Abstract
Immediately after spinal cord injury (SCI), a devastating paralysis results from the loss of brain stem and cortical innervation of spinal neurons that control movement, including a loss of serotonergic (5-HT) innervation of motoneurons. Over time, motoneurons recover from denervation and function autonomously, exhibiting large persistent calcium currents (Ca PICs) that both help with functional recovery and contribute to uncontrolled muscle spasms. Here we systematically evaluated which 5-HT receptor subtypes influence PICs and spasms after injury. Spasms were quantified by recording the long-lasting reflexes (LLRs) on ventral roots in response to dorsal root stimulation, in the chronic spinal rat, in vitro. Ca PICs were quantified by intracellular recording in synaptically isolated motoneurons. Application of agonists selective to 5-HT2B and 5-HT2C receptors (including BW723C86) significantly increased the LLRs and associated Ca PICs, whereas application of agonists to 5-HT1, 5-HT2A, 5-HT3, or 5-HT4/5/6/7 receptors (e.g., 8-OH-DPAT) did not. The 5-HT2 receptor agonist–induced increases in LLRs were dose dependent, with doses for 50% effects (EC50) highly correlated with published doses for agonist receptor binding (Ki) at 5-HT2B and 5-HT2C receptors. Application of selective antagonists to 5-HT2B (e.g., RS127445) and 5-HT2C (SB242084) receptors inhibited the agonist-induced increase in LLR. However, antagonists that are known to specifically be neutral antagonists at 5-HT2B/C receptors (e.g., RS127445) had no effect when given by themselves, indicating that these receptors were not activated by residual 5-HT in the spinal cord. In contrast, inverse agonists (such as SB206553) that block constitutive activity at 5-HT2B or 5-HT2C receptors markedly reduced the LLRs, indicating the presence of constitutive activity in these receptors. 5-HT2B or 5-HT2C receptors were confirmed to be on motoneurons by immunolabeling. In summary, 5-HT2B and 5-HT2C receptors on motoneurons become constitutively active after injury and ultimately contribute to recovery of motoneuron function and emergence of spasms.
INTRODUCTION
After spinal cord injury (SCI), debilitating spasms often develop in the muscles innervated from the spinal cord below the injury, and this is the hallmark of the general spasticity syndrome after SCI (Bennett et al. 2004; Gorassini et al. 2004; Kawamura et al. 1989; Kuhn and Macht 1948). Spasms involve simultaneous contractions of many muscles, lasting anywhere from seconds to minutes, and are triggered by brief innocuous sensory stimuli, such as cutaneous inputs. Upward of 80% of spinal cord–injured humans have spasms and general spasticity that can disrupt residual motor function, cause debilitating pain, and interrupt sleep (Maynard et al. 1990). Treatment of spasms with conventional antispastic drugs such as baclofen is often not adequate or not tolerated because of adverse side effects such as lethargy and weakness (Dario and Tomei 2004). Here we explore whether activity in one or more of the many 5-HT receptor subtypes facilitates spasms and examine whether selectively blocking these receptors may serve as a target for novel antispastic treatment.
The neuronal mechanisms underlying spasms have recently been identified in a rat model of SCI (Bennett et al. 2004; Harvey et al. 2006b; Li et al. 2004a; Murray et al. 2010), and similar mechanisms have been shown to underlie spasms in humans (Gorassini et al. 2004; Norton et al. 2008). Briefly, these studies have shown that, immediately after SCI, motoneuron excitability plummets; in particular, there is a loss of low voltage-activated persistent calcium and sodium currents (persistent inward currents; Ca and Na PICs) that are normally crucial for enabling motoneurons to produce sustained motoneuron discharges in response to synaptic inputs (Heckman et al. 2005; Hultborn et al. 2004; Li et al. 2004a). Many motoneurons are acutely so impaired that they can only fire transiently and can no longer follow synaptic inputs with appropriate firing (Harvey et al. 2006c). However, over the months after injury (chronic injury), PICs spontaneously return, attaining high levels likely only seen in alert awake animals (Button et al. 2008; Harvey et al. 2006c; Hultborn et al. 2004; Li et al. 2004b). Theses PICs are crucial for the recovery of motoneuron firing ability, enabling motoneurons to produce sustained firing and associated muscle contractions in response to transient inputs (Li et al. 2004a). However, these PICs are permanently facilitated and, whenever these voltage-dependent currents are activated by a brief synaptic depolarization, they are difficult to terminate because of the loss of normal descending control of inhibitory inputs to motoneurons with SCI (Holstege and Bongers 1991; Jankowska and Hammar 2002; Nielsen et al. 2007; Rekling et al. 2000). Thus, activity in PICs causes uncontrolled motoneuron firing and associated muscle spasms (Bennett et al. 2004; Li et al. 2004a). To make matters worse, after SCI, there is a general loss of inhibition over sensory afferent transmission (disinhibition) (Hochman et al. 2001; Lundberg 1982; Schmidt and Jordan 2000), leading to unusually large and prolonged polysynaptic excitatory postsynaptic potentials (EPSPs) on motoneurons (Baker and Chandler 1987; Bennett et al. 2004; Li et al. 2004a). These EPSPs readily trigger the large PICs in chronic SCI, which in turn produce many seconds of uncontrolled motoneuron firing. Thus spasms are readily triggered by transient sensory stimulation (Bennett et al. 2004).
Brain stem–derived serotonin (5-HT) plays a critical role in regulating normal levels of spinal cord excitability by several receptors (Hochman et al. 2001; Schmidt and Jordan 2000), including facilitating Ca PICs on motoneurons by activating 5-HT2 receptors (Harvey et al. 2006a; Heckman et al. 2005; Perrier and Delgado-Lezama 2005) and by inhibiting sensory afferent transmission by activating 5-HT1 receptors (Hochman et al. 2001; Millan 2002; Schmidt and Jordan 2000; Yoshimura and Furue 2006). Thus, the immediate loss of motoneuron excitability and emergence of large EPSPs acutely after spinal transection is partly explained by a loss of 5-HT. The spontaneous recovery of motoneuron excitability (e.g., PICs) over the months after injury (chronic spinal rat) is more difficult to explain, but recently Harvey et al. (2006b) suggested that it involves re-activation of 5-HT2 receptors. Furthermore, Murray et al. (2010) have recently shown that this activation of the 5-HT2 receptors occurs in the absence of 5-HT after injury; that is, the receptors become constitutively active, as described in other systems (Aloyo et al. 2009; Seifert and Wenzel-Seifert 2002). These two studies do not clearly define which specific 5-HT2 receptor is involved, although both suggest it could be the 5-HT2C receptor, and Harvey (2006b) speculated that 5-HT2A receptors may be involved as well. Little is known about the role of other 5-HT receptors in motoneuron function and spasms after injury. Furthermore, because mostly nonselective pharmacological agents (e.g., 5-HT2A/2B/2C receptor agonist DOI) have been used to study 5-HT modulation of normal motoneuron function, it even remains unclear which receptors are specifically involved in normal animals, although these seem to include the Gq-protein coupled 5-HT2A, 5-HT2B, and 5-HT2C receptors (Elliott and Wallis 1992; Harvey et al. 2006a; Holohean and Hackman 2004; Perrier and Hounsgaard 2003; Wang and Dun 1990), Gi coupled 5-HT1A receptors (Holohean et al. 1990; Perrier et al. 2003; Wang and Dun 1990), Gs-coupled 5-HT receptors like 5-HT7 receptors that increase cAMP (Inoue et al. 2002; Larkman and Kelly 1997), and possibly ionotropic 5-HT3 receptors (Ziskind-Conhaim et al. 1993).
In this study, we use numerous 5-HT agonists and highly selective antagonists to determine which receptors facilitate motoneuron function (Ca PICs) and associated long-lasting reflexes (spasms) after SCI, by correlating agonist potency at facilitating the spasms with the published binding affinity of these agonists to all 5-HT receptor types (Barnes and Sharp 1999; Boess and Martin 1994). We determine whether spontaneous activity in these receptors contributes to recovery of PICs and emergence of spasms and examine whether this activity results from either residual 5-HT in the caudal spinal cord or constitutive receptor activity (Murray et al. 2010; Seifert and Wenzel-Seifert 2002).
METHODS
Recordings were made from motoneurons and associated ventral roots of the sacrocaudal spinal cord of spastic adult rats with chronic SCI (3.5–5 mo old) (Bennett et al. 2004). Adult female rats were transected at the S2 sacral level at about 2 mo of age (adult rat), and recordings were made after their affected muscles became spastic (1.5–3 mo after injury), as detailed previously (Bennett et al. 1999, 2004). All recordings were made from the whole sacrocaudal spinal cord that was removed from the rat with an S2 sacral transection and maintained in vitro (Li et al. 2004b). This transection was made just rostral to the chronic spinal injury, to not further damage the sacrocaudal cord. All experimental procedures were approved by the University of Alberta Animal Care and Use Committee: Health Sciences.
In vitro preparation
Details of the in vitro experimental procedures have been described in previous publications (Harvey et al. 2006c; Li et al. 2004a,b; Murray et al. 2010). Briefly, all the rats were anesthetized with urethane (0.18 g/100 g; with a maximum dose of 0.45 g), and the sacrocaudal spinal cord was removed and transferred to a dissection chamber containing modified artificial cerebrospinal fluid (mACSF). Spinal roots were removed, except the sacral S4 and caudal Ca1 ventral roots and the Ca1 dorsal roots. After 1.5 h in the dissection chamber (at 20°C), the cord was transferred to a recording chamber containing normal ACSF (nACSF) maintained near 24°C, with a flow rate >5 ml/min. A 1-h period in nACSF was given to wash out the residual anesthetic and mACSF before recording, at which time the nACSF was recycled in a closed system with a peristaltic pump (Harvey et al. 2006b).
Ventral root reflex recording and averaging
Dorsal and ventral roots were mounted on silver-silver-chloride wires above the nASCF of the recording chamber and covered with a 1:1 mixture of petroleum jelly and mineral oil (as for intracellular recording) for monopolar stimulation and recording (Li et al. 2004b). We evoked ventral root reflexes with a low-threshold Ca1 dorsal root stimulation (single pulse, 0.1 ms, 0.02 mA, corresponding to 3x afferent threshold, 3×T; afferent and reflex threshold are similar; Bennett et al. 2004) using a constant current stimulator (Isoflex, Israel). This stimulation intensity (3×T) is compatible with activation of low-threshold group I and II (Aβ) afferents. Because the Ca1 dorsal root innervates the distal third of the tail, which lacks large muscles (Bennett et al. 2004), this stimulation activates largely cutaneous or joint afferents. However, there are small intrinsic muscles in the tail with group Ia and II muscle afferents (Steg 1964), and thus to a lesser extent, muscle afferents may be activated. The stimulation was repeated five times at 10-s intervals for each trial. The ventral root recordings were amplified (×2,000), high-pass filtered at 100 Hz, low-pass filtered at 3 kHz, and recorded with a data-acquisition system sampling at 6.7 kHz (Axonscope 8, Axon Instruments). Ventral root reflexes were quantified using custom written software (Matlab, MathWorks, Natick, MA). That is, data were high pass filtered at 800 Hz and rectified to allow averaging (Fig. 1A, bottom). We quantified the long-latency, long-lasting reflex (LLR) by averaging the rectified response 500–4,000 ms after stimulus, a period where the response is mainly determined by the motoneuron Ca PIC activity and not by stimulus evoked synaptic inputs, which end by about 500 ms. Average ventral root activity was averaged for all five stimuli in a trial. This recording procedure was repeated at 12-min intervals, and 5-HT receptor agonists were added immediately after each recording, giving them time to fully act by the next recording session. Cumulative dose–response relations were computed by increasing agonist doses at these 12-min intervals (0.003, 0.01, 0.03. 0.1,…, 30 μM doses used). Antagonists took longer to act, and responses reached near steady state typically >20 min after application, at which time responses were averaged. The effect of agonists on the reflexes were reversible on washout of the agonist, but full recovery to baseline only occurred after several hours, likely because of the large size of the whole cord preparation. Thus washout of agonists was not feasible between doses of the agonists used in the construction of dose–response relations.
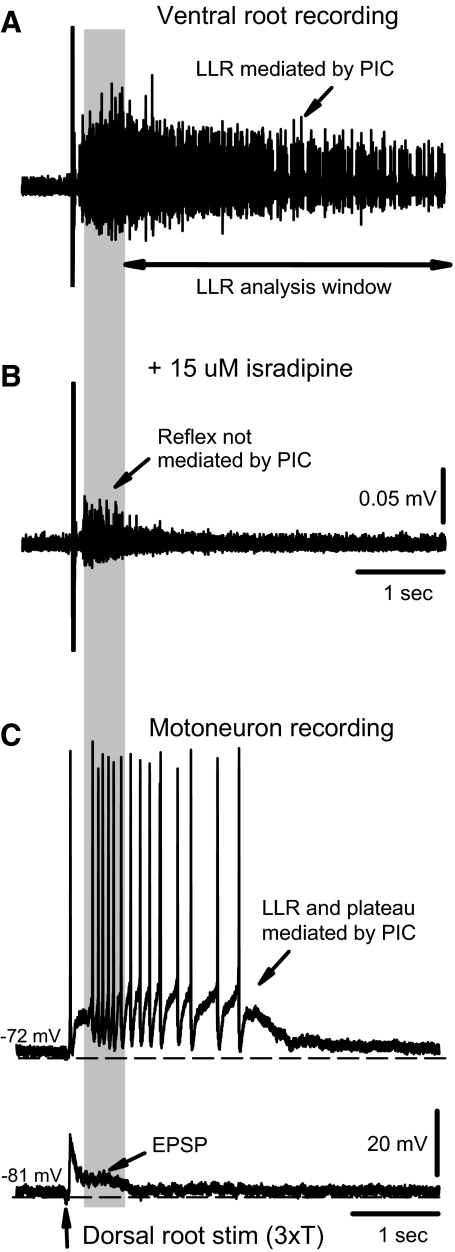
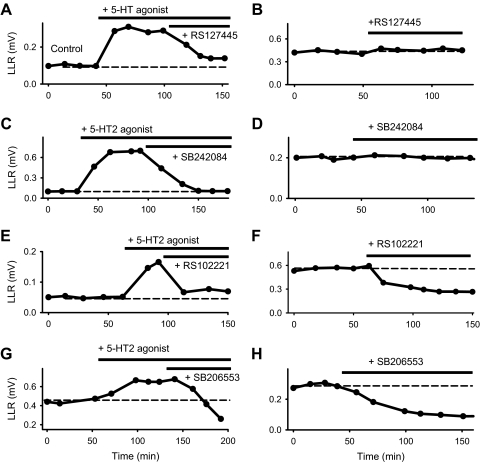
Fig. 1.
Long-lasting reflexes (LLRs), the counterpart of muscle spasms, are mediated by the persistent calcium currents (Ca PICs) in motoneurons in chronic spinal rats. A: LLR triggered by dorsal root stimulation (0.1 ms pulse, 3xT) and recorded from the ventral roots (top). LLR quantified by rectifying the ventral root activity (bottom) and averaging over window indicated. B: elimination of LLR after blocking the L-type Ca2+ channel with isradipine (15 μM). C: PIC-mediated plateau potential and sustained firing (LLR) evoked by dorsal root stimulation (3xT) in motoneuron at rest (without injected current; top). With a hyperpolarizing bias current to prevent PIC activation, the same stimulation only evoked polysynaptic excitatory postsynaptic potentials (EPSPs) that ended within 0.5 s (in gray shaded region), with no synaptic activity during the subsequent period where the LLR was computed (bottom).
Intracellular recording
Sharp intracellular electrodes were made from glass capillary tubes (1.5 mm OD, Warner GC 150F-10) using a Sutter P-87 micropipette puller and filled with a combination of 1 M potassium acetate and 1 M KCl. Electrodes were beveled down from an initial resistance of 40–80 to 26–32 MΩ using a rotary beveller (Sutter BV-10). A stepper-motor micromanipulator (660, Kopf) was used to advance the electrodes through the ventral cord surface into motoneurons. Large 30-μm forward and backward steps were initially required to breach the white matter (about 100 μm thick). Multiple tracks were made in the gray matter with 2-μm steps, without coming completely out of the white matter, by moving the electrode sideways in the white matter before each new tract (to further weaken pia and improve stability). Penetrations were made with capacitance-over-compensation ringing. After penetration, motoneuron identification was made with antidromic ventral root stimulation, and noting ventral horn location, input resistance and time constant (>6 ms for motoneurons) (Li and Bennett 2007; Li et al. 2007). Cells were always held below rest (< −70 mV) with a small continuous bias current to assure that the Ca PICs were not even partly activated before testing, because the Ca PIC slowly deactivates if it is activated for a few minutes and does not recover from this deactivation for many minutes (unpublished data). Data were collected with an Axoclamp 2b intracellular amplifier (Axon Instruments, Burlingame, CA) running in discontinuous current clamp (DCC, switching rate 4–6 kHz, output bandwidth 3.0 kHz, sample rate of 6.7 kHz) or discontinuous single-electrode voltage clamp (SEVC; gain, 0.8–2.5 nA/mV) modes.
Slow triangular voltage ramps (3.5 mV/s voltage clamp) were applied to the motoneurons to measure their electrical properties, as detailed previously (Harvey et al. 2006c; Li et al. 2004a). The input resistance (Rm) was measured during the voltage ramps over a 5-mV range near rest and subthreshold to PIC onset. Resting potential (Vm) was recorded with 0-nA bias current, after the cell had been given about 15 min to stabilize after penetration. The slow triangular voltage ramps were used to directly measure the PICs as follows (shown in Fig. 5A). During the upward portion of this ramp, the current response initially increased linearly with voltage in response to the passive leak conductance. A linear relation was fit in the region just below the PIC onset (5 mV below) and extrapolated to the whole range of the ramp (leak current; thin line in Fig. 5A). At depolarized potentials above the PIC onset threshold, there was a downward deviation from the extrapolated leak current, and the PIC was estimated as the difference between the leak current and the total current (initial peak current at arrow in Fig. 5A; leak-subtracted current). The onset voltage for the PIC (Von) was defined as the voltage at which the slope of the current response initially reached zero (Li and Bennett 2003). Finally, the excitatory postsynaptic potential (EPSP) and associated reflexes were measured in motoneurons by stimulating the Ca1 dorsal roots (at 3xT, as in ventral root reflex recording) while applying hyperpolarizing bias currents to block the PICs (Fig. 1C, bottom).
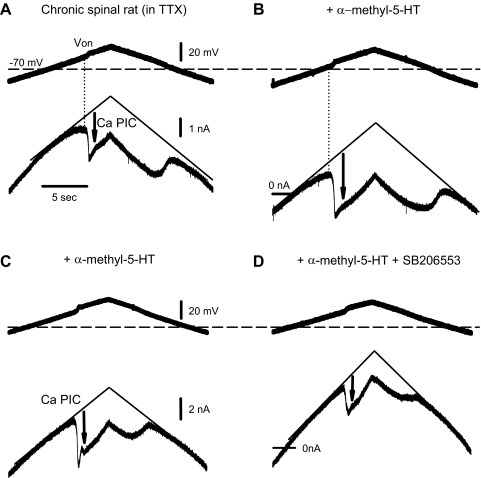
Fig. 5.
5-HT2B and 5-HT2C receptors facilitate the motoneuronal Ca PIC, as they do the LLR. A: Ca PIC in motoneuron of chronic spinal rat, activated by slowly increasing the membrane potential under voltage clamp, and quantified at its initial peak, where it produced a downward deflection in the recorded current (at arrow) relative to the leak current (thin line). Von represents the voltage at which the Ca PIC was activated. Horizontal dashed line indicates resting potential. Recording in presence of TTX (2 μM) to block the Na PIC and synaptic input. B: the 5-HT2 receptor agonist α-methyl-5-HT (0.1 μM) increased the Ca PIC and reduced Von. C and D: in another motoneuron, the selective 5-HT2B/2C antagonist SB206553 (5 μM) reduced the agonist induced increase in the Ca PIC.
Drugs and solutions
Two kinds of ACSF were used in these experiments: mACSF in the dissection chamber before recording and nACSF in the recording chamber. The mACSF was composed of (in mM) 118 NaCl, 24 NaHCO3, 1.5 CaCl2, 3 KCl, 5 MgCl2, 1.4 NaH2PO4, 1.3 MgSO4, 25 d-glucose, and 1 kynurenic acid. nACSF was composed of (in mM) 122 NaCl, 24 NaHCO3, 2.5 CaCl2, 3 KCl, 1 MgCl2, and 12 d-glucose. Both types of ACSF were saturated with 95% O2-5% CO2 and maintained at pH 7.4. The drugs added to the ACSF were 5-HT, DOI[(−)(R)-DOI hydrochloride] (from Sigma-Aldrich), 2-methyl-5-HT, 8-OH-DPAT, AC90179, α-methyl-5-HT, BW723C86, clozapine, cisapride, cyproheptadine, EMD386088, granisetron, isradipine, ketanserin, LP44, LY344864, methylergonovine, methysergide, MK212, prazosin, RS102221, RS127445, SB204741, SB206553, SB224289, SB242084, tryptamine (all from Tocris), TTX (TTX-citrate; Alomone), and zolmitriptan (kindly donated by Astra Zeneca). All drugs were first dissolved as a 10–50 mM stock in water before final dilution in ACSF, with the exception of BW723C86, RS102221, RS127445, cisapride, clozapine, SB204741, and prazosin, which were dissolved in minimal amounts of DMSO (final concentration in ACSF <0.04%; by itself, DMSO had no effect on the LLR in vehicle controls). During intracellular recording, we usually first blocked the sodium currents (fast and persistent) and synaptic transmission with 2 μM TTX and then examined the Ca PIC sensitivity to 5-HT ligands. The Ca PIC was verified to be L-type calcium channel mediated by its blockage by dihydropiridines (nimodipine or isradipine; 15 μM).
Immunolabeling
Rats were killed with Euthanyl (Bimeda-MTC; 700 mg/kg) and perfused intracardially with 100 ml saline followed by 400 ml 2% paraformaldehyde (PFA; in phosphate buffer at room temperature; over about 15 min). Spinal cords were postfixed in PFA for 90 min at 4°C, cryoprotected in 20% sucrose and 2% ethylene-glycol, frozen, and cut on a cryostat in horizontal or transverse 25- to 40-μm sections. Spinal cord sections were incubated overnight at 4°C with primary antibodies: goat anti-5-HT2B receptors (1:500, Santa Cruz SC-15081, Santa Cruz) and mouse anti-SMI32 (1:1,000, Convance SMI-32R) in PBS. This specificity of this 5-HT2B receptor antibody has previously been shown (Moran et al. 2008; Tadros et al. 2007). Antibody labeling was visualized with fluorescent secondary antibodies (5-HT2B: donkey anti-goat conjugated with Alexa488, 1:400, Invitrogen A11055; SMI32: donkey anti-mouse conjugated with Alexa568, 1:500, Invitrogen A11037) for 90 min at room temperature. Image acquisition used a Zeiss LSM 510 laser-scanning confocal microscope: Plan-Apochromat 63× 1.4 oil objective, BP 505–520, BP 560–615, HFT 488/543 with lasers at 488 and 543 nm, acquisitions in multi-track mode, optical slice thickness 0.14 μm. Controls in which the primary antibody was preabsorbed with the antigen that it was raised against were used to verify the selectivity of the antibody labeling. Also, controls in which the primary antibody was omitted were used to confirm that the secondary antibody produced no labeling by itself. SMI32 labels nonphosphorylated neurofilament H and does not change with SCI (Anelli et al. 2007). We have previously shown that SMI32 labels motoneurons and not interneurons in the ventral horn, although it also labels large cells elsewhere in the brain. Thus SMI32 provides a fairly selective marker of motoneurons in the ventral horn (Anelli et al. 2007), and in particular, labels the intracellular space (neurofilament).
Data analysis
Data were analyzed in Clampfit 8.0 (Axon Instruments) and Sigmaplot (Jandel Scientific) and expressed as mean ± SD. A Student's t-test was used to test for statistical differences before and after drug applications, with a significance level of P < 0.05. A Kolmogorov-Smirnov test for normality was applied to each data set, with a P = 0.05 level set for significance. Most data sets were found to be normally distributed, as is required for a t-test. For those that were not normal, a Wilcoxon signed rank test was instead used with a significance level of P < 0.05.
Standard sigmoidal curves were fit to the relation between agonist dose and reflex responses, with doses expressed in log units and with a Hill slope of unity. The dose that produced 50% effect (EC50) was measured from the curve, and –log(EC50) was used to quantify the drug potency: pEC50 = –log(EC50). Also, the maximum drug-induced response (efficacy) was computed from the curve (peak of curve). For comparison to our computed potencies (pEC50), the binding affinity of each drug at the rat 5-HT receptors was also reported, with values taken from the literature (Table 1). The binding of an agonist to a receptor is expressed in terms of its Ki value (nM), which corresponds to the dose that produces 50% binding to that receptor (Knight et al. 2004). This is typically measured by the agonist's ability to displace a standard radio-labeled ligand, such as [3H]-5-HT, from the receptor expressed in isolated cells. Binding affinity is computed as pKi = –log(Ki) (Knight et al. 2004). When possible, binding affinities of different drugs for a given receptor were taken from large studies or summary reviews (Boess and Martin 1994), usually using isolated cloned receptors. Also, high affinity agonist-preferring binding sites were always used, measured with radioactive agonists (usually [3H]-5-HT), rather than radioactive antagonists that bind to a low-affinity site (Egan et al. 2000; Knight et al. 2004). If rat receptor Ki values were not available, human values were used instead, because these are similar for most receptors (Boess and Martin 1994).
Table 1.
5-HT receptor agonists and their receptor binding affinity
| Receptor | Agonist | Ki (nM) | pKi | Receptor | Agonist | Ki (nM) | pKi |
|---|---|---|---|---|---|---|---|
| 5-HT1A | 5-HT | 1.65 | 8.78Hoy | 5-HT2B | 2-Methyl-5-HT | 316.23 | 6.50Boe |
| 8-OH-DPAT | 0.97 | 9.01Boe | 5-HT | 10.23 | 7.99Boe | ||
| LP44 | 52.48 | 7.28Leo | α-Methyl-5-HT | 10.47 | 7.98Boe | ||
| BW723C86 | 12.59 | 7.90Bax | |||||
| DOI | 27.54 | 7.56Boe | |||||
| 5-HT1B | 5-HT | 24.49 | 7.61Hoy | Methylergon | 0.50 | 9.30Kni | |
| BW723C86 | 125.89 | 6.60Bax | Tryptamine | 112.20 | 6.95Boe | ||
| Zolmitriptan | 5.01 | 8.30Mar | |||||
| EMD386088 | 179.88 | 6.75Mat | 5-HT2C | 5-HT | 10.99 | 7.96Ega | |
| α-Methyl-5-HT | 2.69 | 8.57Kni | |||||
| BW723C86 | 125.89 | 6.90Bax | |||||
| 5-HT1D | 5-HT | 2.51 | 8.60Boe | DOI | 9.30 | 8.03Ega | |
| BW723C86 | 125.89 | 6.30Bax | MK212 | 97.72 | 7.01Kni | ||
| Zolmitriptan | 0.63 | 9.20Mar | Methylergon | 4.57 | 8.34Kni | ||
| EMD386088 | 109.90 | 6.96Mat | Tryptamine | 62.0 | 7.21Ega | ||
| 5-HT1E | 5-HT | 6.16 | 8.21Boe | 5-HT3 | 2-Methyl-5-HT | 85.11 | 7.07Mil |
| α-Methyl-5-HT | 120.22 | 6.92Boe | BW723C86 | 316.23 | 6.50Ken | ||
| Methylergon | 89.12 | 7.05Boe | EMD386088 | 34.00 | 7.47Mat | ||
| MK212 | 29.00 | 7.54Gle | |||||
| 5-HT1F | 5-HT | 67.60 | 7.17Boe | ||||
| α-methyl-5-HT | 181.97 | 6.74Boe | 5-HT4 | 5-HT | 6.31 | 8.20Adh | |
| LY344864 | 6.02 | 8.22Phe | α-Methyl-5-HT | 263.03 | 6.58Adh | ||
| Methylergon | 30.90 | 7.51Boe | Cisapride | 25.00 | 7.60Adh | ||
| Zolmitriptan | 63.09 | 7.20Mar | |||||
| 5-HT5A | 5-HT | 7.94 | 8.10Boe | ||||
| 5-HT2A | 5-HT | 5.75 | 8.24Boe | 8-OH-DPAT | 50.12 | 7.30Boe | |
| α-Methyl-5-HT | 127.05 | 6.90Eng | |||||
| BW723C86 | 251.18 | 6.60Bax | 5-HT6 | 2-Methyl-5-HT | 52.48 | 7.28B97 | |
| DOI | 0.79 | 9.10Boe | 5-HT | 56.23 | 7.25Boe | ||
| Methylergon | 0.35 | 9.45Kni | BW723C86 | 398.11 | 6.40Ken | ||
| EMD386088 | 7.41 | 8.13Mat | |||||
| 5-HT7 | 5-HT | 1.51 | 8.82Boe | ||||
| 8-OH-DPAT | 34.67 | 7.46Boe | |||||
| LP44 | 0.22 | 9.66Leo |
Agonists Ki and binding affinity (pKi = −log Ki) obtained from high affinity agonist radioligand bindings studies (Adham et al. 1996; Baxter 1996; Boess and Martin 1994; Boess et al. 1997; Egan et al. 2000; Engel et al. 1986; Glennon et al. 1989; Hoyer et al. 1985; Kennett et al. 1997a; Knight et al. 2004; Leopoldo et al. 2007; Martin et al. 1997; Mattsson et al. 2005; Milburn and Peroutka 1989; Phebus et al. 1997), with references abbreviated by the first few letters of authors' names, except for Boess et al. 1997, which is abbreviated as B97. Methylergonovine is abbreviated as methylergon. Each agonist is considered to activate a receptor if Ki < 400 nM and is listed with that receptor. Cisapride binds to other receptors but was only used after blocking all but 5-HT4 receptors (Table 2), and so is only listed with that receptor.
AQ: Au please spell out DOI.
DOI is one of the few 5-HT2 agonists that is very selective to only 5-HT2 receptors (it activates just 5-HT2A/2B/2C) (Boess and Martin 1994), and accordingly, its dose–response relation was a simple sigmoidal relation. In contrast, other agonists such as α-methyl-5-HT bind nonselectively to other 5-HT receptors, although with much less affinity than to 5-HT2 receptors (Table 1). With these drugs, the dose–response relation was mostly sigmoidal, until higher doses were reached, at which point the LLR tended to decrease with increasing dose, consistent with a nonselective action on other receptors. To minimize the effect of this nonselective receptor activation, only the main part of the dose–response relation with increasing responses to increasing dose (over 2 decades) was fit to the sigmoidal dose–response relation to obtain an estimate of the EC50. We used a Hill slope of 1.0 (see above) to force the sigmoid to have a fixed width of about 2 decades (2 orders of magnitude in dose, as is expected for the response to a single receptor). This approach caused the low dose–responses of agonists (e.g., selective 5-HT2B agonists) to dominate the sigmoidal fit where they are most selective and assured that the EC50 was not affected (e.g., underestimated) by the nonselective action of other 5-HT receptors that may have affected (e.g., inhibited) the LLR at high doses.
RESULTS
Use of LLRs as a measure of Ca PICs and spasms in chronic spinal rats
When the dorsal roots of the in vitro sacrocaudal spinal cord were stimulated with a low-threshold current (3×T), a long-lasting reflex (LLR; 2- to 10-s duration) was evoked in motoneurons, as recorded both from ventral roots (Fig. 1A) and from single motoneurons measured intracellularly (Fig. 1C, top). The many-second-long portion of this LLR is mediated by a large persistent inward calcium current (Ca PIC) that activates a plateau potential and associated sustained firing in motoneurons of chronic spinal rats (Li et al. 2004a), which we verified with two methods. First, directly blocking the Ca PIC (with isradipine or nimodipine; 15 μM) largely eliminated the LLR (significantly reduced by 83.9 ± 7.9%, n = 9, P < 0.05; Fig. 1B), and second, hyperpolarizing motoneurons to prevent activation of the voltage-dependent Ca PIC eliminated the LLR and associated PIC-mediated plateau (Fig. 1C, bottom; n = 10/10). In both cases, eliminating the Ca PIC left an ∼0.5-s-long response that was caused by a polysynaptic EPSP in the motoneurons (Fig. 1, B and C, bottom). For this study, we focused on the Ca PIC and use the LLR reflex measured at times >0.5 s as an indirect estimate of this Ca PIC on motoneurons (Fig. 1A, bottom; rectified response averaged in a 0.5- to 4-s window). This enabled us to efficiently screen many 5-HT drugs and doses with simple ventral root, rather than intracellular motoneuron, recordings. Previous work has shown that the LLR we record in vitro also occurs in vivo in the awake chronic spinal rat, where it produces muscle spasms (Bennett et al. 2004; Murray et al. 2010). Thus the LLR recorded in vitro is also a convenient assay of spasms, and we used it to assess which 5-HT receptors modulate spasms.
Only 5-HT2B and 5-HT2C receptors facilitate the LLR
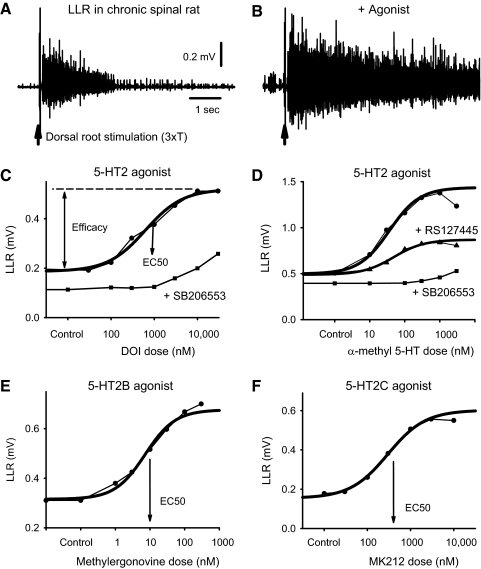
Application of 5-HT2 receptor agonists (α-methyl-5-HT, DOI, BW723C86, methylergonovine, MK212, 2-methyl-5-HT, tryptamine, and 5-HT) increased the LLR (Fig. 2), with increasing doses generally producing larger responses over about a 100-fold change in dose (e.g., 10–1,000 nM for α-methyl-5-HT; Fig. 2D). This agonist dose–response relation was well approximated by a sigmoidal curve (Fig. 2, C and D), from which we computed the agonist dose to produce 50% maximal effect (EC50; Fig. 2D), agonist potency [pEC50 = –log(EC50)], and efficacy (maximal response; Fig. 2C). All 5-HT2 agonists tested exhibited a significant efficacy in facilitating the LLR (Table 2), with efficacy reported as a percent of mean 5-HT-induced LLR, which was 0.393 ± 0.19 mV. This 5-HT–induced LLR was three times greater than the predrug LLR of 0.11 ± 0.06 mV (100% in Table 2 represents a 3-fold change in LLR). Agonists that are known to bind to both 5-HT2B and 5-HT2C receptors (Ki < 50 nM; α-methyl-5-HT, DOI, 5-HT) consistently had the highest efficacies, including 5-HT. Agonists that bind relatively selectively to either 5-HT2B receptors (BW723C86, methylergonovine, 2-methyl-5-HT; i.e., Ki for 5-HT2B about 10 times higher than 5-HT2C; Table 2) or 5-HT2C receptors (MK212; or agonists made selective by antagonists, e.g., α-methyl-5-HT acts as a selective 5-HT2C agonist when given with the 5-HT2C antagonist RS127445 present; Fig. 2C; Table 2) had significantly lower efficacies. However, the sum of the effects of selective 5-HT2B and 5-HT2C agonists was similar to the effect of broad-spectrum 5-HT2 agonists (α-methyl-5-HT and 5-HT, Table 2), suggesting that both 5-HT2B and 5-HT2C receptors are required to maximally facilitate the LLR.
Fig. 2.
5-HT2B and 5-HT2C receptor agonists increase the LLRs in chronic spinal rats. A: LLR triggered by dorsal root stimulation (0.1 ms pulse, 3xT) and recorded from the ventral roots. B: facilitation of LLR with application of the 5-HT2 receptor agonist, α-methyl-5-HT (0.1 μM), quantified over time window indicated in Fig. 1 (0.5–4 s). C–F: facilitation of LLR with increasing doses of broad spectrum 5-HT2 receptor agonists (DOI and α-methyl-5-HT; C and D) and relatively selective 5-HT2B (methylergonovine; E) and 5-HT2C (MK212; F) receptor agonists (increase over ∼100-fold change in dose). Best-fit sigmoidal curves and subsequent estimation of EC50 are shown with thick lines. Prior application of a single blocking dose of the selective 5-HT2B/2C receptor antagonist SB206553 (5 μM) or the 5-HT2B receptor antagonist RS127445 (3 μM) inhibited these agonists induced changes in the LLR (bottom plots in C and D). Each plot shows the typical response from a single rat, with a different rat for each condition, because agonists are not feasible to wash out and repeat after antagonist application (taking many hours to wash).
Table 2.
Facilitation of LLR by 5-HT2B and 5-HT2C receptor agonists
| Agonist | Pretreatment With Antagonists | Selectivity/Receptors Activated with Ki < 50 nM and (Ki = 50–400 nM) | Potency (−logEC50) | EC50 (nM) | Efficacy (% of 5-HT effect) |
|---|---|---|---|---|---|
| 2-Methyl-5-HT | None | (5-HT2B, 3, 6) | 5.41 ± 0.32 | 3852 | 70 ± 38* |
| 5-HT | None | 5-HT1-7 | 7.48 ± 0.23 | 33 | 100 ± 43* |
| 5-HT | SB206553† | 5-HT1, 2A, 3–7 | ND | ND | 1.0 ± 8.2 |
| α-Methyl-5-HT | None | 5-HT2A,2B,2C (5-HT1E, 1F; 5-HT4) | 7.24 ± 0.20 | 58 | 116 ± 49* |
| α-Methyl-5-HT | SB206553 | 5-HT2A (5-HT1E, 1F; 5-HT4) | ND | ND | 18.4 ± 29.8 |
| α-Methyl-5-HT | RS102 + AC901 | 5-HT2B (5-HT1E, 1F; 5-HT4) | 7.12 ± 0.31 | 76 | 77 ± 30* |
| α-Methyl-5-HT | RS127 + AC901 | 5-HT2C (5-HT1E, 1F; 5-HT4) | 7.25 ± 0.26 | 56 | 74 ± 46* |
| BW723C86 | None | 5-HT2B (5-HT1B, 1D, 2A, 2C, 3, 6) | 6.53 ± 0.31 | 295 | 49 ± 48* |
| BW723C86 | SB204741 | (5-HT1B, 1D, 2A,2C,3,6) | 5.84 ± 0.17‡ | 1445 | 31 ± 23* |
| DOI | None | 5-HT2A, 2B, 2C | 6.19 ± 0.17 | 646 | 119 ± 37* |
| DOI | SB206553 | 5-HT2A | ND | ND | 5.8 ± 15.7 |
| Methylergonovine | None | 5-HT2A, 2B, 2C (5-HT1E, 1F) | 7.96 ± 0.25 | 11 | 69 ± 28* |
| MK212 | None | (5-HT2C, 3) | 6.35 ± 0.24 | 447 | 47 ± 29* |
| Tryptamine | SB224289 | 5-HT2A (5-HT2B) | 6.04 ± 0.39 | 912 | 51 ± 24* |
Agonists with varying selectivity for the different 5-HT receptors were applied, sometimes after prior application of 5-HT receptor antagonists to effectively make the agonist action more selective (column 2, pretreatment, starting > 30 min before agonist). The receptors that can be activated by this agonist/antagonist combination are indicated in column 3, with high binding affinity for the agonist listed first (with Ki about 1–50 nM), followed by moderate affinity (in brackets; Ki = 50–400 nM; see Table 1). Antagonist abbreviations and receptors blocked are as follows: RS127445 (RS127, 5-HT2B); RS102221 (RS102, 5-HT2C); AC90179 (AC901, 5-HT2A); SB206553 (5-HT2B/2C); SB204741 (5-HT2B), SB224289 (5-HT1B), prazosin (α1 adrenergic); granisetron (5-HT3); methysergide (5-HT2), and clozapine (5-HT2). Antagonist doses are given in Table 4, except for prazosin (1 μM), granisetron (0.3 μM) and SB224289 (1 μM). The efficacy (maximal effect) of these agonists in facilitating the LLR is indicated in column 6 (max mean rectified LLR, normalized by 5-HT efficacy). When a significant facilitation (efficacy) was detected, the mean agonist potency was computed, from which the EC50 was computed (potency = −logEC50). In addition to the agonists shown, no detectable facilitation (ND, P > 0.05) occurred with application of the agonists: 8-OH-DPAT, cisapride (RS), EMD386088, LP44, LY34864, and zolmitriptan when given up to 3–10 μM doses, ruling out the involvement of the receptors that these drugs selectively activate [see Table 1 for Ki values; cisapride given after pretreatment with antagonists RS127445, RS1022221, granisetron and prazosin to make it selective to 5-HT4 receptors, which is abbreviated cisapride (RS)]. Also after some antagonist pre-treatments (SB206553), there was no detectable (indicated ND) facilitation of the LLR at all but the highest doses, making it impossible to estimate EC50. + between antagonists indicates they were given in combination.
Efficacy significantly different from zero (LLR significantly increased), P < 0.05.
Pretreatment with methysergide, clozapine, or RS127445 + RS102221 also eliminated the facilitation of the LLR by 5-HT (ND), like SB206553. ‡Potency significantly lowered by SB204741, P < 0.05.
Data shown as mean ± SD, with n > 8 per drug (or combination).
Prior application of antagonists that blocked both 5-HT2B and 5-HT2C receptors (including SB206553, clozapine, cyproheptadine, methysergide, or a combination of RS127445 and RS102221) inhibited the 5-HT2 agonist-induced increase in LLR (Fig. 2, C and D; Table 2), eliminating responses to all but the highest doses and significantly reducing the LLR induced by the agonist dose that maximally facilitated the LLR before the antagonists (SB206553 reduced LLR to 15.9 ± 25.7, 4.1 ± 13.2, and 1.0 ± 8.2% of preantagonist efficacy for α-methyl-5-HT, DOI, and 5-HT, respectively). The antagonist application shifted the dose–response relation to such high doses that the standard sigmoidal curve with 100-fold dose range could no longer be fit to the few responsive high doses tested, and the usual EC50 measurements were thus not detectable (ND; Table 2). This thorough inhibitory action of these antagonists suggests that 5-HT2B and/or 5-HT2C receptors facilitate the LLR. Furthermore, the finding that the facilitation of the LLR by 5-HT itself is blocked by these combined 5-HT2B and 5-HT2C antagonists indicates that all other 5-HT receptors (including 5-HT2A) are likely not involved in facilitating the LLR or PIC.
Prior application of the selective 5-HT2B receptor antagonist SB204741 significantly inhibited the effect of the selective 5-HT2B agonist BW723C86 (decreasing the potency, doubling the EC50, and decreasing the efficacy; Table 2), consistent with the involvement of 5-HT2B receptors. However, the response to BW723C86 was not completely eliminated by SB204741, likely because of the weak action of BW723C86 on the 5-HT2C receptor (We thus consider BW723C86+SB204741 a weak 5-HT2C agonist in Table 3, described below.) Blocking all 5-HT2 receptors except the 5-HT2B receptor (with 5-HT2C and 5-HT2A antagonists) did not eliminate the facilitation of the LLR by subsequent application of the 5-HT2 receptor agonist α-methyl-5-HT (Table 2) and did not significantly shift the dose–response curve (EC50 not altered significantly), although the efficacies were lowered (Table 2). Similarly, blocking all but the 5-HT2C receptor did not eliminate the α-methyl-5-HT–induced LLR or significantly shift the EC50 (Table 2). This is consistent with the involvement of both the 5-HT2B and 5-HT2C receptors in facilitating the LLR; the EC50 was not significantly shifted with a block of either 5-HT2B or 5-HT2C receptors, because α-methyl-5-HT binds with similar affinity to 5-HT2B and 5-HT2C receptors (Table 1).
Table 3.
Relative potency of agonists at facilitating the LLR
| Receptor | Agonist | pEC50 - pKi | Receptor | Agonist | pEC50 - pKi |
|---|---|---|---|---|---|
| 5-HT1A | 5-HT | −1.30 ± 0.33* | 5-HT2B | 2-Methyl-5-HT | −1.09 ± 0.32* |
| 5-HT (SB206) | ND | 5-HT | −0.51 ± 0.33* | ||
| 8-OH-DPAT | ND | α-Me-5-HT | −0.74 ± 0.20* | ||
| LP44 | ND | α-Me-5-HT (RS102) | −0.86 ± 0.31* | ||
| BW723C86 | −1.37 ± 0.31* | ||||
| 5-HT1B | 5-HT | −0.13 ± 0.33 | DOI | −1.37 ± 0.31* | |
| 5-HT (SB206) | ND | Methylergon | −1.34 ± 0.25* | ||
| BW723C86 | −0.07 ± 0.31 | Tryptamine (SB224) | −0.91 ± 0.39* | ||
| EMD386088 | ND | ||||
| Zolmitriptan | ND | 5-HT2C | 5-HT | −0.48 ± 0.33* | |
| α-Me-5-HT | −1.33 ± 0.20* | ||||
| 5-HT1D | 5-HT | −1.12 ± 0.33* | α-Me-5-HT (RS127) | −1.32 ± 0.26* | |
| 5-HT (SB206) | ND | BW723C86 (SB204) | −1.06 ± 0.17* | ||
| BW723C86 | +0.23 ± 0.31 | DOI | −1.84 ± 0.17 | ||
| EMD386088 | ND | MK212 | −0.66 ± 0.24* | ||
| Zolmitriptan | ND | Tryptamine (SB224) | −1.17 ± 0.39* | ||
| 5-HT1E | 5-HT | −0.73 ± 0.33* | 5-HT3 | 2-Methyl-5-HT | −1.66 ± 0.32 |
| 5-HT (SB206) | ND | BW723C86 | +0.03 ± 0.31 | ||
| α-me-5-HT | +0.32 ± 0.20 | EMD386088 | ND | ||
| Methylergon | +0.91 ± 0.25 | MK212 | −1.19 ± 0.24* | ||
| 5-HT1F | 5-HT | +0.31 ± 0.33 | 5-HT4 | 5-HT | −0.72 ± 0.33* |
| 5-HT (SB206) | ND | 5-HT (SB206) | ND | ||
| α-Me-5-HT | +0.50 ± 0.20 | α-Me-5-HT | +0.66 ± 0.20 | ||
| LY344864 | ND | Cisapride (RS) | ND | ||
| Methylergon | −0.45 ± 0.25 | ||||
| Zolmitriptan | ND | 5-HT5 | 5-HT | −0.62 ± 0.33* | |
| 5-HT (SB206) | ND | ||||
| 5-HT2A | 5-HT | −1.76 ± 0.33 | 8-OH-DPAT | ND | |
| 5-HT (SB206) | ND | ||||
| α-Me-5-HT | +0.34 ± 0.20 | 5-HT6 | 2-Methyl-5-HT | −1.87 ± 0.32 | |
| α-Me-5-HT (SB206) | ND | 5-HT | +0.23 ± 0.33 | ||
| BW723C86 | −0.07 ± 0.31 | BW723C86 | +0.13 ± 0.31 | ||
| DOI | −2.91 ± 0.17 | EMD (SB216+) | ND | ||
| Methylergon | −1.49 ± 0.25* | ||||
| 5-HT7 | 5-HT | −1.34 ± 0.33* | |||
| 5-HT (SB206) | ND | ||||
| 8-OH-DPAT | ND | ||||
| LP44 | ND |
Relative potency computed as the difference between potency (Table 2) and affinity (Table 1): pEC50 - pKi. Pretreatment with antagonists used to make agonist more selective, indicated in brackets, abbreviated as in Table 2. ND, no detected inhibition of the reflex in Table 2. Relative potency not computed for methylergonovine at the 5-HT2C receptor, because this drug has a much higher affinity for 5-HT2B receptors, which dominate its response. Bold indicates receptors with relative potency values mostly within confidence interval.
relative potency within 2 SD of −1.0 (or the mean for 5-HT2B receptors), the confidence interval for similarity, which is about 0.4–0.6.
Application of selective agonists (or agonist/antagonist combinations) for other 5-HT receptors (5-HT1, 5-HT2A, 5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7) produced no significant facilitation of the LLR even at high doses (3–10 μM; Table 2), again suggesting that only 5-HT2B and 5-HT2C receptors facilitate the LLR.
Agonist potency is correlated with receptor binding affinity at 5-HT2B and 5-HT2C receptors
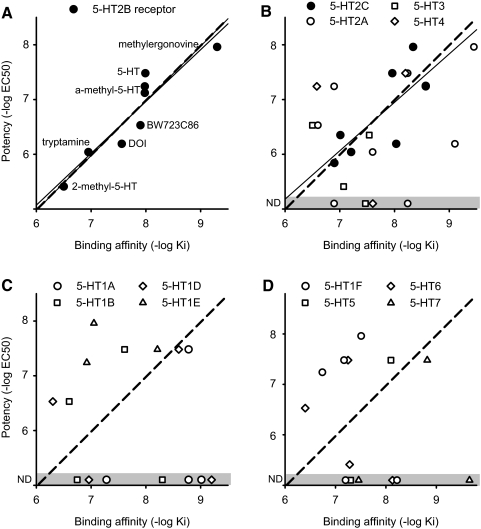
The effective 5-HT2 agonist doses required to facilitate the LLR (EC50 values and associated potencies, pEC50) varied by orders of magnitude between the different agonists (or agonist/antagonist combinations). However, this variation was largely accounted for by the differing binding affinity of these drugs to 5-HT2B and 5-HT2C receptors (pKi; see description of binding affinity in methods). That is, we found that the agonist potencies in facilitating the LLR (pEC50 and associated EC50) were significantly correlated with the binding affinity of these agonists (pKi) for 5-HT2B (r = 0.93, slope = 0.95, n = 8 drugs combinations tested, P < 0.05) and 5-HT2C (r = 0.77, slope = 0.89, n = 8, P < 0.05) receptors, as shown in Fig. 3, B and C. Interestingly, a line with unity slope (pEC50 = pKi + C, where C is a fixed value) was equally well fit to the data, with r = 0.92 and r = 0.77 for 5-HT2B and 5-HT2C receptors, respectively (Fig. 3, A and B), a useful finding that we use in the next section to model the potency–affinity relation to further predict which receptors modulate the LLR. In contrast, there was no significant correlation with the potency and the binding affinity for 5-HT2A receptors (r = 0.31, n = 8, P > 0.05) or any other receptor (Fig. 3), and points were scattered far from the unity slope line that fit the 5-HT2B and 5-HT2C receptors.
Fig. 3.
Potency of 5-HT receptor agonists at facilitating the LLR is only related to binding to 5-HT2B and 5-HT2C receptors. A: 5-HT2B receptor agonist potency [pEC50 = −log(EC50)] for facilitating the LLR plotted against the agonist binding affinity to that receptor (pKi), with each agonist name indicated next to its point. α-methyl-5-HT potency with and without block of 5-HT2C receptor with RS102221 plotted. Thin line: linear correlation between potency and affinity. Dashed line: best fit line with unit slope (potency = binding affinity + C, where C ∼ –1). B–D: similar potency-affinity scatter plots for the remaining 5-HT receptors. Agonists used for each receptor are listed in Table 1, with agonists assumed to act at a receptor only if Ki <400 nM. Potencies are from Table 2. We used the potency of BW723C86 after treatment with SB204741 for the 5-HT2C receptor plot to avoid dominant 5-HT2B receptor action of this drug. Thin line: correlation between agonist potency and affinity for 5-HT2C receptors. Dashed line: best fit unity slope relation for the 5-HT2C receptor, copied into C and D to show lack of similar relation for other receptors. Other receptors had no significant correlation between potency and affinity (open circles; P > 0.05). ND and gray zone: no detected effect of agonist on the LLR.
Potency of each agonist can be quantitatively predicted from its receptor binding affinity
To directly compensate for the variable receptor binding affinity of different agonists, we computed the potency of each agonist in facilitating the LLR relative to its binding affinity at each receptor, which we term the relative potency = pEC50 − pKi. As we just mentioned, this difference is a fixed constant (C = relative potency) for the receptors involved in modulating the LLR (5-HT2B and 5-HT2C), and thus we expect that it should be invariant when computed with pKi values for functional receptors in the spinal cord (5-HT2B) but variable for other receptors. This relative potency should account for all factors that affect potency (e.g., drug diffusion, receptor signaling) other than receptor binding. As shown in Table 3, the relative potency computed for all agonists of the 5-HT2B receptor was indeed very similar, all falling within the 99% confidence interval (±0.58) about the mean relative potency [–1.02 ± 0.32 (SD)], and within 2 SD of this mean, which we set as the similarity criterion. The relative potency of agonists at the 5-HT2C receptor was likewise invariant, clustered remarkably near the mean value of –1.03 ± 0.49 and not significantly different from the relative potency at the 5-HT2B receptor (Table 3). Again, most 5-HT2C agonists had relative potencies within the 99% confidence interval about the mean (±0.53), and within 2 SD of the mean, consistent with the interpretation that 5-HT2C receptors also play a role in modulating the LLR (see also Fig. 3, squares). The only exception was DOI, which had a response that was dominated by the 5-HT2B, rather than the 5-HT2C, receptor. These findings suggest that, after taking into account variable binding affinity, all these 5-HT2 agonists appeared to diffuse equally well to common targets (the 5-HT2B and 5-HT2C receptors) and have similar actions (facilitating the Ca PIC underlying the LLR). Receptor binding affinities (pKi and Ki values) are typically measured in homogenized membranes from isolated cells (transfected with cloned receptors, Boess and Martin 1994; Knight et al. 2004). In contrast, we measured the functional effects of these agonists (the potency or EC50) in a whole spinal cord preparation, where there are substantial barriers to drug diffusion to the receptors. Furthermore, downstream actions of receptor binding may further alter the potency (see discussion). Thus it is not unexpected that the EC50 exceeds the Ki dose value (potency < affinity) and the relative potency (potency − affinity) ∼ = −1.0. Another way to interpret relative potency arises from the law of differences of logarithms
| (1) |
Thus the ratio EC50/Ki = 10–C, which again should be a fixed number for a receptor involved in modulating the LLR. For the 5-HT2B receptor, the overall EC50/Ki was 10.01, indicating that the EC50 in the present whole sacral spinal cord preparation is about 10 times the Ki value, which is likely because of common diffusion barriers.
Our consistent findings of fixed relative potencies for 5-HT2B and 5-HT2C receptors suggest that perhaps, in general, we can predict the EC50 and potency of drugs at a particular receptor from the published Ki values (EC50 = 10 × Ki and potency = affinity – 1); receptors that do not consistently fit this prediction are unlikely to be functional (assuming similar diffusion to each receptor). The power of this modeling approach is that the lack of involvement of a given receptor in the LLR can be proven by the potency measured from as little as one agonist, if that potency is far from the affinity (relative potency different from –1, and corresponding point far from the pEC50 = pKi – 1 line in Fig. 3; e.g., 5-HT2A cannot be involved because DOI exhibits a relative potency of –3 at this receptor). Indeed, we found that the relative potency at all other 5-HT receptors was highly variable, usually significantly different from the relative potency at the 5-HT2B/2C receptors (>2 SD away from –1.0; Table 3; and scattered away from pEC50 = pKi – 1 line in Fig. 3), thus further ruling out the involvement of all but the 5-HT2B and 5-HT2C receptors. Occasionally, the relative potency fell close to –1.0 for a particular agonist, like methylergonovine at the 5-HT2A receptor, but this was simply by chance, because the relative potency of other agonists at these receptors was not consistently –1.0. For example, MK212 has an impossibly high relative potency of +0.36 at the 5-HT2A receptor, with a corresponding EC50 less than Ki (Table 3; MK212 is a poor 5-HT2A agonist). Furthermore, these receptors with inconsistent relative potencies often had substantial binding affinity for agonists (like zolmitriptan at the 5-HT1B receptor) that caused no detectable facilitation of the LLR (ND; Table 3). Thus we again conclude that only the 5-HT2B and 5-HT2C receptors are involved in facilitating the LLR.
Antagonist dose determination
The experiments described above partly rely on using a blocking dose of selective 5-HT2 receptor antagonists before adding agonists, to identify the receptors involved in facilitating the LLR. The antagonist dose that we used was always chosen to be ≥10 times the published Ki value of that antagonist at the target receptor (e.g., 5-HT2; Table 4, column 2), to account for drug diffusion into the whole cord preparation, as described above for agonists (assuming that the EC50 of the antagonist can be predicted from its Ki value, as for agonists: EC50 = 10 × Ki) and typically set ≥100 times the Ki to maximally block the receptor. Furthermore, to be sure that we did not affect other receptors nonselectively, we tried to use an antagonist dose that was less than about 10 times the Ki value of that antagonist at other receptors known to bind this antagonist (Table 4, column 3 shows the receptor most likely to be nonselectively activated, with the highest published Ki value of all other receptors tested). Satisfying this latter criterion was not always possible for nonselective antagonists like methysergide.
Table 4.
5-HT2 receptor antagonists that inhibit the LLR
| Antagonists | Receptors Antagonized, Ki (nM) |
Non selective Binding to Receptor and, Ki (nM) | Refences for Ki | Antagonist dose (nM) | Antagonist Inhibition of Agonist-Induced LLR (% change) | Antagonist Inhibition of Basal LLR (% change) |
|---|---|---|---|---|---|---|
| RS127445 | 5-HT2B (1.1) | 5-HT2C (470) | Knight04 | 3,000 | −45.2 ± 17.8* | −1.9 ± 5.3 |
| SB242084 | 5-HT2B (45) | 5-HT2A (850) | Cussac02 | 3,000 | −89.0 ± 18.2* | 0.3 ± 26.9 |
| 5-HT2C (0.48) | Knight04 | |||||
| Methysergide | 5-HT2B (0.36) | 5-HT1,2A,5,6,7 | Knight04 | 10,000 | −98.8 ± 13.3* | 1.0 ± 56.3 |
| 5-HT2C (4.4) | Boess94 | |||||
| SB204741 | 5-HT2B (51) | 5-HT2C (2100) | Cussac02 | 30,000 | — | −33.1 ± 21.1* |
| RS102221 | 5-HT2C (5.0) | 5-HT2B (2900) | Knight04 | 3000–10000 | −40.9 ± 21.3* | −28.1 ± 8.6* |
| SB206553 | 5-HT 2B (5.5) | 5-HT2A (2300) | Cussac02 | 3000–10000 | −79.1 ± 28.2* | −68.6 ± 18.2* |
| 5-HT2C (3.2) | Knight04 | |||||
| Cyprohept | 5-HT2B (1.5) | 5-HT2A,5,6,7 | Yoshio01 | 10,000 | — | −92.5 ± 10.0* |
| 5-HT2C (2.2) | Bonhaus97 | |||||
| alpha1 (25) | Boess94 | |||||
| Clozapine | 5-HT2B (10) | 5-HT2A,3,6,7 | Knight04 | 10,000 | — | — |
| 5-HT2C (13) | Boess94 | |||||
| Ketanserin | 5-HT2A (8.1) | 5-HT2B (740) | Knight04 | 10,000 | — | −52.8 ± 9.9* |
| 5-HT2C (62) | Yoshio01 | |||||
| alpha1 (6.3) | ||||||
| AC90179 | 5-HT2A (0.20) | 5-HT2C (2.9) | Vanover04 | 30 | 0.1 ± 6.6 | 1.4 ± 9.7 |
The receptors for which the antagonists are selective are shown in column 2, along with Ki values (parentheses). Receptors that the antagonists nonselectively bind at higher doses are shown in column 3, along with Ki values. Ki values were obtained from the following references, abbreviated in column 4 (Boess and Martin 1994; Bonhaus et al. 1999; Cussac et al. 2002; Knight et al. 2004; Vanover et al. 2004; Yoshio et al. 2001). Selectivity is defined as the ratio of Ki's for two receptors (e.g., 470/1.1 = 427-fold selectivity of RS127445 at 5-HT2B over 5-HT2C receptors). For the nonselective antagonists methysergide, cyproheptadine, and clozapine, only Ki values for target receptors are shown, and other receptors affected only listed. Antagonists were used during LLR reflex testing at the doses indicated in column 5, with choice of dose based on Ki values. Following application of the 5-HT2 receptor agonist α-methyl-5-HT (0.3 μM), antagonists were applied (Fig 4A), and the inhibition of the agonist-induced LLR was measured (% change in LLR, relative to agonist-induced LLR; column 6; 0% no effect), with significant effects found for all 5-HT2B or 5-HT2C antagonists tested. Antagonists were also tested without agonists present to examine their negative efficacy (inverse agonist) action (e.g. Fig 4F), and the inhibition of the control/basal LLR measured (% change in LLR; column 7), with significant effects found only for inverse agonists. The first 3 antagonists listed are neutral 5-HT2B/2C receptor antagonists (no effect without agonist present, column 7), whereas the next 6 antagonists act as inverse agonists at 5-HT2B/2C receptors (shaded rows; negative efficacy without agonist present, column 7). Data shown as mean ± SD, with n > 8 per drug (or combination). –, not tested.
Significant inhibition induced by antagonist, P < 0.05.
5-HT2B and 5-HT2C receptor antagonists block the action agonists
To verify that we used 5-HT2 receptor antagonists at an appropriate dose to block the 5-HT2B/2C receptors in the experiments described above (e.g., Table 2), we directly tested the efficacy of most antagonists in inhibiting a prior dose of the 5-HT2 receptor agonist α-methyl-5-HT (0.3 μM). A submaximal agonist dose (near agonist EC50; dose vs. time) was used to assure that the agonist only activated 5-HT2 receptors. As shown in Fig. 3 and Table 4, all 5-HT2B/2C antagonists tested (selective and nonselective drugs) significantly decreased a prior agonist-induced increase in LLR. Importantly, the selective 5-HT2B receptor antagonists (RS127445 and SB204741) and 5-HT2C receptor antagonist (RS102221) each inhibited the LLR induced by the 5-HT agonist (Table 4), confirming that both 5-HT2B and 5-HT2C receptors modulate the LLR and associated Ca PIC. In contrast, the 5-HT2A receptor antagonist AC90179 had no effect (Table 4).
5-HT2B and 5-HT2C receptors are not activated by endogenous 5-HT
Some of the 5-HT2 receptor antagonists had no effect when given by themselves, including drugs selective to 5-HT2B receptors (RS127445; Fig. 4B) and 5-HT2B/2C receptors (SB242084; Fig. 4D) and the nonselective 5-HT2 receptor antagonist methysergide (Table 4, column 7). All these drugs have previously been classified as neutral antagonists (Bonhaus et al. 1999; Chanrion et al. 2008; Westphal and Sanders-Bush 1994). Neutral antagonists, by definition, only act to inhibit the action of 5-HT (endogenous or applied) or other agonists and have no effect when given by themselves (no intrinsic efficacy), because they do not block constitutive receptor activity (as we see in Table 4) (Chanrion et al. 2008; Seifert and Wenzel-Seifert 2002). Thus the lack of effect of these drugs (when given alone) shows that there is no functional residual 5-HT activating 5-HT2B or 5-HT2C receptors in the spinal cord after chronic spinal injury, consistent with our recent report for 5-HT2C receptors (Murray et al. 2010).
Fig. 4.
5-HT2B and 5-HT2C receptors are constitutively active after spinal cord injury. Plots of mean LLR sampled over time with agonists and antagonist applications. A and B: the neutral antagonist RS127445 (3 μM; selective for 5-HT2B receptors) inhibited the increase in the LLR induced by 5-HT2 receptor agonist α-methyl-5-HT (0.3 μM; A), whereas when applied alone RS127445 had no effect on the LLR (B). C and D: likewise, the neutral antagonist SB242084 (3 μM; selective for 5-HT2B and 5-HT2C receptors) inhibited that same agonist induced LLR (C), whereas SB242084 had no effect when applied alone (D). E–H: in contrast, the inverse agonists RS102221 (3 μM, selective for 5-HT2C receptors) and SB206553 (5 μM, selective for 5-HT2B and 5-HT2C receptors) inhibited the agonist-induced LLR (E and G) and inhibited the spontaneously occurring LLR when given alone (F and H). Considering that inverse agonists block constitutive activity and agonist-induced activity, whereas neutral antagonists only block agonist induced activity, these results indicate that the 5-HT2B and 5-HT2C receptors exhibit constitutive activity and are not activated by endogenous residual 5-HT. See statistics in Table 4.
5-HT2B and 5-HT2C receptors are constitutively active
The remaining 5-HT2 receptor antagonists that we used, including those that bind selectively to 5-HT2B receptors (SB204741), 5-HT2C receptors (RS102221 and ketanserin), or both (SB206553), significantly inhibited the LLR when they were given by themselves (without prior agonist application; Table 4; Fig. 4, F and H), thus suggesting that these compounds acted as inverse agonists, which by definition can block constitutive receptor activity (receptor activity in the absence of 5-HT), in addition to their conventional ability to antagonize 5-HT–induced receptor activity (Aloyo et al. 2009; Berg et al. 1999; Chanrion et al. 2008; Seifert and Wenzel-Seifert 2002; Weiner et al. 2001; Westphal and Sanders-Bush 1994). Taken together with the lack of functional 5-HT in the chronic spinal rat, this inhibition of the LLR by such inverse agonists suggests that there is substantial constitutive activity in 5-HT2B and 5-HT2C receptors that endogenously facilitates the LLR. The magnitude of the inhibition of the LLR by SB204741 and RS102221 was similar (20–30% reduction in LLR; Table 4), and thus the endogenous facilitation of the LLR is likely equally produced by constitutive activity in both 5-HT2B and 5-HT2C receptors. Furthermore, the inverse agonist SB206553, which selectively binds both 5-HT2B and 5-HT2C receptors (but no other receptors), reduced the PIC by approximately the amount predicted by the sum of the action of the selective 5-HT2B and 5-HT2C inverse agonist SB204741 and RS102221 (Table 4), suggesting that the action of 5-HT2B and 5-HT2C receptors is additive in facilitating the endogenous LLR (and associated LLR). The broad spectrum inverse agonists cyproheptadine and ketanserin also inhibited LLR (Table 4). We have previously reported this action of SB206553 and cyproheptadine, but at the time we were only able to conclude that either 5-HT2B or 5-HT2C receptors may be constitutively active (Murray et al. 2010). Here we show that both receptors are constitutively active.
Notably, cyproheptadine was the most effective of all inverse agonists tested, significantly exceeding the inhibition of the LLR produced by the 5-HT2B/2C inverse agonist SB206553 (Table 4). This is likely because cyproheptadine potently inhibits both 5-HT2 receptors and α1 adrenergic receptors (Westphal and Sanders-Bush 1994; Yoshio et al. 2001) (Table 4; α1 receptors, like 5-HT2 receptors, are known to facilitate the LLR; Li et al. 2004b). Also, the 5-HT2C inverse agonist ketanserin produced significantly more inhibition of the LLR than the selective 5-HT2C inverse agonist RS102221 (Table 4). Again, this is likely because of the known potent binding of ketanserin to α1 receptors (Yoshio et al. 2001), in addition to its 5-HT2C receptors action (Cussac et al. 2002) (Table 4; ketanserin also binds potently to 5-HT2A receptors, but these are not involved in modulating the LLR; see above). Together these data indicate that there is endogenous activity in α1 receptors, likely mediated by constitutive activity (consistent with Harvey et al. 2006b), as with 5-HT2 receptors; we address this issue in a separate paper (M. Rank and D. J. Bennett., unpublished data).
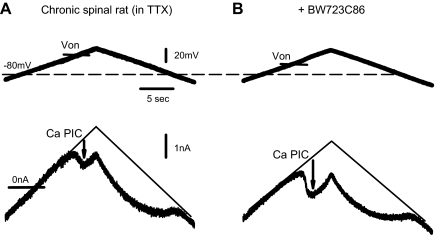
5-HT2B and 5-HT2C receptors regulate the Ca PIC in motoneurons
Considering that the LLR is mediated by the Ca PIC, it is likely that the 5-HT2B and 5-HT2C receptors modulate the Ca PIC, as they do to the LLR. To confirm this, we directly measured the Ca PIC in motoneurons using slow voltage ramps to inactivate transient currents and in the presence of TTX to synaptically isolate the motoneuron (by stopping spike-mediated transmission) and block the Na PIC, which can otherwise obscure the Ca PIC, as previously described (Harvey et al. 2006c). During this slow voltage ramp (under voltage-clamp conditions), the Ca PIC was activated at, on average, −49.6 ± 6.41 mV (Von, n = 46 motoneurons), producing a downward deflection in the recorded current of 1.98 ± 1.07 nA, which we took as an estimate of the Ca PIC amplitude (Fig. 5A, arrow; previously verified to be mediated by L-type calcium channels; nimodipine-sensitive) (Li et al. 2004c). Application of the 5-HT2B/2C agonists α-methyl-5-HT (0.3 μM), DOI (1 μM), BW723C86 (1–2 μM), and 5-HT (1 μM) each significantly increased the Ca PIC amplitude (by −0.71 ± 0.82 nA, n = 11; −0.60 ± 0.75, n = 9; −0.42 ± 0.43, n = 15; and −0.58 ± 0.68 nA, n = 11; respectively; Figs. 4B and 5B), and lowered the onset threshold of the PIC, Von (by −7.9 ± 8.0, −5.6 ± 3.8, −3.8 ± 2.2, and −6.3 ± 4.7 mV, respectively). The 5-HT2B/2C antagonist SB206553 (5 μM) significantly inhibited the facilitation of the Ca PIC by α-methyl-5-HT (by –1.77 ± 0.48 nA; Fig. 5, C and D). Whereas the selective 5-HT2B agonist BW723C86 increased the Ca PIC, its mean effect was one half that of the 5-HT2B/2C agonist α-methyl-5-HT (see above), and subsequent application of the 5-HT2B/2C agonist α-methyl-5-HT (0.3 μM) after BW723C86 further significantly facilitated the Ca PIC (PIC increased by −0.43 ± 0.55 nA; and Von lowered by −3.3 ± 3.7 mV, n = 9; Fig. 6), suggesting involvement of both 5-HT2B and 5-HT2C receptors. The agonists 8-OH-DPAT (3 μM) and zolmitriptan (1 μM) had no significant effects on motoneuron properties (Ca PIC unaffected; n = 5, P > 0.05), confirming that 5-HT1A/1B/1D/1F, 5-HT5, and 5-HT7 receptors have no influence on the Ca PIC. Together these results verify that both 5-HT2B and 5-HT2C receptors are involved in facilitating Ca PIC in motoneurons, whereas other 5-HT receptors are not likely involved.
Fig. 6.
5-HT2B receptor activation alone facilitates the Ca PIC on motoneurons. A: Ca PIC recorded in motoneuron of chronic spinal rat, quantified at arrow, as detailed in Fig. 5 (in TTX, 2 μM). B: the moderately selective 5-HT2B receptor agonist BW723C86 (1 μM) increased the size of the Ca PIC and lowered its onset voltage.
The 5-HT2 receptor agonists α-methyl-5-HT, DOI, and BW723C86 did not significantly change the resting potential (changes: 2.4 ± 9.2, −3.2 ± 8.0, and −1.2 ± 6.1 mV, respectively), consistent with previous reports that resting membrane potential is increased by 5-HT via a non-5-HT2 receptor (Harvey et al. 2006a). The input resistance was significantly increased by these 5-HT2 receptor agonists (by 2.7 ± 2.4, 0.79 ± 0.89, and 0.85 ± 1.15 MΩ, corresponding to 69, 21, and 18% increases, respectively).
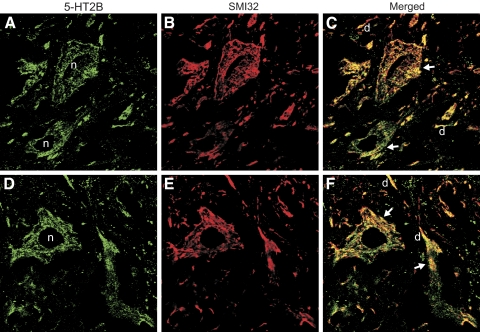
5-HT2B receptors are seen on motoneurons with immunolabeling
Considering that 5-HT2B and 5-HT2C receptor agonists increase the Ca PIC in the presence of TTX, it is likely that motoneurons of chronic spinal rats have both these receptors. Previously, we confirmed the presence of 5-HT2C receptors on these motoneurons using immunolabeling (Murray et al. 2010), and here we repeat this for 5-HT2B receptors (Fig. 7). Motoneurons were labeled with an antibody to a type of neurofilament H (SMI32; Fig. 7, red) that we have previously shown labels motoneurons and not interneurons in the ventral horn (Anelli et al. 2007). Double labeling with a 5-HT2B receptor antibody (green) showed a high degree of colocalization with SMI32 (yellow and orange), indicating the presence of this receptor on motoneurons, in all chronic spinal (n = 6/6; Fig 7, A–C). As with the 5-HT2C receptor (Murray et al. 2010), a large fraction of the 5-HT2B receptor labeling was intracellular to the motoneurons (perinuclear), consistent with the tendency for constitutively active receptor isoforms to become internalized (Chanrion et al. 2008). Similar 5-HT2B labeling was seen on SMI32-labeled motoneurons in normal rats (n = 7/7 rats; Fig. 7, D–F). However, we did not try to quantify the difference between the labeling in normal and injured rats, because cords of injured rats generally perfuse less well with PFA, and this affects the antibody staining.
Fig. 7.
5-HT2B receptor localization on motoneurons of the rat sacrocaudal spinal cord. A: typical immunofluorescence labeling of 5-HT2B receptors (green) in the S4 sacral ventral horn of a chronic spinal rat, showing extensive receptor distribution, including labeling of the soma of motoneurons (with nuclei labeled with n), as visualized with 0.14-μm thin optical sections with confocal microscopy (n = 6). B: double-labeling of the same tissue with SMI32 (red), a selective marker of motoneurons in the ventral horn, which specifically labels neurofilament in the intracellular space. C: an overlay of 5-HT2B and SMI32-labeling shows extensive co-localization (yellow and orange), indicating 5-HT2B receptors on motoneuron soma (arrow) and dendrites (d). D–F: typical immunolabeling of 5-HT2B receptors SMI32 in the sacral ventral horn (below injury) of normal rats (n = 7 rats). Note again the 5-HT2B receptors on motoneurons, with yellow/orange co-localization with SMI32.
DISCUSSION
Our results showed two novel concepts: first, the 5-HT2B receptor modulates motoneuron excitability (Ca PICs) after SCI, and second, the 5-HT2B receptor plays an essential role in the spontaneous recovery of motoneuron function after SCI, by exhibiting constitutive receptor activity. Constitutive 5-HT receptor activity has previously been shown in the CNS for 5-HT2A (Harvey et al. 1999) and 5-HT2C (De Deurwaerdere et al. 2004; Murray et al. 2010) receptors but not previously for 5-HT2B receptors. The 5-HT2B and 5-HT2C receptors that we describe are on the motoneurons themselves, because receptor agonists increased the Ca PICs when synaptic transmission was blocked with TTX. In addition, immunolabeling confirms 5-HT2B (Fig. 7) and 5-HT2C (Murray et al. 2010) receptors on motoneurons in our preparation.
Considering that the Ca PIC is known to cause long-lasting motoneuron discharges evoked by brief stimuli (LLRs) (Li et al. 2004a,b), we used these LLRs as an assay of the Ca PIC to efficiently estimate the dose–response relations for the Ca PIC/LLR for numerous drugs and to perform a thorough analysis of all 5-HT receptors, ruling out the involvement of all but the 5-HT2B and 5-HT2C receptors. We do not know why other receptors, including 5-HT1A, 5-HT2A, 5-HT3, and 5-HT7, do not facilitate the LLR, considering that these receptors are present in the spinal cord (Hochman et al. 2001; Millan 2002; Schmidt and Jordan 2000) and have previously been suggested to have multiple actions on motoneurons, reflexes, or locomotion (Dougherty et al. 2005; Hammar et al. 2007; Holohean et al. 1995; Shay et al. 2005; Ziskind-Conhaim et al. 1993) (see Introduction). The relatively selective 5-HT3 receptor agonist 2-methyl-5-HT (3-fold selectivity in Table 1) (Alexander et al. 2004) and 5-HT2A receptor agonist DOI (10-fold selectivity over other 5-HT2 receptors; Table 1) did increase the LLR, but both these drugs worked at doses too high to be consistent with binding to these receptors and instead acted by their lower affinity binding to the 5-HT2B receptor (Table 3; relative potency near –1) (Wainscott et al. 1993). This underscores the importance of considering the nonselective lower affinity binding of putative agonists, with quantitative comparisons to receptor binding affinity, which requires testing agonists at multiple doses and comparing across multiple agonists. Finally, in this study, we only examined the 5-HT receptors that facilitate the LLR and associated PIC; in a separate paper, we examine the receptors that inhibit sensory afferent transmission in the LLR pathway, which include the 5-HT1B receptor (K. C. Murray, unpublished data).
We did not expect a prominent role of the 5-HT2B receptor, which is a relatively obscure receptor, first described in the stomach fundus and not widely expressed in the brain (Barnes and Sharp 1999; Hoyer et al. 2002; Nichols and Nichols 2008; Wainscott et al. 1993). In the brain (and heart), this receptor is mainly associated with developmental plasticity and pathology (knockouts of the 5-HT2B receptor gene is lethal, unlike for other 5-HT receptors) (Barnes and Sharp 1999; Nichols and Nichols 2008). Outside of reports of 5-HT2B receptors modulating frog N-methyl-d-aspartate (NMDA) receptor function on spinal motoneurons (Hackman and Holohean 2010; Holohean and Hackman 2004), little is known about the role of this receptor in the adult spinal cord, even though it is present in the spinal cord (Hochman et al. 2001; Holohean et al. 1992; Millan 2002). Interestingly, 5-HT2B receptor gene expression (mRNA) appears in rat sacral motoneurons, and this expression persists unchanged with long-term SCI (Wienecke et al. 2010). Considering its novelty, we used several approaches to prove the presence and functionality of the 5-HT2B receptor, including showing that 1) 5-HT2B agonists consistently facilitated motoneuron excitability (LLR and PICs), even when they were made highly selective by a prior block of other receptors known to be activated by the agonists (e.g., 5-HT2C and 5-HT2A receptors for DOI), 2) selective 5-HT2B receptor antagonists overcame the agonist action, when given after a low dose of the agonist titrated to just activate 5-HT2 receptors, 3) agonist action was highly correlated with 5-HT2B binding affinity for the agonists at the 5-HT2B receptor and indeed agonist potency could be quantitatively predicted from binding affinity, and 4) 5-HT2B receptors are on the motoneurons (immunolabeling). The same methods were used to show that the 5-HT2C receptor was also present and functional. In contrast, 5-HT2A receptors were not functional in modulating the PICs, contrary to our previous erroneous conclusion that they modulated the PICs, based on data from the nonselective agonist DOI (Harvey et al. 2006a). The 5-HT2A receptors likely have other functions on motoneurons, because they are on motoneurons and are upregulated with injury (Kong et al. 2010). Interestingly, both 5-HT2B and 5-HT2C receptors seem to have equally large effects, based on the action of selective agonists and antagonists.
An advantage of studying the action of 5-HT receptor agonists after chronic spinal transection or severe contusion is that 5-HT terminals and associated high-affinity serotonin reuptake transporters (SERTs) are mostly eliminated in the spinal cord by the injury (Hayashi et al. 2010; Murray et al. 2010; Newton and Hamill 1988), so they cannot interfere with the action of exogenously applied 5-HT or other agonists [although there may remain some minor low-affinity plasma membrane monoamine transport (PMAT)] (Engel et al. 2004). SERT is almost exclusively located on 5-HT neurons in the brain (explaining its loss with injury), and it regulates the amount of 5-HT in the synaptic cleft (Blakely et al. 1994). Accordingly, SERT dramatically affects the dose of exogenously applied 5-HT needed to activate receptors in the synapse, and serotonin reuptake inhibitors that block SERT (citalopram) decrease the dose of 5-HT or other 5-HT agonists needed to facilitate motoneuron function by a factor of 30 (Elliott and Wallis 1992) (Bennett and Murray, unpublished data). Thus the lack of synaptic SERT in the chronic spinal rat allows 5-HT to reach receptors unimpeded, and our estimates of drug potency (and EC50) therefore are much more accurate. Indeed, our previous work has shown that the in vitro dose of 5-HT needed to affect motoneurons in spinal cords from normal rats is dramatically higher (by a factor of 30; 10 vs. 0.3 μM) than that in chronic spinal rats (Harvey et al. 2006a). We suggest that this supersensitivity in chronic spinal rats is largely caused by the absence of SERT in chronic spinal rats, whereas in normal rats, SERT efficiently prevents much of the exogenously applied 5-HT from reaching the 5-HT receptors. This lack of SERT after injury may also explain the supersensitity seen to other 5-HT receptors agonists (Harvey et al. 2006a), because SERT is rather promiscuous, transporting tryptamine-like compounds (Adkins et al. 2001; Henry et al. 2006), including many common agonists that we use, such as α-methyl-5-HT, 2-Methyl-5-HT, 5-HT, 5-CT, and tryptamine (Adkins et al. 2001; Helfman et al. 1995; Henry et al. 2006) (α-methyl-5-HT is abbreviated α-MST in Henry et al. 2006). In summary, our quantitative analysis of 5-HT receptor agonist potency and correlation to receptor binding affinity would not have been possible had we not been studying the fully transected rat that lacks SERT, because SERT interferes with agonists reaching the receptors in the normal spinal cord, effectively making the normal spinal cord much less sensitive to 5-HT agonists compared with the spinal cord of chronic spinal rats.
We found a remarkably consistent relation between agonist potency (–logEC50) and receptor binding affinity (–logKi). Basically, effective agonist doses (EC50) were consistently only about 10 times the binding Ki values of that agonist to 5-HT2B receptors. Receptor binding affinities are typically measured in homogenized membranes from isolated cell culture systems where agonists are applied directly to the receptors (Boess and Martin 1994), whereas in our whole spinal cord preparations, agonists must diffuse substantial distances to reach receptors (see results). Likely, this diffusion barrier in part helps explain the factor of 10 that relates EC50 to Ki values. Even disregarding the pia, diffusion in brain and spinal cord tissue is somewhat restricted, where diffusion coefficients for small molecules are 4 times lower than in saline (D* = 5 × 10−7 cm2/s)(Nicholson and Tao 1993; Sykova and Nicholson 2008), because of the convoluted and small extracellular space (only 20% of total volume) (Sykova and Nicholson 2008). Considering that the motoneuron cell bodies in the S4–S3 sacral spinal cord are about 0.25 mm below the surface and that we waited about 10 min for drugs to diffuse into the cord before recording, the diffusion equation indicates that the drug concentration at the motoneuron cell bodies should be only about 0.31 times the applied ACSF concentration (assuming D* = 5 × 10−7 cm2/s, and 1D diffusion paths) (using Eq. 9 in Sykova and Nicholson 2008). If we also consider that the 5-HT receptors may be on motoneuron dendrites that can be even deeper in the tissue (say 0.4 mm), the concentration at these deep dendrites should be about 1/10th the applied surface concentration. In summary, diffusion can contribute substantially to our factor of 10 difference between EC50 and Ki. Interestingly, had we waited longer times between the drug application and recording, the drug concentrations in the cord would have been higher, and the EC50 and Ki would have been closer, although computing the EC50 would have been difficult because of slow rundown of reflexes over the hours needed to construct such a dose–response relation.
Other factors like receptor reserve (pool of receptors) and downstream G protein signaling (Boess and Martin 1994) may also contribute to the higher EC50 values (lower potency) in our system. Additionally, we compared our EC50 values to Ki values obtained from competitive binding between our agonists and radiolabeled agonists (e.g., [3H]-5-HT; see methods). This generally labels a high affinity binding state that occurs in response to receptor activation. Had we instead compared with the low affinity binding obtained with radiolabeled antagonists, which do not activate the receptor (Egan et al. 2000; Knight et al. 2004), the EC50 and Ki might have been closer.
Assuming that diffusion is the predominant factor in increasing our observed EC50 and that diffusion affects all 5-HT receptor activation similarly (similar receptor depths), our observed factor-of-10 relation between EC50 and Ki may ultimately help us predict doses of agonist and antagonists needed to effectively activate any particular 5-HT receptor. Indeed, we used this to choose doses of antagonists (Table 4) and independently verified those doses by their action alone or against an agonist. We also used this simple relation to help rule out the involvement of receptors other than 5-HT2B and 5-HT2C; these other receptors had EC50/Ki ratios far from 10. With this method and direct use of agonists to other receptors (8-OH-DPAT, etc.), it is clear that no other 5-HT receptor is involved in modulating the Ca PIC and associated LLR. Different agonists have variable functional efficacy (partial or full agonists) (Kenakin 1996), but we avoided this issue by focusing on binding affinity and potency rather than efficacy. However, a caveat that must be noted is that we do not know for certain that all drugs that bind to a receptor act as an agonist at that receptor, and indeed, compounds like 8-OH-DPAT have poor efficacy (partial agonists) at some receptors to which they bind (e.g., 5-HT7 for 8-OH-DPAT) (Eglen et al. 1997); thus we also tested more effective agonists to these receptors (LP44).
Both 5-HT2B and 5-HT2C receptors are Gq protein–coupled receptors that generally activate the classic PLC (phospholipase C) pathways involved in the synthesis of inositol phosphates (IP) and mobilization of intracellular Ca2+ stores (Hoyer et al. 2002; Kenakin 1996; Lucaites et al. 1996; Mizuno and Itoh 2009). These pathways facilitate PICs (and NMDA receptors) in spinal motoneurons (Holohean and Hackman 2004; Mejia-Gervacio et al. 2004; Perrier et al. 2000). Thus it is likely that the 5-HT2B and 5-HT2C receptors modulate the PICs via IP pathways in our preparation. Both these receptor types are known to exhibit a substantial degree of constitutive activity (Seifert and Wenzel-Seifert 2002; Villazon et al. 2003; Weiner et al. 2001), usually observed in the form of IP production in the absence of 5-HT (or other agonists), which produces a basal level of IP (Chanrion et al. 2008; Kennett et al. 1997b; Knight et al. 2004). Thus, PICs may in part be facilitated by this basal IP production (without 5-HT), and this may explain the emergence of the large PICs seen after SCI when there is an absence of 5-HT, as we discuss below. Importantly, the subclass of antagonists known as inverse agonists inhibit this basal IP production (or associated functions) in 5-HT2B and 5-HT2C receptors, including classic antagonists such as cyproheptadine, clozapine, and ketanserin (Chanrion et al. 2008; Villazon et al. 2003; Weiner et al. 2001; Westphal and Sanders-Bush 1994). In contrast, neutral antagonists like methysergide and RS127445 have no effect when given alone (Bonhaus et al. 1999; Chanrion et al. 2008; Westphal and Sanders-Bush 1994). Therefore, a combination of these drugs is useful in identifying constitutive activity. Both inverse agonists and neutral antagonists block the action of agonists, and thus the action of an inverse agonist alone is not sufficient to prove the presence of constitutive receptor activity, if there is any chance that the receptor is activated by 5-HT (e.g., residual 5-HT in the spinal cord). Definitive proof of constitutive activity only comes by also showing that neutral antagonists have no effect (as in Table 4) (Seifert and Wenzel-Seifert 2002).
Brain stem–derived 5-HT normally serves a critical role in tuning the excitability of motoneurons, facilitating Ca PICs that amplify and prolong responses to synaptic input (Heckman et al. 2005). Thus, when SCI eliminates this major source of 5-HT, motoneurons are initially left in a depressed state and are not able to produce adequate muscle contractions. However, over the weeks after injury, Ca PICs in motoneurons spontaneously recover, to the point where they are permanently large, and not only help with residual motor functions but also contribute to unwanted contractions (muscle spasms) (Bennett et al. 2004; Li et al. 2004a). Recently, we showed that a novel form of plasticity in 5-HT2 receptors causes this recovery of Ca PICs: these receptors become spontaneously active in the absence of 5-HT (constitutively active) (Murray et al. 2010). However, at the time, we were uncertain which particular 5-HT2 receptor modulates the Ca PIC. The present results resolve this issue, showing that both 5-HT2B and 5-HT2C receptors are constitutively active after SCI, because selective 5-HT2B and 5-HT2C inverse agonists that block this constitutive activity decrease motoneuron excitability (LLRs and associated Ca PICs), whereas neutral antagonists do not. As discussed above, the lack of effect of neutral antagonists shows that there is no residual 5-HT below the chronic injury that activates the 5-HT2 receptors. Thus, even though the inverse agonists we tested can block the action of 5-HT, in addition to blocking constitutive receptor activity, their action can only be attributed to constitutive 5-HT2B and 5-HT2C receptor activity. Considering that most known antagonists can act as inverse agonists at 5-HT2B and 5-HT2C receptors in various model systems, whereas neutral antagonists are rare (Weiner et al. 2001; Westphal and Sanders-Bush 1994), we were not surprised that the selective 5-HT2B and 5-HT2C receptor antagonists (SB204741 and RS102221, respectively) could act as inverse agonists in inhibiting the LLR. However, we had not anticipated such prominent constitutive activity in 5-HT2B receptors, as shown by these compounds, because constitutive activity in this receptor has not previously been shown in the CNS, although it has been seen in the stomach fundus (Villazon et al. 2003).
The constitutive 5-HT2 receptor activity that we observed after SCI arises from at least two mechanisms. First, there is an increase in expression of 5-HT2C receptor isoforms that exhibit a high degree of constitutive activity (Murray et al. 2010). Most 5-HT2C receptor isoforms have some probability of being constitutively active, but certain isoforms, like the INI isoform, have a very high degree of constitutive activity (with activity approaching that produced by maximal activation with 5-HT) (Berg et al. 2008; Herrick-Davis et al. 1999; Marion et al. 2004; Niswender et al. 1999; Weiner et al. 2001), and these isoforms increase in expression with SCI (Murray et al. 2010). Second, the total number of 5-HT2C receptors increases with SCI (Hayashi et al. 2010), which increases overall constitutive activity because all isoforms exhibit some constitutive activity (Berg et al. 2008; Herrick-Davis et al. 1999). We do not know whether the 5-HT2B receptor isoform types or total receptor numbers change with injury, although we do know that the 5-HT2B receptor gene expression is unchanged with long-term SCI (Wienecke et al. 2010).
In summary, we performed a systematic analysis of the 5-HT receptors that facilitate motoneuron PICs and associated LLRs (spasms) after SCI and found that both 5-HT2B and 5-HT2C receptors are involved, whereas 5-HT1, 5-HT2A, and 5-HT3/4/5/6/7 receptors are not. Although both 5-HT2B and 5-HT2C receptors are present before injury, they exhibit dramatic plasticity after chronic spinal transection, presumably as a compensation for the near-complete loss of 5-HT innervation. That is, both receptors become constitutively active, producing large Ca PICs in the absence of 5-HT (whereas acutely after injury, PICs are small). These large Ca PICs are essential for the recovery of normal motoneuron firing ability after chronic injury (sustained firing and amplification of synaptic input) (Harvey et al. 2006c; Heckman et al. 2005) and thus must play an important role in all motor recovery, including locomotion (Murray et al. 2010). However, unlike with normal brain stem control over receptor activation, these receptors are permanently active, leading to permanently large PICs and ultimately hyperexcitability of motoneurons associated with muscle spasms. Thus controlling excess constitutive activity in 5-HT2 receptors, especially 5-HT2B receptors, offers a new approach in managing spasticity after SCI.
GRANTS
Funding was provided by National Institute of Neurological Disorders and Stroke Grant NS-47567, Canadian Foundation for Innovation, the Canadian Institutes of Health Research, and the Alberta Heritage Foundation for Medical Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank L. Sanelli for expert technical assistance.
REFERENCES
- Adham N, Gerald C, Schechter L, Vaysse P, Weinshank R, Branchek T. [3H]5-hydroxytryptamine labels the agonist high affinity state of the cloned rat 5-HT4 receptor. Eur J Pharmacol 304: 231–235, 1996 [DOI] [PubMed] [Google Scholar]
- Adkins EM, Barker EL, Blakely RD. Interactions of tryptamine derivatives with serotonin transporter species variants implicate transmembrane domain I in substrate recognition. Mol Pharmacol 59: 514–523, 2001 [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels. Br J Pharmacol 141 Suppl. 1: S1–S126, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA. Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol Ther 121: 160–173, 2009 [DOI] [PubMed] [Google Scholar]
- Anelli R, Sanelli L, Bennett DJ, Heckman CJ. Expression of L-type calcium channel alpha(1)-1.2 and alpha(1)-1.3 subunits on rat sacral motoneurons following chronic spinal cord injury. Neuroscience 145: 751–763, 2007 [DOI] [PubMed] [Google Scholar]
- Baker LL, Chandler SH. Characterization of postsynaptic potentials evoked by sural nerve stimulation in hindlimb motoneurons from acute and chronic spinal cats. Brain Res 420: 340–350, 1987 [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152, 1999 [DOI] [PubMed] [Google Scholar]
- Baxter GS. Novel discriminatory ligands for 5-HT2B receptors. Behav Brain Res 73: 149–152, 1996 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma 16: 69–84, 1999 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke C, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol 91: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Berg KA, Dunlop J, Sanchez T, Silva M, Clarke WP. A conservative, single-amino acid substitution in the second cytoplasmic domain of the human Serotonin2C receptor alters both ligand-dependent and -independent receptor signaling. J Pharmacol Exp Ther 324: 1084–1092, 2008 [DOI] [PubMed] [Google Scholar]
- Berg KA, Stout BD, Cropper JD, Maayani S, Clarke WP. Novel actions of inverse agonists on 5-HT2C receptor systems. Mol Pharmacol 55: 863–872, 1999 [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol 196: 263–281, 1994 [DOI] [PubMed] [Google Scholar]
- Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology 33: 275–317, 1994 [DOI] [PubMed] [Google Scholar]
- Boess FG, Monsma FJ, Jr, Carolo C, Meyer V, Rudler A, Zwingelstein C, Sleight AJ. Functional and radioligand binding characterization of rat 5-HT6 receptors stably expressed in HEK293 cells. Neuropharmacology 36: 713–720, 1997 [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Flippin LA, Greenhouse RJ, Jaime S, Rocha C, Dawson M, Van Natta K, Chang LK, Pulido-Rios T, Webber A, Leung E, Eglen RM, Martin GR. RS-127445: a selective, high affinity, orally bioavailable 5-HT2B receptor antagonist. Br J Pharmacol 127: 1075–1082, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button DC, Kalmar JM, Gardiner K, Marqueste T, Zhong H, Roy RR, Edgerton VR, Gardiner PF. Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection? J Physiol 586: 529–544, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanrion B, Mannoury la Cour C, Gavarini S, Seimandi M, Vincent L, Pujol JF, Bockaert J, Marin P, Millan MJ. Inverse agonist and neutral antagonist actions of antidepressants at recombinant and native 5-hydroxytryptamine2C receptors: differential modulation of cell surface expression and signal transduction. Mol Pharmacol 73: 748–757, 2008 [DOI] [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Quentric Y, Carpentier N, Poissonnet G, Parmentier JG, Goldstein S, Millan MJ. Characterization of phospholipase C activity at h5-HT2C compared with h5-HT2B receptors: influence of novel ligands upon membrane-bound levels of [3H]phosphatidylinositols. Naunyn Schmiedebergs Arch Pharmacol 365: 242–252, 2002 [DOI] [PubMed] [Google Scholar]
- Dario A, Tomei G. A benefit-risk assessment of baclofen in severe spinal spasticity. Drug Saf 27: 799–818, 2004 [DOI] [PubMed] [Google Scholar]
- De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24: 3235–3241, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KJ, Bannatyne BA, Jankowska E, Krutki P, Maxwell DJ. Membrane receptors involved in modulation of responses of spinal dorsal horn interneurons evoked by feline group II muscle afferents. J Neurosci 25: 584–593, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors. Synapse 35: 144–150, 2000 [DOI] [PubMed] [Google Scholar]
- Eglen RM, Jasper JR, Chang DJ, Martin GR. The 5-HT7 receptor: orphan found. Trends Pharmacol Sci 18: 104–107, 1997 [DOI] [PubMed] [Google Scholar]
- Elliott P, Wallis DI. Serotonin and L-norepinephrine as mediators of altered excitability in neonatal rat motoneurons studied in vitro. Neuroscience 47: 533–544, 1992 [DOI] [PubMed] [Google Scholar]
- Engel G, Gothert M, Hoyer D, Schlicker E, Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol 332: 1–7, 1986 [DOI] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem 279: 50042–50049, 2004 [DOI] [PubMed] [Google Scholar]
- Glennon RA, Ismaiel AE, McCarthy BG, Peroutka SJ. Binding of arylpiperazines to 5-HT3 serotonin receptors: results of a structure-affinity study. Eur J Pharmacol 168: 387–392, 1989 [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Hackman JC, Holohean AM. The effects of polyamine agonists and antagonists on N-methyl-D-aspartate-induced depolarizations of amphibian motoneurons in situ. Brain Res 1325: 10–18, 2010 [DOI] [PubMed] [Google Scholar]
- Hammar I, Stecina K, Jankowska E. Differential modulation by monoamine membrane receptor agonists of reticulospinal input to lamina VIII feline spinal commissural interneurons. Eur J Neurosci 26: 1205–1212, 2007 [DOI] [PubMed] [Google Scholar]
- Harvey JA, Welsh SE, Hood H, Romano AG. Effect of 5-HT2 receptor antagonists on a cranial nerve reflex in the rabbit: evidence for inverse agonism. Psychopharmacology (Berl) 141: 162–168, 1999 [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96: 1171–1186, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Jacob-Vadakot S, Dugan EA, McBride S, Olexa R, Simansky K, Murray M, Shumsky JS. 5-HT precursor loading, but not 5-HT receptor agonists, increases motor function after spinal cord contusion in adult rats. Exp Neurol 221: 68–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005 [DOI] [PubMed] [Google Scholar]
- Helfman CC, Anderson GF, Barraco RA. Presynaptic displacement of serotonin by alpha-methyl-5-hydroxytryptamine in the nucleus tractus solitarius of the rat. Neurosci Lett 189: 65–68, 1995 [DOI] [PubMed] [Google Scholar]
- Henry LK, Field JR, Adkins EM, Parnas ML, Vaughan RA, Zou MF, Newman AH, Blakely RD. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem 281: 2012–2023, 2006 [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Niswender CM. Serotonin 5-HT2C receptor RNA editing alters receptor basal activity: implications for serotonergic signal transduction. J Neurochem 73: 1711–1717, 1999 [DOI] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Machacek DW, Shay BL. 5-HT receptors and the neuromodulatory control of spinal cord function. In: Neurobiology of the Spinal Cord, edited by Cope T. Washington, DC: CRC, 2001, p. 47–87 [Google Scholar]
- Holohean AM, Hackman JC. Mechanisms intrinsic to 5-HT2B receptor-induced potentiation of NMDA receptor responses in frog motoneurones. Br J Pharmacol 143: 351–360, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holohean AM, Hackman JC, Davidoff RA. Changes in membrane potential of frog motoneurons induced by activation of serotonin receptor subtypes. Neuroscience 34: 555–564, 1990 [DOI] [PubMed] [Google Scholar]
- Holohean AM, Hackman JC, Davidoff RA. Modulation of frog spinal cord interneuronal activity by activation of 5-HT3 receptors. Brain Res 704: 184–190, 1995 [DOI] [PubMed] [Google Scholar]
- Holohean AM, Hackman JC, Shope SB, Davidoff RA. Serotonin1A facilitation of frog motoneuron responses to afferent stimuli and to N-methyl-D-aspartate. Neuroscience 48: 469–477, 1992 [DOI] [PubMed] [Google Scholar]
- Holstege JC, Bongers CM. A glycinergic projection from the ventromedial lower brainstem to spinal motoneurons. An ultrastructural double labeling study in rat. Brain Res 566: 308–315, 1991 [DOI] [PubMed] [Google Scholar]
- Hoyer D, Engel G, Kalkman HO. Molecular pharmacology of 5-HT1 and 5-HT2 recognition sites in rat and pig brain membranes: radioligand binding studies with [3H]5-HT, [3H]8-OH-DPAT, (-)[125I]iodocyanopindolol, [3H]mesulergine and [3H]ketanserin. Eur J Pharmacol 118: 13–23, 1985 [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71: 533–554, 2002 [DOI] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res 143: 77–95, 2004 [DOI] [PubMed] [Google Scholar]
- Inoue T, Itoh S, Wakisaka S, Ogawa S, Saito M, Morimoto T. Involvement of 5-HT7 receptors in serotonergic effects on spike afterpotentials in presumed jaw-closing motoneurons of rats. Brain Res 954: 202–211, 2002 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I. Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Res Brain Res Rev 40: 19–28, 2002 [DOI] [PubMed] [Google Scholar]
- Kawamura J, Ise M, Tagami M. The clinical features of spasms in patients with a cervical cord injury. Paraplegia 27: 222–226, 1989 [DOI] [PubMed] [Google Scholar]
- Kenakin T. The classification of seven transmembrane receptors in recombinant expression systems. Pharmacol Rev 48: 413–463, 1996 [PubMed] [Google Scholar]
- Kennett GA, Ainsworth K, Trail B, Blackburn TP. BW 723C86, a 5-HT2B receptor agonist, causes hyperphagia and reduced grooming in rats. Neuropharmacology 36: 233–239, 1997a [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 36: 609–620, 1997b [DOI] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol 370: 114–123, 2004 [DOI] [PubMed] [Google Scholar]
- Kong XY, Wienecke J, Hultborn H, Zhang M. Robust upregulation of serotonin 2A receptors after chronic spinal transection of rats: an immunohistochemical study. Brain Res 1320: 60–68, 2010 [DOI] [PubMed] [Google Scholar]
- Kuhn RA, Macht MB. Some manifestations of reflex activity in spinal man with particular reference to the occurrence of extensor spasm. Bull Johns Hopkins Hosp 84: 43–75, 1948 [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. Modulation of IH by 5-HT in neonatal rat motoneurones in vitro: mediation through a phosphorylation independent action of cAMP. Neuropharmacology 36: 721–733, 1997 [DOI] [PubMed] [Google Scholar]
- Leopoldo M, Lacivita E, Contino M, Colabufo NA, Berardi F, Perrone R. Structure-activity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides, a class of 5-HT7 receptor agents. II. J Med Chem 50: 4214–4221, 2007 [DOI] [PubMed] [Google Scholar]
- Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol 97: 3314–3330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 97: 1236–1246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004a [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes in the chronic spinal rat, studied in vitro. J Neurophysiol 91: 2236–2246, 2004b [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol 92: 2694–2703, 2004c [DOI] [PubMed] [Google Scholar]
- Lucaites VL, Nelson DL, Wainscott DB, Baez M. Receptor subtype and density determine the coupling repertoire of the 5-HT2 receptor subfamily. Life Sci 59: 1081–1095, 1996 [DOI] [PubMed] [Google Scholar]
- Lundberg A. Inhibitory control from the brainstem of transmission from primary afferents to motoneurons, primary afferent terminals and ascending pathways. In: Brainstem Control of Spinal Mechanisms, edited by Sjolund B, Bjorklund A. New York: Elsevier, 1982, p. 179–224 [Google Scholar]
- Marion S, Weiner DM, Caron MG. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J Biol Chem 279: 2945–2954, 2004 [DOI] [PubMed] [Google Scholar]
- Martin GR, Robertson AD, MacLennan SJ, Prentice DJ, Barrett VJ, Buckingham J, Honey AC, Giles H, Moncada S. Receptor specificity and trigemino-vascular inhibitory actions of a novel 5-HT1B/1D receptor partial agonist, 311C90 (zolmitriptan). Br J Pharmacol 121: 157–164, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson C, Sonesson C, Sandahl A, Greiner HE, Gassen M, Plaschke J, Leibrock J, Bottcher H. 2-Alkyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indoles as novel 5-HT6 receptor agonists. Bioorg Med Chem Lett 15: 4230–4234, 2005 [DOI] [PubMed] [Google Scholar]
- Maynard FM, Karunas RS, Waring WP., III Epidemiology of spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil 71: 566–569, 1990 [PubMed] [Google Scholar]
- Mejia-Gervacio S, Hounsgaard J, Diaz-Munoz M. Roles of ryanodine and inositol triphosphate receptors in regulation of plateau potentials in turtle spinal motoneurons. Neuroscience 123: 123–130, 2004 [DOI] [PubMed] [Google Scholar]
- Milburn CM, Peroutka SJ. Characterization of [3H]quipazine binding to 5-hydroxytryptamine3 receptors in rat brain membranes. J Neurochem 52: 1787–1792, 1989 [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol 66: 355–474, 2002 [DOI] [PubMed] [Google Scholar]
- Mizuno N, Itoh H. Functions and regulatory mechanisms of Gq-signaling pathways. Neurosignals 17: 42–54, 2009 [DOI] [PubMed] [Google Scholar]
- Moran A, Ortiz de Urbina AV, Martin ML, Garcia M, Rodriguez-Barbero A, Dorado F, San Roman L. Characterization of contractile 5-hydroxytryptamine receptor subtypes in the in situ autoperfused kidney in the anaesthetized rat. Eur J Pharmacol 592: 133–137, 2008 [DOI] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D'Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT(2C) receptors. Nat Med 16: 694–700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton BW, Hamill RW. The morphology and distribution of rat serotoninergic intraspinal neurons: an immunohistochemical study. Brain Res Bull 20: 349–360, 1988 [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. Serotonin receptors. Chem Rev 108: 1614–1641, 2008 [DOI] [PubMed] [Google Scholar]
- Nicholson C, Tao L. Hindered diffusion of high molecular weight compounds in brain extracellular microenvironment measured with integrative optical imaging. Biophys J 65: 2277–2290, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity–from a basic science point of view. Acta Physiol (Oxf) 189: 171–180, 2007 [DOI] [PubMed] [Google Scholar]
- Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J Biol Chem 274: 9472–9478, 1999 [DOI] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain 131: 1478–1491, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Alaburda A, Hounsgaard J. 5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle. J Physiol 548: 485–492, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Delgado-Lezama R. Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J Neurosci 25: 7993–7999, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol 89: 954–959, 2003 [DOI] [PubMed] [Google Scholar]
- Perrier JF, Mejia-Gervacio S, Hounsgaard J. Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J Physiol 528: 107–113, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phebus LA, Johnson KW, Zgombick JM, Gilbert PJ, Van Belle K, Mancuso V, Nelson DL, Calligaro DO, Kiefer AD, Jr, Branchek TA, Flaugh ME. Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci 61: 2117–2126, 1997 [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 80: 767–852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53: 689–710, 2000 [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 366: 381–416, 2002 [DOI] [PubMed] [Google Scholar]
- Shay BL, Sawchuk M, Machacek DW, Hochman S. Serotonin 5-HT2 receptors induce a long-lasting facilitation of spinal reflexes independent of ionotropic receptor activity. J Neurophysiol 94: 2867–2877, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steg G. Efferent muscle innervation and rigidity. Acta Physiol Scand Suppl 225, 61: 1–53, 1964 [PubMed] [Google Scholar]
- Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev 88: 1277–1340, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros SF, D'Souza M, Zettel ML, Zhu X, Lynch-Erhardt M, Frisina RD. Serotonin 2B receptor: upregulated with age and hearing loss in mouse auditory system. Neurobiol Aging 28: 1112–1123, 2007 [DOI] [PubMed] [Google Scholar]
- Vanover KE, Harvey SC, Son T, Bradley SR, Kold H, Makhay M, Veinbergs I, Spalding TA, Weiner DM, Andersson CM, Tolf BR, Brann MR, Hacksell U, Davis RE. Pharmacological characterization of AC-90179 [2-(4-methoxyphenyl)-N-(4-methyl-benzyl)-N-(1-methyl-piperidin-4-yl)-aceta mide hydrochloride]: a selective serotonin 2A receptor inverse agonist. J Pharmacol Exp Ther 310: 943–951, 2004 [DOI] [PubMed] [Google Scholar]
- Villazon M, Enguix MJ, Tristan H, Honrubia MA, Brea J, Maayani S, Cadavid MI, Loza MI. Different pharmacological properties of two equipotent antagonists (clozapine and rauwolscine) for 5-HT2B receptors in rat stomach fundus. Biochem Pharmacol 66: 927–937, 2003 [DOI] [PubMed] [Google Scholar]
- Wainscott DB, Cohen ML, Schenck KW, Audia JE, Nissen JS, Baez M, Kursar JD, Lucaites VL, Nelson DL. Pharmacological characteristics of the newly cloned rat 5-hydroxytryptamine2F receptor. Mol Pharmacol 43: 419–426, 1993 [PubMed] [Google Scholar]
- Wang MY, Dun NJ. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol 430: 87–103, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, Spalding TA, Gibson DF, Krebs-Thomson K, Powell SB, Geyer MA, Hacksell U, Brann MR. 5-Hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther 299: 268–276, 2001 [PubMed] [Google Scholar]
- Westphal RS, Sanders-Bush E. Reciprocal binding properties of 5-hydroxytryptamine type 2C receptor agonists and inverse agonists. Mol Pharmacol 46: 937–942, 1994 [PubMed] [Google Scholar]
- Wienecke J, Westerdahl AC, Hultborn H, Kiehn O, Ryge J. Global gene expression analysis of rodent motor neurons following spinal cord injury associates molecular mechanisms with development of postinjury spasticity. J Neurophysiol 103: 761–778, 2010 [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci 101: 107–117, 2006 [DOI] [PubMed] [Google Scholar]
- Yoshio R, Taniguchi T, Itoh H, Muramatsu I. Affinity of serotonin receptor antagonists and agonists to recombinant and native alpha1-adrenoceptor subtypes. Jpn J Pharmacol 86: 189–195, 2001 [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Seebach BS, Gao BX. Changes in serotonin-induced potentials during spinal cord development. J Neurophysiol 69: 1338–1349, 1993 [DOI] [PubMed] [Google Scholar]