Abstract
Stroke is characterized by massive inflammation in areas surrounding the injury that magnifies damage to the brain. The Liver X Receptors (LXRs) are nuclear receptors that regulate cholesterol, lipid, and glucose metabolism. Synthetic LXR agonists have potent anti-inflammatory properties in a variety of settings, including neuroinflammation. However, the ability of LXR agonists to suppress stroke-associated inflammation has not been evaluated. Here, we have used time-lapse magnetic resonance imaging (MRI) to show that a single dose of an LXR ligand administered post-injury dramatically reduces brain damage in a model of acute brain ischemia. Neuroprotection was associated with suppression of neuroinflammation.
Keywords: acute cerebral ischemia, experimental therapy, neuroprotection, nuclear receptors, transcriptional activators
1. Introduction
Every year, close to a million people in the US alone suffer a stroke [1]. A fifth of them die from the episode and a large proportion of survivors are left with permanent mental and physical disabilities. During stroke, irreversible neuronal injury occurs only in a small area [2]. A much larger volume of the brain surrounding this ischemic core can be salvaged if cerebral blood flow is promptly restored. Tissue survival is determined by a variety of cell growth, survival, and death signals emanating from multiple cell types. Current therapies for stroke consist primarily of fibronilytic agents such as tPA, and surgery, though only a fraction of patients are eligible for these treatments. In addition, anticoagulants are typically used to prevent recurrence of stroke. A dire need exists to discover new treatments for stroke and to minimize its sequela. Recently, anti-inflammatory agents such as indomethacin have shown promise to restraint inflammation during a stroke, enhancing neurogenesis and tissue repair [3]. Hence, agents that can curtail neuroinflammation may have utility in the treatment of stroke.
The LXRs (LXRα and LXRβ) are ligand-activated transcription factors of the nuclear receptor superfamily that regulate cholesterol, lipid, and glucose metabolism [4]. Synthetic LXR ligands have beneficial effects in animal models of atherosclerosis, diabetes, and various diseases with an inflammatory component [5]. LXRα is primarily expressed in liver, fat, macrophages, and to some extent in the brain. In contrast, LXRβ is broadly expressed across tissues and is highly expressed in brain. Recent studies have shown that the LXRs regulate brain cholesterol homeostasis, and that synthetic LXR agonists can ameliorate the functional deficits associated with Alzheimer’s disease, perhaps via their ability to modulate cholesterol metabolism and suppress neuroinflammation [6–8]. These findings prompted us to examine the effect of LXR ligands in acute models of stroke.
2. Materials and Methods
2.1 Animal Experiments
Male Sprague-Dawley rats underwent permanent MCAO as previously described [9,10] and were treated 10 min or 2 h later with vehicle or a single dose of GW3965 (20 mpk i.p.) (n=8/group). MRI measurements were performed 2, 24 and 48 h after MCAO using a 4.7T vertical superwidebore magnet of a Bruker AvanceII spectrometer with micro imaging accessory. Animal preparation, image acquisition, trace of the diffusion tensor [Tr(D)] map computation, ischemic volume determination, and progression of the ischemic damage over time was done as described in reference 14. The experiment was performed twice (n=16 total/group) with identical results. Animal procedures were approved by the Department of Pharmacological Sciences, University of Milan.
2.2 Gene Expression Analysis
RNA from the indicated frozen brain sections was prepared using Nucleospin 96 RNA kits (Macherey-Nagel, Germany). Gene expression was analyzed by TaqMan qRT-PCR using the one-step Superscript III platinum reagent (Invitrogen, Carlsbad, CA). Samples were run in triplicate as multiplexed reactions with a normalizing internal control (36B4). Probe and primer sequences were designed in-house or obtained as pre-made Gene Expression Assays from ABI (Foster City, CA). Sequences for assays designed in-house are available on request. Statistical analysis was performed by using a two-tailed Student’s t test.
2.3 Neurological deficits
Neurological deficits were evaluated using the foot fault, Bederson, and De Ryck tests, as previously detailed [11]. Tests were repeated 24 and 48 h after MCAO in rats treated either with vehicle or with GW3965 at 2 or 6 h after ischemic insult. Statistical differences were evaluated with a Mann-Whitney test.
3. Results
To evaluate the effect of synthetic LXR ligands in stroke, we used the middle cerebral artery occlusion (MCAO) rat model, a well established model to evaluate neuroprotective effects after focal cerebral ischemia. This model has been widely used to assess the ability of compounds such as glucocorticoids, indomethacin, statins, and others to reduce infarct volume and enhance post-injury neurogenesis [3,9,12–14]. Time-lapse noninvasive MRI was used to monitor the evolution of brain lesions in rats where focal cerebral ischemia was induced by permanent middle cerebral artery occlusion. This technique enables evaluation of onset, progression, and outcome of brain damage within the same animal. Male Sprague-Dawley rats underwent monolateral permanent middle cerebral artery occlusion and were treated ten minutes later with either vehicle or a single dose of the LXR ligand GW3965 (20mpk i.p.). MRI of developing damage, detected as hypointense areas in the cerebral cortex, showed that in vehicle treated rats (n=8), brain infarct volume in the injured hemisphere increased significantly between 2 and 48 hours post-MCAO (Figure 1a). In contrast, treatment with the LXR agonist post-injury (n=8) completely prevented increase of damage at 24 and 48 h. The infarct volume area in LXR-ligand-treated animals at 2 and 48 h remained the same, indicating that LXR activation in this model of stroke has dramatic neuroprotective effects (Figure 1b). Identical results were obtained when the LXR ligand was administered 2 hours post-injury, which suggests that the therapeutic window for treatment with LXR activators may be clinically useful (Figure 1c). Treatment with a single dose of LXR agonist after ischemic injury improved sensorimotor function recovery (De Ryck test), and the ability of the animals to integrate motor response (foot fault test). A positive trend was observed also in the postural reflex test of Bederson (Figure 2).
Figure 1. LXR ligands block ischemia-induced brain damage.
a. Male rats underwent MCAO and were treated 10 min later with vehicle or a single dose of GW3965 (20 mpk i.p.) (n=8/group). Infarct volume was monitored by time-lapse MRI. Images are of 3 contiguous slices of the same animal visualized at 3 different time points; 3 representative rats per group are shown. Arrows indicate damaged area. b. Progression of ischemic damage over time was evaluated by ANOVA. For each animal, the ischemic area at 2 hours after ischemia induction was set to 100%, and the extension of ischemic area at all other time points was proportionally calculated, thus providing an internal control of ischemia development [12] c. Progression of ischemic damage in rats treated with LXR ligand 2 hours post-injury. Note that at this time point the LXR ligand continues to provide complete protection. Data are expressed as mean values ± s.e.m.;* P < 0.05, ** P < 0.01.
Figure 2. Treatment with LXR ligands reduces stroke-induced neurological deficits.
Neurological deficits were evaluated by the De Ryck, Bederson, and foot fault tests in rats receiving vehicle or GW3965 administered 2 or 6 h after ischemic insult. Tests were performed at 24 h (white columns) and 48 h (black columns) after MCAO. Statistical differences were evaluated with a Mann-Whitney test. Data are expressed the mean score of 5 rats; & P < 0.05 vs. vehicle at 48 h; * p<0.05 vs vehicle at 24 h; ** p<0.01 vs. vehicle at 24 h; # p<0.05 vs. vehicle at 48 h.
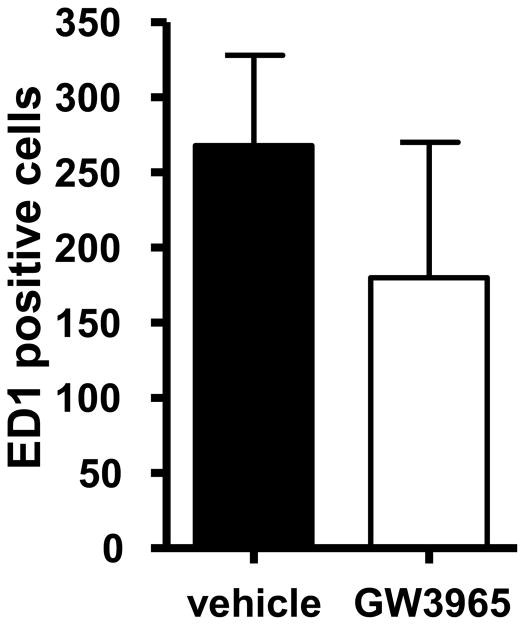
To explore the mechanism of neuroprotection of LXR ligands, we evaluated expression of pro-inflammatory genes in the brain. Analysis of gene expression in the ipsilateral and controlateral areas of the striatum and of the cortex harvested from treated rats at 24 h showed that pro-inflammatory genes such as iNOS, MCP-1, IL-1β, Rantes and COX-2 were strongly upregulated in the ipsilateral part, the damaged area, especially in the cortex (Figure 3) confirming that this is the area most affected by ischemia, as can be seen in Figure 1a. The single dose treatment with the LXR ligand virtually abolished increase in expression of all pro-inflammatory genes induced by ischemia. This dramatic effect of GW3965 was not observed in genes whose expression was not affected by the injury. The reduction in expression of pro-inflammatory markers was reflected in a clear but not statistically significant decrease in the number of ED1 positive cells (present in activated macrophages and microglia), suggesting that LXR activation may inhibit recruitment of immune cells (Figure 4). In addition to their anti-inflammatory properties, the LXRs are also known to regulate expression of vascular endothelial growth factor (VEGF) in macrophages [15]. Because VEGF has been shown to reduce infarct size and improve functional outcome after experimental cerebral brain ischemia [16,17], we examined the expression of VEGF in rats treated with the LXR agonist. Expression of both VEGF-A and VEGF-B was increased in response to GW3965 throughout the brain, suggesting that induction of VEGF expression may contribute to the neuroprotective effects of LXR activation (Figure 2). Expression of direct LXRs target genes, such as ABCA1 and SREBP-1c, was increased after GW3965 treatment in all areas harvested, confirming the efficacy of ligand delivery. Ischemic injury did not result in any significant changes in mRNA expression for either LXR isoform (data not shown).
Figure 3. Gene expression analysis post-MCAO.
Tissues from rats (n=5) treated as in Figure 1a were harvested at 24 h and gene expression analyzed by TaqMan-based qRT-PCR. White bars (vehicle), black bars (GW3965). Ipl, ipsilateral; Col, controlateral. Data are expressed as mean values ± std.* P < 0.05, ** P < 0.01. Statistical analysis was performed by using a two-tailed Student’s t test.
Figure 4. Gene expression analysis post-MCAO.
Rats treated 10 min after MCAO with vehicle or GW3965 were sacrificed 72 h after induction of damage. Paraffin-embedded brain sections were stained with an antibody against ED1 (Serotec, Oxford UK) that detects macrophage/monocyte infiltration, and examined by light microscopy. ED1 positive cells were counted in the ipsilateral hemisphere to damage in four different coronal brain slices from 6 different animals.
4. Discussion
Previous work has established that LXR ligands attenuate inflammatory responses in macrophages, suppressing expression of inflammatory genes induced by pathogens and pro-inflammatory cytokines, such as interleukin 1β (IL-1β), cycloxygenase-2 (COX2), inducible nitric oxide synthase (iNOS), interleukin 6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and matrix metalloprotease 9 (MMP-9) [5,18,19]. The mechanism whereby LXR ligands suppress pro-inflammatory gene expression is thought to involve SUMOylation of the LXRs, and interference of ligand-activated LXRs with NF-κB signaling, a critical pathway for the inflammatory response [20–22]. More recently, it has been shown that LXR agonists can restrain the response of the primary microglia and primary astrocytes to inflammatory stimuli such as lipopolysaccharide (LPS) and fibrils of β-amyloid protein [8,23]. Given the established role of many of these pro-inflammatory genes in the pathogenesis of cerebral ischemia, we decided to examine the neuroprotective potential of synthetic LXR agonists in experimental stroke.
In contrast to previous studies in a variety of settings that demonstrated anti-inflammatory properties of LXR ligands primarily in prophylactic mode, we have found that a single dose of an LXR agonist delivered 2 hours post-injury can effectively block ischemia-induced brain damage. Using time-lapse MRI to evaluate brain damage within the same animal, we have shown that LXR activation can dramatically ameliorate the extent of cytotoxic edema that results from ischemic insult. LXR activation may reduce infarct size by restraining neuroinflammation, and promoting VEGF expression. Because there are several synthetic LXR ligands in clinical development, our findings suggest that LXR activation may constitute a novel approach to minimize stroke-induced brain damage.
Acknowledgments
N.M. is a postdoctoral fellow of the American Heart Association. E.S. is the recipient of a Career Development Award from the American Diabetes Association. Work supported by NIH DK081003.
Abbreviations
- LXR
Liver X receptor
- ABCA1
ATP-binding cassette A1
- SREBP
sterolregulated-element binding protein
- MRI
magnetic resonance imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein LB. Acute ischemic stroke treatment in 2007. Circulation. 2007;116:1504–14. doi: 10.1161/CIRCULATIONAHA.106.670885. [DOI] [PubMed] [Google Scholar]
- 3.Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–24. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- 4.Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annual Review of Physiology. 2006;68:159–91. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- 5.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. Journal of Clinical Investigation. 2006;116:607–14. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefterov I, Bookout A, Wang Z, Staufenbiel M, Mangelsdorf D, Koldamova R. Expression profiling in APP23 mouse brain: inhibition of Abeta amyloidosis and inflammation in response to LXR agonist treatment. Mol Neurodegener. 2007;2:20. doi: 10.1186/1750-1326-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitney KD, et al. Regulation of cholesterol homeostasis by the liver X receptors in the central nervous system. Molecular Endocrinology. 2002;16:1378–85. doi: 10.1210/mend.16.6.0835. [DOI] [PubMed] [Google Scholar]
- 8.Zelcer N, Khanlou N, Clare R, Jiang Q, Reed-Geaghan EG, Landreth GE, Vinters HV, Tontonoz P. Attenuation of neuroinflammation and Alzheimer’s disease pathology by liver x receptors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10601–6. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sironi L, et al. Treatment with statins after induction of focal ischemia in rats reduces the extent of brain damage. Arteriosclerosis, Thrombosis & Vascular Biology. 2003;23:322–7. doi: 10.1161/01.atv.0000044458.23905.3b. [DOI] [PubMed] [Google Scholar]
- 10.Tamura A, Graham DI, McCullogh J, Teasdale MG. Focal cerebral ischemia in the rat: 1: Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- 11.Villa P, et al. The interleukin-8 (IL-8/CXCL8) receptor inhibitor reparixin improves neurological deficits and reduces long-term inflammation in permanent and transient cerebral ischemia in rats. Molecular Medicine. 2007;13:125–33. doi: 10.2119/2007-00008.Villa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciana P, et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO Journal. 2006;25:4615–27. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimino M, Gelosa P, Gianella A, Nobili E, Tremoli E, Sironi L. Statins: multiple mechanisms of action in the ischemic brain. Neuroscientist. 2007;13:208–13. doi: 10.1177/1073858406297121. [DOI] [PubMed] [Google Scholar]
- 14.Guerrini U, Sironi L, Tremoli E, Cimino M, Pollo B, Calvio AM, Paoletti R, Asdente M. New insights into brain damage in stroke-prone rats: a nuclear magnetic imaging study. Stroke. 2002;33:825–30. doi: 10.1161/hs0302.104111. [DOI] [PubMed] [Google Scholar]
- 15.Walczak R, Joseph SB, Laffitte BA, Castrillo A, Pei L, Tontonoz P. Transcription of the vascular endothelial growth factor gene in macrophages is regulated by liver X receptors. Journal of Biological Chemistry. 2004;279:9905–11. doi: 10.1074/jbc.M310587200. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. Journal of Clinical Investigation. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YQ, Guo X, Qiu MH, Feng XY, Sun FY. VEGF overexpression enhances striatal neurogenesis in brain of adult rat after a transient middle cerebral artery occlusion. Journal of Neuroscience Research. 2007;85:73–82. doi: 10.1002/jnr.21091. [DOI] [PubMed] [Google Scholar]
- 18.Castrillo A, Tontonoz P. Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annual Review of Cell & Developmental Biology. 2004;20:455–80. doi: 10.1146/annurev.cellbio.20.012103.134432. [DOI] [PubMed] [Google Scholar]
- 19.Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem. 2003;278:10443–9. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 20.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Molecular Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph SB, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–9. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 23.Kim OS, Lee CS, Joe EH, Jou I. Oxidized low density lipoprotein suppresses lipopolysaccharide-induced inflammatory responses in microglia: oxidative stress acts through control of inflammation. Biochemical & Biophysical Research Communications. 2006;342:9–18. doi: 10.1016/j.bbrc.2006.01.107. [DOI] [PubMed] [Google Scholar]