Abstract
Sensory neurons are generally tuned to a subset of stimulus qualities within their sensory domain and manifest this tuning by the relative size of their responses to stimuli of equal intensity. However, response size alone cannot unambiguously signal stimulus quality, since response size also depends on stimulus intensity. Thus a common problem faced by sensory systems is that response size (e.g., spike count) confounds stimulus quality and intensity. Here, using the gustatory system as a model, we asked whether temporal firing characteristics could disambiguate these axes. To address this question, we recorded taste responses of single neurons in the nucleus of the solitary tract (NTS, the first central gustatory relay) in anesthetized rats to a range of concentrations of NaCl and HCl and their binary mixtures. To assess the contribution of the temporal characteristics of the response to discrimination among tastants, a family of metrics that quantifies the similarity of two spike trains in terms of spike count and spike timing was used. Results showed that the spike count produced by different taste qualities and different concentrations overlapped in most cells, implying that information conveyed by spike count is imprecise. Multidimensional scaling analysis of taste responses using similarity of temporal characteristics showed that different taste qualities, intensities, and mixtures formed distinct clusters in this “temporal coding” taste space and were arranged in a logical order. Thus the temporal structure of taste responses in single cells in the NTS can simultaneously convey information about both taste quality and intensity.
INTRODUCTION
In all sensory systems, individual cells are tuned to respond selectively to a certain set of stimuli. The variety of tuning curves across cells spans and defines the broader stimulus domain and enables the identification and discrimination of different stimuli. However, changes in stimulus intensity generally broaden those tuning curves and may produce confusion between a change in stimulus intensity and a change in identity. When the tuning (specificity) is narrow, the identity of the neuron can signal the identity of the stimulus (e.g., pitch, color, taste quality, etc.) and the relative firing rate can indicate intensity (e.g., loudness, brightness, concentration). In a system such as gustation, where most cells respond well to more than one taste quality (sweet, sour, salty, bitter, and perhaps umami), stimuli of different taste qualities can evoke equivalent firing rates if the concentrations are just right. As a result, in most cases firing rate alone cannot convey an unambiguous message about taste quality, especially in broadly tuned neurons.
In many studies of taste-responsive cells in the CNS, groups of cells are defined by the stimulus that evokes the “best” or most robust response when exemplars of each basic taste quality are presented at moderate concentrations. Even though most cells are multisensitive across taste qualities, several researchers have pointed out that the intensity–response function can be steeper for a cell's best stimulus compared with its nonbest or “sideband” stimuli (Nakamura and Norgren 1991; St John and Smith 2000). These results imply that a cell's best stimulus defines the taste quality about which that cell conveys the most information. However, the problem remains that for almost every cell, there are suprathreshold, moderate concentrations of different taste qualities for which a cell will respond with equal vigor. Thus the gustatory system makes an excellent model for the study of how the nervous system disentangles intensity and identity in single cells.
In a series of studies of taste-responsive cells in the nucleus of the solitary tract (NTS), the first central relay of the gustatory system, we have shown that information about taste quality conveyed by increases in firing rate can be supplemented by information conveyed by spike timing (Di Lorenzo and Victor 2003, 2007; Di Lorenzo et al. 2009; Roussin et al. 2008). The contribution of spike timing was particularly significant when two tastants evoked nearly equal firing rates (Roussin et al. 2008). In our most recent study, we showed that the temporal dynamics of taste responses in broadly tuned NTS cells can disambiguate tastants of different qualities that evoke equal firing rates (Di Lorenzo et al. 2009), even when they are presented as mixtures. Specifically, we showed that responses to binary taste mixtures were linear combinations of rate envelopes of responses to the components and that the rate envelopes corresponding to the four tastants generated a consistent, logical mapping of taste quality. However, to sample a sufficient number of responses to repeated presentations of the four primary tastants and their six pairwise combinations, it was necessary to restrict our analysis to a single concentration of each. This leaves open the question of whether temporal coding can contribute to discrimination of taste quality, when intensity is varied over a wide range, and when primaries are combined in different relative concentrations. To address this requires obtaining a sufficient number of replicate responses to primaries and binary mixtures across a range of concentrations; it was therefore necessary to focus on two primary tastants, as we do here. We chose NaCl and HCl for two reasons. From a practical perspective, there is an extensive literature showing that these two stimuli evoke significant responses in nearly all NTS cells in anesthetized rats. From a theoretical viewpoint, since both stimuli elicit transient responses, it is a strong test of the notion that subtle temporal features can support discrimination of quality. As our results show, even when intensity and relative concentration are varied, the temporal aspects of the response contribute substantially to the signaling of taste quality.
METHODS
Subjects
Thirty-three male, Sprague–Dawley rats (300–450 g) served as subjects for these experiments. All rats were pair-housed in plastic cages and maintained on a 12-h light/dark schedule with lights on at 7:00 a.m. Food and water were available without restriction. All procedures were in accord with the National Institutes of Health Animal Welfare Guide and were approved by the Institutional Animal Care and Use Committee of Binghamton University.
Surgery
Rats were deeply anesthetized with urethane (1.5 g/kg, administered intraperitoneally in two equal doses spaced 30 min apart) and prepared for electrophysiological recording in the NTS. Briefly, rats were tracheotomized and their head mounted in a stereotaxic instrument (Kopf Instruments, Tujunga, CA), with the tooth bar positioned 5.0 mm below the interaural line. The occipital bone was removed and uvular and nodular portions of the cerebellum were aspirated gently to expose the surface of the brain stem just above the NTS. Several stainless steel screws were embedded in the skull and a nontraumatic head holder was cemented to the screws using dental acrylic cement. The ear and tooth bars could then be removed without perturbing the head position and orientation. Core temperature was maintained at 37°C by a heating pad coupled to an anal thermistor probe (FHC, Bowdoinham, ME).
Recording
Etched tungsten microelectrodes (18–20 MΩ, 1 V at 1 kHz; FHC) were lowered slowly into the NTS. Signals were fed to an amplifier (Model P511; Grass Technologies, West Warwick, RI) and sent to a PC computer via an analog/digital interface (CED, Cambridge, UK). Waveforms arising from single neurons were identified using the Spike2 program (CED; sampling rate 25 kHz). A 3:1 signal-to-noise ratio was required for isolation of NTS cells. Taste responses were recorded as long as the cell remained well isolated.
Previous work from our lab has demonstrated that the taste-responsive portion of the NTS is located at about 2.7 mm rostral and 1.8 mm lateral to the obex and 700–1,400 μm ventral to the surface of the brain stem. As the electrode was lowered into the area near the NTS, the tongue was periodically bathed with 0.1 M NaCl (followed by a distilled water rinse) to test for a background taste response. NaCl was chosen as the probe stimulus because many studies of the NTS in anesthetized rats have shown that nearly every taste-responsive cell responds to it. To avoid sampling bias, every cell that was isolated was tested with all four basic taste stimuli.
Once a taste-responsive cell was isolated, testing began. Initially, each tastant was presented in individual trials. A trial consisted of a 10 s baseline (spontaneous activity), 10 s distilled water prerinse, 5 s tastant presentation, 5 s pause, and 20 s distilled water rinse. The interstimulus interval was 2 min. Stimulus delivery tubes were flushed well with distilled water when the stimulus to be delivered was changed.
Taste stimuli
Taste stimuli were NaCl and HCl, both presented at various concentrations indicated in the following text, and 0.5 M sucrose and 0.01 M quinine HCl. Binary mixtures consisted of components whose final concentration in the mixture equaled that of the single-component taste stimuli. Concentrations of sucrose and quinine and medium concentrations of NaCl (0.1 M) and HCl (0.01 M) were chosen because they produce half-maximal responses in the chorda tympani nerve of the rat, which innervates taste buds on the rostral two thirds of the tongue (Ganchrow and Erickson 1970; Ogawa et al. 1974). All tastants were made with reagent grade chemicals, dissolved in distilled water, and presented at room temperature. Taste stimuli were bathed over the tongue through a specially designed stimulus delivery system described previously (Di Lorenzo and Victor 2003). Briefly, this device consisted of a bundle of stainless steel tubes that were perforated on top and bottom so that fluids were delivered to the entire tongue and palate simultaneously. Using a solution of methylene blue dye instead of a tastant, the entire mouth, including the incisor ducts and foliate papillae, were stained when the stimulus delivery stem was activated. Flow rate was 5 ml/s.
Data analysis
GENERAL RESPONSE CHARACTERISTICS.
Taste responses were measured as the average firing rate in spikes/s (sps) ± SE over the first 2 s of the taste stimulus presentation minus the average firing rate over the final 5 s of water prerinse (baseline). Significant responses were defined by firing rates that were ≥2.58SDs above the baseline firing rate, calculated on a trial-by-trial basis. Breadth of tuning was calculated using the Uncertainty measure (Smith and Travers 1979) with the following formula
where Pi is the proportion of the total number of spikes elicited by n stimuli that are evoked by stimulus i, and K is a scaling constant. For four stimuli K = 1.661, which results in U ranging from 0 to 1.0. Values close to 0 result when a cell responds to few stimuli, indicating narrow tuning. Conversely, values near 1.0 result when the cell responds equally well to all stimuli, indicating broad tuning. For each cell, the average evoked firing rate for each stimulus across repetitions was used.
Cells were classified according to their “best” stimulus based on the relative response magnitudes evoked by 0.1 M NaCl, 0.01 M HCl, 0.5 M sucrose, and 0.01 M quinine.
ANALYSES OF TEMPORAL CHARACTERISTICS OF TASTE RESPONSES.
To examine the information conveyed by the temporal structure of taste responses, spike trains were analyzed using metric space analysis (Victor and Purpura 1996, 1997; reviewed in Victor 2005). This approach provides an index of the similarity of two spike trains by calculating the “cost” of transforming one spike train into another through a series of elementary steps. These include moving spikes in time and inserting or deleting spikes. First, the cost of inserting or deleting a spike is set at 1. Next, the cost of moving a spike per unit time is defined as q, in units of 1/s. The cost of moving a spike by an amount of time t is thus counted as qt. Then, the distance between any two spike trains is simply defined as the “minimum total cost” of transforming one spike train into the other via these elementary steps and is denoted as Dspike[q]. Note that when q is zero, the spikes are free to move and the distance (minimum cost) between spike trains is simply the difference of the number of spikes between them. In this case when q = 0, i.e., Dspike[0] is denoted as Dcount. For larger values of q, Dspike[q] is sensitive to the temporal arrangement of spikes at a resolution of 1/q.
After calculating Dspike[q] for all pairs of a neuron's responses, we next determined the extent to which pairs of responses to the same stimulus tended to be closer to each other than pairs of responses to different stimuli. A spike train S was classified as belonging to the response class R if the average distance Dspike[q] from S to each of the spike trains elicited by the stimulus R was shorter than the average distance from S to the responses elicited by any other stimulus. We then calculated information H from the confusion matrix between the actual stimulus that elicited each response and the response class to which it was assigned by this proximity rule. The value of the information H thus indicates the performance of stimulus-dependent clustering based on the temporal patterns of taste responses. For example, perfect classification of responses to the four primary taste qualities (sucrose, NaCl, HCl, quinine) corresponds to H = 2 bits (log2 4 = 2). If the classification is totally random, H = 0. The preceding analysis was carried out for each cell for a range of values of q in half-octave steps from 0.0625 to 256 and for q = 0. When q = 0, H is denoted as H0 and indicates information conveyed by spike count alone (i.e., a rate code). The value of q where H reaches its maximum was denoted by qmax and the maximum information was denoted by Hmax.
To validate the performance of this quantitative measure, three additional information-theoretic analyses were carried out as controls. 1) To control for the statistical effects of a finite data sample (see Di Lorenzo and Victor 2003; Roussin et al. 2008), the values of H calculated from observed responses were compared with the values of H calculated from a data set in which the observed responses were randomly assigned to the various clusters of tastant, called Hshuffle. 2) To distinguish between the influence of firing rate envelope and detailed firing pattern, we applied metric space analysis to surrogate data sets created by “exchange” resampling. These surrogate data sets were created by randomly exchanging spikes between individual responses belonging to the same tastant. Surrogate and actual responses had identical poststimulus time histograms and an identical number of spikes elicited on each trial. If the value of H for the original data was above the value of Hexchange (mean ± 2SD) obtained from exchange-resampled surrogates, we concluded that the information contributed by temporal coding was not merely contained in the average rate envelope of the taste response nor in spike count alone and that spike timing patterns in individual trials must also contribute to taste coding. 3) We created surrogate data sets consisting of inhomogeneous Poisson processes. These surrogate data sets were created by random draws from the set of observed spike times for each stimulus, rather than by exchanging spike times between pairs of responses, so they matched the observed responses in rate envelope but not in the number of spikes elicited on each trial. In general, information estimates from these surrogate data sets (Hpoisson) were nearly identical to those of the exchange-resampled surrogate data sets (Hexchange).
GEOMETRICAL REPRESENTATION OF TEMPORAL FIRING FEATURES: MULTIDIMENSIONAL SCALING ANALYSES.
To visualize the manner in which temporal characteristics of the response differed among the tastants, we applied standard multidimensional scaling (MDS; Kruskal and Wish 1978) to the distances among taste responses, defined by Dspike[q]. For all taste responses, Dspike[q] at qmax was used. In an MDS analysis, “objects” (taste-evoked spike trains) were placed in a “taste-space” so that the distance between objects in the MDS space was closely proportional to the dissimilarity/similarity, as measured by Dspike[q].
The axes in this taste space are abstract: they are defined by the criterion that each successive axis accounts for as much as possible of the variance that is not accounted for by the other axes. Consequently, the axes are not guaranteed to have a direct physiological interpretation, although we noted that the first axis was generally correlated with firing rate. Note also that since the distances Dspike[q] are non-Euclidean (Aronov and Victor 2004), there was no guarantee that an MDS embedding was possible. However, this difficulty generally did not arise among the first three dimensions extracted by MDS, which generally accounted for >95% of power of the distances between the centroids.
RESULTS
Overall, results of this study showed that the temporal dynamics of the responses in single NTS cells significantly contributed to taste quality discrimination across the entire intensity–response function as well as when tastants were part of a mixture. Although responses to individual taste qualities generally increased as the concentration increased, there was considerable trial-to-trial variability. Importantly, responses to NaCl and HCl were highly correlated within and across cells and showed overlap in spike count, indicating that the firing rate was a poor indicator of taste quality. However, analyses of temporal coding in NTS cells showed that the temporal structure of the responses can disambiguate taste quality in the face of variations in concentration and overlap of the number of spikes in the response. Further, results extend previous findings (Di Lorenzo et al. 2009) showing that the temporal features of responses to different taste qualities can disentangle their identities when they are part of a binary mixture, even when their concentrations varied.
General response characteristics
Taste responses not only to the four basic taste stimuli but also to different concentrations of NaCl and HCl were recorded from 52 cells in the NTS. Forty-two cells were presented with NaCl at 0.6 M, 0.1 M, and 0.01 M and HCl at 0.06 M, 0.01 M, and 0.001 M. Of these, 19 were also presented with the binary mixtures as follows: 0.6 M NaCl + 0.06 M HCl, 0.1 M NaCl + 0.01 M HCl, 0.01 M NaCl + 0.001 M HCl, 0.6 M NaCl + 0.001 M HCl, and 0.001 M NaCl + 0.6 M HCl. In an additional 10 cells, an expanded array of concentrations (in M) was tested as follows: for NaCl, 0.5, 0.2, 0.1, 0.06, and 0.03; for HCl, 0.04, 0.02, 0.01, 0.005, and 0.0025. For all cells, repeated trials of each stimulus (range = 5–15 trials per stimulus) were presented. Average spontaneous firing rate across all 52 cells was 3.47 ± 0.47 SE spikes/s (sps).
Taste-responsive NTS cells generally responded to more than one of the basic taste stimuli. Average Uncertainty was 0.81 ± 0.02 SE. All 52 (100%) cells responded to 0.1 M NaCl, 47 (90%) responded to 0.01 M HCl, 36 (69%) responded to 0.5 M sucrose, and 46 (88%) responded to 0.01 M quinine. Thirty cells responded to all four taste stimuli at these “standard” concentrations, 15 cells responded to three stimuli, and 7 cells responded to two stimuli. There were no cells that responded to a single stimulus at the standard concentrations. Thirty-nine of 52 cells (75%) were NaCl best, 5 cells (10%) were HCl best, and 7 cells (15%) were sucrose best. Fifty-one cells (98%) responded to both NaCl and HCl at the highest concentrations tested; one cell (cell 13) responded to NaCl but not to HCl at any concentration tested. Figure 1 shows the raw responses of one cell to two concentrations of NaCl and HCl as well as their binary mixtures. The number and proportion of cells that responded to different tastants at different concentrations are summarized in Table 1. It can be seen that higher concentrations of NaCl or HCl activated more cells and that even at the lowest concentrations tested, the majority of cells showed significant responses.
Fig. 1.
Reponses of one cell to 2 concentrations each of NaCl and HCl and to their binary mixtures. Thin line under each response indicates water presentation. Thick line indicates 5-s taste stimulus presentation.
Table 1.
Number of responses to NaCl and HCl.
| Concentration, M | Number of Cells (Total) | Percentage |
|---|---|---|
| NaCl | ||
| 0.6 | 42 (42) | 100 |
| 0.5 | 10 (10) | 100 |
| 0.2 | 10 (10) | 100 |
| 0.1 | 52 (52) | 100 |
| 0.06 | 9 (10) | 90 |
| 0.03 | 9 (10) | 90 |
| 0.01 | 28 (42) | 67 |
| HCl | ||
| 0.06 | 41 (42) | 98 |
| 0.04 | 10 (10) | 100 |
| 0.02 | 10 (10) | 100 |
| 0.01 | 47 (52) | 90 |
| 0.005 | 9 (10) | 90 |
| 0.0025 | 9 (10) | 90 |
| 0.001 | 28 (42) | 67 |
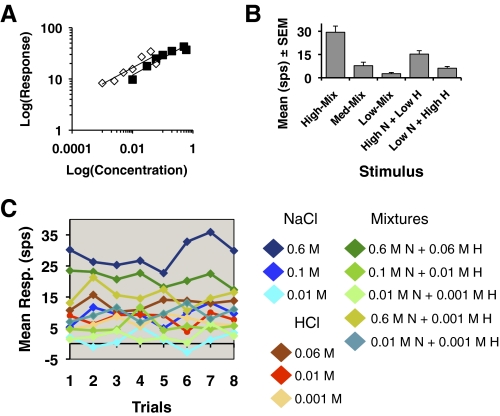
On average, response magnitude to NaCl and HCl monotonically increased as the concentration increased. The mean response magnitudes across cells in response to NaCl and HCl at different concentrations are shown in Fig. 2. Figure 2 shows the log–log plot of responses from 52 cells to all concentrations of NaCl and HCl. The exponent of the fitted power function (slope) is 0.33 (r2 = 0.91) for NaCl and 0.32 (r2 = 0.79) for HCl. It is apparent that average response magnitudes for NaCl and HCl were equivalent at most midrange concentrations. For example, on average, 0.1 M NaCl evoked the same response magnitude as 0.06 M HCl. In a subset of cells (n = 19), responses to mixtures of varying concentrations of NaCl and HCl were also recorded. Average response magnitudes for these stimuli are shown in Fig. 2B. It can be seen that the range of response magnitudes to NaCl or HCl presented as mixtures also overlapped the response magnitudes for these stimuli presented alone. Figure 2C shows an example of response magnitudes of a single cell across blocks of trials to different concentrations of NaCl and HCl and their binary mixtures. Note that across blocks of trials, the magnitudes of the responses to the two qualities overlap. Consequently, taste quality cannot be determined from response magnitude.
Fig. 2.
A: intensity (concentration) –response functions for NaCl (filled squares) and HCl (open squares). Responses from all 52 cells were used. B: mean response ± SE to various mixtures of NaCl and HCl. High-Mix, 0.6 M NaCl + 0.06 M HCl; Med-Mix, 0.1 M NaCl + 0.01 M HCl; Low-Mix, 0.01 M NaCl + 0.001 M HCl; Hi N + Low H, 0.6 M NaCl + 0.001 M HCl; Low N + High H, 0.01 M NaCl + 0.06 M HCl. C: mean responses (spikes/s [sps]) for 3 concentrations of NaCl and HCl and various NaCl–HCl mixtures across 8 trials in one cell. Under Mixtures: N, NaCl; H, HCl.
In Fig. 3, the intensity-based response patterns across the sample of cells were examined. In Fig. 3A, response magnitudes to three different concentrations of NaCl across 42 cells are shown. Cells were first sorted by their response magnitudes to 0.6 M NaCl. Generally, monotonic increases in response magnitudes were observed as stimuli increased in concentration. Similar results were found in Fig. 3B, in which response magnitudes of these cells to three different concentrations of HCl (sorted by their response magnitudes to 0.06 M HCl) are shown. Those cells that responded vigorously to NaCl usually also generated vigorous responses to HCl. In fact, the average correlation of responses to various concentrations of NaCl with responses to various concentrations of HCl was 0.65 ± 0.02 (P < 0.01). Table 2 shows the correlations of across-unit responses for all three concentrations of NaCl and HCl and for sucrose and quinine.
Fig. 3.
Mean response magnitude for individual cells for each concentration tested. A: High N, 0.6 M NaCl; Med N, 0.1 M NaCl; Low N, 001 M NaCl. B: High H, 0.06 M HCl; Med H, 0.01 M HCl; Low H, 0.001 M HCl. C, left: High N, 0.5 M NaCl; High-Med N, 0.2 M NaCl; Med N, 0.1 M NaCl; Med-Low N, 0.06 M NaCl; Low N, 0.03 M NaCl; High H, 0.04 M HCl; High-Med H, 0.02 M HCl; Med H, 0.01 M HCl; Med-Low H, 0.005 M HCl; Low H, 0.0025 M HCl.
Table 2.
Pearson product–moment correlations for all taste stimuli
| NaCl, M |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stimuli | 0.6 | 0.5 | 0.2 | 0.1 | 0.06 | 0.03 | 0.01 | ||
| NaCl, M | |||||||||
| 0.6 | 1.00 | ||||||||
| 0.5 | 1.00 | ||||||||
| 0.2 | 0.82 | 1.00 | |||||||
| 0.1 | 0.95 | 0.82 | 0.98 | 1.00 | |||||
| 0.06 | 0.83 | 1.00 | 0.98 | 1.00 | |||||
| 0.03 | 0.80 | 0.90 | 0.91 | 0.90 | 1.00 | ||||
| 0.01 | 0.81 | 0.84 | 1.00 | ||||||
| HCl, M | |||||||||
| 0.06 | 0.74 | 0.71 | 0.62 | ||||||
| 0.04 | 0.79 | 0.86 | 0.89 | 0.87 | 0.70 | ||||
| 0.02 | 0.56 | 0.68 | 0.70 | 0.67 | 0.51 | ||||
| 0.01 | 0.91 | 0.53 | 0.65 | 0.86 | 0.66 | 0.43 | 0.75 | ||
| 0.005 | 0.50 | 0.54 | 0.53 | 0.54 | 0.38 | ||||
| 0.0025 | 0.82 | 0.54 | 0.58 | 0.54 | 0.66 | ||||
| 0.001 | 0.74 | 0.67 | 0.54 | ||||||
| Sucrose (S), M | 0.09 | 0.28 | 0.42 | 0.17 | 0.45 | 0.40 | 0.13 | ||
| Quinine (Q), M | 0.89 | 0.63 | 0.90 | 0.90 | 0.90 | 0.71 | 0.76 | ||
| HCl, M |
S, M | Q, M | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Stimuli | 0.06 | 0.04 | 0.02 | 0.01 | 0.005 | 0.0025 | 0.001 | 0.5 | 0.01 |
| HCl, M | |||||||||
| 0.06 | 1.00 | ||||||||
| 0.04 | 1.00 | ||||||||
| 0.02 | 0.90 | 1.00 | |||||||
| 0.01 | 0.84 | 0.88 | 0.95 | 1.00 | |||||
| 0.005 | 0.75 | 0.90 | 0.92 | 1.00 | |||||
| 0.0025 | 0.59 | 0.51 | 0.47 | 0.63 | 1.00 | ||||
| 0.001 | 0.57 | 0.79 | 1.00 | ||||||
| Sucrose (S), M | 0.07 | 0.20 | 0.12 | 0.08 | 0.00 | −0.05 | 0.06 | 1.00 | |
| Quinine (Q), M | 0.77 | 0.91 | 0.75 | 0.89 | 0.55 | 0.33 | 0.62 | 0.23 | 1.00 |
Trial-to-trial variability was characterized by the coefficient of variation (CV) for all cells. This measure is the SD divided by the mean response across trials. Within cells, the CV was calculated separately for each stimulus that produced a significant response. Average CVs across cells ranged from 0.11 to 0.35 for NaCl and 0.19 to 0.35 for HCl, generally increasing as the concentration decreased. This is likely due to a positive correlation of response magnitude with the SD (r = 0.67, P < 0.01), as shown in Fig. 4.
Fig. 4.
Plot of mean responses (sps) across trials vs. SD (sps) for all cells and all concentrations of NaCl (filled diamonds) and HCl (open diamonds). Dotted line indicates the condition where the mean = the SD.
When the response magnitude to NaCl–HCl mixtures was compared with the response magnitude evoked by its more effective component (MEC), mixture suppression (mixture < MEC, Student's t-test, P < 0.05) was commonly observed. For mixtures of high concentrations (0.6 M NaCl–0.06 M HCl), mixture suppression was noted in 11 cells (of 19, 58%). For all but two cells the MEC was NaCl at these concentrations. At midrange concentrations (0.1 M NaCl–0.01 M HCl), mixture suppression was found more frequently (18/19 cells, 95%) and NaCl was also the MEC in all but one cell. At the lowest concentrations tested (0.01 M NaCl–0.001 M HCl), mixture suppression was found almost as frequently (16/19 cells, 84%), even though in 10 cells (of 19, 53%) HCl evoked a greater response than that evoked by NaCl. Interestingly, when a mixture of a high concentration of NaCl (0.6 M) was mixed with a low concentration of HCl (0.001) M, mixture suppression was found in 16 cells (of 17, 94%), even though the low concentration of HCl produced no responses in 6 of these cells. Mixture suppression was somewhat less frequent (13/17 cells, 76%) when a high concentration of HCl (0.06 M) was mixed with a low concentration of NaCl (0.001 M), but also occurred in 3 cells when the low concentration of NaCl produced no response. In all, these data suggest that HCl had a more powerful suppressive effect on NaCl than vice versa.
Data analysis: temporal characteristics of taste responses
To assess the extent to which the temporal characteristics of taste responses can signal distinctions among taste qualities and intensities, metric space analyses (Victor and Purpura 1996, 1997) were applied to taste-evoked spike trains. Initially, the information contributed by spike count (firing rate; Hcount) and by spike count plus the temporal characteristics of the response (Hmax) was calculated for response intervals ranging from 100 ms to 5.0 s. (The difference between Hmax and Hcount is indicative of the relative contribution of the temporal characteristics of the response.) The results of these analyses are shown in Fig. 5. Each panel in this figure shows a different discrimination among stimuli: three concentrations each of NaCl and HCl (top), NaCl versus HCl collapsed across concentrations (middle), and three concentrations each of NaCl and HCl and five NaCl–HCl mixtures (bottom). There are two noteworthy points illustrated by this figure common to all three analyses. First, as expected, the information conveyed by both spike count and the temporal characteristics of the response is very small at short response intervals, but increases at longer response times. Second, the contribution of the temporal characteristics of response to the total amount of information conveyed by the response also increases as the response is elaborated over time; however, the relative contribution of the temporal characteristics of the response to the total information remains about the same. Additional analyses of temporal coding focused on the first 2 s of the response.
Fig. 5.
Mean ± SE information conveyed by spike count (firing rate, H0) and a combination of spike count and the temporal characteristics of response (Hmax) at various response intervals. These intervals were (in ms): 100, 125, 200, 250, 500, 1,000, 1,500, 2,000, and 5,000. Each panel in this figure shows a different discrimination among stimuli: 3 concentrations each of NaCl and HCl (top), NaCl vs. HCl collapsed across concentrations (middle), and 3 concentrations each of NaCl and HCl and 5 NaCl–HCl mixtures (bottom). Arrow at the bottom of each plot indicates 2.0 s, the response interval that was chosen for more detailed analyses of temporal coding.
In general, results of metric space analyses showed that, in cases where taste quality and intensity were confounded by spike count alone, consideration of the temporal characteristics of the responses significantly improved the ability to disentangle these two stimulus properties. That is, when two stimuli evoked similar spike counts over the 2.0-s response interval, information conveyed either by spike timing or by the rate envelope of response could be used to aid in the discrimination. Figure 6 illustrates the contribution of information conveyed by spike timing and spike count combined, in relation to that contributed by spike count alone. Responses to NaCl and HCl presented at various concentrations, as well as various mixtures of NaCl–HCl, were analyzed in different subsets of the data set. Each panel plots H0, the amount of information conveyed by spike count alone, against Hmax, the maximum information conveyed by both spike timing and spike count. The diagonal dashed line in each plot shows the condition where spike timing adds no information to that conveyed by spike count alone. Filled squares in each panel indicate cells for which spike timing contributes a significantly larger amount of information about stimulus identity than spike count alone. The top two panels show results of analyses that assessed how well cells could simultaneously discriminate both taste quality and concentration; the bottom two panels included the task of identifying taste mixtures in addition to concentration and quality. In general, when either three or five concentrations of NaCl and HCl were presented, roughly the same proportion of cells showed significantly improved stimulus identification when temporal characteristics were considered [15 of 42 cells (36%) for three concentrations each; 4 of 10 cells (40%) for five concentrations each]. When three binary mixtures were added (0.6 M NaCl–0.00 M HCl; 0.1 M NaCl–0.01 M HCl; 0.01 M NaCl–0.001 M HCl) to three concentrations of each taste quality, 10 of 19 cells tested (53%) showed a significant contribution of spike timing. When responses to all five NaCl–HCl mixtures in addition to three concentrations of each stimulus were analyzed, 9 of 17 cells (53%) showed a significant contribution of spike timing to the total amount of information. It should be noted that in all cases where Hmax was greater than H0, the combination of the temporal characteristics of the responses and their spike count was better at identifying stimuli than spike count alone, even when the contribution of spike timing was not statistically significant. In those cases, the rate envelope was more informative than the number of spikes.
Fig. 6.
Results of temporal coding analysis of responses to 3 or 5 concentrations each of NaCl and HCl, and for 3 concentrations each of NaCl and HCl and either 3 or 5 mixtures. Three mixtures were the High-, Med-, and Low-mix as defined in Fig. 2. Five mixtures were as defined in Fig. 2. Information conveyed by spike count (H0) is plotted against the maximum amount of information conveyed by spike count plus the temporal characteristics of the responses (Hmax). Dashed line in each graph shows the condition where H0 = Hmax. Filled squares indicate cells where Hmax > Hexchange + 2SD. Open squares indicate cells where Hmax ≤ Hexchange + 2SD.
To illustrate the contribution of temporal coding to the task of identification of NaCl versus HCl across concentrations, MDS analyses were performed using Dspike[q] as a measure of similarity. This metric is an index of how dissimilar two spike trains are in terms of both spike count and spike timing. The value of Dspike[q] at qmax was chosen for this analysis. A “taste space” was then constructed where proximity was based on similarity of the temporal characteristics of responses. Figures 7 and 8 show examples of this type of analysis in two cells, along with the results of metric space analyses. Figure 7 shows results from a cell tested with five concentrations each of NaCl and HCl and Fig. 8 shows results from a cell tested with three concentrations each of NaCl and HCl and five binary mixtures of NaCl and HCl. As a reference for the analysis of temporal pattern, MDS spaces based only on spike count are shown (Figs. 7A and 8A). Since spike count is a scalar quantity, these are one-dimensional spaces. Each symbol represents the spike count from a single response trial; asterisks represent the average spike count across all trials for each stimulus. For both cells, spike counts for different stimuli were nearly equal on many, if not most, occasions. Figures 7B and 8B show the three-dimensional MDS space based on similarity of temporal characteristics. In these panels, each stimulus occupies a “cloud,” or relatively circumscribed region of the space, distinct from other stimuli. This implies that the temporal characteristics of the responses, unlike spike count, convey information that can be used to disambiguate taste quality and intensity (concentration). In Figs. 7C and 8C, results of metric space analyses are shown. Each graph shows the amount of information conveyed by the temporal characteristics of the responses at various levels of temporal precision (q). The value of information at q = 0 (i.e., H0) represents the contribution of spike count alone. For each cell, the information conveyed by spike timing is higher than that contributed by spike count alone, as illustrated by the maximum value of H at a value of q higher than that at q = 0. For the cell shown in Fig. 7, spike timing offers a significant advantage over both spike count and the rate envelope in conveying information about stimulus identity. This is indicated by the fact that the information conveyed by the responses peaks at a value of q >0 and is greater than the information present in the Exchange or Poisson control analyses, which control for rate envelope. In Fig. 8C, in contrast, the additional information conveyed by spike timing is accounted for by the rate envelope; this is indicated by the peak value of H at a value of q >0, although this value of H is not significantly greater than the value obtained from the Exchange or Poisson control analyses.
Fig. 7.
Temporal coding analyses of one cell tested with 5 concentrations each of NaCl and HCl. For all plots, dots indicate the location of individual responses; asterisks indicate the centroid of the clusters of responses to a given taste stimulus. Axes are labeled in arbitrary units. Color coding of the stimuli is indicated in the top right of the figure. A: the one-dimensional response space created by multidimensional scaling (MDS) of the spike count distances, Dcount. B: the 3-dimensional response space created by the MDS of the spike time distances Dspike[qmax]. For this cell, qmax = 8 in B. C: analysis of temporal coding in the first 2 s of response using metric space analysis. Plot shows temporal precision, as measured by q (1/s) vs. information (H) in bits. For 10 stimuli, the maximum amount of information that can be conveyed is 3.32 bits; Hmax for this cell was 2.98. Black line: information conveyed by the neural response. Red line indicates the information conveyed by “shuffled” data sets, created by randomly assigning the observed responses to the various tastants. Blue line shows the information conveyed by “exchange” data sets, created by randomly exchanging spikes between individual responses to the same tastants so that the peristimulus time histograms (PSTHs) and the number of spikes elicited on each trial matched that of the original data. Pink line shows the information conveyed by “Poisson” data sets, created by random draws from the set of observed spike times for each stimulus; these surrogates matched the observed responses in rate envelope but not in the number of spikes elicited on each trial. The Poisson and exchange analyses identify the contribution of the firing rate envelope to the total amount of information conveyed by the responses. Results of these 2 analyses are nearly identical. For the surrogate data sets (shuffle, exchange, Poisson), the error bars represent SDs across 40 surrogates.
Fig. 8.
Temporal coding analyses of one cell tested with 3 concentrations each of NaCl and HCl and 5 mixtures. Results displayed as in Fig. 7. For this cell, qmax = 11.3 in C. For 11 stimuli, the maximum amount of information that can be conveyed is 3.46 bits; Hmax for this cell was 2.88.
In Figs. 7 and 8, the analyses were blind to the relationship between the stimuli—i.e., that they consisted of three different concentrations of two primary tastants. Nevertheless, the MDS plots reveal that stimuli of the same quality at different concentrations are located in the taste space in a logical arrangement with respect to each other. Additionally, responses to NaCl are segregated from responses to HCl, except at the lowest concentrations where psychophysical confusion might be expected. Thus it appears that the temporal structure of the response can signal tastant quality, despite large variations in concentration.
To quantify this, we applied the metric space analyses to these responses, collapsed across concentrations. That is, we asked whether the temporal features of the response could discriminate between the two tastants, in the face of wide variations in their intensities. Results are summarized in Fig. 9 (top), and further illustrated in Fig. 10, which shows an example of MDS plots in which the responses to NaCl and HCl at various concentrations are collapsed for each taste quality. To the left of the MDS plot is the mean (±SE) response for each stimulus. To the right, the MDS plot depicts responses to NaCl and HCl, arranged according to the similarity of their temporal characteristics such that responses that show similar temporal characteristics are placed close to each other. In this example, responses to NaCl and HCl occupy separate regions of the response space, even though the range of firing rates overlaps extensively. Again, this indicates that distinguishing between a response to NaCl and HCl can be carried out on the basis of the temporal characteristics of the response.
Fig. 9.
Results of temporal coding analysis of responses to NaCl vs. HCl, collapsed across concentrations (top) and for different concentrations of NaCl (middle) and HCl (bottom). Plots for 3 concentrations of each stimulus are shown on the left and plots using 5 concentrations of each stimulus are shown on the right. Information conveyed by spike count (H0) is plotted against the maximum amount of information conveyed by spike count plus the temporal characteristics of the responses (Hmax). Dashed line in each graph shows the condition where H0 = Hmax. Filled squares indicate cells where Hmax > Hexchange + 2SD. Open squares indicate cells where Hmax ≤ Hexchange + 2SD.
Fig. 10.
Comparison of responses to NaCl and HCl collapsed across concentrations in one cell. Left: mean response magnitude (sps) ± SE for all stimuli across trials. Right: the 3-dimensional response space created by MDS of the spike time distances Dspike[qmax]. Dots indicate the location of individual responses; asterisks indicate the centroid of the clusters of responses to a given taste stimulus. Axes are labeled in arbitrary units. Responses to NaCl are indicated in blue; responses to HCl are indicated in red. For this cell, qmax = 5.7.
We carried out a parallel analysis of discrimination of concentration, by considering, in separate analyses, the responses to each of the tastants at each of the three to five concentration levels tested. Results (Fig. 9, middle and bottom) show that the temporal characteristics of responses can also contribute information about stimulus intensity, above and beyond that contributed by spike count alone, in most cells. However, the contribution of temporal structure was less critical: there are more points closer to the diagonal, corresponding to cells in which temporal structure added nothing to the information that was already available from firing rate. Correspondingly, one can see from Fig. 7 that, as concentration varies, the locus of responses corresponding to a single tastant trace out an orderly path in the response space.
Collectively, results of the MDS analyses clearly showed that 1) firing rate was a poor indication of taste quality since changes in concentration produced equivalent spike counts in response to both NaCl and HCl and 2) the temporal features of responses to NaCl and HCl maintain a consistent difference across a range of concentrations and within NaCl–HCl mixtures. Figure 11 illustrates both the continuity and systematic change of different aspects of the temporal pattern of responses as concentration is varied and as NaCl and HCl are mixed in different ratios. It can be seen that NaCl and HCl both show persistent initial transient responses across concentrations, although the transient in HCl responses is briefer. Responses to both stimuli also show later, tonic components, and for both stimuli, the tonic component shows proportionately more change as the concentration varies. The result is a concentration-dependent change in the rate envelope for both taste stimuli. When NaCl and HCl are mixed, mixture suppression is apparent.
Fig. 11.
PSTHs of responses to 3 concentrations each of NaCl (left) and HCl (right) and various NaCl–HCl mixtures in one cell. Each PSTH shows the sum of 5 presentations of each stimulus. Time bin = 50 ms. Arrow under each plot indicated the onset of the stimulus presentation.
A summary of the results of metric space analyses across all cells is presented in Table 3. From these data it is evident that, on average, spike count generally contributed about half or less of the information necessary for perfect discrimination of stimuli in any of the analyses conducted. In contrast, the temporal characteristics of responses increased the information conveyed by the responses overall by about 50%. For both subsets of data (cells tested with three or five concentrations of NaCl and HCl) the temporal characteristics of responses had the greatest impact on information about taste quality regardless of intensity, nearly doubling the maximum information. With respect to concentration, temporal characteristics played a smaller role, especially for NaCl. Within a given cell, the degree of temporal precision that produced the maximum amount of information was generally higher when the task was more difficult (i.e., more stimuli to discriminate from each other). That is, in both subsets of data, the information used to discern NaCl from HCl, collapsed across concentrations, peaked at lower levels of q than when the task was to discern various concentrations of NaCl, HCl, and their mixtures, for example. This implies that the more demanding the discrimination among taste stimuli, the finer the level of temporal precision is necessary for maximum information.
Table 3.
Summary of metric space analyses, with respect to quality and intensity of NaCl and HCl concentrations
| Factor | qmax | H0 | Hmax | % incr. |
|---|---|---|---|---|
| Quality and intensity | ||||
| 3 concentrations each of NaCl and HCl (n = 42; max info = 2.58) | ||||
| Mean | 8.30 | 1.43 | 1.93 | 37 |
| SE | 1.13 | 0.04 | 0.05 | 3 |
| Median | 6.83 | 1.44 | 1.94 | 35 |
| 5 concentrations each of NaCl and HCl (n = 10; max info = 3.32) | ||||
| Mean | 6.13 | 1.72 | 2.43 | 43 |
| SE | 1.45 | 0.09 | 0.10 | 6 |
| Median | 4.83 | 1.75 | 2.39 | 38 |
| 3 concentrations each of NaCl and HCl plus 3 mixtures (n = 19; max info = 3.17) | ||||
| Mean | 6.78 | 1.72 | 2.43 | 43 |
| SE | 0.78 | 0.05 | 0.08 | 4 |
| Median | 5.66 | 1.71 | 2.25 | 33 |
| 3 concentrations each of NaCl and HCl plus 5 mixtures (n = 17; max info = 3.46) | ||||
| Mean | 6.58 | 1.75 | 2.18 | 36 |
| SE | 0.93 | 0.06 | 0.09 | 3 |
| Median | 5.66 | 1.77 | 2.33 | 31 |
| Quality | ||||
| 3 concentrations of NaCl vs. 3 concentrations of HCl (n = 42; max info = 1.00) | ||||
| Mean | 4.17 | 0.31 | 0.59 | 1,033 |
| SE | 1.34 | 0.04 | 0.03 | 625 |
| Median | 1.00 | 0.28 | 0.58 | 102 |
| 5 concentrations of NaCl vs. 5 concentrations of HCl (n = 10; max info = 1.00) | ||||
| Mean | 5.03 | 0.25 | 0.62 | 3,251 |
| SE | 1.48 | 0.08 | 0.08 | 2,796 |
| Median | 4.83 | 0.14 | 0.73 | 223 |
| Intensity | ||||
| 3 concentrations of NaCl (n = 42; max info = 1.58) | ||||
| Mean | 7.78 | 1.11 | 1.35 | 28 |
| SE | 2.64 | 0.05 | 0.04 | 4 |
| Median | 1.41 | 1.05 | 1.40 | 19 |
| 5 concentrations of NaCl (n = 10; max info = 2.32) | ||||
| Mean | 9.15 | 1.17 | 1.17 | 39 |
| SE | 4.11 | 0.10 | 0.07 | 9 |
| Median | 4.83 | 1.14 | 1.52 | 37 |
| 3 concentrations of HCl (n = 42; max info = 1.58) | ||||
| Mean | 8.33 | 0.70 | 1.10 | 131 |
| SE | 4.25 | 0.06 | 0.05 | 32 |
| Median | 2.83 | 0.66 | 1.13 | 59 |
| 5 concentrations of HCl (n = 10; max info = 2.32) | ||||
| Mean | 8.92 | 1.14 | 1.67 | 54 |
| SE | 2.69 | 0.11 | 0.11 | 11 |
| Median | 6.00 | 1.21 | 1.69 | 51 |
% incr. = (Hmax − H0)/H0, the percentage of the maximum information (max info) that is contributed by the temporal characteristics of the response.
Figure 12 shows the coding strategies that maximize information on a cell-by-cell basis. It is evident that for most cells, the rate envelope of the response conveys the most information about the taste stimuli tested, although in roughly one quarter to one third of the cells spike timing is more informative that either spike count or the rate envelope of the response. For the distinction among different concentrations of either NaCl or HCl, spike count was more often the coding strategy of choice for more cells than temporal coding for NaCl. However, the opposite was true for HCl. This result was not unexpected given that the intensity–response function for NaCl was steeper than that for HCl among the 42 cells presented with only three concentrations of each stimulus. Thus there were relatively large differences in response magnitude for NaCl across concentrations but small differences for different concentrations of HCl. In the group of 10 cells presented with five concentrations of NaCl and HCl, the intensity–response functions were parallel. Among those cells, where differences among responses to various concentrations of both NaCl and HCl were not that different (i.e., where spike count was similar), temporal coding enhanced the information conveyed by the responses. For those cells tested with different concentrations of NaCl and HCl as well as various mixtures, temporal coding always maximized the information conveyed.
Fig. 12.
Incidence of temporal coding by spike timing (blue squares), rate envelope (yellow squares), and spike count (pink squares). Gray squares indicate that no data are available. Numbers indicate the value of q at Hmax for cells that show a significant contribution of temporal coding. Top set of rows: cells tested with 3 concentrations each of NaCl and HCl and their mixtures. Bottom set of rows: cells tested with 5 concentrations each of NaCl and HCl.
For those cells that used spike count alone to signal differences in stimulus concentration, the amount of information conveyed by spike count was nearly the amount needed to convey a perfect discrimination. (In these cells, it is possible that we were unable to observe a contribution of temporal coding because of a ceiling effect and one might have emerged had additional concentrations been tested.) Specifically, to discriminate among three concentrations of a single stimulus, 1.58 bits of information are needed. Among those cells that used spike count alone to convey information, the mean amount of information was 1.48 ± 0.03 bits (1.52 ± 0.03 bits for NaCl, n = 12; 1.36 ± 0.09 bits for HCl, n = 4). These values were significantly larger (Student's t-test, P < 0.01) than those for cells that used temporal characteristics to convey information about intensity. The average maximum information conveyed among cells that showed a contribution of temporal coding (either spike timing or rate envelope) was 1.16 ± 0.09 bits (1.28 ± 0.04 bits for NaCl, n = 30; 1.07 ± 0.05 bits for HCl, n = 38). Interestingly, there were no cells that used spike count to distinguish among concentrations of both NaCl and HCl.
DISCUSSION
The most significant finding of the present study is that single cells in the NTS can use the temporal characteristics of responses to taste stimuli to simultaneously convey information about taste quality and intensity (concentration). Moreover, data show that the temporal signature of taste quality is preserved when the stimulus is presented at different intensities and as part of a mixture of different taste qualities at different intensities. In the context of previous work (Di Lorenzo and Victor 2003; Di Lorenzo et al. 2009; Roussin et al. 2008), these results confirm that the taste system relies on the temporal structure of a response to convey information when the task is more difficult, that is, whenever two tastants evoke similar spike counts. Thus as tastants are simultaneously varied along several dimensions, e.g., quality (Roussin et al. 2008), number of component qualities (Di Lorenzo et al. 2009; present study), and intensity (present study), an increasing proportion of taste-responsive cells rely on the temporal characteristics of responses to convey information. Furthermore, evidence suggests that resolving difficult distinctions among taste stimuli uses finer levels of temporal precision.
Sensory systems have evolved a number of strategies to resolve the confusion between stimulus identity and intensity. One strategy might be to segregate coding of identity and intensity so that different subsets of cells would encode each aspect of the stimulus. However, this would require that tuning is an all-or-none situation, which is rarely encountered. A possible exception is the peripheral taste system in which it is thought that individual taste qualities are encoded by nonoverlapping sets of receptor cells (Yarmolinsky et al. 2009). Another strategy might be to encode stimulus identity in very narrowly tuned cells that simply fire more rapidly as stimulus intensity increases. This type of scheme can be seen in the inferior colliculus (Ehret and Merzenich 1985, 1988). Still another strategy is that of encoding stimulus intensity in ensemble activity rather than in individual cells. This mechanism has been observed in the auditory (Chatterjee and Zwislocki 1998), somatosensory (Bensmaia 2008), and olfactory (Bathellier et al. 2008; Stopfer et al. 2003) systems. In the taste system, as is often the case in sensory systems, many neurons have broad tuning across the sensory domain. The present data show that individual taste cells are capable of multiplexing information about changes in stimulus identity and intensity through the temporal characteristics of their responses.
Response interval
Much of the analyses of taste responses in the present study examined the first 2.0 s of response, even though it has been shown that rats can identify taste quality in much shorter intervals. Specifically, evidence shows that rats can decide whether to continue licking a conditioned taste stimulus after only a single lick, i.e., within 200 ms (Gutierrez et al. 2010; Halpern and Tapper 1971). Consistent with these behavioral data, Stapleton et al. (2001) showed that taste responses in the gustatory cortex are present within 100 ms of stimulus presentation in awake rats. In the present study, we showed that, across cells, the amount of information conveyed in this early interval was quite small. That is, the amount of information conveyed by a single cell at this response interval was not nearly enough to discriminate either taste quality or stimulus concentration. It is reasonable to suppose that, at these short response intervals, information from multiple cells is required to accomplish these discriminations. However, it should be noted that there is no evidence at this time that a taste stimulus can be identified within 200 ms in the face of the confounding effects of large changes in concentration and/or mixtures. As the response unfolded over time, the total amount of information conveyed by a response from a single cell increased and the relative contribution of the temporal characteristics of the response remained about the same. This implies that individual cells may become more competent over time in making fine distinctions among taste stimuli with similar characteristics.
Taste coding based on spike count
Classically, an increase in the intensity of a sensory stimulus produces an increase in the firing rate of a sensitive neuron. When cells respond to a single stimulus, such as a taste quality, spike count might provide a good description of stimulus intensity. However, considering the substantial amount of trial-to-trial variability in response magnitude seen here and in previous studies (Di Lorenzo and Victor 2003; Di Lorenzo et al. 2009), discriminating among different stimulus intensities, especially those that evoke similar response magnitudes can be near impossible. In spite of the problems associated with neural encoding of taste stimulus intensity, behavioral acuity for discrimination among different concentrations of taste stimuli in rats is impressive. For example, Scott and Giza (1987) have shown that rats can discriminate differences of as little as 0.029 M NaCl and 0.0009 M of HCl. Such small differences would certainly result in very small, in some cases barely perceptible, differences in evoked firing rate.
In the taste system, most cells respond to more than one stimulus and responses to different stimuli can evoke nearly equal response rates depending on the particular concentrations tested. In the present study, for example, many cells responded nearly equally well to subsets of stimulus intensities such that at some points in their intensity–response (I–R) functions, a change in concentration was, on average, imperceptible based on firing rate. For nearly all cells, the I–R functions produced by NaCl and HCl overlapped at some point, making the identification of taste quality based on spike count nearly impossible.
Because different cells show different I–R functions, stronger stimuli often excite more cells than weaker ones. In that case, the number of responsive cells can signal stimulus intensity. In the present study, both NaCl and HCl generally evoked a monotonically increasing function as stimulus intensity increased, but individual cells showed idiosyncratic I–R functions. The low concentrations of both NaCl and HCl tested in the majority of these cells evoked only minimal firing, although >60% showed a significant response compared with baseline firing rates. Thus in the present study, and in other studies of taste responses in the rodent CNS (e.g., Di Lorenzo et al. 1994), the majority of neurons respond at very low concentrations of tastes, so the number of responsive cells does not provide much information about intensity.
Taste coding based on the temporal characteristics of responses
In the majority of cells, the amount of information conveyed by spike timing exceeded that for spike count for all stimulus comparisons. For example, when the comparison was taste quality (i.e., salty vs. sour), regardless of intensity, 48 cells (of 52, 92%) conveyed more information through the temporal characteristics of their responses than by spike count; only 4 cells relied on spike count. In all 4 of these cells, the responses to the highest concentration of NaCl were much stronger than the responses to all other stimuli and in 2 of these the responses to both the highest (0.6 M) and medium (0.1 M) concentrations of NaCl were larger than responses to all other tastants. In their study of the gustatory cortex in awake rats, Stapleton et al. (2006) reported that the majority of cortical neurons responded to changes in stimulus concentration with nonmonotonic I–R functions. They suggested that coding taste intensity might use both changes in firing rate and the temporal dynamics of the response. Present data are entirely consistent with that idea.
Although one might predict that changes in stimulus intensity would naturally be encoded by spike count, with more intense stimuli evoking more spikes, this was true of less than half of the sample of cells [12 of 42 (29%) for NaCl; 4 of 42 (10%) for HCl] that were tested with three concentrations of each stimulus. On the other hand, spike timing was better than either the rate envelope or spike count in more cells for both taste qualities tested [14 of 42 (33%) for NaCl; 11 of 42 (26%) for HCl]. When more points were sampled on the I–R function, resulting in responses that were more similar in terms of response magnitude, spike timing as a mechanism for conveying information was much more prevalent [7 of 10 cells (70%) for both NaCl and HCl]. Moreover, when different concentrations as well as mixtures were presented, every cell evidenced some form of temporal coding, either through the rate envelope or spike timing.
Collectively, these results support the idea that when stimuli evoke large differences in firing rate, spike count conveys more information than the temporal characteristics of the response, but when stimuli evoke nearly equal firing rates, the temporal dynamics of the response convey more information than spike count. Furthermore, these data underscore the idea that responses to different taste qualities evoke responses with distinctive temporal characteristics that are retained across changes in stimulus intensity. An important additional observation is that the components of binary mixtures of NaCl and HCl could be identified by the temporal characteristics of responses, even when their concentrations were varied (see Figs. 8 and 12). This result extends our previous study showing that the components of binary mixtures of tastants could be disambiguated by the temporal characteristics of their responses, especially in the most broadly tuned cells (Di Lorenzo et al. 2009). Interestingly, as in the previous study, neural coding by both firing rate and temporal characteristics of response sometimes showed evidence of mixture suppression and in some cases mixture enhancement. The addition of data using a range of concentrations shows that these phenomena are not simply an effect of intensity alone, but rather a true interaction of the taste qualities tested.
Caveats and conclusions
Undoubtedly, our sample of taste-responsive NTS cells was biased toward broadly tuned cells, a fact that certainly affected our results. Since we focused our investigation on cells that responded to both NaCl and HCl, we obviously excluded cells that responded specifically to either tastant or to sucrose or quinine for that matter. The mean Uncertainty measure of our sample was slightly higher than that reported in other studies from our lab: 0.81 ± 0.02 in the present study versus 0.76 ± 0.02 in Di Lorenzo et al. (2009), respectively. However, tastant-specific cells are rare in the rat NTS, generally comprising <10% of the population (Chen and Di Lorenzo 2008). Thus our results, although not comprehensive, do characterize 90% of the taste-responsive cells in the NTS.
The observation that single cells can convey information about both taste quality and intensity does not preclude the possibility, perhaps even the likelihood, that a population (across neuron pattern) code is used to encode these characteristics. On the contrary, the existence of consistent temporal profiles of response among the responsive neurons for a given taste stimulus enhances the uniqueness of the across-neuron pattern of response by adding a dynamic dimension. Thus the spatial pattern produced by a tastant is sculpted as the response unfolds over time. This type of mechanism may enable multiplexing of different aspects of the taste stimulus such as quality and hedonic value. In addition, consistent temporal patterns of response for different stimuli increase the likelihood of synchrony among responsive neurons, a mechanism that has been shown to contribute to taste coding in the NTS (Adachi et al. 1989), PbN (Yamada et al. 1990), and the gustatory cortex (Katz et al. 2002; Stapleton et al. 2007).
GRANTS
This work was supported by National Institutes of Health Grants R01-DC-006914 to P. Di Lorenzo and R01-MH-68012 to D. Gardner.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Present address for J.-Y. Chen: University of California, Riverside, Department of Cell Biology and Neuroscience, Riverside, CA 92521.
REFERENCES
- Adachi M, Ohshima T, Yamada S, Satoh T. Cross-correlation analysis of taste neuron pairs in rat solitary tract nucleus. J Neurophysiol 62: 501–509, 1989 [DOI] [PubMed] [Google Scholar]
- Aronov D, Victor JD. Non-Euclidean properties of spike train metric spaces. Phys Rev E Stat Nonlin Soft Matter Phys 69: 061905, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathellier B, Derek L, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron 57: 586–598, 2008 [DOI] [PubMed] [Google Scholar]
- Bensmaia SJ. Tactile intensity and population codes. Behav Brain Res 190: 165–173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Zwislocki JJ. Cochlear mechanisms of frequency and intensity coding. II. Dynamic range and the code for loudness. Hear Res 124: 170–181, 1998 [DOI] [PubMed] [Google Scholar]
- Chen JY, Di Lorenzo PM. Responses to binary taste mixtures in the nucleus of the solitary tract: neural coding with firing rate. J Neurophysiol 99: 2144–2157, 2008 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Chen JY, Victor JD. Quality time: representation of a multidimensional sensory domain through temporal coding. J Neurosci 29: 9227–9238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM, Monroe S, Hecht GS. Information processing in the parabrachial nucleus of the pons. In: Olfaction and Taste XI, edited by Kurihara K, Suzuki N, Ogawa H. New York: Springer-Verlag, 1994, p. 402–404 [Google Scholar]
- Di Lorenzo PM, Victor JD. Taste response variability and temporal coding in the nucleus of the solitary tract of the rat. J Neurophysiol 90: 1418–1431, 2003 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Victor JD. Neural coding mechanisms for flow rate in taste-responsive cells in the nucleus of the solitary tract of the rat. J Neurophysiol 97: 1857–1861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G, Merzenich MM. Auditory midbrain responses parallel spectral integration phenomena. Science 227: 1245–1247, 1985 [DOI] [PubMed] [Google Scholar]
- Ehret G, Merzenich MM. Complex sound analysis (frequency resolution, filtering and spectral integration) by single units in the inferior colliculus of the cat. Brain Res Rev 13: 139–163, 1988 [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Simon SA, Nicolelis MAL. Licking-induced synchrony in the taste–reward circuit improves cue discrimination during learning. J Neurosci 30: 287–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern BP, Tapper DN. Taste stimuli: quality coding time. Science 171: 1256–1258, 1971 [DOI] [PubMed] [Google Scholar]
- Katz DB, Simon SA, Nicolelis MA. Taste-specific neuronal ensembles in the gustatory cortex of awake rats. J Neurosci 22: 1850–1857, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal JB, Wish M. Multidimensional Scaling. Beverly Hills, CA: Sage, 1978 [Google Scholar]
- Ogawa H, Yamashita S, Sato M. Variation in gustatory nerve fiber discharge pattern with change in stimulus concentration and quality. J Neurophysiol 37: 443–457, 1974 [DOI] [PubMed] [Google Scholar]
- Roussin AT, Victor JD, Chen JY, Di Lorenzo PM. Variability in responses and temporal coding of tastants of similar quality in the nucleus of the solitary tract of the rat. J Neurophysiol 99: 644–655, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TR, Giza BK. A measure of taste intensity discrimination in the rat through conditioned taste aversions. Physiol Behav 41: 315–320, 1987 [DOI] [PubMed] [Google Scholar]
- Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Senses 4: 215–229, 1979 [Google Scholar]
- Stapleton JR, Lavine ML, Nicolelis MA, Simon SA. Ensembles of gustatory cortical neurons anticipate and discriminate between tastants in a single lick. Front Neurosci 1: 161–174, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JR, Lavine ML, Wolpert RL, Nicolelis MA, Simon SA. Rapid taste responses in the gustatory cortex during licking. J Neurosci 26: 4126–4138, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John SJ, Smith DV. Neural representation of salts in the rat solitary nucleus: brain stem correlates of taste discrimination. J Neurophysiol 84: 628–638, 2000 [DOI] [PubMed] [Google Scholar]
- Stopfer M, Vivek Jayaraman V, Laurent G. Intensity versus identity coding in an olfactory system. Neuron 39: 991–1004, 2003 [DOI] [PubMed] [Google Scholar]
- Travers JB, Travers SP, Norgren R. Gustatory neural processing in the hindbrain. Annu Rev Neurosci 10: 595–632, 1987 [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Giza BK, Scott TR. Responses to taste stimulation in the ventroposteromedial nucleus of the thalamus in rats. J Neurophysiol 89: 265–275, 2003 [DOI] [PubMed] [Google Scholar]
- Victor JD. Spike train metrics. Curr Opin Neurobiol 15: 585–592, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor JD, Purpura KP. Nature and precision of temporal coding in visual cortex: a metric-space analysis. J Neurophysiol 76: 1310–1326, 1996 [DOI] [PubMed] [Google Scholar]
- Victor JD, Purpura KP. Metric-space analysis of spike trains: theory, algorithms and application. Network 8: 127–164, 1997 [Google Scholar]
- White MP, Fisher LJ. Degree of light damage to the retina varies with time of day of bright light exposure. Physiol Behav 39: 607–613, 1987 [DOI] [PubMed] [Google Scholar]
- Yamada S, Ohshima T, Oda H, Adachi M, Satoh T. Synchronized discharge of taste neurons recorded simultaneously in rat parabrachial nucleus. J Neurophysiol 63: 294–302, 1990 [DOI] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell 139: 234–244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Manchaiah VK, French D, Price SM. Music exposure and hearing disorders: an overview. Int J Audiol 49: 54–64, 2010 [DOI] [PubMed] [Google Scholar]