Abstract
Paired-pulse transcranial magnetic stimulation (ppTMS) is a noninvasive method to measure cortical inhibition in vivo. Long interpulse interval (50–500 ms) ppTMS (LI-ppTMS) provokes intracortical inhibitory circuits and can reveal pathologically impaired cortical inhibition in disorders such as epilepsy. Adaptation of ppTMS protocols to rodent disease models is highly desirable to facilitate basic and translational research. We previously adapted single-pulse TMS (spTMS) methods to rats, but ppTMS has yet to be applied. Specifically, whether ppTMS elicits an inhibitory response in rodents is unknown. ppTMS in rats also requires anesthesia, a setting under which the preservation of these measures is undetermined. We therefore tested, in anesthetized rats, whether anesthetic choice affects spTMS-motor-evoked potentials (MEPs), LI-ppTMS in rats, as in humans, elicits intracortical inhibition of the MEP, and rat LI-ppTMS inhibition is acutely impaired in a seizure model. Rats were anesthetized with pentobarbital (PB) or ketamine-atropine-xylazine (KAX) and stimulated unilaterally over the motor cortex while recording bilateral brachioradialis MEPs. LI-ppTMS was applied analogous to human long interval intracortical inhibition (LICI) protocols, and acute changes in inhibition were evaluated following injection of the convulsant pentylenetetrazole (PTZ). We find that spTMS-evoked MEPs were reliably present under either anesthetic, and that LI-ppTMS elicits inhibition of the conditioned MEP in rats, similar to human LICI, by as much as 58 ± 12 and 71 ± 11% under PB and KAX anesthesia, respectively. LI-ppTMS inhibition was reduced to as much as 53% of saline controls following PTZ injection, while spTMS-derived measures of corticospinal excitability were unchanged. Our data show that regional inhibition, similar to human LICI, is present in rats, can be elicited under PB or KAX anesthesia, and is reduced following convulsant administration. These results suggest a potential for LI-ppTMS as a biomarker of impaired cortical inhibition in murine disease models.
INTRODUCTION
Transcranial magnetic stimulation (TMS) is a noninvasive and well-tolerated method for focal cortical stimulation that is emerging as a useful tool for measures of cortical excitability in humans (Kobayashi and Pascual-Leone 2003; Pascual-Leone et al. 1998, 2000; Tormos et al. 1999). When the TMS coil is positioned over the scalp in the region of the motor cortex, it can reliably induce muscle contraction in a contralateral limb. With focal motor cortex stimulation, the thresholds (motor threshold, MT) and amplitude of motor-evoked potentials (MEPs) recorded by surface electromyography (EMG) in the contralateral target muscle are used to derive measures of regional cortical excitability. The recorded MEPs provide an in vivo real-time assessment of corticospinal excitability and connectivity when provoked by spTMS (Kobayashi and Pascual-Leone 2003; Ziemann et al. 2001).
TMS applied as a pair of pulses to the same cortical location separated by variable interstimulus intervals (ISIs) further allows for the noninvasive assessment of excitatory and inhibitory cortical circuits (Kujirai et al. 1993; Valls-Sole et al. 1992; Ziemann et al. 1996a,b). In ppTMS protocols, a conditioning stimulus (CS) precedes each successive test stimulus (TS) to provoke excitatory or inhibitory cortical circuits (Chen 2004; Chen et al. 1998; Ilic et al. 2002; Peurala et al. 2008; Ziemann et al. 1996b). In a common paired-pulse TMS (ppTMS) paradigm, long interpulse interval ppTMS (LI-ppTMS), ISIs of 50 ms to beyond 300 ms with suprathreshold (i.e., 120% MT) CS and TS produce a reliable reduction in the conditioned MEP, a process referred to as long interval intracortical inhibition (LICI) (Chu et al. 2008; McCormick 1989; McDonnell et al. 2006; Nakamura et al. 1995; Valls-Sole et al. 1992; Wassermann et al. 1996; Werhahn et al. 1999). Notably, in diseases such as epilepsy, characterized by excess cortical excitability, LICI is reduced, and thus ppTMS may be a valuable provocative test and biomarker for disorders of impaired cortical inhibition (Badawy et al. 2009; Brodtmann et al. 1999; Chen 2004; Molnar et al. 2006; Valzania et al. 1999).
In recent years, single-pulse TMS (spTMS) methods have been adapted for use in anesthetized rats. We previously demonstrated that relatively focal MEPs, restricted to the limb contralateral to the site of stimulation, can be obtained by spTMS lateralized over one hemisphere under pentobarbital anesthesia (Aydin-Abidin et al. 2008; Luft et al. 2001, 2002; Nielsen et al. 2007; Rotenberg et al. 2008, 2010; Zhang et al. 2007). However, ppTMS, which may permit more thorough investigations of cortical inhibition in rat disease models and, with the use of rat subjects, may provide insight into the biological mechanisms of human paired-pulse cortical inhibition, has not yet been adapted to rodent models.
To expand available rat TMS methods and to facilitate future translational rat TMS research, we conducted the present study to test whether ppTMS applied over a range of long ISIs (LI-ppTMS), similar to human LICI protocols, leads to detectable intracortical inhibition. We tested whether regional inhibition of the MEP is preserved in rats anesthetized by one of two common injectable anesthetics, as anesthesia is necessary in rat TMS research. We utilized multiple anesthetics to address rat LI-ppTMS mechanisms and to anticipate further rat TMS research, which may require selective anesthetic utilization. In establishing whether a phenomenon similar to human LICI is present in rats, we also aimed to test the hypothesis that the induced electrical currents in rat TMS, as those in human TMS, are generating action potentials in the cerebral cortex rather than diffusely throughout the corticospinal tract (Rotenberg et al. 2010).
Last, we investigated the capacity of rat LI-ppTMS measurements to detect altered cortical inhibition in an acute chemoconvulsant rat seizure model. As an early step toward evaluating rat disease models by ppTMS, we tested the hypothesis that the chemoconvulsant pentylenetetrazole (PTZ) will preferentially suppress LI-ppTMS inhibition while leaving spTMS measures of corticospinal excitability unchanged.
METHODS
Animals
Twenty-eight adult male Long-Evans rats [269 ± 26 (SD) g] were used for the present experiments. All animals were housed in a temperature-controlled animal care facility with a 12-h light-dark cycle. All procedures were approved by and in accordance with the guidelines of the Animal Care and Use Committee at Children's Hospital (Boston, MA) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of rats used in the present experiments.
Anesthesia
To evaluate the dependence of rat TMS measures on the choice of anesthetic, we divided rats into two groups to receive intraperitoneal (ip) sodium pentobarbital (PB; n = 7; 50 mg/kg) or ip ketamine-atropine-xylazine (KAX; n = 8; 30 mg/ml ketamine, 0.2 mg/ml atropine, and 4 mg/ml xylazine in 1.1 ml/kg). The anesthesia was adjusted until a clinical state of deep sedation with preserved withdrawal from foot pinch was achieved. PB or KAX was administered in increments equal to 20–30% of the initial dose as needed. Rat body temperature was maintained on a heating blanket operating at 42°C.
Electromyography
MEPs were recorded with monopolar uninsulated 27G stainless steel needle electrodes (Axon Systems, Hauppauge, NY) inserted into the belly of each brachioradialis muscle. Location of the brachioradialis was determined by palpation of the extended forelimb. A reference electrode was positioned distally in the paw between the second and third digit. Each animal was electrically grounded via a single needle electrode placed at the tail base. The EMG signal was band-pass filtered at 300–1,000 Hz and amplified 1,000 times (AM Systems Model 1700; Sequim, WA). The EMG signal was digitized with 40 kHz sampling and stored for post hoc analysis (AD Instruments Colorado Springs, CO).
Transcranial magnetic stimulation
Once anesthetized, rats were placed in a stereotaxic frame and focal TMS was delivered with a monophasic current waveform applied to the rat motor cortex through a figure-eight double 40 mm coil using two Magstim 200 magnetic stimulators and a BiStim Module (Magstim, Wales, UK). These methods were adapted from spTMS methods previously described in our laboratory (Rotenberg et al. 2010). TMS intensity was documented as percent machine output (% MO) with 100% corresponding to the maximal amplitude electrical current conducted through the magnetic coil and to the maximal strength of the generated magnetic field. The TMS coil was positioned in a relatively constant location with the center 9.0 ± 0.8 mm lateral (mirrored either left or right) to midline and 3.4 ± 1.1 mm anterior to the interaural line according to the methods of Whishaw and colleagues as we used in a prior publication (Rotenberg et al. 2010; Whishaw et al. 1977). The optimal coil position was defined as that with the lowest stimulation intensity required to elicit lateralized MEPs exclusively in the forelimb contralateral to the TMS coil location. With the TMS coil positioned, the stimulator intensity was systematically adjusted to find motor threshold (MT) in steps of 5% MO increasing from 30% MO until MEPs were observed. Once MEPs in the forelimb contralateral to the coil were apparent, the stimulator intensity was adjusted in finer increments (1% MO) to obtain the lowest stimulus intensity able to elicit MEPs of ≥20 μV peak-to-peak amplitude in 5 of 10 consecutive trials.
spTMS and LI-ppTMS
To approximate human LICI protocols, we tested six ISIs (50, 100, 200, 300, 400, and 500 ms) as well as a spTMS condition for a total of seven conditions (n = 7 rats per anesthetic group). Each experiment included 10 trials per condition presented in a randomized order with an intertrial interval (ITI) of 8 s. Both the CS and TS were set to 120% MT. spTMS was implemented using an unconditioned TS. We specifically selected these ISIs to correspond with those most commonly used in human LICI protocols (50–200 ms). Further, we chose to include ISIs >200 ms in efforts to capture the waning inhibition seen in humans for longer ISIs between 200 and 800 ms for a more complete characterization of rat LI-ppTMS (Chu et al. 2008; McDonnell et al. 2006; Nakamura et al. 1995; Valls-Sole et al. 1992; Wassermann et al. 1996; Werhahn et al. 1999).
Eelectroencephalography and rat PTZ seizure model
To test whether rat LI-ppTMS inhibition is altered in a nonconvulsive rat seizure model, two additional groups were anesthetized with pentobarbital (50 mg/kg ip) and administered either PTZ (70 mg/kg ip; n = 7) or an equivalent volume of normal (0.9%) saline (n = 8). Preliminary experiments demonstrated that under KAX anesthesia, rats exhibited convulsive seizures with PTZ injection. Thus to avoid the confounding effects of spontaneous ictal muscle activation and to provide nonconvulsive seizure induction, we chose PB anesthesia for these experiments. To confirm nonconvulsive seizure induction, continuous electroencephalography (EEG) was acquired with two thin silver/silver-chloride Teflon-coated EEG subdermal wire electrodes (Ives EEG Solutions, Ontario, Canada), with a reference positioned midline at the interorbital line and an active electrode unilaterally placed over the parietal region. The raw EEG signal was processed with a slew rate limiting preamplifier as previously described to allow for real-time assessment of ictal patterns amid periodic TMS artifact (Rotenberg et al. 2008). The EEG signal was digitized at 200 Hz, band-pass filtered at 1–70 Hz, and displayed in a single channel montage (Gamma Reviewer, Grass Telefactor, Providence, RI).
Baseline EEG was recorded for 5 min prior to a LI-ppTMS protocol in which three ISIs were tested (50, 100, and 200 ms). As in the preceding text, 10 trials per condition were presented in a randomized order with an 8 s ITI and CS and TS of 120% MT. Immediately following baseline EEG and ppTMS, rats were administered PTZ or normal saline. ppTMS was repeated at 5, 30, and 45 min following PTZ or saline injection. For the duration of these experiments, EEG was collected for real-time verification and post hoc confirmation of PTZ-induced EEG paroxysms.
Data analysis
Per MEP, the peak-to-peak voltage (Vpp), maximal voltage (Vmax), and absolute MEP integral were acquired. To measure conditioned MEP inhibition, changes in MEP size (μV) following ppTMS were expressed as ratios of the average conditioned MEP to average unconditioned MEP, per ISI, per rat. That is, conditioned:unconditioned ratios >100% represent MEP facilitation while those <100% represent MEP inhibition.
All MEP values that were expressed as ratios of baseline were log10 transformed before statistical testing. The resultant ratios at each ISI were averaged across subjects within each anesthetic group and compared with a mean of 1 using a one-sample t-test to determine the significance of conditioning. Two-way ANOVA with Bonferroni posttests for anesthetic and ISI was performed to determine separately the contributions of the ISI, the choice of anesthetic, and their interaction.
Epileptiform activity following PTZ administration was confirmed by visual inspection and by fast Fourier transform (FFT) of 240 s EEG epochs for each rat at baseline, 5, and 30 min post injection. The signal power (μVpp2) was binned by frequency at 1 Hz resolution and the percent change from baseline power was then computed for each time point and frequency combination and averaged across rats in each group. Repeated two-way ANOVA with Bonferroni posttests was utilized to compare changes in power as a function of treatment and frequency at each time point.
To evaluate the changes in LI-ppTMS inhibition over time after the injection of PTZ or saline, the baseline (preinjection) LI-ppTMS inhibition was averaged across rats within each treatment group. These baseline values were then used to normalize LI-ppTMS inhibition at each time point following either PTZ or saline injection. To highlight the difference in inhibition among the treatment groups, the ppTMS inhibition for each group was normalized to the average inhibition for the saline group at each respective time point. Statistical analysis of the changes in LI-ppTMS inhibition for ISIs of 50, 100, and 200 ms attributable to PTZ-induced seizures was performed using two-way ANOVA to evaluate the effects of treatment, time, and their interaction. Bonferroni posttests were utilized to compare treatment groups for a given ISI at each individual time point to clarify specific temporal significance. In addition, before and after normalizing all data to the average percent inhibition of the saline group, a two-way ANOVA was run on each treatment group to evaluate the effects of ISI, time, and interaction on the changes in inhibition within each group. GraphPad Prism v. 5.0c and JMP v. 8.0.1 statistical programs were used for analysis.
RESULTS
Effect of anesthetic on motor threshold and single-pulse TMS measures
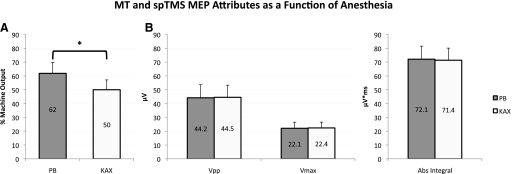
MT was significantly reduced in the KAX group (50 ± 7% MO, mean ± SD) relative to the PB group (62 ± 8% MO; t = 3.776, P = 0.001; Fig. 1A). However, other MEP values evoked by spTMS at a suprathreshold TMS intensity (120% MT) were unaffected by anesthetic selection [F(1,36) = 0.00004, P = 0.995], including the MEP Vpp (PB: 44.2 ± 25.0 μV, KAX: 44.5 ± 23.2 μV), Vmax (PB: 22.1 ± 11.7 μV, KAX: 22.4 ± 11.1 μV), and the absolute integral of the MEP (PB: 72.1 ± 25.9 μV*ms, KAX: 71.4 ± 19.3 μV*ms; Fig. 1B).
Fig. 1.
Single-pulse TMS (spTMS) measures. A: motor threshold (MT) comparison in the pentobarbital (PB) and ketamine-atropine-xylazine (KAX) groups. MT (mean ± SD) is expressed as percent machine output (% MO) averaged across 14 trials from 7 rats per each anesthetic. MT for PB and KAX are 62 ± 8% MO and 50 ± 7% MO, respectively; * P = 0.001. B: comparison of MEP attributes. (spTMS) at 120% MT was used to evoke an MEP and obtain the peak-to-peak voltage (Vpp), maximum voltage (Vmax), and the absolute integral (Abs Integral) of the MEP for all rats (n = 7 rats per anesthetic group). For Vpp, Vmax, and Abs Integral the PB group achieved 44.2 ± 25 μV, 22.1 ± 11.7 μV, and 72.1 ± 25.9 μV*ms, respectively. Similarly, the KAX group yielded 44.5 ± 23.2 μV, 22.4 ± 11.1 μV, and 71.4 ± 19.3 μV*ms. The difference in these attributes among anesthetic groups failed to reach significance [F(1,36) = 0.00004, P = 0.995].
LI–ppTMS inhibition is preserved in anesthetized rats
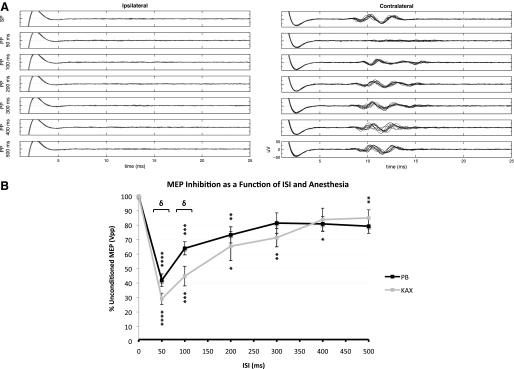
LI-ppTMS inhibition was demonstrable under both anesthetics. LI-ppTMS with either KAX or PB anesthesia produced significant inhibition at nearly all ISIs (Fig. 2, A and B) with maximal inhibition at an ISI of 50 ms; for ISIs of 50, 100, 200, 300, 400, and 500 ms under KAX, t = 13.01, 5.485, 2.876, 3.565, 2.334, 3.504, and P < 0.0001, P = 0.0015, 0.0282, 0.0118, 0.0584, 0.0128, respectively, whereas under PB, t = 7.967, 6.008, 4.223, 2.082, 3.217, 2.862, and P = 0.0002, 0.001, 0.0055, 0.1288, 0.0487, and 0.0645, respectively. Comparison of LI-ppTMS under KAX and PB using two-way ANOVA revealed a significant effect of anesthetic [F(1,75) = 6.494, P = 0.0129] and ISI [F(6,75) = 25.36, P < 0.0001]. These data show the strict dependence of the degree of inhibition on ISI and also suggest that at the two tested doses, greater inhibition may be achievable under KAX relative to PB. Subsequent Bonferroni posttests revealed that the effect of anesthetic was largely driven by a significant difference in inhibition among the KAX and PB groups at an ISI of 50 and 100 ms (t = 2.959, P < 0.05 for both ISIs). At an ISI of 50 ms, LI-ppTMS under PB anesthesia inhibited the conditioned MEP by 58.1 ± 11.6% (t = 7.967, P = 0.0002), while inhibition under KAX reached 70.9 ± 10.5% (t = 13.01, P < 0.0001). Further, at 300 and 500 ms ISIs, LI-ppTMS failed to achieve significant inhibition (300 ms: 18.6 ± 18.7%, t = 2.082, P = 0.1288; 500 ms: 20.7 ± 13.0%, t = 2.862, P = 0.0645) with PB anesthesia, whereas a lack of significant LI-ppTMS inhibition was observed at the 400 ms ISI (16.2 ± 20.9%, t = 2.334, P = 0.0584) for the KAX group.
Fig. 2.
Long interval intracortical inhibition (LICI) in rats. A: MEPs elicited by spTMS (top row; SP) and paired-pulse TMS (ppTMS, rows 2–7; PP). Left and right: ipsi- and contralateral EMG from the brachioradialis muscle, respectively. Intercolumn comparison demonstrates robust lateralization of MEPs. Rows 2–7 correspond to ppTMS with interstimulus intervals (ISIs) of 50, 100, 200, 300, 400, and 500 ms, respectively. Note the near complete inhibition seen at an ISI of 50 ms with diminishing inhibition seen for longer ISIs. Overall findings for LI-ppTMS in rat match the phenomenon of human LICI with the same ppTMS paradigm. Tracings include 10 superimposed stimulus trials. Conditioning stimulus (CS) and test stimulus (TS) were both set to 120% MT. B: conditioned MEP size as function of anesthetic and ISI. The graph shows the conditioned MEP peak-to-peak amplitude normalized to unconditioned MEP peak-to-peak amplitude, expressed as the percent of unconditioned MEP Vpp. All values >100% represent signal facilitation while values <100% represent inhibition. CS and TS were set to 120% MT. 10 stimulus trials for each condition were run on 7 rats for each anesthetic (total = 70 signals per condition per anesthetic) with the exception of 300, 400, and 500 ms ISIs in the PB group for which 4 rats were used. Data points marked with asterisks achieved significant inhibition relative to baseline unconditioned MEP values (*P < 0.05, **P < 0.02, ***P < 0.002, ****P ≤ 0.0002). Bracketed ISIs denote significant intergroup differences among anesthetics (δ <0.05). Values denote means ± SE.
Effect of seizures on spTMS measures
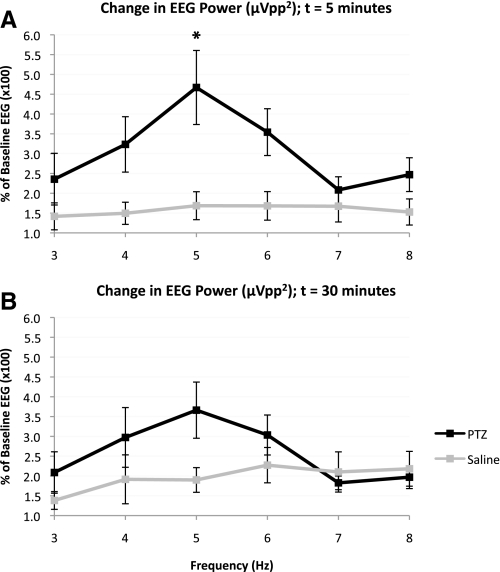
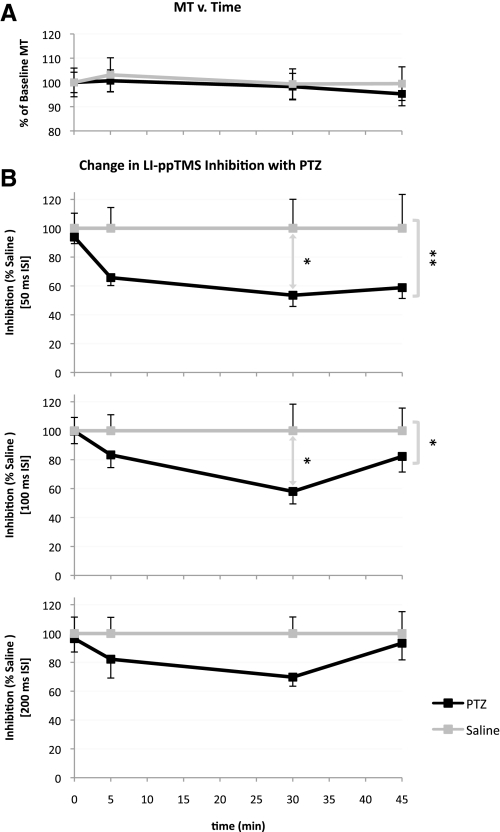
Electrographic seizure activity following PTZ injection was observed in all rats in the PTZ treated group. Digital analysis of the EEG confirmed a significant PTZ-induced increase in signal power relative to control saline treated rats using two-way ANOVA for treatment and frequency in the 3–8 Hz band at 5 min [treatment effect: F(1,66) = 22.71, P < 0.0001] and 30 min [treatment effect: F(1,66) = 4.796, P = 0.0321] following injection (Fig. 3, A and B). Bonferroni posttests demonstrated that this treatment effect was largely driven by differences in EEG signal power at 5 Hz (5 min: t = 3.922, P < 0.01; 30 min: t = 2.484, P > 0.05). The MT was unaffected by the treatment and did not change significantly over time [treatment effect: F(1,48) = 0.1271, P = 0.723; time effect: F(3,48) = 0.222, P = 0.8806; interaction effect: F(3,48) = 0.0488, P = 0.9856]. For the saline group, the percent of baseline MT at time 0, 5, 30, and 45 min was 100.0 ± 15.7, 103.1 ± 18.6, 99.4 ± 16.5, and 99.5 ± 17.0% (mean ± SD), respectively. Likewise, the percent of baseline MT for the PTZ group at time 0, 5, 30, and 45 min was 100.0 ± 11.2, 100.7 ± 11.9, 98.3 ± 14.5, and 95.3 ± 12.0%, respectively. These findings suggest a stable MT in the acute phase of PTZ-mediated enhancement of cortical excitability (Fig. 4A). Further, analysis of the change in MEPs obtained by single-pulse conditions at 120% MT relative to baseline MEPs at each subsequent time point failed to demonstrate a significant intergroup difference for Vpp [treatment effect: F(1,48) = 0.0898, P = 0.7658; time effect: F(3,48) = 0.8666, P = 0.4649; interaction effect: F(3,48) = 0.1505, P = 0.9289], Vmax [treatment effect: F(1,48) = 0.0117, P = 0.9142; time effect: F(3,48) = 0.8766, P = 0.4598; interaction effect: F(3,48) = 0.0996, P = 0.9598] and absolute integral [treatment effect: F(1,48) = 0.0028, P = 0.9577; time effect: F(3,48) = 1.014, P = 0.3949; interaction effect: F(3,48) = 0.2589, P = 0.8546]. At 5 min post injection, the percent of baseline Vpp, Vmax, and MEP absolute integral for the control saline group were 105.4 ± 46.7, 106.5 ± 44.5, and 100.1 ± 34.9%, respectively, while those for the PTZ group were 111.0 ± 53.3, 114.8 ± 59.1, and 115.3 ± 48.1%, respectively. These metrics demonstrate similar negligible change at 30 and 45 min following injection. Additionally, a two-way ANOVA within each treatment group was performed for MEP attribute (i.e., Vpp, Vmax, Abs Integral) and time, failing to demonstrate a significant effect of either of these variables on intragroup changes [saline: MEP attribute effect: F(2,75) = 0.0012, P = 0.9988, time effect: F(3,75) = 2.24, P = 0.0905, interaction effect: F(6,75) = 0.0117, P = 1.000; PTZ: MEP attribute effect: F(2,69) = 0.0519, P = 0.9494, time effect: F(3,69) = 0.8241, P = 0.4851, interaction effect: F(6,69) = 0.0046, P = 1.000].
Fig. 3.
Change in electroencephalographic (EEG) signal power following PTZ injection. Three to 8 Hz fast Fourier transform (FFT) shows change in EEG power (μVpp2) 5 min (A) and 30 min (B) following PTZ or saline injection relative to baseline (% of baseline EEG power). There is a significant shift toward increased power in the PTZ group relative to saline group driven by the treatment effect at 5 [F(1,66) = 22.71, P < 0.0001] and 30 [F(1,66) = 4.796, P = 0.0321] minutes following injection. Bonferroni posttests further revealed a significant intergroup difference at 5 min for 5 Hz (* P < 0.01). Values denote means ± SE.
Fig. 4.
spTMS and ppTMS measures following PTZ injection. A: change in MT over time following PTZ or saline administration. Graph shows the change in MT as a percent of baseline MT (mean ± SE). The changes in MT did not reach significance across treatment groups [treatment effect: F(1,48) = 0.1271, P = 0.723; time effect: F(3,48) = 0.222, P = 0.8806; interaction effect: F(3,48) = 0.0488, P = 0.9856]. In the PTZ group, n = 7, 7, 7, and 6 at 0, 5, 30, and 45 min respectively. Likewise, in the control group n = 8, 6, 8, and 7 rats for each corresponding time point. B: the change in LI-ppTMS inhibition over time after PTZ administration (mean ± SE). Values were normalized to the average level of inhibition in the saline (control) group at each time point for each tested ISI (50, 100, and 200 ms). Values <100% represent reduced LI-ppTMS inhibition. All ISIs demonstrate clear separation between treatment groups with PTZ causing reduced inhibition for all time points with the exception of the 200 ms ISI at 45 min. Asterisks bracketing curves represent significant intergroup differences (2-way ANOVA, treatment effect), whereas asterisks between curves represent significant intergroup differences at specific ISIs (Bonferroni posttests); * P < 0.05, ** P < 0.002.
LI-ppTMS inhibition is suppressed following PTZ injection
While PTZ-induced seizures showed little effect on spTMS outcomes, LI-ppTMS inhibition was significantly reduced following PTZ injection. On average, LI-ppTMS inhibition was reduced in the PTZ treated rats for all ISIs and time points except for the 200 ms ISI at 45 min (Fig. 4B). Tracings representative of this phenomenon are shown in Fig. 5, A and B. Two-way ANOVA for treatment and time demonstrated significant intergroup differences for LI-ppTMS percent inhibition normalized to saline group averages using ISIs of 50 ms [treatment effect: F(1,48) = 10.93, P = 0.0018; time effect: F(3,48) = 2.42, P = 0.0775; interaction effect: F(3,48) = 1.169, P = 0.3314] and 100 ms [treatment effect: F(1,48) = 5.856, P = 0.0194; time effect: F(3,48) = 2.458, P = 0.0742; interaction effect: F(3,48) = 1.516, P = 0.2223]. Bonferroni posttests demonstrated that this difference was largely driven by the significant treatment effect observed at 30 min post injection [50 ms ISI: t = 2.649, P < 0.05; 100 ms ISI: t = 2.952, P < 0.05]. The PTZ group inhibition as a percentage of the average saline group inhibition for ISIs of 50, 100, and 200 ms is as follows: 50 ms ISI: of 65.8 ± 14.6, 53.5 ± 20.5, and 58.8 ± 18.4: for t = 5, 30, and 45 min post injection, respectively; 100 ms ISI: 83.3 ± 23.2, 58.0 ± 22.7, and 82.2 ± 26.4% for t = 5, 30, and 45 min, respectively; 200 ms ISI: 82.2 ± 34.6, 69.7 ± 16.6, and 93.3 ± 28.3% for t = 5, 30, and 45 min, respectively. In addition, prior to normalizing percent inhibition to the saline group averages, a two-way ANOVA was performed within treatment groups for ISI and time. This demonstrated a lack of intragroup ISI or time effect for both the PTZ [ISI effect: F(2,69) = 0.2198, P = 0.8032; time effect: F(3,69) = 1.089, P = 0.3595; interaction effect: F(6,69) = 0.0969, P = 0.9965] and saline groups [ISI effect: F(2,75) = 1.014, P = 0.3675; time effect: F(3,75) = 2.307, P = 0.0834; interaction effect: F(6,75) = 0.3696, P = 0.896]. After normalizing the percent inhibition to the saline group averages this two-way ANOVA for ISI and time was rerun, revealing a significant effect for time [F(3,69) = 10.01, P < 0.0001] and ISI [F(2,69) = 4.596, P = 0.0134] for the PTZ group. This suggests temporal importance when considered referential to the control group. Thus within each group, significant changes in inhibition relative to baseline (t = 0) were not achieved but only realized when the groups were considered referentially; that is, when the PTZ group was considered in reference to its proper control. Bonferroni posttests further clarified that the significant ISI effect was driven by the difference in 50 and 200 ms ISIs at 45 min (t = 2.696, P < 0.05) near extremes of the measured PTZ effect. This suggests perhaps a more robust measure of PTZ effects with ISIs of 50 and 100 ms at times under 45 min after administration in this particular chemoconvulsant protocol. Overall, these data suggest that LI-ppTMS may be used to obtain a reliable marker of pathologically reduced cortical inhibition in rats.
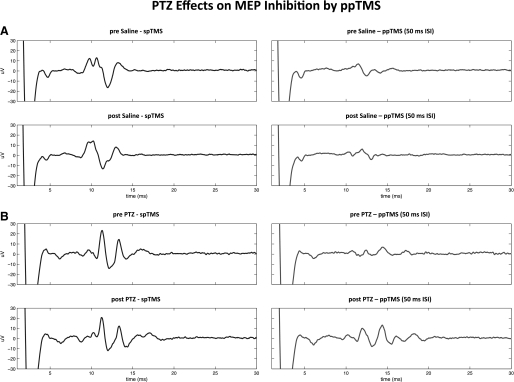
Fig. 5.
Reduced LI-ppTMS inhibition following PTZ administration. Representative MEP tracings show preserved LI-ppTMS inhibition in the saline group with reduced inhibition in the PTZ group. A, top: spTMS-evoked MEP average (left) and ppTMS-evoked MEP average (right) at 50 ms ISI before saline injection. Bottom: same conditions repeated 30 min following saline injection. B: the same conditions as in A except that these occur before and after PTZ injection. Note the reduced LI-ppTMS inhibition 30 min after PTZ injection (B, bottom). Tracings are the average of 4 sweeps obtained from a representative rat from each treatment group.
DISCUSSION
ppTMS may have potential as a clinical measure of cortical inhibition in a variety of patient populations (Chen 2004; Kobayashi and Pascual-Leone 2003). Experimental animal models will be required to evaluate the accuracy of this test and examine how known perturbations of network function will affect ppTMS-derived markers such as LICI. Notably, for both ethical and logistical reasons, such experimental circumstances may require that ppTMS be administered to animal subjects under anesthesia. Therefore to validate the application of ppTMS in rat disease models, we determined whether a measure of cortical inhibition similar to human LICI is present in anesthetized rats, whether the extent of MEP inhibition by ppTMS changes predictably as a function of the ISI in rats, as it does in humans, and whether injection of a chemoconvulsant, which impairs cortical inhibition, leads to reduced rat LI-ppTMS inhibition.
Feasibility of measuring rat regional cortical inhibition
We demonstrate for the first time that LI-ppTMS-derived measures of cortical inhibition can be obtained in anesthetized rats by protocols analogous to human LICI. As an extension of our prior work, showing that regional spTMS is feasible in rats, we find that a lateralized rat forelimb MEP can be modulated by a conditioning stimulus in a LI-ppTMS protocol (Rotenberg et al. 2010). LICI protocols in humans reliably demonstrate inhibition over a range of ISIs from 50 ms to beyond 200 ms that achieves nearly complete to around 50% inhibition of the unconditioned response, which tapers off for higher ISIs (Chen 2004; Nakamura et al. 1995; Udupa et al. 2009; Valls-Sole et al. 1992). In the anesthetized rat, the extent of intracortical inhibition and the LI-ppTMS inhibitory profile as a function of ISI were in the range of 60–70% inhibition for 50 ms and diminished toward 20% for longer ISIs (300–500 ms), similar to ranges recorded for human LICI (Chen 2004; Chen et al. 1999; Inghilleri et al. 1993; McDonnell et al. 2006; Nakamura et al. 1995, 1997; Udupa et al. 2009; Valls-Sole et al. 1992; Wassermann et al. 1996). Thus we show that inhibitory ppTMS protocols may be translated from humans to rats and perhaps may now provide a robust noninvasive in vivo measure of cortical inhibitory circuit activity common to human studies. The similarities in response patterns in rats and in humans support the plausible use of rat LI-ppTMS inhibition as a noninvasive and rapidly obtained biomarker for future therapeutic development using rat disease models, particularly for treatments aimed to modulate cortical inhibition.
Human data demonstrate, by cervical epidural recordings of descending corticospinal volleys, that the circuits probed by ppTMS techniques are of cortical origin (Di Lazzaro et al. 1998, 1999). Thus if LICI is indeed a cortical phenomenon, then our data may also address an important issue in rat TMS: whether the MEP recorded in a rat peripheral muscle is of cortical or extracortical origin. This is an open question given the incompletely understood distribution of intracranial electrical currents in the rat brain during TMS (Zheng et al. 2005). The morphology, latency, and strong lateralization of the brachioradialis MEPs obtained here as well as in our prior experiments with spTMS suggest that the signal is of telencephalic origin and that our rat stimulation protocols are sufficiently focal to restrict suprathreshold induced currents to one hemisphere (Rotenberg et al. 2010). With the present demonstration of a rat LICI-like process by LI-ppTMS, presumably a cortical process, we have further support for cortical activation by TMS in rats. Particularly, the similarities between rat and human LICI profiles as a function of ISI suggest that rat MEP inhibition may be intracortically mediated because the vast difference in length of the corticospinal tracts and the accompanying differences in corticospinal conduction times would likely have contributed to distinct LICI-ISI profiles if extracortical processes were critically involved. Certainly more experimental work, perhaps with microelectrode cortical stimulation and corticospinal descending volley recordings will be required to confirm the cortical origin of this inhibitory process in rats. Of particular interest in subsequent experiments will be a determination of whether this inhibitory phenomenon is primarily affecting directly or indirectly (cortical interneuron-mediated) activated corticospinal volleys (Luft et al. 2001). However, the present data broadly support the notion that rat TMS may be capable of activating the brain with sufficient anatomic resolution to identify cortical inhibitory deficits in modeled neuropathology.
Differential effects of anesthetics on rat LI-ppTMS inhibition
Prior rat TMS studies suggested that the choice of anesthetic might affect MEPs in rats (Luft et al. 2001). This is of particular relevance to translational work since rats must be anesthetized for focal spTMS or ppTMS, and modulation of specific neurotransmitter receptor families by the anesthetic may confound findings, especially in rat disease models. We therefore chose to evaluate two commonly used anesthetics known to work by distinct mechanisms: PB primarily at the GABAA receptor and KAX at the N-methyl-d-aspartate (NMDA) glutamate, muscarinic acetylcholine, and α2 adrenergic receptors (Liu et al. 2006; Schwartz and Clark 1998). We find a reduction in the MT for KAX treated rats in comparison to the PB group (Fig. 1A), but overall similar spTMS-derived MEP attributes in both anesthetic conditions (Fig. 1B). The lowered MT under KAX anesthesia is consistent with published human data, which show that ketamine reduces MT and enhances neuronal excitability via hypothesized non-NMDA receptor dependent processes (Di Lazzaro et al. 2003; Potez and Larkum 2008; Sloan 1998).
Encouragingly, the LI-ppTMS inhibitory phenomenon in rats was preserved with both anesthetics with greater inhibition seen under KAX anesthesia for 50–100 ms ISIs (Fig. 2B). The reduced inhibition seen under PB relative to KAX may be the result of a more profound saturation of inhibition by PB with less room to appreciate the inhibition via LI-ppTMS and/or other pharmacodynamic differences between PB and KAX. In the present study, we used these two anesthetics at doses that delivered light general anesthesia. Our rationale for conducting the present study with PB and KAX was that these are common rodent anesthetics that have been used in prior rat TMS studies (Luft et al. 2001; Nielsen et al. 2007). However, it is likely that other anesthetics will need to be tested in a similar manner before being employed in future translational ppTMS studies. It is also important to note that these experiments were conducted with a single preselected dose of each anesthetic. A more extensive test of a range of doses for each anesthetic may be necessary to precisely compare TMS measures under PB and KAX but is beyond the scope of this report and reserved for a follow-up study.
With the rat LI-ppTMS preparation, our data also may provide additional insight into the pharmacology of LICI, which in humans remains an important gap in knowledge. In humans, the exact mechanisms underlying LICI are incompletely understood but may relate to GABAB receptor (GABABR) activation as suggested by limited data from healthy volunteers exposed to baclofen, a GABABR agonist. Notably, while in one trial by McDonnell and colleagues the investigators observed baclofen enhancement of LICI by ∼35%, others have failed to replicate this result, suggesting a more complex mechanism (Florian et al. 2008; McDonnell et al. 2006). Our data also suggest that LICI may not be a strictly GABABR-dependent process, as we find an acute reduction in inhibition with the administration of the GABAAR antagonist PTZ. However, as the mechanisms of rat LI-ppTMS and human LICI are not fully understood and thus cannot be equated, we cannot definitively generalize such effects. The use of baclofen in this study to further test the GABABR dependence of rat LI-ppTMS inhibition was not feasible as pilot experiments demonstrated that the sedative effects of baclofen in anesthetized rats enhanced the level of sedation sufficiently to impair reliable TMS-evoked MEPs. Optimization of baclofen delivery during anesthetization is beyond the scope of this initial report aimed to identify a rat LICI-like phenomenon and to test whether rat LI-ppTMS inhibition is reduced by PTZ. Although further pharmacologic manipulation will certainly be necessary, our data are a starting point toward elucidating LICI-like mechanisms with the use of rat models.
Suppression of LI-ppTMS inhibition following PTZ administration
Systemic injection of PTZ, a GABAA receptor antagonist, is widely used to model primary generalized seizures in rodents (Konishi et al. 2001; Olsen 1981). Its proconvulsant activity has largely been attributed to selective antagonism of GABA-mediated postsynaptic inhibition (Macdonald and Barker 1978; Olsen 1981). In using this acute chemoconvulsant model, we sought to validate the utility of rat LI-ppTMS to detect changes in cortical inhibition and evaluate LICI for the first time during acute ictal activity. We chose to use pentobarbital anesthesia for these experiments because initial trials using KAX rendered the subjects too sensitive to the convulsive effects of PTZ, and the resultant tonic seizures confounded MEP measurements. This finding in rats is consistent with human TMS data showing enhanced recruitment of excitatory motor cortical networks with reduced motor thresholds and larger EMG responses under subanesthetic ketamine doses (Di Lazzaro et al. 2003). Convulsive episodes were not observed in any subject under pentobarbital anesthesia, although distinct epileptiform EEG changes were observed in all.
Here we find that PTZ significantly reduces rat LI-ppTMS inhibition, but despite the provocation of epileptiform discharges on EEG, PTZ failed to cause a significant change in motor threshold or other attributes of MEPs obtained by spTMS. The selective sensitivity of ppTMS measures to PTZ, in contrast to spTMS measures, suggests a failure of inhibitory mechanisms rather than a change in principal neuronal excitability in this seizure model. In support of this finding, prior human studies have shown isolated interictal reductions in LICI measured in patients newly diagnosed with idiopathic generalized epilepsy and juvenile myoclonic epilepsy (Badawy et al. 2009; Brodtmann et al. 1999; reviewed in Rotenberg 2010). Notably, although suppression of LICI has been demonstrated in humans with epilepsy, fluctuation of LICI with acute seizures has not been shown. Thus whether suppressed LICI reflects a constitutive impairment of inhibitory mechanisms or whether LICI may rise and fall to reflect ongoing changes in inhibition and seizure susceptibility remains unknown. Our finding that, after PTZ injection, the reduction of rat LI-ppTMS inhibition accompanies epileptiform EEG changes suggests that the inhibitory mechanisms underlying LICI and related ppTMS measures may fluctuate acutely with seizures. However, given that all rats in the PTZ group demonstrated electrographic epileptiform activity, we cannot rule out the potential for seizure-independent influences of PTZ on LI-ppTMS inhibition. Yet our data suggest the potential for ppTMS and LICI as a biomarker to measure ongoing changes in cortical inhibition, and perhaps may provide a platform for seizure prediction and/or risk assessment in vulnerable subjects.
Conclusions and practical significance
We report an advance in the translation of human TMS protocols to rodent models. For the purposes of future studies, we show that lateralized MEPs can be obtained under two different anesthetics with differences noted only in motor threshold. This report also documents the first application LI-ppTMS protocols in rats as well as the first in vivo application of these techniques in an acute seizure model. We demonstrate that ppTMS can be applied in rats to achieve predictable MEP inhibition whether under PB or KAX anesthesia and that a measure of impaired inhibition can be obtained after chemoconvulsant administration. These new tools may allow for a more thorough investigation of the mechanisms of ppTMS via biochemical and pharmacologic animal studies.
We anticipate that further translational work with ppTMS protocols in rats and in rat disease models will permit the development of this technique as a practical biomarker in epilepsy as well as other disease states such as Parkinson's disease, schizophrenia, traumatic brain injury, and stroke, which are characterized by a pathologic alteration of intracortical inhibition (Berardelli et al. 1996; Blicher et al. 2009; Butefisch et al. 2003, 2008; Chu et al. 2009; Cicinelli et al. 2003; Daskalakis et al. 2002; Manganotti et al. 2008; Wittenberg et al. 2007). Ultimately, the use of ppTMS in rodents may improve research with animal disease models by enabling noninvasive longitudinal in vivo assessment of cortical excitability and may also provide a venue for deeper investigation of the neurophysiology underlying common TMS phenomena.
GRANTS
This work was supported by National Institutes of Health NS K08 NS055895 (AR), National Institutes of Health K24RR018875 (APL), IDDRC P30-HD18655 (AR, FEJ), the Center for Integration of Medicine and Innovative Technology (CIMIT) (AV, APL, AR), and the Howard Hughes Medical Institute Medical Research Training Fellowship program (AV).
DISCLOSURES
Investigator-initiated grant from Lundbeck Pharmaceuticals to F. Jensen, no relation to the present project; investigator: Frances Jensen.
REFERENCES
- Aydin-Abidin S, Trippe J, Funke K, Eysel UT, Benali A. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp Brain Res 188: 249–261, 2008 [DOI] [PubMed] [Google Scholar]
- Badawy RA, Macdonell RA, Jackson GD, Berkovic SF. Why do seizures in generalized epilepsy often occur in the morning? Neurology 73: 218–222, 2009 [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rona S, Inghilleri M, Manfredi M. Cortical inhibition in Parkinson's disease. A study with paired magnetic stimulation. Brain 119: 71–77, 1996 [DOI] [PubMed] [Google Scholar]
- Blicher JU, Jakobsen J, Andersen G, Nielsen JF. Cortical excitability in chronic stroke and modulation by training: a TMS study. Neurorehabil Neural Repair 23: 486–493, 2009 [DOI] [PubMed] [Google Scholar]
- Brodtmann A, Macdonell RA, Gilligan AK, Curatolo J, Berkovic SF. Cortical excitability and recovery curve analysis in generalized epilepsy. Neurology 53: 1347–1349, 1999 [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V. Remote changes in cortical excitability after stroke. Brain 126: 470–481, 2003 [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair 22: 4–21, 2008 [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res 154: 1–10, 2004 [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res 128: 539–542, 1999 [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol 80: 2870–2881, 1998 [DOI] [PubMed] [Google Scholar]
- Chu J, Gunraj C, Chen R. Possible differences between the time courses of presynaptic and postsynaptic GABAB mediated inhibition in the human motor cortex. Exp Brain Res 184: 571–577, 2008 [DOI] [PubMed] [Google Scholar]
- Chu J, Wagle-Shukla A, Gunraj C, Lang AE, Chen R. Impaired presynaptic inhibition in the motor cortex in Parkinson disease. Neurology 72: 842–849, 2009 [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke 34: 2653–2658, 2003 [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry 59: 347–354, 2002 [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Pilato F, Zito G, Dileone M, Nicoletti R, Pasqualetti P, Tonali PA. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J Physiol 547: 485–496, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 119: 265–268, 1998 [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res 129: 494–499, 1999 [DOI] [PubMed] [Google Scholar]
- Florian J, Muller-Dahlhaus M, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol 586: 495–514, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol 545: 153–167, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol 466: 521–534, 1993 [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2: 145–156, 2003 [DOI] [PubMed] [Google Scholar]
- Konishi Y, Matsu-ura T, Mikoshiba K, Tamura T. Stimulation of gene expression of NeuroD-related factor in the mouse brain following pentylenetetrazol-induced seizures. Brain Res Mol Brain Res 97: 129–136, 2001 [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ji XQ, Zhu XZ. Comparison of psychic emergence reactions after (+/−)-ketamine and (+)-ketamine in mice. Life Sci 78: 1839–1844, 2006 [DOI] [PubMed] [Google Scholar]
- Luft AR, Kaelin-Lang A, Hauser TK, Buitrago MM, Thakor NV, Hanley DF, Cohen LG. Modulation of rodent cortical motor excitability by somatosensory input. Exp Brain Res 142: 562–569, 2002 [DOI] [PubMed] [Google Scholar]
- Luft AR, Kaelin-Lang A, Hauser TK, Cohen LG, Thakor NV, Hanley DF. Transcranial magnetic stimulation in the rat. Exp Brain Res 140: 112–121, 2001 [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Barker JL. Specific antagonism of GABA-mediated postsynaptic inhibition in cultured mammalian spinal cord neurons: a common mode of convulsant action. Neurology 28: 325–330, 1978 [DOI] [PubMed] [Google Scholar]
- Manganotti P, Acler M, Zanette GP, Smania N, Fiaschi A. Motor cortical disinhibition during early and late recovery after stroke. Neurorehabil Neural Repair 22: 396–403, 2008 [DOI] [PubMed] [Google Scholar]
- McCormick DA. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol 62: 1018–1027, 1989 [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res 173: 86–93, 2006 [DOI] [PubMed] [Google Scholar]
- Molnar GF, Sailer A, Gunraj CA, Cunic DI, Wennberg RA, Lozano AM, Chen R. Changes in motor cortex excitability with stimulation of anterior thalamus in epilepsy. Neurology 66: 566–571, 2006 [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol 498: 817–823, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H, Takano H, Nakatoh S. Intracortical facilitation and inhibition after paired magnetic stimulation in humans under anesthesia. Neurosci Lett 199: 155–157, 1995 [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Perez MA, Oudega M, Enriquez-Denton M, Aimonetti JM. Evaluation of transcranial magnetic stimulation for investigating transmission in descending motor tracts in the rat. Eur J Neurosci 25: 805–814, 2007 [DOI] [PubMed] [Google Scholar]
- Olsen RW. The GABA postsynaptic membrane receptor-ionophore complex. Site of action of convulsant and anticonvulsant drugs. Mol Cell Biochem 39: 261–279, 1981 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol 15: 333–343, 1998 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience–virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol 10: 232–237, 2000 [DOI] [PubMed] [Google Scholar]
- Peurala SH, Muller-Dahlhaus JF, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF). Clin Neurophysiol 119: 2291–2297, 2008 [DOI] [PubMed] [Google Scholar]
- Potez S, Larkum ME. Effect of common anesthetics on dendritic properties in layer 5 neocortical pyramidal neurons. J Neurophysiol 99: 1394–1407, 2008 [DOI] [PubMed] [Google Scholar]
- Rotenberg A. Prospects for clinical applications of transcranial magnetic stimulation and real-time EEG in epilepsy. Brain Topogr 22: 257–266, 2010 [DOI] [PubMed] [Google Scholar]
- Rotenberg A, Muller P, Birnbaum D, Harrington M, Riviello JJ, Pascual-Leone A, Jensen FE. Seizure suppression by EEG-guided repetitive transcranial magnetic stimulation in the rat. Clin Neurophysiol 119: 2697–2702, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg A, Muller PA, Vahabzadeh-Hagh AM, Navarro X, Lopez-Vales R, Pascual-Leone A, Jensen F. Lateralization of forelimb motor evoked potentials by transcranial magnetic stimulation in rats. Clin Neurophysiol 121: 104–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DD, Clark TP. Affinity of detomidine, medetomidine and xylazine for alpha-2 adrenergic receptor subtypes. J Vet Pharmacol Ther 21: 107–111, 1998 [DOI] [PubMed] [Google Scholar]
- Sloan TB. Anesthetic effects on electrophysiologic recordings. J Clin Neurophysiol 15: 217–226, 1998 [DOI] [PubMed] [Google Scholar]
- Tormos JM, Catala MD, Pascual-Leone A. [Transcranial magnetic stimulation]. Rev Neurol 29: 165–171, 1999 [PubMed] [Google Scholar]
- Udupa K, Ni Z, Gunraj C, Chen R. Interactions between short latency afferent inhibition and long interval intracortical inhibition. Exp Brain Res 199: 177–183, 2009 [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85: 355–364, 1992 [DOI] [PubMed] [Google Scholar]
- Valzania F, Strafella AP, Tropeani A, Rubboli G, Nassetti SA, Tassinari CA. Facilitation of rhythmic events in progressive myoclonus epilepsy: a transcranial magnetic stimulation study. Clin Neurophysiol 110: 152–157, 1999 [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res 109: 158–163, 1996 [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol 517: 591–597, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Cioe JD, Previsich N, Kolb B. The variability of the interaural line vs the stability of bregma in rat stereotaxic surgery. Physiol Behav 19: 719–722, 1977 [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Bastings EP, Fowlkes AM, Morgan TM, Good DC, Pons TP. Dynamic course of intracortical TMS paired-pulse responses during recovery of motor function after stroke. Neurorehabil Neural Repair 21: 568–573, 2007 [DOI] [PubMed] [Google Scholar]
- Zhang YP, Shields LB, Zhang Y, Pei J, Xu XM, Hoskins R, Cai J, Qiu MS, Magnuson DS, Burke DA, Shields CB. Use of magnetic stimulation to elicit motor evoked potentials, somatosensory evoked potentials, and H-reflexes in non-sedated rodents. J Neurosci Methods 165: 9–17, 2007 [DOI] [PubMed] [Google Scholar]
- Zheng J, Li L, Huo X. Analysis of electric field in real rat head model during transcranial magnetic stimulation. Conf Proc IEEE Eng Med Biol Soc 2: 1529–1532, 2005 [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res 109: 127–135, 1996a [DOI] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain 124: 1171–1181, 2001 [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol 496: 873–881, 1996b [DOI] [PMC free article] [PubMed] [Google Scholar]