown to be related to lower PD risk in previous epidemiologic studies.
Methods:
We prospectively examined whether use of ibuprofen or other NSAIDs is associated with lower PD risk among 136,197 participants in the Nurses' Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) free of PD at baseline (1998 for NHS and 2000 for HPFS). NSAIDs use was assessed via questionnaire. Results were combined in a meta-analysis with those of published prospective investigations.
Results:
We identified 291 incident PD cases during 6 years of follow-up. Users of ibuprofen had a significantly lower PD risk than nonusers (relative risk [RR], adjusted for age, smoking, caffeine, and other covariates = 0.62; 95% confidence interval [CI] 0.42–0.93; p = 0.02). There was a dose–response relationship between tablets of ibuprofen taken per week and PD risk (p trend = 0.01). In contrast, PD risk was not significantly related to use of aspirin (RR = 0.99; 95% CI 0.78–1.26), other NSAIDs (RR = 1.26; 95% CI 0.86–1.84), or acetaminophen (RR = 0.86; 95% CI 0.62–1.18). Similar results were obtained in the meta-analyses: the pooled RR was 0.73 (95% CI 0.63–0.85; p < 0.0001) for ibuprofen use, whereas use of other types of analgesics was not associated with lower PD risk.
Conclusions:
The association between use of ibuprofen and lower PD risks, not shared by other NSAIDs or acetaminophen, suggests ibuprofen should be further investigated as a potential neuroprotective agent against PD.
Neuroinflammation may contribute to the progressive dopaminergic neuronal loss in the substantia nigra that has been observed in some experimental models of PD.1,2 In 2003, we first reported that use of nonaspirin nonsteroidal anti-inflammation drugs (NSAIDs), but not of aspirin, was associated with a lower risk of PD in 2 large prospective cohorts: the Health Professionals Follow-up Study (HPFS, 1986–2000) and the Nurses' Health Study (NHS, 1980–1998).3 Later, using data from the Cancer Prevention Study II Nutrition cohort, we reported that the lower PD risk among nonaspirin NSAIDs users was largely explained by use of ibuprofen, but not other NSAIDs.4 A lower PD risk among users of NSAIDs has been also reported in a few other investigations,5,6 but most of these did not differentiate ibuprofen from other nonaspirin NSAIDs. Here we present new results on NSAID use and PD risk based on recent surveys of the HPFS and the NHS cohorts, which assessed ibuprofen separately from aspirin or other NSAIDs. These analyses are independent of our previous publication3 as we only included new cases that were diagnosed after the completion of our previous study. Further, we investigated the relationship between use of ibuprofen or other types of NSAIDs and PD in a meta-analysis combining the results of the current study with those obtained in previous prospective investigations.
METHODS
Study population.
The HPFS was established in 1986, when 51,529 male US health professionals (dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians) aged 40–75 completed a mailed questionnaire regarding their medical history and lifestyle. The NHS cohort was established in 1976, when 121,700 female registered nurses responded to a similar questionnaire. In both cohorts, follow-up questionnaires have been mailed to participants every 2 years to update information on potential risk factors and to ascertain newly diagnosed diseases. The overall response rate is greater than 94% in the HPFS and the NHS follow-up has been 95% of potential person-years in the overall cohort. Because our previous publication on NSAIDs and PD was based on follow-up through 2000 in HPFS and 1998 in NHS, for the current analysis we used the 2000 survey as baseline for HPFS and the1998 survey for NHS, including a total of 37,305 men and 98,892 women.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Human Research Committees at the Harvard School of Public Health and the Brigham and Women's Hospital, with receipt of each questionnaire accepted as participant's consent.
Assessment of NSAID use and covariates.
In the 2000 HPFS questionnaire, men were asked whether they regularly (2+ times/week) took each type of the following analgesics: aspirin or aspirin-containing products (e.g., Alka-Seltzer with aspirin), ibuprofen (e.g., Advil, Motrin, Nuprin), other anti-inflammatory analgesics (e.g., Aleve, Naprosyn, Relafen, Ketofen, Anaprox, referred to as other NSAIDs in the text), and acetaminophen (e.g., Tylenol). Acetaminophen, though not an NSAID, was included in the analysis as it is a commonly used for similar clinical indications. Regular users of aspirin, ibuprofen, or acetaminophen were further asked for the frequency and tablets of use per week. Similar questions were included in the NHS 1998 questionnaire. Information on these analgesics uses was updated biennially in both cohorts. The reasons for using these analgesics and reproducibility of self-reported analgesic uses are shown in appendix e-1 on the Neurology® Web site at www.neurology.org.
In both cohorts, dietary intakes were assessed every 4 years with validated semi-quantitative food frequency questionnaires.7,8 Information on age, ethnicity, body weight, height, and smoking status was collected through biennial questionnaires. Body mass index (BMI) was calculated as weight (kg)/height (m).2
Ascertainment of PD.
Procedures for PD ascertainment have been described previously.9,10 Briefly, physician-diagnosed PD was first reported in the biennial questionnaires. After obtaining permission from potential patients with PD, we asked their treating neurologists (or internist if there were no neurologist) to complete a questionnaire to confirm or refute PD diagnosis and to send us a copy of the medical records. The medical records were reviewed by a movement disorder specialist (M.A.S.), blind to the exposure status. A case was confirmed if a diagnosis of PD was considered definite or probable by the treating neurologist or internist, or if the medical record included either a final diagnosis of PD made by a neurologist, or evidence of at least 2 of the 3 cardinal signs (rest tremor, rigidity, bradykinesia) in the absence of features suggesting other diagnoses. Overall, the diagnosis was confirmed by the movement disorder specialist based on review of the medical record or by the treating neurologist in 92.0% of the cases who consented to the release of their medical information. A signed consent form could not be obtained from 24% of the participants who reported a diagnosis of PD. The effect of including these individuals as PD cases was tested in sensitivity analyses.
Statistical analyses.
We computed person-time of follow-up for each participant from the return of the 2000 (HPFS) or 1998 (NHS) questionnaires to the date of first PD symptoms, death, or the end of follow-up (2006 for HPFS and 2004 for NHS), whichever came first. In the primary analyses, we categorized participants into regular users vs nonusers of each analgesic and calculated relative risks (RRs) using a Cox proportional hazards model, as there was no evidence of nonproportional hazards for major exposures and covariates (p > 0.1 for all). We controlled for age (in months), smoking status (never smoker, past smoker, current smokers [cigarettes/day] 1–14 or ≥15), BMI (kg/m2; <23, 23–24.9, 25–26.9, 27–29.9, or ≥30), and intakes of caffeine (quintiles), lactose (quintiles), and alcohol (0, 1–4.9, 5–9.9, 10–14.9, or ≥15 g/day for women; 0, 1–9.9, 10–19.9, 20–29.9, or ≥30 g/day for men), which have been suggested to be potential risk factors of PD. We also examined association between dosages of aspirin, ibuprofen, and acetaminophen use and PD risk. Tests for trend were assessed by assigning consecutive integers (1 to 4) to the categories of the NSAIDs dosage. No dose-response analysis was conducted for other NSAIDs as such information was not collected in the HPFS. Log RRs from the 2 cohorts were pooled by a fixed-effects model, weighted by the inverse of their variances as significance tests did not suggest heterogeneity between cohorts (p > 0.1 for all tests).11
We conducted several sensitivity analyses to examine the robustness of our findings. We excluded baseline gout patients as they were more likely to use NSAIDs than individuals without gout and also had a lower PD risk probably due to high plasma urate.12–14 Further, we conducted a lag analysis by excluding cases identified in the first 2 years of follow-up to alleviate concerns about the possibility that early PD symptoms might alter their habits of NSAID use. Finally, we examined whether long-term use of ibuprofen was associated with lower risk of PD. Because the duration of ibuprofen use was not directly asked in these cohorts, it was calculated based on the biennially updated surveys; in this calculation, we assumed that users of nonaspirin NSAIDs before 1998 for the NHS and 1996 for the HPFS were ibuprofen users, because ibuprofen was not assessed separately from other nonaspirin NSAIDs in those surveys.
We also explored potential interactions between NSAID uses and age (< vs ≥70 y, approximately medians of PD onset), smoking status (never vs ever), and caffeine intake (low vs high, based on median intake) in relation to PD risk by adding multiplicative terms in the Cox models.
Finally, we conducted a meta-analysis of the current study together with published prospective data on NSAIDs and PD risk. Relevant studies were identified by searching the Medline, PubMed, and OMIM databases for all published studies from 1966 through July 2009, using the following search algorithm: (nonsteroidal anti-inflammation drugs or aspirin or ibuprofen or acetaminophen) and (PD or Parkinson). We also manually searched the reference lists of relevant publications to identify additional studies. To be included in our meta-analysis, studies had to 1) use information on NSAIDs use collected prior to PD onset (or diagnosis) (referred to as prospective study), and 2) report RRs or odds ratios (ORs) separately for aspirin and ibuprofen or nonaspirin NSAIDs. Six studies were found eligible and included in the meta-analysis (see Results). Unlike previous meta-analyses on NSAIDs and PD risk,5,6 our meta-analysis considered use of ibuprofen separately from use of other NSAIDs, and included only prospective studies due to concern of recall bias and change of behavior caused by PD. We used Q statistic to examine heterogeneity among the studies and the significant level was set at 0.1. We used fixed-effects models to calculate the summary RRs as no significant heterogeneity was identified. Publication bias was examined with the Begg and Egger tests.
We used the SAS statistical package (version 9: SAS Institute, Cary, NC) for the cohort analyses, and STATA (version 9.0; College Station, TX) for the meta-analysis.
RESULTS
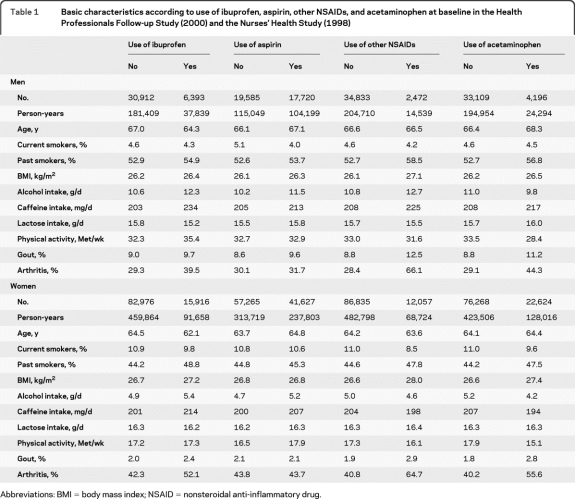
We identified 291 incident PD cases (156 men and 135 women) during the 6 years of follow-up. Participants who reported use of analgesics had higher BMI, consumed slightly larger amounts of caffeine, were more likely to be ex-smokers and less likely to be current smokers, and had a higher prevalence of gout and arthritis relative to nonusers (table 1).
Table 1.
Basic characteristics according to use of ibuprofen, aspirin, other NSAIDs, and acetaminophen at baseline in the Health Professionals Follow-up Study (2000) and the Nurses' Health Study (1998)
Abbreviations: BMI = body mass index; NSAID = nonsteroidal anti-inflammatory drug.
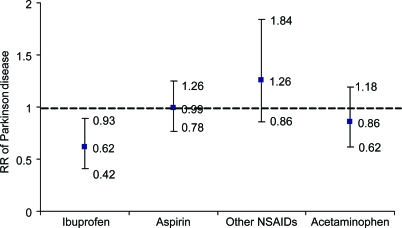
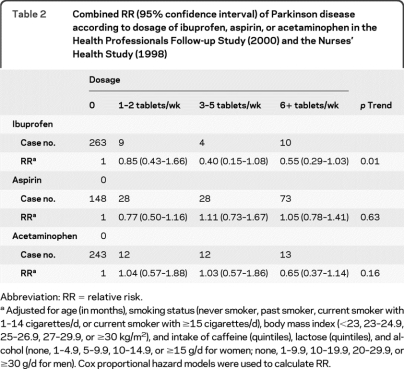
Ibuprofen use was significantly associated with a lower risk of developing PD. The combined multivariate RR of PD was 0.62 (95% confidence interval [CI] 0.42–0.93; p = 0.02); in contrast, no significant associations were observed for uses of aspirin, other NSAIDs, or acetaminophen (figure 1; table e-1). In the dose-response analysis, more tablets of ibuprofen use, but not aspirin or acetaminophen, were associated with lower risk of PD (p for trend = 0.01) (table 2). Further adjustment for self-reported gout, use of other individual types of analgesics, sleep duration, bowel movement, and use of antidepressants did not materially change these results.
Figure 1. Combined relative risks (RRs) of Parkinson disease according to use of each type of nonsteroidal anti-inflammatory drug (NSAID) or acetaminophen.
Adjustment for age (in months), smoking status (never smoker, past smoker, current smoker with 1–14 cigarettes/day, or current smoker with ≥15 cigarettes/day), body mass index (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), and intake of caffeine (quintiles), lactose (quintiles), and alcohol (none, 1–4.9, 5–9.9, 10–14.9, or ≥15 g/day for women; none, 1–9.9, 10–19.9, 20–29.9, or ≥30 g/day for men). Cox proportional hazard models were used to calculate RR. Case number: 28 for ibuprofen users and 263 for nonusers; 143 for aspirin users and 148 for nonusers; 32 for other NSAIDs users and 259 for nonusers; and 38 for acetaminophen users and 243 for nonusers.
Table 2.
Combined RR (95% confidence interval) of Parkinson disease according to dosage of ibuprofen, aspirin, or acetaminophen in the Health Professionals Follow-up Study (2000) and the Nurses' Health Study (1998)
Abbreviation: RR = relative risk.
Adjusted for age (in months), smoking status (never smoker, past smoker, current smoker with 1–14 cigarettes/d, or current smoker with ≥15 cigarettes/d), body mass index (<23, 23–24.9, 25–26.9, 27–29.9, or ≥30 kg/m2), and intake of caffeine (quintiles), lactose (quintiles), and alcohol (none, 1–4.9, 5–9.9, 10–14.9, or ≥15 g/d for women; none, 1–9.9, 10–19.9, 20–29.9, or ≥30 g/d for men). Cox proportional hazard models were used to calculate RR.
This association remained in all sensitivity analyses. The multivariate RR for ibuprofen use was 0.64 (95% CI 0.43–0.96) after excluding patients with gout, and 0.63 (95% CI 0.39–1.01) after excluding patients with PD identified within 2 years of the follow-up. Excluding ibuprofen use reported 2 years prior to onset of PD generated similar results (multivariate RR = 0.65; 95% CI 0.43–0.99; p = 0.04). Compared with nonusers, the RR was 1.00 (95% CI 0.73–1.38) for users with 2–4 years of cumulative ibuprofen use, 0.74 (95% CI 0.24–2.29) for 6–8 years, and 0.43 (95% CI 0.15–1.28) for 10 or more years. A similar association between ibuprofen and PD risk remained in analyses including PD cases not confirmed by treating physicians or review of medical record (RR = 0.62; 95% CI 0.42–0.91). There were no significant interactions between use of any analgesics and age, smoking, or caffeine intake.
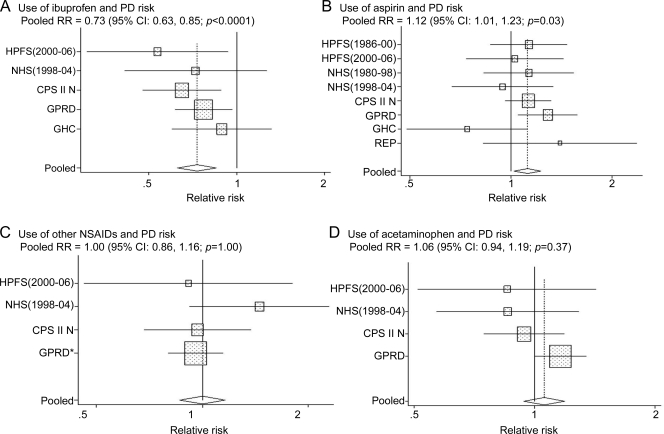
We identified a total of 12 published studies that had examined the association between NSAIDs use and PD risk (table e-2).3,4,15–23 Six studies were excluded because of retrospective data collection15,17,22 or not differentiating nonaspirin NSAIDs from aspirin.16,18,21 The remaining 6 studies3,4,19,20,23 were then included in the meta-analysis with the current one, including a total of 2,779 PD cases. The pooled RR was 0.73 (95% CI 0.63–0.85; p < 0.0001) for ibuprofen use (figure 2 A) and 1.12 (95% CI 1.01–1.23, p = 0.03) (figure 2B) for aspirin use. Use of other NSAIDs or acetaminophen was not associated with PD risk (figure 2, C and D). We identified no significant heterogeneity (p > 0.20 for all) nor evidence of publication bias (p > 0.3 for the Begg and Egger tests) among these studies.
Figure 2. Pooled relative risks (RRs) of Parkinson disease (PD) according to each type of nonsteroidal anti-inflammatory drug (NSAID) or acetaminophen in the meta-analysis.
(A) Ibuprofen; (B) aspirin; (C) other NSAIDs; and (D) acetaminophen. Rectangles indicate RRs from individual studies; error bars indicate 95% confidence intervals (CIs); unshaded diamond indicates the pooled RR from the random-effects model and 95% CI. Pooled RR: ibuprofen, 0.73 (95% CI 0.63–0.85; p < 0.0001); aspirin, 1.12 (95% CI 1.01–1.23, p = 0.03); other NSAIDs, 1.00 (95% CI 0.86–1.16; p = 1.0); acetaminophen, 1.06 (95% CI 0.94–1.19; p = 0.37). *In the General Practice Research Database (GPRD) study, RR for other NSAIDs was calculated by pooling the RR estimates for the different types of other NSAIDs (i.e., diclofenac, naproxen, and others), weighted by inverse of the variance within the study. CPS = Cancer Prevention Study; GHC = Group Health Cooperative; HPFS = Health Professionals Follow-up Study; NHS = Nurses' Health Study; REP = Rochester Epidemiology Project.
DISCUSSION
In this large prospective study, ibuprofen use was associated with a lower future risk of PD. In contrast, we did not find significant associations between use of aspirin, acetaminophen, or other NSAIDs and PD risk. The meta-analyses of all available prospective studies also showed that ibuprofen users had approximately 30% lower PD risk than nonusers; this relation had not been examined in previous meta-analyses of NSAIDs and PD risk.
Strengths of this study include its large sample size and high follow-up rate. Because we included only incident PD cases, our results are unlikely to be significantly affected by recall or selection bias. Further, our assessment of NSAID use was previously shown to be reliable (see appendix e-1) and intended to cover both prescription and over-the-counter uses. Studies based on prescription records16,18,19,21 may have had exposure misclassification as ibuprofen is primarily used over the counter in both the United States and Europe. For example, in the third US National Health and Nutrition Examination Survey (1988–1994), 29.7% of US adults reported monthly use of ibuprofen, as compared to only 1.9% prescription use.24 A few limitations of the current study should also be considered. The reproducibility of NSAIDs use was assessed in the NHS, but not in the HPFS. NSAID use was self-reported and thus subject to error; however, because of the prospective design, this error would most likely be nondifferential with respect to the future risk of PD, and thus would tend to attenuate the underlying association between NSAID use and PD. The validity of exposure assessment was supported by previous findings from these cohorts on other chronic diseases for which the associations of NSAIDs were consistent with their pharmacologic effects.25,26 Our cohorts do not represent random samples of US men and women; therefore, the patterns of use of NSAIDs cannot be taken to reflect the general population. Nevertheless, the biological effects of ibuprofen on PD in these cohorts should be the same as those among men and women in general, as suggested by the results obtained from the meta-analysis.
Accumulating postmortem and experimental evidence suggests that inflammatory mechanisms may contribute to the progressive loss of dopaminergic neurons in the substantia nigra of the brain.1,2 Concentrations of several proinflammatory cytokines were elevated in the brain and CSF of patients with PD and also in prediagnostic blood samples of patients with PD.1,2 While neuroinflammation may have some beneficial effects, uncontrolled inflammatory reactions may contribute to a self-perpetuating cycle that eventually leads to the degeneration of dopaminergic neurons.27,28 Experimentally, several NSAIDs alleviate the loss of dopaminergic neurons but the effect of individual drugs is inconsistent across parkinsonian models, and the underlying mechanisms remain uncertain.27,29 NSAIDs may confer benefit by inhibiting cyclooxygenase (COX), scavenging reactive oxygen and nitrite radicals, or inhibiting the activation of TNFα.29,30 Some NSAIDs, such as ibuprofen and indomethacin, can also activate the peroxisome proliferator-activated receptor γ (PPARγ) pathway.27,29,30 Further, NSAIDs use was examined in relation to PD in several epidemiologic studies.5 However, the finding that ibuprofen use, but not use of aspirin or other NSAIDs, was associated with lower PD risk suggests mechanisms other than a generic anti-inflammatory activity of NSAIDs.
NSAIDs are a heterogeneous group of compounds with different structures and pharmacologic properties.27 Because the neuroprotective effects of individual compounds rank differently across parkinsonian models, no specific NSAIDs emerge from experimental studies as an unequivocal neuroprotectant for PD. However, in inflammatory and oxidative stress models of PD, ibuprofen can display protective properties not shared by aspirin or other NSAIDs.31,32 Ibuprofen acts as a ligand for PPARγ, a novel therapeutic target for PD.29,30 PPARγ can inhibit apoptosis and oxidative damage and counteract the activity of NF-κB, Ap-1, of the signal transducer and activator of transcription-1, and of the nuclear factor of activated T cells.33 In a recent animal study, ibuprofen, but not aspirin, significantly attenuated the reduction of PPARγ expression and dopamine transporter–positive signals, and controlled the accumulation of activated microglial cells induced by methamphetamine.31 Further, in the MPTP mouse model, ibuprofen dose-dependently alleviated the loss of striatal dopamine without toxicity to dopaminergic neurons, while some other NSAIDs (e.g., indomethacin) appeared to be toxic at high doses.34 Interestingly, the dose equivalency for ibuprofen:naproxen in CNS (10:1) is much higher than in peripheral inflammatory assays (10:7), although the mechanisms underlying this difference remain unclear.35 Further support for a potential neuroprotective effect of ibuprofen comes from epidemiologic studies of dementia, where long-term use of ibuprofen, among several individual commonly used NSAIDs, was most strongly associated with a reduced risk of AD.36 Experimental studies also showed that ibuprofen, but not aspirin, naproxen, or celecoxib, lowered β-amyloid 42 levels, a main culprit in the AD pathogenesis.37
Two alternative interpretations of our results should be also considered. The first is confounding by indication: the possibility that ibuprofen was used to treat conditions that themselves are associated with lower PD risk. However, the primary reason for ibuprofen use was muscle/joint pain (accounting for 84% use in our previous study38) and there is no evidence that these symptoms are associated with lower risk of PD. On the contrary, because pathologically confirmed cases of PD commonly present with or are preceded by painful musculoskeletal symptoms,39 one might have actually expected an increased risk of PD in those taking ibuprofen, suggesting that the observed reduced risk of PD among ibuprofen users may have been underestimated. Further, we showed similar results after excluding participants with gout, a condition which could be associated with both ibuprofen use and PD. The other alternative explanation is that individuals at high risk of PD have a more stoic personality,40 and are thus less likely to use analgesics. This personality hypothesis, however, was based primarily on anecdotal clinical observations and would not easily explain why only ibuprofen use, but not use of other analgesics, was associated with a lower risk of PD.
The lower risk of developing PD among users of ibuprofen found in this large prospective study is consistent with previous findings and suggests that ibuprofen has potential neuroprotective effects not shared by aspirin or other commonly used analgesics. This hypothesis could be tested in clinical trials of patients with early PD.
Supplementary Material
- BMI
- body mass index
- CI
- confidence interval
- HPFS
- Health Professionals Follow-up Study
- NHS
- Nurses' Health Study
- NSAID
- nonsteroidal anti-inflammatory drug
- OR
- odds ratio
- PD
- Parkinson disease
- PPAR γ
- peroxisome proliferator-activated receptor γ
- RR
- relative risk
Editorial, page 854
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Gao.
DISCLOSURE
Dr. Gao has received research support from the NIH/NINDS. Dr. Chen has received intramural funding from the NIH. Dr. Schwarzschild has received research support from the NIH/NINDS, the US Department of Defense, the Michael J. Fox Foundation, the Parkinson Disease Foundation, the RJG Parkinson's Disease Foundation, the American Parkinson Disease Association, and the American Federation for Aging Research. Dr. Ascherio serves on a scientific advisory board for the Michael J. Fox Foundation; serves on the editorial boards of Neurology®, Annals of Neurology, and the American Journal of Epidemiology; has received speaker honoraria from Merck Serono; and receives research support from the NIH, the US Department of Defense, and the Michael J. Fox Foundation.
REFERENCES
- 1. Smith PF. Inflammation in Parkinson's disease: an update. Curr Opin Investig Drugs 2008;9:478–484 [PubMed] [Google Scholar]
- 2. Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol 2009;8:382–397 [DOI] [PubMed] [Google Scholar]
- 3. Chen H, Zhang SM, Hernan MA, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol 2003;60:1059–1064 [DOI] [PubMed] [Google Scholar]
- 4. Chen H, Jacobs E, Schwarzschild MA, et al. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol 2005;58:963–967 [DOI] [PubMed] [Google Scholar]
- 5. Samii A, Etminan M, Wiens MO, Jafari S. NSAID use and the risk of Parkinson's disease: systematic review and meta-analysis of observational studies. Drugs Aging 2009;26:769–779 [DOI] [PubMed] [Google Scholar]
- 6. Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology 2010;74:995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–796 [DOI] [PubMed] [Google Scholar]
- 8. Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–867 [DOI] [PubMed] [Google Scholar]
- 9. Ascherio A, Zhang SM, Hernan MA, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol 2001;50:56–63 [DOI] [PubMed] [Google Scholar]
- 10. Gao X, Simon KC, Han J, Schwarzschild MA, Ascherio A. Genetic determinants of hair color and Parkinson's disease risk. Ann Neurol 2009;65:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology 1993;4:218–228 [DOI] [PubMed] [Google Scholar]
- 12. Weisskopf MG, O'Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol 2007;166:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alonso A, Rodriguez LA, Logroscino G, Hernan MA. Gout and risk of Parkinson disease: a prospective study. Neurology 2007;69:1696–1700 [DOI] [PubMed] [Google Scholar]
- 14. Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson's disease risk in men. Am J Epidemiol 2008;167:831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Powers KM, Kay DM, Factor SA, et al. Combined effects of smoking, coffee, and NSAIDs on Parkinson's disease risk. Mov Disord 2008;23:88–95 [DOI] [PubMed] [Google Scholar]
- 16. Etminan M, Carleton BC, Samii A. Non-steroidal anti-inflammatory drug use and the risk of Parkinson disease: a retrospective cohort study. J Clin Neurosci 2008;15:576–577 [DOI] [PubMed] [Google Scholar]
- 17. Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology 2007;69:1836–1842 [DOI] [PubMed] [Google Scholar]
- 18. Bornebroek M, de Lau LM, Haag MD, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Neuroepidemiology 2007;28:193–196 [DOI] [PubMed] [Google Scholar]
- 19. Hernan MA, Logroscino G, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and the incidence of Parkinson disease. Neurology 2006;66:1097–1099 [DOI] [PubMed] [Google Scholar]
- 20. Ton TG, Heckbert SR, Longstreth WT, Jr, et al. Nonsteroidal anti-inflammatory drugs and risk of Parkinson's disease. Mov Disord 2006;21:964–969 [DOI] [PubMed] [Google Scholar]
- 21. Etminan M, Suissa S. NSAID use and the risk of Parkinson's disease. Curr Drug Saf 2006;1:223–225 [DOI] [PubMed] [Google Scholar]
- 22. Hancock DB, Martin ER, Stajich JM, et al. Smoking, caffeine, and nonsteroidal anti-inflammatory drugs in families with Parkinson disease. Arch Neurol 2007;64:576–580 [DOI] [PubMed] [Google Scholar]
- 23. Bower JH, Maraganore DM, Peterson BJ, Ahlskog JE, Rocca WA. Immunologic diseases, anti-inflammatory drugs, and Parkinson disease: a case-control study. Neurology 2006;67:494–496 [DOI] [PubMed] [Google Scholar]
- 24. Paulose-Ram R, Hirsch R, Dillon C, Losonczy K, Cooper M, Ostchega Y. Prescription and non-prescription analgesic use among the US adult population: results from the third National Health and Nutrition Examination Survey (NHANES III). Pharmacoepidemiol Drug Saf 2003;12:315–326 [DOI] [PubMed] [Google Scholar]
- 25. Iso H, Hennekens CH, Stampfer MJ, et al. Prospective study of aspirin use and risk of stroke in women. Stroke 1999;30:1764–1771 [DOI] [PubMed] [Google Scholar]
- 26. Dedier J, Stampfer MJ, Hankinson SE, Willett WC, Speizer FE, Curhan GC. Nonnarcotic analgesic use and the risk of hypertension in US women. Hypertension 2002;40:604–608, discussion 601–603 [DOI] [PubMed] [Google Scholar]
- 27. Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson's disease. Exp Neurol 2007;205:295–312 [DOI] [PubMed] [Google Scholar]
- 28. Orr CF, Rowe DB, Halliday GM. An inflammatory review of Parkinson's disease. Prog Neurobiol 2002;68:325–340 [DOI] [PubMed] [Google Scholar]
- 29. Asanuma M, Miyazaki I. Nonsteroidal anti-inflammatory drugs in experimental parkinsonian models and Parkinson's disease. Curr Pharm Des 2008;14:1428–1434 [DOI] [PubMed] [Google Scholar]
- 30. Asanuma M, Miyazaki I. Common anti-inflammatory drugs are potentially therapeutic for Parkinson's disease? Exp Neurol 2007;206:172–178 [DOI] [PubMed] [Google Scholar]
- 31. Tsuji T, Asanuma M, Miyazaki I, Miyoshi K, Ogawa N. Reduction of nuclear peroxisome proliferator-activated receptor gamma expression in methamphetamine-induced neurotoxicity and neuroprotective effects of ibuprofen. Neurochem Res 2009;34:764–774 [DOI] [PubMed] [Google Scholar]
- 32. Casper D, Yaparpalvi U, Rempel N, Werner P. Ibuprofen protects dopaminergic neurons against glutamate toxicity in vitro. Neurosci Lett 2000;289:201–204 [DOI] [PubMed] [Google Scholar]
- 33. Chaturvedi RK, Beal MF. PPAR: a therapeutic target in Parkinson's disease. J Neurochem 2008;106:506–518 [DOI] [PubMed] [Google Scholar]
- 34. Kurkowska-Jastrzebska I, Czlonkowski A, Czlonkowska A. Ibuprofen and the mouse model of Parkinson's disease. Ann Neurol 2006;59:988–989 [DOI] [PubMed] [Google Scholar]
- 35. Milatovic D, Zaja-Milatovic S, Montine KS, Horner PJ, Montine TJ. Pharmacologic suppression of neuronal oxidative damage and dendritic degeneration following direct activation of glial innate immunity in mouse cerebrum. J Neurochem 2003;87:1518–1526 [DOI] [PubMed] [Google Scholar]
- 36. Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 2008;70:1672–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Helmuth L. NSAIDS for prevention? Protecting the brain while killing pain? Science 2002;297:1262–1263 [DOI] [PubMed] [Google Scholar]
- 38. Curhan GC, Knight EL, Rosner B, Hankinson SE, Stampfer MJ. Lifetime nonnarcotic analgesic use and decline in renal function in women. Arch Intern Med 2004;164:1519–1524 [DOI] [PubMed] [Google Scholar]
- 39. Williams DR, Lees AJ. How do patients with parkinsonism present? A clinicopathological study. Intern Med J 2009;39:7–12 [DOI] [PubMed] [Google Scholar]
- 40. Menza MA, Golbe LI, Cody RA, Forman NE. Dopamine-related personality traits in Parkinson's disease. Neurology 1993;43:505–508 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.