Abstract
The purpose of this study was to compare the discharge characteristics of motor units recruited during an isometric contraction that was sustained with the elbow flexor muscles by older adults at target forces that were less than the recruitment threshold force of each isolated motor unit. The discharge times of 27 single motor units were recorded from the biceps brachii in 11 old adults (78.8 ± 5.9 yr). The target force was set at either a relatively small (6.6 ± 3.7% maximum) or large (11.4 ± 4.5% maximum) difference below the recruitment threshold force and the contraction was sustained until the motor unit was recruited and discharged action potentials for about 60 s. The time to recruitment was longer for the large target-force difference (P = 0.001). At recruitment, the motor units discharged repetitively for both target-force differences, which contrasts with data from young adults when motor units discharged intermittently at recruitment for the large difference between recruitment threshold force and target force. The coefficient of variation (CV) for the first five interspike intervals (ISIs) increased from the small (18.7 ± 7.9) to large difference (35.0 ± 10.2%, P = 0.008) for the young adults, but did not differ for the two target force differences for the old adults (26.3 ± 14.7 to 24.0 ± 13.1%, P = 0.610). When analyzed across the discharge duration, the average CV for the ISI decreased similarly for the two target-force differences (P = 0.618) in old adults. These findings contrast with those of young adults and indicate that the integration of synaptic input during sustained contractions differs between young and old adults.
INTRODUCTION
When a muscle contraction is sustained at a force that is less than the upper limit of motor unit recruitment, not all motor units are activated at the onset of the contraction. As the contraction progresses, the force exerted by motor units active from the beginning of the contraction decreases due to a decline in motor unit discharge rate (Bigland-Ritchie et al. 1986; Carpentier et al. 2001; Enoka et al. 1989; Garland et al. 1994; Gatev et al. 1986; Mottram et al. 2005; Person and Kudina 1972; Rudroff et al. 2010) and a decrease in the force-generating capacity of the muscle fibers (Allen et al. 2008; Westerblad et al. 2002). To compensate for the reduction in motor unit force, the net muscle force is maintained by a gradual increase in the descending drive that recruits previously inactive motor units (Löscher et al. 1996). Once activated, the newly recruited motor units discharge action potentials either repetitively or intermittently (Bawa et al. 2006; Carpentier et al. 2001; Fallentin et al. 1993; Garland et al. 1994; Maton and Gamet 1989; Miller et al. 1996) and the previously active motor units may either continue to discharge action potentials or cease to discharge action potentials for the remainder of the contraction (Person 1974) or until the motor unit has recovered sufficiently to resume activity (Bawa et al. 2006).
Riley et al. (2008) found that the discharge characteristics of motor units at recruitment could differ substantially, depending on the difference between the recruitment threshold of the motor unit and the target force for the sustained contraction. When the target force was roughly 5% of maximum less than the recruitment threshold of an isolated motor unit in biceps brachii, the discharge was less variable than when the target force was about 10% of maximum below its recruitment threshold. Because recordings obtained from the same motor unit in a second set of data (n = 12) were consistent with the larger set of data made from separate motor units (n = 53), the difference in discharge characteristics for the two tasks was attributed to differences in the integration of synaptic input received by the motor neuron (Riley et al. 2008). Given age-associated changes in both the synaptic input that is delivered to motor neurons (Boxer et al. 1988; Eisen et al. 1996; Oliviero et al. 2006) and in the intrinsic properties of the motor neurons (Engelhardt et al. 1989; Morales et al. 1987; Piotrkiewicz et al. 2007; Rossini et al. 1992), it seems likely that the discharge characteristics of motor units at recruitment will differ for older adults when they perform such tasks. The purpose of the study was to compare the discharge characteristics of motor units recruited during an isometric contraction that was sustained with the elbow flexor muscles by older adults at target forces that were less than the recruitment threshold force of each isolated motor unit. Some of these data were previously presented in abstract form (Holmes et al. 2010; Pascoe et al. 2008).
METHODS
Eleven healthy adults (78.8 ± 5.9 yr; range, 71–88 yr; 8 men) who were free from cardiovascular and neurological disorders volunteered for the study and participated in one to six experimental sessions. Informed consent was obtained from all the participants and the experimental procedures were approved by the Institutional Review Board at the University of Colorado (Protocol 0109.11) and were in accordance with the Declaration of Helsinki.
Experimental setup
Subjects were seated upright in an adjustable chair, with the left upper arm vertical and slightly abducted from the trunk. The elbow was flexed to 1.57 rad and secured in a padded brace. The forearm was oriented in a neutral position midway between supination and pronation. The hand and forearm were secured with a modified wrist–hand orthosis (Orthomerica, Newport Beach, CA). The force exerted by the elbow flexor muscles was measured in the upward direction at the wrist with a force–moment sensor (900-N range, 182 N · V−1; JR3, Woodland, CA). The orthosis was attached to the transducer at the level of the wrist (Fig. 1A). Visual feedback of elbow flexion force was provided on a 17-in. computer monitor located at eye level about 1.2 m in front of the subject. Force was digitized with a Power 1401 (Cambridge Electronic Design [CED], Cambridge, UK) at 200 samples/s and stored on a computer.
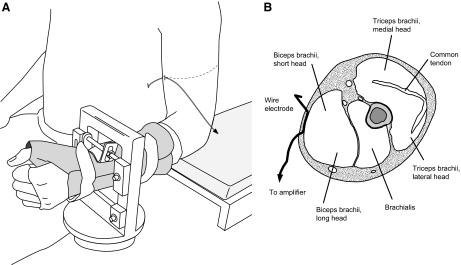
Fig. 1.
A: position of the left arm in the experimental setup. Subjects were seated with the arm slightly abducted from the body and with the elbow resting on a padded platform. Vertical force was measured as the elbow flexor muscles contracted and the left wrist pulled up against a force transducer. A subcutaneous wire electrode was placed over the short head of the biceps brachii muscle. The broken line denotes the level of a transverse section (inset B), through the upper arm to show the location of the electrode as it passes from the medial to the lateral aspect of the arm, overlaying the muscle fascia of the biceps brachii, and continues to the amplifier.
Electromyographic recordings
Single motor unit recordings were obtained from the short head of biceps brachii using a branched bipolar electrode (stainless steel, diameter: 50-μm, Formvar insulated; California Fine Wire, Grover Beach, CA; Enoka et al. 1988; Gydikov et al. 1986; Mottram et al. 2005). The electrode comprised two insulated fine wires that were glued together with three approximately 1-mm regions of the insulation removed; two regions approximately 1 mm on one wire, separated by 3 mm; and a single exposed approximately 1-mm region on the other wire positioned in between the two regions of the other wire. The lateral margin of the short head of biceps brachii and the intermarginal septum of the biceps brachii were identified and a 25-gauge, 3.81-cm disposable hypodermic needle was used to insert the wires subcutaneously across the muscle belly of the short head of biceps brachii without penetrating the muscle fascia and approximately perpendicular to the orientation of the muscle fibers (Fig. 1B). The needle was removed prior to recording motor unit activity. The location of the recording sites was adjusted by pulling on the exposed ends of the electrode and its position was optimized to provide the greatest signal-to-noise ratio during 10-s ramps to a target force of 50% maximal voluntary contraction (MVC) force. A reference electrode was placed on the lateral epicondyle of the humerus. Single motor unit recordings were amplified ×5,000 and band-pass filtered between 300 Hz and 8.5 kHz (S-series; Coulbourn Instruments, Allentown, PA). The motor unit signal was sampled at 20K samples/s with a Power 1401 (CED) and stored on a computer (Spike2, v.5.20, CED).
Surface electromyographic (EMG) recordings were made using a bipolar configuration of circular Ag/AgCl electrodes (8-mm diameter) placed on one side of the innervation zone for the short and long heads of biceps brachii and the lateral head of the triceps brachii (interelectrode distance of ∼20 mm). Reference electrodes were placed over the acromion process of the ipsilateral scapula. Smaller electrodes (4-mm diameter) were placed over the brachioradialis muscle. The surface EMG signals were amplified ×1,000, band-pass filtered between 13 Hz and 1 kHz (S-series; Coulbourn Instruments), sampled at 2K samples/s, and stored on a computer (Spike2, v.5.20, CED).
Protocol
The experimental protocol replicated the previous study with young adults (Riley et al. 2008). The following six tasks were performed on the elbow flexor muscles of the left arm of the volunteers in each experimental session: 1) assessment of the MVC force; 2) identification of a single motor unit; 3) measurement of the recruitment and derecruitment thresholds of the motor unit; 4) performance of the sustained isometric contractions at 5–10% below the recruitment threshold of the motor unit; 5) evaluation of the recruitment and derecruitment thresholds of the motor unit immediately after the final sustained contraction; and 6) completion of another MVC with the elbow flexor muscles. Each experiment lasted about 2 h.
The experimental session began with a minimum of two MVCs in the flexion direction and at least one in the extension direction. The MVC task involved increasing the force from zero to maximum over 3 s and then holding the maximum for a further 1–2 s. To minimize fatigue, subjects rested for 90–120 s between trials. If the peak MVC force for the two elbow flexor trials were within 5% of each other, the larger of the two values was recorded as the maximum and used as a reference value for the recruitment threshold force of the motor unit. Otherwise, additional trials were performed until the 5% criterion was met. Efforts that the subject did not regard as maximal were rejected and the visual gain of the force feedback was varied across trials to minimize the subjects' awareness of differences in performance.
Motor units were identified in the subcutaneous EMG recording by asking the subjects to slowly increase elbow flexor force from rest to 50% MVC force in about 10 s and then gradually relax during the subsequent 10 s to produce a triangular force profile. Subjects were instructed to change force at a constant rate. Once a potential unit was identified, subjects performed three ramp contractions that were separated by 60 s of rest. The recruitment threshold was estimated during the experiment by noting the force after the third interspike interval (ISI) and averaging the values from the ramp contractions.
The target force for the sustained contraction was set at either 5 or 10% MVC force below the recruitment threshold force of the motor unit, which was denoted as small and large differences between the two forces, respectively. The absolute force difference was scaled to the recruitment threshold force of the motor unit. A line was set at the target force on the monitor and the subject was asked to increase the force up to the line gradually (∼15 s) and to maintain the target force as steadily as possible until instructed by the investigator to relax. The gain of the display was adjusted to keep both the target and rest force lines consistent across subjects. Subjects observed the two force traces during the task. The time to recruitment was defined as the time from target acquisition to the first action potential discharged by the isolated motor unit. The task was terminated when the motor unit became active and discharged action potentials repetitively for about 60 s. After a brief rest period (∼180 s), the contraction at the other target force was performed. The order in which the two target forces (small and large differences) were performed was counterbalanced across subjects. The presence of the same motor unit after each sustained contraction was later verified off-line by comparing waveform overdraws created with Spike2 software.
In four separate sessions, subjects maintained target forces that were ≫10% MVC below recruitment threshold force (range: 15.2–27.2% MVC). Furthermore, when a subject was willing and the experimental conditions remained viable, the position of the subcutaneous electrode was adjusted to obtain recordings from a second motor unit, which was possible in four experimental sessions.
Data analysis
Template matching with Spike2 software was performed off-line to discriminate individual motor unit action potentials. The accuracy of the discrimination was verified by close visual inspection of each discriminated spike and by reviewing the ISIs: ISIs >250 ms (<4 pulses/s [pps]; n = 42, 0.10% of discharges) or <10 ms (>100 pps; n = 6, 0.01% of discharges) were excluded from the calculations of discharge rate. Long ISIs likely arose from the brief cessation of motor-unit discharge, whereas very short intervals exceed the rates normally observed during these types of contractions for human motor units (Bigland-Ritchie and Lippold 1954; De Luca et al. 1982; Kanosue et al. 1979; Tanji and Kato 1973) and likely resulted from discrimination error or a double discharge.
Recruitment threshold force was determined with an algorithm that involved advancing a 500-ms window in 1-ms steps across the discharge times of the motor unit until the coefficient of variation (CV) for the ISI in the window was <50% (Moritz et al. 2005). The force corresponding to the time of the first discharge in the window was taken as the recruitment threshold force. The same method was used to define the derecruitment threshold force of the motor unit. The discharge rate and CV for the ISI were determined for a 500-ms window at recruitment and derecruitment. A linear trend was fit through the ascending and descending force trajectories to calculate the rate of force development and relaxation, respectively.
Because the duration of the contraction differed across individuals, each contraction after recruitment was divided into five epochs of equal duration. The discharge rates during the sustained contractions were averaged for each 20% interval and the CV for the ISI was calculated from the first five ISIs in each 20% interval. Force fluctuations were calculated as the normalized measure of variability, the CV [(SD/mean force) × 100]. Surface EMG values are reported as the root-mean-square (RMS) amplitude of the signal normalized to the RMS for a 500-ms epoch centered about the peak force during the MVC. Coactivation ratios were quantified as the quotient of the averaged, rectified, and normalized EMG values of the elbow extensors (lateral head of triceps brachii) relative to that for the pooled average of the elbow flexors (brachioradialis, short and long head of biceps brachii).
Statistical analysis
Paired-samples t-tests were used to compare MVC force before and after the end of the protocol; recruitment threshold force and derecruitment threshold force to one another and also to assess the change after the end of the protocol; discharge rate and the CV for the ISI at recruitment and derecruitment threshold; the duration of the two sustained contractions; and the rate of force development and relaxation during the ramp contractions. Independent-samples t-tests were used to compare recruitment threshold forces between old adults of this study and the young adults of Riley et al. (2008). The mean discharge rate and CV for the ISI in each 20% epoch of discharge duration were compared with repeated-measures two-way ANOVAs (target-force × time).
The target force, difference between target force and recruitment threshold force, and the time to recruitment of the motor unit were compared with a subset of 12 motor units from young adults (Riley et al. 2008) with repeated-measures two-way ANOVAs (age × target-force). In addition, the mean discharge rate and CV for the first five ISIs obtained at recruitment during the sustained contractions were compared using two-way ANOVAs (age × target-force) with repeated measures on the target-force factor. Post hoc analysis with paired-samples t-tests were used when appropriate to compare the mean discharge rate between each of the 20% epochs of discharge duration. The CV for force, average RMS amplitude of the surface EMG, and coactivation ratios were compared for the first and last thirds of discharge duration between young and old subjects using multivariate three-way ANOVAs (age × target-force × time). An alpha level of P < 0.05 was used to identify significant differences. All statistical analyses were performed using SPSS (v.16.0, Chicago, IL). Data are presented in the text as means ± SDs and in figures as means ± SEs.
RESULTS
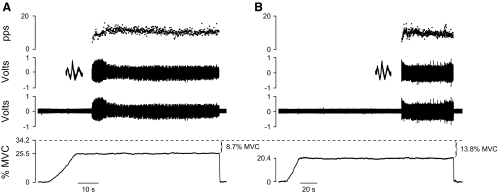
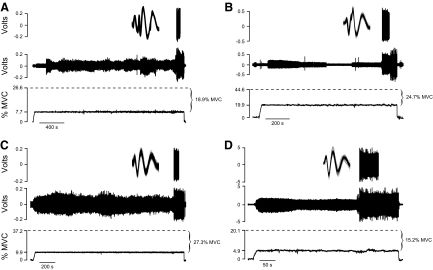
The results comprise recordings for 27 motor units from the short head of biceps brachii when 11 older adults sustained an isometric contraction with the elbow flexor muscles at a target force that was less than the recruitment threshold of the isolated motor unit. The discharge of each motor unit was recorded during two contractions that differed in the magnitude of the difference between the target force and the recruitment threshold force. An example of the sustained contractions at two different target forces below recruitment threshold force (34.2% MVC) from the same motor unit is shown in Fig. 2. The time from the start of the contraction to the recruitment of this motor unit was 7.3 s when the target was set at 8.7% MVC force below recruitment threshold force (Fig. 2A) and 118 s when the target force was set at 13.8% MVC force below recruitment threshold (Fig. 2B). MVC force for the old subjects did not change from the beginning (158 ± 64 N) to the end of the protocol (154 ± 53 N, P = 0.489), which indicates that the two tasks involved minimal fatigue. In the four experiments in which more than one motor unit recording could be obtained, the decline in MVC force from the beginning to the end of the first experiment was 3.1%, which indicated that recording of the second motor unit began with a minimal amount of fatigue.
Fig. 2.
A: representative data from a sustained contraction depicting (from top to bottom) the instantaneous discharge rate of the motor unit (pulses/s [pps]), discriminated action potentials with waveform overlay, interference electromyogram from the wire electrode, and the vertical force exerted against the force transducer by the wrist. The motor unit had a recruitment threshold force of 34.2% maximal voluntary contraction (MVC). The time to recruitment was 7.3 s for a target set at 8.7% MVC force below recruitment threshold force. B: time to recruitment for the same motor unit increased to 118 s when the target was set at 13.8% MVC force below recruitment threshold force.
Recruitment threshold
The average recruitment threshold force for the motor units from the old adults was 25.4 ± 10.2% MVC (range: 9.5–48.3% MVC) and this was unchanged (23.8 ± 10.1% MVC) at the end of the protocol (P = 0.08). Identifiable derecruitment threshold forces were obtained for 15 of the 27 motor units examined. The derecruitment threshold forces (16.6 ± 9.8% MVC) were less than the recruitment threshold forces for the set of ramp contractions that preceded (27.7 ± 11.4% MVC, P < 0.0001) the sustained contractions. The rate of force development (5.00 ± 2.72% MVC/s) was not significantly different from the rate of force relaxation (6.92 ± 9.53% MVC/s) during the ramp contractions (P = 0.290). The discharge rate at recruitment (10.0 ± 2.5 pps) was significantly greater than that at derecruitment (8.01 ± 2.7 pps, P = 0.001). The CV for the ISIs at recruitment (19.3 ± 6.8%) was significantly less than that at derecruitment (25.6 ± 7.2%, P = 0.026). The recruitment thresholds were lower for old adults (25.4 ± 10.2% MVC) when compared with both the group of 53 units (31.1 ± 12.3% MVC, P = 0.043) and the separate subset of 12 units (32.8 ± 9.6% MVC, P = 0.04) from the young adults in Riley et al. (2008).
Sustained contractions
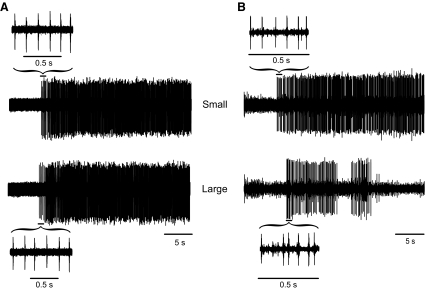
The duration over which discharges were recorded did not differ for the two differences in target force (64.0 ± 10.8 s, P = 0.340). Also, the motor units discharged repetitively at recruitment for both target-force differences (Fig. 3A), which contrasts with a previous observation for young adults when motor units discharged intermittently at recruitment with a large difference between recruitment threshold force and target force (Fig. 3B).
Fig. 3.
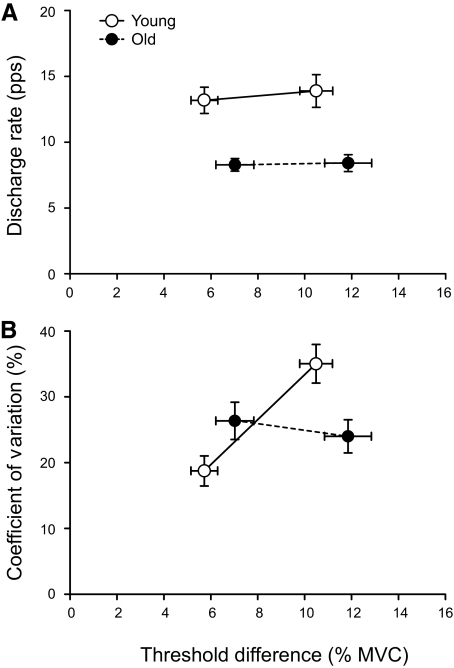
A: representative trains of action potentials at the time of recruitment for a motor unit in biceps brachii of an old adult for both the small and large differences between target force and recruitment threshold force. B: trains of action potentials for a motor unit in the biceps brachii of a young adult. The variability of the first 5 interspike intervals (ISIs) was increased in the young adult for the large difference between the target force and recruitment threshold force, but not in the old adult.
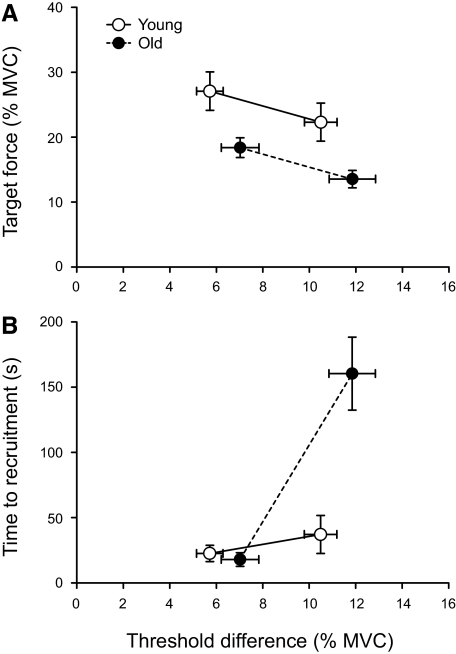
The target forces were greater for young adults (age main effect: P = 0.004) for both small and large differences in target force (27.1 ± 10.3 and 22.3 ± 10.1% MVC force) compared with old adults (18.4 ± 7.9 and 13.5 ± 7.0% MVC force; Fig. 4A). The difference between target force and recruitment threshold force was less for the small difference in both age groups (age × target-force interaction: P = 0.923) and increased from 6.62 ± 3.7 to 11.4 ± 4.5% MVC force for the large difference (target-force main effect: P < 0.001). The small and large target-force differences for the older adults were 7.01 ± 4.2 and 11.9 ± 5.2% MVC, respectively. The time to recruitment was longer for the large difference (target-force main effect: P = 0.001, Fig. 4B), especially for the old adults (age main effect: P = 0.015). There was a significant interaction (age × target-force interaction: P = 0.004) as the time to recruitment increased from 17.9 ± 27.0 to 160 ± 145 s for the old adults compared with 22.5 ± 21.7 to 37.1 ± 50.4 s for the young adults.
Fig. 4.
Task characteristics for the 27 motor units recorded from old adults (filled circles) and the 12 motor units from young adults (open circles). Young data are from Riley et al. (2008). A: target force (% MVC) was greater for young adults than that for old adults (P = 0.004) for both the small and large difference between target force and recruitment threshold force. The target force was greater for the small difference for both ages (P = 0.923) and decreased for the large difference (P < 0.001). B: the time to recruitment (s) increased for the large difference between target force and recruitment threshold force (P = 0.001), but more so for the old adults (P = 0.004).
The mean discharge rate at recruitment was greater in younger adults (age main effect: P < 0.001; Fig. 5A) and did not differ for the two target-force differences for either the young (small difference 13.2 ± 3.5 pps; large difference 13.9 ± 4.3 pps) or old adults (small difference 8.3 ± 2.5 pps; large difference 8.4 ± 3.3 pps). The CV for the first five ISIs did not differ between young and old adults (age main effect: P = 0.610; Fig. 5B); however, a main effect for target-force difference (P = 0.008) and a significant interaction (P < 0.001) indicated that the CV increased for the young adults (small difference 18.7 ± 7.9%; large difference 35.0 ± 10.2%) but did not change for the old adults (small difference 26.3 ± 14.7%; large difference 24.0 ± 13.1%).
Fig. 5.
Discharge characteristics at recruitment for the 27 motor units recorded from old adults (filled circles) and the 12 motor units from young adults (open circles). Young data are from Riley et al. (2008). A: mean discharge rate (pps) for the first 5 ISIs was greater for the young adults (age main effect: P < 0.001) and did not differ between tasks for both the young and old adults (target force main effect: P = 0.458). B: the coefficient of variation (CV, %) for the first 5 ISIs did not differ between young and old adults (age main effect: P = 0.610); however, the CV increased with the difference between target force and recruitment threshold force for the young, but not for the old adults (age × target force interaction: P = 0.001).
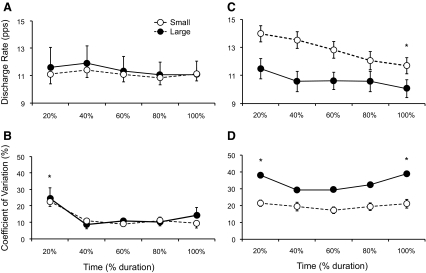
Mean discharge rate for the old adults was similar for all 20% epochs (target-force main effect: P = 0.179); discharge duration was 64.0 ± 10.8 s for the old adults and 63.2 ± 27.9 s for the young adults. When the data were collapsed across target-force differences, a paired comparison indicated there was no difference between the first 20% (11.8 ± 3.1 pps) and the last 20% (11.0 ± 2.0 pps) epoch of discharge duration (P = 0.091, Fig. 6A). The average CV for the ISI also decreased similarly for the two target-force differences (time × target force interaction: P = 0.618, Fig. 6B), decreasing from 25.2 ± 13.8% at the start of the contractions to 10.8 ± 5.6% at the end of the contractions (time main effect: P < 0.0001). These findings contrast with those for young adults (Riley et al. 2008) when the mean discharge rate (Fig. 6C) and CV for the ISI (Fig. 6D) changed in opposite directions for the two target-force differences.
Fig. 6.
Discharge characteristics for the motor units during the sustained contractions for young (right column) and old (left column) adults. Young data are from Riley et al. (2008). A: mean discharge rate (pps) for the 27 units of old adults for the small (filled circles) and large (open circles) differences between target force and recruitment threshold force (% MVC) for each 20% of discharge duration. Discharge rate did not change from the first to the last 20% of discharge duration (P = 0.091). B: CV for first 5 ISIs was greater during the first 20% of discharge duration for old adults (* time main effect: P < 0.0001) and decreased to the same extent for both differences in target force (P = 0.618). C: mean discharge rate for young adults decreased (* time main effect: P = 0.04) for the small (open circles) difference in target force, but not the large difference (filled circles). D: the average CV for the first 5 ISIs for the young adults was less for the small difference in target force (target-force difference main effect: P < 0.001) and it was elevated for the first and last 20% of discharge duration (* time main effect) compared with the middle time points for the large difference in target force.
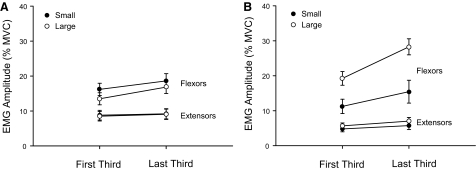
The CV value for force did not differ between young and old adults (age main effect: P = 0.328), but the value was greater for the large difference (target force main effect: P = 0.002) and increased over time (time main effect: P < 0.0001), from 1.63 ± 0.96% in the first third to 2.01 ± 0.94% in the final third of discharge duration. Surface EMG amplitude for all elbow flexors pooled together did not differ between young and old adults (age main effect: P = 0.161; Fig. 7), but the amplitude was greater for the large target-force difference during the first third of the contraction for young adults (target-force main effect: P = 0.013) and increased significantly over time (time main effect: P = 0.004), from 15.5 ± 9.71 to 20.8 ± 13.0% maximum. Surface EMG amplitude for the antagonist (triceps brachii) was minimal and did not change over time (age × time interaction: P = 0.680), but was significantly greater for old adults (8.89 ± 6.11%; age main effect: P < 0.0001) compared with young adults (5.89 ± 5.01%; Fig. 7). The coactivation ratios did not differ between young (0.60 ± 1.7) and old adults (0.60 ± 0.38; age main effect: P = 0.795), between target-force differences (target-force main effect: P = 0.124), or from the beginning to the end of the task (time main effect: P = 0.749).
Fig. 7.
Changes in amplitude of the surface electromyogram (EMG) recorded from the elbow flexors and extensor (triceps brachii) muscles from the first to the last third of contraction duration, normalized to maximum EMG (% MVC) for young (A) and old adults (B). Young data are from Riley et al. (2008). Surface EMG amplitude for all elbow flexors did not differ between young and old adults (age main effect: P = 0.161). Agonist EMG amplitude for the old adults (A) did not change from the first one third (14.8 ± 7.6% maximum) to the last one third (17.7 ± 8.5% maximum) of the contraction (total duration: 154.8 ± 122.6 s). Agonist EMG amplitude for the young adults (B) was greater for the large target-force difference during the first one third of the contraction (target-force main effect: P = 0.013) and increased significantly over time (time main effect: P = 0.004), from 15.9 ± 11.0 to 23.0 ± 15.1% maximum. Surface EMG amplitude for triceps brachii was significantly greater for old adults (8.89 ± 6.11%; age main effect: P < 0.0001) compared with young adults (5.89 ± 5.01%) and did not change over time (time main effect: P = 0.330) for either age group (age × time interaction: P = 0.680).
When the experiment involved a target force that was ≫10% below recruitment threshold force (range: 15.2–27.3% MVC), the four motor units from four different subjects all discharged repetitively for 60 s following recruitment (Fig. 8, A–D). The time to recruitment for the four motor units was 2,287, 1,005, 1,677, and 323 s. The mean discharge rate and CV for the ISI for the first five intervals at recruitment for the four motor units were 14.2, 13.0, 14.7, and 15.9 pps and 10.9, 48.3, 37.4, and 42.4%, respectively.
Fig. 8.
Data from the 4 sustained contractions held at target forces much lower than recruitment threshold force. Each panel depicts the discriminated action potentials, interference electromyogram (EMG), and the vertical force produced by the elbow flexors during these contractions. Despite the substantial difference between the target force and the recruitment threshold force for A–D (18.9, 24.7, 27.3, and 15.2% MVC, respectively), the unit discharged repetitively once recruited.
DISCUSSION
The purpose of the study was to compare the discharge characteristics of motor units recruited during an isometric contraction sustained with the elbow flexor muscles by older adults at target forces set lower than the recruitment threshold force of each isolated motor unit. The main finding of this study was that discharge characteristics of motor units at recruitment in old adults were not influenced by the relative target force; this finding contrasts with the results observed in young adults (Riley et al. 2008). The results suggest that the integration of synaptic input by motor neurons during sustained contractions differs for young and old adults.
Discharge characteristics of motor units in old adults
Motor unit recruitment thresholds extended across a wide range of forces (9.5–48.3% MVC). Although not statistically significant (P = 0.08), the recruitment threshold forces tended to decrease after the sustained contractions. Because recruitment threshold forces typically decrease following a fatiguing contraction (Adam and De Luca 2003; Baudry et al. 2009; Carpentier et al. 2001; Enoka et al. 1989; Garland et al. 1994; Suzuki et al. 1990), the protocol used in the current study involved minimal amounts of muscle fatigue. In addition, there was no difference (P = 0.489) in MVC forces before (158 ± 64 N) and after (154 ± 53 N) the 60-s sustained contractions, consistent with the two tasks involving minimal muscle fatigue.
The derecruitment threshold forces were lower than the recruitment threshold forces for the ramp contractions. Although a lower derecruitment threshold force has been observed in some studies (Baudry et al. 2009; Denier van der Gon et al. 1985; Garland et al. 1994; Romaiguère et al. 1993; Suzuki et al. 1990), many have also reported a lower recruitment threshold force (De Luca et al. 1982; Freund et al. 1975; Jesunathadas et al. 2010; Milner-Brown et al. 1973; Patten and Kamen 2000). Because both ramp contractions involved a gradual linear change in force, differences in threshold forces must be accompanied by differences in discharge rate to achieve comparable changes in force. Accordingly, the discharge rate at derecruitment was lower in the current study as also reported in other studies (Christova and Kossev 1998; De Luca et al. 1982; Denier van der Gon et al. 1985; Gorassini et al. 2002; Oya et al. 2009; Romaiguère et al. 1993). Because lower discharge rates are usually associated with higher discharge variability (Barry et al. 2007; Moritz et al. 2005), the CV value for the ISI in the current study was greater at derecruitment compared with recruitment.
A decrease in mean discharge rate during a sustained contraction is well documented in young adults (Christova and Kossev 1998; Gantchev et al. 1986; Garland et al. 1994; Gatev et al. 1986; Person and Kudina 1972). Relatively few studies have recorded motor unit discharge rate during sustained contractions performed by older adults and have either indicated no reduction in discharge rate for both young and old adults during a sustained submaximal contraction (Christie and Kamen 2009) or a similar decline in discharge rate for young and old adults during intermittent contractions (Rubinstein and Kamen 2005). In agreement with previous findings, the mean discharge rate in the current study did not change from the first 20% to the last 20% epoch of the nearly 60-s contraction (P = 0.091).
However, there was a marked decrease in discharge rate variability in the current study from 25.2% during the first 20% of the contraction to 10.8% for the remainder of the contraction. This result is consistent with previous findings that at relatively low levels of synaptic input (e.g., motor unit recruitment) the motor neuron experiences fluctuations in membrane potential, resulting in variable discharge times (Berg et al. 2007, 2008; Calvin and Stevens 1968; Matthews 1996, 1999; Stein et al. 2005). Previous studies have reported a rapid reduction in discharge rate variability with an increase in contraction intensity (Barry et al. 2007; Baudry and Enoka 2009; Moritz et al. 2005), presumably due to a decline in synaptic noise at higher levels of synaptic input (Matthews 1996, 1999), which would occur progressively during sustained submaximal contractions (Löscher et al. 1996).
Age-related differences in motor unit discharge
The most striking difference between the current results and those obtained from young adults (Riley et al. 2008) was the influence of the two target forces on the discharge characteristics of motor units in biceps brachii at recruitment. Old adults exhibited the same discharge characteristics for the two conditions, whereas the motor units recorded in young adults discharged repetitively for the small difference and intermittently for the large difference. Indirect evidence for an age-associated difference in discharge characteristics at recruitment during sustained contractions was suggested previously by the marked depression in bursts of activity in the surface EMG recordings from the elbow flexor muscles in old adults compared with young adults during fatiguing contractions that were sustained 20% MVC force (Hunter et al. 2005).
The average recruitment threshold force for motor units recorded from old adults was lower than that from young adults, which was similar to reports from previous studies (Erim et al. 1999; Fling et al. 2009; Klass et al. 2005, 2008). The age-associated difference in the current study, however, was attributed to random sampling because only a small portion of the entire motor unit pool could be recorded using selective fine-wire electromyography. The current study found that the biceps brachii motor units of old adults had lower mean discharge rates for the first five ISIs (∼8.4 pps) when compared with young adults (∼13.6 pps) at recruitment (Fig. 5A, P = 0.001). Similarly, others have described lower mean discharge rates for old adults (Barry et al. 2007; Connelly et al. 1999; Dalton et al. 2010; Kamen and Knight 2004; Kamen et al. 1995; Knight and Kamen 2007; Nelson et al. 1984; Newton et al. 1988; Patten et al. 2001), which can be attributed to such age-related changes as prolongation of the afterhyperpolarization (AHP) phase (Piotrkiewicz et al. 2007; Rossini et al. 1992) and motor unit twitch contraction times (Bellemare et al. 1983; Connelly et al. 1999; Newton et al. 1988; Roos et al. 1999). Unlike young adults, the CV for the first five ISIs did not vary across the two target forces for old adults (Fig. 5B) and this may indicate greater levels of synaptic noise at the time of recruitment for the young adults (Matthews 1999).
The discharge characteristics at recruitment depend on how the intrinsic properties of the motor neurons integrate synaptic input (Kernell 1965; Schwindt and Calvin 1972). Studies have shown age-related reductions in the amount of synaptic input received by motor neurons from corticomotor (Eisen et al. 1996; Oliviero et al. 2006), brain stem (Johnson et al. 1993), and spinal reflex (Boxer et al. 1988; Earles et al. 2001; Kido et al. 2004) pathways. Reductions in the number of synaptic inputs from these various pathways may represent differences in the strength and the relative balance of excitatory and inhibitory inputs received by the motor unit pools of old adults. Evidence suggests, for example, that changes in the balance between excitatory and inhibitory inputs can influence the discharge characteristics of neurons (Abbott and Chance 2005; Berg et al. 2007; Stein 2010), which may explain the age-associated difference in the discharge characteristics of motor units observed in the current study.
However, the intrinsic properties of motor neurons also differ between young and old experimental animals. For example, motor neurons from older animals exhibit greater input resistance (Chase et al. 1985; Kalmar et al. 2009; Morales et al. 1987), reduced rheobase current (Kalmar et al. 2009; Morales et al. 1987), and either longer (Cameron et al. 1991; Kalmar et al. 2009) or unchanged (Engelhardt et al. 1989; Morales et al. 1987) AHP duration. Such parameters can be estimated only with indirect approaches in humans, such as the interval death rate analysis of single motor unit ISIs (Matthews 1996) or evaluation of the relation between the mean and SD of ISIs within a train of single motor unit action potentials (Piotrkiewicz et al. 2007). Based on the interval death rate analysis technique, which has been validated in animal experiments (Powers and Binder 2000) and used in several human studies (Macdonell et al. 2007, 2008, 2010), the AHP duration appears to be prolonged in old adults (Christie and Kamen 2010). If such age-associated adaptations can be confirmed, then the intrinsic properties of motor neurons could also contribute to the difference in discharge characteristics of motor units in young and old humans.
The observations by Riley et al. (2008) that motor units in young adults discharged action potentials intermittently when there was a large difference between the target force and recruitment threshold force (Fig. 3B) and that mean discharge rate declined over time (Fig. 6C) were interpreted to indicate the absence of a significant role for persistent inward currents during this task. In contrast, motor units in old adults discharged repetitively at recruitment for both target forces and there was no reduction in mean discharge rate during the 60-s contractions, which is consistent with a significant contribution by persistent inward currents to the net excitation of the motor neurons. Because the proportion of motor neurons that demonstrate persistent inward currents may increase with age (Bae et al. 2008; Kalmar et al. 2009), the different discharge characteristics of the two groups of participants may reflect an age-associated change in the contribution of persistent inward currents during such tasks. Nonetheless, the putative persistent inward currents were not substantial enough to influence the relative values for recruitment and derecruitment thresholds across the two age groups. Additionally, the relative influence of persistent outward currents (Hamm et al. 2010; Turkin et al. 2010) may change with age.
In summary, the discharge characteristics of motor units in the biceps brachii of old adults that were recruited during a sustained contraction were not influenced by the difference between target force and recruitment threshold force of a motor unit. This result contrasts with previous observations on young adults when the discharge characteristics varied with the force difference (Riley et al. 2008). In addition, mean discharge rates were lower for the old adults and did not vary with time across the contraction. Taken together, these results suggest that the integration of synaptic input during sustained contractions changes with age. The lack of modulation in discharge characteristics for motor units of old adults during the constant-force contraction indicates reduced diversity in the output from the spinal cord as a consequence of aging.
GRANTS
This work was supported by National Institute on Aging Grants AG-09000 to R. M. Enoka and T32 AG-000279-08 to R. Schwartz, which supported M. A. Pascoe.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank M. Gaw and B. Hewes for assistance with data analysis and electrode fabrication and Dr. Katrina Maluf for comments on a draft of the manuscript.
REFERENCES
- Abbott LF, Chance FS. Drivers and modulators from push-pull and balanced synaptic input. Prog Brain Res 149: 147–155, 2005 [DOI] [PubMed] [Google Scholar]
- Adam A, De Luca CJ. Recruitment order of motor units in human vastus lateralis muscle is maintained during fatiguing contractions. J Neurophysiol 90: 2919–2927, 2003 [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008 [DOI] [PubMed] [Google Scholar]
- Bae JS, Sawai S, Misawa S, Kanai K, Isose S, Shibuya K, Kuwabara S. Effects of age on excitability properties in human motor axons. Clin Neurophysiol 119: 2282–2286, 2008 [DOI] [PubMed] [Google Scholar]
- Barry BK, Pascoe MA, Jesunathadas M, Enoka RM. Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol 97: 3206–3218, 2007 [DOI] [PubMed] [Google Scholar]
- Baudry S, Enoka R. Influence of load type on presynaptic modulation of Ia afferent input onto two synergist muscles. Exp Brain Res 199: 83–88, 2009 [DOI] [PubMed] [Google Scholar]
- Baudry S, Rudroff T, Pierpoint LA, Enoka RM. Load type influences motor unit recruitment in biceps brachii during a sustained contraction. J Neurophysiol 102: 1725–1735, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P, Pang MY, Olesen KA, Calancie B. Rotation of motoneurons during prolonged isometric contractions in humans. J Neurophysiol 96: 1135–1140, 2006 [DOI] [PubMed] [Google Scholar]
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol 50: 1380–1392, 1983 [DOI] [PubMed] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science 315: 390–393, 2007 [DOI] [PubMed] [Google Scholar]
- Berg RW, Ditlevsen S, Hounsgaard J. Intense synaptic activity enhances temporal resolution in spinal motoneurons. PLoS ONE 3: e3218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Cafarelli E, Vøllestad NK. Fatigue of submaximal static contractions. Acta Physiol Scand Suppl 556: 137–148, 1986 [PubMed] [Google Scholar]
- Bigland-Ritchie B, Lippold OC. Motor unit activity in the voluntary contraction of human muscle. J Physiol 125: 322–335, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer PA, Morales FR, Chase MH. Alterations of group Ia-motoneuron monosynaptic EPSPs in aged cats. Exp Neurol 100: 583–595, 1988 [DOI] [PubMed] [Google Scholar]
- Calvin WH, Stevens CF. Synaptic noise and other sources of randomness in motoneuron interspike intervals. J Neurophysiol 31: 574–587, 1968 [DOI] [PubMed] [Google Scholar]
- Cameron WE, Jodkowski JS, Fang H, Guthrie RD. Electrophysiological properties of developing phrenic motoneurons in the cat. J Neurophysiol 65: 671–679, 1991 [DOI] [PubMed] [Google Scholar]
- Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol 534: 903–912, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MH, Morales FR, Boxer PA, Fung SJ. Aging of motoneurons and synaptic processes in the cat. Exp Neurol 90: 471–478, 1985 [DOI] [PubMed] [Google Scholar]
- Christie A, Kamen G. Motor unit firing behavior during prolonged 50% MVC dorsiflexion contractions in young and older adults. J Electromyogr Kinesiol 19: 543–552, 2009 [DOI] [PubMed] [Google Scholar]
- Christie A, Kamen G. Short-term training adaptations in maximal motor unit firing rates and afterhyperpolarization duration. Muscle Nerve 41: 651–660, 2010 [DOI] [PubMed] [Google Scholar]
- Christova P, Kossev A. Motor unit activity during long-lasting intermittent muscle contractions in humans. Eur J Appl Physiol Occup Physiol 77: 379–387, 1998 [DOI] [PubMed] [Google Scholar]
- Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol 87: 843–852, 1999 [DOI] [PubMed] [Google Scholar]
- Dalton BH, Jakobi JM, Allman BL, Rice CL. Differential age-related changes in motor unit properties between elbow flexors and extensors. Acta Physiol 200: 45–55, 2010 [DOI] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329: 113–128, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denier van der Gon JJ, ter Haar Romeny BM, van Zuylen EJ. Behaviour of motor units of human arm muscles: differences between slow isometric contraction and relaxation. J Physiol 359: 107–118, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earles D, Vardaxis V, Koceja DM. Regulation of motor output between young and elderly subjects. Clin Neurophysiol 112: 1273–1279, 2001 [DOI] [PubMed] [Google Scholar]
- Eisen A, Entezari-Taher M, Stewart H. Cortical projections to spinal motoneurons: changes with aging and amyotrophic lateral sclerosis. Neurology 46: 1396–1404, 1996 [DOI] [PubMed] [Google Scholar]
- Engelhardt JK, Morales FR, Yamuy J, Chase MH. Cable properties of spinal cord motoneurons in adult and aged cats. J Neurophysiol 61: 194–201, 1989 [DOI] [PubMed] [Google Scholar]
- Enoka RM, Robinson GA, Kossev A. A stable, selective electrode for recording single motor-unit potentials in humans. Exp Neurol 99: 761–764, 1988 [DOI] [PubMed] [Google Scholar]
- Enoka RM, Robinson GA, Kossev A. Task and fatigue effects on low-threshold motor units in human hand muscle. J Neurophysiol 62: 1344–1359, 1989 [DOI] [PubMed] [Google Scholar]
- Erim Z, Beg MF, Burke D, De Luca CJ. Effects of aging on motor-unit control properties. J Neurophysiol 82: 2081–2091, 1999 [DOI] [PubMed] [Google Scholar]
- Fallentin N, Jørgensen K, Simonsen EB. Motor unit recruitment during prolonged isometric contractions. Eur J Appl Physiol Occup Physiol 67: 335–341, 1993 [DOI] [PubMed] [Google Scholar]
- Fling B, Knight C, Kamen G. Relationships between motor unit size and recruitment threshold in older adults: implications for size principle. Exp Brain Res 197: 125–133, 2009 [DOI] [PubMed] [Google Scholar]
- Freund HJ, Büdingen HJ, Dietz V. Activity of single motor units from human forearm muscles during voluntary isometric contractions. J Neurophysiol 38: 933–946, 1975 [DOI] [PubMed] [Google Scholar]
- Gantchev G, Gatev P, Ivanova T, Tankov N. Motor unit activity during different functional states of the neuromuscular system. Biomed Biochim Acta 45: S69–S75, 1986 [PubMed] [Google Scholar]
- Garland SJ, Enoka RM, Serrano LP, Robinson GA. Behavior of motor units in human biceps brachii during a submaximal fatiguing contraction. J Appl Physiol 76: 2411–2419, 1994 [DOI] [PubMed] [Google Scholar]
- Gatev P, Ivanova T, Gantchev G. Changes in the firing pattern of high-threshold motor units due to fatigue. Electromyogr Clin Neurophysiol 26: 83–93, 1986 [PubMed] [Google Scholar]
- Gorassini MA, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002 [DOI] [PubMed] [Google Scholar]
- Gydikov AA, Kossev A, Trayanova N, Radicheva N. Selective recording of motor unit potentials. Electromyogr Clin Neurophysiol 26: 273–281, 1986 [PubMed] [Google Scholar]
- Hamm TM, Turkin VV, Bandekar NK, O'Neill D, Jung R. Persistent currents and discharge patterns in rat hindlimb motoneurons. J Neurophysiol 104: 1566–1577, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MR, Pascoe MA, Enoka RM. Discharge characteristics of motor units at recruitment in older adults. Towards Translational Research in Motoneurons, Proceedings of Paris Motoneuron Meeting, July 9–13, 2010 [Google Scholar]
- Hunter SK, Critchlow A, Enoka RM. Muscle endurance is greater for old men compared with strength-matched young men. J Appl Physiol 99: 890–897, 2005 [DOI] [PubMed] [Google Scholar]
- Jesunathadas M, Marmon AR, Gibb JM, Enoka RM. Recruitment and derecruitment characteristics of motor units in a hand muscle of young and old adults. J Appl Physiol 108: 1659–1667, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H, Ulfhake B, Dagerlind A, Bennett GW, Fone KC, Hökfelt T. The serotoninergic bulbospinal system and brainstem-spinal cord content of serotonin-, TRH-, and substance P-like immunoreactivity in the aged rat with special reference to the spinal cord motor nucleus. Synapse 15: 63–89, 1993 [DOI] [PubMed] [Google Scholar]
- Kalmar J, Button D, Gardiner K, Cahill F, Gardiner P. Caloric restriction does not offset age-associated changes in the biophysical properties of motoneurons. J Neurophysiol 101: 548–557, 2009 [DOI] [PubMed] [Google Scholar]
- Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci 59: 1334–1338, 2004 [DOI] [PubMed] [Google Scholar]
- Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol 79: 1908–1913, 1995 [DOI] [PubMed] [Google Scholar]
- Kanosue K, Yoshida M, Akazawa K, Fujii K. The number of active motor units and their firing rates in voluntary contraction of human brachialis muscle. Jpn J Physiol 29: 427–443, 1979 [DOI] [PubMed] [Google Scholar]
- Kernell D. High-frequency repetitive firing of cat lumbosacral motoneurones stimulated by long-lasting injected currents. Acta Physiol Scand 65: 74–86, 1965 [DOI] [PubMed] [Google Scholar]
- Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol 82: 238–248, 2004 [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Contractile properties of single motor units in elderly. Comp Methods Biomech Biomed Eng 8, Suppl. 1: 167–168, 2005 [Google Scholar]
- Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol 104: 739–746, 2008 [DOI] [PubMed] [Google Scholar]
- Knight CA, Kamen G. Modulation of motor unit firing rates during a complex sinusoidal force task in young and older adults. J Appl Physiol 102: 122–129, 2007 [DOI] [PubMed] [Google Scholar]
- Löscher WN, Cresswell AG, Thorstensson A. Excitatory drive to the alpha-motoneuron pool during a fatiguing submaximal contraction in man. J Physiol 491: 271–280, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonell CW, Ivanova TD, Garland SJ. Reliability of the interval death rate analysis for estimating the time course of the motoneurone afterhyperpolarization in humans. J Neurosci Methods 162: 314–319, 2007 [DOI] [PubMed] [Google Scholar]
- Macdonell CW, Ivanova TD, Garland SJ. Afterhyperpolarization time-course and minimal discharge rate in low threshold motor units in humans. Exp Brain Res 189: 23–33, 2008 [DOI] [PubMed] [Google Scholar]
- Macdonell CW, Ivanova TD, Garland SJ. Changes in the estimated time course of the motoneuron afterhyperpolarization induced by tendon vibration. J Neurophysiol 104: 3240–3249, 2010 [DOI] [PubMed] [Google Scholar]
- Maton B, Gamet D. The fatigability of two agonistic muscles in human isometric voluntary submaximal contraction: an EMG study. II. Motor unit firing rate and recruitment. Eur J Appl Physiol Occup Physiol 58: 369–374, 1989 [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol 492: 597–628, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Properties of human motoneurones and their synaptic noise deduced from motor unit recordings with the aid of computer modelling. J Physiol (Paris) 93: 135–145, 1999 [DOI] [PubMed] [Google Scholar]
- Miller KJ, Garland SJ, Ivanova TD, Ohtsuki T. Motor-unit behavior in humans during fatiguing arm movements. J Neurophysiol 75: 1629–1636, 1996 [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol 230: 359–370, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales FR, Boxer PA, Fung SJ, Chase MH. Basic electrophysiological properties of spinal cord motoneurons during old age in the cat. J Neurophysiol 58: 180–194, 1987 [DOI] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93: 2449–2459, 2005 [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Jakobi JM, Semmler JG, Enoka RM. Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol 93: 1381–1392, 2005 [DOI] [PubMed] [Google Scholar]
- Nelson RM, Soderberg GL, Urbscheit NL. Alteration of motor-unit discharge characteristics in aged humans. Phys Ther 64: 29–34, 1984 [DOI] [PubMed] [Google Scholar]
- Newton JP, Yemm R, McDonagh MJ. Study of age changes in the motor units of the first dorsal interosseous muscle in man. Gerontology 34: 115–119, 1988 [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neurosci Res 55: 74–77, 2006 [DOI] [PubMed] [Google Scholar]
- Oya T, Riek S, Cresswell A. Recruitment and rate coding organisation for soleus motor units across entire range of voluntary isometric plantar flexions. J Physiol 587: 4737–4748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe MA, Holmes MR, Gaw ME, Enoka RM. Motor unit recruitment in the biceps brachii of older adults during a fatiguing contraction. Soc Neurosci Abstr 859.14, 2008 [Google Scholar]
- Patten C, Kamen G. Adaptations in motor unit discharge activity with force control training in young and older human adults. Eur J Appl Physiol 83: 128–143, 2000 [DOI] [PubMed] [Google Scholar]
- Patten C, Kamen G, Rowland DM. Adaptations in maximal motor unit discharge rate to strength training in young and older adults. Muscle Nerve 24: 542–550, 2001 [DOI] [PubMed] [Google Scholar]
- Person RS. Rhythmic activity of a group of human motoneurones during voluntary contraction of a muscle. Electroencephalogr Clin Neurophysiol 36: 585–595, 1974 [DOI] [PubMed] [Google Scholar]
- Person RS, Kudina L. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32: 471–483, 1972 [DOI] [PubMed] [Google Scholar]
- Piotrkiewicz M, Kudina, Mierzejewska, Jakubiec, Hausmanowa-Petrusewicz I. Age-related change in duration of afterhyperpolarization of human motoneurones. J Physiol 585: 483–490, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Relationship between the time course of the afterhyperpolarization and discharge variability in cat spinal motoneurones. J Physiol 528: 131–150, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley ZA, Maerz A, Litsey J, Enoka RM. Motor unit recruitment in human biceps brachii during sustained voluntary contractions. J Physiol 586: 2183–2193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaiguère P, Vedel JP, Pagni S. Comparison of fluctuations of motor unit recruitment and de-recruitment thresholds in man. Exp Brain Res 95: 517–522, 1993 [DOI] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve 22: 1094–1103, 1999 [DOI] [PubMed] [Google Scholar]
- Rossini PM, Desiato MT, Caramia MD. Age-related changes of motor evoked potentials in healthy humans: non-invasive evaluation of central and peripheral motor tracts excitability and conductivity. Brain Res 593: 14–19, 1992 [DOI] [PubMed] [Google Scholar]
- Rubinstein S, Kamen G. Decreases in motor unit firing rate during sustained maximal-effort contractions in young and older adults. J Electromyogr Kinesiol 15: 536–543, 2005 [DOI] [PubMed] [Google Scholar]
- Rudroff T, Jordan K, Enoka JA, Matthews SD, Baudry S, Enoka RM. Discharge of biceps brachii motor units is modulated by load compliance and forearm posture. Exp Brain Res 202: 111–120, 2010 [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Calvin WH. Membrane-potential trajectories between spikes underlying motoneuron firing rates. J Neurophysiol 35: 311–325, 1972 [DOI] [PubMed] [Google Scholar]
- Stein PSG. Alternation of agonists and antagonists during turtle hindlimb motor rhythms. Ann NY Acad Sci 1198: 105–118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Gossen ER, Jones KE. Neuronal variability: noise or part of the signal? Nat Rev Neurosci 6: 389–397, 2005 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Hayami A, Suzuki M, Watanabe S, Hutton RS. Reductions in recruitment force thresholds in human single motor units by successive voluntary contractions. Exp Brain Res 82: 227–230, 1990 [DOI] [PubMed] [Google Scholar]
- Tanji J, Kato M. Firing rate of individual motor units in voluntary contraction of abductor digiti minimi muscle in man. Exp Neurol 40: 771–783, 1973 [DOI] [PubMed] [Google Scholar]
- Turkin VV, O'Neill D, Jung R, Iarkov A, Hamm TM. Characteristics and organization of discharge properties in rat hindlimb motoneurons. J Neurophysiol 104: 1549–1565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG, Lännergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? News Physiol Sci 17: 17–21, 2002 [DOI] [PubMed] [Google Scholar]